
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr. , 07 April 2020
Sec. Pediatric Immunology
Volume 8 - 2020 | https://doi.org/10.3389/fped.2020.00148
 Yoshihiro Azuma1
Yoshihiro Azuma1 Yasuo Suzuki1*
Yasuo Suzuki1* Seigo Okada1
Seigo Okada1 Chie Matsuguma1
Chie Matsuguma1 Hiroyuki Wakiguchi1
Hiroyuki Wakiguchi1 Yuji Ohnishi1
Yuji Ohnishi1 Takashi Furuta1
Takashi Furuta1 Akiko Miyake1
Akiko Miyake1 Hiroki Yasudo1
Hiroki Yasudo1 Kiyoshi Ichihara2
Kiyoshi Ichihara2 Shouichi Ohga1,3
Shouichi Ohga1,3 Shunji Hasegawa1
Shunji Hasegawa1Objective: Intravenous immunoglobulin (IVIG) therapy is a useful first-line treatment for Kawasaki disease (KD); however, 10–20% of patients fail to respond and require additional IVIG. Soluble CD163 (sCD163) is considered a biomarker for macrophage activation. There are no reports measuring serum sCD163 in KD patients. This study aimed to explore its possible utility in the clinical management of patients with KD.
Methods: Eighty-seven patients with well-defined KD were retrospectively enrolled together with 19 healthy individuals with comparable ages. KD patients were classified into three groups, Group A (initial IVIG responders), Group B (additional IVIG responders), and Group C (additional IVIG non-responders). Serum sCD163 together with complete blood counts, C-reactive protein, d-dimer, albumin, aspartate aminotransferase (AST), and alanine aminotransferase (ALT) were measured before the initial IVIG treatment in all cases, and afterward in a fraction of cases.
Results: Serum sCD163 in KD patients before initial IVIG was generally much higher than the control group. The median (interquartile range) of sCD163 was as follows: Control 446 (385–521) ng/mL; Group A, 699 (478–1,072); Group B, 1,349 (1,116–1,390); and Group C, 665 (544–1,094). In general, sCD163 showed close positive correlation with ALT and AST, but none with other markers. Among the KD groups, Group B showed the highest sCD163: Group B vs. A: p = 0.0003; B vs. C: p = 0.035). Serum sCD163 was significantly increased after IVIG in Group A, while no change occurred in others.
Conclusion: The serum sCD163 levels could be a useful biomarker in the clinical management of KD, especially for predicting responsiveness to IVIG.
Kawasaki disease (KD) is an early childhood acute inflammatory illness characterized by prolonged fever, diffuse mucosal inflammation, indurative edema of the hands and feet, polymorphous skin rash, and non-suppurative lymphadenopathy (1). Although high-dose intravenous immunoglobulin (IVIG, 2 g/kg/dose) with oral aspirin (30 mg/kg/day) is the first-line therapy for patients with KD in Japan as per Japanese guideline for treatment of acute Kawasaki disease (2), 10–20% of patients with KD are resistant to initial IVIG treatment. Coronary artery lesions (CALs) are the most severe complications of KD, and IVIG non-responders have a higher risk of CALs than IVIG responders (3, 4). For predicting the initial IVIG response, several scoring systems including Gunma score, Osaka score, and Kurume score, have been published in Japan (5–7). Nevertheless, the feasibility of these scores varied among races (8). Repeat IVIG therapy for refractory cases has been applied in practice. However, there is little information on the use of a predictive marker for additional IVIG responsiveness. The active mechanisms of IVIG resistance in children with KD are not understood fully.
CD163 is a scavenger receptor for haptoglobin-hemoglobin, expressed on monocytes/macrophages (9). Shedding of CD163 is regulated by a disintegrin and metalloproteinase-17 that mediate shedding of tumor necrosis factor-α, a cytokine produced mainly by activated monocytes/macrophages (10). Reports show sCD163 as a useful biomarker for macrophage activation in several inflammatory diseases, such as sepsis, inflammatory bowel disease, and influenza-associated encephalopathy (11–13). We previously reported an increased number of peripheral CD14+CD16+ cells, activated monocytes/macrophages in acute KD, and activated monocytes/macrophages role in the pathophysiology of KD (14). Therefore, it is of interest to evaluate the pathophysiological implication of sCD163 in KD as a maker of excessive macrophage activation. So far, there has been no primary report that measured serum sCD163 in KD.
This study was conducted to explore clinical utility of sCD163 in the management of KD in comparison with other laboratory tests. From our recent clinical experiences in measuring sCD163 in KD, we had a specific interest in seeking its possible utility in predicting responsiveness to IVIG among KD patients.
The patients were diagnosed with KD following the 5th revision of Diagnostic Guidelines (15). KD was classified into complete or incomplete type. The complete type defined patients fulfilling 5/6 or all six major symptoms or 4/6 major symptoms combined with CAL as confirmed by coronary angiography or two-dimensional echocardiography, during the disease course. Incomplete type defined patients who did not fulfill the diagnostic criteria but suspected with KD, for example, a patient with only 4/6 major symptoms without CAL, and presence of other diseases excluded.
Acute-phase CALs defined as >3 mm expansion of coronary artery diameter in patients younger than 5 years old, or 1.5 times expansion compared with the coronary artery diameter at diagnosis in five or more years of age. If these changes did not normalize over one month of illness, it defined late-phase CALs. However, no patients suffered from the late-phase CALs in this study. We identified the patients as IVIG non-responders, who had a persistent or recrudescent fever (>37.5°C) at 48 h after IVIG infusion and who required additional therapies (16).
This retrospective observational study included 112 patients with KD treated in Yamaguchi University Hospital between January 2005 and December 2015 (Figure 1). Twenty-five patients were excluded because of defervescence after aspirin therapy alone, unavailable acute-phase samples, febrile flare-up after more than 48 h of an initial IVIG, defervescence before additional-IVIG, a complication of encephalopathy, and received pulsed methylprednisolone as additional therapy. We analyzed 87 patients who received IVIG (2 g/kg/dose) with oral aspirin (30 mg/kg/day) as initial therapy. All initial IVIG non-responders received additional-IVIG (2 g/kg/dose) as the second-line therapy. Eleven patients required the third-line therapy (infliximab; 7, cyclosporine; 3, plasma exchange; 1) (Figure 1). Firstly, we dichotomized them into initial IVIG responders and initial IVIG non-responders. Secondarily, to analyze the patients in detail, we classified them into three subgroups. Group A composed of initial IVIG responders. Group B composed of patients who responded to the additional IVIG. Group C composed of patients who did not respond to the repeated IVIGs and required a third-line therapy (additional IVIG non-responders). Control subjects included 19 healthy children without febrile illness.
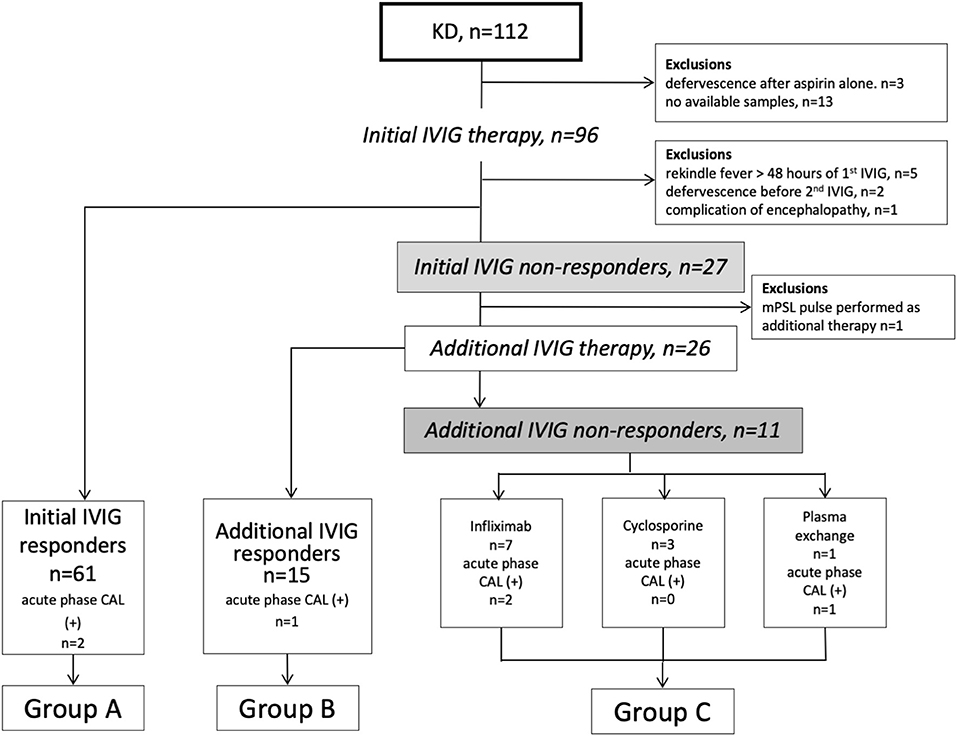
Figure 1. Flow chart of KD patients in this study. KD, Kawasaki disease; IVIG, intravenous immunoglobulin; CAL, coronary arterial lesion; mPSL, methylprednisolone.
We measured serum sCD163 levels in patients during acute-phase of KD and collected other laboratory findings before initial IVIG and clinical data from the medical records at the time of KD diagnosis. Laboratory findings included complete blood count, serum levels of sodium, aspartate aminotransferase (AST), alanine aminotransferase (ALT), C-reactive protein (CRP), albumin, and plasma levels of d-dimer. Serum sCD163 were determined using an sCD163 enzyme-linked immunosorbent assay kit (R&D Systems, Minneapolis, MN, USA) with a detection limit of 1.56 ng/mL, following the manufacturer's instructions. Acute phase samples in all groups were collected within 2 days before the initial-IVIG start. For evaluation of temporal changes in laboratory parameters, serum sCD163 and related parameters were measured again, where possible, within 2 days after initial IVIG. As a limitation of retrospective nature of this study, the number of cases (n) that were valid for the paired analysis was 29 of 87 cases (Group A: n = 9 of 61, Group B: n = 10 of 15, Group C: n = 10 of 11). All serum samples for sCD163 were stored at −20°C after collection.
The Institutional Review Board at Yamaguchi University Hospital (H29-158) approved the retrospective study. Informed consent obtained from the parents of all the patients enrolled in this study.
Statistical analyses for comparison were by Fisher's exact probability test for the categorical data. For multiple comparisons among the three groups, the Kruskal-Wallis test and Steel-Dwass test were used for numerical data. Spearman's correlation coefficient (rs) was used to analyze associations between numerical parameters. Wilcoxon singed-rank test were performed for evaluating differences in paired data. P-value of <0.05 considered statistically significant. These statistical analyses were performed using JMP® 13 (SAS Institute Inc., Cary, NC, USA).
Clinical characteristics of patients with KD are shown in Table 1. The number of patients with acute-phase CALs and high Gunma score (≥5 points) was significantly deferent in the three groups. There was no significant difference in febrile days until diagnosis in three groups.
Median (interquartile range) of serum sCD163 levels in initial-IVIG non-responders was 1,269 (665–1,385) ng/mL, higher than initial-IVIG responders: 699 (478–1,072) ng/mL (Figure 2A).
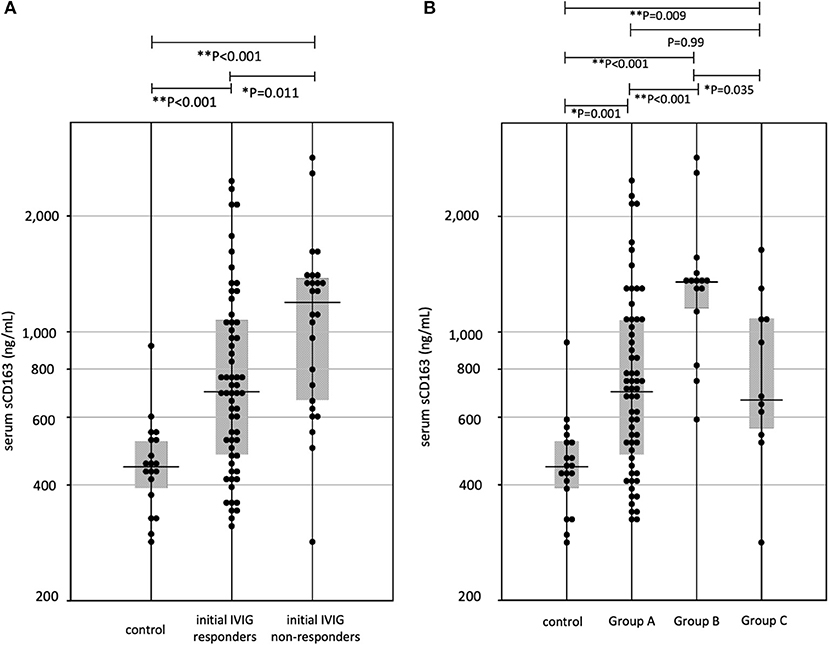
Figure 2. (A) The sCD163 levels in Kawasaki disease (KD). IVIG, intravenous immunoglobulin, *P < 0.05, **P < 0.01 (B) The relationship three groups divided for effective therapies and sCD163 levels. Group A, initial IVIG responders; Group B, additional IVIG responders; Group C, patients who require 3rd line therapy, *P < 0.05, **P < 0.01.
Figure 2B shows serum levels of sCD163 in the three groups. Median (interquartile range) values of serum sCD163 levels were, Group A: 699 (478–1,072) ng/mL, Group B: 1,349 (1,116–1,390) ng/mL, and Group C: 665 (544–1,094) ng/mL. The median level in Group B was the highest among all three groups, however the levels did not differ between Groups A and C. Clinical variables in acute-phase were compared among the three groups. The p-values were as follows, sCD163: P < 0.001, WBC: P = 0.87, hemoglobin: P = 0.70, platelets: P = 0.03, AST: P = 0.002, ALT: P = 0.019, CRP: P = 0.21, sodium: P = 0.001, albumin: P = 0.15. For further analysis, we compared laboratory data among each group by Steel-Dwass method (Table 2). The serum AST levels of Group B was highest in three groups; however, they did not differ between Groups A and C. Serum levels of sCD163, AST, and ALT showed significant positive correlations (sCD163 vs. AST: rs = 0.41, P < 0.001, sCD163 vs. ALT: rs = 0.48, P < 0.001, and AST vs. ALT: rs = 0.88, P < 0.001). Other laboratory parameters were not significantly correlated with sCD163.
Figure 3 shows comparison of parameters before and after initial IVIG among IVIG responders and IVIG non-responders. In Group A, the serum sCD163 significantly increased after IVIG, while WBC, neutrophil counts, monocyte counts, hemoglobin, and CRP significantly decreased. In Group B, sCD163 did not show changes after the initial IVIG, while WBC, neutrophil counts, and hemoglobin decreased significantly. In contrast, in Group C, neither sCD163 nor other parameters showed any change except for slight lowering of hemoglobin.
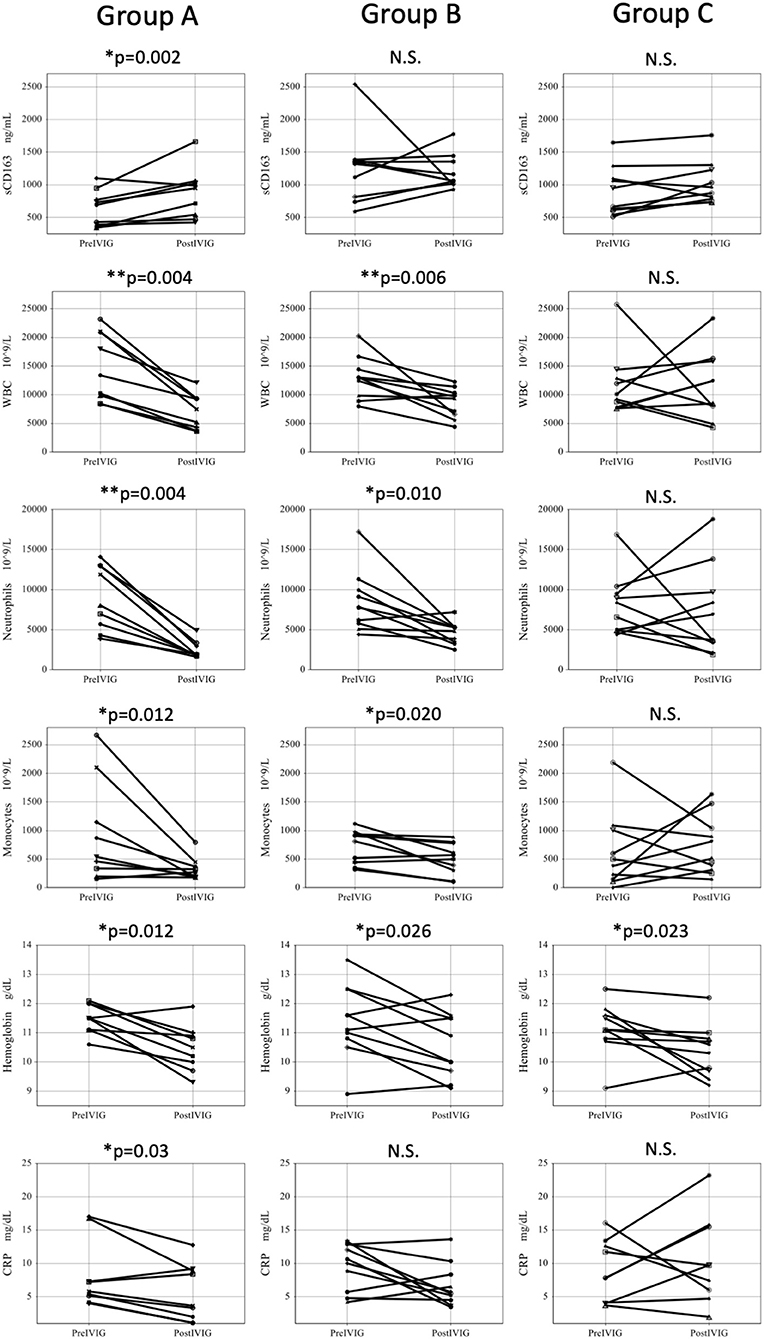
Figure 3. Comparison of parameters before and after initial IVIG among three groups. Pre IVIG, before initial Intravenous immunoglobulin; Post IVIG, after initial intravenous immunoglobulin; Group A, initial IVIG responders; Group B, additional IVIG responders; Group C, patients who require 3rd line therapy; WBC, white blood cell; N.S., not significant, *P < 0.05, **P < 0.01.
In the present study, we first revealed that the serum levels of sCD163, a macrophage activation marker, are elevated in patients with acute-phase KD, and indicated that serum sCD163 can be used as a predictive marker for IVIG responsiveness.
The increased number and activation of CD14+CD16+ monocytes/macrophages via nuclear factor-κB (NF-κB) pathway during acute KD supported our previously published results (14, 17, 18).
We evaluated serum levels of monocyte chemotactic protein-1 (MCP-1) as an activation marker for monocytes/macrophages using the same samples; however, there were no significant differences among groups A: 517 (59–1,311) pg/mL, B: 765 (338–1,129) pg/mL, and C: 633.1 (302–1,609) pg/mL. (Supplementary Figure 1). These results suggest that sCD163 is obviously more useful in predicting additional IVIG responsiveness, compared with other monocytes/macrophages activation markers, such as MCP-1.
In present study, serum sCD163 showed correlation with AST and ALT. Liver function abnormalities have been reported as possible markers for predicting IVIG resistance (19). The mechanism of liver abnormality in KD is not clear. However, it has been reported that Kupffer cells, acting as liver macrophage, proliferate and/or swell in liver of acute KD patients (20). These findings suggest that the liver function abnormality is a result of hyperactivation of macrophages.
After the initial IVIG, significant reduction was observed for WBC and CRP among the initial IVIG responders. These results match with those previously reported (21). Regarding post-treatment changes in sCD163, initial IVIG responders (Group A) showed significant elevation. In contrast, initial IVIG non-responders (Group B and C) did not show any change, but monocyte counts were decreased significantly. This discrepancy may be explained by presumable detachment of CD163 on the macrophage surface induced by IVIG treatment in Group A. In support for this assumption, Samuelsson et al. reported that IVIG treatment causes morphological change in Fcγ receptor on macrophages (22). Furthermore, Sulahian et al. (23) reported that cross-linking of Fcγ receptors triggered shedding of CD163 from macrophage surface via ADAM17. On the other hand, changes in sCD163 were observed neither in Group B nor in Group C, which may reflect the failure of IVIG to act on macrophages for induction of any therapeutic effect.
In the comparison between Group B and C regarding the paired changes, WBC, neutrophil, and monocyte decreased significantly in Group B, but not in Group C. This discrepancy is attributable to the fact that Group B had higher levels of serum sCD163 before IVIG compared to Group C. These findings imply that the profiles of immunological responses are diverse among the IVIG resistance cases. Although the detailed therapeutic mechanism of IVIG is unclear, several in vitro studies showed IVIG inhibition of monocyte/macrophage activation controlled by the NF-κB pathway (24, 25). A retrospective study in China showed elevated serum tumor necrosis factor-α levels in KD, especially in IVIG non- responders (26). These studies suggested an over-activation of macrophages as one of the mechanisms for IVIG-resistant KD. In contrast, several studies showed T cell activation in the pathophysiology of IVIG-resistant KD. CD8+ T cells were present in the media of coronary artery aneurysm by pathological findings (27). Recently, we reported the association of human leukocyte antigen (HLA)-DR expressing T cells with IVIG-resistance in children with KD (28). Excessive CD8+ T cell activation and an imbalance in CD8+ T cell activation and inhibition contribute to the pathogenesis of KD (29). In this setting, in addition to the hyperactivation of macrophages, other mechanisms may account for the pathophysiology of IVIG-resistance in KD.
Previous predictive scores, such as Gunma score, Osaka score, and Kurume score, were constructed for predicting initial IVIG responsiveness. Although several treatments are proposed for initial IVIG non-responders, additional IVIG is most common in the United States and Japan (4, 30). However, at present additional IVIG or infliximab is the treatment choice for initial IVIG non-responders. For this reason, the prediction of additional IVIG responsiveness may provide utility as a second-line treatment. A prospective study in China suggests serum procalcitonin as a marker for predicting additional IVIG responsiveness (31), however there are few reports regarding potential marker for predicting additional IVIG responsiveness.
On the other hand, for predicting initial IVIG responsiveness, there have been reports on the utility of white blood cell count, hemoglobin, CRP, albumin, and d-dimer (32–34). However, we failed to predict the responsiveness of additional IVIG by those conventional markers as shown in Table 2. The present study suggests that serum sCD163 before initial IVIG may be useful as a predicting marker for additional IVIG responsiveness among patients non-responsive to initial IVIG, although further study is needed to confirm this hypothesis. The present study has some limitations. Firstly, the study population was small, and all recruited patients were Japanese. Regional or ethnic differences are associated with the pathophysiology of KD (8). Therefore, additional surveys are required to confirm these results in other ethnic groups. Secondly, we did not consider which therapy is suitable for each additional IVIG non-responder case, because the present study was a retrospective study. The choice of third-line treatment for additional IVIG non-responders was entrusted to the discretion of each pediatrician.
In conclusion, this study clearly showed that serum levels of sCD163, macrophage activation marker, are elevated in patients with acute-phase KD. After initial IVIG, sCD163 was increased in initial IVIG-responders but not in non-responders. Close correlation of serum sCD163 with ALT and AST suggest that liver function abnormality commonly seen in KD may be a result of macrophage activation. Among the initial IVIG non-responders, sCD163 was higher in patients who responded to the additional IVIG. This finding indicates diversity of immunological profiles among the IVIG resistant cases and may be used for predicting the failure to the additional IVIG.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation, to any qualified researcher.
This study was carried out in accordance with the Declaration of Helsinki and the recommendations of the institutional review board of Yamaguchi University Hospital. The Institutional Review Board at Yamaguchi University Hospital (H29-158) approved the retrospective study. Informed consent obtained from the parents of all the patients enrolled in this study.
YA, YS, and SH were the principal investigators taking primary responsibility for the manuscript. YA, YS, SOh, and SH designed this study. SOk, CM, HW, YO, TF, and AM performed the clinical management with helpful discussion for the completion of the study. YA and YS took responsibility for the diagnosis and data collection. KI completed the statistical analysis. YA, YS, HY, and SH wrote the paper.
Support for part of this research was from the JSPS KAKENHI Grant Number JP16K19647.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors are particularly grateful for the technical assistance and support provided by Yoko Mori, Midori Wakabayashi, and Takako Waniishi. We would like to thank Editage (www.editage.com) for English language editing.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2020.00148/full#supplementary-material
Supplementary Figure 1. (a) The monocyte chemotactic protein-1 levels in Kawasaki disease (KD). IVIG: intravenous immunoglobulin (b) The relationship three groups divided for effective therapies and monocyte chemotactic protein-1 levels. Group A, initial IVIG responders; Group B, additional IVIG responders; Group C, patients who require 3rd line therapy.
1. Kawasaki T, Kosaki F, Okawa S, Shigematsu I, Yanagawa H. A new infantile acute febrile mucocutaneous lymph node syndrome (MLNS) prevailing in Japan. Pediatrics. (1974) 54:271–6.
2. Research Committee of the Japanese Society of Pediatric Cardiology, Cardiac Surgery Committee for Development of Guidelines for Medical Treatment of Acute Kawasaki Disease. Guidelines for medical treatment of acute Kawasaki disease: report of the Research Committee of the Japanese Society of Pediatric Cardiology and Cardiac Surgery (2012 revised version). Pediatr Int. (2014) 56:135–58. doi: 10.1111/ped.12317
3. Nakamura Y, Yashiro M, Uehara R, Sadakane A, Tsuboi S, Aoyama Y, et al. Epidemiologic features of Kawasaki disease in Japan: results of the 2009-2010 nationwide survey. J Epidemiol. (2012) 22:216–21. doi: 10.2188/jea.JE20110126
4. Makino N, Nakamura Y, Yashiro M, Ae R, Tsuboi S, Aoyama Y, et al. Descriptive epidemiology of Kawasaki disease in Japan, 2011-2012: from the results of the 22nd nationwide survey. J Epidemiol. (2015) 25:239–45. doi: 10.2188/jea.JE20140089
5. Kobayashi T, Inoue Y, Takeuchi K, Okada Y, Tamura K, Tomomasa T, et al. Prediction of intravenous immunoglobulin unresponsiveness in patients with Kawasaki disease. Circulation. (2006) 113:2606–12. doi: 10.1161/CIRCULATIONAHA.105.592865
6. Sano T, Kurotobi S, Matsuzaki K, Yamamoto T, Maki I, Miki K, et al. Prediction of non-responsiveness to standard high-dose gamma-globulin therapy in patients with acute Kawasaki disease before starting initial treatment. Eur J Pediatr. (2007) 166:131–7. doi: 10.1007/s00431-006-0223-z
7. Egami K, Muta H, Ishii M, Suda K, Sugahara Y, Iemura M, et al. Prediction of resistance to intravenous immunoglobulin treatment in patients with Kawasaki disease. J Pediatr. (2006) 149:237–40. doi: 10.1016/j.jpeds.2006.03.050
8. Sleeper LA, Minich LL, McCrindle BM, Li JS, Mason W, Colan SD, et al. Evaluation of Kawasaki disease risk-scoring systems for intravenous immunoglobulin resistance. J Pediatr. (2011) 158:831–5. doi: 10.1016/j.jpeds.2010.10.031
9. Fabriek BO, Dijkstra CD, van den Berg TK. The macrophage scavenger receptor CD163. Immunobiology. (2005) 210:153–60. doi: 10.1016/j.imbio.2005.05.010
10. Etzerodt A, Maniecki MB, Møller K, Møller HJ, Moestrup SK. Tumor necrosis factor α-converting enzyme (TACE/ADAM17) mediates ectodomain shedding of the scavenger receptor CD163. J Leukoc Biol. (2010) 88:1201–5. doi: 10.1189/jlb.0410235
11. Ingels C, Møller HJ, Hansen TK, Wouters PJ, Vanhorebeek I, Van den Berghe G. Circulating levels of the shed scavenger receptor sCD163 and association with outcome of critically ill patients. J Clin Immunol. (2013) 33:619–29. doi: 10.1007/s10875-012-9830-9
12. Dige A, Støy S, Thomsen KL, Hvas CL, Agnholt J, Dahlerup JF, et al. Soluble CD163, a specific macrophage activation marker, is decreased by anti-TNF-α antibody treatment in active inflammatory bowel disease. Scand J Immunol. (2014) 80:417–23. doi: 10.1111/sji.12222
13. Hasegawa S, Matsushige T, Inoue H, Takahara M, Kajimoto M, Momonaka H, et al. Serum soluble CD163 levels in patients with influenza-associated encephalopathy. Brain Dev. (2013) 35:626–9. doi: 10.1016/j.braindev.2012.10.005
14. Katayama K, Matsubara T, Fujiwara M, Koga M, Furukawa S. CD14+CD16+ monocyte subpopulation in Kawasaki disease. Clin Exp Immunol. (2000) 121:566–70. doi: 10.1046/j.1365-2249.2000.01321.x
15. Ayusawa M, Sonobe T, Uemura S, Ogawa S, Nakamura Y, Kiyosawa N, et al. Revision of diagnostic guidelines for Kawasaki disease (the 5th revised edition). Pediatr Int. (2005) 47:232–4. doi: 10.1111/j.1442-200x.2005.02033.x
16. Ogata S, Ogihara Y, Honda T, Kon S, Akiyama K, Ishii M. Corticosteroid pulse combination therapy for refractory Kawasaki disease: a randomized trial. Pediatrics. (2012) 129:e17–23. doi: 10.1542/peds.2011-0148
17. Matsubara T, Ichiyama T, Furukawa S. Immunological profile of peripheral blood lymphocytes and monocytes/macrophages in Kawasaki disease. Clin Exp Immunol. (2005) 141:381–7. doi: 10.1111/j.1365-2249.2005.02821.x
18. Ichiyama T, Yoshitomi T, Nishikawa M, Fujiwara M, Matsubara T, Hayashi T, et al. NF-kappaB activation in peripheral blood monocytes/macrophages and T cells during acute Kawasaki disease. Clin Immunol. (2001) 99:373–7. doi: 10.1006/clim.2001.5026
19. Eladawy M, Dominguez SR, Anderson MS, Glodé MP. Abnormal liver panel in acute kawasaki disease. Pediatr Infect Dis J. (2011) 30:141–4. doi: 10.1097/INF.0b013e3181f6fe2a
20. Bader-Meunier B, Hadchouel M, Fabre M, Arnoud MD, Dommergues JP. Intrahepatic bile duct damage in children with Kawasaki disease. J Pediatr. (1992) 120:750–2. doi: 10.1016/S0022-3476(05)80239-2
21. Lee KY, Rhim JW, Kang JH. Kawasaki disease: laboratory findings and an immunopathogenesis on the premise of a “protein homeostasis system”. Yonsei Med J. (2012) 53:262–75. doi: 10.3349/ymj.2012.53.2.262
22. Samuelsson A, Towers TL, Ravetch JV. Anti-inflammatory activity of IVIG mediated through the inhibitory Fc receptor. Science. (2001) 291:484–6. doi: 10.1126/science.291.5503.484
23. Sulahian TH, Pioli PA, Wardwell K, Guyre PM. Cross-linking of FcgammaR triggers shedding of the hemoglobin-haptoglobin scavenger receptor CD163. J Leukoc Biol. (2004) 76:271–7. doi: 10.1189/jlb.1003523
24. Ichiyama T, Ueno Y, Hasegawa M, Niimi A, Matsubara T, Furukawa S. Intravenous immunoglobulin inhibits NF-kappaB activation and affects Fcgamma receptor expression in monocytes/macrophages. Naunyn Schmiedebergs Arch Pharmacol. (2004) 369:428–33. doi: 10.1007/s00210-004-0877-x
25. Zhou C, Huang M, Xie L, Shen J, Xiao T, Wang R. IVIG inhibits TNF-α-induced MMP9 expression and activity in monocytes by suppressing NF-κB and P38 MAPK activation. Int J Clin Exp Pathol. (2015) 8:15879–86.
26. Hu P, Jiang GM, Wu Y, Huang BY, Liu SY, Zhang DD, et al. TNF-α is superior to conventional inflammatory mediators in forecasting IVIG nonresponse and coronary arteritis in Chinese children with Kawasaki disease. Clin Chim Acta. (2017) 471:76–80. doi: 10.1016/j.cca.2017.05.019
27. Brown TJ, Crawford SE, Cornwall ML, Garcia F, Shulman ST, Rowley AH. CD8 T lymphocytes and macrophages infiltrate coronary artery aneurysms in acute Kawasaki disease. J Infect Dis. (2001) 184:940–3. doi: 10.1086/323155
28. Wakiguchi H, Hasegawa S, Suzuki Y, Kudo K, Ichiyama T. Relationship between T-cell HLA-DR expression and intravenous immunoglobulin treatment response in Kawasaki disease. Pediatr Res. (2015) 77:536–40. doi: 10.1038/pr.2015.12
29. Ye Q, Gong FQ, Shang SQ, Hu J. Intravenous immunoglobulin treatment responsiveness depends on the degree of CD8+ T cell activation in Kawasaki disease. Clin Immunol. (2016) 171:25–31. doi: 10.1016/j.clim.2016.08.012
30. Ghelani SJ, Pastor W, Parikh K. Demographic and treatment variability of refractory Kawasaki disease: a multicenter analysis from 2005 to 2009. Hosp Pediatr. (2012) 2:71–6. doi: 10.1542/hpeds.2011-00112
31. Shao S, Luo C, Zhou K, Hua Y, Wu M, Liu L, et al. Predictive value of serum procalcitonin for both initial and repeated immunoglobulin resistance in Kawasaki disease: a prospective cohort study. Pediatr Rheumatol Online J. (2019) 17:78. doi: 10.1186/s12969-019-0379-5
32. Muto T, Masuda Y, Numoto S, Kodama S, Yamakawa K, Takasu M, et al. White blood cell and neutrophil counts and response to intravenous immunoglobulin in Kawasaki Disease. Glob Pediatr Health. (2019) 25:2333794X19884826. doi: 10.1177/2333794X19884826
33. Xie T, Wang Y, Fu S, Wang W, Xie C, Zhang Y, et al. Predictors for intravenous immunoglobulin resistance and coronary artery lesions in Kawasaki disease. Pediatr Rheumatol Online J. (2017) 15:17. doi: 10.1186/s12969-017-0149-1
Keywords: intravenous immunoglobulin, Kawasaki disease, macrophage, soluble CD163, sCD163
Citation: Azuma Y, Suzuki Y, Okada S, Matsuguma C, Wakiguchi H, Ohnishi Y, Furuta T, Miyake A, Yasudo H, Ichihara K, Ohga S and Hasegawa S (2020) Utility of Soluble CD163 in the Clinical Management of Patients With Kawasaki Disease. Front. Pediatr. 8:148. doi: 10.3389/fped.2020.00148
Received: 13 September 2019; Accepted: 16 March 2020;
Published: 07 April 2020.
Edited by:
Kyung-Yil Lee, The Catholic University of Korea, South KoreaReviewed by:
Christoph Kessel, Universitätsklinikum Münster, GermanyCopyright © 2020 Azuma, Suzuki, Okada, Matsuguma, Wakiguchi, Ohnishi, Furuta, Miyake, Yasudo, Ichihara, Ohga and Hasegawa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yasuo Suzuki, eS5zdXp1a2lAeWFtYWd1Y2hpLXUuYWMuanA=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.