
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pediatr., 27 March 2018
Sec. Pediatric Critical Care
Volume 6 - 2018 | https://doi.org/10.3389/fped.2018.00068
This article is part of the Research TopicPediatric Critical Care in Resource-Limited SettingsView all 16 articles
It may be difficult to predict the consequences of provision of high-cost pediatric care (HCC) in low- and middle-income countries (LMICs), and these consequences may be different to those experienced in high-income countries. An evaluation of the implications of HCC in LMICs must incorporate considerations of the specific context in that country (population age profile, profile of disease, resources available), likely costs of the HCC, likely benefits that can be gained versus the costs that will be incurred. Ideally, the process that is followed in decision making around HCC should be transparent and should involve the communities that will be most affected by those decisions. It is essential that the impacts of provision of HCC are carefully monitored so that informed decisions can be made about future provision medical interventions.
Ideally, every child in the world should have access to an intensive care unit with facilities for endotracheal intubation and mechanical ventilation. No ethical justification exists for providing these treatments to children in rich countries while denying them to children in poor countries (Shann, 2011)
While there is no ethical justification for differences in health-care access for children across the world, dealing with the realities of those differences remains profoundly challenging. During 2016, 5.6 million children under the age of 5 years died across the world (this is equivalent to 15,000 under-5 deaths per day), mostly in low- (LICs) and low- and middle-income countries (LMICs). The majority of those deaths could have been prevented using simple and affordable interventions (1). There are huge differences in under-5 mortality between high- and LICs as well as between higher- and lower-income groups within individual countries (2), in both high- (3) and LICs (4). Addressing intra-country differences could make nearly as much difference to child mortality as addressing the differences between countries. There have been substantial improvements in child survival across the world with the implementation of the millennium development goals, and with those gains, the role and importance of critical care in low- and middle-income countries (LMICs) has increased (5). However, the decision to provide high-cost care in low-income regions is a complex issue where the outcomes and consequences of that provision may be unpredictable and substantially different to those experienced in high-income countries.
High-cost medical care (HCC) incorporates a wide range of medical interventions, and the “value” that is associated with the provision of HCC must take into account many factors including costs, and benefits and the priorities of the people living in those areas. The “high cost” has to be viewed in relation to the available resources and in relation to the value delivered by the care (it is possible to have both high cost and high value, and high cost and low value). Some have argued that there are no “universal” bioethics (6, 7), but perhaps there may be validity in creating some transparency around the processes that are involved in making decisions as to how resources are allocated and on what basis.
In this review, I will consider the health-care context of LICs, the costs and benefits associated with the provision of HCC (in general, but more specifically directed at pediatric critical care), the way in which different people may be affected by HCC, and consider how the process of decision making around resource allocation could be addressed in LICs. Resources are limited in all regions, but there are substantial differences in resource availability, specific contexts, and particular demands on health-care systems across the world.
Decision making around the utilization of resources for HCC is deeply affected by a wide number of issues including the underlying context (particularly in terms of disease profile and morbidity and mortality data), the resources available (both absolute and relative amounts), and the potential benefits associated with the HCC (to the child, the family, the community, and the overall health services). An underlying concern with the process of “lumping” countries and groups of countries or communities into simple categories (such as LICs) is the fact that there are huge differences in context between (and within) countries, even within similar income groupings. It is not only the current reality but the “trajectory” of development in a country that may affect which health-care services can and should be provided.
As shown in Table 1, approximately 60% of the world’s children live in low- and LMICs, with some 275 million children living in low-income regions of the world. In those regions, children making up a very substantial proportion of the population have limited access to health-care services and extremely limited access to high-cost health-care services. Health-care expenditure in these regions averages US$35 per capita per annum, and the under-5 mortality is approximately 70 per 1000 live births. The majority of childhood deaths occur in these countries, and various authors have highlighted the fact that the majority of these deaths could be prevented by the implementation of relatively low-cost (and affordable) interventions.
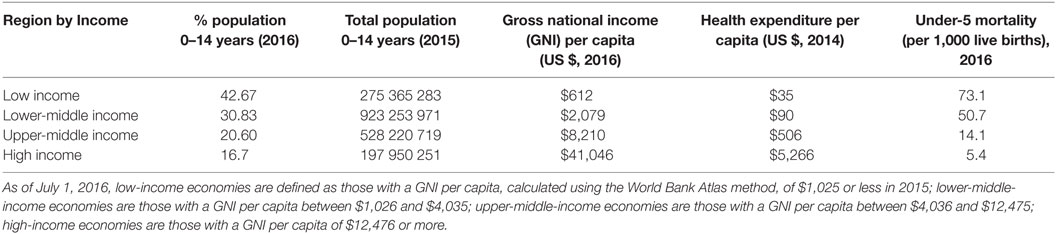
Table 1. Data on population, mortality, and health expenditure by income region (8).
LICs have many features which make the delivery of health care challenging including limited financial and personnel resources for health care, geographical features which may make transport and access challenging, political instability, limited infrastructure (water, electricity, sanitation, transport services, and transport infrastructure), and limited organizational and administrative infrastructure. The large number of displaced people and refugees also complicates decision making for care delivery.
The disease profile in LICs may be substantially different to that in HICs (9–11) and is also changing. In general, there is more trauma (including burns), and there are more infections (including more non-bacterial infections such as dengue, malaria, trypanosomiasis, etc.), and more infections with antibiotic-resistant organisms. Particular concerns relate to infections such as drug-resistant tuberculosis. Data on non-infectious disease are limited, but there is no reason to believe that there is a lower incidence in poorer countries.
As a consequence of multiple factors, patients often present late for therapy (12, 13) with the result that disease processes are often substantially advanced at the time of presentation. A high proportion of deaths occur soon after admission (14). Resource limitations translate into a low number of health-care workers and particularly health-care workers with specialist skills in areas such as pediatrics and pediatric surgery (see Table 2). These workers are deployed in environments that may be overcrowded and poorly maintained (and thus difficult to keep clean), uncomfortable (including heat and humidity or even cold), with very limited equipment and medication. The health-care facilities are often situated in contexts where there may be limited and unreliable sources of clean water, sanitation, and power (particularly electricity and lighting). In a recent review of hospitals in sub-Saharan Africa, the percentage of hospitals with dependable running water and electricity ranged from 22 to 46%, and in countries analyzed, only 19–50% of hospitals had the ability to provide 24-h emergency care (15).
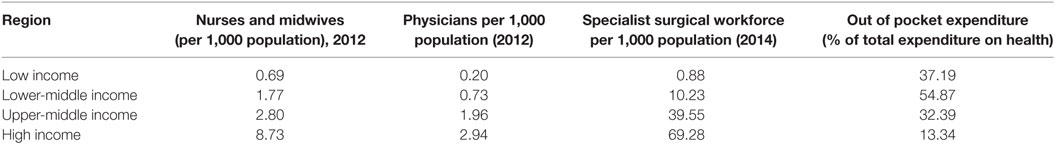
Table 2. Resources for health care by income region (8).
Limited and often dysfunctional supply chains contribute to nonavailability of resources that would be taken for granted and assumed in HICs. All of this may be aggravated by political instability and limited personal safety. In the event of natural disasters or outbreaks of infectious disease, these systems may fail spectacularly as has been demonstrated during recent outbreaks of Ebola virus infection in West Africa (16).
All the factors above need to be taken into consideration when contemplating resource allocation for high-cost care.
In considering the ethics of high-cost care in LICs, it is essential to balance the resources that are available for that care with the outcomes that can be achieved using those resources.
The financial resources available for health care can be considered from a number of perspectives (see Figures 1 and 2). There may be complex interactions between multiple factors.
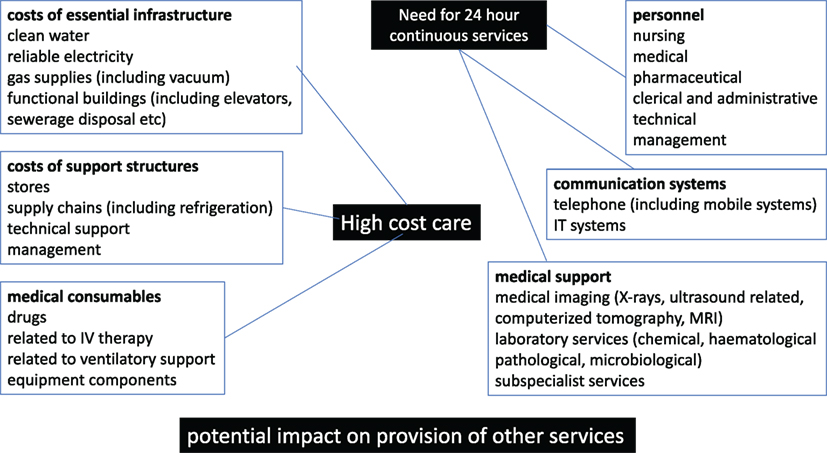
Figure 1. Components of the costs related to high-cost care (HCC) in low- and middle-income countries and low-income countries.
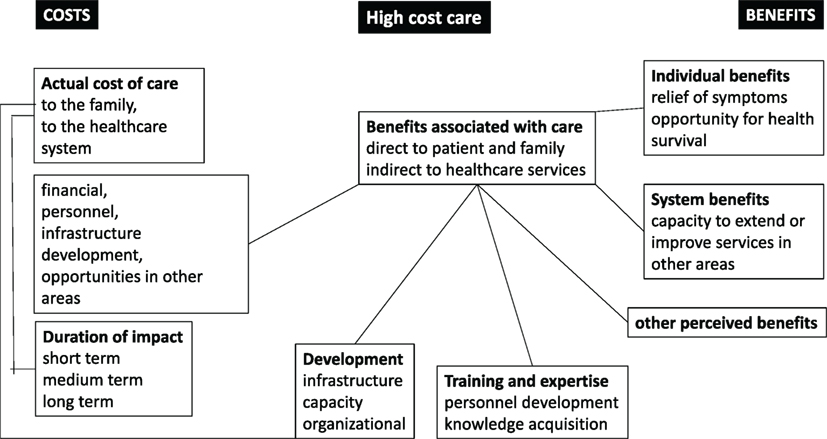
Figure 2. The balances of costs and benefits for high-cost care (HCC) in low- and middle-income countries and low-income countries.
As shown in Table 1, there is a >10-fold difference in the annual per capita health-care expenditure between LMICs and high middle-income countries and >100-fold difference between LMICs.
In LICs, a significant proportion of the funding available for health care may come from outside the country (see Table 2). That funding may be useful but is usually to be used in ways that are defined by the sources of the funding. Increasingly, funding may also be sourced from research projects that are based on high-income countries. This means that there are external drivers of how that funding can and should be used for health-care provision.
There are different ways of assessing the relative costs of high-cost services. When considering the costs of intensive care in 2009, Baker et al. estimated the costs of a day in intensive care in rich countries to be of the order of $1,000 (17). From a national perspective, that day cost would be approximately 20% of the annual per capita expenditure on health in a rich country but approximately 30 times the annual per capita expenditure on health in an LIC.
The costs of delivering some aspects of critical care in poorer countries may be substantially lower than this, as an example the cost of a day in a private ICU for cancer patients in India was reported as $57, However, in settings where a substantial proportion of health-care costs are covered by out-of-pocket expenditure by families (Table 2), that amount has to be related to the family income. In this setting, $57 was approximately 100× the average per capita household income (18). Thus, the provision of expensive health-care services to individuals in these settings has the potential to devastate the financial structure of a family with severe impact on other family members (including siblings) (19).
Significant as this overall difference is, it may actually mask other differences in costs for HCC. The HCC costs in rich countries reflect the costs of providing that care in the context of systems with well-established and functional infrastructure. In LICs, the infrastructure required (including transport, electricity, water provision and sewerage disposal, medical gas supply systems, technical maintenance support, etc) to support complex medical care may be profoundly deficient. The real cost therefore of providing HCC in “austere conditions” may be substantially higher than currently estimated. This may, however, be offset by the lower salaries that are paid to health-care workers in poorer countries. As another example, it has been argued in HIC that catheter closure of ostium secundum atrial septal defects is substantially cheaper than surgical closure, but in fact this was not the case in Guatemala (20).
Many of the resources required for more expensive therapies (including equipment and medication) are developed and manufactured in high-income countries. Access to these resources may be limited by the direct costs, which are exacerbated by indirect costs such as transportation, import duties and taxes, and adverse financial exchange rates.
In many LMICs, the differentials in access to high-cost health care between rich and poor people may vary substantially. In many countries, a small proportion of the population have access to state-of-the-art medical services while the majority of people within the same country may have limited or virtually nonexistent access to health-care services (21). In South Africa in 2015, approximately 49.8% of total health-care expenditure was from private sources, and only approximately 16% of the population have private health-care insurance (22). This difference in financial resources translates into major differences in access to facilities such as intensive care beds (21, 23).
The widespread corruption in some LICs and LMICs may have a profound effect on the resources actually available for health-care services (24).
Thus, any analysis of the resource implications for HCC in LICs must include a review of all the multiple factors and details in specific environments that may profoundly affect both the absolute and the relative costs of HCC in these countries.
Not only are health-care systems in LICs limited by financial resources but they also have profound challenges as regards the availability of trained and skilled health-care providers. Some years ago, the WHO estimated that the world faced a global shortage of almost 4.3 million doctors, midwives, nurses, and other health-care professionals (25). There are particular shortages in the area of surgery [including pediatric surgery (26, 27)] and anesthesia (28) and rehabilitation personnel (29) in poorer countries. The availability of these health-care workers (particularly to patients who cannot afford private health care) may be profoundly affected by the different remuneration patterns and the fact that many health-care workers have to work in several sectors in order to obtain an adequate income (30).
The standard of care that is (and can be) expected from health-care workers may vary substantially. In most HICs, it would be assumed that staff on out-of-hours duty would be fully awake and present throughout their working time. In many LICs, there is an expectation that afterhours staff will be heavily reduced in numbers relative to the day and that they will be expected to sleep for at least some of the time on duty (after all, many have day jobs that they also have to attend to).
When 24-h services are essential for the provision of HCC (such as pediatric intensive care), then personnel may be both a limiting factor as regards availability, but also as regards costs (four to five people have to be employed for each position that needs to be filled on a 24-h basis).
With substantial differences between financial resources in private and government sectors in LMICs, there are major pressures for health-care workers to move into the more affluent areas either full-time or part-time. This process may substantially decrease the access of less affluent members of society to health-care services that are high cost and often require high levels of training and expertise.
Decisions about HCC in LMIC and LICs may also have an effect on medical emigration. As an example, individuals with training in areas such as surgery or cardiac surgery are likely to emigrate or to move into the private sector if they are consistently unable to operate because of the lack of availability of theater time or PICU beds.
Many of the structures in terms of policies, programs, and training infrastructure that are required to support intensive care therapies are simply not present in LICs, as shown in a study completed in Tanzania (31). Thus, time and effort will have to be expended to put all those structures in place before particular services can be provided.
The outcomes of high-cost services are not always simple to establish and may relate to the underlying conditions (see Table 3): the specific interventions undertaken, and the expertise and experience of the teams undertaking those interventions. Some high-cost interventions may be associated with excellent outcomes and minimal long-term costs. At the other extreme, high-cost interventions may be associated with poor outcomes and high long-term costs.
There is evidence that relatively low-cost interventions in the care of critically ill children such as the provision of antibiotics at community level to neonates (40), the provision of oxygen therapy to children with pneumonia (41), the provision of high-flow humidified nasal oxygen or nasal CPAP (42, 43), and the improvement of organization of emergency services or of pediatric services within a hospital (44) may be associated with substantial reductions in mortality without significant added expense. Recent neonatal data suggested that focused implementation of nasal CPAP in Nicaragua could provide improved outcomes while reducing invasive mechanical ventilation (45).
Mechanical ventilation has been described in many reports from LICs as being associated with relatively high mortality (46, 47). Thus, in many situations, HCC such as ventilation may not improve overall outcomes as much as simpler and less-resource intensive care modalities.
Where intensive care services have been implemented in LICs, there is some evidence that the strict application of quality control programs can substantially improve the outcomes of pediatric intensive care (48, 49).
Having reviewed the context, the resources available, and the potential impact of HCC such as pediatric intensive care, it is important to consider ethical principles that could be considered in making decisions about the provision of HCC (see Table 4) as well as the characteristics of the processes used in decision making (Table 5). Turner et al. (14) have highlighted the principles of global justice, resource allocation, and local cultural preferences. Clearly, there is no ethical justification for the global differences in access of children to HCC (50), and there is a huge need to provide advocacy for additional resources from HIC to be allocated to LIMCs, but that will (at best) take time. However, there is a need to review both the basis and the processes used for resource allocation both within and between countries.
The principles generally considered in biomedical ethics (51) include respect for autonomy, non-maleficence, beneficence, and justice. It may be useful to consider the implications of these principles when applied at different levels of the health service delivery (Table 4).
Kass (52) proposed that the following questions should be asked when evaluating potential public health interventions:
What are the goals of the proposed program (this includes both the overall goals and the short-term goals) and in particular who will benefit from this intervention?
How effective is the program at achieving the stated goals?
What are the known or the potential burdens of the program?
Can the burdens be minimized or are there alternative approaches?
Is the program administered fairly?
How can the benefits and burdens of the program be fairly balanced?
If one considers the possibility of pediatric intensive care in LICs, the overall goal would be a reduction in mortality, and the short-term goal would be more effective resuscitation of critically ill children presenting to the health-care services. The costs of pediatric intensive care may be particularly high (including the entire system of staffing, equipment, and support structures). At best, the introduction of pediatric intensive care services will benefit the small number of children who have already accessed health-care services and have access to the intensive care. By contrast, a wider definition of critical care (53), which includes the process of providing care to all children with life-threatening injury and/or illness, would have the potential to affect a much wider group of children and potentially at a much lower cost per child.
It is possible to extrapolate from international data to the possible impact of HCC, but outcomes may be very different in LIMCs. In the setting of cardiac surgery in Guatemala, the outcomes were initially worse than expected and took time and considerable investment to improve, despite the presence of surgeons with considerable experience and training in the USA (54). There is also evidence that the establishment of international quality intervention program may lead to substantial improvements in outcomes from high-cost procedures as demonstrated in pediatric cardiac surgery (55).
The provision of PICU is very unlikely to make a substantial impact on the mortality of children in LICs where there are numerous deaths and at best extremely limited resources available for PICU. In middle-income countries, there is much more likelihood of PICU making an impact on pediatric mortality. There is also the consideration that the training of personnel for services such as PICU may take many years, and thus it may be worth considering the introduction of PICU and investing in personnel training some time before it is likely to be implemented within a region.
There is certainly evidence that over time, the introduction of relatively HCC has improved outcomes in a number of settings (56, 57), particularly for oncological problems. There is also evidence that HCC in LICs can improve over a period of time (47).
A particular challenge for HCC is the requirement of most HCCs for ancillary disciplines including anesthesia (58, 59), pediatric surgery (59), medical imaging, etc.—each of these services is frequently required to implement HCC, and all are under pressure, individual HCC may be difficult to implement.
It is also essential to consider the place of the HCC within the overall context of the health services within that country. Particular in areas such as the care of critically ill children, the overall outcome will depend on the entire “pathway” of care and not simply on the intensive care component (13, 60).
One of the potential consequences of the differentials between private and public health care in LMICs is that both sectors may have a limited benefit from HCC. In the private sector, the limited number of people having access may limit the experience and expertise that can be achieved by therapeutic teams, while the majority of patients simply do not have access. In addition, the movement of expertise for HCC from the public to the private sector may further compromise the quality of care in the public sector.
The direct burden of HCC such as pediatric intensive care relates to the consumption of resources (financial and personnel) within the context of limited resources. An indirect burden of HCC such as pediatric intensive care is the large burden of illness and handicap that may affect both the children who survive PICU (61–63) and their parents (64). This may be extremely problematic in settings where long-term support and rehabilitation are poorly available. Thus, decision making within the HCC environment may include the need to limit interventions at levels that would not usually be considered appropriate in HICs.
A significant potential burden of HCC is the impact of that allocation of resources on other services. In situations where there are relatively rich resources, the implementation of HCC may have a very little impact on other services. Sadly, in LICs, the development of HCC is inevitably going to compromise care in some other area of the system, and it is essential that that impact is recognized, assessed, and included in the overall evaluation process.
A major factor in providing pediatric intensive care services within LMICs [or during disaster events (65)] is the process of developing reasonable strategies to allocate those resources (66), and it is important to consider how this problem will be addressed.
The burdens of HCC can be limited or minimized by strict definitions and decision making around what services can and should be provided and to whom. Ideally, the process of decision making should be transparent and public (66). One potential approach to the process of resource allocation, and particularly the allocation of resources to relatively expensive services such as intensive care is the accountability for reasonableness (A4R) process that was initially described in Canada (67).
As suggested above, it is also important to consider whether in fact less expensive care could be utilized to achieve the same or better outcomes. It has been well demonstrated in several settings that the use of “lower” technology such as nasal cPAP for severe bronchiolitis was associated with both lower costs and better outcomes (68).
This question can be addressed at different phases. Initially, it may be important to carefully consider whether there are individuals or groups of people who are not receiving an equitable opportunity to access the HCC services. It may initially be possible to identify groups of people who for particular reasons (including geography) will not be able to access certain services as easily as others. There are also situations where it is clearly not possible to provide all care to all people (69, 70), and decisions have to made as to what therapy can and should be made available. Ideally, the processes for the allocation of the resources should be agreed to in a process involving the communities affected by those services (66). Generally, those processes will need to have characteristics such as accountability and transparency (see Table 5).
In the longer term, the question can only be fully answered if data are collected on who actually utilized the services and what outcomes were achieved. Carefully collected data provide the only real way in which the impact of a service can be assessed. Inherently, data capture should include not only information on those who accessed a service but also data on those who perhaps could or should have accessed those services.
The benefits of the program can be considered at the individual level. Guidelines for the provision of life-sustaining care in neonates (71), children (72), and adults (73) in HICs have highlighted the need to act in the best interests of the patient at all times. Clearly, there are situations where the application of HCC may actually prolong suffering, and there would be a substantial (although not universal) agreement that this should be avoided.
The training of health-care workers may be substantially affected by decisions made regarding HCC that will be offered in a particular region. The training required to offer HCC such as intensive care is potentially both time-consuming and expensive. The provision of training to health-care workers without a commitment to support the HCC is likely to lead to frustration and in many cases the migration of health-care workers to richer parts of the world. However, lack of opportunities to undergo training and implement therapies (such as surgery and anesthesia) may also lead to medical migration-associated health-care problems.
Decision making around the utilization of high-cost care may affect a number of people including the patient and the family, health-care workers, hospital and health-care managers, and politicians and people involved with the formulation and administration of policy at a provincial and/or national level. With the exponential growth of global health programs in areas such as North America, there are also an increasing number of people outside of the countries who have an interest and sometimes incentives (financial and otherwise) in changing the provision of HCC in LICs and LMICs.
A crucial aspect of balancing burdens and benefits relates to the standards that are expected and applied in the development of the HCC. Programs that have implemented oncology training and services in LIMCs have had to make decisions about the level of services that could safely be provided in those settings (74). The only way in which the impact of interventions can be monitored is by the collection of the appropriate data (both in the HCC service and in the services that are potentially affected) (45, 75). Bhutta (76) has highlighted the need (in research) to consider the realities and constraints in the countries where the research or clinical services being considered have to be implemented. In the setting of resource constraints, the actual care currently available and the risks that would be acceptable in clinical care may be very different to those where other resources are available.
The only way to address potential and actual ethical concerns in this setting is to make sure that information regarding who is involved in the process of providing the HCC must be clearly available to all the people involved in the process. In addition, the allocation of resources to data collection and interpretation is the only way in which it is possible to assess the impact of HCC and interventions. The allocation of limited resources to this monitoring may seem a relatively low priority, but in the longer term, it is the only way in which interventions can be assessed and thus provides a rational basis for ongoing decision making.
In an ideal system, decisions regarding the implementation of health-care policies would be made: in a coherent way at every level of policy making (Table 4), by people with a clear understanding of both the costs and the achievable benefits of high-cost interventions (particularly relative to other lower cost interventions), and in a way that involves and takes into account the wishes and concerns of the people who are affected by those decisions and policies. Particular concerns in LICs are processes required to develop the expertise and organization structures (77) that are locally available. International groupings and organizations may be able to make a contribution in this regard.
It may be particularly challenging to address the relative contributions of managers in health-care systems to decision making. In the South African context, it has been pointed out that while there are substantially higher resources per capita in the private health-care system, much of those resources come from the contributions of the people who benefit from that system. If in an LIC, richer people are effectively paying for their own health services, is there a reasonable case to allow that? In that setting, it may be crucial to develop a detailed understanding of the proportion of the real costs that the public sector is bearing (including training costs, tax rebates, etc). It may also be critical to consider collaborative efforts to bring about mutually beneficial outcomes (78, 79), although these may be associated with risks.
Internationally, support for the collection of accurate data regarding the costs and outcomes of HCC for children in LMICs could provide a substantial evidence base for decision making in those areas. Importantly, the data collection should include the specifics of local contexts as far as possible.
A number of authors have addressed the processes that may be involved, with particular support for the use of the A4R approach (66, 80–83). There is strong evidence that this approach may be useful in addressing a range of health-care dilemmas (including access to HCC such as dialysis). However, there is a real need for ongoing research into decision-making processes in LIMCs (84, 85). Underlying that research is the need for a deeper understanding of the values that need to be incorporated into decision making (and which are not universally agreed to) (6, 7).
Ethical decision making around the provision of HCC pediatric care in LMICs and LICs may be a complex process which requires a deep understanding of the context and the implications of any intervention. At the very least, the process needs to incorporate a realistic assessment of the context, the resources (both available and required), and the likely impact of the provision of that care. There is a very real risk that the implementation of high-cost pediatric care may have relatively poor outcomes, and even worse, the utilization of resources in this way may compromise other services with adverse outcomes for many children.
Ideally, all decision making should be transparent and should involve all the communities who are likely to be affected by those decisions. Frameworks from the public health environment may provide a useful addition to the standard bioethical approach.
The author confirms being the sole contributor of this work and approved it for publication.
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. World Health Organisation. Children: Reducing Mortality (Fact sheet Updated October 2017) [Online]. World Health Organisation (2017). Available from: http://www.who.int/mediacentre/factsheets/fs178/en/ (accessed October 27, 2017).
2. Amouzou A, Kozuki N, Gwatkin DR. Where is the gap?: the contribution of disparities within developing countries to global inequalities in under-five mortality. BMC Public Health (2014) 14:216. doi:10.1186/1471-2458-14-216
3. Blendon RJ, Schoen C, DesRoches CM, Osborn R, Scoles KL, Zapert K. Inequities in health care: a five-country survey. Health Aff (Millwood) (2002) 21(3):182–91. doi:10.1377/hlthaff.21.3.182
4. Braveman P, Tarimo E. Social inequalities in health within countries: not only an issue for affluent nations. Soc Sci Med (2002) 54(11):1621–35. doi:10.1016/S0277-9536(01)00331-8
5. Dondorp AM, Iyer SS, Schultz MJ. Critical care in resource-restricted settings. JAMA (2016) 315(8):753–4. doi:10.1001/jama.2016.0976
6. Engelhardt HT Jr. Critical care: why there is no global bioethics. J Med Philos (1998) 23(6):643–51. doi:10.1076/jmep.23.6.643.2555
7. Engelhardt HT Jr. Critical care: why there is no global bioethics. Curr Opin Crit Care (2005) 11(6):605–9. doi:10.1097/01.ccx.0000184166.10121.c1
8. World Bank Database [Online]. (2017). Available from: http://databank.worldbank.org/data/reports.aspx?source=health-nutrition-and-population-statistics::x00023 (accessed October 26, 2017).
9. Dunser MW, Festic E, Dondorp A, Kissoon N, Ganbat T, Kwizera A, et al. Recommendations for sepsis management in resource-limited settings. Intensive Care Med (2012) 38(4):557–74. doi:10.1007/s00134-012-2468-5
10. Dondorp AM, Haniffa R. Critical care and severe sepsis in resource poor settings. Trans R Soc Trop Med Hyg (2014) 108(8):453–4. doi:10.1093/trstmh/tru099
11. Meier B, Staton C. Sepsis resuscitation in resource-limited settings. Emerg Med Clin North Am (2017) 35(1):159–73. doi:10.1016/j.emc.2016.08.004
12. Papali A, McCurdy MT, Calvello EJ. A "three delays" model for severe sepsis in resource-limited countries. J Crit Care (2015) 30(4):e869–814. doi:10.1016/j.jcrc.2015.04.003
13. Hodkinson P, Argent A, Wallis L, Reid S, Perera R, Harrison S, et al. Pathways to care for critically Ill or injured children: a cohort study from first presentation to healthcare services through to admission to intensive care or death. PLoS One (2016) 11(1):e0145473. doi:10.1371/journal.pone.0145473
14. Turner EL, Nielsen KR, Jamal SM, von Saint Andre-von Arnim A, Musa NL. A review of pediatric critical care in resource-limited settings: a look at past, present, and future directions. Front Pediatr (2016) 4:5. doi:10.3389/fped.2016.00005
15. Hsia RY, Mbembati NA, Macfarlane S, Kruk ME. Access to emergency and surgical care in sub-Saharan Africa: the infrastructure gap. Health Policy Plan (2012) 27(3):234–44. doi:10.1093/heapol/czr023
16. Leligdowicz A, Fischer WA II, Uyeki TM, Fletcher TE, Adhikari NK, Portella G, et al. Ebola virus disease and critical illness. Crit Care (2016) 20(1):217. doi:10.1186/s13054-016-1325-2
17. Baker T. Critical care in low-income countries. Trop Med Int Health (2009) 14(2):143–8. doi:10.1111/j.1365-3156.2008.02202.x
18. Kulkarni AP, Divatia JV. A prospective audit of costs of intensive care in cancer patients in India. Indian J Crit Care Med (2013) 17(5):292–7. doi:10.4103/0972-5229.120321
19. Miljeteig I, Johansson KA, Sayeed SA, Norheim OF. End-of-life decisions as bedside rationing. An ethical analysis of life support restrictions in an Indian neonatal unit. J Med Ethics (2010) 36(8):473–8. doi:10.1136/jme.2010.035535
20. Vida VL, Barnoya J, O’Connell M, Leon-Wyss J, Larrazabal LA, Castaneda AR. Surgical versus percutaneous occlusion of ostium secundum atrial septal defects: results and cost-effective considerations in a low-income country. J Am Coll Cardiol (2006) 47(2):326–31. doi:10.1016/j.jacc.2005.06.086
21. Beerenahally TS. No free bed with ventilator: experience of a public health specialist. Indian J Med Ethics (2017) 2(1):56–7. doi:10.20529/ijme.2017.011
22. Dalal A, Czosek RJ, Kovach J, von Alvensleben JC, Valdes S, Etheridge SP, et al. Clinical presentation of pediatric patients at risk for sudden cardiac arrest. J Pediatr (2016) 177:191–6. doi:10.1016/j.jpeds.2016.06.088
23. Bhagwanjee S, Scribante J. National audit of critical care resources in South Africa—unit and bed distribution. S Afr Med J (2007) 97(12 Pt 3):1311–4.
24. Mostert S, Njuguna F, Olbara G, Sindano S, Sitaresmi MN, Supriyadi E, et al. Corruption in health-care systems and its effect on cancer care in Africa. Lancet Oncol (2015) 16(8):e394–404. doi:10.1016/s1470-2045(15)00163-1
25. Aluttis C, Bishaw T, Frank MW. The workforce for health in a globalized context—global shortages and international migration. Glob Health Action (2014) 7:23611. doi:10.3402/gha.v7.23611
26. Lalchandani P, Dunn JC. Global comparison of pediatric surgery workforce and training. J Pediatr Surg (2015) 50(7):1180–3. doi:10.1016/j.jpedsurg.2014.11.032
27. Toobaie A, Emil S, Ozgediz D, Krishnaswami S, Poenaru D. Pediatric surgical capacity in Africa: current status and future needs. J Pediatr Surg (2017) 52(5):843–8. doi:10.1016/j.jpedsurg.2017.01.033
28. Hoyler M, Finlayson SR, McClain CD, Meara JG, Hagander L. Shortage of doctors, shortage of data: a review of the global surgery, obstetrics, and anesthesia workforce literature. World J Surg (2014) 38(2):269–80. doi:10.1007/s00268-013-2324-y
29. Jesus TS, Landry MD, Dussault G, Fronteira I. Human resources for health (and rehabilitation): six rehab-workforce challenges for the century. Hum Resour Health (2017) 15(1):8. doi:10.1186/s12960-017-0182-7
30. Bertone MP, Witter S. The complex remuneration of human resources for health in low-income settings: policy implications and a research agenda for designing effective financial incentives. Hum Resour Health (2015) 13:62. doi:10.1186/s12960-015-0058-7
31. Baker T, Lugazia E, Eriksen J, Mwafongo V, Irestedt L, Konrad D. Emergency and critical care services in Tanzania: a survey of ten hospitals. BMC Health Serv Res (2013) 13:140. doi:10.1186/1472-6963-13-140
32. Tyler A, McLeod L, Beaty B, Juarez-Colunga E, Birkholz M, Hyman D, et al. Variation in inpatient croup management and outcomes. Pediatrics (2017) 139(4):e20163582. doi:10.1542/peds.2016-3582
33. Sri-udomkajorn S, Suwannachote S. Demographics, clinical features, outcome and prognostic factors of Guillain–Barre syndrome in Thai children. J Med Assoc Thai (2014) 97(Suppl 6):S101–7.
34. Barzegar M, Toopchizadeh V, Maher MHK, Sadeghi P, Jahanjoo F, Pishgahi A. Predictive factors for achieving independent walking in children with Guillain–Barre syndrome. Pediatr Res (2017) 82(2):333–9. doi:10.1038/pr.2017.67
35. van der Pijl J, Wilmshurst JM, van Dijk M, Argent A, Booth J, Zampoli M. Acute flaccid paralysis in South African children: causes, respiratory complications and neurological outcome. J Paediatr Child Health (2017) 54(3):247–53. doi:10.1111/jpc.13709
36. English L, Kumbakumba E, Larson CP, Kabakyenga J, Singer J, Kissoon N, et al. Pediatric out-of-hospital deaths following hospital discharge: a mixed-methods study. Afr Health Sci (2016) 16(4):883–91. doi:10.4314/ahs.v16i4.2
37. Chisti MJ, Graham SM, Duke T, Ahmed T, Faruque AS, Ashraf H, et al. Post-discharge mortality in children with severe malnutrition and pneumonia in Bangladesh. PLoS One (2014) 9(9):e107663. doi:10.1371/journal.pone.0107663
38. Nasr VG, Faraoni D, Valente AM, DiNardo JA. Outcomes and costs of cardiac surgery in adults with congenital heart disease. Pediatr Cardiol (2017) 38(7):1359–64. doi:10.1007/s00246-017-1669-7
39. Zuhlke L, Karthikeyan G, Engel ME, Rangarajan S, Mackie P, Cupido-Katya Mauff B, et al. Clinical outcomes in 3343 children and adults with rheumatic heart disease from 14 low- and middle-income countries: two-year follow-up of the global rheumatic heart disease registry (the REMEDY study). Circulation (2016) 134(19):1456–66. doi:10.1161/circulationaha.116.024769
40. Bhutta ZA, Zaidi AK, Thaver D, Humayun Q, Ali S, Darmstadt GL. Management of newborn infections in primary care settings: a review of the evidence and implications for policy? Pediatr Infect Dis J (2009) 28(1 Suppl):S22–30. doi:10.1097/INF.0b013e31819588ac
41. Duke T, Wandi F, Jonathan M, Matai S, Kaupa M, Saavu M, et al. Improved oxygen systems for childhood pneumonia: a multihospital effectiveness study in Papua New Guinea. Lancet (2008) 372(9646):1328–33. doi:10.1016/s0140-6736(08)61164-2
42. Chisti MJ, Salam MA, Smith JH, Ahmed T, Pietroni MA, Shahunja KM, et al. Bubble continuous positive airway pressure for children with severe pneumonia and hypoxaemia in Bangladesh: an open, randomised controlled trial. Lancet (2015) 386(9998):1057–65. doi:10.1016/s0140-6736(15)60249-5
43. Wilson PT, Baiden F, Brooks JC, Morris MC, Giessler K, Punguyire D, et al. Continuous positive airway pressure for children with undifferentiated respiratory distress in Ghana: an open-label, cluster, crossover trial. Lancet Glob Health (2017) 5(6):e615–23. doi:10.1016/s2214-109x(17)30145-6
44. Molyneux E, Ahmad S, Robertson A. Improved triage and emergency care for children reduces inpatient mortality in a resource-constrained setting. Bull World Health Organ (2006) 84(4):314–9. doi:10.2471/BLT.04.019505
45. Rezzonico R, Caccamo LM, Manfredini V, Cartabia M, Sanchez N, Paredes Z, et al. Impact of the systematic introduction of low-cost bubble nasal CPAP in a NICU of a developing country: a prospective pre- and post-intervention study. BMC Pediatr (2015) 15:26. doi:10.1186/s12887-015-0338-3
46. Mukhtar B, Siddiqui NR, Haque A. Clinical characteristics and immediate-outcome of children mechanically ventilated in PICU of pakistan. Pak J Med Sci (2014) 30(5):927–30. doi:10.12669/pjms.305.5159
47. Khanal A, Sharma A, Basnet S. Current state of pediatric intensive care and high dependency care in Nepal. Pediatr Crit Care Med (2016) 17(11):1032–40. doi:10.1097/pcc.0000000000000938
48. Pollach G, Namboya F. Preventing intensive care admissions for sepsis in tropical Africa (PICASTA): an extension of the international pediatric global sepsis initiative: an African perspective. Pediatr Crit Care Med (2013) 14(6):561–70. doi:10.1097/PCC.0b013e318291774b
49. Rosenthal VD, Pawar M, Leblebicioglu H, Navoa-Ng JA, Villamil-Gomez W, Armas-Ruiz A, et al. Impact of the International Nosocomial Infection Control Consortium (INICC) multidimensional hand hygiene approach over 13 years in 51 cities of 19 limited-resource countries from Latin America, Asia, the Middle East, and Europe. Infect Control Hosp Epidemiol (2013) 34(4):415–23. doi:10.1086/669860
50. Shann F, Argent AC, Ranjit S. Pediatric intensive care in developing countries. 4th ed. In: Fuhrman BP, Zimmerman JJ, editors. Pediatric Critical Care. Philadelphia: Elsevier Health Science (2011). p. 164–78.
51. Beauchamp TL, Childress JF. Principles of Biomedical Ethics. Oxford: Oxford University Press (2013).
52. Kass NE. An ethics framework for public health. Am J Public Health (2001) 91(11):1776–82. doi:10.2105/AJPH.91.11.1776
53. Kissoon N, Argent A, Devictor D, Madden MA, Singhi S, van der Voort E, et al. World federation of pediatric intensive and critical care societies-its global agenda. Pediatr Crit Care Med (2009) 10(5):597–600. doi:10.1097/PCC.0b013e3181a704c6
54. Leon-Wyss JR, Veshti A, Veras O, Gaitan GA, O’Connell M, Mack RA, et al. Pediatric cardiac surgery: a challenge and outcome analysis of the Guatemala effort. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu (2009) 12(1):8–11. doi:10.1053/j.pcsu.2009.01.003
55. Jenkins KJ, Castaneda AR, Cherian KM, Couser CA, Dale EK, Gauvreau K, et al. Reducing mortality and infections after congenital heart surgery in the developing world. Pediatrics (2014) 134(5):e1422–30. doi:10.1542/peds.2014-0356
56. Samudio A, Figueredo D, Lassaletta A, Zelada O, Peris A, Bogado Yinde L, et al. Building a national pediatric cancer center and network in Paraguay: lessons for addressing challenges in a low-income country. J Pediatr Hematol Oncol (2015) 37(5):383–90. doi:10.1097/mph.0000000000000338
57. Molyneux E, Scanlan T, Chagaluka G, Renner L. Haematological cancers in African children: progress and challenges. Br J Haematol (2017) 177(6):971–8. doi:10.1111/bjh.14617
58. Notrica MR, Evans FM, Knowlton LM, Kelly McQueen KA. Rwandan surgical and anesthesia infrastructure: a survey of district hospitals. World J Surg (2011) 35(8):1770–80. doi:10.1007/s00268-011-1125-4
59. Butler M, Drum E, Evans FM, Fitzgerald T, Fraser J, Holterman AX, et al. Guidelines and checklists for short-term missions in global pediatric surgery: recommendations from the American Academy of Pediatrics Delivery of Surgical Care Global Health Subcommittee, American Pediatric Surgical Association Global Pediatric Surgery Committee, Society for Pediatric Anesthesia Committee on International Education and Service, and American Pediatric Surgical Nurses Association, Inc. Global Health Special Interest Group. J Pediatr Surg (2017). doi:10.1016/j.jpedsurg.2017.11.037
60. Jones CH, Ward A, Hodkinson PW, Reid SJ, Wallis LA, Harrison S, et al. Caregivers’ experiences of pathways to care for seriously Ill children in Cape Town, South Africa: a qualitative investigation. PLoS One (2016) 11(3):e0151606. doi:10.1371/journal.pone.0151606
61. Als LC, Picouto MD, Hau SM, Nadel S, Cooper M, Pierce CM, et al. Mental and physical well-being following admission to pediatric intensive care. Pediatr Crit Care Med (2015) 16(5):e141–9. doi:10.1097/pcc.0000000000000424
62. Aspesberro F, Mangione-Smith R, Zimmerman JJ. Health-related quality of life following pediatric critical illness. Intensive Care Med (2015) 41(7):1235–46. doi:10.1007/s00134-015-3780-7
63. Ong C, Lee JH, Leow MK, Puthucheary ZA. Functional outcomes and physical impairments in pediatric critical care survivors: a scoping review. Pediatr Crit Care Med (2016) 17(5):e247–59. doi:10.1097/pcc.0000000000000706
64. Balluffi A, Kassam-Adams N, Kazak A, Tucker M, Dominguez T, Helfaer M. Traumatic stress in parents of children admitted to the pediatric intensive care unit. Pediatr Crit Care Med (2004) 5(6):547–53. doi:10.1097/01.pcc.0000137354.19807.44
65. Burkle FM Jr, Argent AC, Kissoon N. The reality of pediatric emergency mass critical care in the developing world. Pediatr Crit Care Med (2011) 12(6 Suppl):S169–79. doi:10.1097/PCC.0b013e318234a906
66. Argent AC, Ahrens J, Morrow BM, Reynolds LG, Hatherill M, Salie S, et al. Pediatric intensive care in South Africa: an account of making optimum use of limited resources at the Red Cross War Memorial Children’s Hospital*. Pediatr Crit Care Med (2014) 15(1):7–14. doi:10.1097/pcc.0000000000000029
67. Daniels N. Decisions about access to health care and accountability for reasonableness. J Urban Health (1999) 76(2):176–91. doi:10.1007/bf02344674
68. Essouri S, Laurent M, Chevret L, Durand P, Ecochard E, Gajdos V, et al. Improved clinical and economic outcomes in severe bronchiolitis with pre-emptive nCPAP ventilatory strategy. Intensive Care Med (2014) 40(1):84–91. doi:10.1007/s00134-013-3129-z
69. Jeena PM, McNally LM, Stobie M, Coovadia HM, Adhikari MA, Petros AJ. Challenges in the provision of ICU services to HIV infected children in resource poor settings: a South African case study. J Med Ethics (2005) 31(4):226–30. doi:10.1136/jme.2003.004010
70. Thiagraj Soobramoney v. Minster Of Health (Kwazulu-Natal). South African Constitutional Court. Case CCT 32/97. (1998).
71. Nuffield Council on Bioethics. Critical Care Decisions in Fetal and Neonatal Medicine: Ethical Issues. London: Nuffield Council on Bioethics (2006).
72. Inwald D. The best interests test at the end of life on PICU: a plea for a family centred approach. Arch Dis Child (2008) 93(3):248–50. doi:10.1136/adc.2006.111120
73. Curtis JR, Vincent JL. Ethics and end-of-life care for adults in the intensive care unit. Lancet (2010) 376(9749):1347–53. doi:10.1016/s0140-6736(10)60143-2
74. Gupta S, Rivera-Luna R, Ribeiro RC, Howard SC. Pediatric oncology as the next global child health priority: the need for national childhood cancer strategies in low- and middle-income countries. PLoS Med (2014) 11(6):e1001656. doi:10.1371/journal.pmed.1001656
75. Zonneveld R, Holband N, Bertolini A, Bardi F, Lissone NPA, Dijk PH, et al. Improved referral and survival of newborns after scaling up of intensive care in Suriname. BMC Pediatr (2017) 17(1):189. doi:10.1186/s12887-017-0941-6
76. Bhutta ZA. Ethics in international health research: a perspective from the developing world. Bull World Health Organ (2002) 80(2):114–20.
77. Swanson RC, Atun R, Best A, Betigeri A, de Campos F, Chunharas S, et al. Strengthening health systems in low-income countries by enhancing organizational capacities and improving institutions. Global Health (2015) 11:5. doi:10.1186/s12992-015-0090-3
78. Kula N, Fryatt RJ. Public-private interactions on health in South Africa: opportunities for scaling up. Health Policy Plan (2014) 29(5):560–9. doi:10.1093/heapol/czt042
79. Rasanathan K, Bennett S, Atkins V, Beschel R, Carrasquilla G, Charles J, et al. Governing multisectoral action for health in low- and middle-income countries. PLoS Med (2017) 14(4):e1002285. doi:10.1371/journal.pmed.1002285
80. Maluka S, Kamuzora P, San Sebastian M, Byskov J, Ndawi B, Hurtig AK. Improving district level health planning and priority setting in Tanzania through implementing accountability for reasonableness framework: perceptions of stakeholders. BMC Health Serv Res (2010) 10:322. doi:10.1186/1472-6963-10-322
81. Maluka S, Kamuzora P, San Sebastian M, Byskov J, Olsen OE, Shayo E, et al. Decentralized health care priority-setting in Tanzania: evaluating against the accountability for reasonableness framework. Soc Sci Med (2010) 71(4):751–9. doi:10.1016/j.socscimed.2010.04.035
82. Maluka S, Kamuzora P, Sansebastian M, Byskov J, Ndawi B, Olsen OE, et al. Implementing accountability for reasonableness framework at district level in Tanzania: a realist evaluation. Implement Sci (2011) 6:11. doi:10.1186/1748-5908-6-11
83. Moosa MR, Maree JD, Chirehwa MT, Benatar SR. Use of the ‘accountability for reasonableness’ approach to improve fairness in accessing dialysis in a middle-income country. PLoS One (2016) 11(10):e0164201. doi:10.1371/journal.pone.0164201
84. Byskov J, Bloch P, Blystad A, Hurtig AK, Fylkesnes K, Kamuzora P, et al. Accountable priority setting for trust in health systems – the need for research into a new approach for strengthening sustainable health action in developing countries. Health Res Policy Syst (2009) 7:23. doi:10.1186/1478-4505-7-23
Keywords: ethics, low- and middle-income countries, high cost, intensive care, children
Citation: Argent AC (2018) Considerations for Assessing the Appropriateness of High-Cost Pediatric Care in Low-Income Regions. Front. Pediatr. 6:68. doi: 10.3389/fped.2018.00068
Received: 04 January 2018; Accepted: 08 March 2018;
Published: 27 March 2018
Edited by:
Ndidiamaka L. Musa, University of Washington, United StatesReviewed by:
Paolo Biban, Azienda Ospedaliera Universitaria Integrata Verona, ItalyCopyright: © 2018 Argent. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Andrew C. Argent, YW5kcmV3LmFyZ2VudEB1Y3QuYWMuemE=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.