
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pain Res. , 06 March 2025
Sec. Musculoskeletal Pain
Volume 6 - 2025 | https://doi.org/10.3389/fpain.2025.1497328
Introduction: Body perception disturbances (BPD) are well documented in certain chronic pain populations [e.g., complex regional pain syndrome (CRPS)], while being far less studied in chronic pain as a general condition. The aims of this scoping review are to identify the self-reported questionnaires used to assess BPD in individuals with chronic non-cancer pain and to refine the definition of the BPD construct as used in these questionnaires.
Methods: A search strategy focusing on the concepts of “chronic pain”, “body perception” and “questionnaire” was used across four databases. Each record was screened for eligibility by two independent reviewers, and data extraction was performed by one reviewer and validated by a second reviewer.
Results: Eighty-seven studies were included, comprising 18 different questionnaires—either directly related to BPD or containing relevant items. The three most commonly used questionnaires were the Bath Body Perception Disturbance Scale, the Fremantle Back Awareness Questionnaire, and the Neurobehavioral Questionnaire. Appraisal of the construct derived from the questionnaire items identified five main facets: size, shape, cognitive neglect-like symptoms, proprioceptive awareness, and agency, along with 11 other less frequently addressed facets. The most represented clinical populations were CRPS (40 studies) and chronic low-back pain (20 studies).
Discussion: A variety of self-reported questionnaires are available to assess BPD, but most are diagnosis- or body-region specific. To better assess BPD in individuals with chronic non-cancer pain, a consensus on the general definition and the key facets of the construct is needed.
Body perception is defined as the way one consciously perceives one's own body, which relies on ongoing sensory input and is thought to be a fluid concept influenced by memories, beliefs, and psychological factors (1). Body perception disturbances (BPD) have been reported in several chronic pain populations (2–5). For example, people with complex regional pain syndrome (CRPS) often report distortions in the perception of their affected limb compared to its actual characteristics (e.g., in terms of size, temperature, pressure) (2). They also have difficulty determining how their limb is positioned (3). Some people feel a sense of foreignness toward their painful limb, while others distrust it (4, 5). Similar disturbances have been described in other chronic pain populations such as phantom limb pain (PLP) (6), chronic low-back pain (CLBP) (7, 8), and chronic knee pain (9). However, evidence regarding the presence or absence of BPD is scarce for many chronic pain populations.
One way to assess the presence of BPD is through self-reported questionnaires. However, most available questionnaires are diagnosis-specific, having been developed for specific pain syndromes. For instance, the Fremantle Back Awareness Questionnaire (FreBAQ) was developed for people with CLBP (10), and the Bath Body Perception Disturbance Scale (BBPDS) was developed for people with CRPS (11). Although some self-reported questionnaires have been adapted for other chronic pain populations [e.g., FreSHAQ for shoulder pain (12), FreKAQ for knee pain (13)], their use remains limited to the specific populations for which they were developed due to the wording of the items. This also makes them unsuitable for pain syndromes affecting other parts of the body (e.g., migraine), pain affecting multiple body areas (e.g., fibromyalgia), or for clinical and research settings involving diverse pain populations. Moreover, the lack of a generic measure of BPD precludes comparisons across various populations, especially when the definition of the construct varies from one questionnaire to another. Thus, clarification of the definition of BPD is essential for its effective assessment in diverse populations. This is especially relevant given that chronic pain is now recognized as a disease in its own right (14, 15), and recent literature shows that pain is associated with BPD in different pain syndromes (16).
Previous work on this topic includes a systematic review that identified available tools for assessing BPD in CRPS (17) and a systematic scoping review that identified available tools for assessing explicit and implicit own's body and space perception in painful musculoskeletal disorders and rheumatic diseases (18). However, both reviews cover only a subset of chronic pain conditions within their target populations. Furthermore, the review by Viceconti et al. addresses constructs (somatoperception, body ownership, space perception) that only partially overlap with body perception, leaving a gap in the literature regarding available self-reported questionnaires to assess BPD in chronic pain as a generic condition, rather than diagnosis- or body part-specific.
To address these gaps, the aims of this scoping review are to identify the self-reported questionnaires used to assess BPD in chronic non-cancer pain populations and to refine the definition of the BPD construct.
This scoping review was conducted in accordance with the Joanna Briggs Institute methodology for scoping reviews (19).
A search strategy was elaborated with the assistance of an academic health librarian, using keywords from the Title, Abstract, Keywords and index terms of relevant articles. A comprehensive search was then conducted in MEDLINE, CINAHL, PsychInfo and Embase (see Supplementary Appendix S1 for a sample search of MEDLINE). The search strategy comprised three key concepts: (1) Chronic pain; (2) Body Perception; (3) Questionnaire.
An initial search of the databases was performed on January 16th, 2023 and reruns were performed on February 22nd, 2024, and January 16th, 2025, with no date limits. All references were uploaded and deduped in Endnote X20 (Clarivate Analytics, Philadelphia, USA). References were then imported into Covidence (Covidence Systematic Review Software, 2021), an online software designed to facilitate the conduct of systematic and scoping reviews. The screening for eligibility was performed by two independent reviewers using the following inclusion criteria: (1) participants had to be adults (≥18 years old) with chronic non-cancer pain (i.e., pain ≥3 months); (2) at least one self-reported questionnaire was used to assess BPD; (3) body perception was broadly defined as the way one consciously perceives their own body (1) (e.g., the perceived characteristics of the painful body part(s), such as shape, size and temperature, one's perceived ability to locate and move one's body parts in a controlled manner, as well as the feelings of disownership and foreignness about these body parts); (4) original peer-reviewed studies published in French or English. For studies conducted in a heterogeneous sample, at least 50% of the participants had to meet criterion #1 for the paper to be included. Studies that only used approaches such as psychophysical assessments (e.g., quantitative sensory testing), but no questionnaire (criterion #2), were excluded. Finally, according to criterion #3, self-reported questionnaires defining body perception in terms of body image satisfaction or interoceptive awareness were excluded.
Pilot testing was conducted on 20 references for the Title and Abstract screening, and 10 references for the Full text screening. In case of disagreement between the two reviewers, a third party (CM) was involved to make the final decision. A manual search (e.g., screening of the reference lists of all included studies) was also performed to identify additional eligible studies.
Data extraction of included studies was performed by a first reviewer and revised by a second reviewer. The following information was extracted from each included study: Authors, Year, Country in which the study was conducted, Aim(s), Study type (e.g., cross-sectional study, questionnaire development and psychometric testing), Population (sample size, age, sex, chronic pain diagnosis/es), Questionnaire(s) used, Questionnaire items, Other relevant information (e.g., Original questionnaire development study).
The search yielded 5,527 studies. Duplicates were identified and removed using Covidence, leaving 4,497 studies to be screened based on titles and abstracts. Of these, 4,167 studies were excluded, leaving 330 full-text studies to be screened for eligibility. Of these, 70 studies were included. Seventeen additional records were included based on manual search. Thus, a total of 87 studies were included in this review (see Figure 1 for PRISMA flowchart). The included studies were either original studies (n = 59) or questionnaire development and/or psychometric validation studies (n = 28) (See Supplementary Table S1 for the detailed extraction table of included articles).
Among the 87 studies included in this review, a total of 18 different self-reported questionnaires assessing BPD were identified. These questionnaires were categorized as follows: 3.1.1 standardized questionnaires specifically addressing BPD (n = 9); 3.1.2 standardized questionnaires with some items relevant to the construct (n = 1); 3.1.3 non-standardized questionnaires specifically addressing BPD (n = 5); and 3.1.4 non-standardized questionnaires with some items relevant to the construct (n = 3). Questionnaires were considered standardized if they had undergone some level of psychometric testing (e.g., content validity, construct validity, internal consistency), whereas questionnaires that had not been tested (e.g., questionnaires or single questions developed for the purpose of a specific study) were categorized as non-standardized. Tables 1, 2 show the classification of questionnaires and report all the chronic pain populations with which questionnaires were used in the included studies.
Standardized questionnaires consisting of whole scales developed specifically for the construct include the Fremantle Back Awareness Questionnaire [FreBAQ, n = 21 studies (10, 20–39)] and its adaptations for the neck [FreNAQ, n = 3 studies (40–42)], shoulder [FreSHAQ, n = 2 studies (12, 43)], knee [FreKAQ, n = 6 studies (9, 13, 44–47)], perineal region [FrePAQ, n = 1 (48)], fibromyalgia [FreBAQ-FM, n = 2 (49, 50)], and the region-generic version [FreBAQ-general, n = 1 study (51)], as well as the Bath Body Perception Disturbance Scale [BBPDS, n = 29 studies (52–80)], and the Neurobehavioral Questionnaire [n = 19 studies (4, 5, 55, 58, 60, 61, 63, 81–92)].
The FreBAQ consists of nine items [six items for the FreBAQ-general (51)] and was developed to assess BPD in CLBP. The authors define body perception as “the feelings we have of our own body”. Disturbances include signs of cognitive and motor neglect, a loss of proprioceptive awareness, and a distorted perception of the back in terms of size and delineation (10). Each item is scored on a 5-point Likert-type scale and the total score corresponds to the sum of all the items (maximum score: 36 for the original FreBAQ and the region-specific adaptations, and 24 for the region-generic adaptation), with higher scores reflecting greater disturbances.
The BBPDS consists of six items and a body drawing to assess BPD in CRPS (11). Body perception is defined as “the subjective perception of the affected body part” and disturbances include a feeling of foreignness toward the painful body part, an altered awareness of limb position, strong negative emotions, and an altered perception of the body part in terms of shape, size, weight, and temperature (11, 52). The original scale also comprises an item that assesses attention to the affected limb—disturbances manifesting as either hypervigilance or neglect toward the affected limb. Note that a revised version of the scale excluding the item on attention has been proposed by Ten Brink and collaborators. The authors based their decision on the corrected item-total correlation for this item, which was found to be insufficient (60). Items 1 to 4 and 6b are scored on a 0–10 numerical rating scale, while items 5 and 6a are dichotomous. Finally, the body drawing is scored on a 3-point scale (0 = no distortion; 1 = distortion; 2 = severe distortion). The final score corresponds to the sum of all items plus the body drawing (maximum score: 57), with higher scores reflecting greater disturbances.
The Neurobehavioral Questionnaire, or Neglect-Like Symptoms Questionnaire, is a 5-item questionnaire developed by Galer and Jensen to assess neglect-like symptoms (NLS) in CRPS (4). According to the authors, NLS include cognitive neglect (i.e., perceiving the affected limb as foreign and not part of the body), motor neglect (i.e., having to mentally and visually focus to move the limb), and the presence of involuntary movements. The original scale consists of dichotomous Yes/No items. The total score corresponds to the sum of all items, with higher scores reflecting greater disturbances.
This category comprises one questionnaire (n = 1 study (93)). The Cambridge Depersonalization Scale (CDS), a standardized questionnaire developed by Sierra and Berrios to assess depersonalization, includes some items related to BPD (relevant items are listed in Supplementary Table S1) (94). Each of the 29 items of the CDS requires a dual-scoring: the “Frequency” (5-point adjectival scale) and “Duration” (6-point adjectival scale) of each phenomenon. The CDS defines depersonalization as “an alteration in the perception or experience of the self so that one feels detached from, and as if one is an outside observer of, one's mental processus or body” and “an alteration in the perception or experience of the external world so that it seems strange or unreal”. Moreover, the scale accounts for phenomena such as “heightened self-observation”, “changes in body experience”, and “changes in the feeling of agency”. Scores are added separately for “Frequency” and “Duration”, then both totals are combined to obtain a final score. Higher scores reflect greater levels of depersonalization.
This category comprises one questionnaire [Questionnaire on Body Feelings, n = 1 study (65)] and four single questions, either open-ended or dichotomous.
The Questionnaire on Body Feelings was developed by Tajadura-Jiménez et al. to assess participants' perceived body behavior. It was originally developed for a study with pain-free individuals (95) and consists of eight items scored on 7-point scales. The first four items use adjectival scales to assess the perceived speed, weight, strength, and extension of the body, while the remaining four items use Likert scales to assess the feelings of agency, vividness, surprise and feet localization. There is no total score for this questionnaire.
Two studies used single questions to assess the perceived size of a body part. Dagsdóttir et al. assessed the perceived distortion of the face by asking participants with oro-facial pain whether they perceived their face to be either swollen or reduced in size (96). In addition, participants rated their perceived distortion on a Magnitude Estimation Scale (MES) ranging from −100% to +100% (−100% = half the size, 0 = no change, + 100% = double the size). Haslam et al. assessed the perceived change in hand size in individuals with chronic post-stroke pain using a yes/no question (“Since your stroke, does it feel like your hand is now a different size?”) (97). If participants answered “yes”, a follow-up question asked whether their hand felt larger or smaller.
One study used a single open-ended question to assess the awareness of limb position in participants with CRPS (“On a daily basis, how aware are you of the position of your limbs?”) (3).
One study used a single open-ended question to assess the feeling of foreignness toward the affected hand in participants with upper-limb CRPS (98). Participants were asked how they felt about their hand. If participants were unsure of the meaning of the question, a series of close-ended questions were asked to determine whether participants perceived their hand as “ill”, “foreign”, “clumsy”, “unsuitable” or “strange”.
This category comprises three questionnaires: the “Changes in body sensation following limb loss” Questionnaire (CIBS-questionnaire, n = 1 study (6)), the “Assessment of neuropathic pain in fibromyalgic patients” (n = 1 study (99)) and the “Questionnaire on the Phantom Limb” (n = 1 study (100)).
The CIBS-questionnaire was developed by Giummarra et al. to explore different aspects of the phantom limb (e.g., perceived size, shape and posture of the phantom limb, ability to move the phantom limb, PLP) following amputation (6). It consists of 84 items, some of which relate to aspects of “body perception” as defined by Lotze and Moseley (relevant items are identified in Supplementary Table S1). The authors defined the purpose of the questionnaire as “an exploration of the perception of somatic and other qualities in the phantom limb following amputation”. There is no total score for this questionnaire.
The “Assessment of neuropathic pain in fibromyalgic patients” is an online survey developed by Viceconti et al. to explore neuropathic pain symptoms in participants with fibromyalgia. It consists of 37 items, including three items assessing body perception disturbances (99). Participants were provided with a list of 19 body parts and had to select all the body parts in which they experienced pain or stiffness. Then, for each symptomatic area, participants were asked whether they experienced illusory perceptions of swelling, shrinkage, asymmetry, a feeling of constriction or heaviness across that area (first item), how long they had experienced these disturbances (second item), and whether they had mentioned this phenomenon to their health care professionals (third item).
The “Questionnaire on the Phantom Limb” was developed by Kooijman et al. to report on phantom sensations (three items), phantom pain (five items), and stump pain (six items) (100). Among the phantom sensations items, one item lists descriptive words to express the quality of the phantom sensations (e.g., movement, abnormal shape, abnormal, position), which is related to BPD and was therefore included in the present review.
Several language versions of the questionnaires were found (see Tables 3, 4). All 18 questionnaires were available in English—the questionnaires were either originally developed in English, or an English version can be found in the included records (e.g., while the FreNAQ is available in Turkish (41) and Japanese (40), the authors also included an English version of the questionnaire in the published paper). For one of the questionnaires included in this review (CDS), the French version of the questionnaire was used in the included record (93). However, the questionnaire was originally developed in English (94) and is therefore available in this language. Some linguistic adaptations were performed through formal forward-backward translation processes, while others were informally translated by research teams for the purposes of their study (these data are available in Supplementary Table S1).
Table 1 outlines the clinical populations in which the questionnaires were used in the included studies. The most represented clinical populations were CRPS (n = 40), CLBP (n = 20), and OA (n = 9; hand OA: n = 2, knee OA: n = 7). Other diagnoses included amputation (n = 4), chronic neck pain (n = 3), fibromyalgia (n = 3), and chronic shoulder pain (n = 2). Finally, lumbo-pelvic pain, perineal pain, chronic pain of unspecified origin, polyneuropathy, oro-facial pain, post-stroke pain, and spinal cord injury were each represented in one study.
Among the studies that recruited participants with CRPS, some also recruited participants with other chronic pain conditions to form a “pain control group”. For example, one study recruited participants with CLBP, fracture, fibromyalgia, and rheumatoid arthritis (82). Another study recruited participants with OA (unspecified site), polyneuropathy, chronic bursitis, enthesopathy, peripheral nerve injury, and “limb pain other than CRPS (unspecified origin)” (90). Two studies recruited pain control participants with OA (unspecified site), carpal tunnel syndrome, rheumatoid arthritis, and “limb pain other than CRPS (unspecified origin)” (58, 83). Finally, five studies included participants with “limb pain other than CRPS”, without further specification.
In order to gain a clearer view of how the construct of “body perception disturbances” is defined in chronic pain, the items of each questionnaire were compiled and sorted according to the underlying aspect of the construct that they assessed. Figure 2 shows the different aspects of the construct and how often they were addressed in the questionnaires included in the present review (see Supplementary Table S2 for a detailed compilation of which aspects were addressed in which questionnaire). The most frequently addressed aspects of the construct were the perceived size of the painful body part (n = 12 questionnaires, e.g., from the FreBAQ: “My back feels like it is enlarged”, “My back feels like it has shrunk”), cognitive NLS (n = 12 questionnaires, e.g., from the Neurobehavioral Questionnaire: “My painful limb feels as though it is not part of the rest of my body”), the perceived shape of the painful body part (n = 11 questionnaires, e.g., from the FreBAQ: “My back feels lopsided (asymmetrical)”), proprioceptive awareness (n = 11 questionnaires, e.g., from the Bath BPDS: “On a scale of 0–10 how aware are you of the physical position of your limb?”), and agency (n = 11 questionnaires, e.g., from the CDS: “When I move it doesn't feel as if I were in charge of the movements, so that I feel “automatic” and mechanical as if I were a “robot” ”). Other aspects of the construct included motor NLS (n = 8 questionnaires, e.g., from the Neurobehavioral Questionnaire: “I need to focus all my attention on my painful limb to make it move the way I want it to”), and perceived weight (n = 4 questionnaires), pressure (n = 3 questionnaires), and temperature (n = 3 questionnaires) of the painful body part (e.g., from the Bath BPDS: “Is there a difference between how your affected limb looks or is on touch compared to how it feels to you in terms of the following: Size, Temperature, Pressure, Weight”). Emotions toward the painful body part were assessed in two questionnaires (e.g., from the Bath BPDS: “On a scale of 0–10 how strong are the emotional feelings that you have about your limb?”).
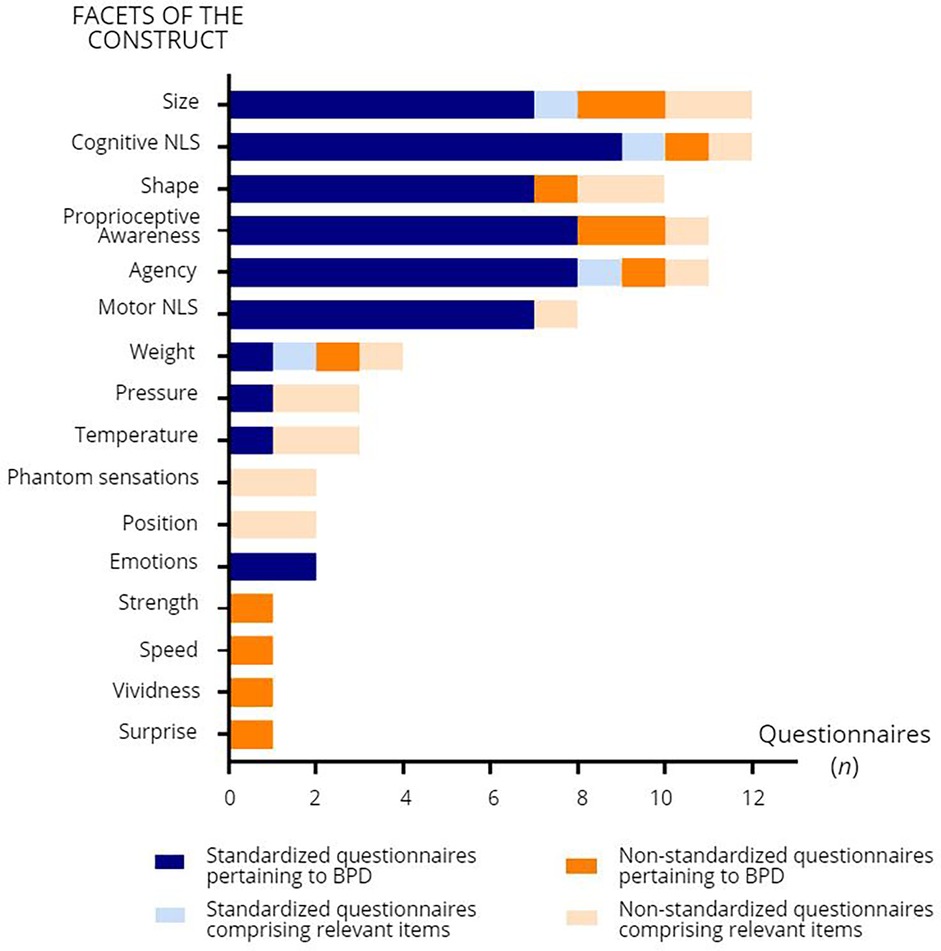
Figure 2. Facets of body perception disturbances (BPD) addressed in the self-reported questionnaires and number of questionnaires (n) comprising items related to these facets. NLS: neglect-like symptoms.
Some aspects of the construct were only addressed in certain clinical populations. For example, the perceived position of the phantom limb (n = 2 questionnaires, e.g., from the CIBS-questionnaire: “Where does your phantom limb usually sit relative to your other limbs?”), and the presence of phantom sensations of itching, touching, electric or vibration sensations (n = 2 questionnaires, e.g., for the CIBS-questionnaire: “Which phantom sensations do you experience?”, response options including “Itching in or on the phantom”, “Something touching the phantom”, and “Electric or vibration sensations”) were explored only in questionnaires specifically designed for amputees. Finally, perceptions of speed (e.g., “I feel slow” to “I feel quick”, 7-point adjectival scale), strength (e.g., “I feel weak” to “I feel strong”, 7-point adjectival scale), vividness (e.g., “It seems the feeling of my body is less vivid than normal”), and surprise (e.g., “The feelings about my body are surprising and unexpected”) were assessed only in the Questionnaire on body feelings, which was used in a study with CRPS participants.
The aims of this scoping review were to identify the self-reported questionnaires used to assess BPD in individuals with chronic non-cancer pain and to refine the definition of the BPD construct as used in these questionnaires. To our knowledge, this is the first literature review that aimed to identify questionnaires assessing BPD in individuals with chronic pain, transcending specific diagnoses. Eighteen questionnaires were identified, with the BBPDS, FreBAQ and Neurobehavioral Questionnaire being the most commonly used. While some pain populations were represented in several studies (e.g., CRPS, CLBP), there was an overall lack of diversity in terms of pain conditions. In addition, this scoping review attempted to circumscribe the main facets of BPD in chronic pain, drawing from identified questionnaires in the literature, in order to find a common terminology and definition for this construct in chronic pain. This process allowed us to identify five main facets at the core of the construct.
The studies included in this review investigated a variety of pain conditions, with CRPS being the most common, followed by CLBP. This is not surprising given the extensive literature on BPD in CRPS (2, 4, 17, 101) and CLBP (7, 8, 102). Quite expectedly, the three most commonly used questionnaires were developed specifically for these pain conditions. As for PLP, the paucity of included studies on this condition may seem surprising given the abundance of literature in this field (1, 103, 104). However, this could be explained by the decision to focus on questionnaires in the present review. Therefore, studies using outcome measures other than questionnaires (e.g., interviews) were excluded. Notably, there is a glaring scarcity of studies focusing on pain conditions other than the three mentioned above. This could be explained by the fact that most standardized questionnaires are body region- or diagnosis-specific (e.g., FreBAQ and its region-specific adaptations, BBPDS). This creates a vicious circle; there is limited evidence for the presence of BPD in non-specific chronic pain, and there are few available questionnaires to assess this phenomenon in a heterogeneous chronic pain population. Yet, chronic pain has recently been recognized as a disease by the International Classification of Diseases (ICD-11) (15). Furthermore, recent evidence suggests that the chronicity of pain is paralleled by the spread of the pain throughout the body (105). Thus, BPD in individuals with chronic pain should be assessed globally, without regard to diagnosis or body region, assuming that BPD also spreads with pain.
Walton et al. assessed BPD in chronic pain without targeting specific conditions by creating a region-generic version of the FreBAQ (51). This version was administered to individuals who self-identified as having chronic pain, with the term “chronic pain” intentionally left vague. This approach allowed for the examination of BPD as an independent construct, not tied to a specific diagnosis or body region. However, Walton et al's study had limitations; chronic pain was investigated in veterans via a survey, and information on pain (onset, intensity, type of pain) and psychosocial factors (e.g., kinesiophobia, catastrophizing, disability) was lacking. This lack of detailed data prevented further analysis of the relationship between BPD and clinical characteristics. In addition, the item wording of the FreBAQ-general is oriented toward the most painful body part, which limits the investigation of BPD to a single body site. This may be of limited use for pain conditions with widespread pain (e.g., fibromyalgia, rheumatoid arthritis). Nevertheless, this questionnaire is a promising first step in the assessment of BPD in chronic pain populations and may allow for the investigation and comparison of the phenomenon across different pain populations in research and clinical settings.
One of the main challenges in assessing BPD in individuals with chronic pain is the lack of a consistent definition for this construct and a standardized terminology to describe it. In fact, authors in the field use different terminologies to refer to the same phenomena, sometimes interchangeably. Notably, some authors speak of “body perception disturbances” (26, 52, 99), “disturbances in body representation” (59), or “impaired self-perception” (10, 46), while others use the terms “maladaptive perceptual awareness” (29), “disturbed body self-awareness” (12, 33) or “distorted body image” (1, 73, 85). Some authors considered NLS to be indicative of the presence of body perceptual disturbances (91) and used the Neurobehavioral Questionnaire (4) or an open-ended question about feelings of foreignness (98) to assess the presence of such symptoms, while others considered NLS to be a subfactor of the construct (along with impaired proprioception and distorted body image) and opted for the FreBAQ (10) or one of its adaptations. In light of this, and to avoid confusion with other constructs (e.g., body awareness, self-perception), we recommend using the terminology “body perception disturbances”.
Despite different terminologies and definitions, certain facets of the construct emerge as essential for a comprehensive understanding of the breadth of BPD in chronic pain. Our findings suggest that the core construct encompasses five main facets: a distorted perception of the size (a) and shape (b) of the painful body part, the presence of cognitive NLS (c), reduced proprioceptive awareness (d), and a disturbed sense of agency (e). Investigation of these facets is paramount to a comprehensive assessment of BPD in individuals with chronic pain. Therefore, studies investigating BPD should select questionnaires that include these facets. Interestingly, however, Walton et al. chose to exclude the two items pertaining to the perceived size of the painful body part from the FreBAQ-general. This decision was based on the results of their exploratory and confirmatory analyses, where the fit for a one-factor model improved after excluding these items (51). One explanation for this could be that these two items [“My (body part) seems larger/smaller than it should be”] were misinterpreted by participants. Indeed, the wording does not necessarily reflect that the painful body part feels smaller/larger than it actually is, which is at the core of BPD. Thus, content validity could be examined to ensure and, if necessary, improve the comprehensibility of these items (106). This limitation has been acknowledged by the authors.
As for motor NLS, and the perceived weight, pressure, and temperature of the painful body part, they were also considered as relevant facets of the construct in some of the questionnaires. Finally, some aspects of the construct were only investigated in specific pain populations (e.g., the presence of phantom sensations was only assessed in PLP, while perceptions of speed and vividness were only assessed in CRPS), raising the question of whether these facets are relevant in some pain conditions, rather than in chronic pain as a global condition.
This scoping review has some limitations. One potential limitation is the use of a key concept related to questionnaires (criterion #2: self-reported questionnaires). While studies on questionnaire development and validation were easily identified with our search strategy, original studies using relevant questionnaires did not always include them in the title, abstract, keywords or index terms. As a result, our search strategy may have missed some relevant records. Nevertheless, a manual search identified 17 additional records. Therefore, it is reasonable to assume that most, if not all, relevant records were included in this review. Another potential limitation lies in the construct appraisal. By adopting the definition proposed by Lotze and Moseley (“the way one's body feels to its owner”) to inform the eligibility of records for this review (criterion #3: body perception), one cannot exclude the possibility of selection bias in identifying key facets of the construct. In fact, we chose to exclude questionnaires pertaining to constructs deemed different from “body perception”. In the interest of consistency, we chose to focus our research on questionnaires developed under a common construct, rather than including different constructs that would only add to the existing confusion around the operational definition of “body perception disturbances”. Finally, the psychometric properties of the identified questionnaires were not assessed, as this was beyond the scope of the present review. Therefore, the next steps should include a systematic critical appraisal of the psychometric properties of each questionnaire to enable clinicians and researchers to make informed choices when selecting self-reported questionnaires.
This scoping review contributes to the field of BPD in chronic pain by identifying available self-reported questionnaires and by attempting to refine the construct definition, while also recommending a unified terminology. As visions of chronic pain evolve, it is paramount to assess how this condition affects body perception and to gain a better understanding of the similarities and differences in the experience of BPD in chronic pain of different origins. The use of common terminology and a generic self-reported questionnaire to assess BPD in chronic pain should allow to portray BPD as a construct independent of specific pain conditions. Further research should include validation studies for the FreBAQ-general in heterogeneous pain populations—including content validity for both the 6-item and the 9-item versions, given that the latter assesses all five main facets of BPD—and descriptive studies in large samples to gain a more complete picture of BPD in chronic pain.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
MD: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing. CP: Data curation, Investigation, Writing – review & editing. TA: Data curation, Investigation, Writing – review & editing. J-SR: Conceptualization, Methodology, Writing – review & editing. CM: Conceptualization, Formal Analysis, Methodology, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by CM's Emeritus research scholar grant from the Fonds de Recherche Québec-Santé (FRQS) (251649) and Canada Research Chair in SensoriMotor Rehabilitation and Pain (CRC-2022-00093). JSR is supported by a FRQS Senior Salary Award (281654). MD and TA are supported by scholarships from the FRQS. CP receives a scholarship from the Center for Interdisciplinary Research in Rehabilitation and Social Integration (Cirris).
The authors would like to thank Chloé Sutter (post-doctoral fellow) for her help with screening and extraction, and Martine Gagnon (academic health librarian) for her help in developing the search strategy.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpain.2025.1497328/full#supplementary-material
1. Lotze M, Moseley GL. Role of distorted body image in pain. Curr Rheumatol Rep. (2007) 9(6):488–96. doi: 10.1007/s11926-007-0079-x
2. Lewis JS, Kersten P, McCabe CS, McPherson KM, Blake DR. Body perception disturbance: a contribution to pain in complex regional pain syndrome (CRPS). Pain. (2007) 133(1–3):111–9. doi: 10.1016/j.pain.2007.03.013
3. Lewis JS, Kersten P, McPherson KM, Taylor GJ, Harris N, McCabe CS, et al. Wherever is my arm? Impaired upper limb position accuracy in complex regional pain syndrome. Pain. (2010) 149(3):463–9. doi: 10.1016/j.pain.2010.02.007
4. Galer BS, Jensen M. Neglect-like symptoms in complex regional pain syndrome: results of a self-administered survey. J Pain Symptom Manage. (1999) 18(3):213–7. doi: 10.1016/S0885-3924(99)00076-7
5. Wittayer M, Dimova V, Birklein F, Schlereth T. Correlates and importance of neglect-like symptoms in complex regional pain syndrome. Pain. (2018) 159(5):978–86. doi: 10.1097/j.pain.0000000000001173
6. Giummarra MJ, Georgiou-Karistianis N, Nicholls MER, Gibson SJ, Chou M, Bradshaw JL. Corporeal awareness and proprioceptive sense of the phantom. Br J Psychol. (2010) 101(4):791–808. doi: 10.1348/000712610X492558
7. Osborn M, Smith JA. Living with a body separate from the self. The experience of the body in chronic benign low back pain: an interpretative phenomenological analysis. Scand J Caring Sci. (2006) 20(2):216–22. doi: 10.1111/j.1471-6712.2006.00399.x
8. Moseley GL. I can’t find it! distorted body image and tactile dysfunction in patients with chronic back pain. Pain. (2008) 140:239–43. doi: 10.1016/j.pain.2008.08.001
9. Tanaka S, Nishigami T, Ohishi K, Nishikawa K, Wand BM, Stanton TR. “ But it feels swollen !”: the frequency and clinical characteristics of people with knee osteoarthritis who report subjective knee swelling in the absence of objective swelling. Pain Rep. (2021) 6(4):e971. doi: 10.1097/PR9.0000000000000971
10. Wand BM, James M, Abbaszadeh S, George PJ, Formby PM, Smith AJ, et al. Assessing self-perception in patients with chronic low back pain: development of a back-specific body-perception questionnaire. J Back Musculoskelet Rehabil. (2014) 27(4):463–73. doi: 10.3233/BMR-140467
11. Lewis J, McCabe C, Lewis J, McCabe C. Body perception disturbance (BPD) in CRPS. Pract Pain Manag. (2010) 10(3):60–6.
12. Koumantakis GA, Sifakis E, Stathis P, Gigourtakis S, Tatsios PI, Paraskevopoulos E, et al. Cross-cultural adaptation, reliability, and validity of the Greek version of the fremantle shoulder awareness questionnaire (FreSHAQ-GR) in patients with chronic shoulder pain. Healthcare (Basel). (2023) 11(18):2512. doi: 10.3390/healthcare11182512
13. Nishigami T, Mibu A, Tanaka K, Yamashita Y, Yamada E, Wand BM, et al. Development and psychometric properties of knee-specific body-perception questionnaire in people with knee osteoarthritis: the fremantle knee awareness questionnaire. PLoS One. (2017) 12(6):1–15. doi: 10.1371/journal.pone.0179225
14. Raffaeli W, Arnaudo E. Pain as a disease: an overview. J Pain Res. (2017) 10:2003–8. doi: 10.2147/JPR.S138864
15. Treede RD, Rief W, Barke A, Aziz Q, Bennett MI, Benoliel R, et al. Chronic pain as a symptom or a disease: the IASP classification of chronic pain for the international classification of diseases (ICD-11). Pain. (2019) 160(1):19–27. doi: 10.1097/j.pain.0000000000001384
16. Budzisz A, Jung A, Adamczyk WM, Szikszay TM, Carvalho GF, Bąbel P, et al. Body image measured via the fremantle awareness questionnaire in individuals with and without pain: a systematic review and meta-analysis. J Pain. (2024) 25(8):1–14. doi: 10.1016/j.jpain.2024.104530
17. Acapo S, Osinski T, Rulleau T, Dupeyron A, Nizard J. Assessment of body perception disturbances in complex regional pain syndrome: a systematic review using the COSMIN guideline. Eur J Pain . (2022) 26(10):2060–73. doi: 10.1002/ejp.2032
18. Viceconti A, Camerone EM, Luzzi D, Pentassuglia D, Pardini M, Ristori D, et al. Explicit and implicit own’s body and space perception in painful musculoskeletal disorders and rheumatic diseases: a systematic scoping review. Front Hum Neurosci. (2020) 14:1–32. doi: 10.3389/fnhum.2020.00083
19. Peters MDJ, Godfrey C, McInerney P, Khalil H, Larsen P, Marnie C, et al. Best practice guidance and reporting items for the development of scoping review protocols. JBI Evid Synth. (2022) 20(4):953–68. doi: 10.11124/JBIES-21-00242
20. Erol E, Yildiz A, Yildiz R, Apaydin U, Gokmen D, Elbasan B. Reliability and validity of the turkish version of the fremantle back awareness questionnaire. Spine (Phila Pa 1976). (2019) 44(9):E549–54. doi: 10.1097/BRS.0000000000002909
21. Goossens N, Janssens L, Brumagne S. Changes in the organization of the secondary somatosensory cortex while processing lumbar proprioception and the relationship with sensorimotor control in low back pain. Clin J Pain. (2019) 35(5):394–406. doi: 10.1097/AJP.0000000000000692
22. Mahmoudzadeh A, Abbaszadeh S, Baharlouei H, Karimi A. Translation and cross-cultural adaptation of the fremantle back awareness questionnaire into Persian language and the assessment of reliability and validity in patients with chronic low back pain. J Res Med Sci. (2020) 25:74. doi: 10.4103/jrms.JRMS_386_19
23. Hu F, Liu C, Cao S, Wang X, Liu W, Li T, et al. Cross-cultural adaptation and validation of the simplified Chinese version of the fremantle back awareness questionnaire in patients with low back pain. Eur Spine J. (2022) 31(4):935–42. doi: 10.1007/s00586-021-07085-8
24. Meier R, Emch C, Gross-Wolf C, Pfeiffer F, Meichtry A, Schmid A, et al. Sensorimotor and body perception assessments of nonspecific chronic low back pain: a cross-sectional study. BMC Musculoskelet Disord. (2021) 22(1):1–10. doi: 10.1186/s12891-021-04269-7
25. Rao P, Jain M, Barman A, Bansal S, Sahu R, Singh N. Fremantle back awareness questionnaire in chronic low back pain (Frebaq-I): translation and validation in the Indian population. Asian J Neurosurg. (2021) 16(01):113–8. doi: 10.4103/ajns.AJNS_359_20
26. Kurashima Y, Nakamura T, Mukaiyama T, Hasegawa K, Kuruma H. Investigation for factors affecting body perception disturbance in patients with low back pain by mechanism-based classification of pain: a cross-sectional study. Pain Res Manag. (2023) 2023:1–7. doi: 10.1155/2023/5083084
27. García-Dopico N, De La Torre-Luque A, Wand BM, Velasco-Roldán O, Sitges C. The cross-cultural adaptation, validity, and reliability of the Spanish version of the fremantle back awareness questionnaire. Front Psychol. (2023) 14(March):1–11. doi: 10.3389/fpsyg.2023.1070411
28. García-Dopico N, de la Torre-Luque A, Sitges C, Velasco-Roldán O. Proprioceptive acuity is core for back awareness in chronic low back pain: further analysis of the content validity of the Spanish version of the fremantle back awareness questionnaire. Front Hum Neurosci. (2022) 16(February):1–11. doi: 10.3389/fnhum.2022.1070402
29. Beales D, Lutz A, Thompson J, Wand BM, O’Sullivan P. Disturbed body perception, reduced sleep, and kinesiophobia in subjects with pregnancy-related persistent lumbopelvic pain and moderate levels of disability: an exploratory study. Man Ther. (2016) 21:69–75. doi: 10.1016/j.math.2015.04.016
30. Janssens L, Goossens N, Wand BM, Pijnenburg M, Thys T, Brumagne S. The development of the Dutch version of the fremantle back awareness questionnaire. Musculoskelet Sci Pract. (2017) 32:84–91. doi: 10.1016/j.msksp.2017.09.003
31. Schafer A, Wand BM, Ludtke K, Ehrenbrusthoff K, Schottker-Koniger T. Validation and investigation of cross cultural equivalence of the fremantle back awareness questionnaire - German version (FreBAQ-G). BMC Musculoskelet Disord. (2021) 22(1):323. doi: 10.1186/s12891-021-04156-1
32. Ehrenbrusthoff K, Ryan CG, Gruneberg C, Wand BM, Martin DJ. The translation, validity and reliability of the German version of the fremantle back awareness questionnaire. PLoS One. (2018) 13(10):e0205244. doi: 10.1371/journal.pone.0205244
33. Nishigami T, Mibu A, Tanaka K, Yamashita Y, Shimizu ME, Wand BM, et al. Validation of the Japanese version of the fremantle back awareness questionnaire in patients with low back pain. Pain Pract. (2018) 18(2):170–9. doi: 10.1111/papr.12586
34. Rabey M, Smith A, Beales D, Slater H, O’Sullivan P. Differing psychologically derived clusters in people with chronic low back pain are associated with different multidimensional profiles. Clin J Pain. (2016) 32(12):1015–27. doi: 10.1097/AJP.0000000000000363
35. Wand BM, Catley MJ, Rabey I, Sullivan BO, Connell NEO, Julia A. Disrupted self-perception in people with chronic low back pain. Further evaluation of the fremantle back awareness questionnaire. J Pain. (2016) 17(9):1001–12. doi: 10.1016/j.jpain.2016.06.003
36. Shigetoh H, Nishi Y, Osumi M, Morioka S. Temporal associations between pain-related factors and abnormal muscle activities in a patient with chronic low back pain: a cross-lag correlation analysis of a single case. J Pain Res. (2020) 13:3247–56. doi: 10.2147/JPR.S286280
37. Yamashita H, Nishigami T, Mibu A, Tanaka K, Manfuku M, Fukuhara H, et al. Perceived body distortion rather than actual body distortion is associated with chronic low back pain in adults with cerebral palsy: a preliminary investigation. Pain Pract. (2019) 19(8):826–35. doi: 10.1111/papr.12815
38. Akl SA, Ahmed El-Sabbahi S, Ewais NF. Validity and Reliability of the Arabic Version of the Fremantle Back Awareness Questionnaire. Available online at: www.ijramr.com
39. Monticone M, Maurandi C, Porcu E, Arippa F, Wand BM, Corona G. The fremantle back awareness questionnaire: cross-cultural adaptation, reliability, and validity of the Italian version in people with chronic low back pain. BMC Musculoskelet Disord. (2024) 25(1):1–11. doi: 10.1186/s12891-024-07420-2
40. Yamashita Y, Nishigami T, Mibu A, Tanaka K, Wand BM, Catley MJ, et al. Development and psychometric testing of the Japanese version of the fremantle neck awareness questionnaire: a cross-sectional study. J Pain Res. (2021) 14:311–24. doi: 10.2147/JPR.S267930
41. Onan D, Gokmen D, Ulger O. The fremantle neck awareness questionnaire in chronic neck pain patients: turkish version, validity and reliability study. Spine (Phila Pa 1976). (2020) 45(3):E163–9. doi: 10.1097/BRS.0000000000003207
42. Koumantakis GA, Nikolaki F, Kefalaki F, Tatsios PI, Paraskevopoulos E, Vrouva S. Cross-Cultural adaptation, reliability, and validity of the Greek version of the fremantle neck awareness questionnaire (FreNAQ-GR) in patients with chronic neck pain. Healthcare. (2024) 12(19):1985. Available online at: https://www.mdpi.com/2227-9032/12/19/1985 doi: 10.3390/healthcare12191985
43. Nishigami T, Watanabe A, Maitani T, Shigetoh H, Mibu A, Wand BM, et al. Development and validation of a shoulder-specific body-perception questionnaire in people with persistent shoulder pain. BMC Musculoskelet Disord. (2021) 22(1):1–11. doi: 10.1186/s12891-021-03944-z
44. Monticone M, Sconza C, Portoghese I, Nishigami T, Wand BM, Sorrentino G, et al. Cross-cultural adaptation, reliability and validity of the fremantle knee awareness questionnaire in Italian subjects with painful knee osteoarthritis. Health Qual Life Outcomes. (2021) 19(1):114. doi: 10.1186/s12955-021-01754-4
45. Hedayati R, Amjadian F, Ebadi A, Ehsani F. Cross-cultural adaptation, validity and reliability of the Persian version of fremantle knee awareness questionnaire. J Bodyw Mov Ther. (2022) 29:257–63. doi: 10.1016/j.jbmt.2021.10.009
46. Toda H, Maruyama T, Fujita K, Yamauchi Y, Tada M. Self-perception of the knee is associated with joint motion during the loading response in individuals with knee osteoarthritis: a pilot cross-sectional study. Sensors. (2021) 21(12):1–11. doi: 10.3390/s21124009
47. Nishigami T, Tanaka S, Mibu A, Imai R, Wand BM. Knee-related disability was largely influenced by cognitive factors and disturbed body perception in knee osteoarthritis. Sci Rep. (2021) 11(1):1–7. doi: 10.1038/s41598-021-85307-1
48. Hardy A, Campbell L, Jones C, Vandyken C, Bond J, Moss P, et al. The development and content validity of the fremantle perineal awareness questionnaire (FrePAQ) for use in people with persistent perineal pain. J Womens Pelvic Health Phys Ther. (2024) 48(3):202–13. doi: 10.1097/JWH.0000000000000307
49. Świdrak J, Arias A, de la Calle ER, Collado Cruz A, Sanchez-Vives MV. Virtual embodiment in fibromyalgia. Sci Rep. (2023) 13(1):1–11. doi: 10.1038/s41598-023-36861-3
50. Świdrak J, Rodriguez T, Polino L, Arias A, Torres X, Sanchez-Vives MV. Drawing the lines of fibromyalgia: a mixed-methods approach to mapping body image, body schema, and emotions in patient subtypes. Psychol Health Med. (2024) 00(00):1–21. doi: 10.1080/13548506.2024.2424997
51. Walton DM, Nazari G, Bobos P, MacDermid JC. Exploratory and confirmatory factor analysis of the new region-generic version of fremantle body awareness—general questionnaire. PLoS One. (2023) 18(3 March):1–14. doi: 10.1371/journal.pone.0282957
52. Lewis JS, Schweinhardt P. Perceptions of the painful body: the relationship between body perception disturbance, pain and tactile discrimination in complex regional pain syndrome. Eur J Pain. (2012) 16(9):1320–30. doi: 10.1002/j.1532-2149.2012.00120.x
53. Brun C, Giorgi N, Pinard AM, Gagné M, McCabe CS, Mercier C. Exploring the relationships between altered body perception, limb position sense, and limb movement sense in complex regional pain syndrome. J Pain. (2019) 20(1):17–27. doi: 10.1016/j.jpain.2018.07.008
54. Echalier A, Borg C, Creac’h C, Laurent B, Michael GA. Spontaneous sensations reveal distorted body perception in complex regional pain syndrome (CRPS). Brain Cogn. (2020) 142:105568. doi: 10.1016/j.bandc.2020.105568
55. Schulte-Goecking H, Azqueta-Gavaldon M, Storz C, Woiczinski M, Fraenkel P, Leukert J, et al. Psychological, social and biological correlates of body perception disturbance in complex regional pain syndrome. Curr Psychol. (2020) 2009:1–11. doi: 10.1007/s12144-020-00635-1
56. Mibu A, Nishigami T, Uematsu H, Tanaka K, Shibata M, Matsuda Y, et al. Validation of the Japanese version of the bath CRPS body perception disturbance scale for CRPS. J Anesth. (2021) 35(1):20–6. doi: 10.1007/s00540-020-02853-0
57. Halicka M, Vittersø AD, Proulx MJ, Bultitude JH. Attention upturned: bias toward and away from the affected side of the body and near space in a case of complex regional pain syndrome. Neuropsychologia. (2021) 163(November):1–16. doi: 10.1016/j.neuropsychologia.2021.108079
58. Reinersmann A, Skinner IW, Lücke T, Massy-Westropp N, Rudolf H, Lorimer Moseley G, et al. Intact tactile anisotropy despite altered hand perception in complex regional pain syndrome: rethinking the role of the primary sensory cortex in tactile and perceptual dysfunction. PeerJ. (2021) 9:1–31. doi: 10.7717/peerj.11156
59. Ten Brink AF, Halicka M, Vittersø AD, Keogh E, Bultitude JH. Ignoring space around a painful limb? No evidence for a body-related visuospatial attention bias in complex regional pain syndrome. Cortex. (2021) 136:89–108. doi: 10.1016/j.cortex.2020.12.007
60. Ten Brink AF, Halicka M, Vittersø AD, Jones HG, Stanton TR, Bultitude JH. Validation of the bath CRPS body perception disturbance scale. J Pain. (2021) 22(11):1371–84. doi: 10.1016/j.jpain.2021.04.007
61. Vittersø AD, Buckingham G, Ten Brink AF, Halicka M, Proulx MJ, Bultitude JH. Normal manual straight ahead pointing in complex regional pain syndrome. PLoS One. (2021) 16(12 December):1–12. doi: 10.1371/journal.pone.0261614
62. Halicka M, Vittersø AD, McCullough H, Goebel A, Heelas L, Proulx MJ, et al. Disputing space-based biases in unilateral complex regional pain syndrome. Cortex. (2020) 127:248–68. doi: 10.1016/j.cortex.2020.02.018
63. Vittersø AD, Buckingham G, Ten Brink AF, Halicka M, Proulx MJ, Bultitude JH. Characterising sensorimotor adaptation in complex regional pain syndrome. Cortex. (2021) 140:157–78. doi: 10.1016/j.cortex.2021.03.028
64. Bultitude JH, Walker I, Spence C. Space-based bias of covert visual attention in complex regional pain syndrome. Brain. (2017) 140(9):2306–21. doi: 10.1093/brain/awx152
65. Tajadura-Jiménez A, Cohen H, Bianchi-Berthouze N. Bodily sensory inputs and anomalous bodily experiences in complex regional pain syndrome: evaluation of the potential effects of sound feedback. Front Hum Neurosci. (2017) 11(July):1–16. doi: 10.3389/fnhum.2017.00379
66. Halicka M, Vittersø AD, McCullough H, Goebel A, Heelas L, Proulx MJ, et al. Prism adaptation treatment for upper-limb complex regional pain syndrome: a double-blind randomized controlled trial. Pain. (2021) 162(2):471–89. doi: 10.1097/j.pain.0000000000002053
67. De Schoenmacker I, Mollo A, Scheuren PS, Sirucek L, Brunner F, Schweinhardt P, et al. Central sensitization in CRPS patients with widespread pain: a cross-sectional study. Pain Med. (2023) 24(8):974–84. doi: 10.1093/pm/pnad040
68. Steenken L, Conde RM, Müller JK, Escolano-Lozano F, Birklein F, Dimova V. Nociceptive two-point discrimination acuity and body representation failure in polyneuropathy. Scand J Pain. (2023) 23(1):66–75. doi: 10.1515/sjpain-2022-0039
69. Beisheim-Ryan EH, Hicks GE, Pohlig RT, Medina J, Sions JM. Body image and perception among adults with and without phantom limb pain. PM R. (2023) 15(3):278–90. doi: 10.1002/pmrj.12750
70. Hwang H, Cho S, Lee JH. The effect of virtual body swapping with mental rehearsal on pain intensity and body perception disturbance in complex regional pain syndrome. Int J Rehabil Res. (2014) 37(2):167–72. doi: 10.1097/MRR.0000000000000053
71. Kotiuk V, Burianov O, Kostrub O, Khimion L, Zasadnyuk I. The impact of mirror therapy on body schema perception in patients with complex regional pain syndrome after distal radius fractures. Br J Pain. (2019) 13(1):35–42. doi: 10.1177/2049463718782544
72. Lewis JS, Kellett S, McCullough R, Tapper A, Tyler C, Viner M, et al. Body perception disturbance and pain reduction in longstanding complex regional pain syndrome following a multidisciplinary rehabilitation program. Pain Med. (2019) 20(11):2213–9. doi: 10.1093/pm/pnz176
73. Osumi M, Okuno H, Nishigami T, Ueta K. Tactile localization training for pain, sensory disturbance, and distorted body image: a case study of complex regional pain syndrome. Neurocase. (2015) 21(5):628–34. doi: 10.1080/13554794.2014.961482
74. Ryan CG, King R, Robinson V, Punt TD, Dinse HR, Grunenberg C, et al. Transcutaneous electrical nerve stimulation using an LTP-like repetitive stimulation protocol for patients with upper limb complex regional pain syndrome: a feasibility study. Hand Ther. (2017) 22(2):52–63. doi: 10.1177/1758998316678588
75. Vittersø AD, Buckingham G, Halicka M, Proulx MJ, Bultitude JH. Altered updating of bodily and spatial representations after tool-use in complex regional pain syndrome. Pain. (2020) 161(7):1609–28. doi: 10.1097/j.pain.0000000000001845
76. Lewis JS, Newport R, Taylor G, Smith M, McCabe CS. Visual illusions modulate body perception disturbance and pain in Complex regional pain syndrome: a randomized trial. Eur J Pain. (2021) 25(7):1551–63. doi: 10.1002/ejp.1766
77. Batalla MAP, Lewis JS. Cognitive multisensory rehabilitation, a novel approach for complex regional pain syndrome: case series. Physiother Theory Pract. (2024) 00(00):1–15. doi: 10.1080/09593985.2024.2393213
78. Halicka M, Cousins OR, Brink AFT, Vittersø AD, Proulx MJ, Bultitude JH. Reduced visuospatial attention in personal space is not limited to the affected limb in complex regional pain syndrome. J Pain Res. (2024) 17(November 2023):1519–29. doi: 10.2147/JPR.S437366
79. Bultitude JH, Petrini K. Altered visuomotor integration in complex regional pain syndrome. Behav Brain Res. (2021) 397(September 2020):112922. doi: 10.1016/j.bbr.2020.112922
80. Brun C, Pinard AM, McCabe CS, Mercier C. Virtual reality-induced sensorimotor conflict evokes limb-specific sensory disturbances in complex regional pain syndrome. Front Virtual Real. (2021) 2(June):1–10. doi: 10.3389/frvir.2021.694293
81. Hayashi K, Nishiwaki K, Kako M, Suzuki K, Hattori K, Sato K, et al. Combination of continuous epidural block and rehabilitation in a case of complex regional pain syndrome. J Nippon Med Sch. (2016) 83(6):262–7. doi: 10.1272/jnms.83.262
82. Kuttikat A, Shaikh M, Oomatia A, Parker R, Shenker N. Novel signs and their clinical utility in diagnosing complex regional pain syndrome (CRPS): a prospective observational cohort study. Clin J Pain. (2017) 33(6):496–502. doi: 10.1097/AJP.0000000000000434
83. Kolb L, Lang C, Seifert F, Maihöfner C. Cognitive correlates of ‘“ neglect-like syndrome “’ in patients with complex regional pain syndrome. Pain. (2012) 153(5):1063–73. doi: 10.1016/j.pain.2012.02.014
84. Michal M, Adler J, Reiner I, Wermke A, Ackermann T, Schlereth T, et al. Association of neglect-like symptoms with anxiety, somatization, and depersonalization in complex regional pain syndrome. Pain Med. (2017) 18(4):764–72. doi: 10.1093/pm/pnw214
85. Reinersmann A, Landwehrt J, Krumova EK, Peterburs J, Ocklenburg S, Güntürkün O, et al. The rubber hand illusion in complex regional pain syndrome: preserved ability to integrate a rubber hand indicates intact multisensory integration. Pain. (2013) 154(9):1519–27. doi: 10.1016/j.pain.2013.03.039
86. Ten Brink AF, Bultitude JH. Predictors of self-reported neglect-like symptoms and involuntary movements in complex regional pain syndrome compared to other chronic limb pain conditions. Pain Med. (2021) 22(10):2337–49. doi: 10.1093/pm/pnab226
87. Reinersmann A, Landwehrt J, Krumova EK, Ocklenburg S, Güntürkün O, Maier C. Impaired spatial body representation in complex regional pain syndrome type 1 (CRPS I). Pain. (2012) 153(11):2174–81. doi: 10.1016/j.pain.2012.05.025
88. Reinersmann A, Haarmeyer GS, Blankenburg M, Frettlöh J, Krumova EK, Ocklenburg S, et al. Left is where the L is right. Significantly delayed reaction time in limb laterality recognition in both CRPS and phantom limb pain patients. Neurosci Lett. (2010) 486(3):240–5. doi: 10.1016/j.neulet.2010.09.062
89. Hirakawa Y, Hara M, Fujiwara A, Hanada H, Morioka S. The relationship among psychological factors, neglect-like symptoms and postoperative pain after total knee arthroplasty. Pain Res Manag. (2014) 19(5):251–6. doi: 10.1155/2014/471529
90. Frettlöh J, Hüppe M, Maier C. Severity and specificity of neglect-like symptoms in patients with complex regional pain syndrome (CRPS) compared to chronic limb pain of other origins. Pain. (2006) 124(1–2):184–9. doi: 10.1016/j.pain.2006.04.010
91. Magni NE, McNair PJ, Rice DA. Sensorimotor performance and function in people with osteoarthritis of the hand: a case-control comparison. Semin Arthritis Rheum. (2018) 47(5):676–82. doi: 10.1016/j.semarthrit.2017.09.008
92. Magni N, Collier J, McNair P, Rice DA. Neuropathic pain in hand osteoarthritis: a cross-sectional study. J Clin Med. (2021) 10(19):1–10. doi: 10.3390/jcm10194439
93. Pozeg P, Palluel E, Ronchi R, Solcà M, Al-Khodairy AW, Jordan X, et al. Virtual reality improves embodiment and neuropathic pain caused by spinal cord injury. Neurology. (2017) 89(18):1894–903. doi: 10.1212/WNL.0000000000004585
94. Sierra M, Berrios GE. The Cambridge depersonalisation scale: a new instrument for the measurement of depersonalisation. Psychiatry Res. (2000) 93(2):153–64. doi: 10.1016/S0165-1781(00)00100-1
95. Tajadura-Jiménez A, Basia M, Deroy O, Fairhurst M, Marquardt N, Bianchi-Berthouze N. As light as your footsteps: altering walking sounds to change perceived body weight, emotional state and gait. Conf Hum Factor Comput Sys Proc. (2015) 2015(May):2943–52. doi: 10.1145/2702123.2702374
96. Dagsdottir LK, Skyt I, Vase L, Baad-Hansen L, Castrillon E, Svensson P. Reports of perceptual distortion of the face are common in patients with different types of chronic oro-facial pain. J Oral Rehabil. (2016) 43(6):409–16. doi: 10.1111/joor.12383
97. Haslam BS, Butler DS, Moseley GL, Kim AS, Carey LM. “My hand is different": altered body perception in stroke survivors with chronic pain. Brain Sci. (2022) 12(1331):1–16. doi: 10.3390/brainsci12101331
98. Förderreuther S, Sailer U, Straube A. Impaired self-perception of the hand in complex regional pain syndrome (CRPS). Pain. (2004) 110(3):756–61. doi: 10.1016/j.pain.2004.05.019
99. Viceconti A, Geri T, De Luca S, Maselli F, Rossettini G, Testa M. Body perception distortions correlate with neuropathic features in Italian fibromyalgic patients: findings from a self-administered online survey. Musculoskelet Sci Pract. (2022) 60(April):1–8. doi: 10.1016/j.msksp.2022.102570
100. Kooijman CM, Dijkstra PU, Geertzen JHB, Elzinga A, Van Der Schans CP. Phantom pain and phantom sensations in upper limb amputees: an epidemiological study. Pain. (2000) 87(1):33–41. doi: 10.1016/S0304-3959(00)00264-5
101. Turton AJ, Palmer M, Grieve S, Timothy P, Lewis J, Mccabe CS. Evaluation of a prototype tool for communicating body perception disturbances in complex regional pain syndrome. Front Hum Neurosci. (2013) 7(August):1–8. doi: 10.3389/fnhum.2013.00517
102. Bray H, Moseley GL. Disrupted working body schema of the trunk in people with back pain. Br J Sports Med. (2011) 45(3):168–73. doi: 10.1136/bjsm.2009.061978
103. Flor H, Elbert T, Knecht S, Wienbruch C, Pantev C, Birbaumers N, et al. Phantom-limb pain as a perceptual correlate of cortical reorganization following arm amputation. Nature. (1995) 375(6531):482–4. doi: 10.1038/375482a0
104. Fraser CM, Halligan PW, Robertson IH, Kirker SGB. Characterising phantom limb phenomena in upper limb amputees. Prosthet Orthot Int. (2001) 25(3):235–42. doi: 10.1080/03093640108726607
105. Tanguay-Sabourin C, Fillingim M, Guglietti G V, Zare A, Parisien M, Norman J, et al. A prognostic risk score for development and spread of chronic pain. Nat Med. (2023) 29:1821–31. doi: 10.1038/s41591-023-02430-4
Keywords: chronic pain, body perception, disturbances, patient-reported questionnaire, adults
Citation: Dagenais M, Proulx C, Augière T, Roy J-S and Mercier C (2025) Self-reported questionnaires assessing body perception disturbances in adults with chronic non-cancer pain: a scoping review. Front. Pain Res. 6:1497328. doi: 10.3389/fpain.2025.1497328
Received: 16 September 2024; Accepted: 19 February 2025;
Published: 6 March 2025.
Edited by:
Emily J. Bartley, University of Florida, United StatesReviewed by:
Hubert Van Griensven, St George's, University of London, United KingdomCopyright: © 2025 Dagenais, Proulx, Augière, Roy and Mercier. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Catherine Mercier, Y2F0aGVyaW5lLm1lcmNpZXJAcmVhLnVsYXZhbC5jYQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.