- 1Department of Pediatrics, University of Tennessee Health Science Center, Le Bonheur Children’s Hospital Memphis, Memphis, TN, USA
- 2Children’s Foundation Research Institute, Le Bonheur Children’s Hospital, Memphis, TN, USA
- 3Department of Performance Improvement and Patient Safety, Children’s Hospital of The King’s Daughters, Norfolk, VA, USA
- 4Department of Quality Improvement, Le Bonheur Children’s Hospital, Memphis, TN, USA
- 5Le Bonheur Children’s Hospital, Memphis, TN, USA
Background: Identifying risk factors related to central venous line (CVL) placement could potentially minimize central line-associated venous thrombosis (CLAVT). We sought to identify the clinical factors associated with CLAVT in children.
Methods: Over a 3-year period, 3733 CVLs were placed at a tertiary-care children’s hospital. Data were extracted from the electronic medical records of patients with clinical signs and symptoms of venous thromboembolism, diagnosed using Doppler ultrasonography and/or echocardiography. Statistical analyses examined differences in CLAVT occurrence between groups based on patient and CVL characteristics (type, brand, placement site, and hospital unit).
Results: Femoral CVL placement was associated with greater risk for developing CLAVT (OR 11.1, 95% CI 3.9–31.6, p < 0.0001). CVLs placed in the NICU were also associated with increased CLAVT occurrence (OR 5.3, 95% CI 2.1–13.2, p = 0.0003). CVL brand was also significantly associated with risk of CLAVT events.
Conclusion: Retrospective analyses identified femoral CVL placement and catheter type as independent risk factors for CLAVT, suggesting increased risks due to mechanical reasons. Placement of CVLs in the NICU also led to an increased risk of CLAVT, suggesting that small infants are at increased risk of thrombotic events. Alternative strategies for CVL placement, thromboprophylaxis, and earlier diagnosis may be important for reducing CLAVT events.
Introduction
The incidence of venous thromboembolism (VTE) is increasing in the hospitalized pediatric population (1–4). This is surprising due to the previously described rarity of this potentially fatal condition (5). Although variable across institutions, an overall VTE incidence of 188/100,000 hospitalized pediatric patients was found in the retrospective KID database (3, 6). The overall incidence of VTE may have increased recently, since there are no current established guidelines for pediatric thromboprophylaxis (4, 7, 8).
A multifactorial process leads to VTE development in hospitalized children, possibly related to their age and race, primary diagnoses, congenital and genetic factors, and mechanical factors (3, 4, 9). Retrospective and prospective studies have identified higher risks for VTE in children below 1 year of age, adolescents, children with specific renal, hematologic, infectious, traumatic, and oncologic diagnoses, and those exposed to mechanical factors (1, 4, 7, 10, 11). Beck et al. found sonographic evidence of venous thrombosis in 18.3% of children requiring a central venous line (CVL) (11).
Central venous line insertion is associated with multiple complications including central line-associated bloodstream infections (CLABSI) and thrombosis (12, 13). Although CLABSI is well characterized in pediatric and adult hospitalized patients, VTE development is less studied in the pediatric population (1, 14). To date, there are no published guidelines for the diagnosis of central line-associated venous thrombosis (CLAVT) in children (14). The diagnosis of CLAVT has depended upon clinical signs and symptoms, the use of Wells’ criteria, along with imaging studies such as Doppler ultrasound and traditional venography (1, 4).
We conducted a retrospective cohort study to identify risk factors associated with CLAVT incidence in a tertiary-care children’s hospital. We hypothesized that CLAVT identified via ultrasound imaging would be increased in children <1 year of age and those with femoral CVL placement.
Materials and Methods
Data Collection
With Institutional Review Board approval, data were collected on 3733 CVLs placed within a 3-year period (2010–2012). The number of charts reviewed did include placement of multiple central lines in the same patient. “Symptomatic” CLAVT was defined as thrombosis associated with a blocked or leaking CVL, progressive distal limb edema, or clinical signs of pulmonary embolism (chest pain, shortness of breath, tachycardia, tachypnea, hypoxia, hemoptysis). Standard nursing assessment of either the presence or absence of venous thrombosis was done every 12 h and CLAVT was characterized by redness, edema, and presence/absence of a distal pulse. CLAVT was confirmed via either Doppler ultrasonography (with increased echogenicity at the site or presence of a filling defect) or with presence of a thrombus on echocardiography. Patients with venous thrombosis either present at hospital admission or unrelated to CVL placement were excluded. The following variables were included hospital location of catheter placement, catheter type, CVL placement site, number of attempts to place CVL, and use of ultrasonography for vascular access.
Statistical Analyses
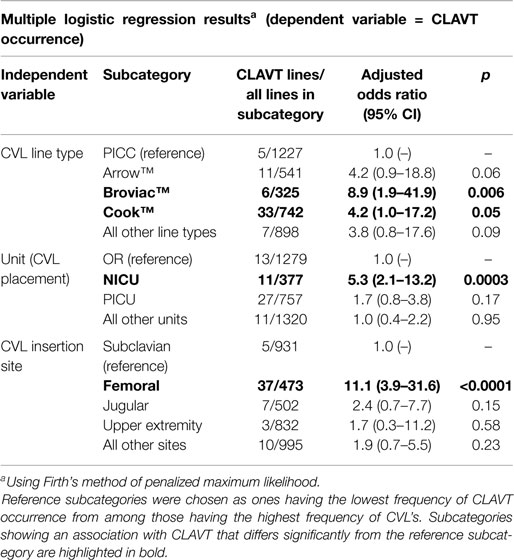
In order to adjust for bias associated with low occurrence of CLAVT in many of the subcategories being examined, the Firth method of multiple logistic regression based on a penalized maximum likelihood function was used to analyze associations between CLAVT and line type (Arrow™, Cook™, Broviac™, PICC, and All Other), CLAVT and unit of insertion (OR, PICU, NICU, All Other), and CLAVT and anatomical insertion site (Femoral, Jugular, Subclavian, Upper Extremity, and All Other). “All other” subcategories represented groupings of all but the categories that were most common in each dataset.
Results
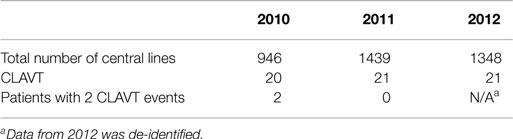
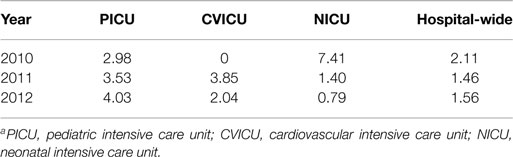
The numbers of CLAVT events are listed in Table 1. The association of total number of central lines placed with the number of CLAVT events shows a slight decline of events in 2011 and 2012, in comparison to data shown for 2010. The number of CLAVT events per total annual catheter number increased in the PICU compared to other hospital locations at the end of the 3-year period (Table 2). Although in 2010, the NICU had the highest percentage of CLAVT events. Additionally, CVL placement in the NICU had a higher odds ratio than other units (OR 5.3, 95% CI 2.1–13.2, p = 0.0003; Table 3).
Risk factor assessment (Table 3) showed that femoral vein placement contributed significantly to CLAVT (OR 11.1, 95% CI 3.9–31.6, p < 0.0001), in comparison to upper extremity lines. Broviac™ and Cook™ catheters were also more commonly associated with CLAVT (Table 3).
Discussion
Central line-associated venous thrombosis is a significant iatrogenic complication of critical illness in our tertiary-care facility. Significant risk factors at our facility include anatomical site, hospital location of CVL placement, and types of lines placed. Variability exists in the diagnosis of CLAVT and its incidence based on clinical risk factors (6). Our finding that small children, specifically NICU patients, have the highest number of CLAVT events is consistent with previous studies of children with central venous catheters (1, 3, 4, 7, 8, 11).
Central venous line characteristics, including type, brand, and anatomic site of insertion and their relationship to number of CLAVT events were evaluated in this study. Our data support the well-established postulate that femoral CVLs predispose pediatric patients to CLAVT, especially in hospitalized children below 1 year of age (4, 15). Higgerson et al. found that femoral DVTs occur in mechanically ventilated PICU patients, whether or not they were CVL-related (7). We found that the highest number of CLAVTs occurred in PICU patients, with a slight decrease in the number of line events in 2012, likely due to a Hawthorne effect. Nonetheless, our findings are congruent with these previously published data, both suggesting that CLAVT is a growing problem in the PICU population. Other CVL characteristics – CVL type and brand – did not have a significant impact on CLAVT development in a study by Male et al. (16). Broviac™ and Cook™ catheters were significantly associated with CLAVT risk in our study; however, a larger, prospective study is needed to assess this risk factor more precisely.
Overall, the incidence of CLAVT minimally increased over a 3-year period. Increased awareness of the study itself could have lead to increased diagnosis in 2011 and 2012. Also, in our patients with symptomatic CLAVT, the probability of screening could potentially be higher (17).
There are several limitations of this retrospective study. CLAVT is associated with increased mortality and increased length of stay, especially in the PICU, but we did not have adequate sample size to examine these outcomes in our study (1, 4, 7, 18). Considering the association of CLAVT with increased mortality, it would have been prudent to assess levels of critical illness. Only information on symptomatic VTEs, based on clinical signs and symptoms, was captured in this study and confirmed by the use of Doppler ultrasonography or echocardiography. Geerts et al. assert that the ratio of asymptomatic to symptomatic DVT ranges from 5:1 to 10:1 and that asymptomatic DVT evolves to symptomatic VTE (17). Asymptomatic CLAVTs were probably undetected in our patients and therefore, we will evaluate CLAVT prospectively in order to gather more data including diagnostic methods and primary diagnoses. CLABSI association was not addressed in this study, as our dataset was limited. Lastly, the use of thromboprophylaxis was not evaluated during this study. Guidelines for use of thromboprophylaxis in pediatric patients have been based upon adult guidelines (19). It is essential to evaluate thromboprophylaxis prospectively, in order to establish which anti-thrombolytic therapies are most effective in pediatric patients (4).
Despite the limitations of this study, we found that ICU patients with femoral CVL placement had the highest incidence of CLAVT in our facility. Based on increased awareness, it would be possible to reach an earlier diagnosis and evaluation interventions to decrease CLAVT rates. Prospective, multicenter studies are warranted to enhance earlier diagnostic strategies and to determine the most effective thromboprophylaxis and antithrombotic interventions required for PICU patients.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Takemoto CM, Sohi S, Desai K, Bharaj R, Khanna A, McFarland S, et al. Hospital-associated venous thromboembolism in children: incidence and clinical characteristics. J Pediatr (2014) 164(2):332–8. doi: 10.1016/j.jpeds.2013.10.025
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
2. Silverstein MD, Heit JA, Mohr DN, Petterson TM, O’Fallon WM, Melton LJ III. Trends in the incidence of deep vein thrombosis and pulmonary embolism: a 25-year population-based study. Arch Intern Med (1998) 158(6):585–93. doi:10.1001/archinte.158.6.585
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
3. Setty BA, O’Brien SH, Kerlin BA. Pediatric venous thromboembolism in the United States: a tertiary care complication of chronic diseases. Pediatr Blood Cancer (2012) 59(2):258–64. doi:10.1002/pbc.23388
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
4. Sandoval JA, Sheehan MP, Stonerock CE, Shafique S, Rescorla FJ, Dalsing MC. Incidence, risk factors, and treatment patterns for deep venous thrombosis in hospitalized children: an increasing population at risk. J Vasc Surg (2008) 47(4):837–43. doi:10.1016/j.jvs.2007.11.054
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
5. Andrew M, David M, Adams M, Ali K, Anderson R, Barnard D, et al. Venous thromboembolic complications (VTE) in children: first analyses of the Canadian registry of VTE. Blood (1994) 83(5):1251–7.
6. Chan AK, Deveber G, Monagle P, Brooker LA, Massicotte PM. Venous thrombosis in children. J Thromb Haemost (2003) 1(7):1443–55. doi:10.1046/j.1538-7836.2003.00308.x
7. Higgerson RA, Lawson KA, Christie LM, Brown AM, McArthur JA, Totapally BR, et al. Incidence and risk factors associated with venous thrombotic events in pediatric intensive care unit patients. Pediatr Crit Care Med (2011) 12(6):628–34. doi:10.1097/PCC.0b013e318207124a
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
8. Raffini L, Huang YS, Witmer C, Feudtner C. Dramatic increase in venous thromboembolism in children’s hospitals in the United States from 2001 to 2007. Pediatrics (2009) 124(4):1001–8. doi:10.1542/peds.2009-0768
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
9. Kenet G, Nowak-Gottl U. Venous thromboembolism in neonates and children. Best Pract Res Clin Haematol (2012) 25(3):333–44. doi:10.1016/j.beha.2012.07.001
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
10. Rana P, Levine MN. Prevention of thrombosis in ambulatory patients with cancer. J Clin Oncol (2009) 27(29):4885–8. doi:10.1200/JCO.2009.23.5481
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
11. Beck C, Dubois J, Grignon A, Lacroix J, David M. Incidence and risk factors of catheter-related deep vein thrombosis in a pediatric intensive care unit: a prospective study. J Pediatr (1998) 133(2):237–41. doi:10.1016/S0022-3476(98)70226-4
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
12. Raad II, Luna M, Khalil SA, Costerton JW, Lam C, Bodey GP. The relationship between the thrombotic and infectious complications of central venous catheters. JAMA (1994) 271(13):1014–6. doi:10.1001/jama.1994.03510370066034
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
13. Timsit JF, Farkas JC, Boyer JM, Martin JB, Misset B, Renaud B, et al. Central vein catheter-related thrombosis in intensive care patients: incidence, risks factors, and relationship with catheter-related sepsis. Chest (1998) 114(1):207–13. doi:10.1378/chest.114.1.207
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
14. Chan AK, Monagle P. Updates in thrombosis in pediatrics: where are we after 20 years? Hematology Am Soc Hematol Educ Program (2012) 2012:439–43. doi:10.1182/asheducation-2012.1.439
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
15. Gray BW, Gonzalez R, Warrier KS, Stephens LA, Drongowski RA, Pipe SW, et al. Characterization of central venous catheter-associated deep venous thrombosis in infants. J Pediatr Surg (2012) 47(6):1159–66. doi:10.1016/j.jpedsurg.2012.03.043
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
16. Male C, Chait P, Andrew M, Hanna K, Julian J, Mitchell L, et al. Central venous line-related thrombosis in children: association with central venous line location and insertion technique. Blood (2003) 101(11):4273–8. doi:10.1182/blood-2002-09-2731
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
17. Geerts WH, Pineo GF, Heit JA, Bergqvist D, Lassen MR, Colwell CW, et al. Prevention of venous thromboembolism: the seventh ACCP conference on antithrombotic and thrombolytic therapy. Chest (2004) 126(3 Suppl):338S–400S. doi:10.1378/chest.126.3_suppl.338S
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
18. Vu LT, Nobuhara KK, Lee H, Farmer DL. Determination of risk factors for deep venous thrombosis in hospitalized children. J Pediatr Surg (2008) 43(6):1095–9. doi:10.1016/j.jpedsurg.2008.02.036
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
19. Monagle P, Chan A, Massicotte P, Chalmers E, Michelson AD. Antithrombotic therapy in children: the seventh ACCP conference on antithrombotic and thrombolytic therapy. Chest (2004) 126(3 Suppl): 645S–87S. doi:10.1378/chest.126.3_suppl.645S
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Keywords: pediatric critical illness, central venous line thrombosis, pediatric thrombosis risk factors, clinical outcomes of pediatric thrombosis, pediatric thrombosis morbidity
Citation: Shah SH, West AN, Sepanski RJ, Hannah D, May WN and Anand KJS (2015) Clinical risk factors for central line-associated venous thrombosis in children. Front. Pediatr. 3:35. doi: 10.3389/fped.2015.00035
Received: 08 December 2014; Accepted: 08 April 2015;
Published: 05 May 2015
Edited by:
Jan Hau Lee, KK Women’s and Children’s Hospital, SingaporeReviewed by:
Christoph P. Hornik, Duke University, USAJacqueline Ong, National University Healthcare System, Singapore
Kozue Shimabukuro, Loma Linda University Children’s Hospital, USA
Copyright: © 2015 Shah, West, Sepanski, Hannah, May and Anand. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Samir H. Shah, Le Bonheur Children’s Hospital, Room 346R, 50 N. Dunlap Street, Memphis, TN 38103, USA,c3NoYWg3QHV0aHNjLmVkdQ==
 Samir H. Shah
Samir H. Shah Alina Nico West
Alina Nico West Robert J. Sepanski3
Robert J. Sepanski3 Kanwaljeet J. S. Anand
Kanwaljeet J. S. Anand