
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Pediatr. , 22 March 2019
Sec. Pediatric Urology
Volume 7 - 2019 | https://doi.org/10.3389/fped.2019.00085
This article is part of the Research Topic Robotic Assisted Laparoscopic Surgery (RALS) in Pediatric Urology View all 14 articles
Robot-assisted laparoscopic (RAL) surgery is a safe, minimally invasive technique that has become more widely used in pediatric urology over recent decades. With several advantages over standard laparoscopy, robotic surgery is particularly well-suited to reconstructive surgery involving delicate structures like the ureter. A robotic approach provides excellent access to and visualization of the ureter at all levels. Common applications include upper ureteral reconstruction (e.g., pyeloplasty, ureteropelvic junction polypectomy, ureterocalicostomy, and high uretero-ureterostomy in duplex systems), mid-ureteral reconstruction (e.g., mid uretero-ureterostomy for stricture or polyp), and lower ureteral reconstruction (e.g., ureteral reimplantation and lower ureter-ureterostomy in duplex systems). Herein, we describe each of these robotic procedures in detail.
Many considerations are involved in choosing surgical approach. Compared to open surgery, roboticsoffer several advantages including smaller incisions and more rapid convalescence. Robot-assisted laparoscopic (RAL) surgery may, however, be difficult or even impossible in very small patients, in whom pure laparoscopic intervention may be preferred. Pure laparoscopy allows for even smaller incisions than robotic surgery, with ports as small as 2–3 mm available. Another disadvantage of robotic surgery is increased cost compared to pure laparoscopic or open approaches. Benefits of robotic surgery include wristed movements and magnified vision, making it the ideal approach for delicate reconstructive procedures. Robotics continue to enjoy expanding applications and growing popularity among urologists and patient families alike.
Pyeloplasty for ureteropelvic junction (UPJ) obstruction is the most common robotic surgery in pediatric urology (1). RAL retroperitoneoscopic pyeloplasty has been described in children (2); however, the transabdominal approach is more frequently utilized, providing a larger working space that facilitates dissection and anastomosis. Transabdominal robotic approach may involve transmesenteric UPJ exposure for left-sided cases to decrease operative time, as previously described for traditional laparoscopic pyeloplasty (3). However, reflecting the colon is usually rapid, and one should not risk limited exposure for potential time savings, particularly in complex cases.
Prior to positioning for the robotic portion of the case, we prefer starting with cystoscopy and retrograde pyelogram to delineate anatomy unless adequately assessed preoperatively with magnetic resonance urogram. A ureteral stent may be placed retrograde if desired. We prefer placing a ureteral stent antegrade during the robotic portion of the case.
Typical patient positioning for transabdominal robotic pyeloplasty is the modified flank/lateral decubitus position with affected side elevated ~45° over a roll, contralateral arm extended on an arm board, and ipsilateral arm straight against the patient's ipsilateral side or extended parallel to the contralateral arm using an elevated armrest or pillows. Alternatively, the patient may be positioned supine with table rotation to elevate the pathologic side (1). One must ensure that all pressure points are adequately padded and the patient is appropriately secured to the table.
The patient is flattened for port placement. The camera port is placed first, usually at the umbilicus, using either open Hasson or Veress needle technique. Some surgeons recommend against the use of Veress needle in children (4). However, we believe that this technique can be applied safely in pediatric cases and have successfully used it for several years at our high volume robotic institution with no complications. For the Si, we use the 8.5 mm robotic camera port. A 10 or 12 mm port (e.g., the Autosuture® balloon trocar) may also be used as the Si robot camera port (5). For the Xi, the camera and working ports are identical, allowing placement of the camera through any port. Robotic working ports are then placed under direct vision. For the Si, 8 and 5 mm robotic ports and instruments are available, while only 8 mm ports/instruments are available for the Xi. We prefer 8 mm robotic ports even with the Si because of the greater variety of instrumentation available. Another advantage of the 8 mm instruments is a shorter intracorporeal length of the wristed segment, with decreased required intracorporeal working distance (5).
For the Si, ideal port placement results in a triangular working field. One working port is placed cephalad to the camera port in the midline or midclavicular line, and the other is placed inferiorly at an ~30° angle rotated from midline toward the kidney of interest (Figure 1A). Ports are ideally spaced ~1 hand's breadth apart, but this may be impossible in smaller children and infants. All ports are instead placed in the midline to maximize the limited working space in infants, as close as 3 cm if necessary (5).
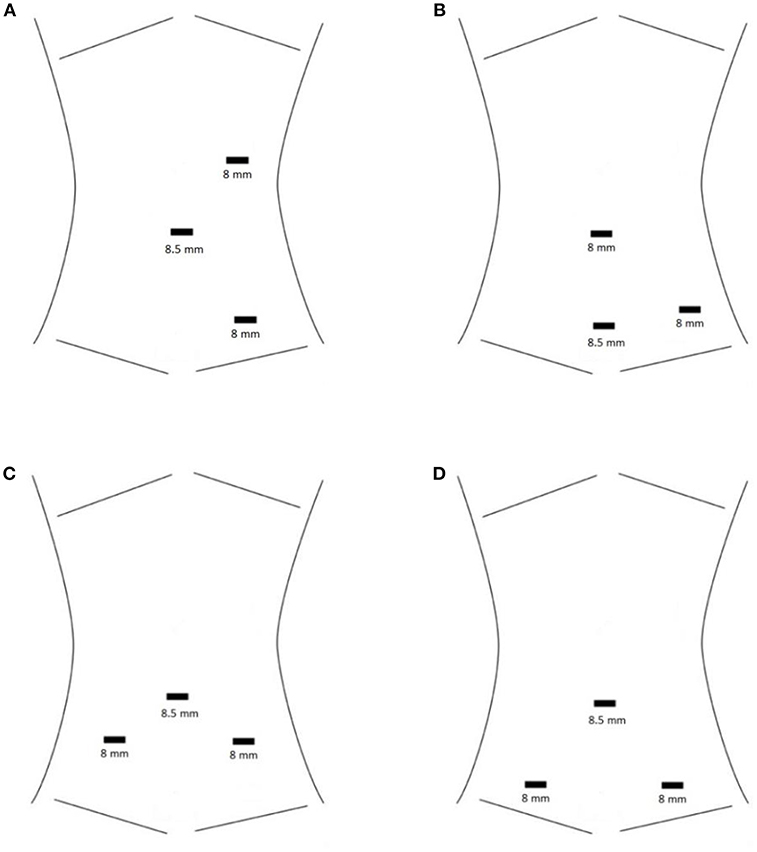
Figure 1. (A) Standard port placement for RAL left pyeloplasty with the Si robot. The camera port is at the umbilicus. (B) HIdES port placement for RAL left pyeloplasty with the Si robot. The camera port is the inferior-most port. The camera port and one working port are hidden at or below the level of a Pfannenstiel incision, while the other working port is hidden in the umbilicus. (C) Standard port placement for RAL ureteral reimplantation with the Si robot. The camera port is at the umbilicus. (D) HIdES port placement for RAL ureteral reimplantation with the Si robot. The camera port is at the umbilicus. Skin incisions for the working ports are lower than in the standard port placement (at or below the level of a Pfannenstiel incision). Fascial entry sites for the working ports may be placed higher than the skin incisions in order to increase working space within the pelvis. This is achieved by applying cephalad traction during port placement.
Optimal port placement for the Xi robot is in a line rather than triangulated. A third robotic port and/or assistant port(s) may be placed if desired. We usually do not find additional ports necessary. With our typical three-port setup, a robotic instrument must be removed to allow the assistant to suction or pass sutures. This positioning and port placement may be used for any renal or upper ureteral procedure.
The hidden incision endoscopic surgery (HIdES) port placement technique was developed to eliminate visible scarring (6). This involves placing the camera port and one robotic working port below the level of a Pfannenstiel incision and placing the second robotic working port infraumbilically (Figure 1B). Incisions are thus hidden beneath the underwear line, which has been shown to be preferable to patients and parents (6, 7).
After port placement, the next step is docking. The bed is rotated to raise the ipsilateral side, and the height of the bed is adjusted as desired. These changes must be made prior to robot docking unless using the Xi system with Trumpf Medical's TruSystem® 7000 dV OR table, which allows “integrated table motion” (OR table movement after docking). With the Si, docking is typically over the ispilateral shoulder at a slight angle or straight in from the side. Docking trajectory is more forgiving with the Xi system, as the robotic arms rotate on the boom into the optimal position when you perform anatomic targeting.
Next, the white line of Toldt is incised, and the colon is reflected medially to expose the retroperitoneum. One may alternatively utilize a transmesenteric approach for left-sided cases. The renal pelvis, UPJ, and ureter are then identified and dissected with limited, low-power cautery use. We routinely use a hitch stitch for traction to facilitate this dissection in the absence of an assistant port. We use a 4–0 Vicryl on an SH needle, which is manually straightened and passed directly through the abdominal wall by the assistant, through-and-through the renal pelvis, then back out the abdominal wall. The assistant may then adjust the tension as desired by the surgeon and snap the stitch in place at the level of the skin. A hitch stitch may not be necessary if the renal pelvis is not too floppy.
Dismemberment is the next step. Depending on UPJ configuration, one may choose an appropriate location and trajectory for renal pelvis transection in order to create an adequately wide pyelotomy for eventual anastomosis. If the UPJ insertion is high, an alternative is to ligate it, transect the proximal ureter, and create a new dependent pyelotomy for anastomosis. Non-dismembered techniques (e.g., Foley Y-V plasty or flap pyeloplasties) are preferred by some authors (8–10). Use of these methods has even been described in the setting of a crossing vessel, with concomitant cephalad translocation of the crossing vessel or Hellström technique (8, 9). Flaps can be particularly useful for long segments of UPJ/ureteral stricture, whereas a Heineke-Mikulicz type pelvotomy (Fenger-plasty) may be sufficient for short strictures (8, 11). We favor the classic Anderson-Hynes dismembered pyeloplasty technique in the majority of cases. Pelvic reduction may be performed if desired; however, this is rarely necessary in our experience.
The proximal ureter is then spatulated. Traditional descriptions favor spatulation along the lateral aspect because the proximal ureteral blood supply arises medially. Spatulation must be continued for an adequate length to ensure a wide anastomosis incorporating healthy ureter. A portion of the proximal ureter may ultimately be excised if it appears unsuitable for reconstruction; however, we recommend leaving such a segment attached for use as a handle until anastomosis is nearly complete. The anastomosis may be performed with running or interrupted fine absorbable suture. Typically, we perform half of the anastomosis with one running 5–0 Vicryl, then place a ureteral stent in antegrade fashion over a wire passed through an angiocatheter advanced directly through the abdominal wall. To confirm appropriate stent positioning, one may have the circulator instill dilute methylene blue solution into the bladder through the Foley catheter, which should reflux up through the stent if the distal coil is in the bladder. The proximal stent coil is then placed within the pelvis, and the anastomosis is completed with a second running suture. Alternative approaches include placing a stent in retrograde fashion or leaving a percutaneous nephrostomy/nephroureterostomy tube or Penrose drain instead of an internal stent. Tubeless procedures have also been described with no short-term complications (12). Long-term success rates of tubeless robotic pyeloplasty have yet to be determined.
Robotic pyeloplasty is effective, with multiple series including ≥50 patients reporting success rates of 94–100% utilizing a transperitoneal or retroperitoneal approach (2, 13–20). A recent retrospective long-term study reported an 8-year failure-free rate of 91.5% after robotic pyeloplasty (21). A meta-analysis from 2014 showed comparable success and complication rates in pediatric patients after minimally invasive or open pyeloplasty (22). A recent retrospective cohort study using the national Premier database revealed that while the total number of pyeloplasties decreased by 7% annually between 2003 and 2015, robotic cases increased by 29% annually, accounting for 40% of all cases in 2015 (23). Increased robot utilization was greatest in the pediatric population. Complication rates were similarly low in open and robotic cases.
Stones and/or UPJ polyps, if present, may be addressed concomitantly with retroperitoneoscopic or transperitoneal robotic pyeloplasty (24–27). Concurrent pyelolithotomy and pyeloplasty is safe and effective, with acceptable stone-free rates (94, 83, and 72% at 1, 3, and 6 months, respectively) (25). Operative time was longer for pyeloplasty with pyelolithotomy (median 151 min) vs. pyeloplasty alone (120 min, p < 0.0001), with no difference in length of hospital stay.
Ureteral fibroepithelial polyps are an uncommon but important source of obstructive hydronephrosis in children and can be challenging to diagnose preoperatively (24). If a polyp is suspected, endoscopy may be the preferred approach. However, in cases of large or multifocal lesions, or if a concurrent UPJ stenosis is thought to be present, robotics provide superior definitive management (26).
Redo (salvage) pyeloplasties present a special challenge. Dense peripelvic fibrosis, longer strictures, and compromised vascularity may all contribute to the increased difficulty in such cases. One recent study looking at laparoscopic redo pyeloplasties found that operative times were longer compared to primary cases (191 vs. 145 min, p = 0.0001), but success rates were comparable at 93.3% (28). Other groups have reported success rates from 77.8 to 100% for small cohorts undergoing redo pyeloplasty (29–32). Use of buccal mucosal onlay grafts for robotic salvage pyeloplasty (33, 34) and complex ureteral stricture repairs (35–37) has been shown to be safe and effective with short-term follow up.
Ureterocalicostomy is an option for renal salvage in cases where pyeloplasty is not feasible. The open procedure was originally described by Neuwirt (38). Indications for ureterocalicostomy are relative and may include UPJ obstruction in with an intrarenal pelvis or recurrent UPJ obstruction with dense scarring making redo pyeloplasty difficult or impossible. It has been considered a last resort for kidney preservation, as an alternative to nephrectomy (39). Robotic ureterocalicostomy was first reported in the pediatric population by Casale et al. with steps based on the open procedure (40). These authors retrospectively studied 9 pediatric patients who underwent transperitoneal robotic ureterocalicostomy in the setting of recurrent UPJ obstruction or intrarenal UPJ. Two patients underwent concomitant ureteroscopic stone treatment. The hilum was mobilized to allow for rapid vascular control; however, hilar clamping was not required in any case. Diuretic renogram confirmed unobstructed systems in all patients 12 months postoperatively (40).
RAL end-to-end UU may be indicated in the setting of mid ureteral stricture. Port placement for mid ureteral reconstruction can be achieved in a fashion similar to that described above for proximal ureteral reconstruction, with the ports shifted slightly inferiorly if needed. The diseased segment may be excised, and both ends spatulated at opposite aspects to achieve a wide anastomosis. For a relatively short stricture, a Heineke-Mikulicz repair may be adequate (41).
For long or multiple mid ureteral strictures, tension-free end-to-end anastomosis may not be possible. In such cases, the use of a graft may obviate the need for more morbid procedures such as ileal ureter, transureteroureterostomy, or autotransplantation. Buccal mucosal grafts may be used for complex pyeloplasties (33, 34) or complex ureteral stricture repairs (35–37). Use of the appendix as a ureteral substitute or as an onlay flap has also been described for complex right mid or upper ureteral stricture repair, initially in the open (42–44) or laparoscopic (45, 46) settings. Recently, Yarlagadda et al published a case report of robotic appendiceal interposition for right-sided ureteral stricture disease (47). In this case, a 5 cm obliterative ureteral stricture secondary to recurrent ureterolithiasis and pyelonephritis was repaired with interposition of the appendix between the proximal and distal healthy ureter. Resolution of hydronephrosis and flank pain was demonstrated at 10 months. Long-term results using this technique are needed.
The most common RAL distal ureteral surgery is extravesical ureteral reimplantation for VUR, following steps of the open Lich-Gregoir technique originally described in the 1960s (48, 49) VUR may also be treated endoscopically or with open or laparoscopic transvesical reimplantation. Open ureteral reimplantation has a reported success of 93.5–98% (50–52). Endoscopic VUR treatment is the least invasive option, but is associated with variable radiographic cure rates of 67–93% (53–57). Success is likely dependent on technique, surgeon experience, and patient factors. The hydrodistention implantation technique (HIT) provides better outcomes than the older subureteric transurethral injection (STING) procedure, and several authors have reported radiographic success rates ≥80% (58–60). The Double HIT has emerged as the most common injection technique in the United States (61), affording the highest endoscopic success rates (57, 62).
Patient positioning for RAL distal ureteral procedures is typically lithotomy for the Si, allowing for cystoscopy (if desired) and robotic surgery in a single prep and drape. The robot is docked between the legs in this scenario. Side-docking is also possible, especially with the Xi, allowing the patient to remain supine. Port placement at our institution involves an 8.5 mm Si robotic camera port at the umbilicus with open Hasson or Veress technique. One may also use a 10 or 12 mm port (e.g., the Autosuture® balloon trocar) for the Si robot (5). Xi camera and working ports are identical, allowing the camera to go through any of the ports.
After camera port placement, working ports are placed on either side of the umbilicus. These are placed inferiorly to the camera port to create a triangular working field for Si (Figure 1C), or in a line for the Xi. One may use 8 or 5 mm working ports for the Si, whereas only 8 mm instruments are available for the Xi. The HIdES port placement technique for lower urinary tract reconstruction involves placing the working ports at or below the level of a typical Pfannenstiel incision (Figure 1D) (6). Assistant port(s) and/or 3rd robotic port may be placed; however, we generally find this unnecessary. Unless the Xi and proprietary OR table are being used, one must adjust table height and position prior to docking.
Once docked, the first steps are opening the peritoneum (Figure 2) and mobilizing the ureter with judicious cautery use. The ureter is then followed distally to the ureterovesical junction (UVJ), taking care to preserve vas or uterine arteries in a boy or girl, respectively. A bladder hitch stitch may be utilized if the bladder is floppy and UVJ not clearly seen. A detrusor tunnel is created in the appropriate trajectory. The ideal location for detrusor tunnel may be more apparent in the absence of a hitch stitch, which may distort the anatomy. Ideal detrusor tunnel length has been described as 5:1 in comparison with the ureteral diameter (10). Flaps are developed on either side of the tunnel in order to prevent obstruction. Lastly, the tunnel is closed over the ureter. We use a running 3–0 V-loc for this, starting at the distal-most aspect and running proximally. Others may use different suture types, interrupted instead of running, and/or may start proximally, according to surgeon preference.
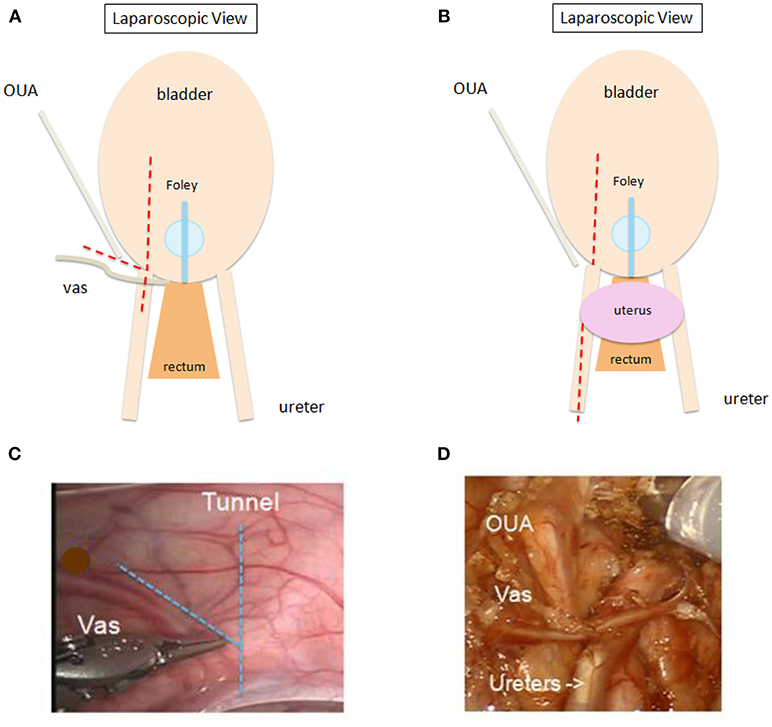
Figure 2. (A,B) Schematic showing sites for opening peritoneum ( ) during RAL ureteral reimplantation. Peritoneum may be opened in line with proposed detrusor tunnel or transversely for wider exposure. “V” flap (A) recommended for adequately exposing vas deferens in boys. One may open peritoneum further cephalad along the ureter to allow for additional ureteral mobilization (B), especially in peri- or post-pubertal girls or in otherwise complex cases. OUA, obliterated umbilical artery. (C,D) Intraoperative view prior to (C) and after (D) opening peritoneum in a male patient. OUA, obliterated umbilical artery.
) during RAL ureteral reimplantation. Peritoneum may be opened in line with proposed detrusor tunnel or transversely for wider exposure. “V” flap (A) recommended for adequately exposing vas deferens in boys. One may open peritoneum further cephalad along the ureter to allow for additional ureteral mobilization (B), especially in peri- or post-pubertal girls or in otherwise complex cases. OUA, obliterated umbilical artery. (C,D) Intraoperative view prior to (C) and after (D) opening peritoneum in a male patient. OUA, obliterated umbilical artery.
VUR resolution rates after extravesical RAL ureteral reimplantation (RALUR) reported in the literature range from 66.7 to 100% in multiple relatively small series (63–73). Overall success upon pooling these series is 91% (74). A multi-institutional retrospective study reported radiographic resolution in 87.9% of 280 ureters (75). More recently, a large prospective multi-institutional study reported 93.8% resolution in 199 ureters (76).
RALUR may be performed bilaterally; however, there is concern that bilateral dissection of the posterior bladder may disrupt the pelvic nerve plexus, resulting in higher rates of postoperative urinary retention. Nerve-sparing dissection has been proposed to reduce this complication (77). In 2008, Casale et al. reported a 97.6% success rate following bilateral nerve-sparing RALUR in 41 patients (65). There were no complications or instances of urinary retention. Herz et al. reported a 91.7% success rate for unilateral RALUR but a success rate of only 77.8% of ureters (72.2% of children) for bilateral cases (78). In this study, complication rates (including ureteral obstruction, readmission, and urinary retention) were higher for bilateral cases. A nerve-sparing technique was not utilized.
Peri-ureteral diverticula (if present) may be reduced/excised during reimplantation (79). In duplex systems, common sheath reimplantation with or without tapering has been described with good outcomes (80). Ureteral tapering may be performed while maintaining the native UVJ in the setting of a non-obstructed, refluxing megaureter (81). For complex reimplants (i.e., those with history of prior anti-reflux surgery, requiring tapering and/or dismembering, or associated duplication or diverticulum), Arlen et al. found comparable success and complication rates for RAL vs. open cases, with shorter hospitalization in the RAL group (82). Older children were more likely to undergo RALUR.
RAL dismembered extravesical ureteral reimplantation with or without tapering may be used for repair of obstructed megaureters (Figures 3A,C–H) (83, 84). The obstructed UVJ is divided, and a new ureteroneocystostomy anastomosis is created. A non-refluxing detrusor tunnel is created as described above. When tapering, we prefer to leave the ureter connected to the bladder during this process to provide retraction. Dismemberment is then performed after tapering is complete, similar to the process described by Khan et al. (85). A non-dismembered technique may also be used to repair obstructed megaureters, using the Heineke-Mikulicz principle (Figure 3B) (86).
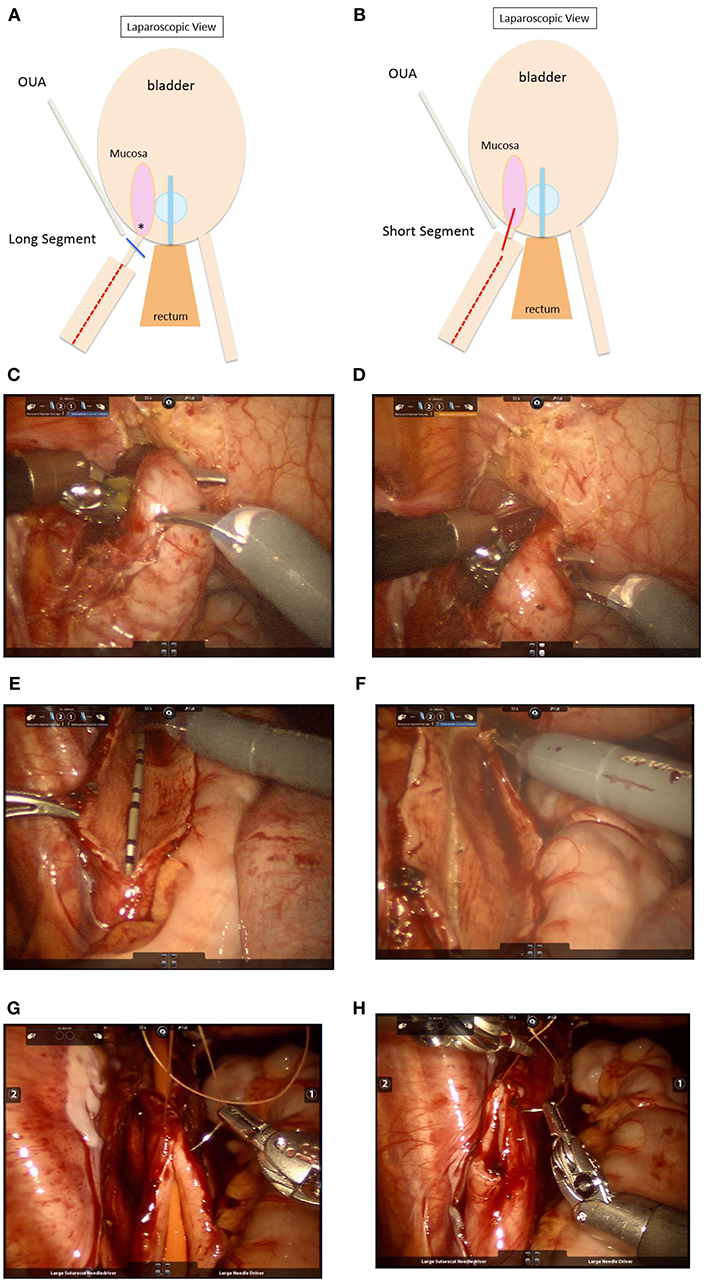
Figure 3. (A) Schematic showing repair of obstructed megaureter with a long segment of stenotic UVJ. Steps: i. Keep ureter attached. ii. Taper megaureter ( ) iii. Ligate UVJ (
) iii. Ligate UVJ ( ). iv. Dismember ureter. v. Anastomosis at new site (*). Stent ± peritoneal closure. OUA, obliterated umbilical artery. (B) Repair of obstructed megaureter with a short stenotic UVJ segment. Steps: i. Keep ureter attached. ii. Taper megaureter (
). iv. Dismember ureter. v. Anastomosis at new site (*). Stent ± peritoneal closure. OUA, obliterated umbilical artery. (B) Repair of obstructed megaureter with a short stenotic UVJ segment. Steps: i. Keep ureter attached. ii. Taper megaureter ( ) iii. Partially dismember (
) iii. Partially dismember ( ). iv. In situ Heineke-Mikulicz anastomosis. Stent ± peritoneal closure. OUA, obliterated umbilical artery. (C) Intraoperative view during robotic repair of a left obstructed megaureter. The ureter has been mobilized circumferentially without devascularizing it. (D) The distally narrowed and obstructed segment is apparent in the view above. (E) A longitudinal ureterotomy has been created to allow for tapering. In this view, the ureter is still attached at the UVJ in order to maintain traction during tapering. (F) The ureter is scored to demarcate excess tissue for excisional tapering. (G,H) After excision of excess tissue, the ureter is closed/tapered using fine absorbable suture (5–0 Vicryl in the case above) over a 10 Fr catheter. The next steps include dismemberment at the UVJ, creation of ureteroneocystostomy, and creation of a detrusor tunnel to achieve a nonobstructed, nonrefluxing reimplantation.
). iv. In situ Heineke-Mikulicz anastomosis. Stent ± peritoneal closure. OUA, obliterated umbilical artery. (C) Intraoperative view during robotic repair of a left obstructed megaureter. The ureter has been mobilized circumferentially without devascularizing it. (D) The distally narrowed and obstructed segment is apparent in the view above. (E) A longitudinal ureterotomy has been created to allow for tapering. In this view, the ureter is still attached at the UVJ in order to maintain traction during tapering. (F) The ureter is scored to demarcate excess tissue for excisional tapering. (G,H) After excision of excess tissue, the ureter is closed/tapered using fine absorbable suture (5–0 Vicryl in the case above) over a 10 Fr catheter. The next steps include dismemberment at the UVJ, creation of ureteroneocystostomy, and creation of a detrusor tunnel to achieve a nonobstructed, nonrefluxing reimplantation.
In appropriate duplex systems, end-to-side ureteroureterostomy (UU) can be performed proximally or distally depending on surgeon preference. We favor a distal approach, eliminating risk of hilar vessel injury, and allowing for intraoperative decision-making regarding performance of UU vs. ureteral reimplantation (vs. both concurrently in select settings). In some cases, it may be safer and more efficacious to perform UU in the mid ureter, thus avoiding both pelvic structures and renal hilar anatomy. Upper-to-lower UU may be performed for obstructed and/or ectopic upper moiety when there is no vesicoureteral reflux (VUR) into the lower moiety. Lower-to-upper UU may be performed in the setting of lower moiety VUR and unobstructed non-ectopic upper moiety (80).
Robot-assisted UU is a safe and effective alternative to open UU in children, with similar operative times and complication rates, and slightly shorter hospitalizations (87). UU has been shown to be safe and effective even in the setting of a minimally functioning/non-functioning moiety (as an alternative to upper moiety heminephrectomy) and irrespective of ureteral size difference (88).
When performing RAL UU, it is imperative to correctly identify each ureter. This can be facilitated with cystourethroscopy and passage of a temporary ureteral stent into one of the ureters. It is our practice to leave a double-J ureteral stent across the anastomosis, which is removed 4–6 weeks postoperatively. A renal-bladder ultrasound is performed ~4 weeks after stent removal, with additional imaging as clinically indicated.
RAL surgery is a safe, minimally invasive technique with various applications in pediatric ureteric reconstruction. A robotic approach allows access to the ureter at all levels. Multiple aspects of robotic surgery, including magnified three-dimensional view and wristed movements with multiple degrees of freedom, are particularly well-suited to these delicate reconstructive procedures. Robotic surgery continues to enjoy growing popularity among urologists and patient families alike.
AB and AK both contributed to deciding the structure, content of the manuscript and to writing, editing the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Van Batavia JP, Casale P. Robotic surgery in pediatric urology. Curr Urol Rep. (2014) 15:402. doi: 10.1007/s11934-014-0402-9
2. Olsen LH, Rawashdeh YF, Jorgensen TM. Pediatric robot assisted retroperitoneoscopic pyeloplasty: a 5-year experience. J Urol. (2007) 178:2137–2141. Discussion 2141. doi: 10.1016/j.juro.2007.07.057
3. Romero FR, Wagner AA, Trapp C, Permpongkosol S, Muntener M, Link RE, et al. Transmesenteric laparoscopic pyeloplasty. J Urol. (2006) 176(6 Pt 1):2526–9. doi: 10.1016/j.juro.2006.07.155
4. Spinoit AF, Nguyen H, Subramaniam R. Role of robotics in children: a brave New World! Eur Urol Focus. (2017) 3:172–80. doi: 10.1016/j.euf.2017.08.011
5. Chang C, Steinberg Z, Shah A, Gundeti MS. Patient positioning and port placement for robot-assisted surgery. J Endourol. (2014) 28:631–8. doi: 10.1089/end.2013.0733
6. Gargollo PC. Hidden incision endoscopic surgery: description of technique, parental satisfaction and applications. J Urol. (2011) 185:1425–31. doi: 10.1016/j.juro.2010.11.054
7. Garcia-Roig ML, Travers C, McCracken C, Cerwinka W, Kirsch JM, Kirsch AJ. Surgical scar location preference for pediatric kidney and pelvic surgery: a crowdsourced survey. J Urol. (2017) 197(3 Pt 2):911–9. doi: 10.1016/j.juro.2016.11.033
8. Amon Sesmero JH, Delgado MC, de la Cruz Martin B, Serrano MR, Mainez Rodriguez JA, Tapia Herrero AM. Laparoscopic pyeloplasty: always dismembered? J Endourol. (2016) 30:778–82. doi: 10.1089/end.2015.0800
9. Szydelko T, Apoznanski W, Koleda P, Rusiecki L, Janczak D. Laparoscopic pyeloplasty with cephalad translocation of the crossing vessel-a new approach to the Hellstrom technique. Wideochir Inne Tech Maloinwazyjne. (2015) 10:25–9. doi: 10.5114/wiitm.2015.48695
10. Wein AJ, Kavoussi LR, Campbell MF. Chapter 41: Management of upper urinary tract obstruction. In: Wein AJ, Kavoussi LR, et al, editors. Campbell-Walsh Urology. 10th ed. Philadelphia, PA: Elsevier Saunders (2012). p. 1122–68.
11. Janetschek G, Peschel R, Bartsch G. Laparoscopic fenger plasty. J Endourol. (2000) 14:889–93. doi: 10.1089/end.2000.14.889
12. Fichtenbaum EJ, Strine AC, Concodora CW, Schulte M, Noh PH. Tubeless outpatient robotic upper urinary tract reconstruction in the pediatric population: short-term assessment of safety. J Robot Surg. (2018) 12:257–60. doi: 10.1007/s11701-017-0722-0
13. Minnillo BJ, Cruz JA, Sayao RH, Passerotti CC, Houck CS, Meier PM, et al. Long-term experience and outcomes of robotic assisted laparoscopic pyeloplasty in children and young adults. J Urol. (2011) 185:1455–60. doi: 10.1016/j.juro.2010.11.056
14. Mufarrij PW, Woods M, Shah OD, Palese MA, Berger AD, Thomas R, et al. Robotic dismembered pyeloplasty: a 6-year, multi-institutional experience. J Urol. (2008) 180:1391–6. doi: 10.1016/j.juro.2008.06.024
15. Singh P, Dogra PN, Kumar R, Gupta NP, Nayak B, Seth A. Outcomes of robot-assisted laparoscopic pyeloplasty in children: a single center experience. J Endourol. (2012) 26:249–53. doi: 10.1089/end.2011.0350
16. Patel V. Robotic-assisted laparoscopic dismembered pyeloplasty. Urology. (2005) 66:45–9. doi: 10.1016/j.urology.2005.01.053
17. Schwentner C, Pelzer A, Neururer R, Springer B, Horninger W, Bartsch G, et al. Robotic Anderson-Hynes pyeloplasty: 5-year experience of one centre. BJU Int. (2007) 100:880–5. doi: 10.1111/j.1464-410X.2007.07032.x
18. Gupta NP, Nayyar R, Hemal AK, Mukherjee S, Kumar R, Dogra PN. Outcome analysis of robotic pyeloplasty: a large single-centre experience. BJU Int. (2010) 105:980–3. doi: 10.1111/j.1464-410X.2009.08983.x
19. Cestari A, Buffi NM, Lista G, Sangalli M, Scapaticci E, Fabbri F, et al. Retroperitoneal and transperitoneal robot-assisted pyeloplasty in adults: techniques and results. Eur Urol. (2010) 58:711–8. doi: 10.1016/j.eururo.2010.07.020
20. Sivaraman A, Leveillee RJ, Patel MB, Chauhan S, Bracho JE II, Moore CR, et al. Robot-assisted laparoscopic dismembered pyeloplasty for ureteropelvic junction obstruction: a multi-institutional experience. Urology. (2012) 79:351–5. doi: 10.1016/j.urology.2011.10.019
21. Hopf HL, Bahler CD, Sundaram CP. Long-term outcomes of robot-assisted laparoscopic pyeloplasty for ureteropelvic junction obstruction. Urology. (2016) 90:106–10. doi: 10.1016/j.urology.2015.12.050
22. Autorino R, Eden C, El-Ghoneimi A, Guazzoni G, Buffi N, Peters CA, et al. Robot-assisted and laparoscopic repair of ureteropelvic junction obstruction: a systematic review and meta-analysis. Eur Urol. (2014) 65:430–52. doi: 10.1016/j.eururo.2013.06.053
23. Varda BK, Wang Y, Chung BI, Lee RS, Kurtz MP, Nelson CP, et al. Has the robot caught up? National trends in utilization, perioperative outcomes, and cost for open, laparoscopic, and robotic pediatric pyeloplasty in the United States from 2003 to 2015. J Pediatr Urol. (2018) 14:336.e1–8. doi: 10.1016/j.jpurol.2017.12.010
24. Dai LN, Chen CD, Lin XK, Wang YB, Xia LG, Liu P et al. Retroperitoneal laparoscopy management for ureteral fibroepithelial polyps causing hydronephrosis in children: a report of five cases. J Pediatr Urol. (2015) 11:257 e251–5. doi: 10.1016/j.jpurol.2015.02.019
25. Jensen PH, Berg KD, Azawi NH. Robot-assisted pyeloplasty and pyelolithotomy in patients with ureteropelvic junction stenosis. Scand J Urol. (2017) 51:323–8. doi: 10.1080/21681805.2017.1300188
26. Osbun N, Ellison JS, Lendvay TS. Robot-Assisted Laparoscopic Excision of ureteral and ureteropelvic junction fibroepithelial polyps in children. J Endourol. (2016) 30:896–900. doi: 10.1089/end.2016.0006
27. Skolarikos A, Dellis A, Knoll T. Ureteropelvic obstruction and renal stones: etiology and treatment. Urolithiasis. (2015) 43:5–12. doi: 10.1007/s00240-014-0736-2
28. Abraham GP, Siddaiah AT, Ramaswami K, George D, Das K. Laparoscopic management of recurrent ureteropelvic junction obstruction following pyeloplasty. Urol Ann. (2015) 7:183–7. doi: 10.4103/0974-7796.150489
29. Powell C, Gatti JM, Juang D, Murphy JP. Laparoscopic pyeloplasty for ureteropelvic junction obstruction following open pyeloplasty in children. J Laparoendosc Adv Surg Tech A. (2015) 25:858–63. doi: 10.1089/lap.2015.0074
30. Basiri A, Behjati S, Zand S, Moghaddam SM. Laparoscopic pyeloplasty in secondary ureteropelvic junction obstruction after failed open surgery. J Endourol. (2007) 21:1045–51. Discussion 1051. doi: 10.1089/end.2006.0414
31. Braga LH, Lorenzo AJ, Skeldon S, Dave S, Bagli DJ, Khoury AE, et al. Failed pyeloplasty in children: comparative analysis of retrograde endopyelotomy versus redo pyeloplasty. J Urol. (2007) 178:2571–5. Discussion 2575. doi: 10.1016/j.juro.2007.08.050
32. Thomas JC, DeMarco RT, Donohoe JM, Adams MC, Pope JCt, Brock JW III. Management of the failed pyeloplasty: a contemporary review. J Urol. (2005) 174:2363–6. doi: 10.1097/01.ju.0000180420.11915.31
33. Zampini AM, Nelson R, Zhang JJH, Reese J, Angermeier KW, Haber GP. Robotic salvage pyeloplasty with buccal mucosal onlay graft: video demonstration of technique and outcomes. Urology. (2017) 110:253–6. doi: 10.1016/j.urology.2017.07.023
34. Ahn JJ, Shapiro ME, Ellison JS, Lendvay TS. Pediatric robot-assisted redo pyeloplasty with buccal mucosa graft: a novel technique. Urology. (2017) 101:56–9. doi: 10.1016/j.urology.2016.12.036
35. Arora S, Campbell L, Tourojman M, Pucheril D, Jones LR, Rogers C. Robotic buccal mucosal graft ureteroplasty for complex ureteral stricture. Urology. (2017) 110:257–8. doi: 10.1016/j.urology.2017.06.037
36. Lee Z, Waldorf BT, Cho EY, Liu JC, Metro MJ, Eun DD. Robotic ureteroplasty with buccal mucosa graft for the management of complex ureteral strictures. J Urol. (2017) 198:1430–5. doi: 10.1016/j.juro.2017.06.097
37. Zhao LC, Yamaguchi Y, Bryk DJ, Adelstein SA, Stifelman MD. Robot-assisted ureteral reconstruction using buccal mucosa. Urology. (2015) 86:634–8. doi: 10.1016/j.urology.2015.06.006
38. Neuwirt K. Implantation of the ureter into the lower calyx of the renal pelvis. Urol Cutaneous Rev. (1948) 52:351.
39. Gite VA, Siddiqui AK, Bote SM, Patil SR, Kandi AJ, Nikose JV. Ureterocalycostomy - final resort in the management of secondary pelvi-ureteric junction obstruction: our experience. Int Braz J Urol. (2016) 42:501–6. doi: 10.1590/S1677-5538.IBJU.2015.0368
40. Casale P, Mucksavage P, Resnick M, Kim SS. Robotic ureterocalicostomy in the pediatric population. J Urol. (2008) 180:2643–8. doi: 10.1016/j.juro.2008.08.052
41. Goel A, Singh D, Sengottayan VK, Sankhwar S. Nondismembered ureteroplasty for congenital midureteral stenosis: a new application of an old technique. Urology. (2010) 76:1004–6. doi: 10.1016/j.urology.2010.03.093
42. Adani GL, Pravisani R, Baccarani U, Bolgeri M, Lorenzin D, Terrosu G, et al. Extended ureteral stricture corrected with appendiceal replacement in a kidney transplant recipient. Urology. (2015) 86:840–3. doi: 10.1016/j.urology.2015.06.010
43. Murai R, Ushida H, Osafune T, Johnin K, Kageyama S, Okada Y. [Repair of right ureteral stenosis by traumatic injury with appendiceal interposition: a case report]. Nihon Hinyokika Gakkai Zasshi. (2013) 104:667–70. doi: 10.5980/jpnjurol.104.667
44. Dagash H, Sen S, Chacko J, Karl S, Ghosh D, Parag P, et al. The appendix as ureteral substitute: a report of 10 cases. J Pediatr Urol. (2008) 4:14–9. doi: 10.1016/j.jpurol.2007.08.004
45. Duty BD, Kreshover JE, Richstone L, Kavoussi LR. Review of appendiceal onlay flap in the management of complex ureteric strictures in six patients. BJU Int. (2015) 115:282–7. doi: 10.1111/bju.12651
46. Reggio E, Richstone L, Okeke Z, Kavoussi LR. Laparoscopic ureteroplasty using on-lay appendix graft. Urology. (2009) 73:928 e927–10. doi: 10.1016/j.urology.2008.06.034
47. Yarlagadda VK, Nix JW, Benson DG, Selph JP. Feasibility of intracorporeal robotic-assisted laparoscopic appendiceal interposition for ureteral stricture disease: a case report. Urology. (2017) 109:201–5. . doi: 10.1016/j.urology.2017.08.017
48. Gregoir W. [The Surgical Treatment of Congenital Vesico-Ureteral Reflux]. Acta Chir Belg. (1964) 63:431–9.
49. Robert Lich Jr., Lonnie WH, and Lawrence AD. Recurrent urosepsis in children. J Urol. (1961) 86:554–8. doi: 10.1016/S0022-5347(17)65219-4
50. Nelson CP, Hubert KC, Kokorowski PJ, Huang L, Prasad MM, Rosoklija I, et al. Long-term incidence of urinary tract infection after ureteral reimplantation for primary vesicoureteral reflux. J Pediatr Urol. (2013) 9:92–8. doi: 10.1016/j.jpurol.2011.12.009
51. Elder JS, Peters CA, Arant BS Jr, Ewalt DH, Hawtrey CE, Hurwitz RS, et al. Pediatric Vesicoureteral Reflux Guidelines Panel summary report on the management of primary vesicoureteral reflux in children. J Urol. (1997) 157:1846–51. doi: 10.1016/S0022-5347(01)64882-1
52. Barrieras D, Lapointe S, Reddy PP, Williot P, McLorie GA, Bigli D, et al. Are postoperative studies justified after extravescial ureteral reimplantation? J Urol. (2000) 164(3 Pt 2):1064–6. doi: 10.1016/S0022-5347(05)67251-5
53. Hacker FM, Frech-Dorfler M, von Rotz M, Rudin C. Endoscopic hyaluronic acid/dextranomer gel implantation is effective as first-line treatment of vesicoureteral reflux (VUR) in children: a single centre experience. Eur J Pediatr Surg. (2011) 21:299–303. doi: 10.1055/s-0031-1279700
54. Elder JS, Diaz M, Caldamone AA, Cendron M, Greenfield S, Hurwitz R, et al. Endoscopic therapy for vesicoureteral reflux: a meta-analysis. I. Reflux resolution and urinary tract infection. J Urol. (2006) 175:716–22. doi: 10.1016/S0022-5347(05)00210-7
55. Friedmacher F, Colhoun E, Puri P. Endoscopic injection of dextranomer/hyaluronic acid as first line treatment in 851 consecutive children with high grade vesicoureteral reflux: efficacy and long-term results. J Urol. (2018) 200:650–5. doi: 10.1016/j.juro.2018.03.074
56. Kaye JD, Srinivasan AK, Delaney C, Cerwinka WH, Elmore JM, Scherz HC, et al. Clinical and radiographic results of endoscopic injection for vesicoureteral reflux: defining measures of success. J Pediatr Urol. (2012) 8:297–303. doi: 10.1016/j.jpurol.2011.02.006
57. Kalisvaart JF, Scherz HC, Cuda S, Kaye JD, Kirsch AJ. Intermediate to long-term follow-up indicates low risk of recurrence after Double HIT endoscopic treatment for primary vesico-ureteral reflux. J Pediatr Urol. (2012) 8:359–65. doi: 10.1016/j.jpurol.2011.07.006
58. Lee EK, Gatti JM, Demarco RT, Murphy JP. Long-term followup of dextranomer/hyaluronic acid injection for vesicoureteral reflux: late failure warrants continued followup. J Urol. (2009) 181:1869–74. Discussion 1874–65. doi: 10.1016/j.juro.2008.12.005
59. Gupta A, Snodgrass W. Intra-orifice versus hydrodistention implantation technique in dextranomer/hyaluronic acid injection for vesicoureteral reflux. J Urol. (2008) 180(4 Suppl.):1589–92. Discussion 1592–83. doi: 10.1016/j.juro.2008.04.073
60. Shim JS, Kim JW, Oh MM, Moon du G. Efficacy of hydrodistention implantation technique in treating high-grade vesicoureteral reflux. Korean J Urol. (2012) 53:194–9. doi: 10.4111/kju.2012.53.3.194
61. Kirsch AJ, Arlen AM, Lackgren G. Current trends in dextranomer hyaluronic acid copolymer (Deflux) injection technique for endoscopic treatment of vesicoureteral reflux. Urology. (2014) 84:462–8. doi: 10.1016/j.urology.2014.04.032
62. Molitierno JA, Scherz HC, Kirsch AJ. Endoscopic treatment of vesicoureteral reflux using dextranomer hyaluronic acid copolymer. J Pediatr Urol. (2008) 4:221–8. doi: 10.1016/j.jpurol.2007.11.015
63. Chan KW, Lee KH, Tam YH, Sihoe JD. Early experience of robotic-assisted reconstructive operations in pediatric urology. J Laparoendosc Adv Surg Tech A. (2010) 20:379–82. doi: 10.1089/lap.2009.0340
64. Lee RS, Sethi AS, Passerotti CC, Peters CA. Robot-assisted laparoscopic nephrectomy and contralateral ureteral reimplantation in children. J Endourol. (2010) 24:123–8. doi: 10.1089/end.2009.0271
65. Casale P, Patel RP, Kolon TF. Nerve sparing robotic extravesical ureteral reimplantation. J Urol. (2008) 179:1987–9. Discussion 1990. doi: 10.1016/j.juro.2008.01.062
66. Marchini GS, Hong YK, Minnillo BJ, Diamond DA, Houck CS, Meier PM, et al. Robotic assisted laparoscopic ureteral reimplantation in children: case matched comparative study with open surgical approach. J Urol. (2011) 185:1870–5. doi: 10.1016/j.juro.2010.12.069
67. Smith RP, Oliver JL, Peters CA. Pediatric robotic extravesical ureteral reimplantation: comparison with open surgery. J Urol. (2011) 185:1876–81. doi: 10.1016/j.juro.2010.12.072
68. Chalmers D, Herbst K, Kim C. Robotic-assisted laparoscopic extravesical ureteral reimplantation: an initial experience. J Pediatr Urol. (2012) 8:268–71. doi: 10.1016/j.jpurol.2011.04.006
69. Kasturi S, Sehgal SS, Christman MS, Lambert SM, Casale P. Prospective long-term analysis of nerve-sparing extravesical robotic-assisted laparoscopic ureteral reimplantation. Urology. (2012) 79:680–3. doi: 10.1016/j.urology.2011.10.052
70. Callewaert PR, Biallosterski BT, Rahnama'i MS, Van Kerrebroeck PE. Robotic extravesical anti-reflux operations in complex cases: technical considerations and preliminary results. Urol Int. (2012) 88:6–11. doi: 10.1159/000332953
71. Gundeti MS, Kojima Y, Haga N, Kiriluk K. Robotic-assisted laparoscopic reconstructive surgery in the lower urinary tract. Curr Urol Rep. (2013) 14:333–41. doi: 10.1007/s11934-013-0328-7
72. Schomburg JL, Haberman K, Willihnganz-Lawson KH, Shukla AR. Robot-assisted laparoscopic ureteral reimplantation: a single surgeon comparison to open surgery. J Pediatr Urol. (2014) 10:875–9. doi: 10.1016/j.jpurol.2014.02.013
73. Akhavan A, Avery D, Lendvay TS. Robot-assisted extravesical ureteral reimplantation: outcomes and conclusions from 78 ureters. J Pediatr Urol. (2014) 10:864–8. doi: 10.1016/j.jpurol.2014.01.028
74. Grimsby GM, Dwyer ME, Jacobs MA, Ost MC, Schneck FX, Cannon GM, et al. Multi-institutional review of outcomes of robot-assisted laparoscopic extravesical ureteral reimplantation. J Urol. (2015) 193(5 Suppl.):1791–5. doi: 10.1016/j.juro.2014.07.128
75. Boysen WR, Ellison JS, Kim C, Koh CJ, Noh P, Whittam B, et al. Multi-institutional review of outcomes and complications of robot-assisted laparoscopic extravesical ureteral reimplantation for treatment of primary vesicoureteral reflux in children. J Urol. (2017) 197:1555–61. doi: 10.1016/j.juro.2017.01.062
76. Boysen WR, Akhavan A, Ko J, Ellison JS, Lendvay TS, Huang J, et al. Prospective multicenter study on robot-assisted laparoscopic extravesical ureteral reimplantation (RALUR-EV): Outcomes and complications. J Pediatr Urol. (2018) 14:262 e261–6. doi: 10.1016/j.jpurol.2018.01.020
77. David S, Kelly C, Poppas DP. Nerve sparing extravesical repair of bilateral vesicoureteral reflux: description of technique and evaluation of urinary retention. J Urol. (2004) 172(4 Pt 2):1617–20. Discussion 1620. doi: 10.1097/01.ju.0000139951.37492.91
78. Herz D, Fuchs M, Todd A, McLeod D, Smith J. Robot-assisted laparoscopic extravesical ureteral reimplant: a critical look at surgical outcomes. J Pediatr Urol. (2016) 12:402 e401–9. doi: 10.1016/j.jpurol.2016.05.042
79. Noh PH, Bansal D. Pediatric robotic assisted laparoscopy for paraureteral bladder diverticulum excision with ureteral reimplantation. J Pediatr Urol. (2013) 9:e28–30. doi: 10.1016/j.jpurol.2012.06.011
80. Herz D, Smith J, McLeod D, Schober M, Preece J, Merguerian P. Robot-assisted laparoscopic management of duplex renal anomaly: comparison of surgical outcomes to traditional pure laparoscopic and open surgery. J Pediatr Urol. (2016) 12:44 e41–7. doi: 10.1016/j.jpurol.2015.04.046
81. Faasse MA, Lindgren BW, Gong EM. Robot-assisted laparoscopic ureteral reimplantation with excisional tailoring for refluxing megaureter. J Pediatr Urol. (2014) 10:773 e771–2. doi: 10.1016/j.jpurol.2014.01.023
82. Arlen AM, Broderick KM, Travers C, Smith EA, Elmore JM, Kirsch AJ. Outcomes of complex robot-assisted extravesical ureteral reimplantation in the pediatric population. J Pediatr Urol. (2016) 12:169 e161–6. doi: 10.1016/j.jpurol.2015.11.007
83. Fu W, Zhang X, Zhang X, Zhang P, Gao J, Dong J, et al. Pure laparoscopic and robot-assisted laparoscopic reconstructive surgery in congenital megaureter: a single institution experience. PLoS ONE. (2014) 9:e99777. doi: 10.1371/journal.pone.0099777
84. Hemal AK, Nayyar R, Rao R. Robotic repair of primary symptomatic obstructive megaureter with intracorporeal or extracorporeal ureteric tapering and ureteroneocystostomy. J Endourol. (2009) 23:2041–6. doi: 10.1089/end.2009.0103
85. Khan A, Rahiman M, Verma A, Bhargava R. Novel technique of laparoscopic extravesical ureteric reimplantation in primary obstructive megaureter. Urol Ann. (2017) 9:150–2. doi: 10.4103/0974-7796.204182
86. Landa-Juarez S, Guerra-Rivas A, Salgado-Sangri R, Castillo-Fernandez AM, de la Cruz-Yanez H, Garcia-Hernandez C. [Laparoscopic ureterovesical repair for megaureter treatment]. Cir Cir. (2017) 85:196–200. doi: 10.1016/j.circir.2016.08.003
87. Lee NG, Corbett ST, Cobb K, Bailey GC, Burns AS, Peters CA. Bi-institutional comparison of robot-assisted laparoscopic versus open ureteroureterostomy in the pediatric population. J Endourol. (2015) 29:1237–41. doi: 10.1089/end.2015.0223
Keywords: robotic surgery, pediatric urology, pyeloplasty, ureteroureterostomy, vesicoureteral reflux, ureteropelvic junction obstruction, ureterovesical junction obstruction, megaureter
Citation: Bilgutay AN and Kirsch AJ (2019) Robotic Ureteral Reconstruction in the Pediatric Population. Front. Pediatr. 7:85. doi: 10.3389/fped.2019.00085
Received: 29 November 2018; Accepted: 27 February 2019;
Published: 22 March 2019.
Edited by:
Mohan S. Gundeti, University of Chicago, United StatesReviewed by:
Yuval Bar-Yosef, Dana-Dwek Children's Hospital, IsraelCopyright © 2019 Bilgutay and Kirsch. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Aylin N. Bilgutay, YXlsaW4uYmlsZ3V0YXlAZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.