- 1Department of Translational Medical Sciences, Federico II University, Naples, Italy
- 2Department of Pediatrics, Federico II University, Naples, Italy
Asthma is the most common chronic disease in children. As suggested by international guidelines, the main goals of asthma treatment are symptoms control and lung function preservation, through a stepwise and control-based approach. The first line therapy based on inhaled corticosteroids may fail to reach control in more than one third of patients, especially adolescents, and in these lung function and quality of life may progressively worsen. Treatment with omalizumab, the first anti-immunoglobulin E recombinant humanized monoclonal antibody, has been definitely approved in pediatric uncontrolled asthma. In this review, we discuss the mechanisms and potential roles of emerging therapies for pediatric severe asthma. Novel biologic drugs (i.e., dupilumab, mepolizumab, reslizumab, and benralizumab) seem to be promising in reducing annual exacerbation rates and steroid-use in glucocorticoid-dependent cases, but available data are few and limited to adolescents and adults. Evidences on the use of the muscarinic antagonist tiotropium as controller medication in pediatric settings are progressively growing, sustaining an application as asthma maintenance treatment in children aged >6 years and in preschool children with persistent asthmatic symptoms, but well powered trials are needed to confirm its safety and efficacy. New inhaled corticosteroids (i.e., ciclesonide and mometasone) are effective as once-daily controller therapy, but long-term studies in the different pediatric ages are needed to compare effectiveness and safety to usual treatments. At present, the role of macrolides in pediatric severe asthma is controversial and their administration is not recommended routinely, but may be considered in children with neutrophilic asthma for reducing daily oral steroids administration and improving lung function. Despite the availability of several novel therapeutic strategies for uncontrolled asthma, future trials targeted at specific pediatric age subgroups are needed to support evidences of safety and efficacy also in children.
Introduction
Asthma is the most common chronic condition of childhood, and its management represents part of the daily activity of most professionals who deal with pediatric care. At any age, the main purposes of asthma treatment are to reduce exacerbations and to limit the progressive loss of lung function, thus decreasing the use of health resources and improving quality of life. In few words, asthma therapy aims to achieve good symptom control (1).
National and international guidelines on asthma management of adults and children agree in indicating inhaled corticosteroids (ICS) as the most effective and safe medications, which may be used alone or associated with other controller therapies, in a stepwise, control-based approach (2–4).
Nevertheless, despite this approach, in more than one third of all patients with asthma the persistence of clinical symptoms, often associated with overt lung function abnormalities, indicate poor control, with the proportion increasing to >50% in adolescents (5, 6). This variability in treatment response is partly genetically determined. Pharmacogenomic studies, through the characterization of genes relevant in asthma treatment response, are paving the way toward the personalization of therapy. This would entail a dramatic change in asthma treatment from the actual “one size fits all” approach to the so called “precision medicine,” that is the tailoring of healthcare to the individual through the identification of clinical, biological, or genetic markers (7). Therefore, the interest in the development of new drugs and in the application of therapies currently used in other conditions for pursuing asthma control is high. This is particularly true in the pediatric setting, due to the lack of adequately designed trials including children. Indeed, even though results from adult studies may be sometimes translated to adolescents, this is not the case for younger asthmatic patients for whom evidence on the safety and efficacy of new treatments is still sparse.
The present review will go through the new therapeutic approaches to pediatric asthma, highlighting available evidence on their efficacy, the related risks, and the areas of uncertainty that in many cases still limit their regular application in the clinical practice.
Biologics
Over the last decades, the development of monoclonal antibodies specifically designed to bind determined targets has deeply changed the approach to a number of conditions, also affecting children (8). In pediatric respiratory medicine, this novelty has been embodied by omalizumab, a recombinant DNA-derived humanized anti-IgE monoclonal antibody, which is the only biologic drug recommended in children with moderate-to-severe asthma (3, 9). Omalizumab, which was approved by the United States (US) and the European Union in 2003 and 2005, respectively, is able to decrease the quantity of cell-bound IgE, to downregulate the IgE receptors on mast cells, basophils, and dendritic cells, thus preventing mediator release from effector cells (10, 11). At present, its use is licensed as an add-on treatment for patients aged > 6 years with severe persistent allergic asthma and positive skin test or specific IgE to perennial aeroallergens, FEV1 < 80% predicted, frequent daytime symptoms, or nighttime awakenings, and multiple severe asthma exacerbations despite traditional maintenance therapy (12, 13). In comparison to the robust body of literature supporting the efficacy and safety of omalizumab in severe, inadequately controlled adult asthma (14, 15), evidence in children is far more limited. Nevertheless, even though no studies have been published to date in preschoolers, only few well-designed clinical trials have addressed the role of omalizumab in uncontrolled asthma affecting children older than 6 years. A large, multicenter randomized controlled trial (RCT) assessed the efficacy of omalizumab in 419 subjects (mean age 10.9 years) with persistent allergic asthma, and showed that anti-IgE treatment increases the number of symptoms-free days and reduces the number of exacerbations and the need for controller therapies (16). Similar results were achieved by other pediatric studies which have strengthened the role of omalizumab in limiting asthma exacerbations, and to a lesser extent, in improving patients' lung function (17–21). Despite reported cases of anaphylaxis in children (22), which warrant hospital administration, omalizumab is basically safe, whereas high cost represents a more relevant issue.
The scenario of biologics applied to asthma treatment has recently added to the more “consolidated” omalizumab, a number of novel drugs for which evidence of safety and efficacy is still very sparse and generally limited to adult studies. Dupilumab, an anti–interleukin-4 receptor α monoclonal antibody blocking both interleukin-4 and interleukin-13 signaling, is probably the most promising for a future application in pediatric asthma, as shown by two recent trials (23, 24). These large, multicenter RCT have enrolled patients aged >12 years with moderate-to-severe uncontrolled asthma (23) or glucocorticoid-dependent severe asthma (24). In such subjects dupilumab has proven effective in decreasing exacerbations and improving asthma control, also resulting in better lung function. Similar results have been provided for mepolizumab, which has been recently approved for severe eosinophilic asthma in adults and adolescents. This anti-interleukin-5 antibody has been shown to reduce severe asthma exacerbations (25) and need for oral steroids (26), even though evidence for its use in children < 12 years is virtually absent. Mepolizumab is not the only anti-interleukin-5 antibody under investigation for improving asthma control. Reslizumab and benralizumab are anti-interleukin-5 antibodies whose efficacy has been evaluated by few recent studies including patients with asthma aged >12 years (27–31). Both treatments have proven safe and effective in improving asthma control and lung function in selected patients with severe uncontrolled asthma and high blood eosinophil count, but, again, available data are few and limited to adolescents and adults.
Muscarinic Antagonists
The increased cholinergic tone typical of asthma makes muscarinic receptors an obvious target for therapeutic strategies aimed at reducing airway hyperresponsiveness. In children with severe asthma exacerbations, inhaled short-acting antimuscarinic agents, namely ipratropium, are a widely accepted therapeutic option whose efficacy on lung function is well documented (32). Used in addition to nebulized albuterol, ipratropium has proven effective in reducing the risk for hospital admission as well as in improving spirometry in children with asthma exacerbations (33). Less frequent is the use of muscarinic antagonists as controller therapy, even though tiotropium, the most widely used long-acting muscarinic antagonist is mentioned as a possible add-on therapeutic option in step 4 of GINA guidelines for children older than 12 years taking the combined treatment of ICS and long-acting beta2-agonists (LABA), but still reporting inadequate asthma control (3). Inhaled tiotropium (Spiriva Respimat®), first indicated in adult COPD treatment, was shown to improve pulmonary function and respiratory symptoms in adult moderate-to-severe asthma (34, 35). Nevertheless, when added to ICS, tiotropium appears to be slightly less effective in improving quality of life in comparison to the traditional combination ICS/LABA (36). Tiotropium was recently approved by the US Federal Drug Administration (FDA) as an asthma maintenance treatment in children aged >6 years (37), whereas in Europe its use is still limited to adults. However, available evidence in children and adolescents is progressively growing, thus making a future wider application in pediatric asthma likely. In particular, school-aged children with severe symptomatic asthma have shown improvement of lung function and good tolerability and safety when tiotropium was added to medium or high-doses of ICS (38, 39). Furthermore, positive trends in asthma control and FEV1 were also observed in adolescents with moderately severe asthma treated with tiotropium as an add-on drug to ICS and other controllers for 3 months (40, 41). Finally, a recent small RCT showed the potential to reduce asthma exacerbation risk in children aged 1–5 years with persistent asthmatic symptoms, with tolerability similar to that of placebo, although mean daytime asthma symptom scores were not significantly different between groups (42). Despite its debated role within the group of controller drugs in childhood asthma, tiotropium remains an attractive option, both for the possibility of once-daily administration and for its peculiar way of delivery. Indeed, the drug is administered by a device named Respimat® in form of a mist of extremely fine particles (diameter < 6 μm), whose main advantages are the lower speed of delivery, the limited pharyngeal deposition and the enhanced pulmonary deposition in comparison to the common metered dose inhalers. Additional well powered trials are needed to further assess the safety and efficacy of tiotropium especially in young children with uncontrolled asthma symptoms.
New Inhaled Corticosteroids
Inhaled corticosteroids represent the cornerstone of asthma therapy at all ages (3). Despite the long and large experience with traditional molecules such as beclometasone, flunisolide, fluticasone, and budesonide, all with high safety and efficacy profiles, some new ICS have been recently approved, but only few of these are allowed for the pediatric use.
Ciclesonide, licensed from age 4 years in the US and from age 12 years in Europe, is a pro-drug activated by esterases within the lung to form the active compound (des-ciclesonide). The possibility of a single daily administration, and evidence supporting its efficacy in improving asthma control and in reducing airway inflammation make this steroid a valid option as asthma controller therapy (43). Nonetheless, given the lack of relevant differences in the efficacy of ciclesonide vs. fluticasone or budesonide, long-term superiority trials are needed to identify the usefulness and safety of ciclesonide compared to other ICS (44). Similarly, mometasone, which has the same FDA approval as ciclesonide and may be used down to the age of 4 years also in Europe, has proven effective as once-daily controller therapy in several pediatric studies (45), with evidence supporting a significant functional improvement in school-aged children with persistent asthma (46).
Macrolide Antibiotics
Macrolides are widely used antibiotics with both antimicrobial and anti-inflammatory activities (47). Indeed, in addition to their well-known antibiotic effect, there is evidence that macrolides modulate the expression of cellular adhesion molecules, may attenuate inflammatory cell migration and inhibit the respiratory burst in polymorphonuclear cells. Furthermore, macrolides clearly affect neutrophil function, even though the exact mechanisms have not been elucidated (48). For these reasons, macrolides have been initially recommended in diffuse panbronchiolitis (49), cystic fibrosis (50), and non-cystic fibrosis bronchiectasis (51). As airways infection is a possible cause for asthma, macrolides have been supposed to be used as long-term treatment for improving the disease control and reducing the need for steroids (52). Actually, the early troleandomycin—no longer recommended because of adverse effects on liver function tests—was reported to work as “steroid-sparing” drug by reducing the catabolism of steroids, but indeed no steroid reduction was demonstrated (53). Most recently, clarithromycin was found to widely suppress severe, steroid-insensitive allergic airways disease in a mouse model through its anti-inflammatory effects on tumor necrosis factor-α/interleukin-17 immune responses that are largely independent of its antimicrobial effects (54). Indeed, macrolides may reduce airway inflammation either by acting on pro-inflammatory cytokines or by controlling intracellular infection which may trigger and maintain inflammation (55).
At present, the role of macrolides in severe asthma is controversial. In a large multicentre RCT azithromycin did not reduce the rate of severe exacerbations and lower respiratory tract infections in a population of severe asthma adults not including children or adolescents (56). Finally, a systematic review and meta-analysis did not show a benefit of macrolides over placebo on rates of exacerbations, quality of life or participants' need for rescue medications (57).
Looking specifically at the pediatric literature, Chlamydia pneumoniae and Mycoplasma pneumoniae have been suggested to play a role in the pathogenesis of severe asthma (58). Preschool-aged children with frequent, severe exacerbations may benefit from sporadic use of azithromycin (59). Regrettably, very few RCTs investigated the role of macrolides in school-age children or adolescents with asthma, and failed to demonstrate a beneficial effect probably because of the low power of the study (60–62). Actually, the possibility that macrolides work well in pediatric severe asthma is not definitely excluded, yet at present the evidence is quite poor. It should be also kept in mind that an inappropriate use of macrolides unavoidably results in antibiotic resistance that is a major worldwide concern, especially in the pediatric population where the respiratory infection rate is as high as in the elderly (63).
Although an official document and a recent pragmatic review do not recommend the routine use of macrolides for children with severe asthma (9, 64), they may be useful for reducing daily oral steroid administration and improving FEV1 (65), and, for this reason, can be proposed as ex-juvantibus trial in children with neutrophilic asthma (66). In conclusion, further well-designed and large RCTs are warranted before routine use of macrolides is recommended or definitely condemned in pediatric severe asthma.
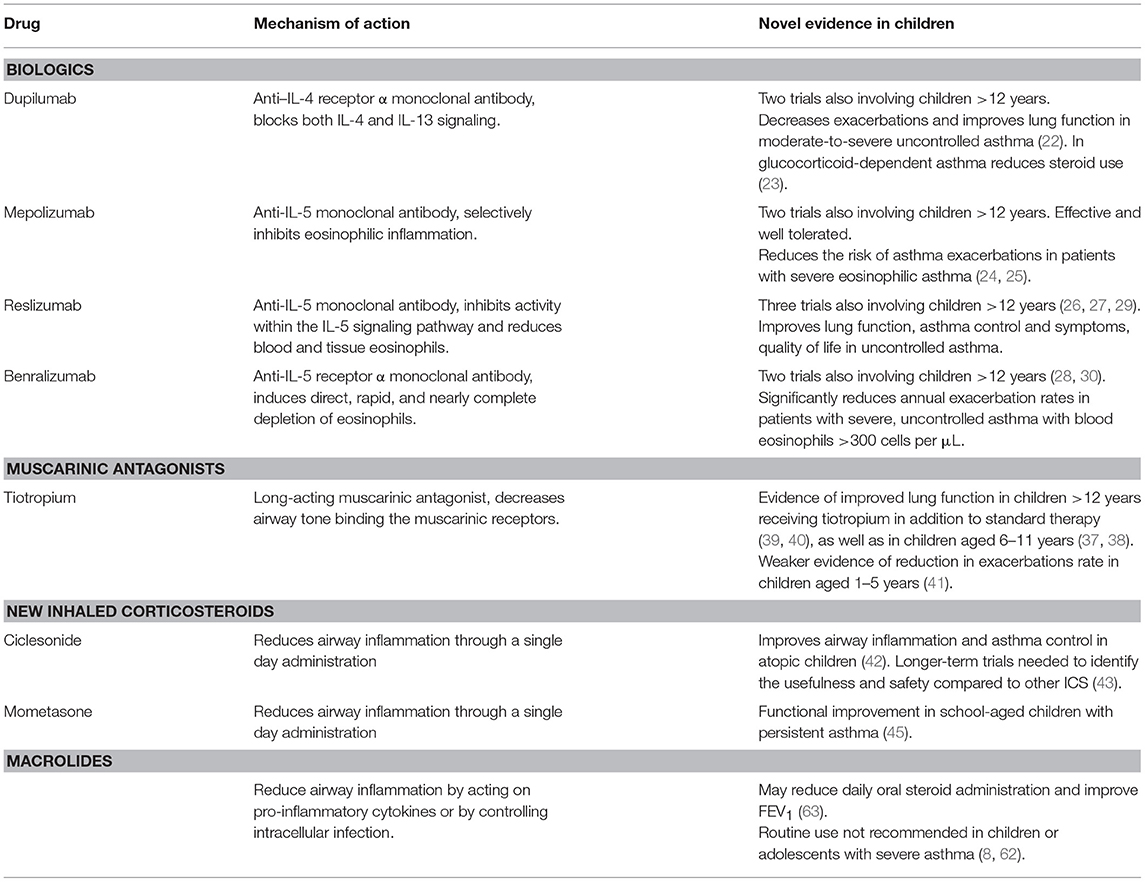
A list of new medications for treating children with severe, uncontrolled asthma, also including the novel evidence for their use, is provided in Table 1.
Other Interventions
Immunosuppressive drugs including cyclosporine, azathioprine and methotrexate, commonly used in several immune-mediated disorders, have been proposed for severe asthma management, but no recommendation may be formulated at present due to the insufficiency of data, particularly in children (3).
Allergen immunotherapy has proven effective in improving symptom control in mild to moderate asthmatic children, especially in the presence of a clear association between symptoms and exposure to a specific allergen, but its applicability in the clinical practice is strongly limited by the requirement for patients to have stable (and not uncontrolled) asthma symptoms due to the risk of severe reaction (67).
A surgical procedure named bronchial thermoplasty, consisting in the ablation of the airway smooth muscle layer, has shown to provide some benefits in adults unresponsive to conventional therapies (68). Nevertheless, evidence in children or adolescents is completely lacking at present, and such intervention is therefore not recommended in these age groups.
Finally, the association between worse symptom control and fungal sensitization in severe asthma has led to studies assessing the efficacy of antifungal drugs on asthma outcomes. However, data are still conflicting and not conclusive and insufficient to support formal recommendations (69).
Conclusions
Severe asthma identifies children or adolescents who need high-dose ICS therapy and a second controller therapy in the previous year, or systemic corticosteroids for 50% of the year, to prevent asthma from being uncontrolled or that remains uncontrolled notwithstanding these medications (9). Unfortunately, once excluded any comorbidity or optimized patients' adherence to treatment and inhalation technique, about 5–10% of the asthmatic pediatric population continue to have severe symptoms or signs and the loss of lung function may be progressive and irreversible (70). The latter point is of paramount importance in view of the fact that a stringent relationship between the childhood insults to the lung and the accelerated aging that can occur in adult chronic obstructive lung disease has been postulated (71). Finally, asthma not responding to treatment may result in significant morbidity and frequent healthcare utilization (1, 72). For all the above, there is considerable need for robust studies of children with uncontrolled asthma confirming the clinical efficacy and safety of medications increasingly used in the adult population, but not allowed in patients < 12 years of age because of the paucity of literature data.
Author Contributions
MM has made substantial contributions to conception and design, has been involved in drafting the manuscript, and has given final approval of the version to be published. MP conceived the idea, has been involved in drafting the manuscript and has given final approval of the version to be published. FS has made substantial contributions to conception and design, has been involved in drafting the manuscript and revising it critically for important intellectual content, and has given final approval of the version to be published.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. O'Byrne PM, Pedersen S, Schatz M, Thoren A, Ekholm E, Carlsson LG, et al. The poorly explored impact of uncontrolled asthma. Chest (2016) 143:511–23. doi: 10.1378/chest.12-0412
2. British Thoracic Society, Scottish Intercollegiate Guidelines Network British guideline on the management of asthma. Thorax (2014) 69(Suppl. 1):1–192.
3. Global Initiative for Asthma. GINA Report: Global Strategy for Asthma Management and Prevention (2017). Available online at: https://ginasthma.org/gina-reports/ (Accessed October 14, 2018).
4. National Heart Lung and Blood Institute National Asthma Education and Prevention Program. Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma (2007). Available online at: http://www.nhlbi.nih.gov/files/docs/guidelines/asthgdln.pdf (Accessed October 03, 2018).
5. Schmier JK, Manjunath R, Halpern MT, Jones ML, Thompson K, Diette GB. The impact of inadequately controlled asthma in urban children on quality of life and productivity. Ann Allergy Asthma Immunol. (2007) 98:245–51. doi: 10.1016/S1081-1206(10)60713-2
6. Licari A, Brambilla I, Marseglia A, De Filippo M, Paganelli V, Marseglia GL. Difficult vs. severe asthma: definition and limits of asthma control in the pediatric population. Front Pediatr. (2018) 6:170. doi: 10.3389/fped.2018.00170
7. Kersten ET, Koppelman GH. Pharmacogenetics of asthma: toward precision medicine. Curr Opin Pulm Med. (2017) 23:12–20. doi: 10.1097/MCP.0000000000000335
8. Katial RK, Bensch GW, Busse WW, Chipps BE, Denson JL, Gerber AN, et al. Changing paradigms in the treatment of severe asthma: the role of biologic therapies. J Allergy Clin Immunol Pract. (2017) 5:S1–14. doi: 10.1016/j.jaip.2016.11.029
9. Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. (2014) 43:343–73. doi: 10.1183/09031936.00202013
10. Djukanović R, Wilson SJ, Kraft M, Jarjour NN, Steel M, Chung KF, et al. Effects of treatment with anti-immunoglobulin E antibody omalizumab on airway inflammation in allergic asthma. Am J Respir Crit Care Med. (2004) 170:583–93. doi: 10.1164/rccm.200312-1651OC
11. Holgate S, Casale T, Wenzel S, Bousquet J, Deniz Y, Reisner C. The anti-inflammatory effects of omalizumab confirm the central role of IgE in allergic inflammation. J Allergy Clin Immunol. (2005) 115:459–65. doi: 10.1016/j.jaci.2004.11.053
12. Federal Drug Administration Advisory for Omalizumab. Available online at: http://www.fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/ApprovalApplications/TherapeuticBiologicApplications/default.htm (Accessed October 14, 2018).
13. European Medicines Agency: assessment report for Xolair. Available online at: https://www.ema.europa.eu/medicines/human/EPAR/xolair (Accessed October 14, 2018).
14. Humbert M, Beasley R, Ayres J, Slavin R, Hébert J, Bousquet J, et al. Benefits of omalizumab as add-on therapy in patients with severe persistent asthma who are inadequately controlled despite best available therapy (GINA 2002 step 4 treatment): INNOVATE. Allergy (2005) 60:309–16. doi: 10.1111/j.1398-9995.2004.00772.x
15. Normansell R, Walker S, Milan SJ, Walters EH, Nair P. Omalizumab for asthma in adults and children. Cochrane Database Syst Rev. (2014) 1:CD003559. doi: 10.1002/14651858.CD003559.pub4
16. Busse WW, Morgan WJ, Gergen PJ, Mitchell HE, Gern JE, Liu AH, et al. Randomized trial of omalizumab (anti-IgE) for asthma in inner-city children. N Engl J Med. (2011) 364:1005–15. doi: 10.1056/NEJMoa1009705
17. Lanier B, Bridges T, Kulus M, Taylor AF, Berhane I, Vidaurre CF. Omalizumab for the treatment of exacerbations in children with inadequately controlled allergic (IgE-mediated) asthma. J Allergy Clin Immunol. (2009) 124:1210–6. doi: 10.1016/j.jaci.2009.09.021
18. Odajima H, Ebisawa M, Nagakura T, Fujisawa T, Akasawa A, Ito K, et al. Omalizumab in Japanese children with severe allergic asthma uncontrolled with standard therapy. Allergol Int. (2015) 64:364–70. doi: 10.1016/j.alit.2015.05.006
19. Milgrom H, Berger W, Nayak A, Gupta N, Pollard S, McAlary M, et al. Treatment of childhood asthma with anti-immunoglobulin E antibody (omalizumab). Pediatrics (2001) 108:E36. doi: 10.1542/peds.108.2.e36
20. Deschildre A, Marguet C, Salleron J, Pin I, Rittié JL, Derelle J, et al. Add-on omalizumab in children with severe allergic asthma: a 1-year real life survey. Eur Respir J. (2013) 42:1224–33. doi: 10.1183/09031936.00149812
21. Teach SJ, Gill MA, Togias A, Sorkness CA, Arbes SJ Jr, Calatroni A, et al. Preseasonal treatment with either omalizumab or an inhaled corticosteroid boost to prevent fall asthma exacerbations. J Allergy Clin Immunol. (2015) 136:1476–85. doi: 10.1016/j.jaci.2015.09.008
22. Limb SL, Starke PR, Lee CE, Chowdhury BA. Delayed onset and protracted progression of anaphylaxis after omalizumab administration in patients with asthma. J Allergy Clin Immunol. (2007) 120:1378–81. doi: 10.1016/j.jaci.2007.09.022
23. Castro M, Corren J, Pavord ID, Maspero J, Wenzel S, Rabe KF, et al. Dupilumab efficacy and safety in moderate-to-severe uncontrolled asthma. N Engl J Med. (2018) 378:2486–96. doi: 10.1056/NEJMoa1804092
24. Rabe KF, Nair P, Brusselle G, Maspero JF, Castro M, Sher L, et al. Efficacy and safety of dupilumab in glucocorticoid-dependent severe asthma. N Engl J Med. (2018) 378:2475–85. doi: 10.1056/NEJMoa1804093
25. Ortega HG, Liu MC, Pavord ID, Brusselle GG, FitzGerald JM, Chetta A, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. (2014) 371:1198–207. doi: 10.1056/NEJMoa1403290
26. Bel EH, Ortega HG, Pavord ID. Glucocorticoids and mepolizumab in eosinophilic asthma. N Engl J Med. (2014) 371:2434. doi: 10.1056/NEJMoa1403291
27. Brusselle G, Germinaro M, Weiss S, Zangrilli J. Reslizumab in patients with inadequately controlled late-onset asthma and elevated blood eosinophils. Pulm Pharmacol Ther. (2017) 43:39–45. doi: 10.1016/j.pupt.2017.01.011
28. Murphy K, Jacobs J, Bjermer L, Fahrenholz JM, Shalit Y, Garin M, et al. Long-term safety and efficacy of reslizumab in patients with eosinophilic asthma. J Allergy Clin Immunol Pract. (2017) 5:1572–81. doi: 10.1016/j.jaip.2017.08.024
29. Bleecker ER, FitzGerald JM, Chanez P, Papi A, Weinstein SF, Barker P, et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting β2-agonists (SIROCCO): a randomised, multicentre, placebo-controlled phase 3 trial. Lancet (2016) 388:2115–27. doi: 10.1016/S0140-6736(16)31324-1
30. Bjermer L, Lemiere C, Maspero J, Weiss S, Zangrilli J, Germinaro M. Reslizumab for inadequately controlled asthma with elevated blood eosinophil levels: a randomized phase 3 study. Chest (2016) 150:789–98. doi: 10.1016/j.chest.2016.03.032
31. FitzGerald JM, Bleecker ER, Nair P, Korn S, Ohta K, Lommatzsch M, et al. Benralizumab, an anti-interleukin-5 receptor α monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet (2016) 388:2128–41. doi: 10.1016/S0140-6736(16)31322-8
32. Qureshi F, Zaritsky A, Lakkis H. Efficacy of nebulized ipratropium in severely asthmatic children. Ann Emerg Med. (1997) 29:205–11. doi: 10.1016/S0196-0644(97)70269-5
33. Rodrigo GJ, Castro-Rodriguez JA. Anticholinergics in the treatment of children and adults with acute asthma: a systematic review with meta-analysis. Thorax (2005) 60:740–6. doi: 10.1136/thx.2005.047803
34. Kerstjens HA, Disse B, Schröder-Babo W, Bantje TA, Gahlemann M, Sigmund R, et al. Tiotropium improves lung function in patients with severe uncontrolled asthma: a randomized controlled trial. J Allergy Clin Immunol. (2011) 128:308–14. doi: 10.1016/j.jaci.2011.04.039
35. Kerstjens HA, Engel M, Dahl R, Paggiaro P, Beck E, Vandewalker M, et al. Tiotropium in asthma poorly controlled with standard combination therapy. N Engl J Med. (2012) 367:1198–207. doi: 10.1056/NEJMoa1208606
36. Kew KM, Evans DJ, Allison DE, Boyter AC. Long-acting muscarinic antagonists (LAMA) added to inhaled corticosteroids (ICS) versus addition of long-acting beta2-agonists (LABA) for adults with asthma. Cochrane Database Syst Rev. (2015) 6:CD011438. doi: 10.1002/14651858.CD011438
37. Boehringer Ingelheim International GmbH. Spiriva Respimat, Highlights of Prescribing Information. (2017). Available online at: http://docs.boehringer-ingelheim.com/PrescribingInformation/PIs/SpirivaRespimat/spirivarespimat.pdf (Accessed October 13, 2018).
38. Vogelberg C, Moroni-Zentgraf P, Leonaviciute-Klimantaviciene M, Sigmund R, Hamelmann E, Engel M, et al. A randomised dose-ranging study of tiotropium Respimat® in children with symptomatic asthma despite inhaled corticosteroids. Respir Res. (2015) 16:20. doi: 10.1186/s12931-015-0175-9
39. Szefler SJ, Murphy K, Harper T III, Boner A, Laki I, Engel M, et al. A phase III randomized controlled trial of tiotropium add-on therapy in children with severe symptomatic asthma. J Allergy Clin Immunol. (2017) 140:1277–87. doi: 10.1016/j.jaci.2017.01.014
40. Hamelmann E, Bernstein JA, Vandewalker M, Moroni-Zentgraf P, Verri D, Unseld A, et al. A randomised controlled trial of tiotropium in adolescents with severe symptomatic asthma. Eur Respir J. (2017) 49:1601100. doi: 10.1183/13993003.01100-2016
41. Hamelmann E, Bateman ED, Vogelberg C, Szefler SJ, Vandewalker M, Moroni-Zentgraf P, et al. Tiotropium add-on therapy in adolescents with moderate asthma: a 1-year randomized controlled trial. J Allergy Clin Immunol. (2016) 138:441–50. doi: 10.1016/j.jaci.2016.01.011
42. Vrijlandt EJLE, El Azzi G, Vandewalker M, Rupp N, Harper T, Graham L, et al. Safety and efficacy of tiotropium in children aged 1–5 years with persistent asthmatic symptoms: a randomised, double-blind, placebo-controlled trial. Lancet Respir Med. (2018) 6:127–37. doi: 10.1016/S2213-2600(18)30012-2
43. Mallol J, Aguirre V, Gallardo A, Cortez E, Sánchez C, Riquelme C, et al. Effect of once-daily generic ciclesonide on exhaled nitric oxide in atopic children with persistent asthma. Allergol Immunopathol. (2016) 44:106–12. doi: 10.1016/j.aller.2015.01.011
44. Kramer S, Rottier BL, Scholten RJ, Boluyt N. Ciclesonide vs. other inhaled corticosteroids for chronic asthma in children. Cochrane Database Syst Rev. (2013) 2:CD010352. doi: 10.1002/14651858.CD010352
45. Milgrom H. Mometasone furoate in children with mild to moderate persistent asthma: a review of the evidence. Paediatr Drugs (2010) 12:213–21. doi: 10.2165/11316220-000000000-00000
46. Amar NJ, Shekar T, Varnell TA, Mehta A, Philip G. Mometasone furoate (MF) improves lung function in pediatric asthma: a double-blind, randomized controlled dose-ranging trial of MF metered-dose inhaler. Pediatr Pulmonol. (2017) 52:310–8. doi: 10.1002/ppul.23563
47. Steel HC, Theron AJ, Cockeran R, Anderson R, Feldman C. Pathogen- and host-directed anti-inflammatory activities of macrolide antibiotics. Mediators Inflamm. (2012) 2012:584262. doi: 10.1155/2012/584262
48. Sharma S, Jaffe A, Dixon G. Immunomodulatory effects of macrolide antibiotics in respiratory disease: therapeutic implications for asthma and cystic fibrosis. Pediatr Drugs (2007) 9:107–18. doi: 10.2165/00148581-200709020-00004
49. Koyama H, Geddes DM. Erythromycin and diffuse panbronchiolitis. Thorax (1997) 52:915–18. doi: 10.1136/thx.52.10.915
50. Equi A, Balfour-Lynn IM, Bush A, Rosenthal M. Long term azithromycin in children with cystic fibrosis: a randomised, placebo-controlled crossover trial. Lancet (2002) 360:978–84. doi: 10.1016/S0140-6736(02)11081-6
51. Choo JM, Abell GCJ, Thomson R, Morgan L, Waterer G, Gordon DL, et al. Impact of long-term erythromycin therapy on the oropharyngeal microbiome and resistance gene reservoir in non-cystic fibrosis bronchiectasis. mSphere (2018) 3:e00103–18. doi: 10.1128/mSphere.00103-18
52. Wong EH, Porter JD, Edwards MR, Johnston SL. The role of macrolides in asthma: current evidence and future directions. Lancet Respir Med. (2014) 2:657–70. doi: 10.1016/S2213-2600(14)70107-9
53. Siracusa A, Brugnami G, Fiordi T, Areni S, Severini C, Marabini A. Troleandomycin in the treatment of difficult asthma. J Allergy Clin Immunol. (1993) 92:677–82. doi: 10.1016/0091-6749(93)90010-D
54. Essilfie AT, Horvat JC, Kim RY, Mayall JR, Pinkerton JW, Beckett EL, et al. Macrolide therapy suppresses key features of experimental steroid-sensitive and steroid-insensitive asthma. Thorax (2015) 70:458–67. doi: 10.1136/thoraxjnl-2014-206067
55. Zimmermann P, Ziesenitz VC, Curtis N, Ritz N. The immunomodulatory effects of macrolides-a systematic review of the underlying mechanisms. Front Immunol. (2018) 9:302. doi: 10.3389/fimmu.2018.00302
56. Brusselle GG, Vanderstichele C, Jordens P, Deman R, Slabbynck H, Ringoet V, et al. Azithromycin for prevention of exacerbations in severe asthma (AZISAST): a multicentre randomised double-blind placebo-controlled trial. Thorax (2013) 68:322–9. doi: 10.1136/thoraxjnl-2012-202698
57. Kew KM, Undela K, Kotortsi I, Ferrara G. Macrolides for chronic asthma. Cochrane Database Syst Rev. (2015) 9:CD002997. doi: 10.1002/14651858.CD002997.pub4
58. Iramain R, De Jesús R, Spitters C, Jara A, Jimenez J, Bogado N, et al. Chlamydia pneumoniae, and mycoplasma pneumoniae: are they related to severe asthma in childhood? J Asthma (2016) 53:618–21. doi: 10.3109/02770903.2015.1116085
59. Bacharier LB, Guilbert TW, Mauger DT, Boehmer S, Beigelman A, Fitzpatrick AM, et al. Early administration of azithromycin and prevention of severe lower respiratory tract illnesses in preschool children with a history of such illnesses: a randomized clinical trial. JAMA (2015) 314:2034–44. doi: 10.1001/jama.2015.13896
60. Kamada AK, Hill MR, Iklé DN, Brenner AM, Szefler SJ. Efficacy and safety of low-dose troleandomycin therapy in children with severe, steroid-requiring asthma. J Allergy Clin Immunol. (1993) 91:873–82.
61. Piacentini GL, Peroni DG, Bodini A, Pigozzi R, Costella S, Loiacono A, et al. Azithromycin reduces bronchial hyperresponsiveness and neutrophilic airway inflammation in asthmatic children: a preliminary report. Allergy Asthma Proc. (2007) 28:194–8. doi: 10.2500/aap.2007.28.2958
62. Strunk RC, Bacharier LB, Phillips BR, Szefler SJ, Zeiger RS, Chinchilli VM, et al. Azithromycin or montelukast as inhaled corticosteroid-sparing agents in moderate-to-severe childhood asthma study. J Allergy Clin Immunol. (2008) 122:1138–44.e4. doi: 10.1016/j.jaci.2008.09.028
63. Malhotra-Kumar S, Lammens C, Coenen S, Van Herck K, Goossens H. Effect of azithromycin and clarithromycin therapy on pharyngeal carriage of macrolide-resistant streptococci in healthy volunteers: a randomised, double-blind, placebo-controlled study. Lancet (2007) 369:482–90. doi: 10.1016/S0140-6736(07)60235-9
64. Barsky EE, Giancola LM, Baxi SN, Gaffin JM. A practical approach to severe asthma in children. Ann Am Thorac Soc. (2018) 15:399–408. doi: 10.1513/AnnalsATS.201708-637FR
65. Mikailov A, Kane I, Aronoff SC, Luck R, Delvecchio MT. Utility of adjunctive macrolide therapy in treatment of children with asthma: a systematic review and meta-analysis. J Asthma Allergy (2013) 6:23–9. doi: 10.2147/JAA.S38652
66. Bush A, Pedersen S, Hedlin G, Baraldi E, Barbato A, de Benedictis F, et al. Pharmacological treatment of severe, therapy-resistant asthma in children: what can we learn from where? Eur Respir J. (2011) 38:947–58. doi: 10.1183/09031936.00030711
67. Cox L, Nelson H, Lockey R, Calabria C, Chacko T, Finegold I, et al. Allergen immunotherapy: a practice parameter third update. J Allergy Clin Immunol. (2011) 127:S1–55. doi: 10.1016/j.jaci.2010.09.034
68. Castro M, Rubin AS, Laviolette M, Fiterman J, De Andrade Lima M, Shah PL, et al. AIR2 Trial Study Group. Effectiveness and safety of bronchial thermoplasty in the treatment of severe asthma: a multicenter, randomized, double-blind, sham-controlled clinical trial. Am J Respir Crit Care Med. (2010) 181:116–24. doi: 10.1164/rccm.200903-0354OC
69. Parulekar AD, Diamant Z, Hanania NA. Antifungals in severe asthma. Curr Opin Pulm Med. (2015) 21:48–54. doi: 10.1097/MCP.0000000000000117
70. Mirra V, Montella S, Santamaria F. Pediatric severe asthma: a case series report and perspectives on anti-IgE treatment. BMC Pediatr. (2018) 18:73. doi: 10.1186/s12887-018-1019-9
71. McGeachie MJ, Yates KP, Zhou X, Guo F, Sternberg AL, Van Natta ML, et al. Patterns of growth and decline in lung function in persistent childhood asthma. N Engl J Med. (2016) 374:1842–52. doi: 10.1056/NEJMoa1513737
Keywords: severe asthma, therapy, biologics, inhaled corticosteroids, muscarinic antagonists, macrolides, children, adolescents
Citation: Maglione M, Poeta M and Santamaria F (2019) New Drugs for Pediatric Asthma. Front. Pediatr. 6:432. doi: 10.3389/fped.2018.00432
Received: 18 October 2018; Accepted: 27 December 2018;
Published: 16 January 2019.
Edited by:
Michael David Shields, Queen's University Belfast, United KingdomReviewed by:
Basil Elnazir, Tallaght Hospital, IrelandIgnacio Tapia, Children's Hospital of Philadelphia, United States
Copyright © 2019 Maglione, Poeta and Santamaria. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Francesca Santamaria, c2FudGFtYXJAdW5pbmEuaXQ=
 Marco Maglione
Marco Maglione Marco Poeta
Marco Poeta Francesca Santamaria
Francesca Santamaria