
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CLINICAL STUDY PROTOCOL article
Front. Pediatr., 19 July 2016
Sec. Pediatric Nephrology
Volume 4 - 2016 | https://doi.org/10.3389/fped.2016.00068
 Jennifer G. Jetton1*†
Jennifer G. Jetton1*† Ronnie Guillet2†
Ronnie Guillet2† David J. Askenazi3
David J. Askenazi3 Lynn Dill3
Lynn Dill3 Judd Jacobs4
Judd Jacobs4 Alison L. Kent5
Alison L. Kent5 David T. Selewski6
David T. Selewski6 Carolyn L. Abitbol7
Carolyn L. Abitbol7 Fredrick J. Kaskel8
Fredrick J. Kaskel8 Maroun J. Mhanna9
Maroun J. Mhanna9 Namasivayam Ambalavanan10
Namasivayam Ambalavanan10 Jennifer R. Charlton11 and the Neonatal Kidney Collaborative‡
Jennifer R. Charlton11 and the Neonatal Kidney Collaborative‡
Introduction: Acute kidney injury (AKI) affects ~30% of hospitalized neonates. Critical to advancing our understanding of neonatal AKI is collaborative research among neonatologists and nephrologists. The Neonatal Kidney Collaborative (NKC) is an international, multidisciplinary group dedicated to investigating neonatal AKI. The AWAKEN study (Assessment of Worldwide Acute Kidney injury Epidemiology in Neonates) was designed to describe the epidemiology of neonatal AKI, validate the definition of neonatal AKI, identify primary risk factors for neonatal AKI, and investigate the contribution of fluid management to AKI events and short-term outcomes.
Methods and analysis: The NKC was established with at least one pediatric nephrologist and neonatologist from 24 institutions in 4 countries (USA, Canada, Australia, and India). A Steering Committee and four subcommittees were created. The database subcommittee oversaw the development of the web-based database (MediData Rave™) that captured all NICU admissions from 1/1/14 to 3/31/14. Inclusion and exclusion criteria were applied to eliminate neonates with a low likelihood of AKI. Data collection included: (1) baseline demographic information; (2) daily physiologic parameters and care received during the first week of life; (3) weekly “snapshots”; (4) discharge information including growth parameters, final diagnoses, discharge medications, and need for renal replacement therapy; and (5) all serum creatinine values.
Ethics and dissemination: AWAKEN was proposed as human subjects research. The study design allowed for a waiver of informed consent/parental permission. NKC investigators will disseminate data through peer-reviewed publications and educational conferences.
Discussion: The purpose of this publication is to describe the formation of the NKC, the establishment of the AWAKEN cohort and database, future directions, and a few “lessons learned.” The AWAKEN database includes ~325 unique variables and >4 million discrete data points. AWAKEN will be the largest, most inclusive neonatal AKI study to date. In addition to validating the neonatal AKI definition and identifying risk factors for AKI, this study will uncover variations in practice patterns related to fluid provision, renal function monitoring, and involvement of pediatric nephrologists during hospitalization. The AWAKEN study will position the NKC to achieve the long-term goal of improving the lives, health, and well-being of newborns at risk for kidney disease.
Over the last decade, the nephrology and critical care communities have repeatedly shown that acute kidney injury (AKI) in children (1, 2) and adults (3–7) portends poor short-term and long-term outcomes independent of severity of illness. The available short-term outcome data in neonates are similar: neonates with AKI have increased rates of mortality and longer hospital stays as compared to those without AKI (Table 1) (8–14). The prevalence of AKI reaches ~30% in neonates admitted to a tertiary level neonatal intensive care unit (NICU) (8–13, 14). However, prevalence estimates and data on short-term outcomes are solely derived from small retrospective, single-center studies. Moreover, little is known about the long-term consequences of neonatal AKI (15). Many significant questions, including how to best define, risk factors for, incidence of, association with other co-morbidities, and the short-term and long-term outcomes after AKI remain unanswered.
Critical to advancing the field of neonatal AKI is collaborative research among neonatologists and pediatric nephrologists. To foster this partnership, the National Institutes of Health (NIH) sponsored a multidisciplinary workshop on neonatal AKI in April 2013 (16, 17). Soon after, the Neonatal Kidney Collaborative (NKC) was formed to address the critical gaps in knowledge highlighted at the workshop. The NKC is an international working group composed of neonatologists and pediatric nephrologists committed to advancing the field of neonatal AKI research. The first mission of the NKC was to develop the infrastructure to function. The second mission of the NKC was the development of the AWAKEN study (Assessment of Worldwide Acute Kidney injury Epidemiology in Neonates), a large, retrospective cohort study designed with the following aims: (1) determine if the Kidney Disease: Improving Global Outcomes (KDIGO) AKI definition, adapted to neonates, is independently associated with mortality, length of stay, and discharge serum creatinine (sCr), (2) define the risk factors associated with neonatal AKI, (3) determine how fluid balance during the first few weeks of life relates to biochemical data and clinical outcomes, and (4) assess the performance of different definitions of AKI in neonates.
Assessment of Worldwide Acute Kidney injury Epidemiology in Neonates is the largest and most inclusive neonatal AKI study to date. Evaluation of these important questions will improve care and stimulate new research questions that will drive this field for many years. Collaborations will foster strong relationships between neonatologists and pediatric nephrologists within and across centers. The infrastructure created to perform the AWAKEN study will position this group to conduct additional clinically relevant, hypothesis-driven studies aimed at improving the lives, health and well-being of patients at risk for kidney disease as the result of events during the newborn period. Here, we describe the formation of the NKC, the establishment of the AWAKEN cohort and database, future directions, and a few “lessons learned” in the process.
The NKC is a voluntary, non-funded group made up of pediatric centers who agreed to ensure: (1) the participation of both a neonatologist and pediatric nephrologist from their institution and (2) local IRB approval. The NKC membership evolved from professional relationships developed through previous clinical and research interactions including the 2013 NIH workshop “Neonatal AKI.” A current roster of participating institutions is included in Table 2.
The NKC infrastructure consists of the Steering Committee overseen by the Director and the co-chairpersons of the four subcommittees (Figure 1). Each subcommittee (Protocol Committee, Manuscript Committee, Database Committee, and Ancillary Studies Committee) is co-chaired by a pediatric nephrologist and a neonatologist, thereby assuring that the clinical expertise of both specialties is represented.
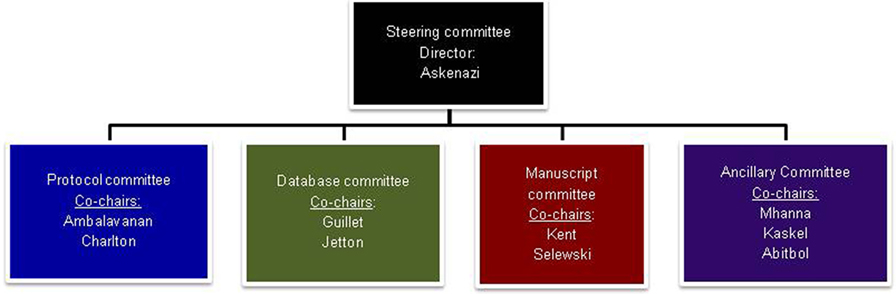
Figure 1. The Steering committee is composed of the director and the co-chairs of each of the sub-committees. The Protocol Committee was tasked to provide oversight and critique of the protocols submitted to the committee, both for the initial retrospective study (AWAKEN) and any future studies. It will also be charged with creating and submitting protocols to funding agencies and Institutional Review Boards. Other responsibilities include establishing rules for Primary Investigator designation and a system for group involvement for the establishment of future protocols. The Database Committee was charged with the development of the database, including the Manual of Procedures and Case Report Forms. Input was solicited from the NKC membership as to the data needed to answer the specific questions of interest for the AWAKEN study. These suggestions were collated and presented to the Steering Committee for final review. The myriad of data potentially available and the number of questions to be answered had the potential for an unwieldy and overwhelming amount of information. Data points that were included were thoroughly vetted by both nephrologists and neonatologists to balance the time of data collection with quality of the data elements. Once agreement was reached, in concert with the Data Management Center at Cincinnati Children’s Hospital Medical Center, electronic data forms were developed, tested, and finalized. The Manuscript Committee is responsible for initiating and developing abstracts for national and international meetings and manuscripts for submission to peer-reviewed journals. This committee will also review these abstracts and manuscripts prior to submission and provide the authors suggestions, as well as determine their suitability. The Ancillary Studies Committee will be responsible for developing rules on requesting use of data for ancillary studies and in developing these ideas into abstracts and manuscripts.
While research interest in neonatal AKI has increased greatly over the last 5 years, advances in clinical recognition, diagnosis, and supportive care for AKI in this population have lagged behind advances seen for adult and older pediatric patient groups (18). One major limitation to moving this field forward has been the lack of a standardized and validated definition of AKI in neonates similar to those used in adult and pediatric patients [e.g., risk, injury, failure, loss and end-stage RIFLE (19), KDIGO (20)]. The lack of a common definition has led to an inability to recognize AKI early and consistently across patients as well as an inability to pool or compare data across studies. In addition, the unique renal physiology of preterm and term infants creates challenges for the use of serum creatinine (sCr) as an AKI biomarker in these patients. Neonatal SCr initially reflects maternal values and then decreases over subsequent weeks after birth at different rates depending on gestational age. In addition, “normal” serum creatinine levels vary widely based on weight and gestational age. Before 2005, the majority of neonatal AKI studies used an arbitrary definition of AKI defined by a serum creatinine concentration (SCr) ≥1.5 mg/dl. In 2012, Jetton and Askenazi (21) proposed a standardized neonatal AKI definition based on the KDIGO definition for adults and children (Table 3) that identifies three levels of AKI severity based on graded changes in both serum creatinine and urine output. This and similar definitions have been utilized successfully in select neonatal patient populations (8, 10–12, 14, 22, 23). Consensus at the NIH neonatal AKI workshop was that this definition, despite its limitations, was currently the most appropriate definition to adopt and validate (Michael Zappetelli, personal communication). The group stressed the need to conduct large multi-center studies to test the performance of this definition against clinically relevant outcomes and further refine the definition based on data generated from these large cohorts. Thus, the AWAKEN study was designed to fulfill this charge and determine the extent to which the definition of AKI is associated with the short-term outcomes of mortality, length of stay, and discharge sCr. Although the association of neonatal AKI with longer term outcomes is beyond the scope of the AWAKEN study, the data generated will provide the foundation for prospective follow-up studies and the groundwork for prospective studies of at-risk populations.
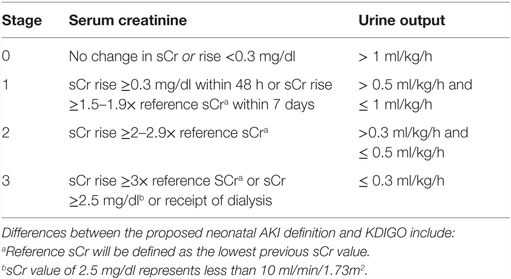
Table 3. Neonatal acute kidney injury KDIGO classification (21).
Similarly, fluid overload has been identified as a major risk factor for morbidity and mortality in adult and pediatric patients with AKI (24, 25), but has not been fully examined in the neonatal population. As with the study of sCr in this group, the unique physiology of neonates, especially premature and low-birth weight infants, creates challenges for applying concepts developed in older patients to this special group. In the first few days after birth, healthy term infants will lose around 7% of their weight. Depending on the amount of fluid provision, ambient temperature and humidity, gestational age, and kidney function, infants admitted to the NICU will have different amounts of weight losses due to changes in fluid balance over the first weeks of life (26).
Appropriate fluid balance is critical to the care of newborns. In premature infants, mild fluid weight losses over the first week of life are desirable as high fluid intake can be associated with patent ductus arteriosus, bronchopulmonary dysplasia, cardiac failure, necrotizing enterocolitis, and intraventricular hemorrhage (27). Alternatively, inadequate fluid provision may cause hemodynamic dysfunction potentially resulting in AKI. However, in the extremely low-birth weight (ELBW) and extremely low gestational age neonate (ELGAN), fluid balance cannot be calculated in the same way as in older, larger neonates, or pediatric patients [e.g., using documented intake and output volumes to calculate fluid overload or fluid deficits (28)]. Transepidermal fluid losses (TEFL) in the first week will vary considerably as a function of ambient humidity and are impossible to measure in clinical practice. Although at birth TEFL in healthy full-term neonates is similar to that in older children and adults (4–8 g/m2/h), it is estimated to be 75 g/m2/h in neonates born at 23 weeks’ gestation (29). Humidified incubators simplify the management of fluids in these infants and help prevent extremes of electrolyte abnormalities. An important objective in AWAKEN will be to determine if variations in body weight are an adequate surrogate for fluid balance in this population rather than the reliance on recorded intake and output for calculation of fluid overload as is used in older patients. We also seek to determine whether fluid overload functions as a sign of kidney injury and increases the risk of morbidity and mortality as has been shown in older patient groups.
The primary hypothesis of AWAKEN is that neonatal AKI is common and associated with increased mortality, longer length of NICU stay and higher discharge serum creatinine, even after controlling for severity of illness, interventions and demographics. The two secondary hypotheses of AWAKEN include (1) maternal and infant risk factors can predict AKI and (2) fluid balance is associated with clinical outcomes (Table 4). Ultimately, the NKC will be positioned to test and validate the proposed neonatal AKI definition and compare it with other AKI definitions to determine which definition is the most accurate. We anticipate that the AWAKEN study can validate these hypotheses because of the large sample size and rich data set. This multicenter study will also uncover variations in practice patterns among NICUs in relation to fluid provision, renal function monitoring, bedside identification of AKI and AKI risk factors, and the involvement of pediatric nephrologists during inpatient hospitalization. Moreover, AWAKEN will provide important information regarding critical time and data points necessary to build a meaningful prospective study in the future.
Assessment of Worldwide Acute Kidney injury Epidemiology in Neonates is a multi-center, international, retrospective cohort study. All neonates who fulfilled the inclusion and exclusion criteria at each of the 24 participating centers from January 1, 2014 until March 31, 2014 were enrolled (Figure 2). Detailed data collection continued until one of the following endpoints was reached: discharge to home, transfer to another facility or out of the NICU for escalating or convalescent care, death or 120 days of age. Limited information was collected between 120 days and final disposition. The study was registered at ClinicalTrials.gov (https://clinicaltrials.gov/ct2/show/NCT02443389?term=kidney+awaken&rank=1).
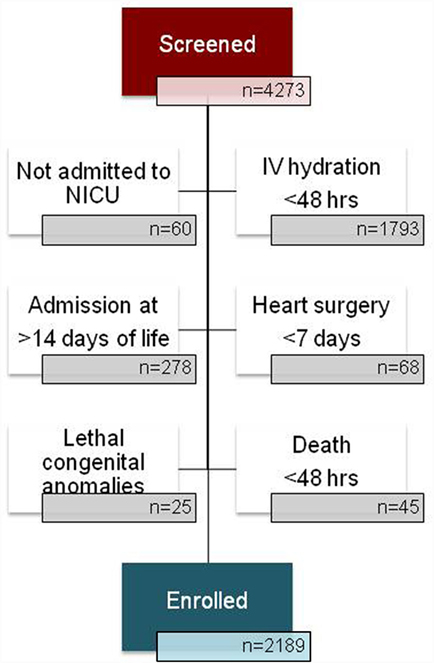
Figure 2. Of the 4273 NICU admissions at the 24 participating institutions during the period 1/1/14–3/31/14, 2162 patients were enrolled. The majority of those not included did not receive at least 48 h of IV hydration and/or nutrition. The second most common reason for exclusion was admission to the NICU at greater than 14 days of age. Subjects may have been excluded for more than one reason and may be counted more than once in the “not enrolled” numbers.
Twenty-four level 2–4 NICUs in the United States (n = 20), Canada (n = 2), India (n = 1), and Australia (n = 1). A broad description of each institution is listed in Table 2, including the number of neonates enrolled in AWAKEN, the percentage of neonates born at that institution and the type of assay used to measure serum creatinine.
All infants admitted from January 1, 2014 to March 31, 2014 who were ≤14 days of age and received at least 48 h of intravenous (IV) fluids as their primary source of hydration or nutrition (Table 5). Neonates who received IV fluids given solely for the administration of medications or line flushes were not included. The inclusion criteria were designed to capture sick neonates at significant risk for AKI and those who had an expected hospitalization of at least 48 h. Infants receiving routine care in the newborn nursery were not included in this study. The exclusion criteria were designed to omit patients with a limited hospitalization in the NICU or who had significant incomplete data particularly in the first 2 weeks of life. This exclusion is based on the belief that the first 2 weeks of life encompass a critical at-risk time for AKI. Because of the variable center-specific practice patterns regarding newborns with congenital heart disease requiring surgical repair (i.e., transfer to the PICU for post-operative care) and because these infants have been the subject of a number of previous AKI studies, neonates who required cardiac surgery within 7 days of birth were excluded. Infants with isolated ventricular septal defect, atrial septal defect, or patent ductus arteriosus were included unless they were transferred out of the NICU <7 days for surgical repair. Premature infants who remained in the NICU for ≥7 days to gain weight and grow in preparation for surgery were also included.
All neonates admitted to the participating NICUs were screened for inclusion criteria. Demographic data including sex, self-reported ethnicity, race, date of birth, and date of NICU admission were collected for all neonates (Supplementary Materials – Data Sheet 1 – Screening Form). Date of birth was required as an “anchor” for the database format and the generation of the appropriate daily and weekly forms and was designated as “day 1.”
The AWAKEN study was designed to acquire information that is most likely related to kidney function and kidney injury in critically ill neonates. All sCr values during the study period were recorded. Data collection was otherwise organized into five components. Case report forms (CRF) and manual of operations are included in Supplementary Materials, Data Sheet 2.
Maternal characteristics including age, gravid, parity, health conditions (chronic and pregnancy-associated), peri-partum infections, complications, and medications received; neonatal characteristics including site of delivery (inborn or outborn), mode of delivery, gestational age and birthweight, length and head circumference, initial temperature, resuscitation data, and reasons for admission.
Weight, blood pressure and heart rate, highest level of respiratory support, fluid intake (intravenous and enteral fluids), fluid output (urine output and total), nephrotoxic medications, and laboratory parameters (hemoglobin, blood urea nitrogen, sodium, albumin, blood cultures, urine cultures, and cerebrospinal fluid cultures).
The first value of the week for all of the variables included in the daily form until the neonate reached the endpoint.
Disposition of the infant at the end of the data collection period (“status” – discharged home prior to 120 days, still in the NICU at ≥120 days, transferred to another facility for convalescent care, transferred to another facility for escalation of care or died on or prior to 120 days), growth parameters, discharge medications if kidney related (urinary tract infection prophylaxis, antihypertensive medications and diuretics), and discharge diagnoses. Detailed information about kidney diagnoses (congenital abnormalities, episodes of AKI and need for nephrology consults) and renal replacement therapy provided were collected.
Date of final disposition and reason for prolonged NICU stay >120 days were collected, as were the highest and last creatinine obtained between 120 days and final disposition.
Because this is a retrospective study, all data reflect current, local standards of care. Definitions of terms listed in the Manual of Procedures were evidence-based whenever possible or consistent with those used in other published research studies.
Data entry of the variables of interest was performed by participating sites using a web-based database, MediData Rave™. Rave™ is a commercially available system designed to capture, manage, and report clinical research data. Through this system, each participating site was assigned a unique code by the database management team. If responses to the initial inclusion and exclusion criteria provided by the individual performing the data entry fulfilled study criteria, the system dynamically generated the remainder of the patient casebook, opening the “gateway” for the site to enter additional data for an enrolled patient. When eligibility was determined, the system guided the data entry personnel at each site to enter the clinical variables of interest. Limited protected health information (date of birth) was required to generate the proper dates for the daily and weekly forms. Release of the date of birth as part of the limited data set was agreed upon in the Data Use Agreement between each site and the primary study site. All participating sites used the same case report forms (CRFs). The electronic CRFs were designed, in partnership with the project’s Database Committee, by the representatives in the Cincinnati Children’s Hospital Medical Center Data Management Center (CCHMC DMC). Data entered by the sites were reviewed by the DMC. Values outside of expected ranges generate queries back to the individual site personnel. Query responses are then reviewed by the DMC personnel as well as physician members of the database subcommittee if clinical expertise was required to adjudicate the responses.
All site primary investigators and site personnel performing data entry completed commercially developed online training prior to being allowed access to MediData Rave™. Additional webinars sponsored by primary site personnel and Database Committee team were conducted to instruct sites on the proper use and interpretation of the Manual of Procedures (including definitions for study variables) and completion of CRFs. Co-chairs of the database committee and the lead coordinator were available for guidance and clarification as needed.
Data management and statistical analysis will be executed at the University of Alabama at Birmingham using SAS software v9.4 (SAS Institute, Cary, NC, USA). Analysis of data will be performed independently for each specific aim. Descriptive statistics will be used to describe the populations of NICU patients overall, with and without AKI. Data will be analyzed to compare the different definitions of neonatal AKI.
No interventions are included in this study protocol.
No conflict with any other ongoing studies as this is a retrospective observational study.
The MediData Rave™ database was piloted by centers from 1/17/15 to 1/28/15. Subsequently final adjustments of the manual of operation were made. Each institution was required to participate in database training on either 2/10/15 or 2/12/15. Additionally, each database entry personnel was required to participate in online training from 2/10/15 to 2/27/15. The database was open for data entry on 3/2/15. The trial was registered at ClinicalTrials.gov on 5/11/15. All institutions completed data entry by 12/12/15.
The AWAKEN study created the largest cohort of neonates assembled to date for exploring AKI in this vulnerable patient population. Across the 24 centers with high acuity NICUs distributed across 3 continents and 4 countries, we screened 4273 and enrolled 2162 newborns during the 3 months’ time-period. As demonstrated in Table 2, the institutions represented in the AWAKEN study include academic medical centers with diverse referral patterns, volumes of patients and creatinine assay methods. By collecting detailed demographic information on these babies, the NKC has created a rich database with which we will be able to describe the prevalence of neonatal AKI in different ethnic, racial, and geographic populations. Analyses can be performed based on birthweight, gestational age, and other criteria. Daily weights can be assessed as a surrogate for “fluid balance” in the first week after birth in ELBW neonates given the difficulty in accurately determining fluid status based solely on intake and measurable output. Although data points were chosen based on relatedness to renal function and injury, the database will be a source of carefully collected information on NICU patients that may be used to compare practices across sites and investigate various best practices in this population, including strategies for fluid provision.
The NKC represents an interdisciplinary group of clinicians and researchers interested in neonatal AKI with the unified goal of improving the lives, health and wellbeing of newborns at risk for kidney disease. Representation of institutions from four countries and three continents and the involvement of both pediatric nephrologists and neonatologists make the unique. Each subspecialty brings its expertise relevant to understanding the problem of neonatal renal development, function, and injury. For example neonatologists have less experience with the long-term ramifications of renal disorders seen in the newborn period, while pediatric nephrologists rarely are involved in the initial care of the extremely premature infants or those with severe perinatal asphyxia, including decisions regarding fluid provision and choice of drugs to treat or prevent various conditions. True collaborations such as this will allow a greater understanding of neonatal renal physiology and pathology and the impact on other organ systems. AWAKEN will provide the infrastructure and experience for gathering evidence-based data on best practices to improve short- and long-term kidney health in neonates.
The AWAKEN study does have several limitations, the most substantial being the retrospective design. The retrospective design will likely result in the absence of data points for many infants. Since the serum creatinine is ordered by the treating neonatologist who may be more likely to do so if there are clinical indicators for AKI, its incidence may be underestimated especially as AKI in neonates may be non-oliguric as in the case of gentamicin nephrotoxicity. However, we will gain insight into current practices for monitoring, diagnosing, and treating kidney function. By comparing practices between NICUs, we will acquire a sense of important time points for data collection for future prospective studies. In addition, we may be able to develop “best practices” standards of care for at-risk neonatal populations.
At the onset of the AWAKEN project the database committee, in conjunction with the Steering committee, scrutinized variables to address the aims of the study, with the goal of balancing the granularity of the data with the time commitment necessary for data extraction and entry. Even after limiting collection to those data points deemed crucial, the time required to complete screening and data entry for excluded patients was ~30 min and an average of 2 h for included patients, depending on the complexity of the medical conditions and length of stay.
As with many clinical studies, retrospective or prospective, much of the data extraction is done by trained research staff and not by physicians. We surveyed the participating centers and discovered the site principal investigator was responsible for a significant proportion of data extraction at only four institutions, and the data was entered by a physician at only two sites. In fact, since this was a study initiated without benefit of external funding, it is likely that the extent of direct physician involvement in the data extraction and entry was higher than if funding were available to hire additional research staff. Given the cross-disciplinary nature of the study, the familiarity of research staff with both neonatal and renal terminology, including definitions of diagnoses, may have been challenging. Several illustrative examples were encountered as the database was “cleaned” and queries generated. This unfamiliarity resulted in additional time and effort on the part of the center PI, the database committee and the data center personnel to ensure the integrity of the data prior to analysis. In future studies, more detail and training will be incorporated in the Manual of Procedures to address this issue.
Even with these limitations, NKC has an unprecedented opportunity to provide better estimates of the incidence and outcomes of neonatal AKI, and it is likely this work will help to refine the definition of neonatal AKI, improve our understanding of the risk factors associated with AKI, and raise awareness of AKI as an important clinical event during the care of critically ill neonates. We will improve our understanding of the contribution of fluid balance to biochemical and clinical outcomes. Additionally, by having a concise documentation of each infant’s hospital course, we will be able to infer the role which AKI and other potential risk factors have on the incidence of CKD in future studies. These collaborations, including the data and infrastructure, will enable the NKC to plan future intervention studies geared toward the reduction of AKI and improved short and long-term renal outcomes, including CKD.
AWAKEN was proposed as human subjects research. The study design allowed for a waiver of informed consent/parental permission. Permission for limited protected health information (PHI) dataset was requested and granted by all sites, specifically to allow the use of date of birth for proper creation of the database as described above. The waiver of consent was pursued based on the following rationale:
• The research involves no more than minimal risk to the subjects.
• The waiver does not adversely affect the rights and welfare of the subjects.
• The research cannot practically be carried out without the waiver or alteration. This is a retrospective cohort study of infants admitted over a year prior to data collection. Requiring informed consent from every eligible patient would cause a significant reduction in enrollment and potentially introduce selection bias into the dataset.
The sites participating in AWAKEN obtained appropriate institutional review board approval from their respective centers and executed data use agreements with the data coordination center (Cincinnati Children’s Hospital Medical Center). Protocol amendments have been generated by the protocol committee and disseminated to each institution.
Deidentified data is maintained at the University of Alabama at Birmingham. NKC investigators plan to disseminate data through peer-reviewed publications and through platform and poster presentations at educational conferences. The ancillary studies committee will provide data sets to NKC members following the approval of the project.
JGJ participated in the design and implementation of the study of the study, the preparation of the initial draft, and has critically reviewed the manuscript. RG participated in the design of the study and implementation of the study, the preparation of the initial draft, and has critically reviewed the manuscript. DA participated in the design and implementation of the study and has critically reviewed the manuscript. LD participated in the implementation of the study and has critically reviewed the manuscript. JJ participated in the design of this study, including the database programing and has critically reviewed the manuscript. AK participated in the design of the study and has critically reviewed the manuscript. DS participated in the design of the study and has critically reviewed the manuscript. CA participated in the implementation of the study and has critically reviewed the manuscript. FK participated in the implementation of the study and has critically reviewed the manuscript. MM participated in the implementation of the study and has critically reviewed the manuscript. NA participated in the implementation of the study and has critically reviewed the manuscript. JC participated in the design of the study, the preparation of the initial draft, and has critically reviewed the manuscript. All authors aided in the drafting of the work or revising of the work it critically for important intellectual content, approve the final version to be published, and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work were appropriately investigated and resolved.
David Askenazi serves on the speaker board for BAXTER, and the AKI Foundation. Stuart L. Goldstein receives grant funding from Baxter Healthcare and is a consultant for Baxter, Bellco, Inc., Akebia, Inc., AM Pharma, Inc., and Astute Medical. Jonathan M. Klein is a consultant for Draeger Medical. Juan C. Kupferman is on the speaker’s Bureau and consultant for Alexion Pharmaceuticals. Subrata Sarkar is a consultant for GW Research Ltd, Cambridge, UK.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Clinical Research Personnel and Colleagues: The authors acknowledge the outstanding work of the following clinical research personnel and colleagues involved in AWAKEN: Ariana Aimani, Montreal Children’s Hospital, McGill University Health Centre, Montreal, QC, Canada; Laila Ajour, BS, University of Colorado, Children’s Hospital Colorado, Aurora, CO, USA; Melissa Bowman, RN, University of Rochester, Rochester, New York, NY, USA; Teresa Cano, RN, Holtz Children’s Hospital, University of Miami, Miami, FL, USA; Sarah Cashman, BS, University of Iowa Children’s Hospital, Iowa City, IA, USA; Jonathan Davis, MD, Floating Hospital for Children at Tufts Medical Center, Tufts University School of Medicine, Boston, MA, USA; Alanna DeMello, British Columbia Children’s Hospital, Vancouver, BC, Canada; Marta G. Galarza, MD, Holtz Children’s Hospital, University of Miami, Miami, Florida; Wendy Glaberson, MD, Holtz Children’s Hospital, University of Miami, Miami, FL, USA; Ellen Guthrie, RN, MetroHealth Medical Center, Case Western Reserve University, Cleveland, OH, USA; Nicholas L. Harris, BS, CCRC, C.S. Mott Children’s Hospital, University of Michigan, Ann Arbor, MI, USA; Susan M. Hieber, MSQM, C.S. Mott Children’s Hospital, University of Michigan, Ann Arbor, Michigan; Katherine Huang, University of Virginia Children’s Hospital, Charlottesville, VI, USA; Nilima Jawale, MD, Maimonides Medical Center, Brooklyn, New York, NY, USA; Emily Kane, Australian National University, Canberra, Australia; Vijay Kher, DM, Medanta Kidney Institute, The Medicity Hospital, Gurgaon, Haryana, India; Ryan Knox, BS, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA; Samantha Kronish, Montreal Children’s Hospital, McGill University Health Centre, Montreal, QC, Canada; Grace Mele, New York College of Osteopathic Medicine, Westbury, New York, NY, USA; Patricia Mele, DNP, NNP, Stony Brook Children’s Hospital, Stony Brook, New York, NY, USA; Aura Arenas Morales, MD, Holtz Children’s Hospital, University of Miami, Miami, Florida; Julie Nicoletta, MD, MA, Floating Hospital for Children at Tufts Medical Center, Tufts University School of Medicine, Boston, MA, USA; Charity Njoku, Texas Children’s Hospital, Baylor College of Medicine, Houston, TX, USA; Ana Palijan, MD, Montreal Children’s Hospital, McGill University Health Centre, Montreal, QC, Canada; Emily Pao, University of Washington, Seattle Children’s Hospital, Seattle, Washington; Tennille Paulsen, Texas Children’s Hospital, Baylor College of Medicine, Houston, TX, USA; Hilary Pitner, MS, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA; Michael Pizzi, Montreal Children’s Hospital, McGill University Health Centre, Montreal, QC, Canada; Becky Selman RN, University of New Mexico Health Sciences Center, Albuquerque, NM, USA; Puneet Sodhi, MBBS, Medanta Kidney Institute, The Medicity Hospital, Gurgaon, Haryana, India; Michele Spear, CCRC, University of New Mexico Health Sciences Center, Albuquerque, NM, USA; Madeleine Stead, BS, University of Iowa Children’s Hospital, Iowa City, IA, USA; Tara Terrell, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA; Denisse Cristina Pareja Valarezo, MD, MPH, Holtz Children’s Hospital, University of Miami, Miami, Florida; Melissa Vega, PA-C, The Children’s Hospital at Montefiore, Bronx, New York, NY, USA; Leslie Walther RN, Washington University, St. Louis, MO, USA; Rosa Waters, University of Virginia Children’s Hospital, Charlottesville, Virginia; Julia Wrona, BS, University of Colorado, Children’s Hospital Colorado, Aurora, Colorado; Sadia Zubair, Texas Children’s Hospital, Baylor College of Medicine, Houston, TX, USA.
The following individuals served as collaborators and site investigators for the AWAKEN study. They participated in protocol development and review, local IRB submission, data collection and reviewed and revised the manuscript:
Ayse Akcan Arikan, Texas Children’s Hospital, Baylor College of Medicine, Houston, TX, USA; Alok Bhutada, Maimonides Medical Center, Brooklyn, New York, NY, USA; Elizabeth Bonachea, Nationwide Children’s Hospital, Columbus, OH, USA; Louis Boohaker, Children’s of Alabama, University of Alabama at Birmingham, Birmingham, AL, USA; Patrick D. Brophy, University of Iowa Children’s Hospital, Iowa City, IA, USA; Aftab S. Chishti, University of Kentucky, Lexington, KY, USA; Tarah T. Colaizy, University of Iowa Children’s Hospital, Iowa City, IA, USA; F. Sessions Cole, Washington University, St. Louis, MO, USA; Carl D’Angio, Golisano Children’s Hospital, University of Rochester, Rochester, New York, NY, USA; T. Keefe Davis, Washington University, St. Louis, MO, USA; Marissa DeFreitas, Holtz Children’s Hospital, University of Miami, Miami, FL, USA; Joshua Dower, Tufts University School of Medicine, Boston, MA, USA; Shahnaz Duara, Holtz Children’s Hospital, University of Miami, Miami, FL, USA; Jeffery Fletcher, Centenary Hospital for Women and Children, Canberra Hospital, Australian National University Medical School, Canberra, Australia; Mamta Fuloria, The Children’s Hospital at Montefiore, Bronx, New York, NY, USA; Jason Gien, University of Colorado, Children’s Hospital Colorado, Aurora, CO, USA; Katja M. Gist, University of Colorado, Children’s Hospital Colorado, Aurora, CO, USA; Stuart L. Goldstein, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA; Russell Griffin, Children’s of Alabama, University of Alabama at Birmingham, Birmingham, AL, USA; Mina H. Hanna, University of Kentucky, Lexington, KY, USA; Sangeeta Hingorani, University of Washington, Seattle Children’s Hospital, Seattle, WA, USA; Susan Ingraham, Nationwide Children’s Hospital, Columbus, OH, USA; Catherine Joseph, University of New Mexico Health Sciences Center, Albuquerque, NM, USA; Surender Khokhar, Apollo Cradle, Gurgaon, Haryana, India; Jonathan M. Klein, University of Iowa Children’s Hospital, Iowa City, IA, USA; Deepak Kumar, MetroHealth Medical Center, Case Western Reserve University, Cleveland, OH, USA; Juan C. Kupferman, Maimonides Medical Center, Brooklyn, New York, NY, USA; John Mahan, Nationwide Children’s Hospital, Columbus, OH, USA; Cherry Mammen, British Columbia Children’s Hospital, Vancouver, BC, Canada; Ayesa Mian, Golisano Children’s Hospital, University of Rochester, Rochester, New York, NY, USA; Lawrence Milner, Floating Hospital for Children at Tufts Medical Center, Tufts University School of Medicine, Boston, MA, USA; Arwa Nada, Nationwide Children’s Hospital, Columbus, OH, USA; Amy T. Nathan, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA; Robin Ohls, University of New Mexico Health Sciences Center, Albuquerque, NM, USA; Sofia Perazzo, Children’s National Medical Center, The George Washington University School of Medicine and the Health Sciences, Washington, DC, USA; Erin Rademacher, Golisano Children’s Hospital, University of Rochester, Rochester, New York, NY, USA; Rupesh Raina, MetroHealth Medical Center, Case Western Reserve University, Cleveland, OH, USA; Shantanu Rastogi, Maimonides Medical Center, Brooklyn, New York, NY, USA; Patricio E. Ray, Children’s National Medical Center, The George Washington University School of Medicine and the Health Sciences, Washington, DC, USA; Kimberly Reidy, The Children’s Hospital at Montefiore, Bronx, New York, NY, USA; Mary Revenis, Children’s National Medical Center, The George Washington University School of Medicine and the Health Sciences, Washington, DC, USA; Christopher J. Rhee, Texas Children’s Hospital, Baylor College of Medicine, Houston, TX, USA; Smriti Rohatgi, Medanta Kidney Institute, The Medicity Hospital, Gurgaon, Haryana, India; Subrata Sarkar, C.S. Mott Children’s Hospital, University of Michigan, Ann Arbor, MI, USA; Sidharth Kumar Sethi, Medanta Kidney Institute, The Medicity Hospital, Gurgaon, Haryana, India; Alexandra Smith, Floating Hospital for Children at Tufts Medical Center, Tufts University School of Medicine, Boston, MA, USA; Danielle E. Soranno, University of Colorado, Children’s Hospital Colorado, Aurora, CO, USA; Shanthy Sridhar, Stony Brook Children’s Hospital, Stony Brook, New York, NY, USA; Amy Staples, University of New Mexico Health Sciences Center, Albuquerque, NM, USA; Michelle Starr, University of Washington, Seattle Children’s Hospital, Seattle, WA, USA; Jonathan R. Swanson, University of Virginia Children’s Hospital, Charlottesville, VI, USA; Anne Synnes, British Columbia Children’s Hospital, Vancouver, BC, Canada; Sanjay Wazir, DM, Apollo Cradle, Gurgaon, Haryana, India; Pia Wintermark, Montreal Children’s Hospital, McGill University Health Centre, Montreal, QC, Canada; Craig S. Wong, University of New Mexico Health Sciences Center, Albuquerque, NM, USA; Robert Woroniecki, Stony Brook Children’s Hospital, Stony Brook, New York, NY, USA; Michael Zappitelli, Montreal Children’s Hospital, McGill University Health Centre, Montreal, QC, Canada.
The Cincinnati Children’s Hospital Center for Acute Care Nephrology (SLG, Director) provided funding to create and maintain the AWAKEN Medidata Rave electronic database. CA was supported by the Micah Batchelor Foundation. AAA and CJR were supported by the Section of Pediatric Nephrology, Department of Pediatrics, Texas Children’s Hospital. DA and NA are supported by the Pediatric and Infant Center for Acute Nephrology (PICAN), which is sponsored by Children’s of Alabama and the University of Alabama at Birmingham’s School of Medicine, Department of Pediatrics and Center for Clinical and Translational Science (CCTS) under award number UL1TR00165. PDB, TTC, JGJ, and JMK were supported by the University of Iowa Institute for Clinical and Translational Science. JRC and JRS were supported by a grant from 100 Women Who Care. FSC and KTD were supported by the Edward Mallinckrodt Department of Pediatrics at Washington University School of Medicine. JF and AK supported by the Canberra Hospital Private Practice Fund. RG and ER were supported by the Department of Pediatrics, Golisano Children’s Hospital, University of Rochester. CJ, RO, AS, and CSW were supported by the University of New Mexico Clinical Translational Science Center UL1TR001449. PER was supported by R01 HL-102497, R01 DK 49419. SS and DTS were supported by the Department of Pediatrics And Communicable Disease, C.S. Mott Children’s Hospital, University of Michigan. SS and RW were supported by Stony Brook Children’s Hospital Department of Pediatrics funding.
The Supplementary Material for this article can be found online at http://journal.frontiersin.org/article/10.3389/fped.2016.00068
AKI, acute kidney injury; AWAKEN, assessment of worldwide acute kidney injury epidemiology in neonates; CKD, chronic kidney disease; CRF, case report forms; CTSI, clinical and translational science institute; ELBW, extremely low birth weight; ELGAN, extremely low gestational age neonate; KDIGO, kidney disease: improving global outcomes; NICU, neonatal intensive care unit; NIH, National Institutes of Health; NKC, neonatal kidney collaborative; RIFLE, risk, injury, failure, loss- and end-stage; SCr, serum creatinine; TEFL, transepidermal fluid losses.
1. Akcan-Arikan A, Zappitelli M, Loftis LL, Washburn KK, Jefferson LS, Goldstein SL. Modified RIFLE criteria in critically ill children with acute kidney injury. Kidney Int (2007) 71(10):1028–35. doi:10.1038/sj.ki.5002231
2. Zappitelli M, Parikh CR, Akcan-Arikan A, Washburn KK, Moffett BS, Goldstein SL. Ascertainment and epidemiology of acute kidney injury varies with definition interpretation. Clin J Am Soc Nephrol (2008) 3(4):948–54. doi:10.2215/CJN.05431207
3. Ricci Z, Cruz D, Ronco C. The RIFLE criteria and mortality in acute kidney injury: a systematic review. Kidney Int (2008) 73(5):538–46. doi:10.1038/sj.ki.5002743
4. Cuhaci B. More data on epidemiology and outcome of acute kidney injury with AKIN criteria: benefits of standardized definitions, AKIN and RIFLE classifications. Crit Care Med (2009) 37(9):2659–61. doi:10.1097/CCM.0b013e3181ad76c2
5. Uchino S. Outcome prediction for patients with acute kidney injury. Nephron Clin Pract (2008) 109(4):c217–23. doi:10.1159/000142931
6. Macedo E, Castro I, Yu L, Abdulkader RR, Vieira JM Jr. Impact of mild acute kidney injury (AKI) on outcome after open repair of aortic aneurysms. Ren Fail (2008) 30(3):287–96. doi:10.1080/08860220701857522
7. Bagshaw SM, George C, Bellomo R; ANZICS Database Management Committee. Changes in the incidence and outcome for early acute kidney injury in a cohort of Australian intensive care units. Crit Care (2007) 11(3):R68. doi:10.1186/cc5949
8. Koralkar R, Ambalavanan N, Levitan EB, McGwin G, Goldstein S, Askenazi D. Acute kidney injury reduces survival in very low birth weight infants. Pediatr Res (2011) 69(4):354–8. doi:10.1203/PDR.0b013e31820b95ca
9. Viswanathan S, Manyam B, Azhibekov T, Mhanna MJ. Risk factors associated with acute kidney injury in extremely low birth weight (ELBW) infants. Pediatr Nephrol (2012) 27(2):303–11. doi:10.1007/s00467-011-1977-8
10. Askenazi DJ, Koralkar R, Hundley HE, Montesanti A, Patil N, Ambalavanan N. Fluid overload and mortality are associated with acute kidney injury in sick near-term/term neonate. Pediatr Nephrol (2013) 28(4):661–6. doi:10.1007/s00467-012-2369-4
11. Gadepalli SK, Selewski DT, Drongowski RA, Mychaliska GB. Acute kidney injury in congenital diaphragmatic hernia requiring extracorporeal life support: an insidious problem. J Pediatr Surg (2011) 46(4):630–5. doi:10.1016/j.jpedsurg.2010.11.031
12. Selewski DT, Jordan BK, Askenazi DJ, Dechert RE, Sarkar S. Acute kidney injury in asphyxiated newborns treated with therapeutic hypothermia. J Pediatr (2013) 162(4):725–29.e1. doi:10.1016/j.jpeds.2012.10.002
13. Mathur NB, Agarwal HS, Maria A. Acute renal failure in neonatal sepsis. Indian J Pediatr (2006) 73(6):499–502. doi:10.1007/BF02759894
14. Carmody JB, Swanson JR, Rhone ET, Charlton JR. Recognition and reporting of AKI in very low birth weight infants. Clin J Am Soc Nephrol (2014) 9(12):2036–43. doi:10.2215/CJN.05190514
15. Abitbol CL, Bauer CR, Montane B, Chandar J, Duara S, Zilleruelo G. Long-term follow-up of extremely low birth weight infants with neonatal renal failure. Pediatr Nephrol (2003) 18(9):887–93. doi:10.1007/s00467-003-1186-1
16. Askenazi DJ, Morgan C, Goldstein SL, Selewski DT, Moxey-Mims MM, Kimmel PL, et al. Strategies to improve the understanding of long-term renal consequences after neonatal acute kidney injury. Pediatr Res (2016) 79(3):502–8. doi:10.1038/pr.2015.241
17. Neonatal Acute Kidney Injury Workshop. (2013). Available from: http://www.niddk.nih.gov/news/events-calendar/Pages/neonatal-acute-kidney-injury-workshop.aspx#tab-event-details
18. Selewski DT, Charlton JR, Jetton JG, Guillet R, Mhanna MJ, Askenazi DJ, et al. Neonatal acute kidney injury. Pediatrics (2015) 136(2):e463–73. doi:10.1542/peds.2014-3819
19. Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P; Acute Dialysis Quality Initiative Workgroup. Acute renal failure – definition, outcome measures, animal models, fluid therapy and information technology needs: the second international consensus conference of the acute dialysis quality initiative (ADQI) group. Crit Care (2004) 8(4):R204–12. doi:10.1186/cc2671
20. Kellum JA, Lameire N; KDIGO AKI Guideline Work Group. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (part 1). Crit Care (2013) 17(1):204. doi:10.1186/cc11454
21. Jetton JG, Askenazi DJ. Update on acute kidney injury in the neonate. Curr Opin Pediatr (2012) 24(2):191–6. doi:10.1097/MOP.0b013e32834f62d5
22. Alabbas A, Campbell A, Skippen P, Human D, Matsell D, Mammen C. Epidemiology of cardiac surgery-associated acute kidney injury in neonates: a retrospective study. Pediatr Nephrol (2013) 28(7):1127–34. doi:10.1007/s00467-013-2454-3
23. Morgan CJ, Zappitelli M, Robertson CM, Alton GY, Sauve RS, Joffe AR, et al. Risk factors for and outcomes of acute kidney injury in neonates undergoing complex cardiac surgery. J Pediatr (2013) 162(1):120–7.e1. doi:10.1016/j.jpeds.2012.06.054
24. Goldstein SL. Fluid management in acute kidney injury. J Intensive Care Med (2012) 29(4):183–9. doi:10.1177/0885066612465816
25. Davison D, Basu RK, Goldstein SL, Chawla LS. Fluid management in adults and children: core curriculum 2014. Am J Kidney Dis (2014) 63(4):700–12. doi:10.1053/j.ajkd.2013.10.044
26. Fenton TR, McMillan DD, Sauve RS. Nutrition and growth analysis of very low birth weight infants. Pediatrics (1990) 86(3):378–83.
27. Bell EF, Acarregui MJ. Restricted versus liberal water intake for preventing morbidity and mortality in preterm infants. Cochrane Database Syst Rev (2014) 12:CD000503. doi:10.1002/14651858.CD000503.pub3
28. Goldstein SL, Currier H, Graf C, Cosio CC, Brewer ED, Sachdeva R. Outcome in children receiving continuous venovenous hemofiltration. Pediatrics (2001) 107(6):1309–12. doi:10.1542/peds.107.6.1309
Keywords: AWAKEN, NKC, neonate, database, KDIGO, acute renal failure
Citation: Jetton JG, Guillet R, Askenazi DJ, Dill L, Jacobs J, Kent AL, Selewski DT, Abitbol CL, Kaskel FJ, Mhanna MJ, Ambalavanan N, Charlton JR and the Neonatal Kidney Collaborative (2016) Assessment of Worldwide Acute Kidney Injury Epidemiology in Neonates: Design of a Retrospective Cohort Study. Front. Pediatr. 4:68. doi: 10.3389/fped.2016.00068
Received: 17 May 2016; Accepted: 20 June 2016;
Published: 19 July 2016
Edited by:
Jacqueline Ho, Children’s Hospital of Pittsburgh of UPMC, USAReviewed by:
Tauqeer Hussain Mallhi, Hospital Universiti Sains MalaysiaCopyright: © 2016 Jetton, Guillet, Askenazi, Dill, Jacobs, Kent, Selewski, Abitbol, Kaskel, Mhanna, Ambalavanan, Charlton and the Neonatal Kidney Collaborative. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jennifer G. Jetton, amVubmlmZXItamV0dG9uQHVpb3dhLmVkdQ==
†Co-first authors.
‡The Neonatal Kidney Collaborative authors are listed at the end of the article.
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.