- 1Institute of Pathology and Neuropathology and Comprehensive Cancer Center Tübingen, University Hospital Tübingen, Eberhard-Karls-University, Tübingen, Germany
- 2Department of Pathology, Konkuk University School of Medicine, Seoul, South Korea
EBV-associated T and NK-cell lymphoproliferative diseases (EBV-T/NK LPDs) are characterized by the transformation and proliferation of EBV-infected T or NK cells. The 2016 revised World Health Organization classification recognizes the following EBV-positive lymphoproliferative disorders (LPD): chronic active EBV infection (CAEBV) of T- and NK-cell type (cutaneous and systemic forms), systemic EBV-positive T-cell lymphoma of childhood, aggressive NK-cell leukemia, extranodal NK/T-cell lymphoma, nasal type, and the new provisional entity primary EBV-positive nodal T/NK-cell lymphoma. EBV-associated hemophagocytic lymphohistiocytosis (HLH), although not included in the WHO classification because it is a reactive, inflammatory disease, is included in this review because it can be life-threatening and may have overlapping features with other EBV+ T/NK LPDs. EBV+ T/NK LPDs are rare diseases difficult to diagnose and manage properly, because some LPDs have unusual presentations, and discrepancies between clinical and histological findings might be encountered. Furthermore, EBV+ T/NK disorders share some clinico-pathological features, and may evolve into other categories during the clinical course, including malignant transformation of CAEBV. Here, we review the EBV+ T/NK LPDs in terms of their definitions, clinical features, histology, immunophenotype, molecular findings, and pathogenesis. This review aims to increase our understanding and awareness of the differential diagnosis among the different EBV+ T/NK LPDs. New insights into the genetic characteristics of these disorders will also be discussed.
Introduction
Epstein-Barr virus (EBV) is a gamma herpesvirus with a double-stranded DNA genome in core. EBV has a tropism for B cells but can infect various types of human cells including T cells, NK cells and even epithelial cells (1). EBV causes chronic latent infection with lifelong persistence in about 95% of the world population (2). Memory B cells are important as the main reservoir of EBV during the latency period. The impairment of balance between host immune response and EBV virus can lead to various EBV-associated lymphoproliferative disorders (LPDs) of B, T, or NK cells. EBV-associated LPDs can be categorized into B or T/NK-cell types based on which cells are infected by the virus.
EBV-associated T and NK-cell LPDs are characterized by the transformation and proliferation of EBV-infected T and NK-lymphocytes that usually carry an EBV-latency type 2. These disorders occur commonly in Asians and Native Americans from Central and South America (3). EBV-associated T and NK-cell LPDs represent a broad spectrum of diseases encompassing various reactive and malignant disorders (4). They are classified into 6 categories, consisting of EBV-associated hemophagocytic lymphohistiocytosis (HLH), chronic active EBV infection (CAEBV) of T- and NK-cell type, systemic EBV-positive T-cell lymphoma of childhood, aggressive NK-cell leukemia, extranodal NK/T-cell lymphoma, nasal type, and primary EBV-positive nodal T/ NK-cell lymphoma, the latter incorporated as a new provisional subgroup within peripheral T-cell lymphoma, not otherwise specified (Table 1). The first three disorders are prevalent in the pediatric and adolescent population, whereas the last three groups usually affect adults (3). Systemic EBV-positive T-cell lymphoma of childhood (previously LPD), aggressive NK-cell leukemia, extranodal NK/T-cell lymphoma, nasal type, and primary EBV-positive nodal T/NK-cell lymphoma are considered malignant proliferations in the 2016 revised WHO classification (3). CAEBV of T- and NK-cell type represents a reactive process of EBV-associated T and NK-cell LPDs with potential to progress into a malignant disorder. CAEBV is divided into one systemic and two cutaneous forms; hydroa vacciniforme (HV)-like LPD (previously lymphoma) and severe mosquito bite allergy. EBV-associated HLH is a clinicopathological syndrome complicated by abnormal hyperinflammatory immune response and is also considered a reactive disorder.
This review aims to describe the clinicopathological features of various EBV-associated T and NK-cell LPDs including reactive lymphoproliferations as well as overt lymphomas. The main features are summarized in Table 2.
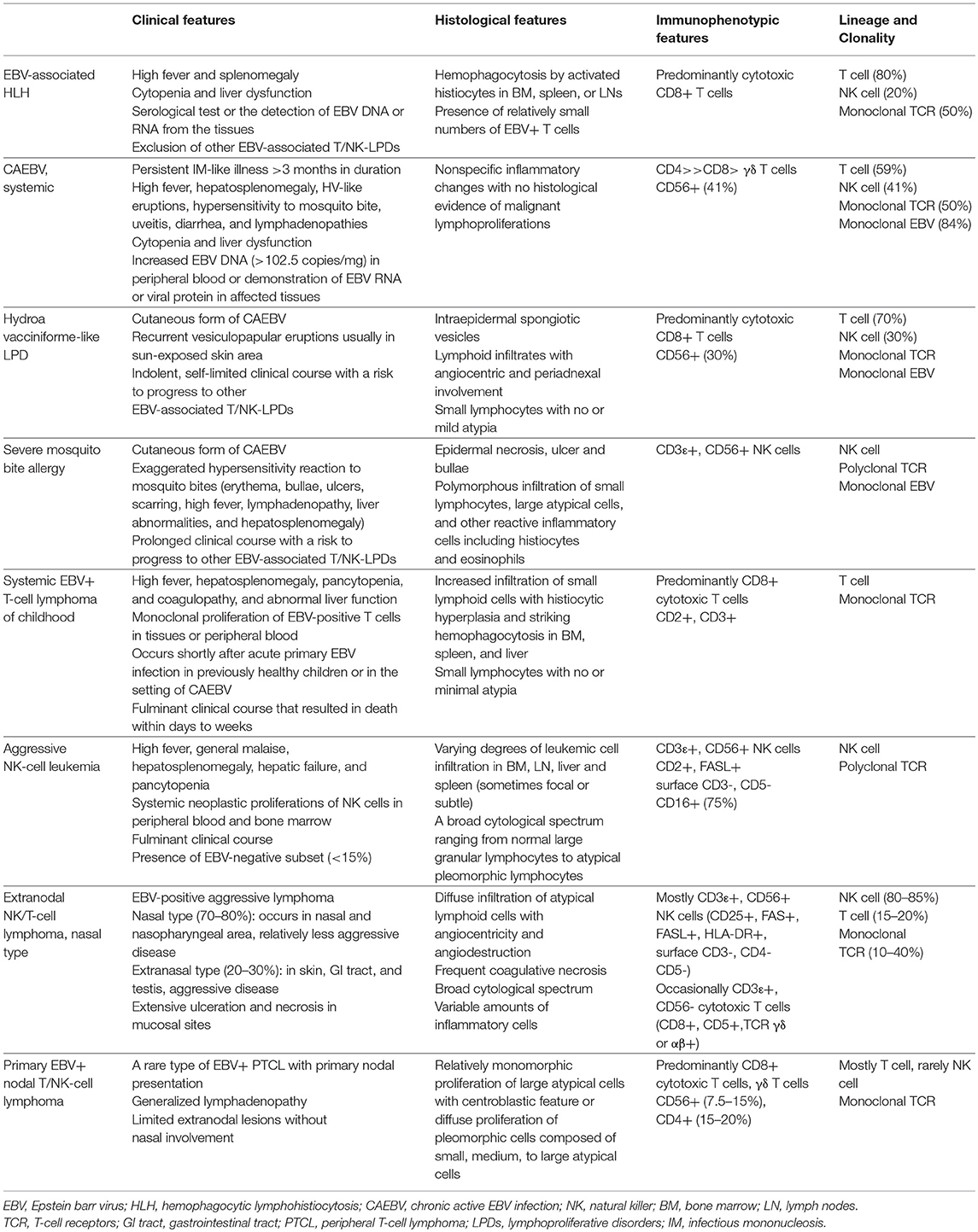
Table 2. Summary of pathological features of EBV-associated T and NK- cell lymphoproliferative diseases.
EBV-Associated Hemophagocytic Lymphohistiocytosis
HLH is a life-threatening inflammatory disease characterized by uncontrolled and overwhelming activation of the immune system. HLH is a clinical syndrome that can be diagnosed when the patient meets diagnostic criteria based on the following findings: (1) clinical features such as fever and splenomegaly; (2) laboratory abnormalities including cytopenias, hyperferritinemia and liver dysfunction; and (3) pathological findings showing hemophagocytosis in bone marrow (BM), spleen, or lymph nodes (LN) (4, 5). HLH can be divided into primary and secondary forms according to underlying causes. The primary or familial type is an inherited disorder with an autosomal recessive inheritance of mutations affecting the cytotoxic function of T and NK cells, and the secondary type corresponds to an acquired type associated with various conditions including infections, autoimmune disorders, and malignancy (6, 7). However, the clinical distinction between primary and secondary HLH may be ambiguous, because patients with HLH-associated gene defects frequently have an infectious event triggering the clinical symptoms (8). Furthermore, advanced molecular techniques including whole-exom sequencing can identify underlying genetic abnormalities that have not been detected by conventional clinical testing in patients with presumed secondary HLH (9). Recent whole-genome sequencing analysis identified several types of genetic defects related with immune dysfunction, including potentially novel ones, in 58% of patients with HLH (9). Therefore, systematic and comprehensive investigations are essential for all patients with newly diagnosed HLH.
Viral infections are the most common cause of secondary HLH, and EBV is the most frequently HLH-associated virus (5). In a nationwide survey performed in Japan, EBV-associated HLH accounted for about 30% of HLH, followed by other infection- or lymphoma-associated HLH (5). EBV infection was reported to be about 75% in patients with HLH in a retrospective study done in China (10). HLH can also occur in the clinical course of other EBV-associated T and NK-cell LPDs including CAEBV of T/NK cell type or systemic EBV-positive T-cell lymphoma of childhood. An independent diagnosis of EBV-associated HLH, as a separate entity, can be made when other EBV-associated LPDs are excluded in the differential diagnosis. EBV-associated HLH is typically associated with EBV infection of T or NK cells rather than B cells.
Among several predisposing genetic conditions, X-linked lymphoproliferative disease (XLP) is often associated with EBV-associated HLH. Especially, HLH arising in patients with XLP type 1 is nearly exclusively linked to EBV (11). In patients with XLP type 2, HLH is commonly associated with EBV, but also arises in response to other infectious agents besides EBV or without an identifiable infectious cause (11). EBV-associated HLH occurs predominantly in children and adolescents. The majority of cases have been reported in East Asians (5, 12). Differences in geographic distribution indicates that there may be some genetic predisposition in the pathogenesis of EBV-associated HLH (13).
Clinical Features
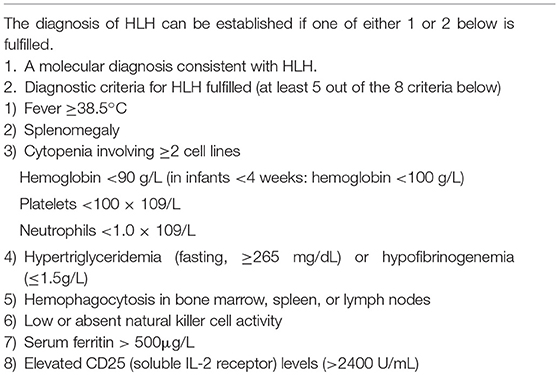
EBV-associated HLH typically presents with continuous high-grade fever and splenomegaly. Other findings including lymphadenopathy, jaundice, edema, and skin rash may also be present. Laboratory tests show cytopenias affecting more than two lineages in the peripheral blood (PB), abnormalities in liver function tests (e.g., hypertriglyceridemia, hypofibrinogenemia, elevated serum transaminases, hyperbilirubinemia, prolonged prothrombin time, and prolonged partial thromboplastin time), hyperferritinemia, cerebral spinal fluid (CSF) pleocytosis, hyponatremia, and hypoproteinemia (6). Among these, cytopenia, hypertriglyceridemia, hypofibrinogenemia, and hyperferritinemia are included in the diagnostic criteria of HLH (Table 3) (14). EBV-HLH tends to show these clinical findings in a more rapid and severe fashion, compared to other HLHs (12). However, clinical manifestations may be highly variable and some patients can have nonspecific presentations by unusual organ involvement, including intestinal perforation, cutaneous lesions, pulmonary infiltrates, and central nervous system (CNS) disease (12, 13, 15). Therefore, clinical suspicion is important for the appropriate diagnosis and treatment of EBV-associated HLH.
EBV-associated HLH induces hypercytokinemia resulting from abnormal hyperactivation of the immune system. Various cytokines including IFN-γ, TNF, sIL-2R, IL-6, IL-10, and IL-18 are secreted by activated macrophages and T-lymphocytes (12). They have been used as biological indicators reflecting the severity of HLH. The level of sIL-2 receptor (sCD25) is used as a diagnostic marker. It is crucial to detect EBV infection in the patient for diagnosing EBV-associated HLH. The presence of EBV can be determined by serological tests or by detection of EBV DNA or RNA from PB or any tissue. Viral load can be estimated by measuring EBV copy numbers using the real time PCR assay, which correlates better with clinical severity than serology (12). The clinical course of EBV-associated HLH varies from mild to severe or fatal.
Morphology and Immunophenotypical Findings
Histologically, activated macrophages engulfing RBCs, leukocytes, platelets, and their precursor cells are scattered in the sinusoids of BM, spleen, liver, and LN, but are not required for diagnosis. EBV-infected T cells are also found with hemophagocytic histiocytes and show a cytotoxic phenotype with expression of CD8 and granzyme B in the majority of cases (4). The characteristics of infiltrating T cells are different between EBV-associated HLH and other EBV-associated T and NK-cell LPDs. Generally, EBV+ T cells frequently show a cytotoxic phenotype with CD8 expression in EBV-associated HLH and systemic EBV-positive T-cell lymphoma, whereas CD4+ cells or NK cells are predominantly infected by EBV in other EBV-associated T and NK-cell LPDs. In addition, the EBV+ cells are present in relatively small amounts in EBV-associated HLH, compared to other LPDs. In situ hybridization (ISH) with the EBV-encoded small RNA (EBER) is used to detect EBV-infected cells. Double staining with EBER ISH and CD20, CD3, or CD56 can be done to identify which cells are infected by EBV. HLH induced by EBV-infected NK cells has been reported to occur uncommonly, accounting for 20% in a previous report (4, 16).
Pathogenesis and Molecular Features
The precise mechanism on how T or NK cells lacking CD21, the primary receptor for EBV, are infected by EBV in EBV-associated HLH is still unknown. A previous report showed that CD21 is synaptically transferred to NK cells through conjugation to CD21+, EBV-infected B cells, thereby allowing EBV binding to NK cells (16, 17). T-cell receptor (TCR) gene rearrangement can be detected in about half of cases with EBV-associated HLH using conventional method (18). Furthermore, with the introduction of Biomed-2 multiplex PCR, the detection rate of T-cell clonality is notably increasing in EBV-associated HLH. It has been suggested that changes in T cell clonality pattern (monoclonal to polyclonal) could be helpful to predict the therapeutic response of patients (18).
Many predisposing genetic conditions of HLH are characterized by impaired cytotoxicity of cytotoxic T or NK cells. Familial HLH 2, 3, 4, and 5 are caused by mutations in PRF1, UNC13D, STX11, and STXBP2, respectively (19–22). In patients with these mutations, cytotoxic cells demonstrate functional impairment in the degranulation process including cytotoxic granule docking, priming, and fusion with the plasma membrane, when encountering susceptible target cells infected with virus (11, 23). As a result, target cells are not eliminated by cytotoxic lymphocytes, and persistently activate immune cells, ultimately leading to a hyperinflammatory HLH (11). Among these mutations, PRF1 mutation induces total deficiency of functional perforin, which results in defective cytotoxicity of cytotoxic T or NK cells (24). The pathogenetic mechanism of XLP-associated HLH is more complicated. Patients with XLP type 1 harbor mutations in SH2D1A (Xq25) encoding signaling lymphocyte activation molecule-associated protein (SAP). Defective SAP induces serious immunological complications including impaired 2B4-mediated cytotoxicity of T or NK cells against EBV-infected cells, vigorous expansion of CD8+ T cells by a failure of T cell reactivation-induced cell death, and defects in the development of NKT cells (25, 26). XLP type 2-induced HLH is pathogenetically different from other genetic HLH, because cytotoxic lymphocyte-mediated cytotoxicity is apparently normal in patients with XLP type 2, which is caused by mutations of XIAP/BIRC4 (27, 28). Instead, defective expression of XIAP increases a susceptibility of lymphocytes to apoptosis in response to CD95 and tumor necrosis factor receptor–related apoptosis-inducing ligand receptor stimulation, and induces defective NOD2 signaling with dysregulation of inflammasome function (27, 29, 30). Due to normal cytotoxicity, the development of HLH in these patients seems to have a less strong association with EBV, compared to patients with XLP type 1.
Chronic Active EBV Infection of T- and NK- Cell Type, Systemic Form
CAEBV of systemic form is characterized by persistent clinical symptoms and signs including fever, hepatosplenomegaly, hepatitis, and lymphadenopathy after infectious mononucleosis (IM). Originally, when first described by Straus et al., the required duration of IM-like symptoms was more than 6 months to fulfill the criteria for CAEBV; however, the revised criteria require now only 3 months (3, 31, 32). The current diagnostic criteria are as follows: (1) IM-like symptoms persisting more than 3 months; (2) increased EBV DNA (>102.5 copies/mg) in PB, (3) histological evidence of organ disease; and (4) demonstration of EBV RNA or viral protein in affected tissues (3). In addition, CAEBV should be diagnosed in patients without known immunodeficiency, malignancy or autoimmune disorders. Most cases have been reported in East Asia including Japan, South Korea, 33–China, and Taiwan (36). Few reports come from Latin America. It appears to occur rarely in Western and African populations (37). CAEBV arises predominantly in pediatric and adolescent patients. If it develops in adults, it shows a more aggressive clinical course (38). No sex predilection is present.
Clinical Features
The typical IM-like manifestations including persistent fever, hepatosplenomegaly and lymphadenopathy are present in about half of the patients. Some patients with CAEBV may have variable and non-specific symptoms according to the organs affected by EBV-induced inflammation, which often causes the diagnosis to be delayed or misdiagnosed. Other relatively common symptoms and signs are severe mosquito bite allergy (33%), skin rash (26%), HV-like eruptions (10%), diarrhea (6%), and uveitis (5%) (39). Pancytopenia and liver dysfunction are common. High titers of anti-VCA IgG and anti-early antigen IgG are found in almost all patients, and IgA antibodies against VCA and early antigen are frequently revealed. Increased copies of EBV DNA are also identified in all patients with CAEBV (39, 40).
The clinical course is variable. Some patients show an indolent clinical course and remain stable over a long period, while other patients progressively deteriorate with serious complications including hemophagocytic syndrome (24%), disseminated intravascular coagulation (16%), hepatic failure (15%), digestive tract ulcer/perforation (11%), coronary artery aneurysm (9%), CNS involvement (9%), interstitial pneumonia (5%), and myocarditis (7%) (4, 33, 39). The variability of clinical behavior generally depends on the viral load of EBV DNA and the host immunity. The prognosis is associated with the predominant cell type infected with EBV. Patients with CAEBV of T-cell type shows significantly poorer survival rate than those with CAEBV of NK-cell type (probability of 5-year survival, 0.59 in CAEBV of T-cell type vs. 0.87 in CAEBV of NK-cell type) (39). Furthermore, both groups tend to have different clinical presentations. Patients with T-cell infection are more likely to show severe systemic symptoms, high titers of EBV specific antibodies and aggressive clinical behavior, whereas those with NK-cell infection commonly have mild systemic symptoms, high concentrations of IgE, relatively lower levels of EBV-specific antibody and skin lesions including rash and hypersensitivity to mosquito bites, probably reflecting the more favorable clinical course of this type (4, 39, 41). However, CAEBV of NK-type (23.1%) might eventually evolve into aggressive NK-cell leukemia or extranodal NK/T cell lymphoma, nasal type (39).
Morphology and Immunophenotypical Findings
The biopsy of affected tissues in CAEBV is characterized by reactive inflammatory changes with no histological evidence of a malignant lymphoproliferation. Because microscopic findings are similar to nonspecific inflammation, pathological diagnosis can be very difficult and overlooked without careful attention to the clinical history. The detection of EBV infection in the infiltrated lymphoid cells using EBER ISH is necessary for tissue confirmation, and very useful especially in patients with no clinical suspicion of having CAEBV, due to nonspecific and unusual presentations. Histological findings of LN include follicular hyperplasia, paracortical hyperplasia, focal necrosis, and small epithelioid granulomas (3). A polymorphic infiltrate can be noted occasionally in interfollicular areas. Atrophy of the white pulp and congestion of the red pulp are seen in the spleen. In liver biopsies, infiltration of small lymphocytes is shown in portal areas or sinusoids like in viral hepatitis. The BM appears to be microscopically normal, except cases with accompanying HLH. According to the affected organs, CAEBV can mimic interstitial pneumonia in lung biopsies, myocarditis in heart biopsies and dermatitis in skin biopsies. EBV-infected cells show either a T-cell immunophenotype (59%) or a NK-cell immunophetype (41%), and rarely EBV-infection in both T and NK cells (3%). T-cells are mostly CD4+ (21%), with a minority being either CD8+ (8%), or γδ T-cell type (5%). Ill-defined T-cell phenotypes occur in 25% of the cases. CAEBV of B-cell type occurs rarely (2–3%) and mainly in Western population (42).
Pathogenesis and Molecular Features
The pathogenesis remains unknown. Although CAEBV develops in immunocompetent hosts by definition, some patients have impaired activity in EBV-specific cytotoxic T cells (43, 44). Strong racial differences in susceptibility to CAEBV suggest that its occurrence is influenced by genetic polymorphisms in host immune response-related genes (34, 45). The viral latency pattern of CAEBV indicates latency type 2, because EBV-infected T or NK cells express only a few EBV-related genes including EBNA1, LMP1 and LMP2A (41, 44). EBV is mononclonal in 84%, oligoclonal in 11%, and polyclonal in 5% of the reported cases (4). The rearrangement pattern of the TCR genes seems to be monoclonal in about 50 % of the cases (4). Therefore, monoclonality of EBV-infected cells does not warrant a diagnosis of malignant lymphoma in EBV-associated T and NK-cell LPDs. Chromosomal abnormalities have been noted in a minority of cases (4). A clinicopathological classification of EBV-associated T and NK-cell LPDs has been proposed according to morphological evaluation and clonality results, which consists of category A1 (polymorphic, polyclonal LPD), category A2 (polymorphic, monoclonal LPD), category A3 (monomorphic, monoclonal LPD), and category B (monomorphic, monoclonal LPD with fulminant course) (46). Categories A1–A3 represents a continuous spectrum of CAEBV and its evolution to overt lymphoma. Category B corresponds to systemic EBV-positive T-cell lymphoma of childhood in the 2016 revised WHO classification (3).
Hydroa Vacciniforme-Like Lymphoproliferative Disease
HV-like LPD is one of the cutaneous forms of CAEBV. HV-like LPD is considered as a chronic EBV+ LPD that affects mainly children, but it carries a risk of progression to clinically overt malignant lymphoma. HV was initially described in Western populations as a benign photodermatosis that presents with recurrent self-limited vesiculopapular eruption, which progresses to crusts with rupture and heals with vacciniform scarring (47). Skin lesions mimicking classic HV were identified with some different features including no association with photosensitivity in children and young adolescents from Asia and Latin America (48–51). These lesions were shown to be associated with EBV infection and frequently contained monoclonal TCR gene rearrangements in subsequent studies (49, 50, 52). Reflecting these results, HV-like lymphoma was included in the 2008 WHO classification as a new type of EBV-positive cutaneous T-cell lymphoma of childhood. However, because this term does not represent the diverse clinical spectrum from classic self-limited HV to HV-like lymphoma, the term HV-like lymphoma was changed to HV-like LPD in the 2016 revised WHO classification (3).
Clinical Features
HV-like LPD shows variable clinical presentations and behaviors (4, 53, 54). HV-like LPD was previously classified into the classic type and severe type, representing the two extremes of the clinical presentation. Patients with the classic HV have light-induced vesiculopapules in sun-exposed skin area including the face and arm without systemic symptoms, whereas the severe form is characterized by the complicated vesicles that progress to large skin ulcers and occasionally leave severe scarring with disfigurement in sun-exposed and unexposed areas, with frequent systemic symptoms such as fever, lymphadenopathy, and hepatosplenomegaly (55). Some mild cases are cured after photoprotection, but the majority of cases usually shows a very indolent clinical course and has multiple recurrences and remissions that may finally develop into a more severe disease including EBV-positive T or NK-cell lymphoma of skin. A seasonal variation is present with increased occurrences of episodes in spring and summer.
Morphology and Immunophenotypical Findings
Intraepidermal spongiotic vesicles are characteristically formed by epidermal reticular degeneration. However, in some case only a very subtle lymphoid infiltration might be observed (Figures 1A–F). These lymphoid infiltrates are predominantly present in the dermis with the frequent extension to the subcutaneous tissue, and often exhibit an angiocentric and periadnexal involvement with the pattern of septal or lobular panniculitis. The infiltrating cells are usually bland-looking small lymphocytes with no or mild atypia, and highlighted by EBV positivity (Figures 1A,B,E). These cells are CD8+ cytotoxic T cells in the majority of cases (Figures 1C,D,F), followed by CD56+ NK cells (about 30%) (4, 56, 57). γδ T cells are found in a few cases in immunophenotyping of skin-infiltrating T cells, whereas flow cytometry of PB shows clonal expansion of γδ T cells in most cases (56, 58, 59). CD30 is frequently positive in the lymphoid infiltrates, and LMP1 is mostly negative (56).
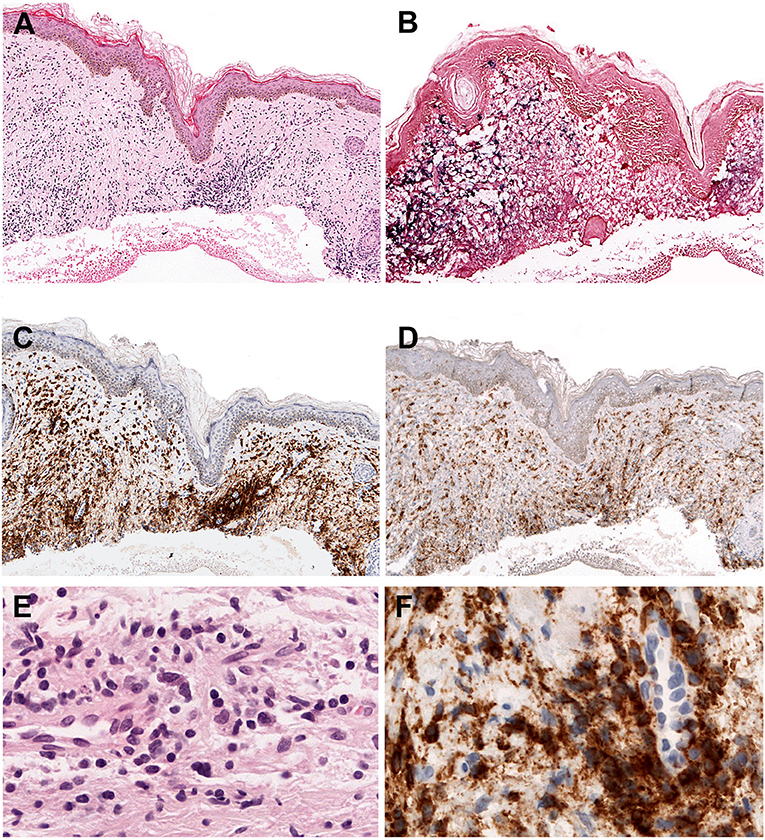
Figure 1. Hydroa vacciniforme-like lymphoproliferative disorder. (A) A skin biopsy with a subtle dermal infiltrate surrounding adnexae and blood vessels (H&E, 25x); (B) The lymphoid cells are EBV positive, as demonstrated by in situ hybridization for EBV-encoded small RNA (EBER) (in situ hybridization, 25x); (C) CD8 is positive in the majority of the infiltrating cells (immunohistochemistry, 25x); (D) The infiltrating cells are negative for CD4. CD4 highlights the abundant histiocytes (immunohistochemistry, 25x); (E) The infiltrating cells are predominantly small, without atypia (H&E, 400×); (F) The infiltrating cells are surrounding a blood vessel highlighted by CD8 stain (immunohistochemistry, 400×).
Pathogenesis and Molecular Features
The pathogenesis is unknown. The geographic and racial distribution pattern suggests that genetic predisposition related to a defective immune response to EBV may contribute to the susceptibility to HV-like LPD, like in other EBV-associated T, and NK-cell LPDs. Monoclonal rearrangement of the TCR genes is found in almost all cases with T-cell immunophenotype (4, 56). EBV-infected cells can be detected by EBER ISH. The number of EBV-positive cells is variable among cases, and there are only a small number of EBV-positive cells in some cases. Pathological parameters including T cell clonality or the quantity of EBV-positive cells are generally not associated with the clinical behavior or related to the risk to develop a systemic lymphoma (3). In contrast to LMP1 negativity shown by immunohistochemistry in tissues, LMP1 expression is mostly identified by PCR in PB, indicating an EBV latency type 2 (60).
Severe Mosquito Bite Allergy
Severe mosquito bite allergy is another cutaneous form of CAEBV. Severe mosquito bite allergy is an EBV+ NK-cell LPD involving skin and characterized by an exaggerated allergic reaction to mosquito bites. Patients with severe mosquito bite allergy may be complicated by HLH, HV-like LPD, or systemic CAEBV in the prolonged clinical course. Furthermore, they have a higher risk of developing overt NK/T-cell lymphoma or aggressive NK-cell leukemia.
Severe mosquito bite allergy is a rare disease, of which most have been described in Japan with a few cases from other East Asia (4, 61–65). It mainly affects children and young adolescents aged 0–18 years (mean onset age, 6.7 years) (66). No sex predilection is reported.
Clinical Features
Hypersensitivity reactions after mosquito bites include localized cutaneous manifestations such as erythema, bullae, ulcers, and scar formation, and systemic findings including high fever, lymphadenopathy, liver abnormalities, and hepatosplenomegaly. Some patients experience similar hypersensitivity reactions at the injection site after vaccination (66). Most patients exhibit a high serum IgE level, high EBV load, and NK-cell lymphocytosis in the PB. After the hypersensitivity reaction resolves, the patients remain asymptomatic until the next mosquito bites.
Morphology and Immunophenotypical Findings
Epidermal necrosis, ulceration and bullae formation are present in the mosquito bite lesion. Skin biopsies from these lesions show a dense infiltrate of lymphoid cells that may extend into the subcutaneous tissue. The lymphoid infiltrate has a polymorphous composition consisting of small lymphocytes, large atypical cells, and other reactive inflammatory cells including histiocytes and eosinophils. The overall appearances are similar to HV-like LPD.
The infiltrating lymphoid cells are immunophenotypically CD3ε+, CD56+ NK cells with the expression of cytotoxic molecules TIA1 and granzyme B. Reactive CD4+ or CD8+ T cells are also present in the infiltrates. EBV-positive cells are often positive for CD30, and rarely positive for LMP1.
Pathogenesis and Molecular Features
The etiology remains unknown. Genetic predispositions and environmental factors may have an influence on the pathogenesis. Mosquito bites can induce the expression of LMP1 in NK cells through the proliferation of mosquito antigen-specific, CD4+ T cells, which are involved in the reactivation of latent EBV in NK cells (67–69). EBV positivity is found only in a percentage of the infiltrating NK cells. EBV is monoclonal in almost all cases by EBV terminal repeat analysis (4). Like HV-like LPD, LMP1 expression is seen in PB by PCR, thereby, indicating type 2 EBV latency.
Systemic EBV-Positive T-Cell Lymphoma of Childhood
Systemic EBV-positive T-cell lymphoma of childhood is a rapidly progressive, fatal disease of children and young adults that is characterized by monoclonal expansion of EBV-positive T cells with an activated cytotoxic phenotype in tissues or PB. Historically, several different terms have been used to describe this disease, including fulminant EBV+ T-cell LPD of childhood, sporadic fatal IM, fulminant hemophagocytic syndrome in children, fatal EBV-associated hemophagocytic syndrome, and severe CAEBV. Systemic EBV-positive T-cell lymphoma is almost always accompanied by HLH, and shows a fulminant clinical course, rapidly progressing to multiple organ failure, sepsis, and finally death, within days to weeks. This disease was first incorporated as a LPD in the 2008 WHO classification; however, in the current 2016 revised WHO classification, it has been renamed as systemic EBV-positive T-cell lymphoma of childhood, reflecting its clinical severity. Systemic EBV-positive T-cell lymphoma of childhood occurs mainly in East Asia including Japan, Taiwan and China (33, 70–72). It also occurs in Latin America but it is rare in Western populations (73).
Clinical Features
Systemic EBV-positive T-cell lymphoma of childhood usually occurs shortly after acute primary EBV infection in previously healthy children or adolescents. Patients present with severe systemic findings such as fever, hepatosplenomegaly, pancytopenia, coagulopathy, and abnormal liver function, within days to weeks after IM symptoms due to primary EBV infection. Lymphadenopathy is occasionally seen. The patients are usually complicated by HLH, sepsis, and multiorgan dysfunction, ultimately leading to death within days to weeks.
In serological tests for EBV, anti-VCA IgM is often absent or barely detectable in the majority of patients, whereas IgG antibodies against VCA are positive. These abnormal results may be misleading and contribute to the delay in diagnosis, considering that they do not indicate acute or active EBV infection (74, 75).
Morphology and Immunophenotypical Findings
Systemic EBV-positive T-cell lymphoma of childhood is histologically characterized by increased infiltration of small lymphoid cells with histiocytic hyperplasia and striking hemophagocytosis in the BM, spleen, and liver. Tumor cells have no or minimal cytological atypia in most cases and may not be distinguishable from normal lymphocytes. However, some cases show atypical lymphoid infiltrates composed of pleomorphic medium to large-sized cells with frequent mitosis (72) (Figure 2). The liver reveals mild to marked infiltration of small lymphocytes in portal and sinusoidal area with cholestasis, steatosis, and focal necrosis (73). The spleen shows the depletion of white pulp with prominent sinusoidal and nodular lymphoid infiltrates. The LNs are usually unremarkable with preserved architecture. Early histological findings of lymph nodes include the depletion of B-cell areas and the expansion of paracortical/interfollicular areas that is infiltrated by a polymorphous population of lymphoid cells consisting of small to medium-sized lymphocytes and large atypical lymphocytes with irregular nuclei (Figures 2A–F). The LNs appear to be more depleted along the disease progression. The infiltrating lymphoid cells are mostly EBV+, CD8+ cytotoxic T cells that express CD2, CD3, TIA-1, and granzyme B, and lack CD56 (71, 73). In contrast, cases associated with CAEBV show CD4+ immunophenotype (73). Rare cases exhibit EBV-positive tumor cells with co- expression of CD4 and CD8 (73). LMP1 is usually negative by immunohistochemistry. EBNA2 is always negative. The confirmation of EBV infection by EBV ISH is very useful for diagnosis.
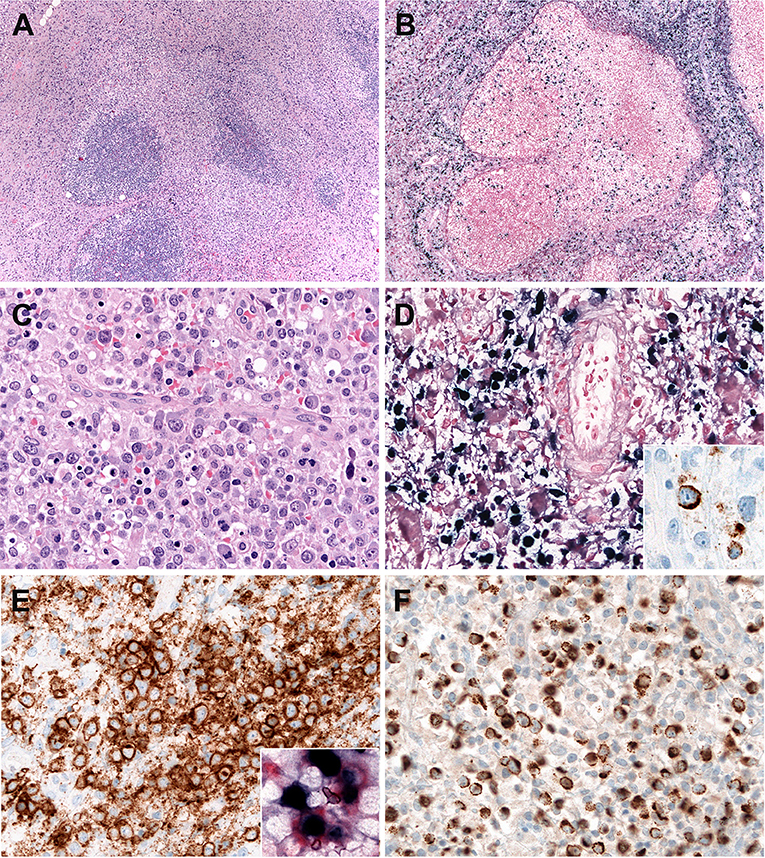
Figure 2. Systemic Epstein-Bar virus (EBV)-positive T-cell lymphoma of childhood. (A) Lymph node with partial preservation of the architecture with depleted germinal centers and expansion of the interfollicular area (H&E, 50×); (B) Many of the lymphoid cells in the interfollicular area are EBV-positive, as demonstrated by in situ hybridization for EBV-encoded small RNA (EBER) (in-situ hybridization 100×); (C) The neoplastic cells are mostly medium to large-sized cells with irregular nuclei. Note the presence of apoptosis (H&E, 400×); (D) Many cells are EBER positive (in-situ hybridization, 400×). LMP1 is positive indicating an EBV latency type 2 (immunohistochemistry, insert, 400×); (E) The neoplastic cells are CD8 positive (immunohistochemistry, 400×). Double stainings show that the CD8-positive cells (red) are EBER-positive (Black) (Immunohistochemistry and in situ hybridization, insert, 400×) (F) TIA1 is positive in the infiltrating cells (immunostaining, 400×).
Pathogenesis and Molecular Features
The etiology of systemic EBV-positive T-cell lymphoma of childhood is unknown. However, its association with primary EBV infection and strong racial predisposition suggest a genetic defect in the host immune response to EBV. The infiltrating T cells show monoclonal rearrangements of the TCR genes. EBV is present in a clonal episomal form in all cases (33, 70, 72, 73, 76). Because systemic T-cell lymphoma of childhood have some clinicopathological characteristics overlapping with EBV-associated HLH, the distinction of both diseases is sometimes difficult. In a literature review of systemic T-cell lymphoma and EBV-associated HLH, the patients with chromosomal aberrations are 100% fatal, whereas cases with evidence for T-cell clonality are fatal in 62% (77). Therefore, karyotypic abnormalities can be more helpful to distinguish systemic T-cell lymphoma of childhood from EBV-associated HLH in ambiguous cases, compared to T cell clonality, which is demonstrated in about half of EBV-associated HLH cases.
Aggressive NK-Cell Leukemia
Aggressive NK-cell leukemia is a very rare, fatal disease characterized by systemic neoplastic proliferations of NK cells in PB and BM and a strong association with EBV. However, leukemic NK cells are variably present, and sometimes can be sparse in the PB and BM. It was originally designated as aggressive NK-cell leukemia/lymphoma to emphasize the variable clinical manifestations, because there are some cases without leukemic phase, presenting with hepatosplenomegaly and peripheral lymphadenopathy (78, 79). To permit a clear distinction, and avoid confusion between this disease and extranodal NK/T-cell lymphoma, nasal type, the term aggressive NK-cell leukemia has been chosen in the WHO classification. Extranodal NK/T-cell lymphoma with systemic involvement at multiple sites has some clinicopathological features similar to those of aggressive NK-cell leukemia, and the distinction between the two diseases may be difficult. Aggressive NK-cell leukemia has been reported mainly in Asians (80). It occurs mostly in young to middle-aged adults with no definite sex predilection.
Clinical Features
Clinical presentations include high fever, general malaise, hepatosplenomegaly, hepatic failure, and pancytopenia. Variable numbers of leukemic NK cells are present in the PB, ranging from <5% to >80% of all leukocytes (75). Laboratory tests show high levels of serum lactate dehydrogenase (LDH) levels and circulating FAS ligand (FASL) (81). Lymphadenopathy is occasionally seen, and skin lesions are uncommon. This disease is frequently complicated by HLH and coagulopathy, and shows a fulminant clinical course with multiple organ failure (75). The overall prognosis is very poor with a median survival <2 months (82). Some cases develop in the setting of CAEBV infection of NK cell type, or evolve from extranodal NK/T-cell lymphoma or chronic LPD of NK cells (4, 33, 83–86). Aggressive NK-cell leukemia shares some clinicopathological features with systemic EBV-positive T-cell lymphoma of childhood, but the immunophenotype of the neoplastic cells is basically different between these two disease entities (CD56+ NK cells vs. CD56- T cells).
Morphology and Immunophenotypical Findings
Leukemic NK cells have a broad cytological spectrum ranging from normal large granular lymphocytes to atypical lymphocytes showing nuclear enlargement, irregular nuclear contours, open chromatin, or conspicuous nucleoli. The cytoplasm is pale or lightly basophilic, and relatively abundant with fine or coarse azurophilic granules. In the BM biopsy, the extent of neoplastic NK cells varies from extensive to focal, subtle infiltration. Some cases show minimal involvement of the BM, indistinguishable from normal BM in conventional H&E stain. Increased infiltration of histiocytes is observed with hemophagocytosis in the BM. The liver, spleen, and LNs show varying degrees of tumor cell infiltration, displaying massive, patchy, or subtle involvement, like the BM (Figures 3A–E). The immunophenotype of the tumor cells is that of a mature CD56+ NK-cell with positivity for CD2, CD3ε, and cytotoxic granules TIA1 and granzyme B, and negative for surface CD3 and CD5. Aggressive NK-cell leukemia frequently expresses CD16 (in 75%), which is different from CD16-negative extranodal NK/T-cell lymphoma (82). The neoplastic cells express FASL, but usually lack CD57. The early diagnosis of aggressive NK-cell leukemia can be difficult due to unusual pathological findings including lack of lymphocytosis in PB, interstitial infiltration pattern in BM, rarely EBER negativity and aberrant immunophenotype such as CD3 negativity by immunohistochemistry (87).
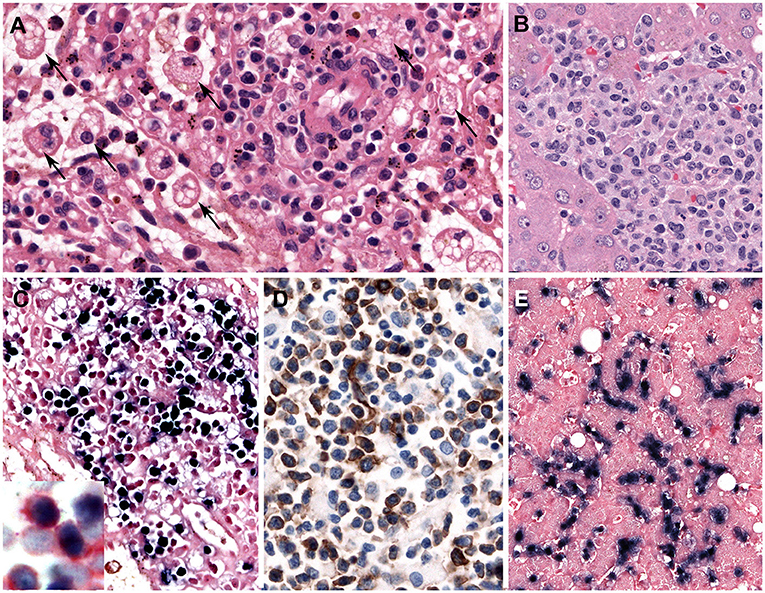
Figure 3. Aggressive NK-cell leukemia. (A) The spleen shows a scant atypical lymphoid infiltrate of small cells with bland cytology surrounding blood vessels. Note the striking erythrophagocytosis (arrows) (H&E, 400×); (B) The liver shows an atypical infiltrate in the sinusoids composed of medium-sized cells with irregular nuclei and pale cytoplasm; (C) Neoplastic cells in the spleen are stained positively with EBER (in-situ hybridization 400×). Insert shows double staining of CD56 (red) and EBER (black) demonstrating that the NK cells are infected by EBV (Immunohistochemistry and in situ hybridization 400×); and (D) The infiltrating cells are CD56 positive (immunohistochemistry 400×); (E) The neoplastic cells in the liver are EBER positive. Note the intrasinusoidal infiltration characteristic of the disease (in-situ hybridization, 400×).
Pathogenesis and Molecular Features
Although the etiology remains unknown, the strong association with EBV has been suggested to be central in pathogenesis. EBV infection has been reported in 85–100% of cases (88–90). EBV exists in clonal episomal form. However, some cases of EBV-negative aggressive NK-cell leukemia have been described, and show the clinicopathological features similar to those of EBV-positive cases, except for the fact that EBV-negative cases tend to occur in older patients with no obvious racial predilection (87, 91). Some of the EBV-negative cases may evolve from chronic LPD of NK cells (82, 91, 92). The genetic comparison of aggressive NK-cell leukemia and extranodal NK/T-cell lymphoma show some significant differences. Aggressive NK-cell leukemia shows gains of 1q23.1–q24.2 and 1q31.3–q44 and losses of 7p15.1–p22.3 and 17p13.1 more frequently than extranodal NK/T-cell lymphoma (93). A previous study reported that EBV-negative aggressive NK-cell leukemia was negative for JAK-STAT pathway-associated gene mutations, known as recurrently mutated in extranodal NK/T-cell lymphoma, suggesting different molecular pathogenesis between these two diseases (87). However, because the mutations of the JAK-STAT pathway-associated genes have been also reported in EBV-positive aggressive NK-cell leukemia (94), further investigations are warranted to reveal the mutational landscape of the NK-cell malignancies.
Extranodal NK/T-Cell Lymphoma, Nasal Type
Extranodal NK/T-cell lymphoma, nasal type, is an EBV-positive aggressive lymphoma characterized by prominent necrosis, angioinvasion, and cytotoxic phenotype. It is derived from NK cells and uncommonly cytotoxic T cells. Extranodal NK/T-cell lymphoma was called “lethal midline granuloma” in the past, because of the destruction of midline facial structures due to vascular damage and subsequent ischemic necrosis. Extranodal NK/T-cell lymphoma, nasal type, occurs commonly in East Asians and the Native Americans in Central and South America, but rarely in Western populations (95). It accounts for ~6–8% of all lymphomas in East Asia and some Latin American countries, but <1% in Western populations (96–100). It affects males more commonly than females. It occurs frequently in middle-aged adults.
Clinical Features
Extranodal NK/T-cell lymphoma arises from extranodal sites in almost all cases, most commonly involving upper aerodigestive tract (UAT) including nasal cavity, paranasal sinuses, nasopharynx, oropharynx, oral cavity, and palates. It also occurs in non-UAT sites including skin, soft tissue, gastrointestinal tract, testis, lung, and CNS. Nasal NK/T-cell lymphoma is defined as a primary tumor involving the nasal and nasopharyngeal region, regardless of dissemination to other sites. Patients with nasal NK/T-cell lymphoma present initially with nonspecific localized symptoms including nasal obstruction, purulent nasal discharge, and epistaxis. In later stages, nasal NK/T-cell lymphoma can extend to adjacent UAT tissues, or cause extensive necrotic lesions in the midline facial area. The BM is infrequently involved. Some patients may be complicated by HLH and extranasal dissemination to various non-UAT sites. The prognosis of nasal NK/T-cell lymphoma has been reported to be poor with the overall survival rate of 30–40%, but has recently improved with the introduction of new chemotherapy regimens such as L-asparaginase-based chemotherapy (95, 101). Extranasal NK/T-cell lymphoma indicates a primary tumor in a non-UAT site at first clinical presentation, and occurs in ~20–30% of cases (Figure 4) (100, 102). These lymphomas usually present with advanced stage at diagnosis, multiple sites of involvement, elevated LDH levels, and poor performance status. They are often refractory to treatment and show an inferior prognosis compared to nasal NK/T-cell lymphoma (101). Skin lesions commonly present as nodular ulcerative lesions. Gastrointestinal involvement frequently results in ulcer, bleeding, or perforation. Lymph nodes can be secondarily involved by dissemination. As some extranasal cases may harbor occult nasal lymphoma, it is important to inspect the UAT regions carefully.
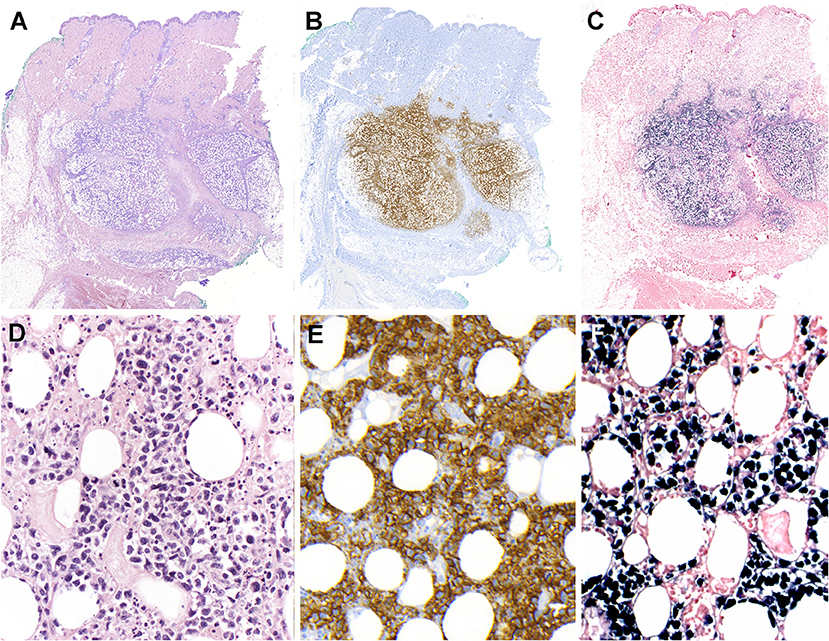
Figure 4. Extranodal, NK/T-cell lymphoma, nasal type in the skin. (A) Panoramic view of a skin biopsy shows a partially circumscribed nodule located in the subcutaneous tissue (H&E, scanned slide); (B) The tumor cells are CD56 positive (immunohistochemistry, scanned slide). (C) The lymphoid cells are positive for EBV-encoded small RNA in situ hybridization (EBER) (in situ hybridization) (D) The infiltrate is composed of large atypical cells with irregular nuclei. The tumor cells surround the adipocytes revealing a “lace-like pattern” mimicking panniculitis-like T-cell lymphoma. Numerous apoptotic bodies are observed (H&E, 400×); (E,F) Higher magnification demonstrates that the neoplastic cells are positive for CD56 and EBER, (immunohistochemistry and in situ hybridization 400×).
Morphology and Immunophenotypical Findings
Involvement of mucosal sites frequently presents with extensive ulceration. Histological findings show diffuse infiltration of atypical lymphoid cells with angiocentricity and angiodestruction, leading to vascular obstruction and the consequent ischemic, coagulative necrosis. Involvement of non-mucosal sites also shows similar morphological changes. Tumor cells have a broad cytological spectrum, ranging from bland-looking small lymphocytes to large pleomorphic cells (Figures 4A–F). Most cases show a relatively monotonous population of medium-sized cells or a polymorphous pattern composed of small and large cells. Variable amounts of reactive inflammatory cells are admixed with tumor cells, mimicking an inflammatory lesion. Tumor cells have often irregularly folded nuclei with indistinct nucleoli and moderate amount of cytoplasm. Mitotic figures are easily identified. The most common immunophenotype is CD3ε+, CD56+, CD2+ and cytotoxic molecules (granzyme B, perforin and TIA1), but lacks surface CD3, CD4 and CD5 (Figures 4, 5). Tumor cells often express CD25, FAS, FASL, and HLA-DR. CD30 expression is identified in about 30–40% of cases (103–106). The minority of cases shows a CD3+, CD56- cytotoxic T-cell phenotype, expressing CD8, CD5,TCR (γδ or αβ type), and cytotoxic molecules. These cases account for 15–20% of all cases and represent a real T-cell phenotype of the tumor cells (104, 107). There are no significant differences in the clinicopathological features between CD56+ and CD56- cases (88). EBV infection should be confirmed in virtually all cases to render a diagnosis of extranodal NK/T-cell lymphoma. Therefore, when CD3ε+, CD56+ nasal lymphomas do not show EBV positivity, other types of T-cell lymphoma should be considered in the diagnosis. Because of a strong association with EBV, the immune microenvironment has some prognostic implication. High quantity of tumor-infiltrating FOXP3+ regulatory T cells or PD-L1 expression on tumor cells independently predict better prognosis, suggesting that inhibitory immunomodulation might suppress lymphoma cells, as well as immune cells related with antitumor immune response (108, 109).
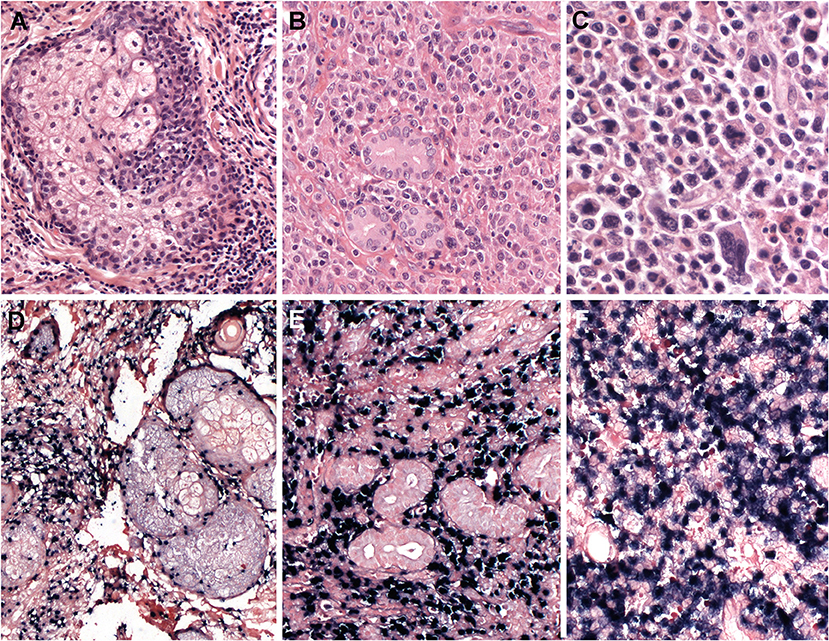
Figure 5. Extranodal, NK/T-cell lymphoma, nasal type. (A–C) Nasal biopsies displaying the morphological spectrum of ENKTCL. (A) Infiltrate of small lymphoid cells with bland cytology surrounding the sebaceous gland, mimicking a reactive lesion (H&E, 400×);(B) Dense lymphoid infiltrate of intermediate-sized cells, showing nuclear irregularity and pale to clear cytoplasm (H&E, 400×); (C) Atypical cell infiltrate, of pleomorphic large cells admixed with intermediate-sized cells (H&E, 400×); (D–F) Lymphoma cells are positive for EBV-encoded small RNA in situ hybridization (EBER, 400×).
Pathogenesis and Molecular Features
EBV has a pathogenetic role in the development of extranodal NK/T-cell lymphoma, nasal type. EBV exists in a clonal episomal form with EBV type 2 latency. Most patients are infected with EBV subtype A with some geographic variations (110–112). EBV has frequently a 30-base pair deletion in LMP1 gene, which may contribute to lymphomagenesis through decrease in immune recognition (113–115). The quantity of circulating EBV DNA reflects the tumor load and activity, because EBV DNA is released into the blood from apoptotic tumor cells. Elevated EBV DNA copies are correlated to adverse clinical parameters, poor response to treatment and inferior clinical outcomes (116, 117). Most cases show a germline configuration of TCR genes. Monoclonal rearrangements of the TCR genes are found in 10–40% of cases, which may be derived from cytotoxic T cells (100, 107, 118). In the gene expression profiling, extranodal NK/T-cell lymphomas cluster together, regardless of NK cell or γδ T-cell phenotype, and show patterns similar to non-hepatosplenic γδ T-cell lymphomas (119). The most frequent chromosomal aberrations in extranodal NK/T-cell lymphoma are deletion of chromosome 6q at q21–23 region, which contains some tumor suppressor gene such as HACE1, PRMD1, FOXO3, and PTPRK (120–122). This deletion is also commonly found in aggressive NK-cell leukemia, suggesting a genetic link between these two diseases. However, it is unknown whether this genetic alteration develops as primary pathogenetic event or secondary progression-related event (123, 124). Mutation analyses have shown that activating mutations of JAK3 (5–35%), STAT3 (6–27%) and STAT5B (2–6%) are commonly found, suggesting that the JAK-STAT pathway can be a therapeutic target (125–127). Other mutations include the RNA helicase DDX3X, the tumor suppressor gene TP53, the transcription corepressor BCOR, and genes involved in epigenetic pathways (MLL2, ASXL3, ARID1A, and EP300) (127, 128).
Primary EBV-Positive Nodal T and NK-Cell Lymphoma
Primary EBV-positive nodal T and NK-cell lymphoma is a rare type of peripheral T-cell lymphoma that primarily involves the LNs without nasal or other extranodal site involvement. It has been included in the current 2016 WHO classification as a new provisional group within peripheral T-cell lymphoma, not otherwise specified (PTCL, NOS). It affects mainly elderly patients with a median age of 61 years, showing an older age distribution than extranodal NK/T-cell lymphoma (129). There is a male predilection (129, 130).
Clinical Features
Most patients present with generalized lymphadenopathy. Extranodal involvement may be present in a limited number of sites, but the nasal cavity and adjacent structures should not be involved by definition. This disease shows an aggressive clinical course with adverse clinical features including advanced stage, systemic symptoms, and high International Prognostic Index scores. The prognosis is very poor with a median survival <4 months (129, 130).
Morphology and Immunophenotypical Findings
Most cases show relatively monomorphic proliferation of large atypical cells with centroblastic features or diffuse proliferation of pleomorphic cells composed of small, medium, to large atypical cells mimicking Hodgkin cells and Reed-Sternberg cells (Figure 6). Necrosis is occasionally seen with granulomatous or epithelioid histiocytic reactions. Angiodestructive pattern is rare. The immunophenotype of the tumor cells is mostly that of a cytotoxic T cell with expression of CD3, CD8, and cytotoxic molecules. Expression of CD56 (7.5–15%) or CD4 (15–20%) is rarely observed (129, 130). The majority of cases are of αβ T cell phenotype (46–64%), followed by TCR-silent T cells (negative for both TCRβF1 and TCRγ; 21–26%) and other T cells (130). Cases with NK-cell phenotype are found in 6.6–15%. CD30 expression is frequently expressed in TCR-silent cases, which raises the differential diagnosis with anaplastic large cell lymphoma (ALCL).
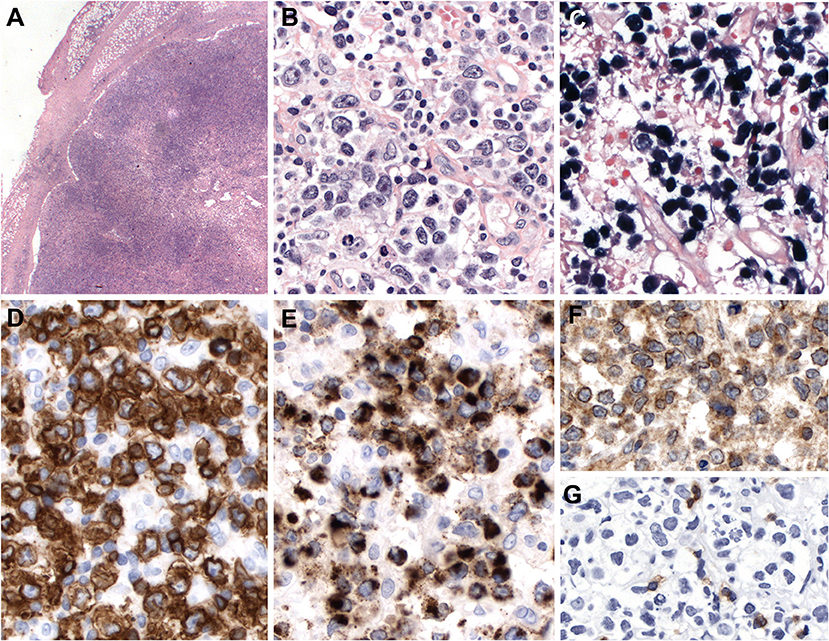
Figure 6. Primary EBV-positive nodal T and NK-cell lymphoma. (A) Lymph node with complete effacement of the architecture by a diffuse infiltrate that extends beyond the capsule and infiltrate the perinodal fat (H&E, 12,5×); (B) Neoplastic cells are large, pleomorphic with irregular nuclei and clear or pale cytoplasm (HE,400×); (C–E) The neoplastic cells EBER, CD56 and TIA-1 positive (in-situ hybridization and immunohistochemistry, 400×); (F) TCR-gamma immunostain demonstrates the gamma-delta derivation of the tumor cells (immunohistochemistry, 400×); (G) TCR alpha-beta (BetaF1) is negative in the tumor cells but positive in the reactive T cells (immunohistochemistry, 400×).
Pathogenesis and Molecular Features
Monoclonal rearrangements of the TCR genes are found in most cases. EBV infection is diffusely detected with high density by EBER ISH, and LMP1 expression indicates EBV latency type 2. In a recent gene expression profiling and cytogenetic analysis, primary EBV-positive nodal T/NK-cell lymphoma showed a distinct molecular signature characterized by upregulation of PD-L1 and T-cell-related genes, including CD2 and CD8, and downregulation of CD56, compared to extranodal NK/T-cell lymphoma (131). A cytogenetic deletion frequently found is loss of chromosome 14q11.2 indicating loss of TCR loci as evidence for the T-cell origin of this lymphoma (131).
Conclusion
EBV-associated T and NK-cell LPDs are a group of diseases including reactive LPDs, as well as overt hematolymphoid malignancies. Some categories have an indolent clinical course with pathological features mimicking a reactive disorder delaying the appropriate diagnosis and treatment. It is crucial to have good clinical information to render the correct diagnosis since some of these disorders have overlapping morphological features. It is recommended to perform EBER ISH in biopsies from extranodal sites with “atypical” infiltrations of T and NK cells regardless of the severity of the infiltration to confirm the presence of EBV infection. EBV+ T and NK cell LPDs frequently express CD30, which might be misleading raising the diagnosis of ALK- ALCL. New genetic studies suggest that the new provisional group of primary nodal T and NK cell lymphomas might be a distinct entity among the EBV+ LPDs.
Author Contributions
WYK performed the literature review and wrote the manuscript. IM-M prepared all photographs and images. FF helped writing the manuscript. LQ-M supervised the work and helped writing the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This paper was written as part of Konkuk University's research support program for its faculty (WYK) on sabbatical leave in 2018. IM-M is supported by funding from the European Union's Horizon 2020 research and innovative Programme under the Marie Sklodowska-Curie grant agreement No. 675712.
References
1. Young LS, Rickinson AB. Epstein-Barr virus: 40 years on. Nat Rev Cancer. (2004) 4:757–68. doi: 10.1038/nrc1452
2. Taylor GS, Long HM, Brooks JM, Rickinson AB, Hislop AD. The immunology of Epstein-Barr virus-induced disease. Annu Rev Immunol. (2015) 33:787–821. doi: 10.1146/annurev-immunol-032414-112326
3. Quintanilla-Martinez L, Ko Y-H, Kimura H, Jaffe ES. EBV-positive T-cell and NK-cell lymphoproliferative diseases of childhood. In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al, editors. Who Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC (2017). p. 355–63.
4. Kimura H, Ito Y, Kawabe S, Gotoh K, Takahashi Y, Kojima S, et al. EBV-associated T/NK-cell lymphoproliferative diseases in nonimmunocompromised hosts: prospective analysis of 108 cases. Blood. (2012) 119:673–86. doi: 10.1182/blood-2011-10-381921
5. Ishii E, Ohga S, Imashuku S, Yasukawa M, Tsuda H, Miura I, et al. Nationwide survey of hemophagocytic lymphohistiocytosis in Japan. Int J Hematol. (2007) 86:58–65. doi: 10.1532/IJH97.07012
6. Verbsky JW, Grossman WJ. Hemophagocytic lymphohistiocytosis: diagnosis, pathophysiology, treatment, and future perspectives. Ann Med. (2006) 38:20–31. doi: 10.1080/07853890500465189
7. Fisman DN. Hemophagocytic syndromes and infection. Emerg Infect Dis. (2000) 6:601–8. doi: 10.3201/eid0606.000608
8. Allen CE, McClain KL. Pathophysiology and epidemiology of hemophagocytic lymphohistiocytosis. Hematol Am Soc Hematol Educ Program. (2015) 2015:177–82. doi: 10.1182/asheducation-2015.1.177
9. Chinn IK, Eckstein OS, Peckham-Gregory EC, Goldberg BR, Forbes LR, Nicholas SK, et al. Genetic and mechanistic diversity in pediatric hemophagocytic lymphohistiocytosis. Blood. (2018) 132:89–100. doi: 10.1182/blood-2017-11-814244
10. Xu XJ, Wang HS, Ju XL, Xiao PF, Xiao Y, Xue HM, et al. Clinical presentation and outcome of pediatric patients with hemophagocytic lymphohistiocytosis in China: a retrospective multicenter study. Pediatr Blood Cancer. (2017) 64. doi: 10.1002/pbc.26264
11. Marsh RA. Epstein-Barr virus and hemophagocytic lymphohistiocytosis. Front Immunol. (2017) 8:1902. doi: 10.3389/fimmu.2017.01902
12. Imashuku S. Clinical features and treatment strategies of Epstein-Barr virus-associated hemophagocytic lymphohistiocytosis. Crit Rev Oncol Hematol. (2002) 44:259–72. doi: 10.1016/S1040-8428(02)00117-8
13. Ishii E. Hemophagocytic lymphohistiocytosis in children: pathogenesis and treatment. Front Pediatr. (2016) 4:47. doi: 10.3389/fped.2016.00047
14. Henter JI, Horne A, Arico M, Egeler RM, Filipovich AH, Imashuku S, et al. HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. (2007) 48:124–31. doi: 10.1002/pbc.21039
15. Min KW, Jung HY, Han HS, Hwang TS, Kim SY, Kim WS, et al. Ileal mass-like lesion induced by Epstein-Barr virus-associated hemophagocytic lymphohistiocytosis in a patient with aplastic anemia. APMIS. (2015) 123:81–6. doi: 10.1111/apm.12308
16. Kasahara Y, Yachie A. Cell type specific infection of Epstein-Barr virus (EBV) in EBV-associated hemophagocytic lymphohistiocytosis and chronic active EBV infection. Crit Rev Oncol Hematol. (2002) 44:283–94. doi: 10.1016/S1040-8428(02)00119-1
17. Tabiasco J, Vercellone A, Meggetto F, Hudrisier D, Brousset P, Fournie JJ. Acquisition of viral receptor by NK cells through immunological synapse. J Immunol. (2003) 170:5993–8. doi: 10.4049/jimmunol.170.12.5993
18. Matsuda K, Nakazawa Y, Yanagisawa R, Honda T, Ishii E, Koike K. Detection of T-cell receptor gene rearrangement in children with Epstein-Barr virus-associated hemophagocytic lymphohistiocytosis using the BIOMED-2 multiplex polymerase chain reaction combined with GeneScan analysis. Clin Chim Acta. (2011) 412:1554–8. doi: 10.1016/j.cca.2011.04.036
19. Feldmann J, Callebaut I, Raposo G, Certain S, Bacq D, Dumont C, et al. Munc13–4 is essential for cytolytic granules fusion and is mutated in a form of familial hemophagocytic lymphohistiocytosis (FHL3). Cell. (2003) 115:461–73. doi: 10.1016/S0092-8674(03)00855-9
20. Stepp SE, Dufourcq-Lagelouse R, Le Deist F, Bhawan S, Certain S, Mathew PA, et al. Perforin gene defects in familial hemophagocytic lymphohistiocytosis. Science. (1999) 286:1957–9. doi: 10.1126/science.286.5446.1957
21. zur Stadt U, Schmidt S, Kasper B, Beutel K, Diler AS, Henter JI, et al. Linkage of familial hemophagocytic lymphohistiocytosis (FHL) type-4 to chromosome 6q24 and identification of mutations in syntaxin 11. Hum Mol Genet. (2005) 14:827–34. doi: 10.1093/hmg/ddi076
22. zur Stadt U, Rohr J, Seifert W, Koch F, Grieve S, Pagel J, et al. Familial hemophagocytic lymphohistiocytosis type 5 (FHL-5) is caused by mutations in Munc18–2 and impaired binding to syntaxin 11. Am J Hum Genet. (2009) 85:482–92. doi: 10.1016/j.ajhg.2009.09.005
23. Bryceson YT, Rudd E, Zheng C, Edner J, Ma D, Wood SM, et al. Defective cytotoxic lymphocyte degranulation in syntaxin-11 deficient familial hemophagocytic lymphohistiocytosis 4 (FHL4) patients. Blood. (2007) 110:1906–15. doi: 10.1182/blood-2007-02-074468
24. Voskoboinik I, Dunstone MA, Baran K, Whisstock JC, Trapani JA. Perforin: structure, function, and role in human immunopathology. Immunol Rev. (2010) 235:35–54. doi: 10.1111/j.0105-2896.2010.00896.x
25. Meazza R, Falco M, Marcenaro S, Loiacono F, Canevali P, Bellora F, et al. Inhibitory 2B4 contributes to NK cell education and immunological derangements in XLP1 patients. Eur J Immunol. (2017) 47:1051–61. doi: 10.1002/eji.201646885
26. Cannons JL, Tangye SG, Schwartzberg PL. SLAM family receptors and SAP adaptors in immunity. Annu Rev Immunol. (2011) 29:665–705. doi: 10.1146/annurev-immunol-030409-101302
27. Rigaud S, Fondaneche MC, Lambert N, Pasquier B, Mateo V, Soulas P, et al. XIAP deficiency in humans causes an X-linked lymphoproliferative syndrome. Nature. (2006) 444:110–4. doi: 10.1038/nature05257
28. Marsh RA, Madden L, Kitchen BJ, Mody R, McClimon B, Jordan MB, et al. XIAP deficiency: a unique primary immunodeficiency best classified as X-linked familial hemophagocytic lymphohistiocytosis and not as X-linked lymphoproliferative disease. Blood. (2010) 116:1079–82. doi: 10.1182/blood-2010-01-256099
29. Yabal M, Muller N, Adler H, Knies N, Gross CJ, Damgaard RB, et al. XIAP restricts TNF- and RIP3-dependent cell death and inflammasome activation. Cell Rep. (2014) 7:1796–808. doi: 10.1016/j.celrep.2014.05.008
30. Damgaard RB, Fiil BK, Speckmann C, Yabal M, zur Stadt U, Bekker-Jensen S, et al. Disease-causing mutations in the XIAP BIR2 domain impair NOD2-dependent immune signalling. EMBO Mol Med. (2013) 5:1278–95. doi: 10.1002/emmm.201303090
31. Straus SE. The chronic mononucleosis syndrome. J Infect Dis. (1988) 157:405–12. doi: 10.1093/infdis/157.3.405
32. Jones JF, Straus SE. Chronic Epstein-Barr virus infection. Annu Rev Med. (1987) 38:195–209. doi: 10.1146/annurev.me.38.020187.001211
33. Kimura H, Hoshino Y, Kanegane H, Tsuge I, Okamura T, Kawa K, et al. Clinical and virologic characteristics of chronic active Epstein-Barr virus infection. Blood. (2001) 98:280–6. doi: 10.1182/blood.V98.2.280
34. Kimura H. Pathogenesis of chronic active Epstein-Barr virus infection: is this an infectious disease, lymphoproliferative disorder, or immunodeficiency? Rev Med Virol. (2006) 16:251–61. doi: 10.1002/rmv.505
35. Hong M, Ko YH, Yoo KH, Koo HH, Kim SJ, Kim WS, et al. EBV-positive T/NK-cell lymphoproliferative disease of childhood. Korean J Pathol. (2013) 47:137–47. doi: 10.4132/KoreanJPathol.2013.47.2.137
36. Wang RC, Chang ST, Hsieh YC, Huang WT, Hsu JD, Tseng CE, et al. Spectrum of Epstein-Barr virus-associated T-cell lymphoproliferative disorder in adolescents and young adults in Taiwan. Int J Clin Exp Pathol. (2014) 7:2430–7.
37. Roth DE, Jones A, Smith L, Lai R, Preiksaitis J, Robinson J. Severe chronic active Epstein-Barr virus infection mimicking steroid-dependent inflammatory bowel disease. Pediatr Infect Dis J. (2005) 24:261–4. doi: 10.1097/01.inf.0000154335.48682.af
38. Isobe Y, Aritaka N, Setoguchi Y, Ito Y, Kimura H, Hamano Y, et al. T/NK cell type chronic active Epstein-Barr virus disease in adults: an underlying condition for Epstein-Barr virus-associated T/NK-cell lymphoma. J Clin Pathol. (2012) 65:278–82. doi: 10.1136/jclinpath-2011-200523
39. Kimura H, Morishima T, Kanegane H, Ohga S, Hoshino Y, Maeda A, et al. Prognostic factors for chronic active Epstein-Barr virus infection. J Infect Dis. (2003) 187:527–33. doi: 10.1086/367988
40. Tosato G, Straus S, Henle W, Pike SE, Blaese RM. Characteristic T cell dysfunction in patients with chronic active Epstein-Barr virus infection (chronic infectious mononucleosis). J Immunol. (1985) 134:3082–8.
41. Kimura H, Hoshino Y, Hara S, Sugaya N, Kawada J, Shibata Y, et al. Differences between T cell-type and natural killer cell-type chronic active Epstein-Barr virus infection. J Infect Dis. (2005) 191:531–9. doi: 10.1086/427239
42. Cohen JI, Jaffe ES, Dale JK, Pittaluga S, Heslop HE, Rooney CM, et al. Characterization and treatment of chronic active Epstein-Barr virus disease: a 28-year experience in the United States. Blood. (2011) 117:5835–49. doi: 10.1182/blood-2010-11-316745
43. Tsuge I, Morishima T, Kimura H, Kuzushima K, Matsuoka H. Impaired cytotoxic T lymphocyte response to Epstein-Barr virus-infected NK cells in patients with severe chronic active EBV infection. J Med Virol. (2001) 64:141–8. doi: 10.1002/jmv.1029
44. Sugaya N, Kimura H, Hara S, Hoshino Y, Kojima S, Morishima T, et al. Quantitative analysis of Epstein-Barr virus (EBV)-specific CD8+ T cells in patients with chronic active EBV infection. J Infect Dis. (2004) 190:985–8. doi: 10.1086/423285
45. Cohen JI, Kimura H, Nakamura S, Ko YH, Jaffe ES. Epstein-Barr virus-associated lymphoproliferative disease in non-immunocompromised hosts: a status report and summary of an international meeting, 8–9 September 2008. Ann Oncol. (2009) 20:1472–82. doi: 10.1093/annonc/mdp064
46. Ohshima K, Kimura H, Yoshino T, Kim CW, Ko YH, Lee SS, et al. Proposed categorization of pathological states of EBV-associated T/natural killer-cell lymphoproliferative disorder (LPD) in children and young adults: overlap with chronic active EBV infection and infantile fulminant EBV T-LPD. Pathol Int. (2008) 58:209–17. doi: 10.1111/j.1440-1827.2008.02213.x
47. Gupta G, Man I, Kemmett D. Hydroa vacciniforme: a clinical and follow-up study of 17 cases. J Am Acad Dermatol. (2000) 42(2 Pt. 1):208–13. doi: 10.1016/S0190-9622(00)90127-0
48. Ruiz-Maldonado R, Parrilla FM, Orozco-Covarrubias ML, Ridaura C, Tamayo Sanchez L, Duran McKinster C. Edematous, scarring vasculitic panniculitis: a new multisystemic disease with malignant potential. J Am Acad Dermatol. (1995) 32:37–44. doi: 10.1016/0190-9622(95)90181-7
49. Barrionuevo C, Anderson VM, Zevallos-Giampietri E, Zaharia M, Misad O, Bravo F, et al. Hydroa-like cutaneous T-cell lymphoma: a clinicopathologic and molecular genetic study of 16 pediatric cases from Peru. Appl Immunohistochem Mol Morphol. (2002) 10:7–14. doi: 10.1097/00129039-200203000-00002
50. Iwatsuki K, Xu Z, Takata M, Iguchi M, Ohtsuka M, Akiba H, et al. The association of latent Epstein-Barr virus infection with hydroa vacciniforme. Br J Dermatol. (1999) 140:715–21. doi: 10.1046/j.1365-2133.1999.02777.x
51. Iwatsuki K, Ohtsuka M, Akiba H, Kaneko F. Atypical hydroa vacciniforme in childhood: from a smoldering stage to Epstein-Barr virus-associated lymphoid malignancy. J Am Acad Dermatol. (1999) 40(2 Pt. 1):283–4. doi: 10.1016/S0190-9622(99)70210-0
52. Magana M, Sangueza P, Gil-Beristain J, Sanchez-Sosa S, Salgado A, Ramon G, et al. Angiocentric cutaneous T-cell lymphoma of childhood (hydroa-like lymphoma): a distinctive type of cutaneous T-cell lymphoma. J Am Acad Dermatol. (1998) 38:574–9. doi: 10.1016/S0190-9622(98)70120-3
53. Cho KH, Lee SH, Kim CW, Jeon YK, Kwon IH, Cho YJ, et al. Epstein-Barr virus-associated lymphoproliferative lesions presenting as a hydroa vacciniforme-like eruption: an analysis of six cases. Br J Dermatol. (2004) 151:372–80. doi: 10.1111/j.1365-2133.2004.06038.x
54. Iwatsuki K, Satoh M, Yamamoto T, Oono T, Morizane S, Ohtsuka M, et al. Pathogenic link between hydroa vacciniforme and Epstein-Barr virus-associated hematologic disorders. Arch Dermatol. (2006) 142:587–95. doi: 10.1001/archderm.142.5.587
55. Ko Y-H. Epstein-Barr virus-positive T/NK-cell lymphoproliferative diseases in children and adolescents. Precis Future Med. (2018) 2:1–7. doi: 10.23838/pfm.2017.00198
56. Quintanilla-Martinez L, Ridaura C, Nagl F, Saez-de-Ocariz M, Duran-McKinster C, Ruiz-Maldonado R, et al. Hydroa vacciniforme-like lymphoma: a chronic EBV+ lymphoproliferative disorder with risk to develop a systemic lymphoma. Blood. (2013) 122:3101–10. doi: 10.1182/blood-2013-05-502203
57. Rodriguez-Pinilla SM, Barrionuevo C, Garcia J, Martinez MT, Pajares R, Montes-Moreno S, et al. EBV-associated cutaneous NK/T-cell lymphoma: review of a series of 14 cases from peru in children and young adults. Am J Surg Pathol. (2010) 34:1773–82. doi: 10.1097/PAS.0b013e3181fbb4fd
58. Hirai Y, Yamamoto T, Kimura H, Ito Y, Tsuji K, Miyake T, et al. Hydroa vacciniforme is associated with increased numbers of Epstein-Barr virus-infected gammadeltaT cells. J Invest Dermatol. (2012) 132:1401–8. doi: 10.1038/jid.2011.461
59. Wada T, Toga A, Sakakibara Y, Toma T, Hasegawa M, Takehara K, et al. Clonal expansion of Epstein-Barr virus (EBV)-infected gammadelta T cells in patients with chronic active EBV disease and hydroa vacciniforme-like eruptions. Int J Hematol. (2012) 96:443–9. doi: 10.1007/s12185-012-1156-0
60. Iwata S, Wada K, Tobita S, Gotoh K, Ito Y, Demachi-Okamura A, et al. Quantitative analysis of Epstein-Barr virus (EBV)-related gene expression in patients with chronic active EBV infection. J Gen Virol. (2010) 91 (Pt. 1):42–50. doi: 10.1099/vir.0.013482-0
61. Ishihara S, Okada S, Wakiguchi H, Kurashige T, Hirai K, Kawa-Ha K. Clonal lymphoproliferation following chronic active Epstein-Barr virus infection and hypersensitivity to mosquito bites. Am J Hematol. (1997) 54:276–81. doi: 10.1002/(SICI)1096-8652(199704)54:4<276::AID-AJH3>3.0.CO;2-S
62. Ishihara S, Ohshima K, Tokura Y, Yabuta R, Imaishi H, Wakiguchi H, et al. Hypersensitivity to mosquito bites conceals clonal lymphoproliferation of Epstein-Barr viral DNA-positive natural killer cells. Jpn J Cancer Res. (1997) 88:82–7. doi: 10.1111/j.1349-7006.1997.tb00305.x
63. Tokura Y, Tamura Y, Takigawa M, Koide M, Satoh T, Sakamoto T, et al. Severe hypersensitivity to mosquito bites associated with natural killer cell lymphocytosis. Arch Dermatol. (1990) 126:362–8. doi: 10.1001/archderm.1990.01670270094016
64. Cho JH, Kim HS, Ko YH, Park CS. Epstein-Barr virus infected natural killer cell lymphoma in a patient with hypersensitivity to mosquito bite. J Infect. (2006) 52:e173–6. doi: 10.1016/j.jinf.2005.08.035
65. Fan PC, Chang HN. Hypersensitivity to mosquito bite: a case report. Gaoxiong Yi Xue Ke Xue Za Zhi. (1995) 11:420–4.
66. Tokura Y, Ishihara S, Tagawa S, Seo N, Ohshima K, Takigawa M. Hypersensitivity to mosquito bites as the primary clinical manifestation of a juvenile type of Epstein-Barr virus-associated natural killer cell leukemia/lymphoma. J Am Acad Dermatol. (2001) 45:569–78. doi: 10.1067/mjd.2001.114751
67. Asada H, Saito-Katsuragi M, Niizeki H, Yoshioka A, Suguri S, Isonokami M, et al. Mosquito salivary gland extracts induce EBV-infected NK cell oncogenesis via CD4 T cells in patients with hypersensitivity to mosquito bites. J Invest Dermatol. (2005) 125:956–61. doi: 10.1111/j.0022-202X.2005.23915.x
68. Tokura Y, Matsuoka H, Koga C, Asada H, Seo N, Ishihara S, et al. Enhanced T-cell response to mosquito extracts by NK cells in hypersensitivity to mosquito bites associated with EBV infection and NK cell lymphocytosis. Cancer Sci. (2005) 96:519–26. doi: 10.1111/j.1349-7006.2005.00076.x
69. Asada H, Miyagawa S, Sumikawa Y, Yamaguchi Y, Itami S, Suguri S, et al. CD4+ T-lymphocyte-induced Epstein-Barr virus reactivation in a patient with severe hypersensitivity to mosquito bites and Epstein-Barr virus-infected NK cell lymphocytosis. Arch Dermatol. (2003) 139:1601–7. doi: 10.1001/archderm.139.12.1601
70. Kikuta H, Sakiyama Y, Matsumoto S, Oh-Ishi T, Nakano T, Nagashima T, et al. Fatal Epstein-Barr virus-associated hemophagocytic syndrome. Blood. (1993) 82:3259–64.
71. Su IJ, Chen RL, Lin DT, Lin KS, Chen CC. Epstein-Barr virus (EBV) infects T lymphocytes in childhood EBV-associated hemophagocytic syndrome in Taiwan. Am J Pathol. (1994) 144:1219–25.
72. Suzuki K, Ohshima K, Karube K, Suzumiya J, Ohga S, Ishihara S, et al. Clinicopathological states of Epstein-Barr virus-associated T/NK-cell lymphoproliferative disorders (severe chronic active EBV infection) of children and young adults. Int J Oncol. (2004) 24:1165–74. doi: 10.3892/ijo.24.5.1165
73. Quintanilla-Martinez L, Kumar S, Fend F, Reyes E, Teruya-Feldstein J, Kingma DW, et al. Fulminant EBV(+) T-cell lymphoproliferative disorder following acute/chronic EBV infection: a distinct clinicopathologic syndrome. Blood. (2000) 96:443–51.
74. Young KH, Zhang D, Malik JT, Williams EC. Fulminant EBV-driven CD8 T-cell lymphoproliferative disorder following primary acute EBV infection: a unique spectrum of T-cell malignancy. Int J Clin Exp Pathol. (2008) 1:185–97.
75. Ko YH, Chan JK, Quintanilla-Martinez L. Virally associated T-cell and NK-cell neoplasms. In: Jaffe E, Arber D, Campo E, Harris N, Quintanilla-Martinez L, editors. Haematopathology. Philadelphia: Elsvier (2016). p. 565–98.
76. Jones JF, Shurin S, Abramowsky C, Tubbs RR, Sciotto CG, Wahl R, et al. T-cell lymphomas containing Epstein-Barr viral DNA in patients with chronic Epstein-Barr virus infections. N Engl J Med. (1988) 318:733–41. doi: 10.1056/NEJM198803243181203
77. Smith MC, Cohen DN, Greig B, Yenamandra A, Vnencak-Jones C, Thompson MA, et al. The ambiguous boundary between EBV-related hemophagocytic lymphohistiocytosis and systemic EBV-driven T cell lymphoproliferative disorder. Int J Clin Exp Pathol. (2014) 7:5738–49.
78. Imamura N, Kusunoki Y, Kawa-Ha K, Yumura K, Hara J, Oda K, et al. Aggressive natural killer cell leukaemia/lymphoma: report of four cases and review of the literature. Possible existence of a new clinical entity originating from the third lineage of lymphoid cells. Br J Haematol. (1990) 75:49–59. doi: 10.1111/j.1365-2141.1990.tb02615.x
79. Quintanilla-Martinez L, Jaffe ES. Commentary: aggressive NK cell lymphomas: insights into the spectrum of NK cell derived malignancies. Histopathology. (2000) 37:372–4. doi: 10.1046/j.1365-2559.2000.01029.x
80. Ruskova A, Thula R, Chan G. Aggressive Natural Killer-Cell Leukemia: report of five cases and review of the literature. Leuk Lymphoma. (2004) 45:2427–38. doi: 10.1080/10428190400004513
81. Kato K, Ohshima K, Ishihara S, Anzai K, Suzumiya J, Kikuchi M. Elevated serum soluble Fas ligand in natural killer cell proliferative disorders. Br J Haematol. (1998) 103:1164–6. doi: 10.1046/j.1365-2141.1998.01095.x
82. Suzuki R, Suzumiya J, Nakamura S, Aoki S, Notoya A, Ozaki S, et al. Aggressive natural killer-cell leukemia revisited: large granular lymphocyte leukemia of cytotoxic NK cells. Leukemia. (2004) 18:763–70. doi: 10.1038/sj.leu.2403262
83. Hasserjian RP, Harris NL. NK-cell lymphomas and leukemias: a spectrum of tumors with variable manifestations and immunophenotype. Am J Clin Pathol. (2007) 127:860–8. doi: 10.1309/2F39NX1AL3L54WU8
84. Oshimi K, Yamada O, Kaneko T, Nishinarita S, Iizuka Y, Urabe A, et al. Laboratory findings and clinical courses of 33 patients with granular lymphocyte-proliferative disorders. Leukemia. (1993) 7:782–8.
85. Soler J, Bordes R, Ortuno F, Montagud M, Martorell J, Pons C, et al. Aggressive natural killer cell leukaemia/lymphoma in two patients with lethal midline granuloma. Br J Haematol. (1994) 86:659–62. doi: 10.1111/j.1365-2141.1994.tb04804.x
86. Zhang Q, Jing W, Ouyang J, Zeng H, George SK, Liu Z. Six cases of aggressive natural killer-cell leukemia in a Chinese population. Int J Clin Exp Pathol. (2014) 7:3423–31.
87. Gao J, Behdad A, Ji P, Wolniak KL, Frankfurt O, Chen YH. EBV-negative aggressive NK-cell leukemia/lymphoma: a clinical and pathological study from a single institution. Mod Pathol. (2017) 30:1100–15. doi: 10.1038/modpathol.2017.37
88. Chan JK, Sin VC, Wong KF, Ng CS, Tsang WY, Chan CH, et al. Nonnasal lymphoma expressing the natural killer cell marker CD56: a clinicopathologic study of 49 cases of an uncommon aggressive neoplasm. Blood. (1997) 89:4501–13.
89. Hart DN, Baker BW, Inglis MJ, Nimmo JC, Starling GC, Deacon E, et al. Epstein-Barr viral DNA in acute large granular lymphocyte (natural killer) leukemic cells. Blood. (1992) 79:2116–23.
90. Kawa-Ha K, Ishihara S, Ninomiya T, Yumura-Yagi K, Hara J, Murayama F, et al. CD3-negative lymphoproliferative disease of granular lymphocytes containing Epstein-Barr viral DNA. J Clin Invest. (1989) 84:51–5. doi: 10.1172/JCI114168
91. Nicolae A, Ganapathi KA, Pham TH, Xi L, Torres-Cabala CA, Nanaji NM, et al. EBV-negative aggressive NK-cell leukemia/lymphoma: clinical, pathologic, and genetic features. Am J Surg Pathol. (2017) 41:67–74. doi: 10.1097/PAS.0000000000000735
92. Park S, Ko YH. Epstein-Barr virus-associated T/natural killer-cell lymphoproliferative disorders. J Dermatol. (2014) 41:29–39. doi: 10.1111/1346-8138.12322
93. Nakashima Y, Tagawa H, Suzuki R, Karnan S, Karube K, Ohshima K, et al. Genome-wide array-based comparative genomic hybridization of natural killer cell lymphoma/leukemia: different genomic alteration patterns of aggressive NK-cell leukemia and extranodal Nk/T-cell lymphoma, nasal type. Genes Chromosomes Cancer. (2005) 44:247–55. doi: 10.1002/gcc.20245
94. Gao LM, Zhao S, Liu WP, Zhang WY, Li GD, Kucuk C, et al. Clinicopathologic characterization of aggressive natural killer cell leukemia involving different tissue sites. Am J Surg Pathol. (2016) 40:836–46. doi: 10.1097/PAS.0000000000000634
95. Chan JKC, Quintanilla-Martinez L, Ferry JA. Extranodal NK/T-cell lymphoma, nasal type. In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al, editors. Who Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC (2017). p. 368–71.
96. Ng CS, Chan JK, Lo ST, Poon YF. Immunophenotypic analysis of non-Hodgkin's lymphomas in Chinese. a study of 75 cases in Hong Kong. Pathology. (1986) 18:419–25. doi: 10.3109/00313028609087562
97. Aozasa K, Ohsawa M, Tajima K, Sasaki R, Maeda H, Matsunaga T, et al. Nation-wide study of lethal mid-line granuloma in Japan: frequencies of wegener's granulomatosis, polymorphic reticulosis, malignant lymphoma and other related conditions. Int J Cancer. (1989) 44:63–6. doi: 10.1002/ijc.2910440112
98. Kim JM, Ko YH, Lee SS, Huh J, Kang CS, Kim CW, et al. WHO Classification of malignant lymphomas in Korea: report of the third nationwide study. J Pathol Transl Med. (2011) 45:254–60. doi: 10.4132/KoreanJPathol.2011.45.3.254
99. Quintanilla-Martinez L, Franklin JL, Guerrero I, Krenacs L, Naresh KN, Rama-Rao C, et al. Histological and immunophenotypic profile of nasal NK/T cell lymphomas from Peru: high prevalence of p53 overexpression. Hum Pathol. (1999) 30:849–55. doi: 10.1016/S0046-8177(99)90147-8
100. Au WY, Weisenburger DD, Intragumtornchai T, Nakamura S, Kim WS, Sng I, et al. Clinical differences between nasal and extranasal natural killer/T-cell lymphoma: a study of 136 cases from the international peripheral T-cell lymphoma project. Blood. (2009) 113:3931–7. doi: 10.1182/blood-2008-10-185256
101. Kim TM, Heo DS. Extranodal NK / T-cell lymphoma, nasal type: new staging system and treatment strategies. Cancer Sci. (2009) 100:2242–8. doi: 10.1111/j.1349-7006.2009.01319.x
102. Lim ST, Hee SW, Quek R, Lim LC, Yap SP, Loong EL, et al. Comparative analysis of extra-nodal NK/T-cell lymphoma and peripheral T-cell lymphoma: significant differences in clinical characteristics and prognosis. Eur J Haematol. (2008) 80:55–60. doi: 10.1111/j.1600-0609.2007.00978.x
103. Li P, Jiang L, Zhang X, Liu J, Wang H. CD30 expression is a novel prognostic indicator in extranodal natural killer/T-cell lymphoma, nasal type. BMC Cancer. (2014) 14:890. doi: 10.1186/1471-2407-14-890
104. Kim WY, Nam SJ, Kim S, Kim TM, Heo DS, Kim CW, et al. Prognostic implications of CD30 expression in extranodal natural killer/T-cell lymphoma according to treatment modalities. Leuk Lymphoma. (2015) 56:1778–86. doi: 10.3109/10428194.2014.974048
105. Kuo TT, Shih LY, Tsang NM. Nasal NK/T cell lymphoma in Taiwan: a clinicopathologic study of 22 cases, with analysis of histologic subtypes, Epstein-Barr virus LMP-1 gene association, and treatment modalities. Int J Surg Pathol. (2004) 12:375–87. doi: 10.1177/106689690401200410
106. Li S, Feng X, Li T, Zhang S, Zuo Z, Lin P, et al. Extranodal NK/T-cell lymphoma, nasal type: a report of 73 cases at MD anderson cancer center. Am J Surg Pathol. (2013) 37:14–23. doi: 10.1097/PAS.0b013e31826731b5
107. Jhuang JY, Chang ST, Weng SF, Pan ST, Chu PY, Hsieh PP, et al. Extranodal natural killer/T-cell lymphoma, nasal type in Taiwan: a relatively higher frequency of T-cell lineage and poor survival for extranasal tumors. Hum Pathol. (2015) 46:313–21. doi: 10.1016/j.humpath.2014.11.008
108. Kim WY, Jeon YK, Kim TM, Kim JE, Kim YA, Lee SH, et al. Increased quantity of tumor-infiltrating FOXP3-positive regulatory T cells is an independent predictor for improved clinical outcome in extranodal NK/T-cell lymphoma. Ann Oncol. (2009) 20:1688–96. doi: 10.1093/annonc/mdp056
109. Kim WY, Jung HY, Nam SJ, Kim TM, Heo DS, Kim CW, et al. Expression of programmed cell death ligand 1 (PD-L1) in advanced stage EBV-associated extranodal NK/T cell lymphoma is associated with better prognosis. Virchows Arch. (2016) 469:581–90. doi: 10.1007/s00428-016-2011-0
110. Gualco G, Domeny-Duarte P, Chioato L, Barber G, Natkunam Y, Bacchi CE. Clinicopathologic and molecular features of 122 Brazilian cases of nodal and extranodal NK/T-cell lymphoma, nasal type, with EBV subtyping analysis. Am J Surg Pathol. (2011) 35:1195–203. doi: 10.1097/PAS.0b013e31821ec4b5
111. Chiang AK, Wong KY, Liang AC, Srivastava G. Comparative analysis of Epstein-Barr virus gene polymorphisms in nasal T/NK-cell lymphomas and normal nasal tissues: implications on virus strain selection in malignancy. Int J Cancer. (1999) 80:356–64. doi: 10.1002/(SICI)1097-0215(19990129)80:3<356::AID-IJC4>3.0.CO;2-D
112. Garcia-Cosio M, Santon A, Mendez MC, Rivas C, Martin C, Bellas C. Nasopharyngeal/nasal type T/NK lymphomas: analysis of 14 cases and review of the literature. Tumori. (2003) 89:278–84. doi: 10.1177/030089160308900309
113. Nagamine M, Takahara M, Kishibe K, Nagato T, Ishii H, Bandoh N, et al. Sequence variations of Epstein-Barr virus LMP1 gene in nasal NK/T-cell lymphoma. Virus Genes. (2007) 34:47–54. doi: 10.1007/s11262-006-0008-5
114. Dirnhofer S, Angeles-Angeles A, Ortiz-Hidalgo C, Reyes E, Gredler E, Krugmann J, et al. High prevalence of a 30-base pair deletion in the Epstein-Barr virus (EBV) latent membrane protein 1 gene and of strain type B EBV in Mexican classical Hodgkin's disease and reactive lymphoid tissue. Human Pathol. (1999) 30:781–7. doi: 10.1016/S0046-8177(99)90138-7
115. Kim JE, Kim YA, Jeon YK, Park SS, Heo DS, Kim CW. Comparative analysis of NK/T-cell lymphoma and peripheral T-cell lymphoma in Korea: clinicopathological correlations and analysis of EBV strain type and 30-bp deletion variant LMP1. Pathol Int. (2003) 53:735–43. doi: 10.1046/j.1320-5463.2003.01552.x
116. Kwong YL, Pang AW, Leung AY, Chim CS, Tse E. Quantification of circulating Epstein-Barr virus DNA in NK/T-cell lymphoma treated with the SMILE protocol: diagnostic and prognostic significance. Leukemia. (2014) 28:865–70. doi: 10.1038/leu.2013.212
117. Au WY, Pang A, Choy C, Chim CS, Kwong YL. Quantification of circulating Epstein-Barr virus (EBV) DNA in the diagnosis and monitoring of natural killer cell and EBV-positive lymphomas in immunocompetent patients. Blood. (2004) 104:243–9. doi: 10.1182/blood-2003-12-4197
118. Hong M, Lee T, Young Kang S, Kim SJ, Kim W, Ko YH. Nasal-type NK/T-cell lymphomas are more frequently T rather than NK lineage based on T-cell receptor gene, RNA, and protein studies: lineage does not predict clinical behavior. Mod Pathol. (2016) 29:430–43. doi: 10.1038/modpathol.2016.47
119. Iqbal J, Weisenburger DD, Chowdhury A, Tsai MY, Srivastava G, Greiner TC, et al. Natural killer cell lymphoma shares strikingly similar molecular features with a group of non-hepatosplenic gammadelta T-cell lymphoma and is highly sensitive to a novel aurora kinase A inhibitor in vitro. Leukemia. (2011) 25:348–58. doi: 10.1038/leu.2010.255
120. Wong KF, Zhang YM, Chan JK. Cytogenetic abnormalities in natural killer cell lymphoma/leukaemia–is there a consistent pattern? Leuk Lymphoma. (1999) 34:241–50. doi: 10.3109/10428199909050949
121. Wong KF, Chan JK, Kwong YL. Identification of del(6)(q21q25) as a recurring chromosomal abnormality in putative NK cell lymphoma/leukaemia. Br J Haematol. (1997) 98:922–6. doi: 10.1046/j.1365-2141.1997.3223139.x
122. Tse E, Kwong YL. The diagnosis and management of NK/T-cell lymphomas. J Hematol Oncol. (2017) 10:85. doi: 10.1186/s13045-017-0452-9
123. Siu LL, Chan V, Chan JK, Wong KF, Liang R, Kwong YL. Consistent patterns of allelic loss in natural killer cell lymphoma. Am J Pathol. (2000) 157:1803–9. doi: 10.1016/S0002-9440(10)64818-3
124. Siu LL, Wong KF, Chan JK, Kwong YL. Comparative genomic hybridization analysis of natural killer cell lymphoma/leukemia. Recognition of consistent patterns of genetic alterations. Am J Pathol. (1999) 155:1419–25. doi: 10.1016/S0002-9440(10)65454-5
125. Koo GC, Tan SY, Tang T, Poon SL, Allen GE, Tan L, et al. Janus kinase 3-activating mutations identified in natural killer/T-cell lymphoma. Cancer Discov. (2012) 2:591–7. doi: 10.1158/2159-8290.CD-12-0028
126. Kucuk C, Jiang B, Hu X, Zhang W, Chan JK, Xiao W, et al. Activating mutations of STAT5B and STAT3 in lymphomas derived from gammadelta-T or NK cells. Nat Commun. (2015) 6:6025. doi: 10.1038/ncomms7025
127. Lee S, Park HY, Kang SY, Kim SJ, Hwang J, Lee S, et al. Genetic alterations of JAK/STAT cascade and histone modification in extranodal NK/T-cell lymphoma nasal type. Oncotarget. (2015) 6:17764–76. doi: 10.18632/oncotarget.3776
128. Jiang L, Gu ZH, Yan ZX, Zhao X, Xie YY, Zhang ZG, et al. Exome sequencing identifies somatic mutations of DDX3X in natural killer/T-cell lymphoma. Nat Genet. (2015) 47:1061–6. doi: 10.1038/ng.3358
129. Kato S, Asano N, Miyata-Takata T, Takata K, Elsayed AA, Satou A, et al. T-cell receptor (TCR) phenotype of nodal Epstein-Barr virus (EBV)-positive cytotoxic T-cell lymphoma (CTL): a clinicopathologic study of 39 cases. Am J Surg Pathol. (2015) 39:462–71. doi: 10.1097/PAS.0000000000000323
130. Jeon YK, Kim JH, Sung JY, Han JH, Ko YH Hematopathology study group of the Korean Society of P. Epstein-Barr virus-positive nodal T/NK-cell lymphoma: an analysis of 15 cases with distinct clinicopathological features. Hum Pathol. (2015) 46:981–90. doi: 10.1016/j.humpath.2015.03.002
Keywords: EBV, lymphoproliferations, hemophagocytic lymphohistiocytosis, chronic active EBV infection, systemic EBV positive T-cell lymphoma, aggressive NK-cell leukemia, extranodal NK/T-cell lymphoma, primary EBV nodal T and NK-cell lymphoma
Citation: Kim WY, Montes-Mojarro IA, Fend F and Quintanilla-Martinez L (2019) Epstein-Barr Virus-Associated T and NK-Cell Lymphoproliferative Diseases. Front. Pediatr. 7:71. doi: 10.3389/fped.2019.00071
Received: 01 November 2018; Accepted: 21 February 2019;
Published: 15 March 2019.
Edited by:
Shigeyoshi Fujiwara, National Center for Child Health and Development (NCCHD), JapanReviewed by:
Fabian Hauck, Ludwig Maximilian University of Munich, GermanySiok Bian Ng, National University of Singapore, Singapore
Copyright © 2019 Kim, Montes-Mojarro, Fend and Quintanilla-Martinez. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Leticia Quintanilla-Martinez, TGV0aWNpYS5RdWludGFuaWxsYS1GZW5kQG1lZC51bmktdHVlYmluZ2VuLmRl
 Wook Youn Kim
Wook Youn Kim Ivonne A. Montes-Mojarro
Ivonne A. Montes-Mojarro Falko Fend1
Falko Fend1 Leticia Quintanilla-Martinez
Leticia Quintanilla-Martinez