- 1Department of Sustainable Design Engineering, Faculty of Industrial Design Engineering, Delft University of Technology, Delft, Netherlands
- 2Department of Parasitology, Leiden University Medical Center, Leiden, Netherlands
Introduction: The INSPIRED project aims to develop inclusive Digital Optical Diagnostic Devices (DODDs) for schistosomiasis, to support disease management by enabling rapid diagnostic results, to improve efficient data management to guide decision-making and to provide healthcare workers with critical health information to facilitate follow-up action. Due to the non-availability of Target Product Profiles (TPPs) for guiding the development of digital diagnostics for schistosomiasis, we explored existing diagnostic TPPs.
Methods: Using a curated open access database (Notion database), we studied a selection of TPPs for diagnosing infectious diseases, focusing on specifications related to digital health products for Neglected Tropical Diseases (NTDs).
Results: Eighteen TPPs originating from 12 documents, covering 13 specific diseases, were selected and their characteristics were labeled and entered into the database. Further exploration of the database revealed several gaps, including a lack of stakeholder input, sustainability, and TPP availability. Other significant gaps related to digital health platform interconnectivity and data stewardship specifically in relation to digital diagnostics, including DODDs.
Discussion: These findings reflect two possible scenarios: (1) there is currently no need for digital diagnostic devices for schistosomiasis and, by extension for other NTDs; or (2) those needs are not yet covered by TPPs. Therefore, we recommend that digital health diagnostics are included in the use cases for schistosomiasis control and elimination, at least in the ideal/desirable scenario, as this will guide research and incentivize investment in digital health diagnostics for schistosomiasis.
1 Introduction
Schistosomiasis is a Neglected Tropical Disease (NTD) prevalent in rural communities in tropical regions and affecting more than 250 million people worldwide (World Health Organization, 2023b). The WHO’s NTD 2021-2030 roadmap sets global targets and milestones to prevent, control, and eliminate diseases and one of the action points is the development of new tools and diagnostics (World Health Organization, 2020a).
In 2021 two use cases for schistosomiasis diagnostics became available, one at the individual patient level (test and treat) and the second for diagnosis at the population-level (monitoring and evaluation) (WHO, 2021). Considering the individual level, accurate diagnostics are mostly needed at the primary health care level, where patients seek medical attention. On the other hand, population-level-based diagnostic strategies involve screening of whole communities or school-age children, and also includes sample pooling procedures. In both situations, Point-of-Care (POC) diagnostic approaches play an essential role (Cavalcanti et al., 2019; Hoekstra et al., 2021).
Currently, diagnostic tests for schistosomiasis include egg detection by microscopy, the measurement of morbidity markers such as haematuria, Nucleic Acid Amplification Tests (NAAT) and immunological tests, such as antibody detection and antigen detection tests, including the urine-based lateral flow test for the detection of Circulating Anodic Antigen (POC-CCA) (Ajibola et al., 2018; Casacuberta-Partal et al., 2019; Hoekstra et al., 2021). Newer digital diagnostic tests are currently undergoing development, focusing on improving the ease of use of current optical and immuno-chemistry-based tests by reducing potential human error and diminishing ergonomic problems (Davies et al., 2017; Privitera et al., 2017; Vojnov et al., 2019; Mewamba et al., 2021). Some of these devices are being implemented for use in NTDs (Mewamba et al., 2021), haematology (Bachar et al., 2021) and cancer diagnostics (Gola & Doyle-Lindrud, 2016; Valvert et al., 2021). Digital technology-based tests can also be implemented with fewer and less technically trained staff within the limitations posed by the current human resource health challenges in Africa (Petti et al., 2006; Davies et al., 2017).
The INSPiRED consortium (http://inspired-diagnostics.info/) is developing Digital Optical Diagnostic Devices (DODDs; Table 1) for schistosomiasis, which include automated digital microscopes and optical readers for lateral flow tests (Meulah et al., 2022b; Oyibo et al., 2022). Other groups are also developing optical digital devices for the detection of helminth eggs in urine or stool samples or the reading of the POC-CCA urine strip test (Bogoch et al., 2014; Holmström et al., 2017; Mewamba et al., 2021; Armstrong et al., 2022; Ward et al., 2022). In parallel, there are initiatives for NAAT-based diagnostics coupled with digital technology which could potentially be applied to NTDs (Cunnington and The Digital Diagnostics for Africa Network, 2022). These digital diagnostic devices are part of a wide range of Digital Health Products (DHPs) that are being explored to support traditional methods of diagnosis, treatment, monitoring and evaluation of diseases.
Digital Health Diagnostics (DHDs) are DHPs using digital data to make or support disease diagnosis. They can be used by patients, health workers and health policymakers to diagnose and manage diseases and health risks, promote wellness, and support health-related decision-making processes (Ronquillo et al., 2022). They have the potential to reduce inefficiencies related to manual procedures, thus improving access and quality of care, and increasing ownership of health by patients through personalized care (Center for Devices and Radiological Health, 2020).
As developers, we envision that DODDs, a subset of DHDs, could play an important role in controlling and eliminating schistosomiasis in low and middle-income countries (population-level use case). The digital capture of optical images from DODDs can substantially improve data quality and aid prompt decision-making by providing digital data to support faster diagnostic decision-making. Notably, our recent literature review demonstrated that most DODDs for schistosomiasis are limited to the proof of concept stage, and almost no devices are implemented and/or commercially available (Meulah et al., 2022a).
Guidance for developers of diagnostic tests, including optimal and minimal parameter specifications, typically in the form of use cases combined with Target Product Profiles (TPPs) endorsed by organizations like the WHO and/or other international organizations, is a key factor to entice product developers, especially in areas like NTDs where the economic attractiveness is limited. TPPs are intended to guide researchers, policymakers, commercial parties, and funders toward the development of health products that fit the particular needs of a given context.
The Diagnostic Technical Advisory Group (DTAG), commissioned by WHO, is the working group with a coordinating role in the development of TPPs (Figure 1) for NTDs to reach the goals described in the 2020-2030 roadmap (World Health Organization, 2020b). The diagnostic TPPs for schistosomiasis, developed by DTAG, are for (1) monitoring and evaluation of control programmes and (2) determining if the transmission has been sufficiently interrupted and when to conduct post-mass drug administration (MDA) surveillance (World Health Organization, 2020b).
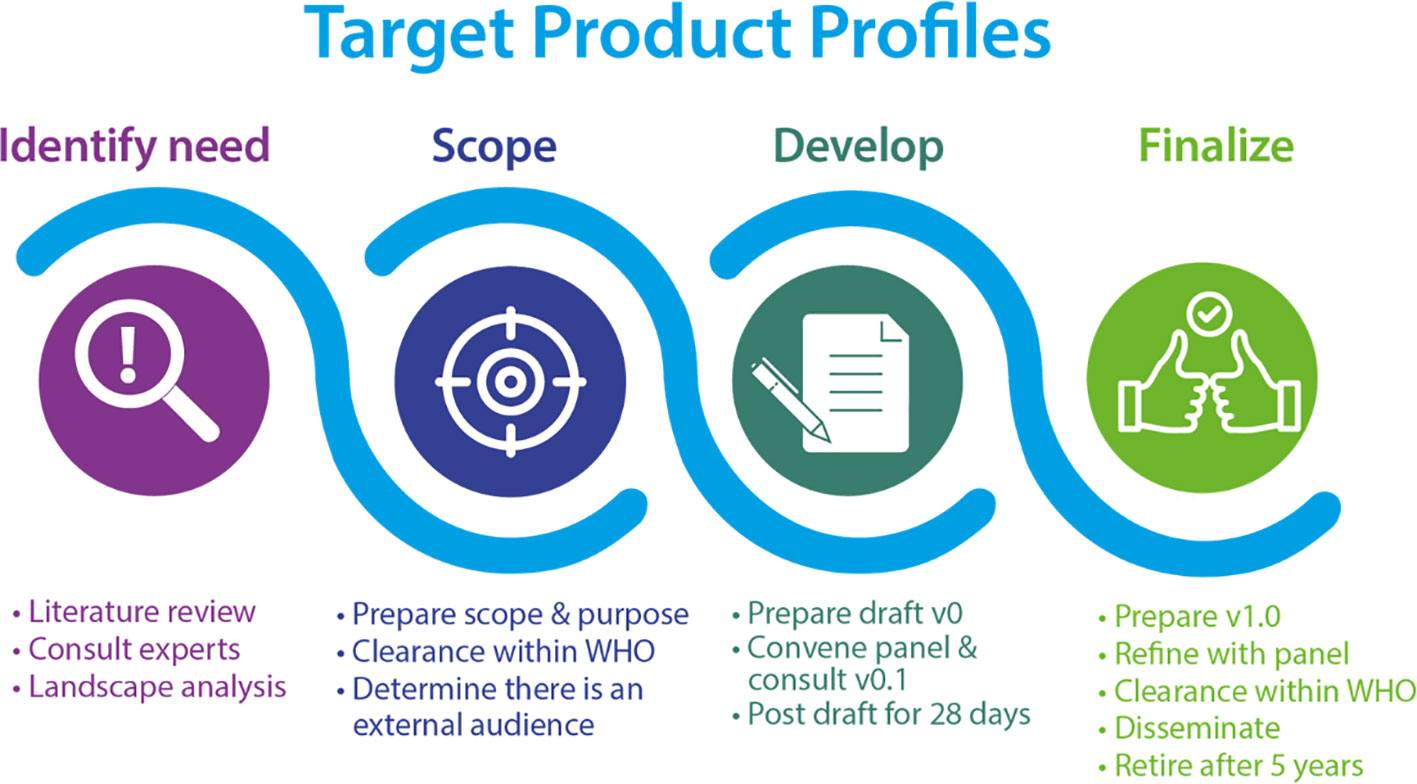
Figure 1 The WHO TPP drafting process (World Health Organization, 2023a) CC BY-NC-SA 3.0 IGO.
Although developing DHDs for schistosomiasis, and by extension, other NTDs, will make a substantial contribution to meeting WHO targets, there are currently no guidelines to support developers in this process. A broad search for TPPs of DHDs for schistosomiasis and other NTDs yielded hardly any information. The WHO Health Product Profile Directory (HPPD), which is a database of currently available health products targeting low-resource settings, includes ‘digital health’ as a product type. However, a search of the directory on June 15, 2023, revealed that DHPs are only available for TB and clinical decision support mainly focused on disease management and awareness. Furthermore, while many TPPs exist in the database, only a few of those TPPs are dedicated to digital products in the context of global health or at least provide some guidance on digital connectivity.
For schistosomiasis specifically and other NTDs broadly, there are no existing TPPs published by health organizations including the WHO that describe the needs for and characteristics of DHPs to facilitate schistosomiasis diagnostics development. This could either indicate there is no perceived need for DHPs and by extension DHDs and DODDs in this context, or it could mean those needs are not specified and described yet. According to the road map for neglected tropical diseases 2021-2030 (World Health Organization, 2020a), digital health platforms for collecting and monitoring data are needed. However, this need is not further specified. While the WHO, has made an effort to include ‘digital health’ as part of the need for NTD elimination, it is still unclear how and what this need should entail.
This paper discusses the need for DHDs for schistosomiasis elimination and highlights the gaps in the current TPPs for schistosomiasis, particularly the absence of guidance for the development of digital diagnostics that can support the elimination of schistosomiasis. The paper addresses ways to improve TPP inclusivity, taking into consideration the importance of specific digital health concepts that do not apply to currently available TPPs.
2 Methodology
To explore what we can learn from existing TPPs concerning the need for DHDs for schistosomiasis, a publicly accessible database consisting of a selection of TPPs was curated (Notion database). Documents for the database were obtained via a general web search using the Google search engine. The Google search was limited to the first six pages of the database. Other websites and databases of NGOs searched include PATH, FIND, MSF and WHO. The search was performed in June 2022 and only non-profit health organizations such as the WHO were included, in order to reduce the potential risk of conflict of interest by TPPs constructed by private-for-profit companies.
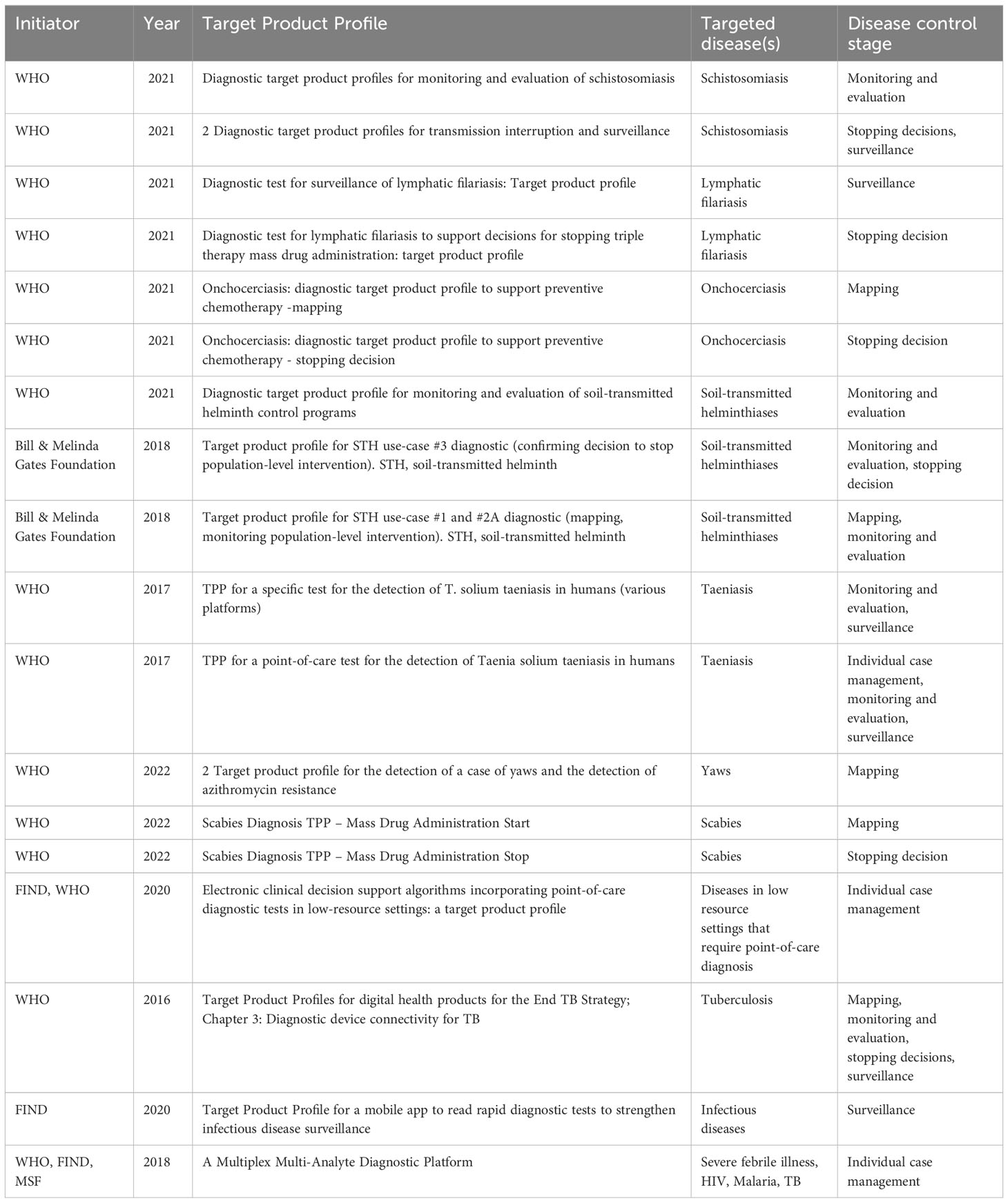
The inclusion criteria for a TPP to be entered into the notion database were based on the year of publication (≥ 2016) and three domains: (1) diagnostic tests using human samples to support mass drug administration programs (MDA) for NTDs; (2) diagnostic tools and digital devices to aid the detection of NTDs, HIV/AIDS, TB and malaria; (3) diagnostic systems such as digital health applications for NTDs, HIV/AIDS, TB and malaria. While there are 21 NTDs, only NTDs that include MDA as a treatment approach were included in the database (World Health Organization, 2020a). HIV/AIDS, TB and malaria were selected based on their global health relevance. Based on this selection criteria, the TPPs for the following NTDs were then included in the database: foodborne trematodiases; lymphatic filariasis; onchocerciasis; scabies and other ectoparasites; schistosomiasis; soil-transmitted helminthiases; taeniasis/cysticercosis; and yaws and other endemic trepanemotoses.
Thereafter, the following search terms were used to find relevant TPPs for analysis in the notion database:
Target Product Profile + Lymphatic filariasis, onchocerciasis, river blindness, schistosomiasis, ascariasis, trichuriasis, hookworm, soil-transmitted helminthiases, trachoma, foodborne trematodiases, taeniasis, cysticercosis, yaws, scabies.
Target Product Profile + Digital device, electronic device, digital health, e-health, smart device.
Target Product profiles + WHO, FIND, GHIT, PATH, Bill & Melinda Gates Foundation, UNICEF.
The contents of each TPP were categorized in the database according to predefined characteristics that are standardized in formal TPPs (i.e., all minimum and ideal characteristics were labeled in the database within a category). In this way, the contents of different TPPs can be easily searched and compared, and metadata can be analyzed. For easy handling, sharing and the functionality of adding and filtering multiple labels, the web-based application Notion was used to analyse the database.
Metadata was added to single characteristics and requirements as stated in the TPP, in the form of labels (e.g. context and user; functionality; performance; market and business). This database allows the user to categorize TPPs based on the specified use case (i.e., individual case management; mapping; monitoring and evaluation; stopping decisions; surveillance).
3 Results
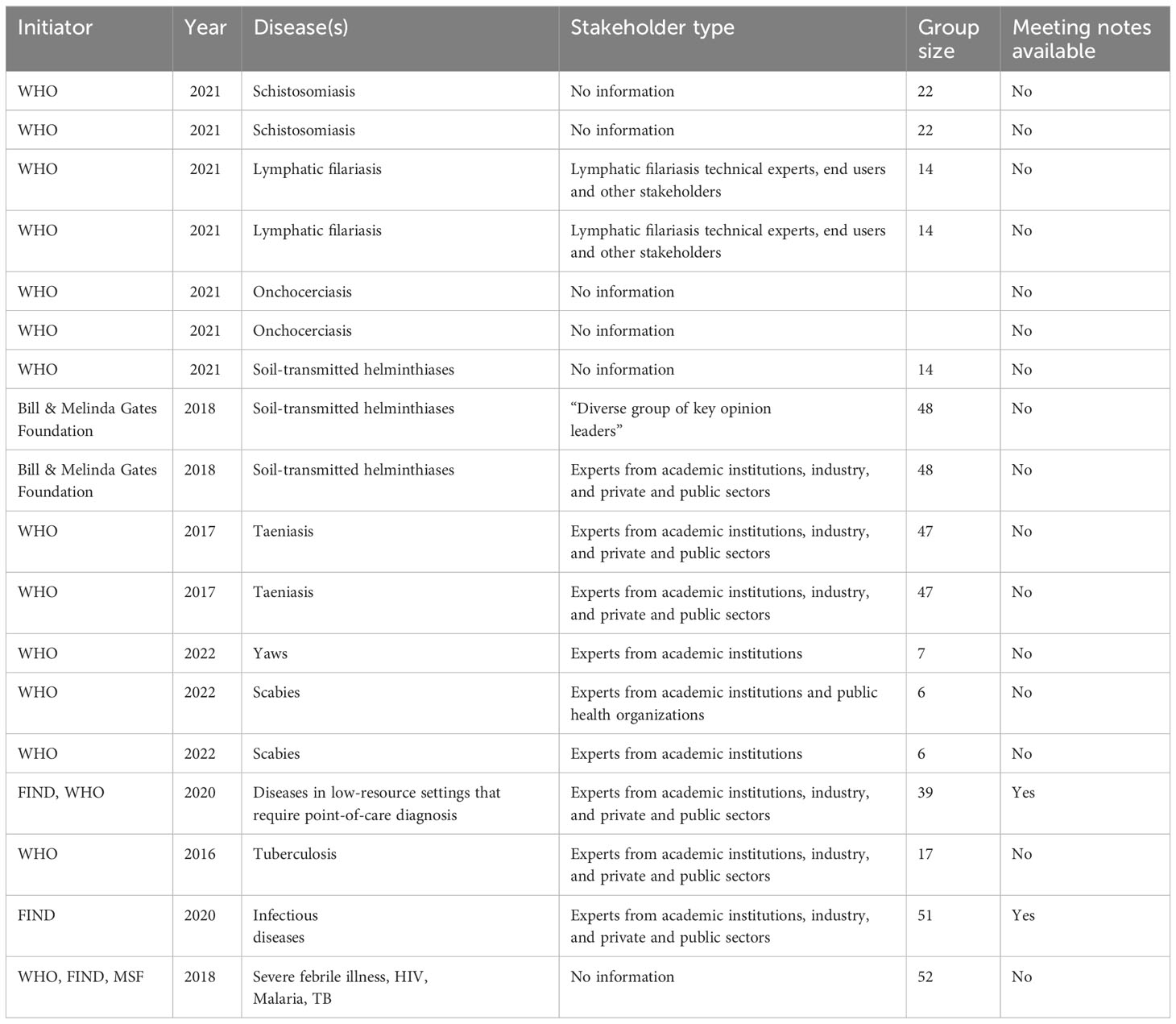
Figure 2 demonstrates the process of data collection for the Notion database which is publicly available and can be explored by users. 18 TPPs that matched the criteria were found. The 18 TPPs originated from 12 documents. Some documents contained more than one TPP, for example, different TPPs for different use scenarios. The WHO was the (co)initiator of 15 of the 18 TPPs and 12 TPPs were published between 2020 to 2022. 14 TPPs were related to the first search terms while the other 4 TPPs were related to digital devices and digital health (search terms 2 & 3), as found in Table 2 and the appendix. Two of those four TPPs target mobile phone use, while the other two describe dedicated digital devices. 12 TPPs mentioned stakeholders involved in the drafting process with a minimum of 6 to a maximum of 52 stakeholders. Only 2 TPP documents had TPP meeting notes available. 8 TPP development processes were sponsored by a funding agency, foundation or fund.
4 Discussion
Based on the content of the database, two broad themes emerged in relation to the gaps and opportunities for improving current TPPs applicable to DHDs. Theme 1 focuses on inclusivity and addresses gaps like TPP availability, stakeholder inclusion, and sustainability. Theme 2 applies mainly to interconnectivity and data stewardship which are DHP-specific considerations that are applicable to both DHDs and DODDs.
4.1 Theme 1: inclusivity
4.1.1 TPP availability
One of the barriers to DHD device development is the lack of availability and accessibility of TPPs. For example, at the time of this study, there were no TPPs for developing DHDs for schistosomiasis within the WHO health product directory. This absence can impact the DHD product development process and lead to a waste of time and financial resources in developing products which may not fit into the context of use or duplication of healthcare products. Conversely, this absence causes a lack of incentive for developers to invest in digital health products as they often rely on the TPPs before product development is initiated.
Unfortunately, the HPPD webpage was not available online during the development of our database (2021-2022), due to upgrades of the HPPD platform (personal communication with WHO representatives). In our view, this creates a lack of clarity for those who are not directly involved in the process of constructing TPPs. The transparency for end users could be improved, not only by regular updating existing TPPs, as they should be living documents, but also by maintaining a version history available online of each TPP and the HPPD webpage. Preferably TPP updates should be performed in parallel with reviewing NTD targets.
4.1.2 Stakeholder inclusion
The TPP drafting process involves consultation with stakeholders. Of the 18 selected, 12 TPPs mentioned the types of stakeholders participating in the drafting process (Table 3). While it is sensible to determine relevant stakeholders per use-case (as TPPs are developed per use case); the extent of stakeholder involvement in the TPP development process is unclear. For instance, we found no published list of stakeholders consulted in the TPP drafting process in the documents reviewed. Only 2 TPPs had meeting notes available (these were both from the NGO database). Although the inclusion of end user involvement in the TPP drafting process is documented in the DTAG process, (WHO, 2021), we found the roles of these end users not clear. Considering and giving preference to stakeholders from schistosomiasis dominant areas is critical to improving contextual fit and uptake.
To improve transparency of the TPP development process, we suggest a list of all consulted stakeholders to be included at the end of the TPP document, with their roles in the process and the organizations they represent. In our opinion it is also important to identify and include a broader representation of stakeholders, such as design engineers, and field-based health workers such as Community Health Extension Workers (CHEWs) which are the last link to communities in sub-Saharan Africa (Onasanya et al., 2020; Banda et al., 2021). Inclusion of CHEWs and other lower cadre healthcare staff, in addition to more proxy end users such as researchers and healthcare managers, can support a successful drafting process of TPPs for DHDs that will potentially be used by these CHEWs.
4.1.3 Sustainability
When exploring the TPPs in the database we noticed that sustainability was hardly mentioned in any of the TPPs reviewed, not even in ideal scenarios. In alignment with the SDGs, environmental sustainability in diagnostic device design is an important aspect to consider in the development of TPPs for digital diagnostic devices and should ideally be part of each TPP. Sustainability covers a broad range of parameters including materials used, product lifecycle, and energy requirements and is not limited to product disposal only (Hinrichs-Krapels et al., 2022). Although the emphasis on sustainability can increase the entry threshold for interested companies and can be a barrier to diagnostic device development due to potential limited economic returns on investment, including sustainability in the TPP parameters will bring the topic to the forefront of the product design process, which is the key to designing sustainable products. In addition, including sustainability upfront can foster the co-creation of device development with stakeholders who understand the use context thereby creating a mutually beneficial product.
Our database revealed that components of several reviewed TPPs vary. Taken as a whole, TPPs by WHO and other NGOs showed variation in the level of detail, with more details on the performance and functionality characteristics and less detail on human operational factors such as ease of use and field applicability in low resource settings and ergonomic design. All these can affect contextual fit and sustainability. Other contextual fit and sustainability challenges for DHDs that need to be considered include internet connectivity and limited or no electricity supply in hard-to- reach areas where NTDs are dominant (Onasanya et al., 2023). Considering the use of alternate sources of power supply such as solar technology and use of Bluetooth, Local Area Network (LAN) and external hard drives as alternate sources to sending and keeping diagnostics data should be explored.
Finally, a lack of inclusion of commercial market perspectives can also have an impact on sustainability. Although market perspectives and commercialization may be purposely omitted from the TPP, and there seems to be no market in the NTD space for DHDs, we believe that funding NTDs and improving country ownership of eliminating schistosomiasis and other NTDs will require finding country-based financing mechanisms for digital diagnostics and other diagnostic tools. This can contribute to more sustainable disease control and elimination strategies. We believe there is a need for DHDs and the absence of this perspective in the TPP eliminates its market potential. Including a commercial perspective will require input from health planning officers, health economists and private sector representatives from disease-dominant regions in the TPP drafting process.
4.2 Theme 2: interconnectivity and data ownership
While it may appear that TPPs for non-digital diagnostic medical devices may also apply to digital diagnostic medical devices, there are specific concerns for DHPs and DHDs which are not addressed by current TPPs. Ideally, digital diagnostics should be interconnected with other established digital health systems such as health management information systems, logistics management information systems and electronic medical records. These ensure interoperability and reusability across different health program areas, supporting a more robust digital health system. Addressing these at an early stage in the TPPs aligns with the WHO digital health strategy (World Health Organization, 2023a) and enhances a systems approach to the control of NTDs. But interconnectivity also leads to questions concerning data stewardship and the ethical aspects of data sharing policies with third parties such as developers and stakeholders involved in the use of these devices. Consequently, more attention should be paid in TPPs to topics such as data privacy, informed consent, data protection and governance, data storage and data ownership.
5 Conclusion
We see great potential for DHPs, DHDs and DODDs that can diagnose schistosomiasis, collate, and can transfer data to digital platforms such as the District Health Information System. DHDs can transform data management systems thereby making healthcare delivery easier and providing decision support for health workers and policymakers. The need for DHDs for NTDs is clear but is mainly driven by the scientific research community, and has not been made actionable by concrete strategies. We found DHDs to be not well embedded in current TPPs, while addressing inclusiveness is important for the further development of DHDs. Our suggestions include the incorporation of end user representatives in the TPP drafting process, more attention to sustainability aspects and addressing interconnectivity gaps and the ethical aspects of data stewardship. Overall, this study highlights the gaps in the current TPPs for diagnostic devices of schistosomiasis, particularly the absence of guidelines for the development of DHPs, surprisingly not even in the ideal scenario. This study also provides an open-access database that others can use to easily search and compare the contents of different diagnostic TPPs for multiple use cases.
Data availability statement
The datasets generated for this study are available here: https://mud-wheel-f86.notion.site/24e3fa2b55564b0e8ef1cdc4526a3899?v=4a5b9b7a6c004498adf5ec6df308574f”Notiondatabase. Further inquiries can be directed to the corresponding author.
Author contributions
AO: Writing – original draft, Writing – review & editing. MB: Project administration, Writing – review & editing. LdG: Data curation, Formal Analysis, Investigation, Methodology, Software, Writing – review & editing. JvE: Writing – review & editing. J-CD: Writing – review & editing. LvL: Conceptualization, Funding acquisition, Methodology, Supervision, Validation, Writing – review & editing.
Funding
The research described has been funded by NWO-WOTRO Science for Global Development program, grant number W 07.30318.009.
Acknowledgments
We thank René Paulussen of Mondialdx and Govert van Dam of the Leiden University Medical Center for helpful discussions and their feedback on the topics covered in this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author LvM declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpara.2023.1255848/full#supplementary-material
References
Ajibola O., Gulumbe B. H., Eze A. A., Obishakin E. (2018). Tools for detection of schistosomiasis in resource limited settings. Med. Sci. (Basel, Switzerland) 6 (2), 39. doi: 10.3390/medsci6020039
Armstrong M., Harris A. R., D’Ambrosio M. V., Coulibaly J. T., Essien-Baidoo S., Ephraim R. K. D., et al. (2022). Point-of-care sample preparation and automated quantitative detection of schistosoma haematobium using mobile phone microscopy. Am. J. Trop. Med. Hygiene 106 (5), 1442–1449. doi: 10.4269/ajtmh.21-1071
Bachar N., Benbassat D., Brailovsky D., Eshel Y., Glück D., Levner D., et al. (2021). An artificial intelligence-assisted diagnostic platform for rapid near-patient hematology. Am. J. Hematol. 96 (10), 1264–1274. doi: 10.1002/ajh.26295
Banda G. T., Deribe K., Davey G. (2021). How can we better integrate the prevention, treatment, control and elimination of neglected tropical diseases with other health interventions? A systematic review. BMJ Global Health 6 (10), e006968. doi: 10.1136/bmjgh-2021-006968
Bogoch I. I., Coulibaly J. T., Andrews J. R., Speich B., Keiser J., Stothard J. R., et al. (2014). Evaluation of portable microscopic devices for the diagnosis of Schistosoma and soil-transmitted helminth infection. Parasitology 141 (14), 1811–1818. doi: 10.1017/S0031182014000432
Casacuberta-Partal M., Hoekstra P. T., Kornelis D., van Lieshout L., van Dam G. J. (2019). An innovative and user-friendly scoring system for standardised quantitative interpretation of the urine-based point-of-care strip test (POC-CCA) for the diagnosis of intestinal schistosomiasis: A proof-of-concept study. Acta Tropica 199, 105150. doi: 10.1016/j.actatropica.2019.105150
Cavalcanti M. G., Cunha A. F. A., Peralta J. M. (2019). The advances in molecular and new point-of-care (POC) diagnosis of schistosomiasis pre- and post-praziquantel use: in the pursuit of more reliable approaches for low endemic and non-endemic areas. Front. Immunol. 10. doi: 10.3389/fimmu.2019.00858
Center for Devices and Radiological Health. (2020). What is digital health? (USA: FDA). Available at: https://www.fda.gov/medical-devices/digital-health-center-excellence/what-digital-health.
Cunnington A., The Digital Diagnostics for Africa Network. (2022). The potential of digital molecular diagnostics for infectious diseases in sub-Saharan Africa. PloS Digit Health 1 (6), e0000064. doi: 10.1371/journal.pdig.0000064
Davies J., Abimiku A., Alobo M., Mullan Z., Nugent R., Schneidman M., et al. (2017). Sustainable clinical laboratory capacity for health in Africa. Lancet Global Health 5 (3), e248–e249. doi: 10.1016/S2214-109X(17)30024-4
Gola S., Doyle-Lindrud S. (2016). The MarginProbe® system: an innovative approach to reduce the incidence of positive margins found after lumpectomy. Clin. J. Oncol. Nursing 20 (6), 598–599. doi: 10.1188/16.CJON.598-599
Hinrichs-Krapels S., Diehl J.-C., Hunfeld N., van Raaij E. (2022). Towards sustainability for medical devices and consumables: The radical and incremental challenges in the technology ecosystem. J. Health Serv. Res. Policy 27 (4), 253–254. doi: 10.1177/13558196221110416
Hoekstra P. T., van Dam G. J., van Lieshout L. (2021). Context-specific procedures for the diagnosis of human schistosomiasis – A mini review. Front. Trop. Dis. 2. doi: 10.3389/fitd.2021.722438
Holmström O., Linder N., Ngasala B., Mårtensson A., Linder E., Lundin M., et al. (2017). Point-of-care mobile digital microscopy and deep learning for the detection of soil-transmitted helminths and Schistosoma haematobium. Global Health Action 10 (sup3), 1337325. doi: 10.1080/16549716.2017.1337325
Meulah B., Bengtson M., Lieshout L. V., Hokke C. H., Kreidenweiss A., Diehl J.-C., et al. (2022a). A review on innovative optical devices for the diagnosis of human soil-transmitted helminthiasis and schistosomiasis: from research and development to commercialisation. Parasitology 150 (2), 137–149. doi: 10.1017/S0031182022001664
Meulah B., Oyibo P., Bengtson M., Agbana T., Lontchi R. A. L., Adegnika A. A., et al. (2022b). Performance evaluation of the schistoscope 5.0 for (Semi-)automated digital detection and quantification of schistosoma haematobium eggs in urine: A field-based study in Nigeria. Am. J. Trop. Med. Hygiene 107 (5), 1047–1054. doi: 10.4269/ajtmh.22-0276
Mewamba E. M., Tiofack A. A. Z., Kamdem C. N., Ngassam R. I. K., Mbagnia M. C. T., Nyangiri O., et al. (2021). Field assessment in Cameroon of a reader of POC-CCA lateral flow strips for the quantification of Schistosoma mansoni circulating cathodic antigen in urine. PloS Negl. Trop. Dis. 15 (7), e0009569. doi: 10.1371/journal.pntd.0009569
Onasanya A., Bengtson M., Agbana T., Oladunni O., van Engelen J., Oladepo O., et al. (2023). Towards inclusive diagnostics for neglected tropical diseases: user experience of a new digital diagnostic device in low-income settings. Trop. Med. Infect. Dis. 8 (3), 3. doi: 10.3390/tropicalmed8030176
Onasanya A., Keshinro M., Oladepo O., Van Engelen J., Diehl J. C. (2020). A stakeholder analysis of schistosomiasis diagnostic landscape in South-West Nigeria: insights for diagnostics co-creation. Front. Public Health 8. doi: 10.3389/fpubh.2020.564381
Oyibo P., Jujjavarapu S., Meulah B., Agbana T., Braakman I., van Diepen A., et al. (2022). Schistoscope: an automated microscope with artificial intelligence for detection of schistosoma haematobium eggs in resource-limited settings. Micromachines 13 (5), 5. doi: 10.3390/mi13050643
Petti C. A., Polage C. R., Quinn T. C., Ronald A. R., Sande M. A. (2006). Laboratory medicine in africa: A barrier to effective health care. Clin. Infect. Dis. 42 (3), 377–382. doi: 10.1086/499363
Privitera M. B., Evans M., Southee D. (2017). Human factors in the design of medical devices—Approaches to meeting international standards in the European Union and USA. Appl. Ergonomics 59 (Pt A), 251–263. doi: 10.1016/j.apergo.2016.08.034
Ronquillo Y., Meyers A., Korvek S. J. (2022). “Digital health,” in StatPearls (Treasure Island (FL): StatPearls Publishing). Available at: http://www.ncbi.nlm.nih.gov/books/NBK470260/.
Valvert F., Silva O., Solórzano-Ortiz E., Puligandla M., Siliézar Tala M. M., Guyon T. (2021). Low-cost transcriptional diagnostic to accurately categorize lymphomas in low- and middle-income countries. Blood Advances 5 (10), 2447–2455. doi: 10.1182/bloodadvances.2021004347
Vojnov L., Taegtmeyer M., Boeke C., Markby J., Harris L., Doherty M., et al. (2019). Performance of non-laboratory staff for diagnostic testing and specimen collection in HIV programs: A systematic review and meta-analysis. PloS One 14 (5), e0216277. doi: 10.1371/journal.pone.0216277
Ward P., Dahlberg P., Lagatie O., Larsson J., Tynong A., Vlaminck J., et al. (2022). Affordable artificial intelligence-based digital pathology for neglected tropical diseases: A proof-of-concept for the detection of soil-transmitted helminths and Schistosoma mansoni eggs in Kato-Katz stool thick smears. PloS Negl. Trop. Dis. 16 (6), e0010500. doi: 10.1371/journal.pntd.0010500
WHO (2021) Diagnostic target product profiles for monitoring, evaluation and surveillance of schistosomiasis control programmes. Available at: https://www.who.int/publications-detail-redirect/9789240031104.
World Health Organization (2020a). Ending the neglect to attain the Sustainable Development Goals – A road map for neglected tropical diseases 2021–2030 (Geneva: World Health Organization). Available at: https://www.who.int/publications/i/item/9789240010352.
World Health Organization (2020b). Report of the first meeting of the WHO diagnostic technical advisory group for neglected tropical diseases: Geneva, Switzerland, 30–31 October 2019 (Geneva: World Health Organization). Available at: https://apps.who.int/iris/handle/10665/331954.
World Health Organization. (2023a) Target product profile directory. Available at: https://www.who.int/our-work/science-division/research-for-health/target-product-profile-directory.
World Health Organization. (2023b) Schistosomiasis. Available at: https://www.who.int/news-room/fact-sheets/detail/schistosomiasis.
Keywords: schistosomiasis, Target Product Profile, digital diagnostics, inclusiveness, design
Citation: Onasanya A, Bengtson M, de Goeje L, van Engelen J, Diehl J-C and van Lieshout L (2023) Developing inclusive digital health diagnostic for schistosomiasis: a need for guidance via target product profiles. Front. Parasitol. 2:1255848. doi: 10.3389/fpara.2023.1255848
Received: 09 July 2023; Accepted: 07 September 2023;
Published: 26 September 2023.
Edited by:
Patricia Cuervo, Oswaldo Cruz Foundation (Fiocruz), BrazilReviewed by:
Catherine A. Gordon, The University of Queensland, AustraliaManuel Ritter, University Hospital Bonn, Germany
Copyright © 2023 Onasanya, Bengtson, de Goeje, van Engelen, Diehl and van Lieshout. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Adeola Onasanya, QS5BLk9uYXNhbnlhQHR1ZGVsZnQubmw=
 Adeola Onasanya
Adeola Onasanya Michel Bengtson
Michel Bengtson Ludo de Goeje2
Ludo de Goeje2 Jan-Carel Diehl
Jan-Carel Diehl Lisette van Lieshout
Lisette van Lieshout