- 1Department of Otolaryngology-Head and Neck Surgery, Asahikawa Medical University, Asahikawa, Japan
- 2Department of Innovative Head and Neck Cancer Research and Treatment, Asahikawa Medical University, Asahikawa, Japan
Extranodal natural killer (NK)/T-cell lymphoma, nasal type (NNKTL) has very unique epidemiological, etiologic, histologic, and clinical characteristics. It is commonly observed in Eastern Asia, but quite rare in the United States and Europe. The progressive necrotic lesions mainly in the nasal cavity, poor prognosis caused by rapid local progression with distant metastases, and angiocentric and polymorphous lymphoreticular infiltrates are the main clinical and histologic features. Phenotypic and genotypic studies revealed that the lymphoma is originated from either NK- or γδ T-cell, both of which express CD56. In 1990, the authors first reported the presence of Epstein-Barr virus (EBV)-DNA and EBV-oncogenic proteins, and EBV has now been recognized to play an etiological role in NNKTL. in vitro studies revealed that a wide variety of cytokines, chemokines, and micro RNAs, which may be produced by EBV-oncogenic proteins in the lymphoma cells, play important roles for tumor progression in NNKTL, and could be therapeutic targets. In addition, it was revealed that the interaction between NNKTL cells and immune cells such as monocytes and macrophages in NNKTL tissues contribute to lymphoma progression. For diagnosis, monitoring the clinical course and predicting prognosis, the measurements of EBV-DNAs and EBV-micro RNAs in sera are very useful. For treatment with early stage, novel concomitant chemoradiotherapy such as DeVIC regimen with local radiotherapy and MPVIC-P regimen using intra-arterial infusion developed with concomitant radiotherapy and the prognosis became noticeably better. However, the prognosis of patients with advanced stage was still poor. Establishment of novel treatments such as the usage of immune checkpoint inhibitor or peptide vaccine with molecular targeting therapy will be necessary. This review addresses recent advances in the molecular understanding of NNKTL to establish novel treatments, in addition to the epidemiologic, clinical, pathological, and EBV features.
Introduction
Extranodal natural killer (NK)/T-cell lymphoma, nasal type (NNKTL) has very unique epidemiological, etiologic, histologic, and clinical characteristics. The lymphoma is commonly observed in Eastern Asia (1–4) and Latin America (4, 5) but quite rare in United States and Europe (6–8). The progressive necrotic lesion mainly in the nasal cavity is one of the main clinical features of this disease, which is often characterized by a poor prognosis because of rapid local progression and distant metastases (2, 9). The histological feature shows angiocentric and polymorphous lymphoreticular infiltrate, and the disease has been previously called polymorphic reticulosis (10, 11). Phenotypic and genotypic studies revealed that the lymphoma is originated from either NK- or γδ T-cell, both of which express CD56 (2, 8, 12–16). In the late 20th century, the authors first demonstrated the presence of Epstein-Barr virus (EBV) DNA, EBV oncogenic proteins, and the clonotypic EBV genome in NNKTL (1, 2, 16, 17). Based on these findings, EBV has been recognized to play an etiological role in NNKTL (16), and EBV DNA has been applied as a clinical progression/recurrence marker (18). Although NNKTL generally occurs in adult patients, a few cases of pediatric NNKTL are reported (19). In this article, the authors summarize the current understandings of clinical, pathological, biological, and virological characteristics of this lymphoma.
Historical Backgrounds
McBride (20) first reported, in 1897, the rapid destruction of the nose and face (midline) with progressing necrotic granuloma. The clinical course was generally aggressive and lethal, this disease was initially termed as “rhinitis gangrenosa progressiva” (21) in Europe or “lethal midline granuloma” (22, 23) in the United States. The histologic features show angiocentric and polymorphous lymphoreticular infiltrates with necrotic granuloma. Therefore, the disease had been called many histopathologic terms such as “reticulum cell sarcoma,” “midline malignant reticulosis”, “polymorphic reticulosis” (11), and “malignant histiocytosis” (24). Since the late twentieth century, this disease had been coined as nasal T-cell lymphoma based on the finding that these tumor cells had a T-cell phenotype (9, 25). Subsequently, the expression of NK-cell marker CD56 was also reported. Accordingly, the term of this lymphoma has been determined as nasal NK/T-cell lymphoma (NNKTL) (26).
Etiologically, Harabuchi et al. (1) first found the presence of EBV-DNA and EBV-determined nuclear antigen (EBNA1) in the lymphoma cells from 5 Japanese patients. These EBV-findings were also verified in Western countries (8, 27–29). Accordingly, NNKTL is now classified as one of the EBV-associated malignancies (16).
Epidemiology
There is a clear geographic deviation in NNKTL prevalence. In Asia and South America, NNKTL consists of 3-10% of non-Hodgkin lymphoma, whereas less than 1% in Western countries (30–33). In Peru, the percentage of NNKTL in non-Hodgkin lymphoma was 8%, respectively (34). Aozasa et al. (35) estimated that the incidence rate of NNKTL is higher in Asia by 10-fold compared to Europe. Because the common race in Asia and South America is mongoloid, the genetic background may play a role in the onset of NNKTL. Although the specific genetic feature of NNKTL patients remains to be elucidated, there is a possibility that a specific type of HLA has the disadvantage to present EBV-associated epitope to T cells. Indeed, HLA-B46 is a risk factor in nasopharyngeal cancer, an EBV-associated malignancy (36).
Another explanation of the skewed distribution of NNKTL could be EBV strain and/or environmental factors. Because the subtype of EBV in NNKTL (Type A/F/C) is similar to healthy donors (37), the high-risk EBV subtype has not been identified in this lymphoma. Nagamine et al. (38) previously demonstrated that the EBV strain in NNKTL has an amino acid mutation in the CD8 T-cell epitope. Furthermore, Nagamine et al. (39) investigated the full length of the LMP1 sequence in NNKTL and found the amino acid changes at codon 126 and 129 in an HLA-A2 restricted CD8 T cell epitope LMP1 125–133 from all patients. Our group (40) previously showed that the frequency of HLA-A*0201 was significantly lower in NNKTL than in the healthy population, suggesting that the HLA-A*0201-restricted CTL responses may inhibit the development of the lymphoma. It is possible that the mutated EBV allows infected cells to escape from cytolysis by immune cells. The precise characteristics of EBV in NNKTL are described below.
The environmental factors also influence the pathogenesis of NNKTL. Our group (41) previously demonstrated that the exposure to pesticides and chemical solvents could be a significant risk factor in NNKTL by a case-control study. Every type of pesticide, herbicide, insecticide, and fungicide was related to the NNKTL incidence. Moreover, the use of gloves or mask to circumvent the pesticide pollution was effective to reduce the incidence of NNKTL. Kojya et al. (42) also reported the case of familial development of NNKTL who are exposed to the pesticide. Taken together, the genetic background, EBV strain, and environmental factors concordantly affect the geographic distribution of NNKTL.
Clinical Features
The lymphoma is initially found as progressive ulceration and necrotic granuloma in the nasal cavity, palate, and nasopharynx (1, 2) (Figure 1). The tumor frequently invades around tissues such as facial skin, paranasal sinus, and orbits, and then develops extensive destruction of midline lesions (1, 20, 23). The most common symptoms at the time of diagnosis are nasal obstruction and bloody rhinorrhea (2, 43). The swelling of cheek or orbit, sore throat, and hoarseness are also major symptoms of NNKTL (2, 43). In addition, systemic symptoms such as prolonged fever and weight loss are commonly seen (2, 43, 44).
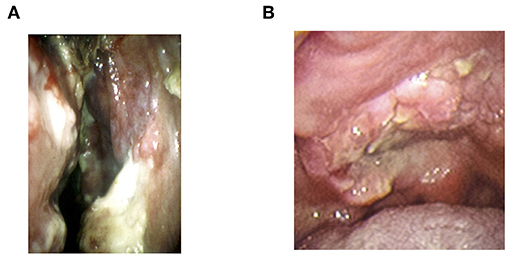
Figure 1. The representative local findings of NNKTL. (A) Necrotic granulation in nasal cavity. (B) Necrotic ulceration in hard palate.
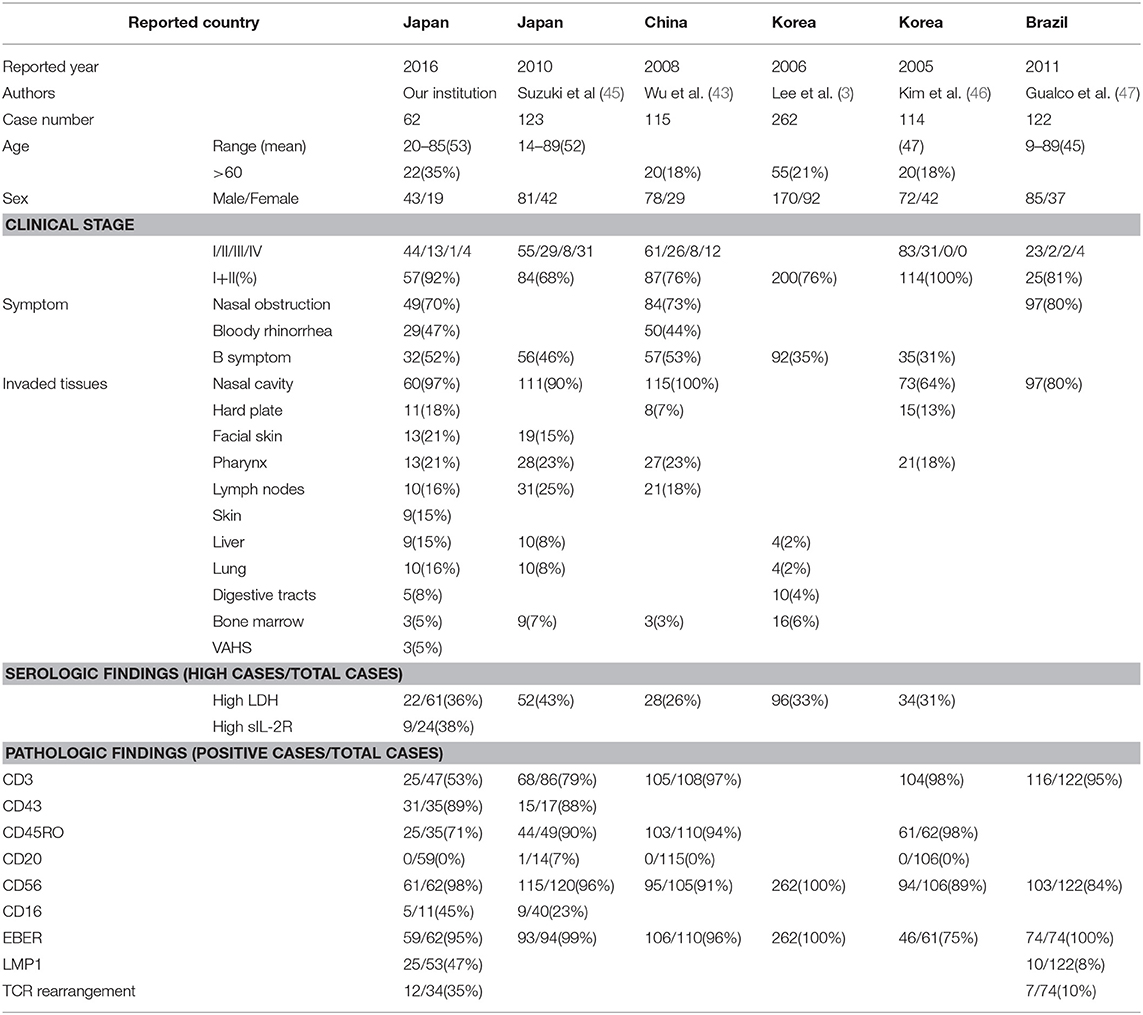
The clinical characteristics of NNKTL from different countries are summarized in Table 1 (3, 43–47). The disease developed around 40 to 50 years of age, and there is no significant difference between the sexes. The patients over 60 years old were not common (18–35%). Most patients (69–100%) were diagnosed in early stages, Stage I or II. As mentioned above, the most common symptoms were nasal obstruction (70–80%), bloody rhinorrhea (44–47%) and B symptoms (31–53%). The tumor directly invaded several tissues including nasal cavity, hard plate, facial skin, and pharynx. Lymph nodes and distant tissues such as liver, lung, digestive tracts, and bone marrow were also involved. Twenty to forty percentages of patients had high lactate dehydrogenase (LDH), whereas 38% of patients had high soluble IL-2 receptor in serum. More than 90% of tumors expressed T cell markers such as CD3 or NK marker CD56 on the surface. EBV LMP1 was found in 47% of patients. T-cell receptor (TCR) gene rearrangement was observed in 10–35% of patients. The precise role of EBV, surface markers, or TCR gene rearrangement is described later in this review.
Pathology
Pathologic characteristics of NNKTL show diffused infiltrates of lymphoma cells, which have a diverse size, pleomorphic large or small cells with mitosis, together with various inflammatory cells such as granulocytes, macrophages and plasma cells, in the necrotic background. The lymphoma had been called as polymorphic reticulosis or angiocentric lymphoma because angiocentric and angio-invasive infiltrates are commonly found (48). The lymphoma cells express T-cell markers such as CD2, cytoplasmic CD3 (CD3ε), and CD45 as well as NK-cell marker CD56. Perforin, Fas ligand, and intercellular adhesion molecule-1 (ICAM-1) are also shown in the NNKTL cells (49).
The lymphoma cell was initially thought to be originated from NK-cells alone by reason that the gene rearrangement of T-cell receptors (TCR) was not found out (50). However, a number of cases with TCR rearrangement reported by Harabuchi et al. (2) and others (51, 52) indicated that some NNKTL are derived from T-cell lineage. This is evidenced by Nagata et al. (13), who succeeded in establishing two NNKTL cell lines from patients, NK-cell lineage without TCR rearrangement and γδ T-cell lineage with γδ TCR rearrangement. Therefore, the current concept of the origin of NNKTL is NK- or γδ T-cells lineage (14, 15) as first proposed by Harabuchi et al. (2).
EBV Characteristics
We first reported the close association of EBV with NKTCL in 1990 (1), because EBV genomic DNA and EBNA1 were identified in the nuclei of the lymphoma cells. Subsequently, we demonstrated clonotypic EBV genome (2), suggesting that EBV plays a role in the lymphomagenesis. The other studies also confirmed the etiological role of EBV for NNKTL (1, 8, 28, 29). The lymphoma cells express EBNA1 but not the other EBNAs (2, 53). We detected the mRNA of LMP 1 in all patients, but found the protein of LMP 1 in only half of the patients, because of the methylation of LMP coding sequences (2, 17, 44). Therefore, NNKTL is categorized to the type II latency infection of EBV. Moreover, we performed a southern blot analysis of terminal repeats of the EBV genome and found a single fused terminal fragment, indicating that EBV infection may occur at the early stage of lymphomageneses, and EBV infection in these cells is not from contamination (2, 17, 53).
To discover the oncogenic strains of EBV, several efforts have been made. In the sequence analysis of LMP1, the 30-bp deletion in the codon 343–352 of the B95-8 strain was found in the vast majority of the patients (39, 54). Moreover, we found that several amino acid changes in the LMP1 and LMP2 sequence coding major HLA-A2 restricted CTL epitopes (38, 39). These data suggest that the mutation in EBV endows EBV-infected cells with the ability to escape from immune surveillance by CTLs, which may play an important role in lymphomagenesis.
Gene Mutations
The genetic abnormalities, which may have pathogenic importance, have been reported in NNKTL. The deletion in the chromosome 6q21–25 was frequently found (55–58). The aberrant activation of the JAK/STAT3 pathway, which supports the growth of tumors, has been reported (59). The gene mutations in the cell surface receptor Fas (Apo-1/CD95), which transmits an apoptosis signal, were detected in more than half of the patients (60, 61). Our group found a different frequency of p53, K-ras, and c-kit mutations between NNKTL in Korea and Japan (62). In NNKTL, the p53 mutations were detected in 20–50% of patients (44, 62–65). However, our group showed that mutations of the Ras, c-kit, and β-catenin were not frequent (44, 62, 64). Regarding relation to prognosis, Takahara et al. (44) showed that the p53 missense mutation had a prognostic value predicting poor survival.
Proliferation and Invasion Factors of NNKTL Cells
Based on the success of establishing two EBV-positive NNKTL cell lines from NK- and γδ T-cell lineage origins (13), the gene or protein expressions of these cell lines have been discovered. Nagato et al. (66) investigated gene expression patterns of these NNKTL cell lines using cDNA arrays, and (66) found that both the IL-9 mRNA and protein were specifically expressed in NNKTL cell lines (66). NNKTL cell lines also expressed IL-9 receptor. Anti-IL-9 neutralizing antibody decreased proliferation of the cells and recombinant IL-9 increased, suggesting that NNKTL cells use IL-9 as a proliferation factor in an autocrine manner. IL-9 was present in clinical specimens and NNKTL patient sera. EBER induces IL-9 expression (67), suggesting that EBV may play a role for IL-9 expression in the lymphoma.
In addition to IL-9, Takahara et al. (68) found that IL-10 was also secreted by NNKTL cells. Exogenous IL-10 increased CD25 (IL-2 receptor) and LMP1 expressions, and then enhanced cell growth of NNKTL. IL-10 treated cells required lower amounts of IL-2 for proliferation. This effect was seen only with the EBV-positive NK-cell lines, in which CD25 and LMP1 were overexpressed, suggesting that IL-10 induces IL-2 receptor expression via enhancement of LMP1 expression, resulting in the proliferation of NNKTL cells.
Chemokines play a huge role in proliferating/recruiting tumor cells and immune cells. Moriai et al. (69) analyzed the expression of chemokines in these NNKTL cell lines using a protein array analysis. We found that the interferon-gamma-inducible protein-10 (IP-10), i.e., CXCL10 was produced in NNKTL cell lines. The amount of IP-10 was significantly larger in NNKTL cell lines than in EBV-negative NK-cell lines. IP-10 was also determined in the biopsy samples and sera from NNKTL patients. The receptor of IP-10, CXCR3, was also expressed in NNKTL cells. In vitro studies showed that exogenous IP-10 enhanced invasion of the NNKTL cells, on the other hand, the neutralizing antibodies to IP-10 and CXCR3 inhibited, suggesting that NNKTL cells use IP-10/CXCR3 to invade in an autocrine manner.
Subsequently, Kumai et al. (70) found that NNKTL cells produced chemokine (C-C motif) ligand (CCL) 17 and CCL22. CCL17 and CCL22 were also observed in the NNKTL patients' sera. Moreover, CCR4, which is the receptor for CCL17 and CCL22, was expressed on the NNKTL cell lines and tissues. Anti-CCR4 antibody efficiently induced antibody-dependent cellular cytotoxicity mediated by NK-cells against NNKTL cell lines. Because anti-CCR4 antibody mogamulizumab has shown clinical efficiency in cutaneous T-cell lymphoma (71), this antibody could also be a useful option in NNKTL treatment.
Metalloelastase is a family of extracellular matrix-degrading enzymes. Metalloelastase degrades several substrates such as elastin, laminin, collagen, fibronectin, and casein. Because MMP-9 was expressed in NNKTL samples (16, 72), NNKTL cells might use this enzyme to invade into surrounding tissues.
CD70, a ligand of CD27, is expressed on activated T-cells, B-cells, and lymphoma. Because lymphoma expressed a higher level of CD70 than lymphocytes, anti-CD70 antibodies might be a possible treatment for CD70 positive lymphomas (73). Yoshino et al. (74) found that NNKTL cell lines specifically expressed CD70, but not EBV-positive NK-cell lines without LMP1 did not. Exogenous soluble CD27, which is the ligand for CD70, enhanced cell proliferation of NNKTL cells in a dose-dependent fashion. In the clinical samples, CD70 was expressed on the NNKTL tissues, and soluble CD27 was detected in patients' sera at higher levels. These results suggest that soluble CD27/CD70 signaling, possibly up-regulated by LMP-1 (75), supports lymphoma progression, and anti-CD70 antibody may be a candidate for the NNKTL treatment.
Intercellular adhesion molecule (ICAM)-1, a ligand for LFA-1, attracts macrophage and create precancerous environment (76). Harabuchi et al. (49) have previously shown that ICAM-1 and soluble ICAM-1 (sICAM-1) was expressed in NNKTL cells and in NNKTL patient sera, respectively. To elucidate the functional role of ICAM-1 in NNKTL, Takahara et al. (77) examined the NNKTL proliferation with sICAM-1. As a result, exogenous sICAM-1 enhanced the proliferation of NNKTL cells, whereas LFA-1/ICAM-1 blockade by anti-ICAM-1 antibody, anti-LFA-1 antibody, or LFA-1 inhibitor simvastatin reduced the number of viable NNKTL cells. In the NNKTL tissues, we confirmed that NNKTL cells also expressed LFA-1. Accordingly, the blockade of LFA-1/ICAM-1 by simvastatin may be a potential agent for NNKTL.
Micro RNAs (miR) play an important role in the carcinogenesis of several malignancies by regulating gene expression. Komabayashi et al. (78) performed MiR array and quantitative RT-PCR analyses and then found that miR-15a was downregulated, while the expression of MYB and cyclin D1 was elevated in NNKTL cells. On the other hand, transfected NNKTL cells with miR-15a precursor downregulated MYB and cyclin D1 levels, resulting in blocking G1/S cell cycle transition and cell proliferation. In NNKTL tissues, we found that the reduced miR-15a expression, which correlated with MYB and cyclin D1 expression, was associated with poor prognosis of NNKTL patients. Knockdown of LMP1 significantly increased miR-15a expression in NNKTL cells, suggesting that LMP1 downregulate miR-15a and then induce cell proliferation via MYB and cyclin D1. Therefore, miR-15a may be useful as a target for anti-tumor therapy as well as a prognostic factor for NNKTL patients.
Together, these results suggest that cytokines, chemokines, and miR, which may be produced by EBV-oncogenic proteins in the lymphoma cells, play important roles for tumor progression in NNKTL (Figure 2), and could be therapeutic targets in NNKTL patients.
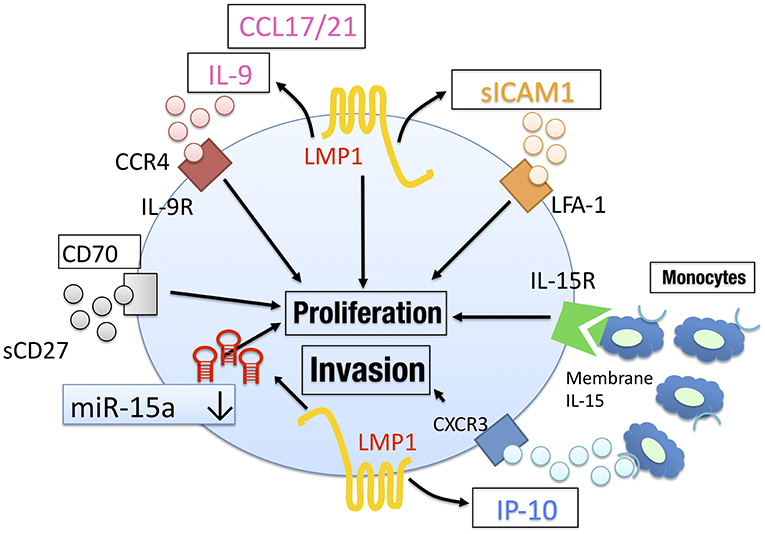
Figure 2. The tumor microenvironment in NNKTL. NNKTL utilize CCL17/21, IL-9, IP-10, and soluble ICAM1 to proliferate/invade in an autocrine manner. These factors may be regulated by EBV LMP1. CD70 activation via soluble CD27 also mediate tumor proliferation. The downregulation of miR-15a mediates tumor progression by regulating surviving. Surrounding immune cells such as monocytes support NNKTL through IL-15 signaling.
Interaction Between NNKTL Cells and Immune Cells
Histological features of NNKTL are characterized by diffused infiltrates of lymphoma cells, together with various inflammatory cells such as granulocytes, monocytes, macrophages, and lymphocytes. Accordingly, it is rational to consider that there is an interaction between NNKTL cells and surrounding immune cells. Ishii et al. (79) co-cultured the NNKTL cells with monocytes or granulocytes to examine whether proliferation, survival and LMP1 expression of NNKTL cells are affected by immune cells. Although granulocytes had no effect on proliferation, survival, or LMP1 expression, co-cultured monocytes enhanced proliferation, LMP1 expression and IP-10 production of NNKTL cells. Being not observed when monocytes were placed in a separate chamber, this interaction was mediated in a contact-dependent manner. Because the monocyte-induced proliferation and LMP1 expression of NNKTL cells were inhibited by anti-IL15 antibody, monocytes might support NNKTL cells via membrane-bound IL-15/IL-15 receptor α complex. Because NNKTL cells secrete IP-10 (69), CCL2 and CCL22 (70) that are monocyte-attractant chemokines, a positive feedback loop by the interaction between NNKTL cells and monocytes may contribute to lymphoma progression in vivo (79).
Recently, the detrimental effects of negative immune checkpoints have been considered as a druggable target (80). Nagato et al. (81) detected the PD-L1 expression on NNKTL cells and PD-1 expression on the macrophages infiltrated the NNKTL tissues. Soluble PD-L1 was also detected in sera of NNKTL patients at higher levels. Patients with higher soluble PD-L1 in sera showed worse prognosis. Furthermore, Nagato et al. (81) elucidated that NNKTL cell lines expressed and secreted PD-L1 in vitro. Because IL-10 converts macrophage to tumor-associated macrophages (82), it is possible that NNKTL cells educate macrophage to be tumor-supportive. The clinical significance of PD-1/PD-L1 blockade is mentioned below.
Diagnosis
Early diagnosis of NNKTL is essential to treat patients promptly (2, 16). Although pathological examination (detection of tumor cells with CD56 and EBER1) is indispensable, the surrounding necrotic tissue may lead to the difficulty of NNKTL diagnosis.
Circulating cell free EBV DNA levels measured by RT-PCR is previously reported to be useful as a tumor marker of EBV-associated malignancies (83). Nagato et al. (18) measured both Bam HI W DNA and LMP1 DNA levels in sera and showed that measurement of both DNAs is more useful as a predictor for prognosis and as a monitoring marker for the clinical course of NNKTL than measurement of Bam HI W DNA alone. These DNAs were decreased with the treatment and increased at relapse in NNKTL patients. Patients with high pre-treatment EBV DNAs showed an aggressive clinical course. Multivariate analysis revealed that high pre-treatment level of both EBV DNAs has the most value as an independent prognostic factor. Because the detection of serum EBV DNA reflects the residual lymphoma cells, further treatment should be considered to achieve a complete remission in NNKTL patients with detectable serum EBV DNAs even after the initial therapy (18). This is supported by prospective measurement of serum EBV-DNA in NNKTL patients (84).
Epstein-Barr virus encodes viral miRNAs (miRs). Komabayashi et al. (85) investigated whether the circulating EBV-miRs level was useful as biomarkers for NNKTL. As a result, the serum levels of miR-BART2-5p, miR-BART7-3p, miR-BART13-3p, and miR-BART1-5p could distinguish NNKTL patients from normal donors. In vitro studies confirmed that these EBV-miRs were secreted from NNKTL cells. In NNKTL patients, these levels significantly decreased after treatment. Moreover, a high circulating miR-BART2-5p level correlated with poor prognosis. Thus, circulating EBV-miRs, particularly miR-BART2-5p, are useful as diagnostic and prognostic biomarkers in NNKTL patients.
As described above, Nagato et al. (81) clearly showed that the level of serum sPD-L1 was elevated and consistent with disease prognosis in NNKTL patients. Collectively, serum EBV DNA, miRs, and sPD-L1 must be useful biomarkers in NNKTL treatment.
NNKTL Treatment
Because a high recurrence rate was reported in the radiation therapy alone (86), chemoradiotherapy is the main strategy to treat NNKTL, but even in early clinical stages, five-year survival rates had been around 50% (44, 87). To improve the treatment outcome, the phase I/II trial (JCOG0211), which consists of three course of DeVIC chemotherapy (dexamethasone, etoposide, ifosfamide, and carboplatin) concomitant with local radiotherapy (50 Gy) for localized NNKTL, conducted in Japan and then showed a good clinical outcome for NNKTL (88). Ifosfamide and carboplatin were chosen because they are not affected by multidrug resistance genes 1, which is frequently expressed in the NNKTL cells (89). Etoposide was used to prevent virus-associated hemophagocytic syndrome (VAHS) (90). Toxicities of the therapy were comparable to those in a previous trial. With a median follow-up of 32 months, 2-year overall survival was 78% (91).
Other regimens such as SMILE (steroid, methotrexate, ifosfamide, L-asparaginase, and etoposide) showed a promising clinical outcome even in a late stage of NNKTL patients (92). Due to the high rate of progression, asparaginase needs to be combined in the regimen with ifosfamide, methotrexate, etoposide, and prednisolone (93). The similar regimen without ifosfamide but sandwiched with radiotherapy also displayed a favorable result (94). The necessity of methotrexate has been examined in the ongoing clinical trial (NCT00283985). The same regimen without radiotherapy has shown satisfactory results (95). Another regimen of the sandwich protocol was reported, Jiang et al. presented the protocol using L-asparaginase, cisplatin, dexamethasone and etoposide sandwiched with radiotherapy (96). The overall response rate in a phase 2 study of sequential radiation therapy followed by gemcitabine, dexamethasone, and cisplatin was 97.5% in early stage NNKTL (97). Another chemotherapy combining gemcitabine (DDPG: cisplatin, dexamethasone, gemcitabine and pegaspargase) without radiotherapy has also shown promising results (98). Li et al. demonstrated that DDPG chemotherapy showed an improved response rate without severe toxicity like the SMILE regimen (99). GELAD (gemcitabine, etoposide, pegaspargase, and dexamethasone, NCT02733458), GDP (gemcitabine, cisplatin, dexamethasone) with radiotherapy (NCT02276248), P-Gemox (gemcitabine, oxaliplatin, and pegaspargase) have been tested in the clinical trials.
Bone marrow transplant is another approach to treat NNKTL. Despite the expectation, the outcome of autologous or allogenic bone marrow transplant is controversial (100–102). Because improved treatment approaches were needed for localized NNKTL exhibiting elevated pretreatment soluble interleukin-2 receptor (103), it is mandatory to develop novel treatment approaches in NNKTL.
Recently, Takahara et al. (104) have developed a novel arterial infusion chemotherapy from a superficial temporal artery in combination with radiotherapy. The regimen for the arterial infusion consists of methotrexate, peplomycin, etoposide, ifosfamide, carboplatin, and prednisolone (MPVIC-P), which is not influenced by multidrug resistance genes 1 (except for etoposide) as well as a DeVIC regimen. Chemotherapy and concomitant radiotherapy were performed for 3 cycles and over 54Gy, respectively. We administered 12 Japanese patients with stage I-II. During the observation period from 39 to 111 months after the treatment (median: 81 months), all 12 patients achieved complete remission and have remained tumor-free. Common adverse effects were mucositis (83%) and myelosuppression (33%), both of which were manageable. Thus, this MPVIC-P regimen using intra-arterial infusion with concomitant radiotherapy is an effective treatment for early stage NNKTL with adaptable toxicity.
Establishment of Novel Treatments for NNKTL
Immune Checkpoint Inhibitor
One of the negative immune checkpoints, the PD-1/PD-L1 pathway, plays an important role in immune evasion of tumor cells through T-cell exhaustion. Because of the expression of PD-L1 on tumor cells (81), PD-1 inhibitor is a promising therapeutic armamentarium in NNKTL. Although the general clinical outcome of NNKTL patients failing chemotherapy is fatal, PD-1 inhibitor has shown remission in these patients (105, 106). The adverse events were tolerable. Several clinical trials with PD-1/PD-L1 blockade are ongoing (NCT03595657, 03107962, 03439501). Thus, PD-1/PD-L1 blockade could be a favorable treatment in chemotherapy-resistant NNKTL patients.
Peptide Vaccine With Molecular Targeting Therapy
Among immunotherapy, peptide vaccine is a promising treatment to target virus-associated malignancy because these types of tumor express non-self viral antigens that are not ignored by immune cells (107). EBV-related proteins are ideal antigens for the peptide vaccine in NNKTL treatment. Kobayashi et al. (108) previously found an epitope peptide, which could bind to promiscuous MHC Class II (HLA-DR9, HLA-DR53, or HLA-DR15), by computer-based peptide algorithm from EBV LMP1. This peptide was naturally processed and expressed on NNKTL cells and could elicit peptide-specific helper T cells, which displayed Th1 phenotype and cytotoxic activity against NNKTL cell. Because this LMP1 epitope peptide overlaps with an HLA-A2–restricted CD8 T cell epitope, this peptide might have the ability to simultaneously induce antitumor CD4 and CD8 T cells against NNKTL cells.
HGF and its receptor c-Met play an essential role in cell proliferation and are involved in various malignancies. Kumai et al. (109) found that both HGF and c-Met were expressed in NNKTL cells and NNKTL tissues, and this pathway activated the proliferation of NNKTL cells in an autocrine manner. c-Met was also responsible for TGF-b production, a negative regulator of immune cells, from NNKTL cells. Kumai et al. (109) further found that several c-Met-derived helper T cell epitope peptides, which are restricted by various HLA-DR molecules. These peptides could elicit c-Met-reactive CD4 T cells that have a cytolytic ability to NNKTL and several solid tumor cells.
Taken together, LMP-1 and c-Met are promising antigens for a peptide vaccine to treat NNKTK patients, and c-Met blockade may augment the antitumor function of peptide-reactive T cells by suppressing TGF-b production from NNKTL cells.
Future Prospective
The pathogenesis and molecular biology of NNKTL have been gradually revealed as mentioned above. Some of these findings have been considered as direct evidence to establish current promising treatment in NNKTL (81, 105, 106). Although prospective clinical trials are required, novel chemotherapeutic approaches such as MPVIC-P and SMILE have shown favorable clinical outcomes (92, 104). Despite these successes, further basic and translational researches are required to improve the prognosis of NNKTL patients. The blockade of cytokine or chemokine (IL-9, IL-10, CCL17, or CCL21) to inhibit NNKTL proliferation can be an attractive method to treat NNKTL. Recently, several antibodies against cytokine have been approved in the clinic. Mogamulizumab, a clinical-grade anti-CCR4 antibody is a promising candidate to treat NNKTL as shown by Kumai et al. (70). It is mandatory to test the safety of these novel agents in an in vivo model. The development of immune deficient mice, in which we succeeded to engraft NNKTL cells, enable us to investigate the effect and safety of these agents (81, 110).
The ligands to pattern-recognition receptors have been recognized as efficient adjuvants in peptide vaccines (111). EBV LMP-1- or c-Met-derived peptide can be a useful peptide vaccine to treat NNKTL patients when combined with adjuvants such as poly-IC or gardiquimod (109, 111). The combination of c-Met or checkpoint blockade with peptide vaccine would be an attracting treatment option in NNKTL. The potential of immunotherapy against NNKTL has been summarized in a review article (112). Because NNKTL is an EBV-related disease, gene therapy to knockout EBV-related proteins or miR would be a fundamental solution to remove NNKTL cells. Since EBV is a widely disseminated virus, the prophylactic vaccine is difficult to establish. However, there is a possibility that high risk EBV subtypes with gene mutation cause EBV-associated malignancies including NNKTL (55–58). Thus, the vaccine that targets high risk EBV can be a preventive vaccine for NNKTL as well as other EBV-associated malignancies.
In conclusion, we demonstrated that recent advances in the molecular understanding of NNKTL have led us to establish novel approaches to treat NNKTL patients (Figure 3). We believe that further investigation will make NNKTL a curable disease.
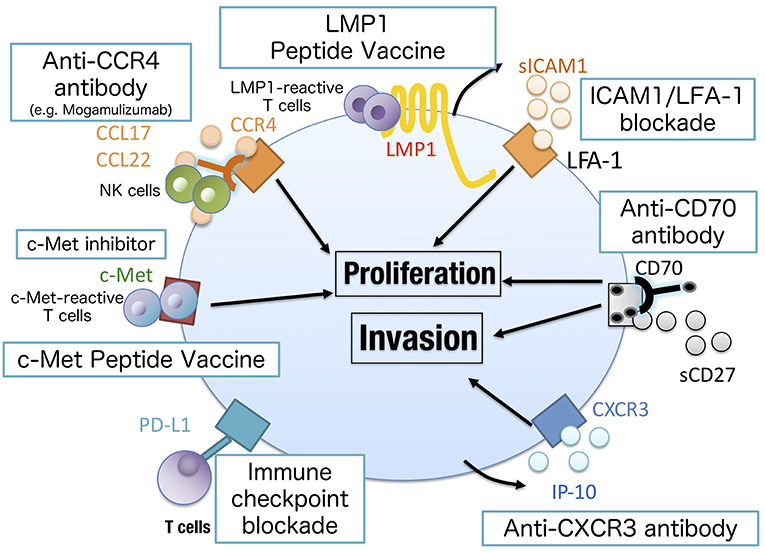
Figure 3. Novel approaches to treat NNKTL. Chemokine/cytokine blockade may inhibit the growth of NNKTL cells (IL-9, IL-10, CXCR3, or LFA-1 blockade) as well as c-Met inhibitor. The antibody against surface markers on NNKTL can directly lyse tumor cells by antibody-dependent cellular cytotoxicity or complement-dependent cytotoxicity. CCR4 or CD70 could be a promising target in this approach. Mogamulizumab, an anti-CCR4 antibody, has been clinically approved to treat cutaneous T cell lymphoma. LMP1 or c-Met peptide vaccine is useful to elicit tumor-specific T cell responses. Because NNKTL cells express PD-L1 to attenuate antitumor T cell responses, immune checkpoint blockades have shown clinical activity in NNKTL patients.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Funding
JSPS KAKENHI Grant Number 24791735 (YH), 16K20223 (TK), 15H04986 (YH), and 18H02948 (YH).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Harabuchi Y, Yamanaka N, Kataura A, Imai S, Kinoshita T, Mizuno F, et al. Epstein-Barr virus in nasal T-cell lymphomas in patients with lethal midline granuloma. Lancet. (1990) 335:128–30. doi: 10.1016/0140-6736(90)90002-M
2. Harabuchi Y, Imai S, Wakashima J, Hirao M, Kataura A, Osato T, et al. Nasal T-cell lymphoma causally associated with Epstein-Barr virus: clinicopathologic, phenotypic, and genotypic studies. Cancer. (1996) 77:2137–49. doi: 10.1002/(SICI)1097-0142(19960515)77:10<2137::AID-CNCR27>3.0.CO;2-V
3. Lee J, Suh C, Park YH, Ko YH, Bang SM, Lee JH, et al. Extranodal natural killer T-cell lymphoma, nasal-type: a prognostic model from a retrospective multicenter study. J Clin Oncol. (2006) 24:612–8. doi: 10.1200/JCO.2005.04.1384
4. Aozasa K, Ohsawa M, Tajima K, Sasaki R, Maeda H, Matsunaga T, et al. Nation-wide study of lethal mid-line granuloma in Japan: frequencies of Wegener's granulomatosis, polymorphic reticulosis, malignant lymphoma and other related conditions. Int J Cancer. (1989) 44:63–66. doi: 10.1002/ijc.2910440112
5. Altemani A, Barbosa AC, Kulka M, Takahashi T, Endo L, Vassallo J, et al. Characteristics of nasal T/NK-cell lymphoma among Brazilians. Neoplasma. (2002) 49:55–60.
6. Gaal K, Sun NC, Hernandez AM, Arber DA Sinonasal NK/T-cell lymphomas in the United States. Am J Surg Pathol. (2000) 24:1511–7. doi: 10.1097/00000478-200011000-00006
7. Vidal RW, Devaney K, Ferlito A, Rinaldo A, Carbone A. Sinonasal malignant lymphomas: a distinct clinicopathological category. Ann Otol Rhinol Laryngol. (1999) 108:411–9. doi: 10.1177/000348949910800417
8. Kanavaros P, Lescs MC, Briere J, Divine M, Galateau F, Joab I, et al. Nasal T-cell lymphoma: a clinicopathologic entity associated with peculiar phenotype and with Epstein-Barr virus. Blood. (1993) 81:2688–95.
9. Yamanaka N, Kataura A, Sambe S, Minase T, Ishii Y. Midfacial T cell lymphoma: Characterization by monoclonal antibodies. Ann Otol Rhinol Laryngol. (1985) 94:207–11. doi: 10.1177/000348948509400223
10. Harris NL, Jaffe ES, Stein H, Banks PM, Chan JK, Cleary ML, et al. A revised European-American classification of lymphoid neoplasms: a proposal from the international lymphoma study group. Blood. (1994) 84:1361–92.
11. Eichel BS, Harrison EG, Devine KD, Scanlon PW, Brown HA. Primary lymphoma of the nose including a relationship to lethal midline granuloma. Am J Surg. (1966) 112:597–605. doi: 10.1016/0002-9610(66)90328-X
12. Emile JF, Boulland ML, Haioun C, Kanavaros P, Petrella T, Delfau-Larue MH, et al. CD5-CD56+ T-cell receptor silent peripheral T-cell lymphomas are natural killer cell lymphomas. Blood. (1996) 87:1466–73.
13. Nagata H, Konno A, Kimura N, Zhang Y, Kimura M, Demachi A, et al. Characterization of novel natural killer (NK)-cell and gammadelta T-cell lines established from primary lesions of nasal T/NK-cell lymphomas associated with the Epstein-Barr virus. Blood. (2001) 97:708–13. doi: 10.1182/blood.V97.3.708
14. Kinney MC. The role of morphologic features, phenotype, genotype, and anatomic site in defining extranodal T-cell or NK-cell neoplasms. Am J Clin Pathol. (1999) 111:S104–18.
15. Suzuki R, Takeuchi K, Ohshima K, Nakamura S. Extranodal NK/T-cell lymphoma: diagnosis and treatment cues. Hematol Oncol. (2008) 26:66–72. doi: 10.1002/hon.847
16. Harabuchi Y, Takahara M, Kishibe K, Moriai S, Nagato T, Ishii H. Nasal natural killer (NK)/T-cell lymphoma: clinical, histological, virological, and genetic features. Int J Clin Oncol. (2009) 14:181–90. doi: 10.1007/s10147-009-0882-7
17. Minarovits J, Hu L, Imai S, Harabuchi Y, Kataura A, Minarovits-Kormuta S, et al. Clonality, expression and methylation patterns of the Epstein-Barr virus genomes in lethal midline granulomas classified as peripheral angiocentric T cell lymphomas. J General Virol. (1994) 75:77–84. doi: 10.1099/0022-1317-75-1-77
18. Ishii H, Ogino T, Berger C, Kochli-Schmitz N, Nagato T, Takahara M, et al. Clinical usefulness of serum EBV DNA levels of BamHI W and LMP1 for Nasal NK/T-cell lymphoma. J Med Virol. (2007) 79:562–72. doi: 10.1002/jmv.20853
19. Huang Y, Xie J, Ding Y, Zhou X. Extranodal natural killer/T-cell lymphoma in children and adolescents: a report of 17 cases in China. Am J Clin Pathol. (2016) 145:46–54. doi: 10.1093/ajcp/aqv010
20. McBride P. Photographs of a case of rapid destruction of the nose and face. J Laryngol Otol. (1897) 12:64–66.
21. Kraus EJ. Uber ein eigenartiges granulom aer nasen-, rachen- und mundhohle. Verh Dtsch Ges Pathol. (1929) 24:43.
22. Williams HL. Lethal granulomatous ulceration, involving midline facial tissues. Ann Otol Rhino Laryngol. (1949) 58:1013–55. doi: 10.1177/000348944905800405
23. Harabuchi Y, Kataura A, Kobayashi K, Yamamoto T, Yamanaka N, Hirao M, et al. Lethal midline granuloma (peripheral T-cell lymphoma) after lymphomatoid papulosis. Cancer. (1992) 70:835–9. doi: 10.1002/1097-0142(19920815)70:4<835::AID-CNCR2820700419>3.0.CO;2-F
24. Michaels L, Gregory M. Pathology of nonhealing (midline) granuloma. J Clin Path. (1977) 30:317–327. doi: 10.1136/jcp.30.4.317
25. Ishii Y, Yamanaka N, Ogawa K, Yoshida Y, Takami T, Matsuura A, et al. Nasal T-cell lymphoma as a type of so-called “lethal midline granuloma”. Cancer. (1982) 50:2336–44. doi: 10.1002/1097-0142(19821201)50:11<2336::AID-CNCR2820501120>3.0.CO;2-C
26. Ng CS, Chan JK, Lo ST. Expression of natural killer cell markers in non-Hodgkin's lymphomas. Hum Pathol. (1987) 18:1257–62. doi: 10.1016/S0046-8177(87)80410-0
27. Ho FC, Srivastava G, Loke SL, Fu KH, Leung BP, Liang R, et al. Presence of Epstein-Barr virus DNA in nasal lymphomas of B and 'T' cell type. Hematol Oncol. (1990) 8:271–81. doi: 10.1002/hon.2900080505
28. Medeiros LJ, Jaffe ES, Chen YY, Weiss LM. Localization of Epstein-Barr viral genomes in angiocentric immunoproliferative lesions. Am J Surg Pathol. (1992) 16:439–47. doi: 10.1097/00000478-199205000-00002
29. Weiss LM, Gaffey MJ, Y.-Chen Y, Frierson HF. Frequency of Epstein-Barr viral DNA in “Western” sinonasal and Waldeyer's ring non-Hodgkin's lymphoma. Am J Surg Pathol. (1992) 16:156–62. doi: 10.1097/00000478-199202000-00008
30. Liu J, Song B, Fan T, Huang C, Xie C, Li J, et al. Pathological and clinical characteristics of 1,248 non-Hodgkin's lymphomas from a regional cancer hospital in Shandong, China. Asian Pac J Cancer Prev. (2011) 12:3055–61. Available online at: http://journal.waocp.org/article_26012_35bfe62aeb1a44a637da13f8398a8e12.pdf
31. Vose J, Armitage J, Weisenburger D, International LP. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. (2008) 26:4124–30. doi: 10.1200/JCO.2008.16.4558
32. Rudiger T, Weisenburger DD, Anderson JR, Armitage JO, Diebold J, MacLennan KA, et al. P. Non-Hodgkin's Lymphoma Classification, Peripheral T-cell lymphoma (excluding anaplastic large-cell lymphoma): results from the Non-Hodgkin's Lymphoma Classification Project. Ann Oncol. (2002) 13:140–9. doi: 10.1093/annonc/mdf033
33. Lima M. Aggressive mature natural killer cell neoplasms: from epidemiology to diagnosis. Orphanet J Rare Dis. (2013) 8:95. doi: 10.1186/1750-1172-8-95
34. Arber DA, Weiss LM, Albujar PF, Chen YY, Jaffe ES. Nasal lymphomas in peru. high incidence of T-cell immunophenotype and Epstein-Barr virus infection [see comments]. Am J Surg Pathol. (1993) 17:392–9. doi: 10.1097/00000478-199304000-00010
35. Aozasa K, Zaki MA. Epidemiology and pathogenesis of nasal NK/T-cell lymphoma: a mini-review. Sci World J. (2011) 11:422–8. doi: 10.1100/tsw.2011.41
36. Goldsmith DB, West TM, Morton R. HLA associations with nasopharyngeal carcinoma in Southern Chinese: a meta-analysis. Clin Otolaryngol Allied Sci. (2002) 27:61–7. doi: 10.1046/j.0307-7772.2001.00529.x
37. Sidagis J, Ueno K, Tokunaga M, Ohyama M, Eizuru Y. Molecular epidemiology of Epstein-Barr virus (EBV) in EBV-related malignancies. Int J Cancer. (1997) 72:72–6. doi: 10.1002/(SICI)1097-0215(19970703)72:1<72::AID-IJC11>3.0.CO;2-C
38. Nagamine M, Kishibe K, Takahara M, Nagato T, Ishii H, Bandoh N, et al. Selected amino acid change encoding Epstein-Barr virus-specific T cell epitope of the LMP2A gene in Japanese nasal NK/T cell lymphoma patients. Intervirology. (2007) 50:319–22. doi: 10.1159/000106462
39. Nagamine M, Takahara M, Kishibe K, Nagato T, Ishii H, Bandoh N, et al. Sequence variations of Epstein-Barr virus LMP1 gene in nasal NK/T-cell lymphoma. Virus genes. (2007) 34:47–54. doi: 10.1007/s11262-006-0008-5
40. Kanno H, Kojya S, Li T, Ohsawa M, Nakatsuka S, Miyaguchi M, et al. Low frequency of HLA-A*0201 allele in patients with Epstein-Barr virus-positive nasal lymphomas with polymorphic reticulosis morphology. Int J Cancer. (2000) 87:195–9. doi: 10.1002/1097-0215(20000715)87:2<195::AID-IJC6>3.0.CO;2-0
41. Xu JX, Hoshida Y, Yang WI, Inohara H, Kubo T, Kim GE, et al. Life-style and environmental factors in the development of nasal NK/T-cell lymphoma: a case-control study in East Asia. Int J Cancer. (2006) 120:406–10. doi: 10.1002/ijc.22313
42. Kojya S, Matsumura J, Ting L, Hongyo T, Inazawa J, Kirihata M, et al. Familial nasal NK/T-cell lymphoma and pesticide use. Am J Hematol. (2001) 66:145–7. doi: 10.1002/1096-8652(200102)66:2<145::AID-AJH1033>3.0.CO;2-V
43. Wu X, Li P, Zhao J, Yang X, Wang F, Yang YQ, et al. A clinical study of 115 patients with extranodal natural killer/T-cell lymphoma, nasal type. Clin Oncol. (2008) 20:619–25.
44. Takahara M, Kishibe K, Bandoh N, Nonaka S, Harabuchi Y. P53, N- and K-Ras, and beta-catenin gene mutations and prognostic factors in nasal NK/T-cell lymphoma from Hokkaido, Japan. Hum Pathol. (2004) 35:86–95. doi: 10.1016/j.humpath.2003.08.025
45. Suzuki R, Suzumiya J, Yamaguchi M, Nakamura S, Kameoka J, Kojima H, et al. Prognostic factors for mature natural killer (NK) cell neoplasms: aggressive NK cell leukemia and extranodal NK cell lymphoma, nasal type. Ann Oncol. (2010) 21:1032–40. doi: 10.1093/annonc/mdp418
46. Kim TM, Park YH, Lee SY, Kim JH, Kim DW, Im SA, et al. Local tumor invasiveness is more predictive of survival than international prognostic index in stage I(E)/II(E) extranodal NK/T-cell lymphoma, nasal type. Blood. (2005) 106:3785–90. doi: 10.1182/blood-2005-05-2056
47. Gualco G, Domeny-Duarte P, Chioato L, Barber G, Natkunam Y, Bacchi CE. Clinicopathologic and molecular features of 122 Brazilian cases of nodal and extranodal NK/T-cell lymphoma, nasal type, with EBV subtyping analysis. Am J Surg Pathol. (2011) 35:1195–203. doi: 10.1097/PAS.0b013e31821ec4b5
48. Aozasa K, Takakuwa T, Hongyo T, Yang WI. Nasal NK/T-cell lymphoma: epidemiology and pathogenesis. Int J Hematol. (2008) 87:110–7. doi: 10.1007/s12185-008-0021-7
49. Harabuchi Y, Kataura A, Imai K. Circulating intercellular adhesion molecule-1 and its cellular expression in head and neck non-Hodgkin's lymphomas, including lethal midline granuloma. Ann Otol Rhinol Laryngol. (1996) 105:634–42. doi: 10.1177/000348949610500809
50. Jaffe ES, Chan JK, Su J-I, Frizzera G, Mori S, Feller A, et al. Report of the workshop on nasal and related extranodal angiocentric T/natural killer cell lymphomas: definitions, differential diagnosis, and epidemiology. Am J Surg Pathol. (1996) 20:103–11. doi: 10.1097/00000478-199601000-00012
51. Gaulard P, Henni T, Marolleau JP, Haioun C, Henni Z, Voisin MC, et al. Lethal midline granuloma (polymorphic reticulosis) and lymphomatoid granulomatosis. evidence for a monoclonal T-cell lymphoproliferative disorder. Cancer. (1988) 62:705–10. doi: 10.1002/1097-0142(19880815)62:4<705::AID-CNCR2820620410>3.0.CO;2-Z
52. Yoon TY, Lee HT, Chang SH. Nasal-type T/natural killer cell angiocentric lymphoma, Epstein-Barr virus-associated, and showing clonal T-cell receptor gamma gene rearrangement. Br J Dermatol. (1999) 140:505–8. doi: 10.1046/j.1365-2133.1999.02718.x
53. Chiang AK, Tao Q, Srivastava G, Ho FC. Nasal NK- and T-cell lymphomas share the same type of Epstein-Barr virus latency as nasopharyngeal carcinoma and Hodgkin's disease. Int J Cancer. (1996) 68:285–90. doi: 10.1002/(SICI)1097-0215(19961104)68:3<285::AID-IJC3>3.0.CO;2-Y
54. Kim JE, Kim YA, Jeon YK, Park SS, Heo DS, Kim CW. Comparative analysis of NK/T-cell lymphoma and peripheral T-cell lymphoma in Korea: clinicopathological correlations and analysis of EBV strain type and 30-bp deletion variant LMP1. Pathol Int. (2003) 53:735–43. doi: 10.1046/j.1320-5463.2003.01552.x
55. Tien HF, Su IJ, Tang JL, Liu MC, Lee FY, Chen YC, et al. Clonal chromosomal abnormalities as direct evidence for clonality in nasal T/natural killer cell lymphomas. Br J Haematol. (1997) 97:621–5. doi: 10.1046/j.1365-2141.1997.752711.x
56. Wong KF, Chan JK, Kwong YL. Identification of del(6)(q21q25) as a recurring chromosomal abnormality in putative NK cell lymphoma/leukaemia. Br J Haematol. (1997) 98:922–6. doi: 10.1046/j.1365-2141.1997.3223139.x
57. Siu LL, Chan V, Chan JK, Wong KF, Liang R, Kwong YL. Consistent patterns of allelic loss in natural killer cell lymphoma. Am J Pathol. (2000) 157:1803–9. doi: 10.1016/S0002-9440(10)64818-3
58. Sun HS, Su IJ, Lin YC, Chen JS, Fang SY. A 2.6 Mb interval on chromosome 6q25.2-q25.3 is commonly deleted in human nasal natural killer/T-cell lymphoma. Br J Haematol. (2003) 122:590–9. doi: 10.1046/j.1365-2141.2003.04419.x
59. Lee S, Park HY, Kang SY, Kim SJ, Hwang J, Lee S, et al. Genetic alterations of JAK/STAT cascade and histone modification in extranodal NK/T-cell lymphoma nasal type. Oncotarget. (2015) 6:17764–76. doi: 10.18632/oncotarget.3776
60. Takakuwa T, Dong Z, Nakatsuka S, Kojya S, Harabuchi Y, Yang WI, et al. Frequent mutations of Fas gene in nasal NK/T cell lymphoma. Oncogene. (2002) 21:4702–5. doi: 10.1038/sj.onc.1205571
61. Shen L, Liang AC, Lu L, Au WY, Kwong YL, Liang RH, et al. Frequent deletion of Fas gene sequences encoding death and transmembrane domains in nasal natural killer/T-cell lymphoma. Am J Pathol. (2002) 161:2123–31. doi: 10.1016/S0002-9440(10)64490-2
62. Hongyo T, Hoshida Y, Nakatsuka S, Syaifudin M, Kojya S, Yang WI, et al. p53, K-ras, c-kit and beta-catenin gene mutations in sinonasal NK/T-cell lymphoma in Korea and Japan. Oncology reports. (2005) 13:265–71. Available online at: https://www.spandidos-publications.com/or/13/2/265
63. Li T, Hongyo T, Syaifudin M, Nomura T, Dong Z, Shingu N, et al. Mutations of the p53 gene in nasal NK/T-cell lymphoma. Lab Invest. (2000) 80:493–9. doi: 10.1038/labinvest.3780055
64. Hoshida Y, Hongyo T, Jia X, He Y, Hasui K, Dong Z, et al. Analysis of p53, K-ras, c-kit, and beta-catenin gene mutations in sinonasal NK/T cell lymphoma in northeast district of China. Cancer Sci. (2003) 94:297–301. doi: 10.1111/j.1349-7006.2003.tb01436.x
65. Quintanilla-Martinez L, Kremer M, Keller G, Nathrath M, Gamboa-Dominguez A, Meneses A, et al. p53 Mutations in nasal natural killer/T-cell lymphoma from Mexico: association with large cell morphology and advanced disease. Am J Pathol. (2001) 159:2095–105. doi: 10.1016/S0002-9440(10)63061-1
66. Nagato T, Kobayashi H, Kishibe K, Takahara M, Ogino T, Ishii H, et al. Expression of interleukin-9 in nasal natural killer/T-cell lymphoma cell lines and patients. Clin Cancer Res. (2005) 11:8250–7. doi: 10.1158/1078-0432.CCR-05-1426
67. Yang L, Aozasa K, Oshimi K, Takada K. Epstein-Barr virus (EBV)-encoded RNA promotes growth of EBV-infected T cells through interleukin-9 induction. Cancer Res. (2004) 64:5332–7. doi: 10.1158/0008-5472.CAN-04-0733
68. Takahara M, Kis LL, Nagy N, Liu A, Harabuchi Y, Klein G, et al. Concomitant increase of LMP1 and CD25 (IL-2-receptor alpha) expression induced by IL-10 in the EBV-positive NK lines SNK6 and KAI3. Int J Cancer. (2006) 119:2775–83. doi: 10.1002/ijc.22139
69. Moriai S, Takahara M, Ogino T, Nagato T, Kishibe K, Ishii H, et al. Production of interferon-{gamma}-inducible protein-10 and its role as an autocrine invasion factor in nasal natural killer/T-cell lymphoma cells. Clin Cancer Res. (2009) 15:6771–9. doi: 10.1158/1078-0432.CCR-09-1052
70. Kumai T, Nagato T, Kobayashi H, Komabayashi Y, Ueda S, Kishibe K, et al. CCL17 and CCL22/CCR4 signaling is a strong candidate for novel targeted therapy against nasal natural killer/T-cell lymphoma. Cancer Immunol Immunother. (2015) 64:697–705. doi: 10.1007/s00262-015-1675-7
71. Kim YH, Bagot M, Pinter-Brown L, Rook AH, Porcu P, Horwitz SM, et al. Mogamulizumab versus vorinostat in previously treated cutaneous T-cell lymphoma (MAVORIC): an international, open-label, randomised, controlled phase 3 trial. Lancet Oncol. (2018) 19:1192–204. doi: 10.1016/S1470-2045(18)30379-6
72. Sakata K, Someya M, Omatsu M, Asanuma H, Hasegawa T, Ichimiya S, et al. The enhanced expression of the matrix metalloproteinase 9 in nasal NK/T-cell lymphoma. BMC Cancer. (2007) 7:229. doi: 10.1186/1471-2407-7-229
73. Israel BF, Gulley M, Elmore S, Ferrini S, Feng WH, Kenney SC. Anti-CD70 antibodies: a potential treatment for EBV+ CD70-expressing lymphomas. Mol Cancer Ther. (2005) 4:2037–44. doi: 10.1158/1535-7163.MCT-05-0253
74. Yoshino K, Kishibe K, Nagato T, Ueda S, Komabayashi Y, Takahara M, et al. Expression of CD70 in nasal natural killer/T cell lymphoma cell lines and patients; its role for cell proliferation through binding to soluble CD27. Br J Haematol. (2013) 160:331–42. doi: 10.1111/bjh.12136
75. Choi IK, Wang Z, Ke Q, Hong M, Qian Y, Zhao X, et al. Signaling by the Epstein-Barr virus LMP1 protein induces potent cytotoxic CD4(+) and CD8(+) T cell responses. Proc Natl Acad Sci USA. (2018) 115:E686–95. doi: 10.1073/pnas.1713607115
76. Liou GY, Doppler H, Necela B, Edenfield B, Zhang L, Dawson DW, et al. Mutant KRAS-induced expression of ICAM-1 in pancreatic acinar cells causes attraction of macrophages to expedite the formation of precancerous lesions. Cancer Discov. (2015) 5:52–63. doi: 10.1158/2159-8290.CD-14-0474
77. Takahara M, Nagato T, Komabayashi Y, Yoshino K, Ueda S, Kishibe K, et al. Soluble ICAM-1 secretion and its functional role as an autocrine growth factor in nasal NK/T cell lymphoma cells. Exp Hematol. (2013) 41:711–8. doi: 10.1016/j.exphem.2013.03.009
78. Komabayashi Y, Kishibe K, Nagato T, Ueda S, Takahara M, Harabuchi Y. Downregulation of miR-15a due to LMP1 promotes cell proliferation and predicts poor prognosis in nasal NK/T-cell lymphoma. Am J Hematol. (2014) 89:25–33. doi: 10.1002/ajh.23570
79. Ishii H, Takahara M, Nagato T, Kis LL, Nagy N, Kishibe K, et al. Monocytes enhance cell proliferation and LMP1 expression of nasal natural killer/T-cell lymphoma cells by cell contact-dependent interaction through membrane-bound IL-15. Int J Cancer. (2012) 130:48–58. doi: 10.1002/ijc.25969
80. Taube JM, Klein A, Brahmer JR, Xu H, Pan X, Kim JH, et al. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res. (2014) 20:5064–74. doi: 10.1158/1078-0432.CCR-13-3271
81. Nagato T, Ohkuri T, Ohara K, Hirata Y, Kishibe K, Komabayashi Y, et al. Programmed death-ligand 1 and its soluble form are highly expressed in nasal natural killer/T-cell lymphoma: a potential rationale for immunotherapy. Cancer Immunol Immunother. (2017) 66:877–90. doi: 10.1007/s00262-017-1987-x
82. Sica A, Saccani A, Bottazzi B, Polentarutti N, Vecchi A, van Damme J, et al. Autocrine production of IL-10 mediates defective IL-12 production and NF-kappa B activation in tumor-associated macrophages. J Immunol. (2000) 164:762–7. doi: 10.4049/jimmunol.164.2.762
83. Lei KI, Chan LY, Chan WY, Johnson PJ, Lo YM. Quantitative analysis of circulating cell-free Epstein-Barr virus (EBV) DNA levels in patients with EBV-associated lymphoid malignancies. Br J Haematol. (2000) 111:239–46. doi: 10.1046/j.1365-2141.2000.02344.x
84. Suzuki R, Yamaguchi M, Izutsu K, Yamamoto G, Takada K, Harabuchi Y, et al. Prospective measurement of Epstein-Barr virus-DNA in plasma and peripheral blood mononuclear cells of extranodal NK/T-cell lymphoma, nasal type. Blood. (2011) 118:6018–22. doi: 10.1182/blood-2011-05-354142
85. Komabayashi Y, Kishibe K, Nagato T, Ueda S, Takahara M, Harabuchi Y. Circulating Epstein-Barr virus-encoded micro-RNAs as potential biomarkers for nasal natural killer/T-cell lymphoma. Hematol Oncol. (2017) 35:655–63. doi: 10.1002/hon.2360
86. Tse E, Kwong YL. The diagnosis and management of NK/T-cell lymphomas. J Hematol Oncol. (2017) 10:85. doi: 10.1186/s13045-017-0452-9
87. Isobe K, Uno T, Tamaru J, Kawakami H, Ueno N, Wakita H, et al. Extranodal natural killer/T-cell lymphoma, nasal type: the significance of radiotherapeutic parameters. Cancer. (2006) 106:609–15. doi: 10.1002/cncr.21656
88. Yamaguchi M, Ogawa S, Nomoto Y. Treatment outcome of nasal NK-cell lymphoma: a case report of 12 consecutively-diagnosed cases and a review of the literature. J Clin Exp Hematopathol. (2001) 41:93–99. doi: 10.3960/jslrt.41.93
89. Yamaguchi M, Kita K, Miwa H, Nishii K, Oka K, Ohno T, et al. Frequent expression of P-glycoprotein/MDR1 by nasal T-cell lymphoma cells. Cancer. (1995) 76:2351–6. doi: 10.1002/1097-0142(19951201)76:11<2351::AID-CNCR2820761125>3.0.CO;2-1
90. Imashuku S. Advances in the management of hemophagocytic lymphohistiocytosis. Int J Hematol. (2000) 72:1–11.
91. Yamaguchi M, Tobinai K, Oguchi M, Ishizuka N, Kobayashi Y, Isobe Y, et al. Phase I/II study of concurrent chemoradiotherapy for localized nasal natural killer/T-cell lymphoma: japan clinical oncology group study JCOG0211. J Clin Oncol. (2009) 27:5594–600. doi: 10.1200/JCO.2009.23.8295
92. Yamaguchi M, Suzuki R, Kwong YL, Kim WS, Hasegawa Y, Izutsu K, et al. Phase I study of dexamethasone, methotrexate, ifosfamide, L-asparaginase, and etoposide (SMILE) chemotherapy for advanced-stage, relapsed or refractory extranodal natural killer (NK)/T-cell lymphoma and leukemia. Cancer Sci. (2008) 99:1016–20. doi: 10.1111/j.1349-7006.2008.00768.x
93. Kim TM, Kim DW, Kang YK, Chung J, Song HS, Kim HJ, et al. A phase II study of ifosfamide, methotrexate, etoposide, and prednisolone for previously untreated stage I/II extranodal natural killer/T-cell lymphoma, nasal type: a multicenter trial of the Korean cancer study group. Oncologist. (2014) 19:1129–30. doi: 10.1634/theoncologist.2014-0305
94. Xu PP, Xiong J, Cheng S, Zhao X, Wang CF, Cai G, et al. A phase II study of methotrexate, etoposide, dexamethasone and pegaspargase sandwiched with radiotherapy in the treatment of newly diagnosed, stage ie to iie extranodal natural-killer/T-cell lymphoma, nasal-type. EBioMed. (2017) 25:41–49. doi: 10.1016/j.ebiom.2017.10.011
95. Liang R, Gao GX, Chen JP, Wang JS, Wang XM, Zeng Y, et al. A phase 2 study of methotrexate, etoposide, dexamethasone, and pegaspargase chemotherapy for newly diagnosed, relapsed, or refractory extranodal natural killer/T-cell lymphoma, nasal type: a multicenter trial in Northwest China. Hematol Oncol. (2017) 35:619–29. doi: 10.1002/hon.2325
96. Jiang M, Zhang L, Xie L, Zhang H, Jiang Y, Liu WP, et al. A phase II prospective study of the “Sandwich” protocol, L-asparaginase, cisplatin, dexamethasone and etoposide chemotherapy combined with concurrent radiation and cisplatin, in newly diagnosed, I/II stage, nasal type, extranodal natural killer/T-cell lymphoma. Oncotarget. (2017) 8:50155–63. doi: 10.18632/oncotarget.16334
97. Qi F, Wang WH, He XH, Chen B, Gui L, Fang H, et al. Phase 2 study of first-line intensity modulated radiation therapy followed by gemcitabine, dexamethasone, and cisplatin for high-risk, early stage extranodal nasal-type nk/t-cell lymphoma: the green study. Int J Radiat Oncol Biol Phys. (2018) 102:61–70. doi: 10.1016/j.ijrobp.2018.05.046
98. Zhang L, Jia S, Ma Y, Li L, Li X, Wang X, et al. Efficacy and safety of cisplatin, dexamethasone, gemcitabine and pegaspargase (DDGP) regimen in newly diagnosed, advanced-stage extranodal natural killer/T-cell lymphoma: interim analysis of a phase 4 study NCT01501149. Oncotarget. (2016) 7:55721–31. doi: 10.18632/oncotarget.10124
99. Li X, Cui Y, Sun Z, Zhang L, Li L, Wang X, et al. DDGP versus SMILE in newly diagnosed advanced natural killer/T-cell lymphoma: a randomized controlled, multicenter, open-label study in China. Clin Cancer Res. (2016) 22:5223–8. doi: 10.1158/1078-0432.CCR-16-0153
100. Takenaka K, Shinagawa K, Maeda Y, Makita M, Kozuka T, Ashiba A, et al. High-dose chemotherapy with hematopoietic stem cell transplantation is effective for nasal and nasal-type CD56+ natural killer cell lymphomas. Leukemia lymphoma. (2001) 42:1297–303. doi: 10.1080/10428190127500
101. Murashige N, Kami M, Kishi Y, Kim SW, Takeuchi M, Matsue K, et al. Allogeneic haematopoietic stem cell transplantation as a promising treatment for natural killer-cell neoplasms. Br J Haematol. (2005) 130:561–7. doi: 10.1111/j.1365-2141.2005.05651.x
102. Suzuki R, Suzumiya J, Nakamura S, Kagami Y, Kameoka JI, Sakai C, et al. Hematopoietic stem cell transplantation for natural killer-cell lineage neoplasms. Bone Marrow Transplant. (2006) 37:425–31. doi: 10.1038/sj.bmt.1705244
103. Yamaguchi M, Suzuki R, Oguchi M, Asano N, Amaki J, Akiba T, et al. Treatments and outcomes of patients with extranodal natural killer/T-cell lymphoma diagnosed between 2000 and 2013: a cooperative study in Japan. J Clin Oncol. (2017) 35:32–39. doi: 10.1200/JCO.2016.68.1619
104. Takahara M, Nagato T, Kishibe K, Ueda S, Komabayashi Y, Yamashina M, et al. Novel treatment for early-stage nasal natural killer/T-cell lymphoma: intra-maxillary arterial infusion chemotherapy with concomitant radiotherapy. Hematol Oncol. (2017) 35:158–62. doi: 10.1002/hon.2273
105. Liu MY, Li YF, Du JW, Dong LH, Wang YS, Gao X, et al. [Combination of PD-1 inhibitor and chemotherapy in the treatment of nasal NK/T cell lymphoma: 5 cases report and literature review]. Zhonghua xue ye xue za zhi = Zhonghua xueyexue zazhi. (2018) 39:420–422. doi: 10.3760/cma.j.issn.0253-2727.2018.05.015
106. Kwong YL, Chan TSY, Tan D, Kim SJ, Poon LM, Mow B, et al. PD1 blockade with pembrolizumab is highly effective in relapsed or refractory NK/T-cell lymphoma failing l-asparaginase. Blood. (2017) 129:2437–42. doi: 10.1182/blood-2016-12-756841
107. Kumai T, Kobayashi H, Harabuchi Y, Celis E. Peptide vaccines in cancer-old concept revisited. Curr Opin Immunol. (2017) 45:1–7. doi: 10.1016/j.coi.2016.11.001
108. Kobayashi H, Nagato T, Takahara M, Sato K, Kimura S, Aoki N, et al. Induction of EBV-latent membrane protein 1-specific MHC class II-restricted T-cell responses against natural killer lymphoma cells. Cancer Res. (2008) 68:901–8. doi: 10.1158/0008-5472.CAN-07-3212
109. Kumai T, Matsuda Y, Ohkuri T, Oikawa K, Ishibashi K, Aoki N, et al. c-Met is a novel tumor associated antigen for T-cell based immunotherapy against NK/T cell lymphoma. Oncoimmunology. (2015) 4:e976077. doi: 10.4161/2162402X.2014.976077
110. Murata T, Iwata S, Siddiquey MN, Kanazawa T, Goshima F, Kawashima D, et al. Heat shock protein 90 inhibitors repress latent membrane protein 1 (LMP1) expression and proliferation of Epstein-Barr virus-positive natural killer cell lymphoma. PloS one. (2013) 8:e63566. doi: 10.1371/journal.pone.0063566
111. Kumai T, Lee S, Cho HI, Sultan H, Kobayashi H, Harabuchi Y, et al. Optimization of peptide vaccines to induce robust antitumor CD4 T-cell responses. Cancer Immunol Res. (2017) 5:72–83. doi: 10.1158/2326-6066.CIR-16-0194
Keywords: nasal natural killer /T-cell lymphoma, cytokine, chemokine, Epstein-Barr virus, ICAM-1, CCR4, PD-L1, MPVIC-P
Citation: Harabuchi Y, Takahara M, Kishibe K, Nagato T and Kumai T (2019) Extranodal Natural Killer/T-Cell Lymphoma, Nasal Type: Basic Science and Clinical Progress. Front. Pediatr. 7:141. doi: 10.3389/fped.2019.00141
Received: 12 December 2018; Accepted: 26 March 2019;
Published: 16 April 2019.
Edited by:
Shigeyoshi Fujiwara, National Center for Child Health and Development (NCCHD), JapanReviewed by:
Lisa Renee Forbes, Baylor College of Medicine, United StatesNaohiro Wakisaka, Kanazawa University, Japan
Copyright © 2019 Harabuchi, Takahara, Kishibe, Nagato and Kumai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Takumi Kumai, dC1rdW1haUBhc2FoaWthd2EtbWVkLmFjLmpw
†These authors have contributed equally to this work
 Yasuaki Harabuchi
Yasuaki Harabuchi Miki Takahara1
Miki Takahara1 Takumi Kumai
Takumi Kumai