
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr. , 11 December 2019
Sec. Pediatric Cardiology
Volume 7 - 2019 | https://doi.org/10.3389/fped.2019.00514
This article is part of the Research Topic Acquired Heart Disease in Children: Pathogenesis, Diagnosis and Management View all 36 articles
 Shu Wu1,2†
Shu Wu1,2† Yuan Long3†
Yuan Long3† Selena Chen4
Selena Chen4 Yaqian Huang1
Yaqian Huang1 Ying Liao1
Ying Liao1 Yan Sun1
Yan Sun1 Qingyou Zhang1
Qingyou Zhang1 Chunyu Zhang1
Chunyu Zhang1 Hui Yan1
Hui Yan1 Jianguang Qi1
Jianguang Qi1 Xueqin Liu1
Xueqin Liu1 Yonghong Chen1
Yonghong Chen1 Yong Zhang3*
Yong Zhang3* Junbao Du1,5*
Junbao Du1,5*Background: Children with Kawasaki disease (KD) under 1-year old are at high risk for intravenous immunoglobulin (IVIG) resistance. The study was designed to explore the predictive measure of IVIG resistance in infants under 1-year old with KD.
Methods: This study enrolled children under 1-year old suffering from KD in Peking University First Hospital and Wuhan Children's Hospital. All infants were divided into IVIG-responsive and IVIG-resistant groups. The differences in demographic characteristics, clinical features, and laboratory examinations were compared and the risk factors of IVIG resistant KD were analyzed. Furthermore, a scoring system was developed for predicting IVIG resistance in KD infants and an external validation was performed.
Result: A total of 282 infants (194 boys, median age of 7.0 months) were enrolled in this study, of whom 23 children were IVIG-resistant. Compared with IVIG-responsive infants, those in the IVIG-resistant group had a high neutrophil-to-lymphocyte ratio (NLR), high platelet-to-lymphocyte ratio (PLR), high mean platelet volume-to-lymphocyte ratio (MPVLR) in peripheral blood, and low serum albumin, and low serum sodium before IVIG therapy (all P < 0.01). Multiple regression analysis indicated that high levels of peripheral NLR and MPVLR, and low levels of serum albumin and serum sodium were independent risk factors for IVIG resistant KD infants. A scoring system, which included peripheral NLR ≥ 2.69 (1 point), MPVLR ≥ 2.78 (1 point), serum albumin ≤ 30.7 g/L (1 point), and serum sodium ≤ 135.2 mmol/L (1 point), was established. A cut-off value of a total score of 2 points or higher yielded a sensitivity of 87.0% and a specificity of 78.4%, with an area under the curve of 0.891. External validation with clinical diagnostic standard showed that a cut-off value of total score of 2 points or higher for predicting the IVIG-resistance yielded a sensitivity of 70.0% and a specificity of 75.1%.
Conclusion: For the first time, we proposed a predictive model of IVIG resistance in KD infants under 1-year old. The scoring system, which accounts for baseline peripheral NLR, MPVLR, and serum albumin and sodium, predicts with relatively high sensitivity and specificity for IVIG-resistant infants with KD under 1-year old.
Kawasaki disease (KD) commonly presents as an acute autoimmune vasculitis in childhood (1). Serious complications include coronary dilatation and coronary aneurysm, which may result in myocardial infarction (2, 3). Intravenous immunoglobulin (IVIG) with oral aspirin can significantly reduce the incidence of coronary artery complications (4). It is a standardized treatment for KD that is widely accepted (5). However, some children are resistant to IVIG therapy and have recurrent or persistent fever 36–48 h after the first dose of IVIG (4). The incidence of IVIG resistance was about 4.9–38.3% in different regions according to particular definition (4, 6–9). IVIG resistance represents severe inflammatory response and it is also an independent predictor for coronary artery lesions (10–12).
The peak incidence of IVIG resistance occurs at ages <1-year old, especially between 9 and 11 months old (9). Kobayashi et al. have shown that ages under 1-year old are an independent risk factor for not only IVIG resistance (13) but also coronary artery lesions (8). In recent years, randomized, open-label, blinded-endpoints trials have confirmed that IVIG therapy combined with other immunosuppressive agents such as glucocorticoid and cyclosporine effectively reduce the incidence of coronary artery complications in children predicted with IVIG resistance before treatment (14, 15). Therefore, it is important to determine an efficient predictive scoring system of IVIG resistance for KD infants under 1-year old.
Classic indicators previously identified to predict IVIG resistance include young ages, a high peripheral neutrophil percentage, high c-reactive protein, serum alanine transaminase (ALT), glutamyl transpeptidase, and total bilirubin levels, and low peripheral hemoglobin, serum albumin, and serum sodium levels (13, 16–21). Investigators have reported several scoring systems predicting IVIG resistance, for instance, the scoring systems by Sano, Kobayashi, and Egami scoring systems in Japan and San Diego scoring system in the United States. However, they showed unsatisfactory predictive abilities when validated externally in Chinese (22, 23). Recent studies showed that the neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and mean platelet volume-to-lymphocyte ratio (MPVLR) in peripheral blood could reflect the severity of inflammatory and cardiovascular disease (24–26). The basic pathological manifestation of KD is systemic vasculitis, and the increase of peripheral NLR and PLR are closely related to IVIG resistance (24, 27). However, at present the relationship between MPVLR and IVIG resistance remains unexplored. A previous study showed that in patients at all ages with KD, NLR ≥ 2.8 was a high risk factor for IVIG resistance (28), but the peripheral lymphocyte count or neutrophil count markedly changes with respect to the age groups in children. This has a significant influence on the predictive value of IVIG-resistant patients with KD.
Therefore, considering the specific impact of the peripheral lymphocyte count or neutrophil count according to age, and understanding that the peak incidence of IVIG resistance occurs at ages younger than 12 months old, the present study was undertaken to explore the predictive indicators of IVIG resistance to establish a Chinese scoring system predicting IVIG resistant KD infants under 1-year old.
This research was a double-center-based retrospective study. The medical data of children under 1-year old diagnosed with KD in the department of pediatrics at Peking University First Hospital from January 2008 to August 2019 and Wuhan Children's Hospital from January 2018 to August 2019 were collected for constructing the predictive scoring system. Furthermore, the medical data of children under 1-year old diagnosed with KD in Wuhan Children's Hospital from January 2016 to December 2017 were used for external validation. All children met the KD diagnostic criteria by the American Academy of Pediatrics and the American Heart Association (29). The first day of illness was defined as the first day of fever. The following cases were excluded: (1) patients with illness days longer than 10 days; (2) patients treated with IVIG before admission; (3) patients without use of IVIG after admission; (4) patients with incomplete data (Figure 1). A total of 469 children were enrolled, receiving IVIG of 2 g/kg combined with oral aspirin of 30–50 mg/kg/d initially. IVIG resistance was defined as infants with KD having persistent or recrudescent fever (≥38°C) 48 h after completion of the first IVIG infusion (18). Two hundred eighty-two infants (259 IVIG-responsive cases and 23 IVIG-resistant cases) were used for the scoring system development to predict IVIG resistance in KD infants, and another 187 infants (177 IVIG-responsive cases and 10 IVIG-resistant cases) for the external validation (Figure 1). This study was approved by the Ethics Committee of Peking University First Hospital, China and the Ethics Committee of Wuhan Children's Hospital.
Data referring to demographic characteristics, clinical manifestations, laboratory examinations before IVIG therapy, and echocardiography results were documented. The peripheral white blood cell count (WBC), neutrophil count, lymphocyte count, hemoglobin, platelet count, mean platelet volume, NLR, PLR, and MPVLR, together with ALT, albumin, and sodium in serum were recorded. We used echocardiography by two-dimensional ultrasound during hospitalization to assess coronary artery lesions. Coronary artery luminal diameters of the left main coronary artery and the right coronary artery were converted to body surface area-adjusted Z-scores. If the maximum Z-score of the coronary artery was >2.5, a coronary artery lesion was determined (29).
Statistical analysis was performed by SPSS version 25.0. We used frequency (percentage) to describe categorical variables and a χ2-test was used to analyze the difference between the 2 groups. For continuous variables, normally distributed variables were expressed as the mean ± standard deviation and assessed by independent sample t-test, and non-normally distributed variables were shown as median (interquartile range) and compared by the Mann-Whitney U test. Univariable analysis was performed to determine the differences in age (months), gender, peripheral WBC, hemoglobin, NLR, PLR, and MPVLR, and serum ALT, albumin, and sodium between two groups, and the continuous variables were converted to categorical variables first. Variables selected by the univariate analysis (p < 0.05) were applied for multivariate logistic regression to screen out independent risk factors for IVIG resistance. To construct the scoring system, the score of independent risk factors were determined by the odd ratios, and each patient obtained a total score. The cut-off point was chosen by the receiver-operator characteristic (ROC) curves and adjusted by the previous classical literature and clinical practice. The cut-off score was chosen at the highest Youden index and the sensitivity and specificity of the scoring system were analyzed. A value of P < 0.05 was considered statistically significant.
One hundred ninety-four boys and 88 girls at a median age of 7.0 (4.0, 9.0) months were analyzed for establishing the scoring system in this study. There were 259 IVIG responders and 23 IVIG non-responders. IVIG resistance occurred in 8.16% of the study subjects. The IVIG-responsive group included 175 boys (67.6%) and 84 girls (32.4%) at a median age of 7.0 (4.0, 9.0) months. The IVIG-resistant group included 19 boys (82.6%) and 4 girls (17.4%) at a median age of 8.0 (5.0, 10.0) months. The percentage of patients with incomplete KD and coronary artery abnormalities between two groups did not differ (P > 0.05, Table 1). Compared with the IVIG-responsive group, the levels of peripheral neutrophil count, NLR, PLR, and MPVLR were significantly increased in IVIG-resistant patients, and the levels of peripheral lymphocyte and platelet count, serum albumin and sodium levels were significantly decreased (P < 0.01, except for the platelet count, P < 0.05; Table 1).
Ten categorical variables were analyzed in the univariate analysis. The cut-off point for each variable was as follows: (1) age ≤6 months; (2) gender, male; (3) peripheral WBC ≥ 14.5 × 109/L; (4) peripheral hemoglobin ≤ 100.5 g/L; (5) peripheral NLR ≥ 2.69; (6) peripheral PLR ≥ 110.92; (7) peripheral MPVLR ≥ 2.78; (8) serum ALT ≥ 60 IU/L; (9) serum albumin ≤ 30.2 g/L; and (10) serum sodium ≤ 135.2 mmol/L. Peripheral NLR, PLR, and MPVLR, and serum albumin and sodium levels were significantly different between the two groups (P < 0.01, Table 2).
Peripheral NLR, PLR, and MPVLR, and serum albumin and sodium were analyzed by multivariate logistic regression. The results indicated that peripheral NLR (≥ 2.69), MPVLR (≥ 2.78), serum albumin (≤ 30.7 g/L), and sodium (≤ 135.2 mmol/L) were independent risk factors for IVIG resistance with OR values of 4.027, 3.860, 3.300, and 3.700, respectively (Table 3).
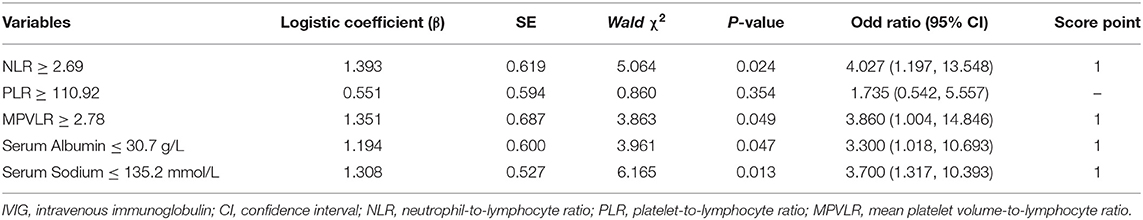
Table 3. Independent factors identified by multiple logistic regression analysis for prediction of IVIG resistance.
To construct the predictive scoring system, peripheral NLR (≥2.69), MPVLR (≥2.78), serum albumin (≤30.7 g/L), and serum sodium (≤135.2 mmol/L) were all given 1 point depending upon the proximity of their odds ratio values. The total scores were calculated for each patient with KD. ROC analysis showed that the area under the curve (AUC) was 0.891 (95% confidence interval, 0.837–0.945; P < 0.001), and a cut-off score of 2 points or higher yielded the sensitivity of 87.0% and specificity of 78.4% to predict IVIG resistance (Figure 2).
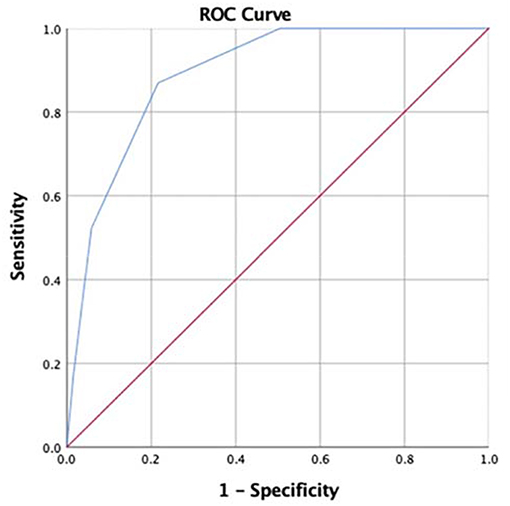
Figure 2. Receiver operating characteristic (ROC) curve of our scoring system for prediction of intravenous immunoglobulin (IVIG) resistance in Kawasaki disease (KD) patients under 1-year old. For the cut-off value of 2 points or more, the sensitivity and specificity were 87.0% and 78.4%, and the area under the curve (AUC) was 0.891 (95% confidence interval 0.837–0.945, p < 0.001).
One hundred eighty-seven infants were enrolled in the externally validated population. External validation with clinical diagnostic standard showed that a cut-off value of total score of 2 points or higher for predicting the IVIG-resistance yielded a sensitivity of 70.0% and a specificity of 75.1% (Table 4).
Patients under 1-year of age diagnosed with KD are prone to be resistant to the initial IVIG treatment and develop coronary artery lesions. Our predictive model is the first scoring system for predicting IVIG-resistant patients with KD under 1-year old. The scoring system includes peripheral NLR ≥ 2.69 (1 point), peripheral MPVLR ≥ 2.78 (1 point), serum albumin ≤ 30.7 g/L (1 point) and serum sodium ≤ 135.2 mmol/L (1 point), and a total score ≥ 2 points yielded a sensitivity and a specificity of 87.0 and 78.4%, respectively, for predicting IVIG-resistance, and in external validation the sensitivity and specificity of predicting IVIG-resistance in KD infants were 70.0% and 75.1%, respectively.
The major pathological changes of KD were systemic vasculitis affecting small and medium-size arteries. Elevated peripheral NLR and MPVLR and decreased serum albumin and sodium represent the severity of inflammation. NLR stands for the ratio of absolute neutrophil count to lymphocyte count in peripheral blood. During systemic inflammation, increased neutrophil production in the bone marrow and circulation into blood, as well as delayed apoptosis, result in neutrophilia. Neutrophils play a critical role in the progression of vascular inflammation by migrating to the site of inflammation and releasing inflammatory cytokines and activating T cells. Meanwhile, accelerated apoptosis results from immunosuppression induced lymphocytopenia (30, 31). In consequence, a high level of peripheral NLR indicates the severity of the clinical course. Peripheral MPVLR represents the ratio of mean platelet volume to lymphocyte count, and high peripheral MPV values have been found in a variety of inflammatory diseases (32). Elevated MPVLR was shown in previous studies to predict the poor prognosis of patients with cardiovascular disease, especially for coronary heart disease (25, 33). This present study is the first to report that high MPVLR (≥2.78) is an independent risk indicator for predicting IVIG resistance in infants with KD under 1-year old.
The mechanisms of hypoalbuminemia consist of the following: first, increased vascular permeability leading to leakage of albumin (34, 35); second, liver dysfunction resulting in decreased albumin synthesis; and third, a lack of essential amino acids due to low nutrient intake or malnutrition, resulting in reduced albumin synthesis (36). IVIG non-responders tend to have more severe vascular leakage and liver damage, inducing lower albumin levels. The cause of hyponatremia is still unknown. Lim et al. found that there was a strong negative correlation between the level of serum sodium and inflammatory factors including c-reactive protein and interleukin-6 (IL-6) in children with KD (37). In addition to KD, studies referring to patients with inflammatory disease such as pneumonia, urinary tract infection, and lupus erythematosus also demonstrated that hyponatremia is an important marker for the severity and prognosis (38–40). The most probable pathophysiological mechanism for hyponatremia is non-osmotic secretion of antidiuretic hormone (ADH). Several studies have confirmed that the release of ADH is promoted by IL-6 and tumor necrosis factor-α (TNF-α) during inflammation (41). IL-6, TNF-α as well as other cytokines participate in inflammation of KD patients in the acute phase (42), suggesting that hyponatremia may be associated with inappropriate release of ADH. The marked increase in plasma IL-6 and TNF-α in IVIG-resistant infants compared with IVIG-responsive patients (43, 44) may explain the significant hyponatremia in IVIG non-responders. More serious inflammatory reactions at the acute phase in IVIG non-responders than in IVIG responders supports our findings that the inflammation-related indicators, including high peripheral NLR and MPVLR, and low serum albumin and sodium, could be used for predicting IVIG-resistant infants with KD under 1-year old effectively.
The indicators in our scoring system for predicting IVIG resistance, which include peripheral NLR and MPVLR and serum albumin and sodium, have significant advantages. They are inexpensive and easy-to-operate as routine examinations. Moreover, the peripheral neutrophil and lymphocyte are less influenced by age during the first 12 months. Our scoring system would have evident practical value for clinical applications due to its relatively high sensitivity and specificity.
There are some limitations to this study. The results may have bias as it was a retrospective study. The sample size was not large enough and a large-scaled external validation of our scoring system will be required in the future. However, the predictive model consisting of peripheral NLR (≥2.69) and MPVLR (≥2.78) and serum albumin (≤30.7 g/L) and sodium (≤135.2 mmol/L) prior to IVIG therapy showed relatively high sensitivity and specificity for the prediction of IVIG-resistant infants with KD under 1-year old.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation, to any qualified researcher.
The studies involving human participants were reviewed and approved by the Ethics Committee of Peking University First Hospital and the Ethics Committee of Wuhan Children's Hospital. Written informed consent for participation was not provided by the participants' legal guardians/next of kin because this is a retrospective study.
SW, YLo, YZ, and JD designed the study and analyzed the data. SW, YLo, YLi, YH, YS, CZ, HY, QZ, JQ, YC, XL, and YZ acquired the data. SW and YZ organized the database. SW, SC, and JD drafted the manuscript. YLo, YLi, YS, CZ, HY, QZ, JQ, YC, XL, YZ, and YH read and revised the manuscript. All authors contributed to all study data, write and approved the final version of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Saguil A, Fargo M, Grogan S. Diagnosis and management of Kawasaki disease. Am Fam Physician. (2015) 91:365–71.
2. Bayers S, Shulman ST, Paller AS. Kawasaki disease: part II. complications and treatment. J Am Acad Dermatol. (2013) 69:513.e1–8; quiz 21–2. doi: 10.1016/j.jaad.2013.06.040
3. Shulman ST, Rowley AH. Kawasaki disease: insights into pathogenesis and approaches to treatment. Nat Rev Rheumatol. (2015) 11:475–82. doi: 10.1038/nrrheum.2015.54
4. McCrindle BW, Rowley AH, Newburger JW, Burns JC, Bolger AF, Gewitz M, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a scientific statement for health professionals from the American Heart Association. Circulation. (2017) 135:e927–99. doi: 10.1161/CIR.0000000000000484
5. Kainth R, Shah P. Kawasaki disease: origins and evolution. Arch Dis Child. (2019). doi: 10.1136/archdischild-2019-317070. [Epub ahead of print].
6. Research Committee of the Japanese Society of Pediatric Cardiology, Cardiac Surgery Committee for Development of Guidelines for Medical Treatment of Acute Kawasaki Disease. Guidelines for medical treatment of acute Kawasaki disease: report of the research committee of the Japanese society of pediatric cardiology and cardiac surgery (2012 revised version). Pediatr Int. (2014) 56:135–58. doi: 10.1111/ped.12317
7. Phuong LK, Curtis N, Gowdie P, Akikusa J, Burgner D. Treatment options for resistant Kawasaki disease. Paediatr Drugs. (2018) 20:59–80. doi: 10.1007/s40272-017-0269-6
8. Chen JJ, Ma XJ, Liu F, Yan WL, Huang MR, Huang M, et al. Epidemiologic features of Kawasaki disease in Shanghai from 2008 through 2012. Pediatr Infect Dis J. (2016) 35:7–12. doi: 10.1097/INF.0000000000000914
9. Makino N, Nakamura Y, Yashiro M, Kosami K, Matsubara Y, Ae R, et al. Nationwide epidemiologic survey of Kawasaki disease in Japan, 2015–2016. Pediatr Int. (2019) 61:397–403. doi: 10.1111/ped.13809
10. Xie LP, Yan WL, Huang M, Huang MR, Chen S, Huang GY, et al. Epidemiologic features of Kawasaki disease in Shanghai from 2013 through 2017. J Epidemiol. (2019). doi: 10.2188/jea.JE20190065. [Epub ahead of print].
11. Hua W, Ma F, Wang Y, Fu S, Wang W, Xie C, et al. A new scoring system to predict Kawasaki disease with coronary artery lesions. Clin Rheumatol. (2018) 38:1099–107. doi: 10.1007/s10067-018-4393-7
12. Tang Y, Gao X, Shen J, Sun L, Yan W. Epidemiological and clinical characteristics of Kawasaki disease and factors associated with coronary artery abnormalities in east China: nine years experience. J Trop Pediatr. (2016) 62:86–93. doi: 10.1093/tropej/fmv077
13. Kobayashi T, Inoue Y, Takeuchi K, Okada Y, Tamura K, Tomomasa T, et al. Prediction of intravenous immunoglobulin unresponsiveness in patients with Kawasaki disease. Circulation. (2006) 113:2606–12. doi: 10.1161/CIRCULATIONAHA.105.592865
14. Kobayashi T, Saji T, Otani T, Takeuchi K, Nakamura T, Arakawa H, et al. Efficacy of immunoglobulin plus prednisolone for prevention of coronary artery abnormalities in severe Kawasaki disease (RAISE study): a randomised, open-label, blinded-endpoints trial. Lancet. (2012) 379:1613–20. doi: 10.1016/S0140-6736(11)61930-2
15. Hamada H, Suzuki H, Onouchi Y, Ebata R, Terai M, Fuse S, et al. Efficacy of primary treatment with immunoglobulin plus ciclosporin for prevention of coronary artery abnormalities in patients with Kawasaki disease predicted to be at increased risk of non-response to intravenous immunoglobulin (KAICA): a randomised controlled, open-label, blinded-endpoints, phase 3 trial. Lancet. (2019) 393:1128–37. doi: 10.1016/S0140-6736(18)32003-8
16. Egami K, Muta H, Ishii M, Suda K, Sugahara Y, Iemura M, et al. Prediction of resistance to intravenous immunoglobulin treatment in patients with Kawasaki disease. J Pediatr. (2006) 149:237–40. doi: 10.1016/j.jpeds.2006.03.050
17. Sano T, Kurotobi S, Matsuzaki K, Yamamoto T, Maki I, Miki K, et al. Prediction of non-responsiveness to standard high-dose gamma-globulin therapy in patients with acute Kawasaki disease before starting initial treatment. Eur J Pediatr. (2007) 166:131–7. doi: 10.1007/s00431-006-0223-z
18. Tremoulet AH, Best BM, Song S, Wang S, Corinaldesi E, Eichenfield JR, et al. Resistance to intravenous immunoglobulin in children with Kawasaki disease. J Pediatr. (2008) 153:117–21. doi: 10.1016/j.jpeds.2007.12.021
19. Tang Y, Yan W, Sun L, Huang J, Qian W, Ding Y, et al. Prediction of intravenous immunoglobulin resistance in Kawasaki disease in an East China population. Clin Rheumatol. (2016) 35:2771–6. doi: 10.1007/s10067-016-3370-2
20. Tan XH, Zhang XW, Wang XY, He XQ, Fan C, Lyu TW, et al. A new model for predicting intravenous immunoglobin-resistant Kawasaki disease in Chongqing: a retrospective study on 5277 patients. Sci Rep. (2019) 9:1722. doi: 10.1038/s41598-019-39330-y
21. Yang S, Song R, Zhang J, Li X, Li C. Predictive tool for intravenous immunoglobulin resistance of Kawasaki disease in Beijing. Arch Dis Child. (2019) 104:262–7. doi: 10.1136/archdischild-2017-314512
22. Song R, Yao W, Li X. Efficacy of four scoring systems in predicting intravenous immunoglobulin resistance in children with Kawasaki disease in a children's hospital in Beijing, North China. J Pediatr. (2017) 184:120–4. doi: 10.1016/j.jpeds.2016.12.018
23. Qian W, Tang Y, Yan W, Sun L, Lv H. A comparison of efficacy of six prediction models for intravenous immunoglobulin resistance in Kawasaki disease. Ital J Pediatr. (2018) 44:33. doi: 10.1186/s13052-018-0475-z
24. Kawamura Y, Takeshita S, Kanai T, Yoshida Y, Nonoyama S. The combined usefulness of the neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios in predicting intravenous immunoglobulin resistance with Kawasaki disease. J Pediatr. (2016) 178:281–4.e1. doi: 10.1016/j.jpeds.2016.07.035
25. Kurtul A, Acikgoz SK. Usefulness of mean platelet volume-to-lymphocyte ratio for predicting angiographic no-reflow and short-term prognosis after primary percutaneous coronary intervention in patients with ST-segment elevation myocardial infarction. Am J Cardiol. (2017) 120:534–41. doi: 10.1016/j.amjcard.2017.05.020
26. Gasparyan AY, Ayvazyan L, Mukanova U, Yessirkepov M, Kitas GD. The platelet-to-lymphocyte ratio as an inflammatory marker in rheumatic diseases. Ann Lab Med. (2019) 39:345–57. doi: 10.3343/alm.2019.39.4.345
27. Yuan YD, Sun J, Li PF, Wei CL, Yu YH. Values of neutrophil-lymphocyte ratio and platelet-lymphocyte ratio in predicting sensitivity to intravenous immunoglobulin in Kawasaki disease. Zhongguo Dang Dai Er Ke Za Zhi. (2017) 19:410–3. doi: 10.7499/j.issn.1008-8830.2017.04.010
28. Hua W, Sun Y, Wang Y, Fu S, Wang W, Xie C, et al. A new model to predict intravenous immunoglobin-resistant Kawasaki disease. Oncotarget. (2017) 8:80722–9. doi: 10.18632/oncotarget.21083
29. Newburger JW, Takahashi M, Gerber MA, Gewitz MH, Tani LY, Burns JC, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the committee on rheumatic fever, endocarditis and Kawasaki disease, council on Cardiovascular disease in the Young, American Heart Association. Circulation. (2004) 110:2747–71. doi: 10.1161/01.CIR.0000145143.19711.78
30. Zahorec R. Ratio of neutrophil to lymphocyte counts–rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl Lek Listy. (2001) 102:5–14.
31. Hwang SY, Shin TG, Jo IJ, Jeon K, Suh GY, Lee TR, et al. Neutrophil-to-lymphocyte ratio as a prognostic marker in critically-ill septic patients. Am J Emerg Med. (2017) 35:234–9. doi: 10.1016/j.ajem.2016.10.055
32. Korniluk A, Koper-Lenkiewicz OM, Kaminska J, Kemona H, Dymicka-Piekarska V. Mean platelet volume (MPV): new perspectives for an old marker in the course and prognosis of inflammatory conditions. Mediat Inflamm. (2019) 2019:9213074. doi: 10.1155/2019/9213074
33. Hudzik B, Szkodzinski J, Lekston A, Gierlotka M, Polonski L, Gasior M. Mean platelet volume-to-lymphocyte ratio: a novel marker of poor short- and long-term prognosis in patients with diabetes mellitus and acute myocardial infarction. J Diabetes Complications. (2016) 30:1097–102. doi: 10.1016/j.jdiacomp.2016.04.010
34. Kuo HC, Liang CD, Wang CL, Yu HR, Hwang KP, Yang KD. Serum albumin level predicts initial intravenous immunoglobulin treatment failure in Kawasaki disease. Acta Paediatr. (2010) 99:1578–83. doi: 10.1111/j.1651-2227.2010.01875.x
35. Soeters PB, Wolfe RR, Shenkin A. Hypoalbuminemia: pathogenesis and clinical significance. JPEN J Parenter Enteral Nutr. (2019) 43:181–93. doi: 10.1002/jpen.1451
36. Arques S. Human serum albumin in cardiovascular diseases. Eur J Intern Med. (2018) 52:8–12. doi: 10.1016/j.ejim.2018.04.014
37. Lim GW, Lee M, Kim HS, Hong YM, Sohn S. Hyponatremia and syndrome of inappropriate antidiuretic hormone secretion in kawasaki disease. Korean Circ J. (2010) 40:507–13. doi: 10.4070/kcj.2010.40.10.507
38. Park SW, Shin SM, Jeong M, Cho DH, Lee KH, Eisenhut M, et al. Hyponatremia in children with respiratory infections: a cross-sectional analysis of a cohort of 3938 patients. Sci Rep. (2018) 8:16494. doi: 10.1038/s41598-018-34703-1
39. Park SJ, Oh YS, Choi MJ, Shin JI, Kim KH. Hyponatremia may reflect severe inflammation in children with febrile urinary tract infection. Pediatr Nephrol. (2012) 27:2261–7. doi: 10.1007/s00467-012-2267-9
40. Il Shin J, Park SJ, Suh CH, Lee GH, Hur MW, Han SY, et al. Hyponatremia in patients with systemic lupus erythematosus. Sci Rep. (2016) 6:25566. doi: 10.1038/srep25566
41. Kim JH, Park JH, Eisenhut M, Yu JW, Shin JI. Inflammasome activation by cell volume regulation and inflammation-associated hyponatremia:a vicious cycle. Med Hypotheses. (2016) 93:117–21. doi: 10.1016/j.mehy.2016.05.018
42. Agarwal S, Agrawal DK. Kawasaki disease: etiopathogenesis and novel treatment strategies. Expert Rev Clin Immunol. (2017) 13:247–58. doi: 10.1080/1744666X.2017.1232165
43. Hu P, Jiang GM, Wu Y, Huang BY, Liu SY, Zhang DD, et al. TNF-alpha is superior to conventional inflammatory mediators in forecasting IVIG nonresponse and coronary arteritis in Chinese children with Kawasaki disease. Clin Chim Acta. (2017) 471:76–80. doi: 10.1016/j.cca.2017.05.019
Keywords: Kawasaki disease, infants under 1-year old, intravenous immunoglobulin resistance, scoring system, prediction
Citation: Wu S, Long Y, Chen S, Huang Y, Liao Y, Sun Y, Zhang Q, Zhang C, Yan H, Qi J, Liu X, Chen Y, Zhang Y and Du J (2019) A New Scoring System for Prediction of Intravenous Immunoglobulin Resistance of Kawasaki Disease in Infants Under 1-Year Old. Front. Pediatr. 7:514. doi: 10.3389/fped.2019.00514
Received: 26 October 2019; Accepted: 27 November 2019;
Published: 11 December 2019.
Edited by:
Fu Lijun, Shanghai Children's Medical Center, ChinaReviewed by:
Mingguo Xu, Shenzhen Children's Hospital, ChinaCopyright © 2019 Wu, Long, Chen, Huang, Liao, Sun, Zhang, Zhang, Yan, Qi, Liu, Chen, Zhang and Du. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yong Zhang, MTUzOTIxMDI5OEBxcS5jb20=; Junbao Du, anVuYmFvZHUxQDEyNi5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.