- Child Health, University of Aberdeen, Aberdeen, UK
Asthma is a common condition, which is associated with atopy and allergic conditions including hay fever, eczema, and food allergies. Asthma and atopy are both complex conditions where genetic and environmental factors are implicated in causation. Interactions between genetic and environmental factors, likely via epigenetic mechanisms, are widely thought to be important in determining the risk for developing asthma and atopy. The nature of the relationship between asthma and atopy is unclear and the answer to the question “does atopy cause asthma?” remains unknown. This review explores the relationship between asthma and atopy from a gene–environment interaction perspective and tackles the question “are similar gene–environment interactions present for asthma and atopy?” The main finding is that gene–environment interactions are described for asthma and atopy in children but these interactions are seldom sought for both asthma and atopy in the same population. In the few instances where a gene–environment interaction is related to both asthma and atopy, there is no consistent evidence that similar interactions are common to asthma and atopy. Many plausible gene–environment interactions for asthma and atopy are yet to be explored. Overall, from the gene–environment interaction perspective, there is absence of evidence to better understand the complex relationship between asthma and atopy.
Introduction
Childhood asthma is a common condition, which affects approximately 1 million children in the UK (1) and six million children in the US (2). Treatment for asthma is available to treat and prevent symptoms, but at present there is no cure for asthma and, therefore, there is a pressing need to understand why some individuals develop asthma. Approximately 50 years of research has yielded a considerable amount of information, which allows us to understand some aspects of asthma pathogenesis.
Asthma has long been associated with atopy [(3), p. 63–70] and is regarded by some individuals as an atopic condition, i.e., one caused by atopy. Asthma is understood to be a complex condition where genetic and environmental factors both contribute to causation, and twin studies suggest that as much as 70% of asthma causation may be explained by hereditary factors [(4), p. 8–14]. The search for “the” asthma gene was called off many years ago with the realization that asthma is a polygenic conditions where approximately ten genes each make a modest contribution to risk [(5), p. 68–74]. There are several environmental exposures, which are associated with childhood asthma and these include exposure to second hand smoke (SHS), inhaled chemicals, mold, ambient air pollutants, some deficiencies in maternal diet, and respiratory viruses (6). Recent work suggests that the relationship between environmental exposures and asthma may change over time; for example, the relationship between SHS and asthma has become slightly stronger over time, perhaps as children become more susceptible (7). Many non-communicable diseases, such as asthma, have both a genetic predisposition and environmental triggers. The gene–environment relationship is nicely captured in the phrase “genetics loads the gun and the environment pulls the trigger.”
Atopy, defined here as production of Immunoglobulin E specific to a common environmental exposure, is a highly prevalent phenomenon in modern children. Some children who are atopic have no symptoms [(8), p. 580–587] and the prevalence of childhood atopy is hard to detect due to it being clinically silent in some individuals, but is likely to be in excess of 30% in Western populations. The prevalence of atopic conditions such as eczema and hay fever is more easily identified due to the presence of symptoms and is close to 30% in many populations despite the use of self-reported diagnosis captured often by different definitions [(9), p. 733–743; (10), p. e008446]. Twin studies of eczema [(11), p. 535–539] and hay fever [(12), p. 2177–2182] suggest that hereditary factors explain up to 80% of causation of these atopic conditions.
The nature of the relationship between asthma and atopy is unclear. While many children with asthma are also atopic and have eczema, hay fever, or food allergies, there are many more children with atopy than with asthma [(9), p. 733–743; (10), p e008446]. In the largest community study of asthma and atopy in the UK, approximately 50% of 6 year olds with asthma (as evidenced by wheeze) were atopic (as evidenced by skin prick positivity) [(13), p. 974–980], i.e., many young children with asthma symptoms are not atopic. The “atopic march,” where at a population level, the prevalences of food allergy, eczema, asthma, and hay fever peak at increasing ages [(14), p. 99–106], has been cited as evidence to support a causal relationship between atopy and asthma but at an individual level, this “march” is very rarely seen [(15), p. e1001748]. While it is possible that atopy may lie on a causal pathway toward asthma, the reverse may also be true (i.e., asthma may lead to the development of atopy) and a third possibility remains that asthma and atopy are independently caused by some other process. The present review is one in a series, which explores the nature of the relationship between asthma and atopy from a number of perspectives. The focus of this review is to review the relationship between asthma and atopy from the gene–environment perspective. Specifically, the hypothesis tested here is: the same gene–environment interactions are associated with both asthma and atopy.
To test this hypothesis, a 2007 review of gene–environment interactions for asthma [(16), p. 1032–1035] was summarized and the literature published after 2007 describing gene–environment interactions for asthma was reviewed. The literature describing gene–environment interactions for atopy and atopic conditions was also summarized. This was not an exhaustive or systematic review, instead, the aim was to identify a number of gene–environment interactions for asthma and for atopy and determine whether there were any common interactions. Papers were included, which described gene–environment interactions for severity of asthma and atopy. Epigenetic mechanisms are covered elsewhere in this series and the interested reader is referred there.
Methodological Issues for Gene–Environment Interactions
The study of gene–environment interactions for asthma and atopy in childhood is challenging for a number of reasons, which are discussed more fully elsewhere [(17), p. 1229–1240]. The reader should be aware of the following issues before considering the evidence:
1. Definitions of asthma and atopy differs between studies, which makes comparison challenging. For asthma, definitions include self-reported symptoms, current, or “ever” doctor diagnosed asthma and also objective measurements of respiratory physiology, e.g., FEV1. For atopy, definitions might include self-reported current or “ever” eczema, hay fever, and food allergy symptoms, and objective measures such as skin prick reactivity and total IgE.
2. Measuring environmental exposures is a challenge and many different methodologies might be applied to the same exposure. Often, exposure is by subjective report, which is known to be potentially unreliable, e.g., exposure to tobacco smoke.
3. Studies require a large sample size to avoid false positive finding and also to detect small effect sizes. Many studies are underpowered. Publication bias means that relatively small studies where associations are seen are published whereas similar sized studies where no associations are seen are not accepted (or even submitted) for publication.
4. Studies require replication in more than one population to be considered generalizable.
5. How are genetic factor(s) of interest selected and related to which environmental exposure(s)? Searching for plausible interactions between candidate genes and environmental exposures can be justified based on current knowledge but this confines research to what is already known.
6. New analytical approaches are required, which can consider large numbers of single-nucleotide polymorphisms (SNPs) and environmental exposures, often which are measured at different ages in the same individual.
Gene–Environment Interactions for Asthma
The 2007 non-systematic review of gene–environment interactions for asthma [(16), p. 1032–1035] found that the majority of the literature had been published since 2000 and was focused in two areas: first, interactions between oxidant exposures (primarily SHS) and variants in genes coding for antioxidant defenses [especially the family of antioxidant enzymes collectively called glutathione-S transferase (GST)]; and second, interactions between exposures to bacteria or bacterial products and variants in genes coding for components of the adaptive and innate immune system (e.g., CD14). See Figure 1.
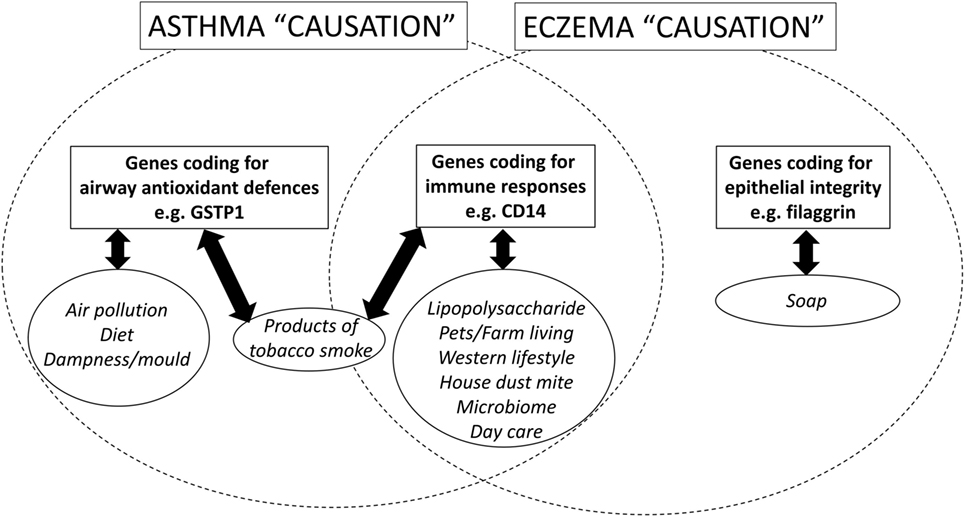
Figure 1. A schematic diagram, which shows the candidate genetic and environmental factors for asthma and eczema (a surrogate for atopy) in children.
Since 2007, interactions between oxidant exposures and variants in GST continue to be described, Table 1. Two papers reported on associations between variants coding for the antioxidant protein glutathione S-transferase P1 (GSTP1) and SHS exposure [(18), p. 125; (19), p. 226–232] and neither found an association although one [(18), p. 125] was able to describe increased risk for asthma among those exposed to SHS and low dietary vitamin E, who also were genetically predisposed to oxidant stress. Two other studies related SNPs in the gene coding for GSTP1 and exposure to dampness [(20), p. e30694] and air pollution [(21), p. e52715]; one study described complicated gene–gene and gene–environment interactions for dampness (but not several other exposures) and asthma [(20), p. e30694] while the second described a small increased risk for asthma among those genetically predisposed to oxidant stress and exposed to nitrogen dioxide (NO2) [(21), p. e52715]. In addition to variants in genes coding for GST, a study from Hungary also observed a twofold increase in asthma risk for children with rare SNPs, which might reduce host antioxidant defenses but only on exposure to high ambient NO2 concentrations [(22), p. 25–33].
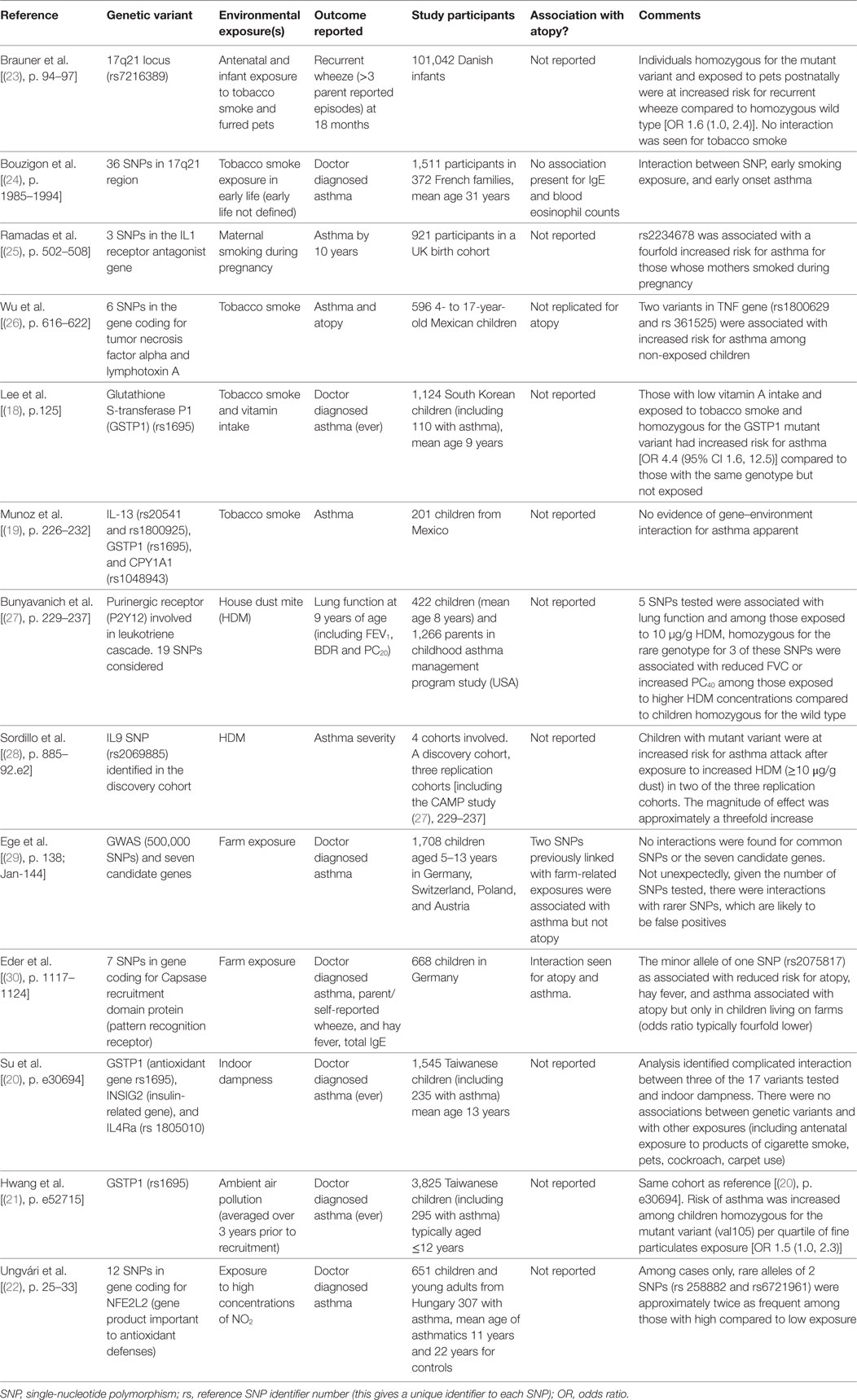
Table 1. Summary of examples of where gene–environment interactions for asthma or asthma outcomes have been sought.
Exposure to products of tobacco smoke is a well-known risk factor for childhood asthma (6) and interaction with genetic variants, which reduce GST have already been discussed, but interactions with variants in other genes might also be important. The ORMDL3 gene is associated with asthma in many populations and is found in the 17q21 region, so not surprisingly, gene–environment interactions have been sought between this area of the genome and exposure to products of tobacco smoke. Two papers [(24), p. 1985–1994; (23), p. 94–97] examined the relationship between many SNPs in the 17q21 region and SHS exposure. One paper found evidence of an interaction between mutant variants and early exposure to SHS and early onset asthma in young adults [(24), p. 1985–1994]. The second paper found no evidence between a single SNP and antenatal exposure to products of tobacco smoke but the mutant variant was associated with a modest increase in risk for early wheeze in association with exposure to pets [(23), p. 94–97]. A third study, from Mexico, reported an unexpected interaction between SNPs in the gene coding for tumor necrosis factor and increased smoking among non-asthmatic children [(26), p. 616–622]. A fourth paper described an interaction between maternal smoking and genetic variant in the IL-1 receptor antagonist for childhood asthma [(25), p. 502–508]. Smoking is an exposure, which is often under reported and which is confounded by many variables including lifestyle and domestic environment, so although not infrequently implicated in gene–environment interactions for asthma, the nature of the relationship cannot be assumed to be causal.
Genetic interactions with house dust mite (HDM) have been sought in two studies [(28), p. 885–92.e2; (27), p. 229–237]; both studies described associations between increased HDM exposure and variants in genes coding for factors associated with the inflammatory response and increased risk for asthma or for respiratory physiological changes associated with asthma. One of these studies was not able to replicate findings in all the populations studied [(28), p. 885–92.e2]. The final exposure considered in gene–environment interactions for asthma in this review is exposure to a farming environment. A study of five populations [(29), p. 138; Jan-144] was not able to replicate interaction between farm exposure and a number of candidate genes for asthma.
Gene–Environment Interactions for Atopy
At first inspection, there seems to be very little to suggest that there may be common gene–environment interactions for both asthma and atopy. First, genome wide association studies have identified different loci for genes associated with asthma and eczema (a surrogate for atopy). Second, while gene–environment interactions for asthma focus on variants in genes coding for host antioxidant mechanisms, gene–environment interactions for eczema (an atopic condition) are focused on genes associated with epithelial integrity [(31), p. 3–21]. However, GWAS studies [(32), p. 1154–1162] and candidate gene studies [(33), p. 704–714] do find some common areas of the genome and specific variants associated with both asthma and atopy and these regions/genes code for components of the immune system, for CD14, IL4, IL4R, IL13, see Figure 1. Arguably, the most well-recognized gene–environment interaction for atopy is the “endotoxin switch” where individuals carrying with the CC genotype for CD14-159/T (rs 2569190) are at increased risk for atopy at lower endotoxin exposures, but this risk reduces as endotoxin exposure rises [(34), p. 386–392]; those homozygous for CC are at increased risk for non-atopic wheeze at higher endotoxin exposures [(34), p. 386–392].
Compared to gene–environment interactions for asthma, there appear to be considerably fewer publications, which describe gene–environment interactions for atopy; for this review, five papers [(29), p. 138; Jan-144; (35), p. 593–602; (36), p. 231–6.e1–5; (37), p. 621–630; (38), p. 430–437] and one letter [(39), p. 1408–11.e1] were identified, Table 2. All the publications describe associations between variants in genes whose products are part of the adaptive or innate immune system. Variants in the gene coding for CD14 are described in three papers [(35), p. 593–602; (36), p. 231–6.e1–5; (38), p. 430–437] of which two describe interactions with pet exposure [(35), p. 593–602; (38), p. 430–437]. Compared to the literature describing gene–environment interactions for eczema, considered for atopy per se is rather sparse. While an interaction between CD14 variants and pets for IgE or eczema is plausible, this need replication in other populations.
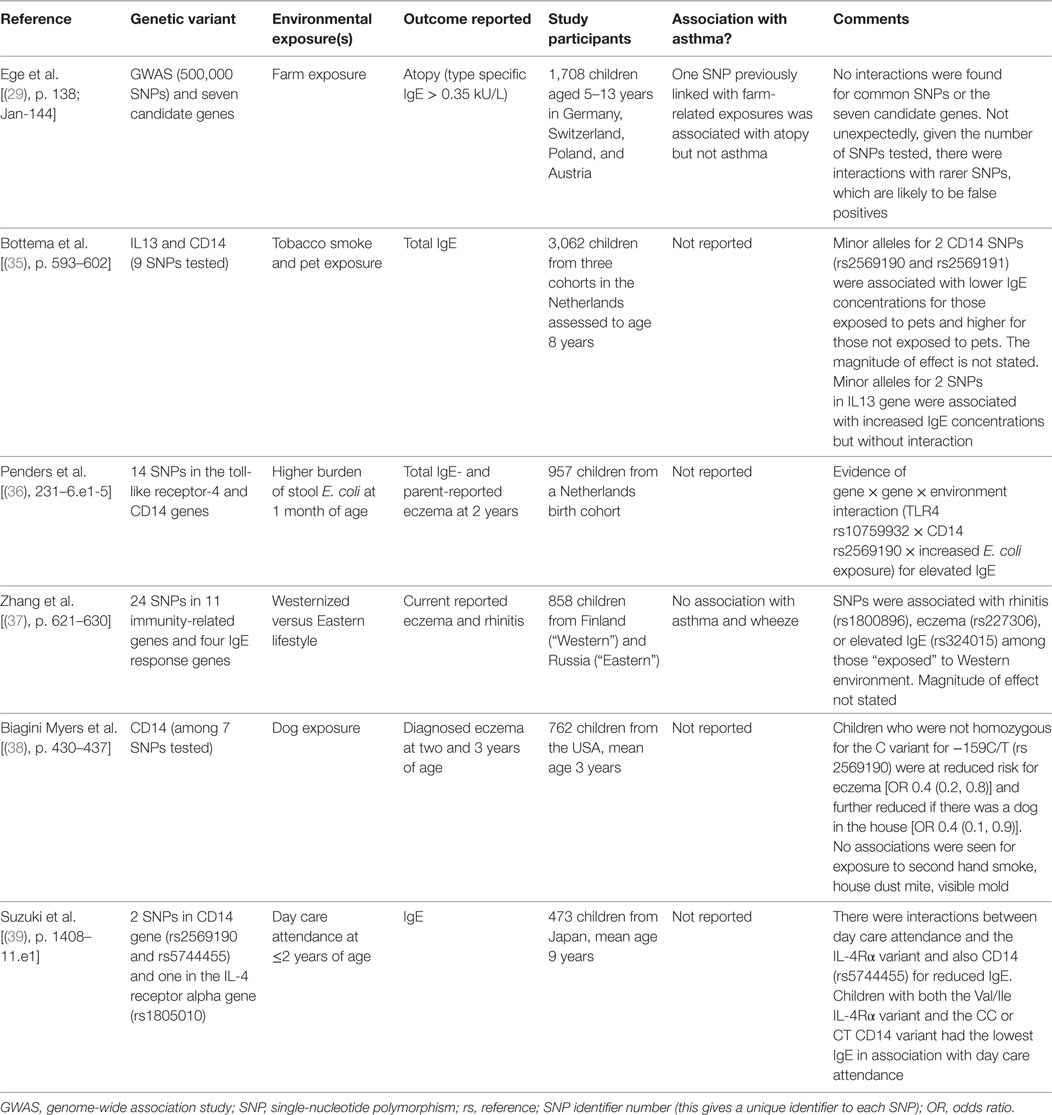
Table 2. Summary of examples of where gene–environment interactions for atopy, eczema, hay fever, or food allergy have been sought.
Conclusion
Gene–environment interactions were the “new kids on the block” during the first 10 years of this century, and during this time, there were many publications and regular review articles. Since 2010, there has been a notable reduction in the number of published original research articles and reviews of gene–environment interactions for asthma and atopy (or eczema to be more precise). The shift of focus away from gene–environment interactions may be partly explained by disappointment in the relatively few interactions described and their apparently small effect size. Technological developments may also have shifted scientific thinking and gene–environment-wide interaction studies (GEWIS) may be the “new new kids on the block” [(40), p. 227–230]. An example of a GEWIS is reported in this review [(29), p. 138; Jan-144].
So what is the evidence that there are common gene–environment interactions for asthma and atopy? One paper describing gene–environment interactions for asthma identified in the earlier review [(16), p. 1032–1035] described an interaction between a variant coding for toll-like receptor 2 (rs 4696480) and living on a farm for both asthma and atopy [(30), p. 1117–1124], but this could not be replicated in other populations subsequently [(29), p. 138; Jan-144]. The present review did identify three studies where a relationship between the same gene–environment interaction was sought for both asthma and atopy but no common interaction was found [(24), p. 1985–1994; (26), p. 616–622; (37), p. 621–630]. There is, therefore, absence of evidence to robustly answer the question “are the same gene–environment interactions associated with both asthma and atopy?” but there is some evidence of absence that the same gene–environment are not related to asthma and atopy.
Studying the relationship between asthma and atopy is not easy due to issues, which include definitions and the coexistence of the two conditions in many individuals in Western populations. The association between asthma and atopy (as evidenced by eczema) may change over time for a population (7), which adds to the challenge in better understanding the nature of the relationship. A paper that is based on epidemiology studies, and which will be frequently cited in this series of reviews, suggests that perhaps as much as 50% of asthma may be attributable to atopy [(41), p. 268–272]. In some countries, many children with asthma are non-atopic [(42), p. 409–416], so the relationship between asthma and atopy is apparently irrelevant for some individuals.
Ultimately, gene–environment interactions are likely to be important to the development of both asthma and atopy but, at this time, do not give a useful insight into the nature of the relationship between asthma and atopy. Other perspectives, for example, intervention studies, may be more helpful in understanding the inter-relationship between asthma and atopy. Some interventions are linked to reduced eczema in preschool children but not asthma [(43), p. 1178–1184; (44), p. 807–813] while other interventions achieve reductions in asthma and eczema into and beyond school age [(45), p. 1046–1051; (46), p. 49–55].
Author Contributions
The author confirms being the sole contributor of this work and approved it for publication.
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
No funding was required for this manuscript.
References
1. Asthma UK. Asthma Facts and FAQs. (2014). Available from: http://www.asthma.org.uk/asthma-facts-and-statistics
2. Centers for Disease Control and Prevention. Asthma. Most Recent Data. (2016). Available from: https://www.cdc.gov/asthma/most_recent_data.htm
3. Zimmerman B, Feanny S, Reisman J, Hak H, Rashed N, McLaughlin FJ, et al. Allergy in asthma. I. The dose relationship of allergy to severity of childhood asthma. J Allergy Clin Immunol (1988) 81(1):63–70. doi: 10.1016/0091-6749(88)90221-7
4. Skadhauge LR, Christensen K, Kyvik KO, Sigsgaard T. genetic and environmental influence on asthma: a population-based study of 11,688 Danish twin pairs. Eur Respir J (1999) 13(1):8–14. doi:10.1183/09031936.99.13100899
5. Carroll W. Asthma genetics: pitfalls and triumphs. Paediatr Respir Rev (2005) 6(1):68–74. doi:10.1016/j.prrv.2004.11.007
6. Dick S, Friend A, Dynes K, Al Kandari F, Doust E, Cowie H, et al. A systematic review of associations between environmental exposures and development of asthma in children aged up to nine years. BMJ Open (2014) 4(11): e006554. doi:10.1136/bmjopen-2014-006554
7. Barnish MS, Tagiyeva N, Devereux G, Aucott L, Turner S. Changes in the relationship between asthma and associated risk factors over fifty years. Paediatr Allergy Immunol (2017) 28(2):162–9. doi:10.1111/pai.12674
8. Pastorello EA, Incorvaia C, Ortolani C, Bonini S, Canonica GW, Romagnani S, et al. Studies on the relationship between the level of specific IgE antibodies and the clinical expression of allergy: I. definition of levels distinguishing patients with symptomatic from patients with asymptomatic allergy to common aeroallergens. J Allergy Clin Immunol (1995) 96(5 Pt 1):580–7. doi:10.1016/S0091-6749(95)70255-5
9. Asher M I, Montefort S, Bjorksten B, Lai CK, Strachan DP, Weiland SK, et al. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC phases one and three repeat multicountry cross-sectional surveys. Lancet (2006) 368(9537):733–43. doi:10.1016/S0140-6736(06)69283-0
10. Barnish MS, Tagiyeva N, Devereux G, Aucott L, Turner S. Diverging prevalences and different risk factors for childhood asthma and eczema: a cross-sectional study. BMJ Open (2015) 5:e008446. doi:10.1136/bmjopen-2015-008446
11. Thomsen SF, Ulrik CS, Kyvik KO, Hjelmborg Jv, Skadhauge LR, Steffensen I, et al. Importance of genetic factors in the etiology of atopic dermatitis: a twin study. Allergy Asthma Proc (2007) 28(5):535–9. doi:10.2500/aap2007.28.3041
12. Thomsen SF, Ulrik CS, Kyvik KO, von Bornemann Hjelmborg J, Skadhauge LR, Steffensen I, et al. Genetic and environmental contributions to hay fever among young adult twins. Respir Med (2006) 100(12):2177–82. doi:10.1016/j.rmed.2006.03.013
13. Henderson J, Granell R, Heron J, Sherriff A, Simpson A, Woodcock A, et al. Associations of wheezing phenotypes in the first 6 years of life with atopy, lung function and airway responsiveness in mid-childhood. Thorax (2008) 63(11):974–80. doi:10.1136/thx.2007.093187
14. Spergel JM. From atopic dermatitis to asthma: the atopic march. Ann Allergy Asthma Immunol (2010) 105(2):99–106. doi:10.1016/j.anai.2009.10.002
15. Belgrave DC, Granell R, Simpson A, Guiver J, Bishop C, Buchan I, et al. Developmental profiles of eczema, wheeze, and rhinitis: two population-based birth cohort studies. PLoS Med (2014) 11(10):e1001748. doi:10.1371/journal.pmed.1001748
16. McLeish S, Turner SW. Gene-environment interactions in Asthma. Arch Dis Child (2007) 92(11):1032–5. doi:10.1136/adc.2006.112185
17. Kauffmann F, Demenais F. Gene-environment interactions in asthma and allergic diseases: challenges and perspectives. J Allergy Clin Immunol (2012) 130(6):1229–40. doi:10.1016/j.jaci.2012.10.038
18. Lee SY, Kim BS, Kwon SO, Oh SY, Shin HL, Jung YH, et al. Modification of additive effect between vitamins and ets on childhood asthma risk according to GSTP1 polymorphism: a cross -sectional study. BMC Pulm Med (2015) 15:125. doi:10.1186/s12890-015-0093-0
19. Muñoz B, Magaña JJ, Romero-Toledo I, Juárez-Pérez E, López-Moya A, Leyva-García N, et al. The relationship among IL-13, GSTP1, and CYP1A1 polymorphisms and environmental tobacco smoke in a population of children with asthma in Northern Mexico. Environ Toxicol Pharmacol (2012) 33(2):226–32. doi:10.1016/j.etap.2011.12.007
20. Su MW, Tung KY, Liang PH, Tsai CH, Kuo NW, Lee YL. Gene-gene and gene-environmental interactions of childhood asthma: a multifactor dimension reduction approach. PLoS One (2012) 7(2):e30694. doi:10.1371/journal.pone.0030694
21. Hwang BF, Young LH, Tsai CH, Tung KY, Wang PC, Su MW, et al. Fine particle, ozone exposure, and asthma/wheezing: effect modification by glutathione S-transferase P1 polymorphisms. PLoS One (2013) 8(1):e52715. doi:10.1371/journal.pone.0052715
22. Ungvári I, Hadadi E, Virág V, Nagy A, Kiss A, Kalmár A, et al. Relationship between air pollution, NFE2L2 gene polymorphisms and childhood asthma in a hungarian population. J Community Genet (2012) 3:25–33. doi:10.1007/s12687-011-0075-8
23. Brauner EV, Loft S, Raaschou-Nielsen O, Vogel U, Andersen PS, Sorensen M. Effects of a 17q21 chromosome gene variant, tobacco smoke and furred pets on infant wheeze. Genes Immun (2012) 13(1):94–7. doi:10.1038/gene.2011.51
24. Bouzigon E, Corda E, Aschard H, Dizier MH, Boland A, Bousquet J, et al. Effect of 17q21 variants and smoking exposure in early-onset asthma. N Engl J Med (2008) 359(19):1985–94. doi:10.1056/NEJMoa0806604
25. Ramadas RA, Sadeghnejad A, Karmaus W, Arshad SH, Matthews S, Huebner M, et al. Interleukin-1R antagonist gene and pre-natal smoke exposure are associated with childhood asthma. Eur Respir J (2007) 29(3):502–8. doi:10.1183/09031936.00029506
26. Wu H, Romieu I, Sienra-Monge JJ, del Rio-Navarro BE, Anderson DM, Dunn EW, et al. Parental smoking modifies the relation between genetic variation in tumor necrosis factor-alpha (TNF) and childhood asthma. Environ Health Perspect (2007) 115(4):616–22. doi:10.1289/ehp.9740
27. Bunyavanich S, Boyce JA, Raby BA, Weiss ST. Gene-by-environment effect of house dust mite on purinergic receptor P2Y12 (P2RY12) and lung function in children with asthma. Clin Exp Allergy (2012) 42(2):229–37. doi:10.1111/j.1365-2222.2011.03874.x
28. Sordillo JE, Kelly R, Bunyavanich S, McGeachie M, Qiu W, Croteau-Chonka DC, et al. Genome-wide expression profiles identify potential targets for gene-environment interactions in asthma severity. J Allergy Clin Immunol (2015) 136(4):885.e–92.e. doi:10.1016/j.jaci.2015.02.035
29. Ege MJ, Strachan DP, Cookson WO, Moffatt MF, Gut I, Lathrop M, et al. Gene-environment interaction for childhood asthma and exposure to farming in Central Europe. J Allergy Clin Immunol (2011) 127(1):138. doi:10.1016/j.jaci.2010.09.041
30. Eder W, Klimecki W, Yu L, von Mutius E, Riedler J, Braun-Fahrländer C, et al. Association between exposure to farming, allergies and genetic variation in CARD4/NOD1. Allergy (2006) 61(9):1117–24. doi:10.1111/j.1398-9995.2006.01128.x
31. Cork MJ, Robinson DA, Vasilopoulos Y, Ferguson A, Moustafa M, MacGowan A, et al. New perspectives on epidermal barrier dysfunction in atopic dermatitis: gene-environment interactions. J Allergy Clin Immunol (2006) 118(1):3–21. doi:10.1016/j.jaci.2006.04.042
32. Ober C, Tsalenko A, Parry R, Cox NJ. A second-generation genomewide screen for asthma-susceptibility alleles in a founder population. Am J Hum Genet (2000) 67(5):1154–62. doi:10.1086/321191
33. Biagini Myers JM, Khurana Hershey GK. Eczema in early life: genetics, the skin barrier, and lessons learned from birth cohort studies. J Pediatr (2010) 157(5):704–14. doi:10.1016/j.jpeds.2010.07.009
34. Simpson A, John SL, Jury F, Niven R, Woodcock A, Ollier WE, et al. Endotoxin exposure, CD14, and allergic disease: an interaction between genes and the environment. Am J Respir Crit Care Med (2006) 174(4):386–92. doi:10.1164/rccm.200509-1380OC
35. Bottema RWB, Reijmerink NE, Kerkhof M, Koppelman GH, Stelma FF, Gerritsen J, et al. Interleukin 13, CD14, pet and tobacco smoke influence atopy in three Dutch cohorts: the allergenic study. Eur Respir J (2008) 32(3):593–602. doi:10.1183/09031936.00162407
36. Penders J, Thijs C, Mommers M, Stobberingh EE, Dompeling E, Reijmerink NE, et al. Host-microbial interactions in childhood atopy: toll-like receptor 4 (TLR4), CD14, and fecal Escherichia coli. J Allergy Clin Immunol (2010) 125(1):.e1–5. doi:10.1016/j.jaci.2009.10.011
37. Zhang G, Candelaria P, Mäkelä JM, Khoo SK, Hayden MC, von Hertzen L, et al. Disparity of innate immunity-related gene effects on asthma and allergy on Karelia. Pediatr Allergy Immunol (2011) 22(6):621–30. doi:10.1111/j.1399-3038.2011.01186.x
38. Biagini Myers JM, Wang N, LeMasters GK, Bernstein DI, Epstein TG, Lindsey MA, et al. Genetic and environmental risk factors for childhood eczema development and allergic sensitization in the CCAAPS cohort. J Invest Dermatol (2010) 130(2):430–7. doi:10.1038/jid.2009.300
39. Suzuki Y, Hattori S, Mashimo Y, Funamizu M, Kohno Y, Okamoto Y, et al. CD14 and IL4R gene polymorphisms modify the effect of day care attendance on serum IgE levels. J Allergy Clin Immunol (2009) 123(6):1408.e–11.e. doi:10.1016/j.jaci.2009.03.035
40. Khoury MJ, Wacholder S. Invited commentary: from genome-wide association studies to gene-environment-wide interaction studies – challenges and opportunities. Am J Epidemiol (2009) 169(2):227–30. doi:10.1093/aje/kwn351
41. Pearce N, Pekkanen J, Beasley R. How much asthma is really attributable to atopy? Thorax (1999) 54(3):268–72. doi:10.1136/thx.54.3.268
42. Moncayo AL, Vaca M, Oviedo G, Erazo S, Quinzo I, Fiaccone RL, et al. Risk factors for atopic and non-atopic asthma in a rural area of Ecuador. Thorax (2010) 65(5):409–16. doi:10.1136/thx.2009.126490
43. Dunstan JA, Mori TA, Barden A, Beilin LJ, Taylor AL, Holt PG, et al. Fish oil supplementation in pregnancy modifies neonatal allergen-specific immune responses and clinical outcomes in infants at high risk of atopy: a randomized, controlled trial. J Allergy Clin Immunol (2003) 112(6):1178–84. doi:10.1016/j.jaci.2003.09.009
44. Peat JK, Mihrshahi S, Kemp AS, Marks GB, Tovey ER, Webb K, et al. Three-year outcomes of dietary fatty acid modification and house dust mite reduction in the childhood asthma prevention study. J Allergy Clin Immunol (2004) 114(4):807–13. doi:10.1016/j.jaci.2004.06.057
45. Scott M, Roberts G, Kurukulaaratchy RJ, Matthews S, Nove A, Arshad SH. Multifaceted allergen avoidance during infancy reduces asthma during childhood with the effect persisting until age 18 years. Thorax (2012) 67(12):1046–51. doi:10.1136/thoraxjnl-2012-202150
Keywords: asthma, atopy, child, eczema, environment, gene, mechanism
Citation: Turner S (2017) Gene–Environment Interactions—What Can These Tell Us about the Relationship between Asthma and Allergy? Front. Pediatr. 5:118. doi: 10.3389/fped.2017.00118
Received: 13 February 2017; Accepted: 03 May 2017;
Published: 22 May 2017
Edited by:
Luis Garcia-Marcos, University of Murcia, SpainReviewed by:
Enrico Lombardi, Meyer Pediatric University-Hospital, ItalyMassimo Pifferi, University Hospital of Pisa, Italy
Copyright: © 2017 Turner. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Steve Turner, s.w.turner@abdn.ac.uk
 Steve Turner
Steve Turner