
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr., 20 February 2020
Sec. Pediatric Nephrology
Volume 8 - 2020 | https://doi.org/10.3389/fped.2020.00046
This article is part of the Research TopicHighlights in Pediatric Nephrology: 2021View all 15 articles
 Corina Nailescu1*
Corina Nailescu1* Raoul D. Nelson2
Raoul D. Nelson2 Priya S. Verghese3
Priya S. Verghese3 Katherine E. Twombley4
Katherine E. Twombley4 Aftab S. Chishti5
Aftab S. Chishti5 Michele Mills6
Michele Mills6 John D. Mahan7
John D. Mahan7 James E. Slaven8
James E. Slaven8 Marcia L. Shew1
Marcia L. Shew1Background: Kidney transplant (KT) recipients have higher incidence of malignancies, including Human Papillomavirus (HPV)-associated cancers. Thus, HPV vaccines may have an important role in preventing HPV-related disease in this population; however, immunogenicity and safety data are lacking.
Objective: To examine the immunological response and tolerability to HPV vaccination in pediatric KT recipients compared to future KT candidates.
Methods: The quadrivalent HPV vaccine was administered to girls and boys age 9–18 recruited from seven centers part of the Pediatric Nephrology Research Consortium. Subjects were recruited for three groups: (1) CKD: chronic kidney disease stages 3, 4, and 5 not on dialysis; (2) Dialysis; (3) KT recipients. The outcome consisted of antibody concentrations against HPV 6, 11, 16, and 18. Geometric mean titers (GMTs) and seroconversion rates were compared. Vaccine tolerability was assessed.
Results: Sixty-five participants were recruited: 18 in the CKD, 18 in the dialysis, and 29 into the KT groups. KT patients had significantly lower GMTs after vaccination for all serotypes. The percentages of subjects who reached seroconversion were overall lower for the KT group, reaching statistical significance for HPV 6, 11, and 18. Comparing immunosuppressed subjects (anyone taking immunosuppression medications, whether KT recipient or not) with the non-immunosuppressed participants, the former had significantly lower GMTs for all the HPV serotypes and lower seroconversion rates for HPV 6, 11, and 18. KT females had higher GMTs and seroconversion rates for certain serotypes. There were no adverse events in either group.
Conclusions: HPV vaccine was well-tolerated in this population. Pediatric KT recipients had in general lower GMTs and seroconversion rates compared to their peers with CKD or on dialysis. Immunosuppression played a role in the lack of seroconversion. Our results emphasize the importance of advocating for HPV vaccination prior to KT and acknowledge its safety post transplantation. Future studies are needed to investigate the effect of a supplemental dose of HPV vaccine in KT recipients who do not seroconvert and to evaluate the long-term persistence of antibodies post-KT.
Solid organ transplant (SOT) recipients are at increased risk for malignancies in both pediatric and adult populations (1–3), with an elevated risk of dying of their cancer, even after adjustment for stage and treatment (4). Pediatric and adult kidney transplant (KT) recipients carry similar risk and malignancies are third most common cause of mortality within this population (5, 6). Specifically, female KT recipients have an increased risk of cervical cancer up to 14-fold, vulvar cancer up to 50-fold and anal cancers up to 100-fold (7, 8). In addition, male SOT recipients have an increased risk for penile cancers (9). Human papillomavirus (HPV) 16 and to a much lesser extent HPV 18 are responsible for the majority of these cancers (10). In addition, HPV 16 is also responsible for a large proportion of oral pharyngeal cancers (11). Not only that HPV infections are the most prevalent sexually transmitted infections in the US (12), but non-carcinogenic HPV types, HPV 6 and 11, can be associated with genital warts and can be difficult to treat in the face of immunosuppression (13). Immunosuppressed individuals, including transplant recipients, have higher rates of HPV infections (14–16), greater likelihood of persistent infections (17, 18) and higher rates of HPV clinical disease, including cancer (9, 19, 20). Thus, preventing HPV infections in this population is critical. HPV-related vaccine trials have been shown to prevent HPV infection in immunocompetent individuals when the vaccine is given prior to viral exposure (21). In addition, more recent studies have demonstrated a true reduction in abnormal Pap smears and cervical cancers with use of HPV vaccine (22, 23). Because HPV is sexually transmitted and often acquired soon after the onset of sexual activity (24), vaccination should ideally occur before sexual debut.
The efficacy and safety of the HPV vaccine in certain immunocompromised populations, such as pediatric chronic kidney disease (CKD), dialysis and KT recipients, has not been well-studied. This is a population at increased risk for infections and HPV related sequelae; in addition, they appear to also be at high risk for not getting vaccinated due to lack of data and less immediate concerns about sexual activity when ill and awaiting transplant, as has been shown in other patients with severe chronic illnesses (25, 26). The goal of this study was to examine the immunological response and tolerability of the quadrivalent HPV vaccine in both female and male pediatric patients with CKD, on dialysis and with a KT. The primary hypothesis was that all these populations will mount an immune response to the vaccine. Our secondary hypothesis was that pediatric patients with CKD 3, 4, 5 and on dialysis, therefore future KT candidates, will respond to the HPV vaccination better than the KT recipients.
Males and females ages 9–18 years were recruited from seven collaborating large medical centers in the Pediatric Nephrology Research Consortium: Riley Hospital for Children in Indianapolis, IN, Primary Children's Hospital in Salt Lake City, UT, Medical University of South Carolina Children's Hospital in Charleston, SC, University of Minnesota Masonic Children's Hospital in Minneapolis, MN, Kentucky Children's Hospital in Lexington, KY, C.S. Mott Children's Hospital in Ann Arbor, MI and Nationwide Children's Hospital in Columbus, OH. Subjects were recruited in three cohorts:
– Group 1 (CKD) included patients with CKD stages 3 [glomerular filtration rate (GFR) 30–59 mL/min per 1.73 m2], 4 (GFR 15–29 mL/min per 1.73 m2) or 5 not on dialysis (GFR <15 mL/min/1.73 m2, but not on dialysis).
– Group 2 (Dialysis) included patients on either chronic hemodialysis or peritoneal dialysis.
– Group 3 (KT) included patients who had received a KT at least 6 months prior to enrollment, in keeping with the recommendations of the American Society of Transplantation that inactivated vaccines may be resumed 3–6 months after a SOT (27).
Exclusion criteria were: pregnancy (self-reported), post-transplant lymphoproliferative disorder, active infectious diseases, fever, bleeding disorders, having received blood products in the past 6 months and hypersensitivity to any of the vaccine components.
All participants who met eligibility criteria and who had not been vaccinated against HPV were approached within the dialysis units or clinics by a study coordinator at each site. Study coordinators discussed the study and consent was obtained from the parent/guardian and assent from the participant. Medical records were used to abstract age, gender, race, and estimated GFR (eGFR) using the modified Schwartz formula (0.413 × height in cm/serum creatinine in mg/dL). For the CKD 3, 4, 5 and dialysis patients, it was documented whether they were on immunosuppression medications for their respective primary kidney diseases. The rationale for doing so was to perform sub-analyses to determine whether patients on immunosuppression medications (including KT recipients, but also patients with CKD 3, 4, and 5 taking immunosuppression medications to treat their primary disease) had a weaker immune response to the HPV vaccine compared to the rest. In addition, for KT patients, type of donor (living vs. deceased), number of transplants and time since most recent transplant were recorded. HPV quadrivalent vaccine (Gardasil®, Merck & Co., Inc.) was administered to each study participant per protocol according to the Advisory Committee on Immunization Practices recommendations at the time (28). Dose 1 of the HPV vaccine was administered to each study participant on day 1 of enrollment, after obtaining a baseline blood sample for antibody testing. Dose 2 was targeted to be administered at month 2 and dose 3 at month 6. The minimum interval between dose 1 and 2 of the vaccine was 4 weeks. The minimum recommended interval between dose 2 and 3 of the vaccine was 12 weeks. No subject received dose 2 or 3 more than 4 weeks later than initially targeted date. The Institutional Review Board of each participating center approved the study.
The primary outcome was the antibody response to each of the four HPV serotypes contained within the vaccine (6, 11, 16, 18). Serum was collected from all subjects on day 1 of enrollment prior to dose 1 of the vaccine administration [Time 1 (T1)] and at month 7 (or 1 month after vaccine series was completed) [Time 2 (T2)]. Anti-HPV responses were measured using a competitive Luminex Immunoassay performed by PPD Vaccines and Biologics (Wayne, PA) and expressed as geometric mean titers (GMTs). A participant was considered to have seroconverted if no antibody titers were found at enrollment and antibody levels post immunization were above the sero-status cutoffs for HPV types 6, 11, 16, and 18, as determined by the assay developer (GMTs ≥ 20, ≥16, ≥20, and ≥24 mM units/mL, respectively).
Secondary analyses evaluated the potential effect of other variables on antibody response to the HPV vaccine, such as age, gender, race, eGFR and use of immunosuppression medications. Within the transplanted group, donor type (living vs. deceased), number of transplants and time from transplant were also evaluated.
In addition, although the sample size was small, data was collected regarding safety of the HPV vaccine in this patient population, since it has not been well-studied. All subjects received a vaccination report card (VRC) after each vaccine dose administration. On the VRC, the parent/guardian was asked to record the subject's oral evening temperature daily for 5 days. In addition, injection-site and systemic adverse events (AEs) for a total of 15 days after each vaccination were recorded by parent/guardian. For injection-site erythema and swelling, subjects were instructed by the VRC to measure an injection-site reaction at its greatest width (“maximum size”) from edge to edge in maximum units ranging from 0 to >7 inches (17.5 cm) on the VRC, rounding up to the next unit if in between 2 units [each unit on the VRC measured ~1 inch (2.5 cm)]. For all AEs, subjects were instructed by the VRC to estimate the severity of AEs as mild (awareness of symptom but easily tolerated), moderate (discomfort enough to cause interference with usual activities), or severe (incapacitating with inability to work or do usual activity). Serious AEs were collected for the whole duration of the study regardless of causality and were followed for outcome. In addition, for KT recipients, data was specifically collected on any biopsy-proven acute rejection episodes after T1 up to 6 months after the last dose of the vaccine administration.
Basic demographic and clinical characteristics are presented as means [standard deviations (SD)] for age, median [interquartile range (IQR)] for eGFR due to non-linearity, and frequencies (percentages) for categorical variables (sex, race, use of immunosuppression medications), with analyses comparing groups performed with ANOVA, Kruskal–Wallis, and Chi–Square tests, respectively. The two main outcomes of serotiter and seroconversion were assessed to determine if there were significant differences between groups at T2. Serotiter values were analyzed using Kruskal–Wallis non-parametric tests and seroconversion proportions using Fisher's Exact tests, with descriptive statistics being given as medians (IQRs) and frequencies (percentages), respectively. Pairwise comparisons were made for serotiter levels using a Bonferroni adjusted p-0.0167 to control for inflated type I error rates. Similar analyses, for demographics and the two outcome variables, were also performed comparing those who were on immunosuppression medications vs. those who were not, with similar analyses, using Student's t-tests, Wilcoxon rank-sum tests, and Fisher's Exact tests. All analyses were performed using SAS v9.4 (SAS Institute, Cary, NC) and all analytic assumptions were verified. Non-inferiority analyses were also performed, but all were non-significant due to the extremely low power due to our small sample size (results not presented).
A total of 72 subjects were enrolled: 28 from Riley Hospital for Children in Indianapolis; 15 from Primary Children's Hospital in Salt Lake City; 9 from University of Minnesota Masonic Children's Hospital in Minneapolis; 9 from Medical University of South Carolina Children's Hospital in Charleston; 5 from Kentucky Children's Hospital in Lexington; 5 from C.S. Mott Children's Hospital in Ann Arbor; 1 from Nationwide Children's Hospital in Columbus. A total of 65 participants completed the study: 18 participants in the CKD group, 18 participants in the dialysis group and 29 participants in the KT group (7 subjects were excluded for either not receiving all three doses of the vaccine or for not doing both serology collections). The average age of enrollment was 13.6 years (SD 2.6) and little over one half were males (N = 34, 54.8%). The majority of participants were white (N = 41, 65.1%) or black (N = 11, 17.5%). Table 1 compares the demographics of the three groups with differences demonstrated among the three groups in regards to sex, race, eGFR and use of immunosuppression medications. All the KT patients were taking immunosuppression medications; in addition, some of the patients in the other two groups were also taking immunosuppression medications to treat the immune-mediated diseases leading to CKD or dialysis). Within the KT group (n = 29) the median (range) time since first transplant was 3.9 years (3.6, 10.5), about half (51.9%) had received a kidney from a living donor, one individual had had two transplants; two KT recipients reported events of rejection during the study enrollment.
All participants were seronegative for all four of the vaccine HPV types at enrollment, except for three participants who were not naïve for HPV 6, and thus excluded from serotiter and seroconversion analyses for that particular HPV type only. Serotiters for all HPV types were detected post-vaccination at T2, with KT patients having significantly lower GMTs for all serotypes; pairwise comparisons indicated the significant associations were driven by the difference between CKD and KT groups, although CKD and dialysis groups were also different for HPV type 11 (Table 2). Examination of the seroconversion rates based on manufacturer cutoffs showed significant heterogeneity between the groups (Table 3). The seroconversion rates post-vaccination were significantly lower for the transplanted group for three of the serotypes (HPV 6, 11, and 18). Although not statistically different, seroconversion rates were also lower for the HPV 16 serotype.
All variables found to be different among the three treatment groups were examined directly with the two outcome variables, GMTs at T2 and seroconversion. Age, sex, race, and eGFR were found to have no association with GMTs at T2 or actual seroconversion. However, when only the KT population was examined, being a female KT-recipient was associated with greater GMTs at T2 for HPV 6 and 11 only (p = 0.020 and 0.046, respectively) and with higher seroconversion rates for HPV 18 only (90.9 vs. 44.4% for females and males, respectively; p = 0.020). Although statistically significant, this is difficult to interpret clinically with the inconsistencies among the various HPV types and small numbers for multiple analyses.
Comparing subjects who were using immunosuppression medications (including KT recipients, as well as CKD 3, 4, and 5 on immunosuppression medications to treat their primary kidney conditions) vs. not, the immunosuppression medication-using group had significantly lower serotiters for all four HPV serotypes at T2 and lower seroconversion rates for HPV 6, 11, and 18 (Table 4). The immunosuppression medications used in the transplant population included prednisone, tacrolimus, cyclosporine A and mycophenolate mofetil. The immunosuppression medications used to treat the primary kidney diseases in the CKD and dialysis patients included prednisone, tacrolimus, mycophenolate mofetil and azathioprine. Unlike in the KT population, when the male vs. female differences were examined specifically in the immunosuppression medication-using group, no differences were seen.
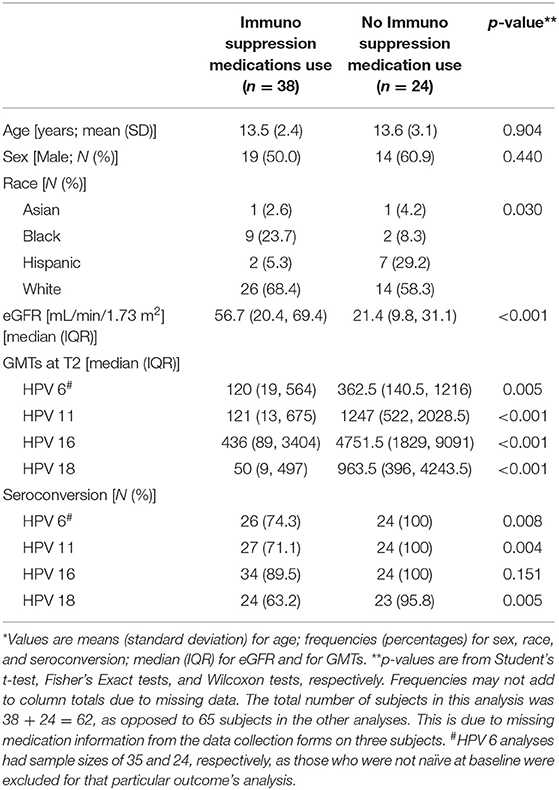
Table 4. Comparison between subjects who used immunosuppression medications vs. subjects not on immunosuppression medications*.
Within the transplant population, neither the donor type, neither time since transplant, nor the number of transplants, made any difference in terms of GMTs at T2 or seroconversion rates.
As for the AEs, they were minimal, limited to only mild local reactions. Two patients underwent acute rejection episodes within 6 months following the last dose of the vaccine. However, upon review of chart data, these events were considered to be likely related to medication non-adherence.
American Society of Transplantation recommends that HPV vaccine be given to all SOT candidates and recipients in the recommended age-group (27). However, the efficacy and tolerability of the HPV vaccine in such populations, as well as the optimal timing to do so, are not completely understood. Our results demonstrated that the HPV vaccine was well-tolerated and an adequate immune response for all four HPV types was observed with CKD and dialysis patients. However, the immune response was slightly lower within the transplanted population and immunosuppressant medications had an effect on lack of seroconversion. HPV vaccine acceptance by patients and their parents/guardians has always been problematic even in the general population (29, 30) and probably more so in these specific groups, where data is scant (26). Our study provides further evidence that patients who are likely to need a KT in the future respond best to the HPV vaccine pre-transplantation, while with CKD or on dialysis. As the majority of causes of end-stage renal disease in pediatrics are congenital diseases in which the kidney function slowly deteriorates over time, pediatric nephrologists often have the advantage of being able to better plan a KT (whether preemptive or not) considering multiple aspects, including vaccinations, compared to their adult nephrologist colleagues (26). This study showed that girls and boys with CKD and on dialysis had stronger immunologic responses to the HPV vaccine compared to those who were already transplanted. Therefore, this study should help pediatric nephrologists and pediatricians in their work with patients and families to pursue and complete the administration of HPV vaccine ideally prior to transplant whenever possible, but also post-transplant in cases where it was not given prior to KT. Is is to be noted that in 2015 the Advisory Committee on Immunization Practices (ACIP) recommended the 9-valent HPV vaccine (9vHPV) (Gardasil 9, Merck and Co., Inc.) as an alternative to the quadrivalent vaccine (31). The 9-valent vaccine was found to be safe, provided more extensive serotype coverage and it was even found to be cost-saving when compared with the quadrivalent vaccine, therefore it is currently more widely used (31). Our study was initiated well before 2015, hence the use of the quadrivalent vaccine.
It is important for the HPV vaccine to be given before HPV infection is acquired, meaning before the onset of sexual activity (28). This is the rationale behind the fact that the HPV vaccine is recommended to be given at 11–12 years of age, although it can be administered starting at age 9 (28). In the US, the proportion of males who reported having sexual intercourse before age 13 years varied from 5 to 25% across metropolitan sites (32), hence the need to vaccinate at a young age, designed to capture most teenagers before becoming sexually active. In our study, the onset of sexual activity was not assessed out of concerns that in a multisite trial, the investigators could not assure patient confidentiality relative to their parents/caregivers for all participants. However, obtaining this information in future studies would be helpful in assessing clinical risk and outcomes in patients with chronic diseases (33, 34). In children with CKD and on dialysis, contrary to what was believed in the past, recent cohort studies have shown that growth and sexual maturation are only slightly delayed compared to healthy subjects (35). Although there is lack of specific data, given the circumstances, it is plausible that adolescents with CKD or on dialysis are not far behind from their peers in terms of their onset of sexual activity. It is therefore of concern that in our study the mean age of subjects was 13.6 (SD 2.6), meaning that our patient population was vaccinated on average later than the recommended age.
Our study showed for the first time both in boys and girls that overall, transplant patients had a decreased response to the HPV vaccine compared to CKD and dialysis patients, likely related to their state of medication-induced immunosuppression. However, specifically for HPV 16, seroconversion rates were not found to be significantly lower for KT patients and neither were they found to be lower for patients taking immunosuppression medications. Although power may have biased this result, it is also somewhat reassuring since the majority of HPV cancers are associated with type 16. At the very least, the data supports ongoing vaccination in this group due to the fact that the majority of individuals did effectively respond to the vaccine. Review of literature revealed that the first studies to look into HPV vaccine responses in immunosuppressed populations was in HIV-positive cohorts, which showed promising results, as 95–100% of the subjects demonstrated seroconversion (36–38). Thus, seroconversion was not much lower than in healthy populations (39). Surprisingly, the first study of the immunogenicity of the HPV vaccine in SOT recipients was done in a cohort of 47 adults (females and males) and reported much lower rates of seroconversion: 63.2, 68.4, 63.2, and 52.6% for HPV 6, 11, 16, and 18, respectively (40). Somewhat similarly, in pediatrics, a study of a cohort of 57 patients (females only) aged 9–21 years old with CKD, on dialysis, or post-KT, showed that among patients with transplants, the percentages achieving seropositivity were significantly lower compared to CKD and dialysis patients: 63.6, 63.6, and 72.7% for HPV 6, 11, and 18, respectively (41). Our study included both girls and boys age 10 to 16 with CKD, on dialysis, or post-KT and showed lower seroconversion rates: 72.4, 69.0, and 62.1% for HPV 6, 11, and 18, respectively, in transplant patients, thus demonstrating similar results for the first time in boys, as well.
In this study, it is likely that the entire population was affected by varying levels of immunosuppression, regardless of the study group. Indeed, patients with CKD and more so the ones on dialysis have been shown to have various degrees of immunosuppression, whether caused by defects in neutrophil function, antigen processing, cell-mediated immunity, or antibody-mediated immunity (42, 43). Antibody responses to certain vaccines have been found to be attenuated in this patient population for certain vaccines, such as the measles, mumps and rubella (44). On the contrary, for other vaccines immune responses have been found to be similar to the general population (45). In the case of HPV vaccine, a study on pediatric and adult patients with CKD 4, 5, and on dialysis showed 98.2, 100, 100, and 98.2% serocoversion for HPV genotypes 6, 11, 16, and 18, respectively (46). These values are comparable to the data generated in healthy adult women large populations, which reported 99.7, 99.5, 99.7, and 99.2% seropositivity for HPV genotypes 6, 11, 16, and 18, respectively (39). Our study also reported robust immune responses in the CKD and dialysis pediatric patients. Although there was a non-statistically significant tendency to less robust responses for dialysis patients compared to CKD, this was probably explained by the fact that half of the dialysis patients were on immunosuppressive medications for their kidney conditions. In addition, we have found no difference in terms of HPV vaccine responses between patients on hemodialysis vs. peritoneal dialysis in our study population (data not presented). It is likely that being on anti-rejection medications creates a more profound state of immunosuppression in transplant recipients than the immune dysregulation seen in CKD or while on dialysis, since KT patients had less robust immune responses to the HPV vaccine in our study. Likewise, patients with CKD or on dialysis who were on immunosuppression medications for their primary kidney diseases, which are in fact similar to anti-rejection medications, also demonstrated poorer seroconversion rates.
It is possible that girls and boys who are KT recipients respond differently to the HPV vaccine. The quadrivalent HPV vaccine was approved in the US in 2006 for use in girls/women only. Subsequently, in 2009, the indication was extended to boys/men as well. The few studies that compared the seroconversion rates to HPV vaccine between males and females in the general population have not shown any differences (47, 48). Our study showed that overall there were no differences between males and females in terms of HPV antibody responses. However, when data was analyzed specifically for the KT recipients, male subjects had lower GMTs at T2 for HPV 6 and 11 (but not 16 or 18) and lower seroconversion rates for HPV 18 (but not for HPV6, HPV11, or HPV16) compared to females. Although statistically significant, this is difficult to interpret clinically with the inconsistencies among the various HPV types and small numbers for multiple analyses. Interestingly enough, unlike in the KT population, when the male vs. female differences were examined specifically in the immunosuppression medication-using group, no differences were seen. Sex-based differences in immune function and responses to vaccination have been described before, where females typically developed higher antibody responses and experienced more adverse reactions following vaccination than males for bacillus Calmette-Guerin, measles, mumps, rubella, yellow fever and influenza vaccines (49, 50). In addition, men SOT recipients have a higher incidence of malignancies, also suggesting the fact that SOT men could become more immunosuppressed than women when treated with anti-rejection medications (51). In any event, the fact that male KT recipients tend to have lower antibody titers and seroconversion rates for certain serotypes following HPV vaccination, while of uncertain significance, still raises questions about optimal vaccination strategies specifically in this patient population, which has been already shown to also have lower vaccination rates compared to females (52).
AEs reported were similar to those noted in prior studies, limited to minor vaccine local skin reactions. Two patients underwent acute rejection episodes within 6 months following dose 3 of the HPV vaccine. In 2014, a study initiated HPV vaccination in 14 pediatric KT recipients; unfortunately six of them developed acute rejection shortly after the initiation of the vaccine series, which lead to an early termination of the study (53). In contrast, in 2016, only two of the 23 pediatric KT recipients of HPV vaccination were reported to have acute rejection episodes (41). In our patient population, we report a similar acute rejection burden; upon chart review, it was felt that our two cases of acute rejection were related to medication non-adherence, as opposed to vaccine administration.
We acknowledge that our study has certain limitations. Like in many pediatric SOT studies, our study was limited by its small sample size. Unfortunately three subjects were missing information on medication use in the data collection form and some were not complete, thus impairing our ability to analyze the effect of specific immunosuppresion medications. Indeed, for specific vaccinations, for example influenza vaccination in KT recipients, it has been demonstrated that different imunosuppression regimens can influence the immune response (54). In addition, our study lacked long-term follow up, as we did not look into long-term HPV-vaccine induced antibody persistence post-transplantation; other studies suggested that there was a decline in titers over time (40). Lastly, we were unable to determine factors beyond immunosuppression that may play a role in preventing seroconversion in transplant recipients. However, this is the largest and the only multicenter study that examined the humoral response to the HPV vaccine in both girls and boys with KTs, compared to CKD and on dialysis. It is also the first study that compared the humoral response between girls and boys in these populations. In addition, we included only data for the subjects who were HPV naïve at baseline, had complete data in terms of antibody titers and completed the entire three dose vaccination series.
In conclusion, this study addresses the immune response to the HPV vaccine in pediatric KT recipients, CKD and dialysis patients, as well as its safety. The HPV vaccine was well-tolerated in these populations. Pediatric KT recipients had in general lower GMTs and seroconversion rates compared to their peers in the CKD and dialysis groups. In addition, immunosuppression (either due to the need to address immune-mediated diseases of the native kidneys or to prevent rejection in KT) played a role in the lack of seroconversion. Our results emphasize the importance of advocating for HPV vaccination prior to KT and acknowledge its safety post transplantation. However, questions still remain. Specifically, studies to identify factors associated with the lack of seroconversion in KT recipients are needed for targeted interventions. In addition, future studies should investigate the effect of a supplemental dose of HPV vaccine in KT recipients who do not seroconvert. It would also be important to evaluate the long-term persistence of antibodies post-KT and the possible need for re-immunization post-KT.
All datasets generated for this study are included in the article/supplementary material.
The studies involving human participants were reviewed and approved by Indiana University Office of Research Administration Institutional Board Review. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
CN generated the hypothesis, obtained funding, designed the study, recruited subjects at her institution, interpreted data, and wrote the manuscript. RN, PV, KT, AC, MM, and JM had solutions for improving design, recruited subjects at their respective institutions, and corrected the manuscript. JS analyzed and presented data, while also helping CN and MS write the statistics paragraph and interpret data. MS mentored and helped CN in all of the above endeavors.
This study was funded by the Merck Investigator Studies Program (Merck GmbH, Whitehouse Station, NJ, USA). IISP ID#: 33600.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank Dr. Alfred Saah for the careful review of the manuscript.
SOT, solid organ transplant; KT, kidney transplant; HPV, human papilloma virus; CKD, chronic kidney disease; GFR, glomerular filtration rate; eGFR, estimated GFR calculated using the modified Schwartz formula (0.413 × height in cm/serum creatinine in mg/dL); T1, time 1 (day 1 of enrollment prior to dose 1 of the vaccine administration); T2, time 2 (one month after vaccine series was completed); VRC, vaccination report card; AEs, adverse events; SD, standard deviation; IQR, interquartile range.
1. Kitchlu A, Dixon S, Dirk JS, Chanchlani R, Vasilevska-Ristovska J, Borges K, et al. Elevated risk of cancer after solid organ transplant in childhood: a population-based cohort study. Transplantation. (2019) 103:588–96. doi: 10.1097/TP.0000000000002378
2. Yanik EL, Smith JM, Shiels MS, Clarke CA, Lynch CF, Kahn AR, et al. Cancer risk after pediatric solid organ transplantation. Pediatrics. (2017) 139:e20163893. doi: 10.1542/peds.2016-3893
3. Hall EC, Pfeiffer RM, Segev DL, Engels EA. Cumulative incidence of cancer after solid organ transplantation. Cancer. (2013) 119:2300–8. doi: 10.1002/cncr.28043
4. D'Arcy ME, Coghill AE, Lynch CF, Koch LA, Li J, Pawlish KS, et al. Survival after a cancer diagnosis among solid organ transplant recipients in the United States. Cancer. (2019) 125:933–42. doi: 10.1002/cncr.31782
5. Smith JM, Martz K, Blydt-Hansen TD. Pediatric kidney transplant practice patterns and outcome benchmarks, 1987-2010: a report of the North American pediatric renal trials and collaborative studies. Pediatr Transplant. (2013) 17:149–57. doi: 10.1111/petr.12034
6. Awan AA, Niu J, Pan JS, Erickson KF, Mandayam S, Winkelmayer WC, et al. Trends in the causes of death among kidney transplant recipients in the United States (1996-2014). Am J Nephrol. (2018) 48:472–81. doi: 10.1159/000495081
7. Hinten F, Meeuwis KA, van Rossum MM, de Hullu JA. HPV-related (pre)malignancies of the female anogenital tract in renal transplant recipients. Crit Rev Oncol Hematol. (2012) 84:161–80. doi: 10.1016/j.critrevonc.2012.02.008
8. Liao JB, Fisher CE, Madeleine MM. Gynecologic cancers and solid organ transplantation. Am J Transplant. (2019) 19:1266–77. doi: 10.1111/ajt.15292
9. Madeleine MM, Finch JL, Lynch CF, Goodman MT, Engels EA. HPV-related cancers after solid organ transplantation in the United States. Am J Transplant. (2013) 13:3202–9. doi: 10.1111/ajt.12472
10. Smith JS, Lindsay L, Hoots B, Keys J, Franceschi S, Winer R, et al. Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions: a meta-analysis update. Int J Cancer. (2007) 121:621–32. doi: 10.1002/ijc.22527
11. Antonsson A, Neale RE, Boros S, Lampe G, Coman WB, Pryor DI, et al. Human papillomavirus status and p16(INK4A) expression in patients with mucosal squamous cell carcinoma of the head and neck in Queensland, Australia. Cancer Epidemiol. (2015) 39:174–81. doi: 10.1016/j.canep.2015.01.010
12. Satterwhite CL, Torrone E, Meites E, Dunne EF, Mahajan R, Ocfemia MC, et al. Sexually transmitted infections among US women and men: prevalence and incidence estimates, 2008. Sex Transm Dis. (2013) 40:187–93. doi: 10.1097/OLQ.0b013e318286bb53
13. Komlos KF, Kocjan BJ, Kosorok P, Luzar B, Meglic L, Potocnik M, et al. Tumor-specific and gender-specific pre-vaccination distribution of human papillomavirus types 6 and 11 in anogenital warts and laryngeal papillomas: a study on 574 tissue specimens. J Med Virol. (2012) 84:1233–41. doi: 10.1002/jmv.23318
14. Orlando G, Bianchi S, Fasolo MM, Mazza F, Frati ER, Rizzardini G, et al. Cervical Human Papillomavirus genotypes in HIV-infected women: a cross-sectional analysis of the VALHIDATE study. J Prev Med Hyg. (2017) 58:E259–e65. doi: 10.15167/2421-4248/jpmh2017.58.4.804
15. Long ME, Chantigian PDM, Weaver AL. Cervical cytology and histology after solid organ transplant: a longitudinal cohort study. J Low Genit Tract Dis. (2018) 22:362–6. doi: 10.1097/LGT.0000000000000414
16. Veroux M, Corona D, Scalia G, Garozzo V, Gagliano M, Giuffrida G, et al. Surveillance of human papilloma virus infection and cervical cancer in kidney transplant recipients: preliminary data. Transplant Proc. (2009) 41:1191–4. doi: 10.1016/j.transproceed.2009.03.015
17. Berkhout RJ, Bouwes Bavinck JN, ter Schegget J. Persistence of human papillomavirus DNA in benign and (pre)malignant skin lesions from renal transplant recipients. J Clin Microbiol. (2000) 38:2087–96.
18. Pietrzak B, Mazanowska N, Ekiel AM, Durlik M, Martirosian G, Wielgos M, et al. Prevalence of high-risk human papillomavirus cervical infection in female kidney graft recipients: an observational study. Virol J. (2012) 9:117. doi: 10.1186/1743-422X-9-117
19. Meeuwis KA, Melchers WJ, Bouten H, van de Kerkhof PC, Hinten F, Quint WG, et al. Anogenital malignancies in women after renal transplantation over 40 years in a single center. Transplantation. (2012) 93:914–22. doi: 10.1097/TP.0b013e318249b13d
20. Busnach G, Piselli P, Arbustini E, Baccarani U, Burra P, Carrieri MP, et al. Immunosuppression and cancer: a comparison of risks in recipients of organ transplants and in HIV-positive individuals. Transplant Proc. (2006) 38:3533–5. doi: 10.1016/j.transproceed.2006.10.144
21. Guerra FM, Rosella LC, Dunn S, Wilson SE, Chen C, Deeks SL. Early impact of Ontario's human papillomavirus (HPV) vaccination program on anogenital warts (AGWs): A population-based assessment. Vaccine. (2016) 34:4678–83. doi: 10.1016/j.vaccine.2016.08.020
22. Niccolai LM, Meek JI, Brackney M, Hadler JL, Sosa LE, Weinberger DM. Declines in Human Papillomavirus (HPV)-associated high-grade cervical lesions after introduction of HPV vaccines in connecticut, United States, 2008-2015. Clin Infect Dis. (2017) 65:884–9. doi: 10.1093/cid/cix455
23. Arbyn M, Xu L. Efficacy and safety of prophylactic HPV vaccines. a Cochrane review of randomized trials. Expert Rev Vaccines. (2018) 17:1085–91. doi: 10.1080/14760584.2018.1548282
24. Collins S, Mazloomzadeh S, Winter H, Blomfield P, Bailey A, Young LS, et al. High incidence of cervical human papillomavirus infection in women during their first sexual relationship. BJOG. (2002) 109:96–8. doi: 10.1111/j.1471-0528.2002.01053.x
25. Castellino SM, Allen KE, Pleasant K, Keyes G, Poehling KA, Tooze JA. Suboptimal uptake of human papillomavirus (HPV) vaccine in survivors of childhood and adolescent and young adult (AYA) cancer. J Cancer Surviv. (2019) 13:730–8. doi: 10.1007/s11764-019-00791-9
26. Fox TG, Nailescu C. Vaccinations in pediatric kidney transplant recipients. Pediatr Nephrol. (2019) 34:579–91. doi: 10.1007/s00467-018-3953-z
27. Danziger-Isakov L, Kumar D. Vaccination in solid organ transplantation. Am J Transplant. (2013) 13 (Suppl. 4):311–7. doi: 10.1111/ajt.12122
28. Centers for Disease Control and Prevention (CDC). Recommendations on the use of quadrivalent human papillomavirus vaccine in males–Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep. (2011) 60:1705–8.
29. Dempsey AF, Patel DA. HPV vaccine acceptance, utilization and expected impacts in the U.S.: Where are we now? Hum Vaccin. (2010) 6:715–20. doi: 10.4161/hv.6.9.12730
30. Donahue KL, Stupiansky NW, Alexander AB, Zimet GD. Acceptability of the human papillomavirus vaccine and reasons for non-vaccination among parents of adolescent sons. Vaccine. (2014) 32:3883–5. doi: 10.1016/j.vaccine.2014.05.035
31. Petrosky E, Bocchini JA Jr, Hariri S, Chesson H, Curtis CR, Saraiya M, et al. Use of 9-valent human papillomavirus (HPV) vaccine: updated HPV vaccination recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep. (2015) 64:300–4.
32. Lindberg LD, Maddow-Zimet I, Marcell AV. Prevalence of sexual initiation before Age 13 years among male adolescents and young adults in the United States. JAMA Pediatr. 2019. doi: 10.1001/jamapediatrics.2019.0458
33. Gambadauro P, Carli V, Hadlaczky G, Sarchiapone M, Apter A, Balazs J, et al. Correlates of sexual initiation among European adolescents. PloS ONE. (2018) 13:e0191451. doi: 10.1371/journal.pone.0191451
34. Kahn NF, Halpern CT. Associations between patterns of sexual initiation, sexual partnering, and sexual health outcomes from adolescence to early adulthood. Arch Sex Behav. (2018) 47:1791–810. doi: 10.1007/s10508-018-1176-9
35. Franke D, Winkel S, Gellermann J, Querfeld U, Pape L, Ehrich JH, et al. Growth and maturation improvement in children on renal replacement therapy over the past 20 years. Pediatr Nephrol. (2013) 28:2043–51. doi: 10.1007/s00467-013-2502-z
36. Wilkin T, Lee JY, Lensing SY, Stier EA, Goldstone SE, Berry JM, et al. Safety and immunogenicity of the quadrivalent human papillomavirus vaccine in HIV-1-infected men. J Infect Dis. (2010) 202:1246–53. doi: 10.1086/656320
37. Levin MJ, Moscicki AB, Song LY, Fenton T, Meyer WA 3rd, Read JS, et al. Safety and immunogenicity of a quadrivalent human papillomavirus (types 6, 11, 16, and 18) vaccine in HIV-infected children 7 to 12 years old. J Acquir Immune Defic Syndr. (2010) 55:197–204. doi: 10.1097/QAI.0b013e3181de8d26
38. Weinberg A, Song LY, Saah A, Brown M, Moscicki AB, Meyer WA 3rd, et al. Humoral, mucosal, and cell-mediated immunity against vaccine and nonvaccine genotypes after administration of quadrivalent human papillomavirus vaccine to HIV-infected children. J Infect Dis. (2012) 206:1309–18. doi: 10.1093/infdis/jis489
39. Brown DR, Garland SM, Ferris DG, Joura E, Steben M, James M, et al. The humoral response to Gardasil over four years as defined by total IgG and competitive Luminex immunoassay. Hum Vaccin. (2011) 7:230–8. doi: 10.4161/hv.7.2.13948
40. Kumar D, Unger ER, Panicker G, Medvedev P, Wilson L, Humar A. Immunogenicity of quadrivalent human papillomavirus vaccine in organ transplant recipients. Am J Transplant. (2013) 13:2411–7. doi: 10.1111/ajt.12329
41. Nelson DR, Neu AM, Abraham A, Amaral S, Batisky D, Fadrowski JJ. Immunogenicity of human papillomavirus recombinant vaccine in children with CKD. Clin J Am Soc Nephrol. (2016) 11:776–84. doi: 10.2215/CJN.09690915
42. Chonchol M. Neutrophil dysfunction and infection risk in end-stage renal disease. Semin Dial. (2006) 19:291–6. doi: 10.1111/j.1525-139X.2006.00175.x
43. Dalrymple LS, Go AS. Epidemiology of acute infections among patients with chronic kidney disease. Clin J Am Soc Nephrol. (2008) 3:1487–93. doi: 10.2215/CJN.01290308
44. Schulman SL, Deforest A, Kaiser BA, Polinsky MS, Baluarte HJ. Response to measles-mumps-rubella vaccine in children on dialysis. Pediatr Nephrol. (1992) 6:187–9. doi: 10.1007/BF00866312
45. Fadrowski JJ, Furth SL. Varicella zoster virus: vaccination and implications in children with renal failure. Expert Rev Vaccines. (2004) 3:291–8. doi: 10.1586/14760584.3.3.291
46. Praditpornsilpa K, Kingwatanakul P, Deekajorndej T, Rianthavorn P, Susantitaphong P, Katavetin P, et al. Immunogenicity and safety of quadrivalent human papillomavirus types 6/11/16/18 recombinant vaccine in chronic kidney disease stage IV, V and VD. Nephrol Dial Transplant. (2017) 32:132–6. doi: 10.1093/ndt/gfv444
47. Block SL, Nolan T, Sattler C, Barr E, Giacoletti KE, Marchant CD, et al. Comparison of the immunogenicity and reactogenicity of a prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in male and female adolescents and young adult women. Pediatrics. (2006) 118:2135–45. doi: 10.1542/peds.2006-0461
48. Ferris D, Samakoses R, Block SL, Lazcano-Ponce E, Restrepo JA, Reisinger KS, et al. Long-term study of a quadrivalent human papillomavirus vaccine. Pediatrics. (2014) 134:e657–65. doi: 10.1542/peds.2013-4144
49. Klein SL, Marriott I, Fish EN. Sex-based differences in immune function and responses to vaccination. Trans R Soc Trop Med Hyg. (2015) 109:9–15. doi: 10.1093/trstmh/tru167
50. Furman D, Hejblum BP, Simon N, Jojic V, Dekker CL, Thiebaut R, et al. Systems analysis of sex differences reveals an immunosuppressive role for testosterone in the response to influenza vaccination. Proc Natl Acad Sci USA. (2014) 111:869–74. doi: 10.1073/pnas.1321060111
51. Bhat M, Mara K, Dierkhising R, Watt KDS. Immunosuppression, race, and donor-related risk factors affect de novo cancer incidence across solid organ transplant recipients. Mayo Clin Proc. (2018) 93:1236–46. doi: 10.1016/j.mayocp.2018.04.025
52. Choi Y, Eworuke E, Segal R. What explains the different rates of human papillomavirus vaccination among adolescent males and females in the United States? Papillomavirus Res. (2016) 2:46–51. doi: 10.1016/j.pvr.2016.02.001
53. Gomez-Lobo V, Whyte T, Kaufman S, Torres C, Moudgil A. Immunogenicity of a prophylactic quadrivalent human papillomavirus L1 virus-like particle vaccine in male and female adolescent transplant recipients. Pediatr Transplant. (2014) 18:310–5. doi: 10.1111/petr.12226
Keywords: HPV—human papillomavirus, vaccination, kidney transplantation, chronic kidney disease, dialysis, pediatric
Citation: Nailescu C, Nelson RD, Verghese PS, Twombley KE, Chishti AS, Mills M, Mahan JD, Slaven JE and Shew ML (2020) Human Papillomavirus Vaccination in Male and Female Adolescents Before and After Kidney Transplantation: A Pediatric Nephrology Research Consortium Study. Front. Pediatr. 8:46. doi: 10.3389/fped.2020.00046
Received: 13 December 2019; Accepted: 28 January 2020;
Published: 20 February 2020.
Edited by:
Agnieszka Swiatecka-Urban, University of Pittsburgh, United StatesReviewed by:
Vera Hermina Koch, University of São Paulo, BrazilCopyright © 2020 Nailescu, Nelson, Verghese, Twombley, Chishti, Mills, Mahan, Slaven and Shew. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Corina Nailescu, Y25haWxlc2NAaXUuZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.