
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pediatr. , 03 April 2018
Sec. Pediatric Critical Care
Volume 6 - 2018 | https://doi.org/10.3389/fped.2018.00078
This article is part of the Research Topic Improving Extracorporeal Life Support Outcomes in Children View all 8 articles
Since the advent of extracorporeal membrane oxygenation (ECMO) over 40 years ago, there has been increasing interest in the use of the extracorporeal circuit as a platform for providing multiple organ support. In this review, we will examine the evidence for the use of continuous renal replacement therapy, therapeutic plasma exchange, leukopheresis, adsorptive therapies, and extracorporeal liver support in conjunction with ECMO.
In 1975, Bartlett et al. supported a newborn with refractory hypoxemic respiratory failure and pulmonary hypertension with extracorporeal membrane oxygenation (ECMO) (1). The success of this case led to a revolution in the support of the patient with refractory, but reversible respiratory and/or cardiac failure. Since then, there has been a great expansion in the types of patients supported with ECMO. We are now placing larger numbers of increasingly sick patients on ECMO (2). Once relegated to neonates, adults now comprise the largest group of patients supported with ECMO (3). Previously, there were strict contraindications to ECMO, but now, it is commonplace to place patients on ECMO with septic shock (4), in active cardiac arrest (5, 6), and with irreversible heart or lung failure as a bridge to transplant (7, 8). As we place more complicated patients on ECMO with multiple organ dysfunction, we are increasingly providing multiple organ support. In the neonatal and pediatric population, patient size is a limiting factor in obtaining adequate vascular access. However, in patients supported with ECMO, the extracorporeal circuit provides a platform in which other forms of organ support can be added. In this review, we will look at some of the evidence for providing multiple organ support in conjunction with ECMO.
Acute kidney injury (AKI) and fluid overload are commonplace in critically ill patients requiring ECMO. Using the RIFLE criteria, previous studies found an incidence of AKI in ECMO patients of approximately 70% (9–11). A recent, multicenter study using the Kidney Disease Improving Global Outcomes consensus definition found AKI to occur in 74% of children supported on ECMO. For these neonatal and pediatric ECMO patients, AKI was strongly associated with increased duration of ECMO and increased mortality (12). The negative impact of AKI, fluid overload, and need for renal support therapy (RST) on morbidity and mortality has been demonstrated in multiple pediatric and adult studies (13–16).
The pathophysiology of AKI in ECMO patients is complex and multifactorial. Although the literature on the etiology and association of AKI and ECMO is limited, the process is likely driven by pre-ECMO morbidity and exacerbated by intrinsic ECMO factors. Critically ill patients requiring ECMO support are at a high risk for AKI prior to initiation of ECMO due to their underlying pathophysiology (hypoxic insult, low cardiac output state, activation of inflammatory mediators) and the common administration of nephrotoxic medications. Inotropes/vasopressors are often needed to support patients both before and around the time of ECMO initiation, and the use of these agents in critically ill patients is associated with an increased risk of AKI (17). In a multicenter study of AKI in pediatric patients who suffered cardiac arrest, time to return of spontaneous circulation was not associated with the development of AKI, but the total number of epinephrine doses given was associated with the development of AKI (18). The initiation of ECMO can then exacerbate the initial insult by provoking reperfusion injury (19) and exacerbation of fluid overload. Another proposed etiology for AKI in patients on ECMO is the non-pulsatile flow while on venoarterial ECMO (20). However, this is a topic of debate. Adademir et al. found lower IL-18 and neutrophil gelatinase-associated lipocalin (NGAL) levels in adults who underwent pulsatile flow on cardiopulmonary bypass (both markers of renal injury) compared to non-pulsatile flow (21), but there is a paucity of data regarding clinical development of AKI using these different modalities. Additionally, the use of venovenous ECMO, which preserves pulsatile flow, is also frequently complicated by AKI (22). This suggests that the lack or decrease of pulsatile flow is not necessarily a key factor in the development of renal injury. There are some factors intrinsic to ECMO that can also aggravate AKI. The systemic inflammation caused by blood exposure to artificial surfaces exacerbates the likely pre-existing stress response and can cause renal inflammation and injury (20, 23). Another aggravating factor is the common development hemolysis. Elevated levels of plasma-free hemoglobin have been associated with the development of hemoglobinuria nephropathy (20, 24). A large multicenter report on the incidence of AKI in pediatric patients on ECMO showed that the development of AKI occurs early in the ECMO course with 51–64% of patients meeting AKI definitions at initiation of ECMO and 86–93% of AKI developing by 48 h (12), suggesting that significant renal injury has already occurred at the time of ECMO initiation.
Continuous renal replacement therapy is commonly used in critically ill patients as a method of solute clearance and treatment of fluid overload that can be tolerated even in patients with hemodynamic instability. Indications for CRRT in patients on ECMO are similar to classic CRRT indications; electrolyte abnormalities, uremia, and fluid overload. However, a survey of ELSO centers on initiation of CRRT showed significant variation among centers, with 23% of centers reporting no use of RST for ECMO patients (25). This survey also revealed that fluid overload is the most common indication for CRRT (43%). Fluid overload has been shown to be a risk factor for increased mortality and prolonged ECMO duration (26–29). A multicenter study of 756 neonatal and pediatric ECMO patients demonstrated that both degree of fluid overload at ECMO initiation and peak fluid overload during ECMO were independently associated with increased mortality. In survivors, both fluid overload at ECMO initiation and peak fluid overload during ECMO were independently associated with increased duration of ECMO (30).
There are multiple modalities to provide CRRT for patients on ECMO. The three most widely used methods are: introducing an in-line hemofilter into the ECMO circuit, introducing a commercially available CRRT device in the ECMO circuit, and performing CRRT via independent venous access (31). In the survey study by Fleming et al., of the responding centers that use CRRT with ECMO, 21.5% of centers exclusively used an in-line hemofilter and 50.8% of centers exclusively used a commercially available CRRT device connected to the ECMO circuit (12).
The addition of a hemofilter into the ECMO circuit is relatively simple and cost-effective. The inlet of the filter is connected after the ECMO pump and its outlet is reconnected to the proximal limb of the ECMO circuit. The volume of replacement fluid, dialysis, and effluent fluid is controlled via an intravenous infusion pump (Figure 1). One of the drawbacks of this technique is the potential inaccuracy of the amount of volume being delivered and removed with intravenous infusion pumps (31, 32), and more precise methods require substantial increase in bedside workload. Another potential pitfall of this method is the lack of monitoring of the pressures in the hemofiltration circuit, which can lead to a lag time in detection of clotting and/or rupture of the filter (31, 33).
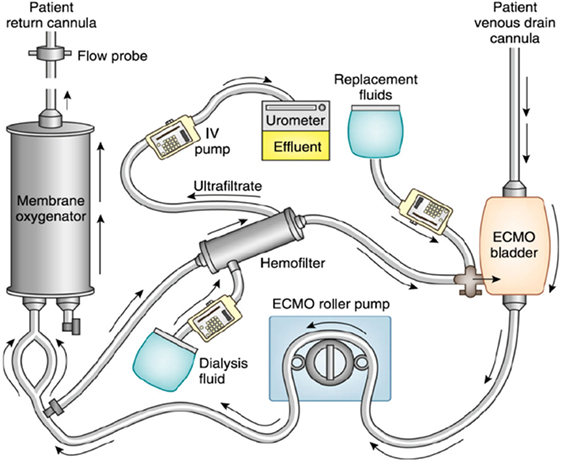
Figure 1. Renal support therapy using an in-line hemofilter with extracorporeal membrane oxygenation (ECMO). Blood from the ECMO circuit is shunted through an in-line hemofilter. The volume of fluid removed by the hemofilter, the ultrafiltrate, can be controlled using an intravenous pump. A filter replacement fluid or dialysis fluid can be used for additional solute clearance. Reprinted with permission from Askenazi et al. (19).
There is evidence that connecting a CRRT device to an ECMO circuit can provide more accurate fluid management. Several techniques have been described for the attachment of a commercially available CRRT device to an ECMO circuit. Santiago et al. described the addition of a CRRT device in series with the ECMO circuit (33). The authors tested their system in a closed circuit, an experimental animal study, and finally in a clinical trial with six children. They found no significant change in the pressures of the ECMO circuit with the introduction of the CRRT device. Other authors have described the addition of a CRRT device in parallel with the ECMO circuit (34), arguing that, in doing so, there is a reduction in blood flow resistance and turbulence after the centrifugal pump. A recent study in a simulated neonatal patient showed no change in the hemodynamic performance using a CRRT circuit in a variety of positions when a centrifugal pump was used. In the case of the roller head pump, the position in which the CRRT device did not have an effect on the hemodynamics of the circuit was in series with the circuit after the pump and before the membrane oxygenator (35). The type of ECMO pump will, therefore, influence the position of the CRRT device. With a centrifugal pump, the device should be connected after the ECMO pump because of the risk of air entrapment due to the negative pressure generated by centrifugal pumps. Regardless of the type of pump, the return blood from the CRRT device should be returned prior to the oxygenator to reduce the risk of air or clot being sent to the patient (19).
While ECMO patients with AKI and/or fluid overload have increased mortality, there has been some concern that the use of CRRT in ECMO patients could increase the risk of developing chronic renal failure. However, these concerns have not been substantiated. Paden et al. showed that in the absence of primary renal disease, 96% of patients had full renal recovery (36). The pathophysiology of AKI in this subset of patients is complex and results from a multitude of factors often working synergistically. Further research into the etiology of AKI and preventive strategies are needed. While most contemporary staging of AKI is based on a change in creatinine from baseline, serum creatinine is an imprecise reflection of renal function, and biomarkers such as NGAL may better reflect renal impairment (37). The use of more accurate biomarkers could better identify patients with AKI and, therefore, the more rapid initiation of CRRT on ECMO. A recent study in which CRRT was implemented in all neonates within 48 h of ECMO cannulation demonstrated a decreased weight gain at the time of CRRT initiation and quicker resolution of fluid overload. However, it did not show significant improvement in duration of ECMO support or mortality (38). A summary of pediatric studies of ECMO and RST is presented in Table 1.
Therapeutic plasma exchange is a technique typically carried out via a centrifugal device to separate and remove plasma from whole blood or with the use of a semipermeable membrane that separates plasma from whole blood. The removed plasma volume is then replaced, and although the composition is not standardized, typical replacement fluids include varied amounts of normal saline, human albumin, and fresh frozen plasma. As the centrifugal pump separates blood components based on density, TPE is non-selective, but carries the potential benefit of removing pro-inflammatory mediators, antibodies, and cytokines (39, 40). Published by the American Society for Apheresis, guidelines offer current literature as well as category and grade recommendations on conditions amenable to TPE (41). Although prior evidence has shown TPE to be effective for the treatment of thrombotic thrombocytopenic purpura (TTP) (42, 43) and Guillain–Barre syndrome (44), recent studies have sought to evaluate the utility of TPE in the setting of sepsis and thrombocytopenia-associated multiple organ failure (TAMOF) (39, 40, 45–47).
Thrombocytopenia-associated multiple organ failure is a syndrome described in critically ill children, and like TTP, it has been associated with decreased levels of a disintegrin-like and metalloprotease with thrombospondin (ADAMTS-13), which can result in von Willebrand factor-mediated thrombotic microangiopathy and confers an increased risk of mortality (40, 45). Nguyen et al. demonstrated that levels of ADAMTS-13 activity were increased after TPE in children with TAMOF and was associated with improvement in organ dysfunction. Their prospective randomized controlled trial also demonstrated reduced ADAMTS-13 activity level when TPE was stopped in three patients and return of ADAMTS-13 activity when it was reinstituted in two of those patients (40). In 2014, Rimmer et al. published a meta-analysis of four trials of plasma exchange in patients with sepsis that included a total of 194 patients. Of the four trials, one included only adult patients, one included adult and pediatric patients, and two included only pediatric patients. This review showed a significant reduction of all-cause mortality in adults after TPE, but the trend in children was not statistically significant (48). A single-center retrospective study of 14 pediatric ECMO patients with sepsis and TAMOF demonstrated an improvement in Organ Failure Index (OFI) and Vasoactive-Inotropic Scores (VIS) after TPE. The treatment group had an estimated survival rate of 40% (based on OFI) but overall survival rate of 71.4% after treatment with conventional therapy and plasma exchange. They also noted a trend toward improved survival compared to the historical control group, which had a survival rate of 50%. Additionally, through subgroup analyses, a trend toward greater improvement in organ recovery (as determined by change in OFI) and improved hemodynamic status (as determined by VIS) was noted in patients who received TPE earlier in their hospital course, although this was not statistically significant (47).
Severe pertussis is a dreaded disease in young infants, with hypoxia, pulmonary hypertension, and cardiopulmonary collapse that can be refractory to traditional management and confers a high mortality despite aggressive interventions. The pathophysiology of the severe hypoxia and pulmonary hypertension that is seen in these infants is poorly understood. However, based on postmortem studies showing leukocyte thrombi in the pulmonary vasculature and the correlation between degree of hyperleukocytosis and poor outcomes, the hypothesis that viscosity and hyperleukocytosis are major players in this disease process has been brought forward (49, 50). In 2004, Romano et al. conducted the first double volume exchange transfusion as a means for leukodepletion in a 3 months old with severe pertussis on mechanical ventilation with escalating oxygen requirements and evidence of pulmonary hypertension on echocardiogram (51). They saw improvement in oxygenation within a few hours of leukopheresis. Two years later, Grzeszczak et al. first described successful leukopheresis in a 5-week-old patient with severe pertussis necessitating ECMO support and showed dramatic temporal association between leukopheresis initiation and cardiovascular function (49).
Since then, there have been several case reports and case series published, the largest by Rowlands et al. where a comparison was made between patients treated prior to and after the adoption of aggressive leukopheresis (50). The patients requiring ECMO support underwent leukopheresis by adding a white blood cell (WBC) filter in the bridge of the ECMO circuit while priming the circuit, and after ECMO flow was established, opening flow to the bridge (approximately 100 mL/min) until the WBC count approached 15,000/μL. The patients not requiring ECMO support underwent exchange transfusion via arterial and central venous lines using 4.5% albumin and packed red blood cells to achieve a WBC count of less than 50,000/μL. The authors found a trend toward higher survival after implementation of aggressive leukopheresis in both the ECMO and non-ECMO supported patients, yet, their results were not statistically significant. However, their quoted 20% mortality for patients requiring ECMO and treated with leukopheresis is significantly lower than the 70% mortality reported in an ELSO database analysis of infants with severe pertussis requiring ECMO support in 2003 (52). More recent reports still describe a high mortality for infants with severe pertussis requiring ECMO support; reports from California in 2015 (53) and Australia and New Zealand in 2016 (54) found a mortality of 98 and 75%, respectively, suggesting that the improved survival seen in Rowlands’s second study period does not reflect improvement in overall care of these infants. The degree of leukocytosis necessitating leukopheresis has not been studied, although the authors from Rowlands et al. recommend leukopheresis for patients on ECMO with WBC counts above 50,000/μL. A recent retrospective, multicenter study of infants requiring ECMO for pertussis showed a survival of 28%. Younger age, lower PaO2/FiO2 ratio, use of vasoactive infusions, pulmonary hypertension, and a decreased time from intubation to need for ECMO, were all associated with higher mortality. However, leukopheresis was independently associated with increased survival in this patient population [OR 3.36 (1.13–11.68); p = 0.03] (55).
Adsorptive therapies have developed as a means of attenuating the effects of severe septic shock. Adsorptive cartridges can be integrated into extracorporeal circuits with the intention of removing endotoxin or inflammatory mediators from systemic circulation (56). This technique allows for the use of polymyxin B, an antibiotic capable of binding and neutralizing endotoxin, but whose systemic use is generally limited due to its toxicity (57, 58). Polymyxin B hemoperfusion (PMX-HP) has been used for over 20 years in Japan with the majority of the literature based on studies of adult patients.
One such study used a national database in Japan to conduct a retrospective analysis of PMX-HP in adults with abdominal septic shock. This review found no significant difference in 28-day mortality between the groups that received PMX-HP in addition to conventional therapies versus conventional therapies alone (58). These findings were consistent with a multicenter randomized control trial in France that also found PMX-HP with conventional therapy compared to conventional therapy alone had no significant difference in the 28-day mortality of patients with septic shock due to peritonitis (56). A systematic review of five trials studying PMX-HP in patients with septic shock noted improvement in mean arterial blood pressures, PaO2/FiO2 ratios, and decreased mortality, although the authors cautioned that few of these studies were planned or powered to assess mortality (59). While the efficacy of PMX-HP in adults with sepsis is undergoing investigation, there are even fewer studies examining its use in pediatric populations. Thus far, the use of adsorptive therapies in conjunction with ECMO has been limited to case reports (60–62).
Extracorporeal liver support has been used as a bridge to recovery or to transplant in patients with acute or acute on chronic liver failure. The types of artificial extracorporeal liver support include single-pass albumin dialysis (SPAD), molecular adsorbent recirculating system (MARS®; Gambro, Germany), and the Prometheus® fractionated plasma separation and adsorption system (Fresenius, Germany). SPAD can be performed using a standard continuous renal replacement device. The patient’s blood flows through a standard hemofilter and dialyzate containing albumin flows counter-current to the blood to allow for the removal of protein bound molecules that are not removed with standard renal support therapies, and the used dialysate fluid is then discarded. In a similar fashion, liver support with the MARS device consists of blood flow through a hemofilter with a countercurrent albumin solution that allows for the removal of protein bound toxins. The used albumin solution then undergoes dialysis to remove water soluble toxins and then passes through an anion exchanger resin adsorber and a charcoal absorber. The replenished albumin is then returned to the primary circuit to be utilized again. With the Prometheus system, the albumin is selectively filtered from the patient’s blood, and this albumin enriched plasma is sent through a resin adsorber column and then to an anion exchanger adsorber column to remove toxins that are bound to the albumin. After passing through the two adsorber columns, this cleansed albumin solution returns to the primary circuit to undergo removal of water soluble compounds via a hemofilter and conventional dialysis (63, 64).
In a case series of adult ECMO patients with severe hyperbilirubinemia, five patients were supported with MARS in addition to venovenous ECMO. Based on historic controls of ECMO patients with severe hyperbilirubinemia, there would be no expected survivors. However, 40% of the patients supported with MARS survived (65). In a single center, retrospective study of adult ECMO patients with acute liver failure, patients supported with ECMO and MARS therapy had a survival to weaning off ECMO of 64% versus a survival to weaning off ECMO of only 21% in the ECMO and standard medical therapies group (p = 0.02). There was a trend in 30-day survival after ECMO in the MARS therapy group versus the standard therapy group (43 versus 14%), but this was not statistically significant (66).
As ECMO continues to evolve, we will continue to push the limits of the support that can be provided with increasing numbers of higher acuity patients. We continue to support critically ill patients while waiting for organ recovery, or when organ recovery is not possible, to bridge to organ transplantation. There are promising results for the use of other organ support therapies in conjunction with ECMO. However, these results must be interpreted with caution, as there is a lack of prospective, randomized studies looking at the use these therapies with ECMO.
BB, MC, and JD made substantial contributions to the writing and revision of this manuscript. Each author approved of the final version of this manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Bartlett RH. Esperanza: the first neonatal ECMO patient. ASAIO J (2017) 63(6):832–43. doi:10.1097/MAT.0000000000000697
2. Zabrocki LA, Brogan TV, Statler KD, Poss WB, Rollins MD, Bratton SL. Extracorporeal membrane oxygenation for pediatric respiratory failure: survival and predictors of mortality. Crit Care Med (2011) 39(2):364–70. doi:10.1097/CCM.0b013e3181fb7b35
3. Thiagarajan RR, Barbaro RP, Rycus PT, McMullan DM, Conrad SA, Fortenberry JD, et al. Extracorporeal life support organization registry international report 2016. ASAIO J (2017) 63(1):60–7. doi:10.1097/MAT.0000000000000475
4. Ruth A, McCracken CE, Fortenberry JD, Hall M, Simon HK, Hebbar KB. Pediatric severe sepsis: current trends and outcomes from the pediatric health information systems database. Pediatr Crit Care Med (2014) 15(9):828–38. doi:10.1097/PCC.0000000000000254
5. Richardson AS, Schmidt M, Bailey M, Pellegrino VA, Rycus PT, Pilcher DV. ECMO cardio-pulmonary resuscitation (ECPR), trends in survival from an international multicentre cohort study over 12-years. Resuscitation (2017) 112:34–40. doi:10.1016/j.resuscitation.2016.12.009
6. Conrad SJ, Bridges BC, Kalra Y, Pietsch JB, Smith AH. Extracorporeal cardiopulmonary resuscitation among patients with structurally normal hearts. ASAIO J (2017) 63(6):781–6. doi:10.1097/MAT.0000000000000568
7. Tsiouris A, Budev MM, Yun JJ. Extracorporeal membrane oxygenation as a bridge to lung transplantation in the United States: a multicenter survey. ASAIO J (2017). doi:10.1097/MAT.0000000000000731
8. Dipchand AI, Mahle WT, Tresler M, Naftel DC, Almond C, Kirklin JK, et al. Extracorporeal membrane oxygenation as a bridge to pediatric heart transplantation: effect on post-listing and post-transplantation outcomes. Circ Heart Fail (2015) 8(5):960–9. doi:10.1161/CIRCHEARTFAILURE.114.001553
9. Gadepalli SK, Selewski DT, Drongowski RA, Mychaliska GB. Acute kidney injury in congenital diaphragmatic hernia requiring extracorporeal life support: an insidious problem. J Pediatr Surg (2011) 46(4):630–5. doi:10.1016/j.jpedsurg.2010.11.031
10. Lin CY, Chen YC, Tsai FC, Tian YC, Jenq CC, Fang JT, et al. RIFLE classification is predictive of short-term prognosis in critically ill patients with acute renal failure supported by extracorporeal membrane oxygenation. Nephrol Dial Transplant (2006) 21(10):2867–73. doi:10.1093/ndt/gfl326
11. Smith AH, Hardison DC, Worden CR, Fleming GM, Taylor MB. Acute renal failure during extracorporeal support in the pediatric cardiac patient. ASAIO J (2009) 55(4):412–6. doi:10.1097/MAT.0b013e31819ca3d0
12. Fleming GM, Sahay R, Zappitelli M, King E, Askenazi DJ, Bridges BC, et al. The incidence of acute kidney injury and its effect on neonatal and pediatric extracorporeal membrane oxygenation outcomes: a multicenter report from the kidney intervention during extracorporeal membrane oxygenation study group. Pediatr Crit Care Med (2016) 17(12):1157–69. doi:10.1097/PCC.0000000000000970
13. Meyer RJ, Brophy PD, Bunchman TE, Annich GM, Maxvold NJ, Mottes TA, et al. Survival and renal function in pediatric patients following extracorporeal life support with hemofiltration. Pediatr Crit Care Med (2001) 2(3):238–42. doi:10.1097/00130478-200107000-00009
14. Hoover NG, Heard M, Reid C, Wagoner S, Rogers K, Foland J, et al. Enhanced fluid management with continuous venovenous hemofiltration in pediatric respiratory failure patients receiving extracorporeal membrane oxygenation support. Intensive Care Med (2008) 34(12):2241–7. doi:10.1007/s00134-008-1200-y
15. Askenazi DJ, Ambalavanan N, Hamilton K, Cutter G, Laney D, Kaslow R, et al. Acute kidney injury and renal replacement therapy independently predict mortality in neonatal and pediatric noncardiac patients on extracorporeal membrane oxygenation. Pediatr Crit Care Med (2011) 12(1):e1–6. doi:10.1097/PCC.0b013e3181d8e348
16. Schneider J, Khemani R, Grushkin C, Bart R. Serum creatinine as stratified in the RIFLE score for acute kidney injury is associated with mortality and length of stay for children in the pediatric intensive care unit. Crit Care Med (2010) 38(3):933–9. doi:10.1097/CCM.0b013e3181cd12e1
17. Cartin-Ceba R, Kashiouris M, Plataki M, Kor DJ, Gajic O, Casey ET. Risk factors for development of acute kidney injury in critically ill patients: a systematic review and meta-analysis of observational studies. Crit Care Res Pract (2012) 2012:691013. doi:10.1155/2012/691013
18. Neumayr TM, Gill J, Fitzgerald JC, Gazit AZ, Pineda JA, Berg RA, et al. Identifying risk for acute kidney injury in infants and children following cardiac arrest. Pediatr Crit Care Med (2017) 18(10):e446–54. doi:10.1097/PCC.0000000000001280
19. Askenazi DJ, Selewski DT, Paden ML, Cooper DS, Bridges BC, Zappitelli M, et al. Renal replacement therapy in critically ill patients receiving extracorporeal membrane oxygenation. Clin J Am Soc Nephrol (2012) 7(8):1328–36. doi:10.2215/CJN.12731211
20. Kilburn DJ, Shekar K, Fraser JF. The complex relationship of extracorporeal membrane oxygenation and acute kidney injury: causation or association? Biomed Res Int (2016) 2016:1094296. doi:10.1155/2016/1094296
21. Adademir T, Ak K, Aljodi M, Elci ME, Arsan S, Isbir S. The effects of pulsatile cardiopulmonary bypass on acute kidney injury. Int J Artif Organs (2012) 35(7):511–9. doi:10.5301/ijao.5000097
22. Roy BJ, Cornish JD, Clark RH. Venovenous extracorporeal membrane oxygenation affects renal function. Pediatrics (1995) 95(4):573–8.
23. Antonucci E, Lamanna I, Fagnoul D, Vincent JL, De Backer D, Silvio Taccone F. The impact of renal failure and renal replacement therapy on outcome during extracorporeal membrane oxygenation therapy. Artif Organs (2016) 40(8):746–54. doi:10.1111/aor.12695
24. Gbadegesin R, Zhao S, Charpie J, Brophy PD, Smoyer WE, Lin JJ. Significance of hemolysis on extracorporeal life support after cardiac surgery in children. Pediatr Nephrol (2009) 24(3):589–95. doi:10.1007/s00467-008-1047-z
25. Fleming GM, Askenazi DJ, Bridges BC, Cooper DS, Paden ML, Selewski DT, et al. A multicenter international survey of renal supportive therapy during ECMO: the Kidney Intervention During Extracorporeal Membrane Oxygenation (KIDMO) group. ASAIO J (2012) 58(4):407–14. doi:10.1097/MAT.0b013e3182579218
26. Swaniker F, Kolla S, Moler F, Custer J, Grams R, Barlett R, et al. Extracorporeal life support outcome for 128 pediatric patients with respiratory failure. J Pediatr Surg (2000) 35(2):197–202. doi:10.1016/S0022-3468(00)90009-5
27. Schmidt M, Bailey M, Kelly J, Hodgson C, Cooper DJ, Scheinkestel C, et al. Impact of fluid balance on outcome of adult patients treated with extracorporeal membrane oxygenation. Intensive Care Med (2014) 40(9):1256–66. doi:10.1007/s00134-014-3360-2
28. Blijdorp K, Cransberg K, Wildschut ED, Gischler SJ, Jan Houmes R, Wolff ED, et al. Haemofiltration in newborns treated with extracorporeal membrane oxygenation: a case-comparison study. Crit Care (2009) 13(2):R48. doi:10.1186/cc7771
29. Selewski DT, Cornell TT, Blatt NB, Han YY, Mottes T, Kommareddi M, et al. Fluid overload and fluid removal in pediatric patients on extracorporeal membrane oxygenation requiring continuous renal replacement therapy. Crit Care Med (2012) 40(9):2694–9. doi:10.1097/CCM.0b013e318258ff01
30. Selewski DT, Askenazi DJ, Bridges BC, Cooper DS, Fleming GM, Paden ML, et al. The impact of fluid overload on outcomes in children treated with extracorporeal membrane oxygenation: a multicenter retrospective cohort study. Pediatr Crit Care Med (2017) 18(12):1126–35. doi:10.1097/PCC.0000000000001349
31. Chen H, Yu RG, Yin NN, Zhou JX. Combination of extracorporeal membrane oxygenation and continuous renal replacement therapy in critically ill patients: a systematic review. Crit Care (2014) 18(6):675. doi:10.1186/s13054-014-0675-x
32. Jenkins R, Harrison H, Chen B, Arnold D, Funk J. Accuracy of intravenous infusion pumps in continuous renal replacement therapies. ASAIO J (1992) 38(4):808–10. doi:10.1097/00002480-199210000-00012
33. Santiago MJ, Sanchez A, Lopez-Herce J, Perez R, del Castillo J, Urbano J, et al. The use of continuous renal replacement therapy in series with extracorporeal membrane oxygenation. Kidney Int (2009) 76(12):1289–92. doi:10.1038/ki.2009.383
34. Ricci Z, Ronco C, Picardo S. CRRT in series with extracorporeal membrane oxygenation in pediatric patients. Kidney Int (2010) 77(5):469–70; author reply 71. doi:10.1038/ki.2009.495
35. Profeta E, Shank K, Wang S, O’Connor C, Kunselman AR, Woitas K, et al. Evaluation of hemodynamic performance of a combined ECLS and CRRT circuit in seven positions with a simulated neonatal patient. Artif Organs (2017) 42(2):155–65. doi:10.1111/aor.12907
36. Paden ML, Warshaw BL, Heard ML, Fortenberry JD. Recovery of renal function and survival after continuous renal replacement therapy during extracorporeal membrane oxygenation. Pediatr Crit Care Med (2011) 12(2):153–8. doi:10.1097/PCC.0b013e3181e2a596
37. Devarajan P. Neutrophil gelatinase-associated lipocalin: a promising biomarker for human acute kidney injury. Biomark Med (2010) 4(2):265–80. doi:10.2217/bmm.10.12
38. Murphy HJ, Cahill JB, Twombley KE, Annibale DJ, Kiger JR. Implementing a practice change: early initiation of continuous renal replacement therapy during neonatal extracorporeal life support standardizes care and improves short-term outcomes. J Artif Organs (2017) 21(1):76–85. doi:10.1007/s10047-017-1000-7
39. McMaster P, Shann F. The use of extracorporeal techniques to remove humoral factors in sepsis. Pediatr Crit Care Med (2003) 4(1):2–7. doi:10.1097/00130478-200301000-00002
40. Nguyen TC, Han YY, Kiss JE, Hall MW, Hassett AC, Jaffe R, et al. Intensive plasma exchange increases a disintegrin and metalloprotease with thrombospondin motifs-13 activity and reverses organ dysfunction in children with thrombocytopenia-associated multiple organ failure. Crit Care Med (2008) 36(10):2878–87. doi:10.1097/CCM.0b013e318186aa49
41. Schwartz J, Padmanabhan A, Aqui N, Balogun RA, Connelly-Smith L, Delaney M, et al. Guidelines on the use of therapeutic apheresis in clinical practice-evidence-based approach from the Writing Committee of the American society for apheresis: the seventh special issue. J Clin Apher (2016) 31(3):149–62. doi:10.1002/jca.21470_c
42. Bell WR, Braine HG, Ness PM, Kickler TS. Improved survival in thrombotic thrombocytopenic purpura-hemolytic uremic syndrome. clinical experience in 108 patients. N Engl J Med (1991) 325(6):398–403. doi:10.1056/NEJM199108083250605
43. Rock GA, Shumak KH, Buskard NA, Blanchette VS, Kelton JG, Nair RC, et al. Comparison of plasma exchange with plasma infusion in the treatment of thrombotic thrombocytopenic purpura. Canadian Apheresis Study Group. N Engl J Med (1991) 325(6):393–7. doi:10.1056/NEJM199108083250604
44. Jayasena YA, Mudalige SP, Manchanayake GS, Dharmapala HL, Kumarasiri RP, Weerasinghe VS, et al. Physiological changes during and outcome following ‘filtration’ based continuous plasma exchange in Guillain Barre syndrome. Transfus Apher Sci (2010) 42(2):109–13. doi:10.1016/j.transci.2010.01.002
45. Nguyen TC, Carcillo JA. Bench-to-bedside review: thrombocytopenia-associated multiple organ failure – a newly appreciated syndrome in the critically ill. Crit Care (2006) 10(6):235. doi:10.1186/cc4561
46. Nguyen TC, Han YY. Plasma exchange therapy for thrombotic microangiopathies. Organogenesis (2011) 7(1):28–31. doi:10.4161/org.7.1.14027
47. Kawai Y, Cornell TT, Cooley EG, Beckman CN, Baldridge PK, Mottes TA, et al. Therapeutic plasma exchange may improve hemodynamics and organ failure among children with sepsis-induced multiple organ dysfunction syndrome receiving extracorporeal life support. Pediatr Crit Care Med (2015) 16(4):366–74. doi:10.1097/PCC.0000000000000351
48. Rimmer E, Houston BL, Kumar A, Abou-Setta AM, Friesen C, Marshall JC, et al. The efficacy and safety of plasma exchange in patients with sepsis and septic shock: a systematic review and meta-analysis. Crit Care (2014) 18(6):699. doi:10.1186/s13054-014-0699-2
49. Grzeszczak MJ, Churchwell KB, Edwards KM, Pietsch J. Leukopheresis therapy for severe infantile pertussis with myocardial and pulmonary failure. Pediatr Crit Care Med (2006) 7(6):580–2. doi:10.1097/01.PCC.0000235253.19315.56
50. Rowlands HE, Goldman AP, Harrington K, Karimova A, Brierley J, Cross N, et al. Impact of rapid leukodepletion on the outcome of severe clinical pertussis in young infants. Pediatrics (2010) 126(4):e816–27. doi:10.1542/peds.2009-2860
51. Romano MJ, Weber MD, Weisse ME, Siu BL. Pertussis pneumonia, hypoxemia, hyperleukocytosis, and pulmonary hypertension: improvement in oxygenation after a double volume exchange transfusion. Pediatrics (2004) 114(2):e264–6. doi:10.1542/peds.114.2.e264
52. Halasa NB, Barr FE, Johnson JE, Edwards KM. Fatal pulmonary hypertension associated with pertussis in infants: does extracorporeal membrane oxygenation have a role? Pediatrics (2003) 112(6 Pt 1):1274–8. doi:10.1542/peds.112.6.1274
53. Winter K, Zipprich J, Harriman K, Murray EL, Gornbein J, Hammer SJ, et al. Risk factors associated with infant deaths from pertussis: a case-control study. Clin Infect Dis (2015) 61(7):1099–106. doi:10.1093/cid/civ472
54. Straney L, Schibler A, Ganeshalingham A, Alexander J, Festa M, Slater A, et al. Burden and outcomes of severe pertussis infection in critically ill infants. Pediatr Crit Care Med (2016) 17(8):735–42. doi:10.1097/PCC.0000000000000851
55. Domico M, Ridout D, MacLaren G, Barbaro R, Annich G, Schlapbach LJ, et al. Extracorporeal membrane oxygenation for pertussis: predictors of outcome including pulmonary hypertension and leukodepletion. Pediatr Crit Care Med (2018) 19(3):254–61. doi:10.1097/PCC.0000000000001454
56. Payen DM, Guilhot J, Launey Y, Lukaszewicz AC, Kaaki M, Veber B, et al. Early use of polymyxin B hemoperfusion in patients with septic shock due to peritonitis: a multicenter randomized control trial. Intensive Care Med (2015) 41(6):975–84. doi:10.1007/s00134-015-3751-z
57. Ronco C, Klein DJ. Polymyxin B hemoperfusion: a mechanistic perspective. Crit Care (2014) 18(3):309. doi:10.1186/cc13912
58. Iwagami M, Yasunaga H, Doi K, Horiguchi H, Fushimi K, Matsubara T, et al. Postoperative polymyxin B hemoperfusion and mortality in patients with abdominal septic shock: a propensity-matched analysis. Crit Care Med (2014) 42(5):1187–93. doi:10.1097/CCM.0000000000000150
59. Stegmayr B, Abdel-Rahman EM, Balogun RA. Septic shock with multiorgan failure: from conventional apheresis to adsorption therapies. Semin Dial (2012) 25(2):171–5. doi:10.1111/j.1525-139X.2011.01029.x
60. Hirakawa E, Ibara S, Tokuhisa T, Hiwatashi S, Hayashida Y, Maede Y, et al. Septic neonate rescued by polymyxin B hemoperfusion. Pediatr Int (2013) 55(3):e70–2. doi:10.1111/ped.12029
61. Itai J, Ohshimo S, Kida Y, Ota K, Iwasaki Y, Hirohashi N, et al. A pilot study: a combined therapy using polymyxin-B hemoperfusion and extracorporeal membrane oxygenation for acute exacerbation of interstitial pneumonia. Sarcoidosis Vasc Diffuse Lung Dis (2015) 31(4):343–9.
62. Bruenger F, Kizner L, Weile J, Morshuis M, Gummert JF. First successful combination of ECMO with cytokine removal therapy in cardiogenic septic shock: a case report. Int J Artif Organs (2015) 38(2):113–6. doi:10.5301/ijao.5000382
63. Jain V, Dhawan A. Extracorporeal liver support systems in paediatric liver failure. J Pediatr Gastroenterol Nutr (2017) 64(6):855–63. doi:10.1097/MPG.0000000000001500
64. Rademacher S, Oppert M, Jorres A. Artificial extracorporeal liver support therapy in patients with severe liver failure. Expert Rev Gastroenterol Hepatol (2011) 5(5):591–9. doi:10.1586/egh.11.59
65. Peek GJ, Killer HM, Sosnowski MA, Firmin RK. Modular extracorporeal life support for multiorgan failure patients. Liver (2002) 22(Suppl 2):69–71. doi:10.1034/j.1600-0676.2002.00014.x
Keywords: extracorporeal membrane oxygenation, continuous renal replacement therapy, therapeutic plasma exchange, adsorptive therapies, extracorporeal liver support
Citation: Canter MO, Daniels J and Bridges BC (2018) Adjunctive Therapies During Extracorporeal Membrane Oxygenation to Enhance Multiple Organ Support in Critically Ill Children. Front. Pediatr. 6:78. doi: 10.3389/fped.2018.00078
Received: 15 January 2018; Accepted: 14 March 2018;
Published: 03 April 2018
Edited by:
James Donald Fortenberry, Emory University, United StatesReviewed by:
Robert Kelly, Children’s Hospital of Orange County, United StatesCopyright: © 2018 Canter, Daniels and Bridges. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Brian C. Bridges, YnJpYW4uYy5icmlkZ2VzQHZhbmRlcmJpbHQuZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.