
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pediatr. , 16 July 2019
Sec. Pediatric Nephrology
Volume 7 - 2019 | https://doi.org/10.3389/fped.2019.00287
This article is part of the Research Topic Nephrotic Syndrome: Etiology, Genetic Testing, and Biomarkers View all 10 articles
Arterial hypertension (HTN) is commonly encountered by clinicians treating children with steroid sensitive (SSNS) and steroid resistant nephrotic syndrome (SRNS). Although the prevalence of HTN in SSNS is less documented than in SRNS, recent studies reported high prevalence in both. Studies have estimated the prevalence of HTN in different patient populations with NS to range from 8 to 59.1%. Ambulatory HTN, abnormalities in BP circadian rhythm, and measures of BP variability are prevalent in patients with NS. Multiple mechanisms and co-morbidities contribute to the pathophysiology of HTN in children with NS. Some contributing factors are known to cause acute and episodic elevations in blood pressure such as fluid shifts, sodium retention, and medication side effects (steroids, CNIs). Others are associated with chronic and more sustained HTN such as renal fibrosis, decreased GFR, and progression of chronic kidney disease. Children with NS are more likely to suffer from other cardiovascular disease risk factors, such as obesity, increased measures of arterial stiffness [increased carotid intima-media thickness (cIMT), endothelial dysfunction, increased pulse wave velocity (PWV)], impaired glucose metabolism, dyslipidemia, left ventricular hypertrophy (LVH), left ventricular dysfunction, and atherosclerosis. Those risk factors have been associated with premature death in adults. In this review on HTN in patients with NS, we will discuss the epidemiology and pathophysiology of hypertension in patients with NS, as well as management aspects of HTN in children with NS.
Nephrotic syndrome (NS) is one of the most common childhood kidney diseases worldwide, with a reported incidence of 2–16.9/100,000 children (1, 2). NS encompasses several primary and secondary renal diseases that have common physical changes in glomerular filtration barrier, which result in a massive leak of serum proteins into the urine. The great majority of cases are steroid responsive, with only <20% of children with NS being steroid resistant (3). Minimal change disease (MCD) is the most common glomerular pathology. Although MCD carries an excellent prognosis with low risk of progression to ESRD, its relapsing nature necessitates that children receive frequent courses of steroid therapy and other steroid-sparing medications, many of which are known to affect blood pressure (BP). NS was once thought to be associated with normal or low blood pressure, as described by Volhard's comment, “One of the most important criteria of nephrosis is absence of blood pressure elevation and absence of cardiac hypertrophy (4).” In fact, now we know that a significant portion of children with NS have HTN (Table 1).
NS in children is classified by The American Heart Association as a Tier II (moderate) cardiovascular risk factor (5). The etiology of HTN in nephrotic syndrome (NS) is multifactorial; it is related to a host of both renal and non-renal intrinsic and extrinsic/environmental factors. Some contributing factors are known to cause acute and episodic elevations in blood pressure such as fluid shifts and medication side effects. Others are associated with chronic and more sustained HTN, including renal fibrosis, decreased GFR and progression of chronic kidney disease.
In this review, we examine the prevalence and pathophysiological factors that contribute to the development of HTN in children with NS. We focus our review on primary NS due to MCD and focal segmental glomerulosclerosis (FSGS). We discuss BP variability, circadian rhythms, and ambulatory blood pressure monitoring (ABPM) in this patient population, and highlight management considerations of HTN in children with NS.
The reported prevalence of HTN in childhood NS varies widely. Studies have used different HTN definitions, included patients at different states of NS disease activity, and recruited heterogeneous NS populations with varying glomerular filtration rates (GFRs) receiving different anti-proteinuric and antihypertensive medications, which has made it hard to discern an accurate prevalence of HTN in children with NS.
The International Study of Kidney Disease in Children (ISKDC) reported diastolic blood pressure > 98th %tile in 25/195 (12.8%) of children with Nil disease (MCD), and in 11/82 (13.4%) children with focal glomerular obsolescence (FSGS) (6, 7). Utilizing the PodoNet registry cohort, Trautmann et al. reported the prevalence of HTN at presentation in steroid-resistant and congenital NS to range from 10.2% in children <3 months to 27.9% in adolescents. Children with the histopathologic diagnosis of diffuse mesangial sclerosis had the highest prevalence of HTN (26.3%) (8). Previously, we have reported the prevalence of HTN in a small cohort of children with FSGS to be 43.8%. Steroid resistant patients had a much higher prevalence compared to steroid sensitive patients: 66.7 and 14.3%, respectively (9). Keshri et al. examined 81 children with SSNS in remission and off steroid therapy for 1–10 years and found 23% with hypertension. Of those, 73% had a positive family history of HTN compared to 32% in the normotensive group. BP was significantly correlated with serum cholesterol and LDL levels (10).
In a study examining the prevalence of hypertension in MCD and other types of NS, Küster et al. reported the pre-steroid treatment (edematous phase) prevalence of HTN (defined as BP > 95% of age) in 57 children to be 95%. After complete remission, the prevalence of HTN in this cohort decreased to 19% (11). Kontchou et al. examined the possible role of family history of HTN on the prevalence of HTN in children with MCD. Forty-nine prepubertal children with NS were included; 65% had systolic and/or diastolic BP > 90th %tile in the first week of edema. After 4 weeks of steroid therapy, 34% of children still had blood pressure > 90th percentile. They reported a much higher prevalence of HTN in children with MCD with family history of essential hypertension compared to children with no family history. This difference was more striking (88 vs. 53%) at disease presentation (edematous stage) but also persisted (52 vs. 34%) after 4 weeks of steroid therapy (12).
Mitsnefes et al. reported the prevalence of HTN (SBP or DBP ≥ 95th percentile for gender, age, and height) in a cohort of 3,834 children registered in NAPRTCS to be 48%. In a subgroup of 546 children with glomerulonephritis/FSGS, the prevalence was 17.5%. Children with HTN were more likely to reach the study endpoints (dialysis or 10-ml/min/1.73 m2 drop from baseline eGFR) compared to the normotensive children. It is important to point out that this cohort was composed of children with impaired kidney function and only 13.8% of the cohort had glomerulonephritis/FSGS (13). In children enrolled in the FSGS Clinical Trial, 41 out of 72 (56.9%) children randomized to cyclosporine, and 39 out of 66 (59.1%) children randomized to MMF/dexamethasone had HTN (14). Baseline blood pressures were not reported in this study. In children with nephrotic proteinuria enrolled in Chronic Kidney Disease in Children (CKiD) Study, 17% (10 subjects of 432) had systolic blood pressure ≥ 95th %tile and 23% (13/432) had diastolic blood pressure ≥ 95th %tile. HTN was more prevalent in children with sub-nephrotic proteinuria: 60% (35/432) for SBP and 54% (31/432) for DBP, respectively (15).
A recent review of 147 children enrolled in the Nephrotic Syndrome Study Network (NEPTUNE), consisting of 69 (46.9%) children with Minimal Change Disease (MCD), 49 (33.3%) with FSGS, 8 (5.4%) with IgA, 2 (0.04%) with Membranous Nephropathy, and 19 (12.9%) other glomerulopathy, showed that 69 (46.9%) had prior history of HTN or blood pressure ≥95th% at baseline visit. Authors reported no statistical difference in prevalence of hypertensive status between MCD (41%), FSGS (46.9%), and IgA (62.5%) subjects (16). This is possibly related to the preservation of kidney disease in the childhood years, lessening the effect of lower GFR usually seen with advancing age in FSGS patients. One other possibility is the fact that childhood MCD tends to have a relapsing course necessitating repeated courses of steroids and other steroid-sparing medications, which may increase prevalence of HTN over time.
Gabban et al. prospectively examined a cohort of 71 children 1–18 years old: 33 (46.5%) with steroid sensitive NS, 28 (39.4%) with steroid dependent NS, and 10 (14.1%) with steroid-resistant NS. HTN was present at diagnosis in 5 patients (7%) with steroid sensitive NS, 9 patients (12.6%) with steroid resistant NS and 14 patients (19.7%) with steroid dependent NS. Family history of HTN was reported in 4 cases (5.6%). In over 7 months of follow-up, the overall prevalence of HTN increased to 39.4% (17).
Inaba et al. followed 69 children 1–5 years of age with idiopathic steroid resistant NS for ≥4 years and found that HTN, defined as the need for anti-hypertensive therapy except when given for renoprotective purpose, affected 31.9% of patients (18).
Sarkar et al. examined in-clinic and ABPM in 99 children with frequently relapsing NS. Clinic blood pressure was >95th percentile in 63 (63.6%) patients. Ambulatory HTN was present in 33.3, 16.1% had masked HTN, and 30.3 % had white coat HTN. Nocturnal non-dipping was seen in 72 and 55 patients had a high nocturnal systolic BP load. Twenty-one patients had increased left ventricular mass index (LVMI), of which 42.9% had ambulatory HTN, 14.3% had masked HTN and 28.6% patients had white coat HTN (19). These circadian BP abnormalities and high prevalence of nighttime HTN were also reported in another smaller cohort of 21 patients with primary NS, 17 with MCD and 4 with FSGS. Of these patients, 8 (38%) had daytime HTN, 13 (62%) had nighttime HTN, and 13 (62%) were non-dippers. The data from repeated ABP measurements, before and after the achievement of remission, showed a marked decrease in the average 24 h ABP after remission (20). Xu et al. demonstrated by ABPM that among 114 children with primary NS, 101 had elevated BP (88.6%), 45 showed high incidence of masked HTN (39.5%), and 80 had (nocturnal) non-dipping BP (70.2%).
Data from the NEPTUNE study showed BP variability (BPV) to be associated with the study pre-determined endpoints (complete remission and composite endpoint of ESRD or loss of ≥40% GFR). Greater systolic and diastolic standard deviation (SD) and average real variability were associated with greater hazard of reaching the composite end point in adults (all P < 0.01). In children, greater BPV was an independent predictor of composite endpoint (determined by systolic SD and average real variability) and complete remission (determined by systolic and diastolic average real variability); all P < 0.05 (16).
Children with NS suffer from comorbidities related to medication side effects and the NS disease pathophysiology, many of which are known to increase cardiovascular disease risk including obesity, left ventricular hypertrophy (LVH), increased measures of arterial stiffness (increased cIMT, endothelial dysfunction), impaired glucose metabolism, and hyperlipidemia.
Several studies have reported on the high prevalence of obesity and overweight in children with NS. In a 10 years follow-up study, Ishikura et al. reported 8% of children with frequently relapsing/steroid dependent NS to be obese (21). In a longterm follow-up study (from childhood to adulthood) of 61 patients with NS, Skrzypczyk et al. reported 23% of patients were overweight, 4.9% obese, and 16.1% were hypertensive as adults (22).
Hyperlipidemia, prevalent while children are having significant proteinuria, generally improves once NS is in remission. In a subset of NS patients with steroid resistance and/or frequent relapses, hyperlipidemia becomes a chronic condition. The disorders of lipid and lipoprotein metabolism in NS contribute to the development and progression of cardiovascular and kidney disease, and has been nicely reviewed elsewhere (23). Studies have shown the effects of lipid dysregulation to last for years after steroid treatment (24).
Patients with NS have increased arterial stiffness; Cungor et al. found significantly higher carotid-femoral pulse wave velocity (PWV) in adults with NS compared to healthy controls. Mean arterial pressure was predictive of arterial stiffness (25). Rahul et al. described a lower flow-mediated dilatation (FMD) in 32 children with NS disease duration of more than 2 years compared to their matched controls. The authors attributed the lower FMD to endothelial dysfunction (26). It is important to point out that the authors did not find significant differences in cIMT between the two groups. On the other hand, Hooman et al. found children with NS to have higher cIMT compared to healthy sex- and age-matched children. In their study, the duration of NS and systolic HTN were significantly correlated with carotid IMT (27).
Candan et al. demonstrated subclinical cardiovascular disease in 37 pediatric patients with steroid resistant NS. Compared with the controls, those patients had significantly higher mean aortic PWV, mean cIMT, and LVMI. Increased aortic PWV was noted in 5% of patients, increased carotid IMT in 22%, and increased LVM index in 19% while nocturnal dipping was absent in 67.6% (28).
In summary, the prevalence of HTN in children with NS is highly variable. This is largely due to the dynamic nature of NS disease process. Patients may be in relapses or remission, some patients require treatment with IV albumin infusion and diuretics, patents can be on different and changing doses of medications known to affect BP, and occasionally patients may develop acute kidney injury (AKI) as a complication of NS. Furthermore, studies did not use the same HTN definition, included patients at different states of the NS disease process, included heterogeneous NS populations with varying GFRs and receiving different anti-proteinuric and antihypertensive medications. Although studies examining arterial stiffness in this patient population have reported conflicting results, the majority have signaled an increased arterial stiffness (assessed by PWV, FMD, and cIMT) and highlighted the need for larger prospective controlled studies to further understand the prevalence and the pathophysiology of arterial stiffness in children with NS.
The pathophysiology of HTN in NS is complex, with multiple renal and extra-renal contributing factors. Renal factors include sodium retention, fibrosis/loss of GFR and progression of kidney disease, and recently, a feed-forward loop between albuminuria and blood pressure has been described (29). On the other hand, extra-renal factors include medication side effects, co-morbid conditions, and genetic predisposition (Figure 1).
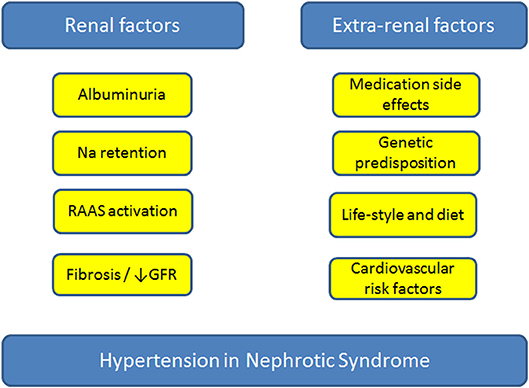
Figure 1. Factors contributing to the development of hypertension in patients with nephrotic syndrome. GFR, glomerular filtration rate; Na, sodium; RAAS, renin-angiotensin-aldosterone-system.
Sodium handling in NS not only has a central role in the development of edema but also plays an important role in blood pressure regulation. Two main hypotheses have been posited to explain the development of sodium retention in NS: the underfill and the overfill hypotheses (30). In the underfill hypothesis, hypovolemia secondary to low oncotic pressure and third-spacing leads to activation of the renin-angiotensin-aldosterone system (RAAS) and sodium retention. In the overfill hypothesis, RAAS is suppressed and it is believed that the sodium retention is related to an intrinsic renal defect in sodium handling. Multiple investigators have examined the role of ENaC activation in sodium and water retention in NS. In those experiments, a defective glomerular filtration barrier allowed the passage of proteolytic enzymes that had the ability to activate ENaC (31, 32). ENaC mediates the absorption of sodium from the distal parts of the nephron. In NS, multiple factors contribute to the activation of these channels; aldosterone, vasopressin, proteases, and urinary plasminogen in tubular ultra-filtrate all appear to contribute to its activation. Activation of the ENaC not only enhances the development of edema, but also has implications on blood pressure regulation (30). To highlight the complexity, heterogeneity, and multifactorial nature of HTN in children with NS, Kuster et al. observed in 57 children with NS and despite administration of steroids, BP fell during treatment even before proteinuria had completely disappeared, suggestive of overfill (altered intrarenal sodium handling) at play (11).
In response to volume expansion, atrial natriuretic peptide (ANP) facilitates diuresis by increasing the GFR while reducing sodium reabsorption in the renal tubules. Investigators have demonstrated a blunted response to elevated serum ANP levels in NS (33–35). This blunted effect has been attributed to abnormal ANP-dependent signaling mechanisms and decreased conversion of pro-ANP to active ANP in proteinuric kidney disease (36).
Investigators have also examined the role of nitric oxide (NO) in sodium retention and the pathogenesis of HTN in animal models of NS. Ni et al. (37) demonstrated reduction in kidney NO synthase and reduced fractional excretion of sodium (FENa) in proteinuric rats. The authors attributed the NO synthase deficiency in their model to the presence of proteinuria, and thus the NO deficiency reduced FENa by augmenting renal tubular sodium reabsorption and preglomerular vasoconstriction.
Many of the commonly used medications in the treatment of NS are known to affect BP and contribute to the development of HTN. Calcineurin inhibitors induce nephrotoxicity and arterial HTN (38). Vasoconstriction, sympathetic excitation and sodium retention by the kidney had been shown to play a role in CNI induced HTN (38, 39). CNIs are usually used in SRNS patients; a subgroup of NS patients that are more likely to have HTN compared to the SDNS patients (9). In a retrospective study examining the effects of tacrolimus in treatment-resistant nephrotic syndrome, 6/15 children developed worsening or new-onset HTN (40). El-Husseini et al. reported the development of HTN in 10% of children with NS who received cyclosporine treatment for more than 2 years and 6% of children developed renal insufficiency (mostly patients with FSGS on renal biopsy) (41).
Synthetic steroids (prednisolone, prednisone, and methyl-prednisone) are central in the treatment regimens of patients with NS. These steroids have slightly different glucocorticoid and mineralocorticoid activities with predominantly glucocorticoid (immunosuppressive, anti-inflammatory properties); traditionally, it is their salt-retaining via mineralocorticoid-receptor properties that have been blamed for the effects on BP. The exact mechanism by which the glucocorticoid effect induces HTN is unclear. The high prevalence of HTN in steroid—treated patients has been reported even in therapy with predominantly glucocorticoid activity. Synthetic steroids may play a role in BP regulation via other mechanisms, such as fluid shifts from interstitial to the intravascular compartment, elevated plasma renin activity, increased sympathetic nerve activity, altered prostaglandin biosynthesis, enhanced vascular smooth muscle responsiveness to catecholamines and angiotensinogen II, impaired vasodilation, and nitric oxide synthase activity (42–47).
The effect of steroid therapy on BP in children with NS is variable. Some NS patients may develop HTN or worsening of HTN with steroid therapy, while others have improved BP after achieving remission despite being on high dose steroid therapy. Klepikov at al. described 7/27 NS patients who developed HTN with steroid therapy. They were more likely to be hypervolemic with severe sodium retention and suppressed renin and aldosterone levels. On the other hand, normo- and hypo-volemic patients had a robust diuresis and natriuresis with steroid therapy (48). Kontchou et al. reported improved BP profiles in children with MCD after 4 weeks of steroid therapy (12). The heterogeneity in the effects of steroids on BP maybe attributed to the heterogeneity of the study populations and the complex interplay between genetic and environmental factors.
Recently, a study by Haas at al. supported the existence of a feed-forward loop between albuminuria and blood pressure and implied that albuminuria could increase the risk of cardiovascular disease through blood pressure. In their study, the authors first performed a genome-wide association study for albuminuria in a large cohort and identified 32 new albuminuria loci. Then they constructed an albuminuria genetic risk scores and tested the score for association with cardiometabolic diseases. Genetically elevated albuminuria was strongly associated with increased risk of HTN. The authors also found that the relationship between BP and albuminuria was bidirectional; genetically elevated albuminuria led to higher BP and higher systolic BP predicted an increase in albuminuria. These findings suggest that pathways leading to albuminuria such as endothelial dysfunction, impaired kidney function and decreased ability to excrete sodium may contribute to the pathogenesis of HTN. Although the study was not conducted in a NS cohort, it does represent an additional significant step toward our understanding of the effects of albuminuria (the primary protein lost in patients with NS) on BP and highlights the need for further studies to better understand this complex interplay between albuminuria and HTN (29).
Uncontrolled HTN is a well-known cardiovascular disease risk factor; in adults it is associated with cardiovascular morbidity and mortality (49, 50). In children, studies have shown HTN to be associated with target-organ damage (TOD), such as LVH, cognitive impairment, and faster progression of chronic kidney disease (15).
Salt restriction and RAAS inhibition are considered integral parts of the management of children with proteinuria and both are known to have a blood pressure reducing effect. RAAS blockade is known to have a reno-protective effect in patients with glomerular disease; some studies have demonstrated a greater reduction of proteinuria with combination ACEi/ARB therapy. This reno-protective effect has been attributed to both BP reduction and BP independent mechanisms (51, 52). Both the AIPRI and the REIN studies support that angiotensin-converting enzyme (ACE) inhibitors have a long-term renoprotective effect. The benefits of ACE inhibitors can be demonstrated even in patients who are not hypertensive (53, 54). It is important to point out that the use of ACEi/ARB therapy in an intravascularly depleted child may precipitate AKI. Treating clinicians should always weigh the risk and benefit before initiating therapy.
Studies examining the effects of blood pressure control in children with idiopathic NS are lacking. On the other hand, the benefit of strict BP control has been demonstrated in children with CKD. The ESCAPE trial (13.5% of study cohort had glomerulopathies) showed intensified blood-pressure control to confer a substantial benefit with respect to renal function (55).
Aldosterone is a known contributor to the sodium retention in patients with NS (56). Recently, effects that are independent of sodium transport have been described too, including increased fibrosis, collagen deposition, inflammation, and remodeling of the heart and blood vessels. These effects are markedly increased in the presence of high sodium intake (57). Studies have shown the addition of spironolactone (an aldosterone antagonist) to ACE inhibitor leads to further reduction of proteinuria, an effect that maybe confounded by BP reduction. In adult patients with CKD, spironolactone, when added ACE/ARB, was found to reduce proteinuria levels as well as the rate of GFR loss (58). Unfortunately, spironolactone treatment is associated with a significant increase in serum potassium levels, which necessities close electrolyte monitoring and limits its use in patients with lower GFRs.
Sparsentan, which combines endothelin receptor type A blockade with angiotensin II inhibition, has been shown to reduce proteinuria in patients with FSGS. It also had a greater effect on lowering BP compared with irbesartan (59).
Diuretics play an important role in the management of children with NS (60). Different classes of diuretics result in net fluid and sodium loss, a desired goal in the management of the edematous and hypertensive child. It is important to caution against aggressive diuresis of the intravascularly deplete child and the treating clinician needs to carefully weigh risks and benefits while using diuretics in combination with other BP-lowering medication classes such as RAAS inhibitors. As previously discussed, ENaC mediates the absorption of sodium from the distal parts of the nephron (30). ENaC inhibition with amiloride is known to reduce edema and improve blood pressure and has been reported to resolve edema and HTN in a patient with NS (61). However, the use of this agent may be limited by the risk of hyperkalemia.
In addition, while salt restriction and RAAS inhibition represent the key pillars in the management of the hypertensive child with proteinuria, other classes of antihypertensive medications have been used. While the use of beta blockers carries the theoretical benefit of lowering BP and indirectly inhibiting the RAAS without impairing the GFR, there are several reports suggesting worsened glycemic control with this class. Vasodilators, when used alone, may result in greater sodium retention. Unlike other vasodilating drugs, calcium channel blockers do not cause renal sodium and water retention, and do not cause hyperkalemia. One perceived advantage of CCBs may be their lack of nephrotoxicity, which makes this drug class an appealing choice for monotherapy or combination therapy in patients with kidney disease. However, the safety of some CCBs (dihydropyridine vs. non-DHP) in patients with proteinuric renal diseases (mostly adults patients with diabetic nephropathy) and renal insufficiency may be questioned because of reported untoward effects on urinary protein excretion (62, 63). Finally, it is important to emphasize the importance of lifestyle interventions (exercise and dietary counseling) as part of HTN management in this patient population.
Given the heterogeneity in intravascular volume status of patients with NS (volume expansion and low renin in a subset of patients while others have intravascular depletion and elevated renin levels), the management of the hypertensive child with NS should aim at addressing possible contributing factors, taking into consideration the child's clinical examination, laboratory findings and current medications.
HTN is prevalent in childhood NS. The increased risk of HTN lasts many years after stopping therapy and remission of the disease. Clinicians should continue to monitor blood pressure in children with a history of NS. Multiple factors are known to affect BP and contribute to the development of HTN in NS. Treatment of HTN should address the underlying disease pathophysiology and include lifestyle modifications. HTN and the cardiovascular disease risk in children with NS may be under recognized. 24-h ABPM can identify abnormalities in blood pressure measurements and patterns in this at-risk population. The majority of existing studies that examined the prevalence of HTN in patients with NS were observational and included heterogeneous NS patient populations, which makes it challenging to draw clear conclusions about the true prevalence and explains the substantial variability in the reported prevalence (Table 1). HTN in children with NS remains poorly understood and there is a pressing need for studies in this patient population to identify the “optimal” safe and efficacious antihypertensive medication class.
Children with HTN and NS are at increased risk for dyslipidemia, LVH, left ventricular dysfunction, atherosclerosis, and aortic stiffness, all factors that have been associated with premature death in adults (64). Future research should aim at exploring specific patient characteristics that predispose a subset of children with NS to develop HTN; this should be carried out using well-defined patient populations and using a standardized BP definition. Children may benefit from already-proven measures to reduce cardiovascular disease risk in adults: diet, exercise, avoidance of smoking/vaping/illicit drugs, stress reduction, lipid lowering agents, cardiac remodeling agents, and renoprotective drugs (65). Another area in need of further research is the role of ABPM in the assessment of BP in children with NS. The current clinical practice guidelines for screening and management of HTN in children do not address ABPM indications in children with SSNS or SRNS with normal GFR (66). The role of immune pathways and their role in the pathogenesis of HTN in NS, a disease that is widely believed to have underlying immune dysregulation, warrant more investigation. Recently, the human microbiome role in BP regulation has been described (67). Future research is needed to better understand the relationship between the human microbiome and virome in BP regulation in children with NS. Currently, a multi-centric prospective cohort study of 70 steroid-resistant, 70 steroid-sensitive, and 70 healthy controls are being recruited to better understand the epidemiology of endothelial dysfunction and associated subclinical cardiovascular co-morbidity in childhood NS (68).
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
The publication of this article was funded by the Qatar National Library.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Warady BA, Chadha V. Chronic kidney disease in children: the global perspective. Pediatr Nephrol. (2007) 22:1999–2009. doi: 10.1007/s00467-006-0410-1
2. Chanchlani R, Parekh RS. Ethnic differences in childhood nephrotic syndrome. Front Pediatr. (2016) 4:39. doi: 10.3389/fped.2016.00039
3. Koskimies O, Vilska J, Rapola J, Hallman N. Long-term outcome of primary nephrotic syndrome. Arch Dis Child. (1982) 57:544–8. doi: 10.1136/adc.57.7.544
4. Arneil GC, Lam CN. Long-term assessment of steroid therapy in childhood nephrosis. Lancet. (1966) 2:819–21. doi: 10.1016/S0140-6736(66)92253-7
5. Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents, National Heart, Lung, and Blood Institute. Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report. Pediatrics. (2011) 128(Suppl. 5):S213–56. doi: 10.1542/peds.2009-2107C
6. The primary nephrotic syndrome in children. Identification of patients with minimal change nephrotic syndrome from initial response to prednisone. A report of the international study of kidney disease in children. J Pediatr. (1981) 98:561–4. doi: 10.1016/S0022-3476(81)80760-3
7. Primary nephrotic syndrome in children: clinical significance of histopathologic variants of minimal change and of diffuse mesangial hypercellularity. A report of the international study of kidney disease in children. Kidney Int. (1981) 20:765–71. doi: 10.1038/ki.1981.209
8. Trautmann A, Bodria M, Ozaltin F, Gheisari A, Melk A, Azocar M, et al. Spectrum of steroid-resistant and congenital nephrotic syndrome in children: the PodoNet registry cohort. Clin J Am Soc Nephrol. (2015) 10:592–600. doi: 10.2215/CJN.06260614
9. Shatat IF, Schoeneman M, Flynn JT, Woroniecki RP. Association of steroid and cyclosporin resistance in focal segmental glomerulosclerosis. Pediatr Nephrol. (2007) 22:834–9. doi: 10.1007/s00467-006-0413-y
10. Keshri S, Sharma S, Agrawal N, Bansal S, Guilliani BP, Aggrawal KC. Hypertension and its severity in children with steroid sensitive nephrotic syndrome during remission. Clin Exp Nephrol. (2018) 22:1157–62. doi: 10.1007/s10157-018-1565-3
11. Küster S, Mehls O, Seidel C, Ritz E. Blood pressure in minimal change and other types of nephrotic syndrome. Am J Nephrol. (1990) 10(Suppl. 1):76–80. doi: 10.1159/000168198
12. Kontchou LM, Liccioli G, Pela I. Blood pressure in children with minimal change nephrotic syndrome during oedema and after steroid therapy: the influence of familial essential hypertension. Kidney Blood Press Res. (2009) 32:258–62. doi: 10.1159/000238823
13. Mitsnefes M, Ho PL, McEnery PT. Hypertension and progression of chronic renal insufficiency in children: a report of the North American Pediatric Renal Transplant Cooperative Study (NAPRTCS). J Am Soc Nephrol. (2003) 14:2618–22. doi: 10.1097/01.ASN.0000089565.04535.4B
14. Hogg RJ, Friedman A, Greene T, Radeva M, Budisavljevic MN, Gassman J, et al. Renal function and proteinuria after successful immunosuppressive therapies in patients with FSGS. Clin J Am Soc Nephrol. (2013) 8:211–8. doi: 10.2215/CJN.08330812
15. Flynn JT, Mitsnefes M, Pierce C, Cole SR, Parekh RS, Furth SL, et al. Blood pressure in children with chronic kidney disease: a report from the chronic kidney disease in children study. Hypertension. (2008) 52:631–7. doi: 10.1161/HYPERTENSIONAHA.108.110635
16. Sethna CB, Meyers KEC, Mariani LH, Psoter KJ, Gadegbeku CA, Gibson KL, et al. Blood pressure and visit-to-visit blood pressure variability among individuals with primary proteinuric glomerulopathies. Hypertension. (2017) 70:315–23. doi: 10.1161/HYPERTENSIONAHA.117.09475
17. Gabban NIAl, Abdullah EA, Abd HN. Nephrotic syndrome and hypertension. Iraqi J Comm Med. (2010) 4:271–6.
18. Inaba A, Hamasaki Y, Ishikura K, Hamada R, Sakai T, Hataya H, et al. Long-term outcome of idiopathic steroid-resistant nephrotic syndrome in children. Pediatr Nephrol. (2016) 31:425–34. doi: 10.1007/s00467-015-3174-7
19. Sarkar S, Sinha A, Lakshmy R, Agarwala A, Saxena A, Hari P, et al. Ambulatory blood pressure monitoring in frequently relapsing nephrotic syndrome. Indian J Pediatr. (2017) 84:31–5. doi: 10.1007/s12098-016-2207-y
20. Haruhara K, Tsuboi N, Koike K, Kanzaki G, Okabayashi Y, Sasaki T, et al. Circadian blood pressure abnormalities in patients with primary nephrotic syndrome. Clin Exp Hypertens. (2017) 39:155–9. doi: 10.1080/10641963.2016.1235179
21. Ishikura K, Yoshikawa N, Nakazato H, Sasaki S, Nakanishi K, Matsuyama T, et al. Morbidity in children with frequently relapsing nephrosis: 10-year follow-up of a randomized controlled trial. Pediatr Nephrol. (2015) 30:459–68. doi: 10.1007/s00467-014-2955-8
22. Skrzypczyk P, Panczyk-Tomaszewska M, Roszkowska-Blaim M, Wawer Z, Bienias B, Zajgzkowska M, et al. Long-term outcomes in idiopathic nephrotic syndrome: from childhood to adulthood. Clin Nephrol. (2014) 81:166–73. doi: 10.5414/CN108044
23. Vaziri ND. Disorders of lipid metabolism in nephrotic syndrome: mechanisms and consequences. Kidney Int. (2016) 90:41–52. doi: 10.1016/j.kint.2016.02.026
24. Kniazewska MH, Obuchowicz AK, Wielkoszynski T, Zmudzinska-Kitczak J, Urban K, Marek M, et al. Atherosclerosis risk factors in young patients formerly treated for idiopathic nephrotic syndrome. Pediatr Nephrol. (2009) 24:549–54. doi: 10.1007/s00467-008-1029-1
25. Gungor O, Demirci MS, Kircelli F, Tatar E, Sipahi S, Hur E, et al. Increased arterial stiffness in patients with nephrotic syndrome. Clin Nephrol. (2013) 79:1–6. doi: 10.5414/CN107760
26. Rahul I, Krishnamurthy S, Satheesh S, Biswal N, Bobby Z, Lakshminarayanan S. Brachial artery flow-mediated dilatation and carotid intima medial thickness in pediatric nephrotic syndrome: a cross-sectional case-control study. Clin Exp Nephrol. (2015) 19:125–32. doi: 10.1007/s10157-014-0958-1
27. Hooman N, Isa-Tafreshi R, Otukesh H, Mostafavi SH, Hallaji F. Carotid artery function in children with idiopathic nephrotic syndrome. Nefrologia. (2013) 33:650–6. doi: 10.3265/Nefrologia.pre2013.May.12036
28. Candan C, Canpolat N, Gökalp S, Yildiz N, Turhan P, Taşdemir M, et al. Subclinical cardiovascular disease and its association with risk factors in children with steroid-resistant nephrotic syndrome. Pediatr Nephrol. (2014) 29:95–102. doi: 10.1007/s00467-013-2608-3
29. Haas ME, Aragam KG, Emdin CA, Bick AG, Hemani G, Davey Smith G, et al. Genetic association of albuminuria with cardiometabolic disease and blood pressure. Am J Hum Genet. (2018) 103:461–73. doi: 10.1016/j.ajhg.2018.08.004
30. Ray EC, Rondon-Berrios H, Boyd CR, Kleyman TR. Sodium retention and volume expansion in nephrotic syndrome: implications for hypertension. Adv Chronic Kidney Dis. (2015) 22:179–84. doi: 10.1053/j.ackd.2014.11.006
31. Andersen RF, Buhl KB, Jensen BL, Svenningsen P, Friis UG, Jespersen B, et al. Remission of nephrotic syndrome diminishes urinary plasmin content and abolishes activation of ENaC. Pediatr Nephrol. (2013) 28:1227–34. doi: 10.1007/s00467-013-2439-2
32. Svenningsen P, Bistrup C, Friis UG, Bertog M, Haerteis S, Krueger B, et al. Plasmin in nephrotic urine activates the epithelial sodium channel. J Am Soc Nephrol. (2009) 20:299–310. doi: 10.1681/ASN.2008040364
33. Perico N, Delaini F, Lupini C, Benigni A, Galbusera M, Boccardo P, et al. Blunted excretory response to atrial natriuretic peptide in experimental nephrosis. Kidney Int. (1989) 36:57–64. doi: 10.1038/ki.1989.161
34. Valentin JP, Qiu C, Muldowney WP, Ying WZ, Gardner DG, Humphreys MH. Cellular basis for blunted volume expansion natriuresis in experimental nephrotic syndrome. J Clin Invest. (1992) 90:1302–12. doi: 10.1172/JCI115995
35. Peterson C, Madsen B, Perlman A, Chan AY, Myers BD. Atrial natriuretic peptide and the renal response to hypervolemia in nephrotic humans. Kidney Int. (1988) 34:825–31. doi: 10.1038/ki.1988.256
36. Polzin D, Kaminski HJ, Kastner C, Wang W, Krämer S, Gambaryan S, et al. Decreased renal corin expression contributes to sodium retention in proteinuric kidney diseases. Kidney Int. (2010) 78:650–9. doi: 10.1038/ki.2010.197
37. Ni Z, Vaziri ND. Downregulation of nitric oxide synthase in nephrotic syndrome: role of proteinuria. Biochim Biophys Acta. (2003) 1638:129–37. doi: 10.1016/S0925-4439(03)00061-9
38. Hošková L, Málek I, Kopkan L, Kautzner J. Pathophysiological mechanisms of calcineurin inhibitor-induced nephrotoxicity and arterial hypertension. Physiol Res. (2017) 66:167–80.
39. Hoorn EJ, Walsh SB, McCormick JA, Zietse R, Unwin RJ, Ellison DH. Pathogenesis of calcineurin inhibitor-induced hypertension. J Nephrol. (2012) 25:269–75. doi: 10.5301/jn.5000174
40. Loeffler K, Gowrishankar M, Yiu V. Tacrolimus therapy in pediatric patients with treatment-resistant nephrotic syndrome. Pediatr Nephrol. (2004) 19:281–7. doi: 10.1007/s00467-003-1370-3
41. El-Husseini A, El-Basuony F, Mahmoud I, Sheashaa H, Sabry A, Hassan R, et al. Long-term effects of cyclosporine in children with idiopathic nephrotic syndrome: a single-centre experience. Nephrol Dial Transplant. (2005) 20:2433–8. doi: 10.1093/ndt/gfi059
42. Saruta T. Mechanism of glucocorticoid-induced hypertension. Hypertens Res. (1996) 19:1–8. doi: 10.1291/hypres.19.1
43. Kornel L, Prancan AV, Kanamarlapudi N, Hynes J, Kuzianik E. Study on the mechanisms of glucocorticoid-induced hypertension: glucocorticoids increase transmembrane Ca2+ influx in vascular smooth muscle in vivo. Endocr Res. (1995) 21:203–10. doi: 10.3109/07435809509030436
44. Whitworth JA. Studies on the mechanisms of glucocorticoid hypertension in humans. Blood Press. (1994) 3:24–32. doi: 10.3109/08037059409101518
45. Sato A, Suzuki H, Nakazato Y, Shibata H, Inagami T, Saruta T. Increased expression of vascular angiotensin II type 1A receptor gene in glucocorticoid-induced hypertension. J Hypertens. (1994) 12:511–6. doi: 10.1097/00004872-199405000-00003
46. Ong SL, Whitworth JA. How do glucocorticoids cause hypertension: role of nitric oxide deficiency, oxidative stress, and eicosanoids. Endocrinol Metab Clin North Am. (2011) 40:393–407, ix. doi: 10.1016/j.ecl.2011.01.010
47. Frey FJ, Odermatt A, Frey BM. Glucocorticoid-mediated mineralocorticoid receptor activation and hypertension. Curr Opin Nephrol Hypertens. (2004) 13:451–8. doi: 10.1097/01.mnh.0000133976.32559.b0
48. Klepikov PV, Kutyrina IM, Tareyeva IE. Steroid-induced hypertension in patients with nephrotic syndrome. Nephron. (1988) 48:286–90. doi: 10.1159/000184944
49. Kannel WB, Wolf PA, Verter J, McNamara PM. Epidemiologic assessment of the role of blood pressure in stroke. The Framingham study. JAMA. (1970) 214:301–10. doi: 10.1001/jama.1970.03180020021004
50. Kannel WB, Castelli WP, McNamara PM, McKee PA, Feinleib M. Role of blood pressure in the development of congestive heart failure. The Framingham study. N Engl J Med. (1972) 287:781–7. doi: 10.1056/NEJM197210192871601
51. Tylicki L, Rutkowski P, Renke M, Rutkowski B. Renoprotective effect of small doses of losartan and enalapril in patients with primary glomerulonephritis. Short-term observation. Am J Nephrol. (2002) 22:356–62. doi: 10.1159/000065227
52. Ljutić D, Kes P. The role of arterial hypertension in the progression of non-diabetic glomerular diseases. Nephrol Dial Transplant. (2003) 18(Suppl. 5):v28–30. doi: 10.1093/ndt/gfg1040
53. Locatelli F, Carbarns IR, Maschio G, Mann JF, Ponticelli C, Ritz E, et al. Long-term progression of chronic renal insufficiency in the AIPRI extension study. The angiotensin-converting-enzyme inhibition in progressive renal insufficiency study group. Kidney Int Suppl. (1997) 63:S63–6.
54. Randomised placebo-controlled trial of effect of ramipril on decline in glomerular filtration rate and risk of terminal renal failure in proteinuric, non-diabetic nephropathy. The GISEN Group (Gruppo Italiano di Studi Epidemiologici in Nefrologia). Lancet. (1997) 349:1857–63. doi: 10.1016/S0140-6736(96)11445-8
55. Wühl E, Trivelli A, Picca S, Litwin M, Peco-Antic A, Zurowska A, et al. Strict blood-pressure control and progression of renal failure in children. N Engl J Med. (2009) 361:1639–50. doi: 10.1056/NEJMoa0902066
56. Shapiro MD, Hasbargen J, Hensen J, Schrier RW. Role of aldosterone in the sodium retention of patients with nephrotic syndrome. Am J Nephrol. (1990) 10:44–8. doi: 10.1159/000168052
57. Schrier RW, Masoumi A, Elhassan E. Aldosterone: role in edematous disorders, hypertension, chronic renal failure, and metabolic syndrome. Clin J Am Soc Nephrol. (2010) 5:1132–40. doi: 10.2215/CJN.01410210
58. Bianchi S, Bigazzi R, Campese VM. Long-term effects of spironolactone on proteinuria and kidney function in patients with chronic kidney disease. Kidney Int. (2006) 70:2116–23. doi: 10.1038/sj.ki.5001854
59. Trachtman H, Nelson P, Adler S, Campbell KN, Chaudhuri A, Derebail VK, et al. DUET: a phase 2 study evaluating the efficacy and safety of sparsentan in patients with FSGS. J Am Soc Nephrol. (2018) 29:2745–54. doi: 10.1681/ASN.2018010091
60. Duffy M, Jain S, Harrell N, Kothari N, Reddi AS. Albumin and furosemide combination for management of edema in nephrotic syndrome: a review of clinical studies. Cells. (2015) 4:622–30. doi: 10.3390/cells4040622
61. Oxlund CS, Buhl KB, Jacobsen IA, Hansen MR, Gram J, Henriksen JE, et al. Amiloride lowers blood pressure and attenuates urine plasminogen activation in patients with treatment-resistant hypertension. J Am Soc Hypertens. (2014) 8:872–81. doi: 10.1016/j.jash.2014.09.019
62. Kloke HJ, Branten AJ, Huysmans FT, Wetzels JF. Antihypertensive treatment of patients with proteinuric renal diseases: risks or benefits of calcium channel blockers? Kidney Int. (1998) 53:1559–73. doi: 10.1046/j.1523-1755.1998.00912.x
63. Holdaas H, Hartmann A, Lien MG, Nilsen L, Jervell J, Fauchald P, et al. Contrasting effects of lisinopril and nifedipine on albuminuria and tubular transport functions in insulin dependent diabetics with nephropathy. J Intern Med. (1991) 229:163–70. doi: 10.1111/j.1365-2796.1991.tb00325.x
64. Danaei G, Ding EL, Mozaffarian D, Taylor B, Rehm J, Murray CJ, et al. The preventable causes of death in the United States: comparative risk assessment of dietary, lifestyle, and metabolic risk factors. PLoS Med. (2009) 6:e1000058. doi: 10.1371/journal.pmed.1000058
65. Reboussin DM, Allen NB, Griswold ME, Guallar E, Hong Y, Lackland DT, et al. Systematic review for the 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Hypertension. (2018) 71:e116–35. doi: 10.1161/HYP.0000000000000067
66. Flynn JT, Kaelber DC, Baker-Smith CM, Blowey D, Carroll AE, Daniels SR, et al. Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics. (2017) 140:e20171904. doi: 10.1542/peds.2017-3035
67. Al Khodor S, Reichert B, Shatat IF. The microbiome and blood pressure:can microbes regulate our blood pressure? Front Pediatr. (2017) 5:138. doi: 10.3389/fped.2017.00138
Keywords: nephrotic syndrome, hypertension, pediatric, ambulatory blood pressure, blood pressure variability
Citation: Shatat IF, Becton LJ and Woroniecki RP (2019) Hypertension in Childhood Nephrotic Syndrome. Front. Pediatr. 7:287. doi: 10.3389/fped.2019.00287
Received: 26 February 2019; Accepted: 26 June 2019;
Published: 16 July 2019.
Edited by:
Rasheed Gbadegesin, Duke University, United StatesReviewed by:
Aditi Sinha, All India Institute of Medical Sciences, IndiaCopyright © 2019 Shatat, Becton and Woroniecki. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ibrahim F. Shatat, aXNoYXRhdEBzaWRyYS5vcmc=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.