- 1Department of Psychiatry, The Institute of Living, Hartford, CT, United States
- 2Department of Psychiatry, Atrium Behavior Health, Charlotte, NC, United States
- 3Department of Psychiatry, University of Connecticut School of Medicine, Farmington, CT, United States
- 4Internal Medicine, Cimpar, S.C., Oak Park, IL, United States
- 5Department of Psychiatry, Boston Medical Center, Boston, MA, United States
- 6Department of Psychiatry, Liaquat College of Medicine and Dentistry, Karachi, Pakistan
- 7Addiction Services Division, Connecticut Valley Hospital, Middletown, CT, United States
- 8Department of Psychiatry, Oregon State Hospital, Salem, OR, United States
- 9Department of Psychiatry, BronxCare Health System, New York, NY, United States
- 10Department of Psychiatry, Maimonides Medical Center, Brooklyn, NY, United States
- 11Department of Psychiatry, University of Wisconsin Hospital, Madison, WI, United States
- 12Department of Medicine, Bogomolets National Medical University, Kyiv, Ukraine
- 13Dhaka Medical College, Dhaka, Bangladesh
- 14Permian Basin Campus, Texas Tech University, Lubbock, TX, United States
- 15Department of Internal Medicine, Suburban Community Hospital, Philadelphia, PA, United States
- 16Centre for Brain Research, Indian Institute of Science, Bengaluru, India
- 17Department of Health Services, Srinagar, India
- 18Department of Psychiatry, Healing Mind and Wellness Initiative Nawab Bazar, Srinagar, India
- 19Department of Psychiatry, Greater Los Angeles VA Medical Center, Los Angeles, CA, United States
- 20Department of Mental Health, ASL 4 Teramo, Contrada Casalena, Teramo, Italy
- 21Department of Psychiatry, Saint Francis Hospital and Medical Center, Hartford, CT, United States
Background: The escalating number of deaths related to opioid usage has intensified the pursuit of non-opioid alternatives for managing chronic pain. It's often observed that psychiatric comorbidities coexist in patients suffering from chronic pain. There are a variety of psychotropic medications that have demonstrated effectiveness in treating both psychiatric symptoms and pain. This systematic review and meta-analysis aim to assess the effectiveness of various psychiatric drugs in managing specific types of chronic pain, including fibromyalgia, neuropathic pain, and chronic low back pain.
Methods: A comprehensive search of five major databases was conducted through February 2023 to identify randomized controlled trials (RCTs) that met our inclusion criteria, focusing on outpatients Over 18 years of age with chronic pain. The study assessed the effectiveness of duloxetine, mirogabalin, pregabalin, gabapentin, and tricyclic antidepressants (TCAs), including serotonin-norepinephrine reuptake inhibitors (SNRIs), across various chronic pain conditions such as fibromyalgia, neuropathic pain, and chronic low back pain. The primary outcome measures included pain reduction, improvement in function, and quality of life. Of the 29 RCTs in the systematic review, 20 studies qualified for the meta-analysis. The analysis was stratified by pain type and treatment duration (short-term ≤14 weeks vs. long-term >14 weeks), using Hedge's g standardized mean differences and a random-effects model, along with sensitivity and subgroup analyses.
Results: The overall short-term intervention effect across all studies was significant (SMD −1.45, 95% CI −2.15 to −0.75, p < 0.001), with considerable heterogeneity (I2 = 99%). For fibromyalgia, both duloxetine and mirogabalin demonstrated substantial efficacy with SMDs of −2.42 (95% CI −3.67 to −1.18, p < 0.0001) and −2.10 (95% CI −3.28 to −0.92, p = 0.0005), respectively. Conversely, treatments for neuropathic pain and chronic low back pain, including those with amitriptyline and desipramine, did not show significant benefits. The effectiveness of gabapentin could not be conclusively determined due to limited representation in the data. Additionally, no consistent long-term benefits were observed for any of the medications.
Conclusions: While the results of this study underscore the importance of exploring non-opioid alternatives for chronic pain management, particularly in light of the opioid crisis, it is crucial to interpret the findings carefully. Our analysis suggests that certain psychiatric medications, such Duloxetine and mirogabalin demonstrated significant short-term efficacy in fibromyalgia patients. However, their effectiveness in treating neuropathic pain and chronic low back pain was not statistically significant. Additionally, the effectiveness of gabapentin and other medications, such as pregabalin for neuropathic pain, could not be conclusively determined due to limited data and high study heterogeneity. No consistent long-term benefits were observed for any of the drugs studied, raising questions about their sustained efficacy in chronic pain management. These findings highlight the need for further research to understand better the role of psychiatric medications in managing specific chronic pain conditions without prematurely concluding that they are ineffective or unsuitable for these purposes.
1 Introduction
While patients find opioids extremely effective for pain control, they carry an associated risk of creating physical dependence, and this has led to the widespread misuse of opioids, and their addiction has developed into a severe public health crisis.
Approximately 21%–29% of individuals administered opioids for chronic pain misuse them, and about 8%–12% develop Opioid Use Disorder (1). Roughly 80% of heroin users began by abusing prescription painkillers (2). Since the year 2000, almost 500,000 people have died as a result of drug overdose (3). Various methods to reduce dependence on opioids, such as restricting their supply, distributing naloxone, and expanding treatment, were found to have only a minor impact on overdose deaths, calling for additional, robust mitigating strategies (4–7). The CDC reported a massive surge in deaths due to opioid overdose between June 2019 and May 2020 (8–10), most likely attributed to increased fentanyl use. Consequently, the USDA has agreed to promote the use of buprenorphine and methadone in patients with opioid use disorder (11, 12). In January 2023, the U.S. government decided to remove the federal requirement for practitioners to possess a DATA Waiver (X-Waiver) to prescribe buprenorphine for the treatment of opioid use disorder (OUD).
Another solution for this opioid epidemic is the utilization of non-opioid medications for pain management. Various studies have found no significant difference in pain management between opioid and non-opioid options (13, 14). There are many non-opioid analgesics on the market now that have better side effect profiles and a lower risk of addiction. Acetaminophen and nonsteroidal anti-inflammatory medicines (NSAIDs) are non-opioid pharmacological choices that have analgesic characteristics for specific illnesses. However, antidepressants and anticonvulsants, the most common adjuvant analgesics (15, 16), not only alleviate pain but also target the underlying psychiatric comorbidities that often accompany chronic pain.
Chronic pain is often highly comorbid with psychiatric disorders (15), such as depression and anxiety, suggesting a shared pathophysiology between these conditions. Psychiatric medications, such as antidepressants and anticonvulsants, work by modulating neurotransmitters like serotonin and norepinephrine. These neurotransmitters play an important role in regulating mood and perceiving pain. Because of this dual effect, these medications could be effective in managing chronic pain, especially in patients who also struggle with psychiatric conditions. For example, studies have shown that duloxetine significantly reduces pain and improves function in patients with fibromyalgia, while pregabalin has been effective in managing pain associated with neuropathy. Psychiatrists often treat patients who suffer from both psychiatric disorders and chronic pain. Addressing the use of psychiatric medications in managing chronic pain is important, as it can help reduce the risk of polypharmacy, improve medication adherence, and minimize the burden of side effects. Involving psychiatry in a multidisciplinary pain management team also allows for better identification of patients who may be developing physical dependence or progressing toward an opioid use disorder. Despite the significant overlap between psychiatric conditions and chronic pain, there are currently no specific guidelines to assist psychiatrists in prescribing these medications for chronic pain management. One considerable advantage of psychiatric medications is their ability to address both the emotional and physical components of pain. Chronic pain often exacerbates conditions like depression and anxiety, which creates a cycle where pain and mental health issues feed into each other. By treating both aspects simultaneously, psychiatric medications can help break this cycle, potentially leading to better overall outcomes for patients. Psychiatric medications tend to have a lower risk profile compared to opioids. These medications are less likely to cause dependence, and their side effect profiles are relatively more favorable than those of long-term opioid use. This makes them a safer alternative, particularly in the context of the ongoing opioid epidemic. Given the serious concerns associated with opioid use, the lower risk profile of psychiatric medications for addiction and overdose makes them a particularly attractive option in the current healthcare landscape. Despite the promising potential of these medications, there remains a significant gap in the guidelines available to psychiatrists for prescribing these drugs specifically for chronic pain management. This study aims to address this gap by systematically reviewing and analyzing the effectiveness of non-opioid psychiatric medications in managing chronic pain conditions such as fibromyalgia, chronic back pain, and neuropathic pain. This study aims to investigate the effectiveness of non-opioid medication in managing certain chronic pain conditions such as fibromyalgia, chronic back pain, and neuropathic pain.
The study focuses on non-opioid psychiatric drugs, including duloxetine, mirogabalin, pregabalin, gabapentin, desipramine, and amitriptyline. By conducting a systematic review and meta-analysis, we seek to update the current knowledge base and contribute to developing safer and more effective pain management strategies.
2 Methods
We conducted a systematic review and meta-analysis adhering to the PRISMA Statement guidelines. We searched five databases, including PubMed, Scopus, EMBASE, Web of Science, and Cochrane Library, for articles published within the past decade up until February 2023. We utilized the following keywords: “neuropathic pain,” “fibromyalgia,” and “back pain.” In addition to the initial search terms, we included “radiculopathy,” “sciatica,” “nerve pain,” “central sensitization,” “nociplastic pain,” “musculoskeletal pain,” “discogenic pain,” and “spinal stenosis.” We elected to include Randomized–Controlled Trials conducted in outpatient settings. We focused only on studies where non-opioid psychiatric medications were used as an intervention to treat pain; these include duloxetine, gabapentin, pregabalin, tricyclic antidepressants (TCAs), and serotonin-norepinephrine reuptake inhibitors (SNRIs). Our analysis excluded non-English publications, systematic reviews, case reports, case studies, commentaries, and grey literature. We only considered studies focused on non-acute chronic pain treatment and excluded studies conducted in inpatient settings and those involving alternative treatments like opioid therapy, cannabis, acupuncture, nerve blocks, anti-inflammatory drugs, or corticosteroids.
To identify and control for potential confounding factors, we focused on variables such as patient demographics (e.g., age, gender), baseline pain severity, duration of pain, psychiatric comorbidities, and prior treatments. Studies were included only if they reported sufficient data on these variables, allowing us to perform subgroup analyses where necessary. We also conducted sensitivity analyses to evaluate the robustness of our findings. We used meta-regression to adjust for these potential confounders, ensuring that the impact of these variables on treatment outcomes was minimized.
Search strategy: The initial search, searching through PubMed, Scopus, EMBASE, Web of Science, and Cochrane Library, generated 11,023 citations. After removing duplicates, we had 1,345 studies to screen based on their titles. This process led to the inclusion of 433 citations. We then scrutinized the abstracts, which resulted in the exclusion of 370 citations. The remaining 63 papers were reviewed for eligibility by three authors (SP, SA, and LJ). In case of any disagreements, the 4th and 5th authors (AB and Sehar) stepped in to resolve the disagreements. After applying our stringent inclusion criteria, we selected 29 full-text articles aligned with our research objectives. In addition to our search through five major databases, we employed the snowballing method to enrich our research further. This approach allowed us to discover additional relevant studies and references related to our topic by examining the references in key papers (such as published systematic reviews), including (17–26) Ferraro et al., 2021, Chou et al., 2017, Qaseem et al., 2017, McDonagh et al., 2020, Walitt et al., 2015, Wang et al., 2022, Finnerup et al., 2015, Ferreira et al., 2021, Caruso et al., 2019, and Tort et al., 2012. The PRISMA (27) flow diagram is given below in Figure 1A.
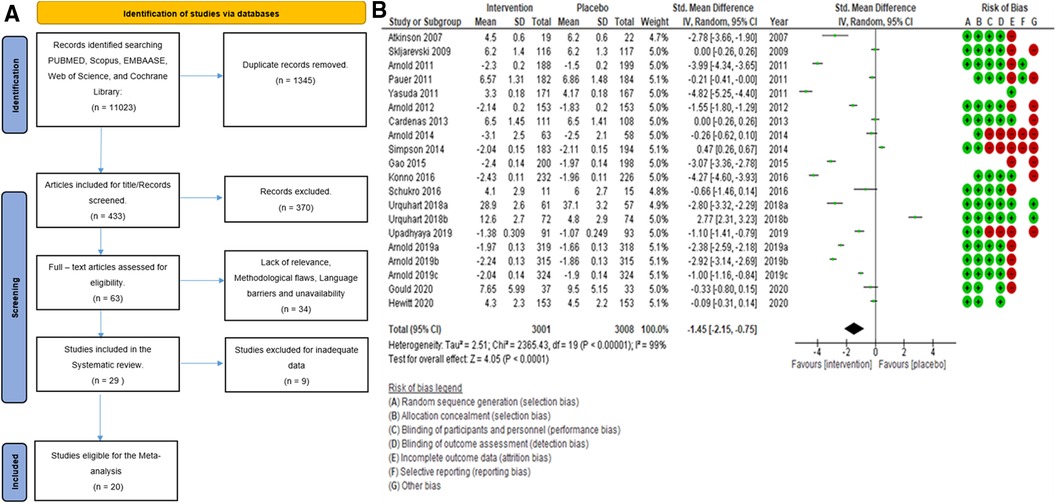
Figure 1. (A) Search flowchart. (B) Forest plot of the primary analysis of 20 RCT studies with the risk of bias assessment. Green and red circles represent low and high risks of bias.
3 Meta-analysis
3.1 Statistical methods
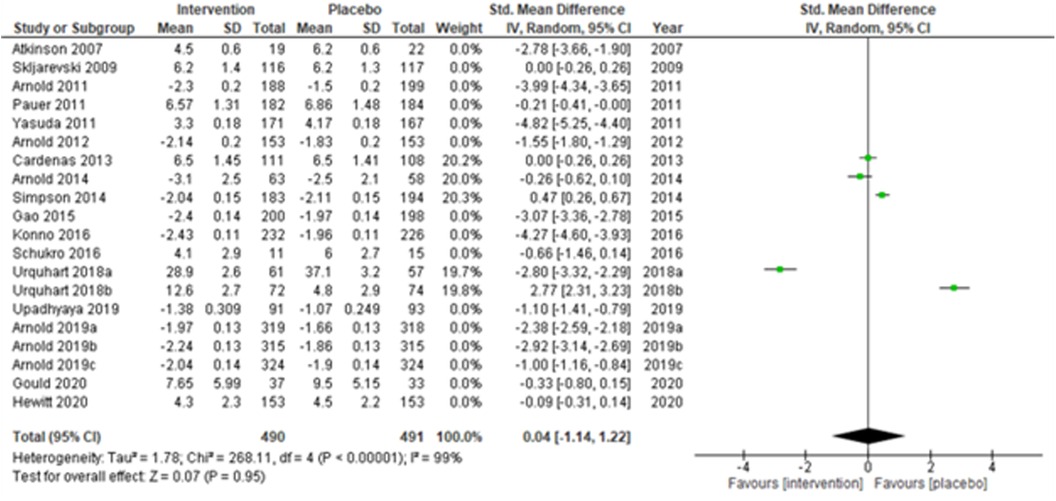
A total of 29 RCTs were included in the systematic review (Table 1A–C), but only 20 studies (28, 30–34, 36, 38, 43, 44, 48, 50–53, 55, 56, 58) (Atkinson et al., 2007; Skljarevski et al., 2009; Arnold et al., 2011; Pauer et al., 2011; Yasuda et al., 2011; Arnold et al., 2012; Cardenas et al., 2013; Arnold et al., 2014; Simpson et al., 2014; Gao et al., 2015; Konno et al., 2016; Schukro et al., 2016; Urquhart et al., 2018a; Urquhart et al., 2018b; Upadhyaya et al., 2019; Arnold et al., 2019a; Arnold et al., 2019b; Arnold et al., 2019c; Gould et al., 2020; and Hewitt et al., 2020) qualified for the meta-analysis due to the availability of the required data. Arnold et al. (2019) (31) reported the results of three studies separately, and thus, these were considered as three independent studies (Arnold et al., 2019a; Arnold et al., 2019b; and Arnold et al., 2019c) during the analysis. Studies were assessed using the PICO (Population, Intervention, Comparison, and Outcomes) format recommended by the Cochrane Collaboration. Hedge's g standardized mean differences (SMDs) and 95% confidence intervals (C.I.s) were calculated as the continuous study outcome, wherein a negative SMD indicated an effective intervention compared with a placebo. We adopted a random-effects model and used inverse-variance-based DerSimonian and Laird's (DL) estimation method for estimating the summary measure and the heterogeneity variance. In addition, sensitivity analyses were performed by excluding (a) studies with active placebo, such as benztropine, (b) studies with sample sizes < 50 in each study arm, and (c) studies with a high risk of bias. Furthermore, subgroup analyses were performed, and the summary measures were compared for (a) multicenter vs. single-center study settings, (b) neuropathic pain, fibromyalgia vs. chronic low back pain, and (c) duration of the study ≤ 14 weeks vs. study duration > 14 weeks. The impact of heterogeneity was measured using the I2-statistic. The funnel plot was used to check for publication bias. Egger's test was used to check small-study effects. The quality of the studies was evaluated using the Cochrane Risk of Bias assessment tool (59). Analyses were undertaken by intention-to-treat. The statistical significance was checked at a 5% level of significance. Analyses were conducted using the Cochrane RevMan 5.3 and Stata version 17 software.
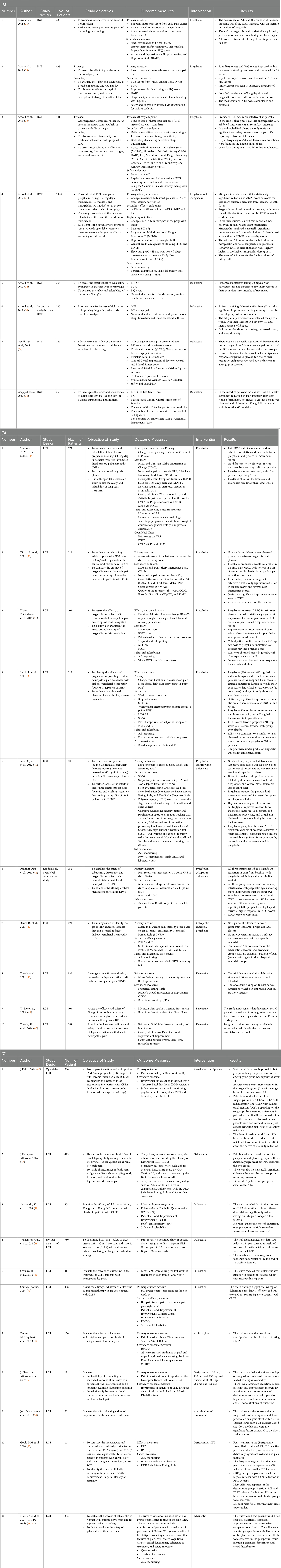
Table 1. (A) Studies evaluating fibromyalgia. (B) Studies evaluating neuropathic pain. (C) Studies evaluating chronic lower back pain.
3.2 The review of the literature
Fibromyalgia is a chronic disorder that is primarily characterized by the presence of generalized pain throughout the body. Pain is only one symptom of its complex, multifaceted symptomatology. When treating fibromyalgia, it is essential to adopt a comprehensive approach. A treatment plan may include thorough assessments, goal-setting, education on the condition, pharmacological and non-pharmacological interventions, alternative, complementary therapies, and lifestyle modifications (60, 61). We have investigated several pharmacological non-opioid treatments for fibromyalgia, such as pregabalin, mirogabalin, and duloxetine. Studies have shown that some of these medications are effective in alleviating pain related to fibromyalgia and also improving overall functioning (28, 29). Pauer et al. (28) conducted a 14-week study investigating the use of pregabalin for its efficacy, safety, and functioning in 736 individuals with fibromyalgia. The research showed that 450 mg/day of pregabalin significantly reduced pain scores and improved sleep and function. Ohta et al. investigated the efficacy of pregabalin in Japanese patients. The subjects started at 150 mg/day and subsequently increased to a maintenance dose of either 300 or 450 mg/day over 15 weeks. The results show that 450 mg/day of pregabalin effectively reduced pain and improved sleep and functioning. There were, however, minor adverse effects, including dizziness and sleepiness. The findings of this study support the potential use of pregabalin as a viable and safe treatment option for fibromyalgia patients. In a centers-based study, Arnold and colleagues (2014) (30) conducted a study to evaluate the safety and efficacy of a controlled-release (C.R.) version of pregabalin in patients with fibromyalgia. The study initially included 441 participants who underwent a 6-week single-blind treatment phase with pregabalin C.R., followed by a 13-week double-blind phase where patients were randomized to continue with pregabalin C.R. or switch to a placebo.
The key outcome measured was the time to loss of therapeutic response (LTR), defined as a less than 30% pain reduction from the single-blind phase baseline or discontinuation due to lack of efficacy or adverse events. The results show that the median time to LTR was significantly longer for patients in the pregabalin C.R. group (58 days) compared to the placebo group (22 days). By the end of the trial, 54.0% of patients in the pregabalin C.R. group experienced LTR, which is lower than the 70.7% in the placebo group, indicating that a more significant proportion of patients in the pregabalin C.R. group maintained their therapeutic response. Dizziness and sleepiness were reported but were generally mild to moderate in their rating.
Another study exploring mirogabalin compared its efficacy with pregabalin in a 13-week randomized trial followed by a 52-week open-label extension (31). In this study, 1,293 patients were given a placebo, 1,270 pregabalin (150 mg twice daily), and 1,301 received mirogabalin (15 mg once or twice daily). The results show that pregabalin significantly reduced pain, while mirogabalin at 15 mg once or twice daily did not produce comparable outcomes. Duloxetine has been studied for its effects on fatigue and pain severity, showing improvements in both outcomes (32, 35). Research studies have investigated duloxetine in adolescents with juvenile fibromyalgia. The results have shown mixed results in reducing pain and higher rates of adverse events (34). Overall, fibromyalgia management requires both pharmacological interventions and non-pharmacological interventions, as well as complementary therapies (Tai Chi, acupuncture, music, hydrotherapy, and massage therapy) and lifestyle modifications.
Neuropathic pain is a complex and debilitating condition that often requires the exploration of innovative treatment approaches to optimize patient outcomes (23, 37). Kim et al. (2011) investigated the effectiveness of pregabalin in treating neuropathic pain among 110 central post-stroke pain (CPSP) patients over 13 weeks (37). The study's results show no significant improvement in pain; however, there was improvement in sleep, anxiety, and overall patient functioning. Satoh et al. (2011) conducted a 14-week trial involving 317 Japanese patients, in which they examined pregabalin's efficacy in alleviating pain secondary to diabetic peripheral neuropathy (39). The study found a significant reduction in pain compared to the placebo group (p < 0.005), with some mild to moderate side effects reported. In another study, Cardenas et al. (2013) evaluated the effectiveness of pregabalin among patients with neuropathic pain associated with spinal cord injuries (38). The study found that pregabalin had a beneficial effect on reducing pain, with mild side effects. Contrary to that, in the treatment of neuropathic pain associated with HIV, Simpson et al. (2014) found no significant difference between pregabalin and a placebo (36). Yasuda et al. (2011) assessed the efficacy and safety of duloxetine in diabetic neuropathy and found it to be more effective than a placebo but noted higher adverse effects (43). Gao et al. (2012) (44) conducted a similar study on 405 Chinese patients with diabetic peripheral neuropathic pain (DPNP), which reported a significant reduction in pain and a good safety profile for duloxetine. Regarding long-term efficacy and safety, Yasuda et al. (2016) examined duloxetine in Japanese patients with DPNP and found significant pain reduction with no significant adverse effects (45). Rauck et al. (2013) investigated the optimal dose of gabapentin for treating DPNP but found no statistically significant difference between various doses and a placebo (42). We also included trials comparing multiple medications. Boyle et al. (2012) (40) evaluated the effects of amitriptyline, duloxetine, and pregabalin on DPNP, finding that all three drugs reduced pain but caused no improvement in quality of life. Padmini et al. (2012) (41) evaluated the effects of the three drugs in a study of 152 patients and reported significant pain reduction in diabetic neuropathy, with pregabalin being the fastest acting. The research studies on pregabalin, gabapentin, and duloxetine have shown positive outcomes in managing neuropathic pain, varying efficacy and tolerability across various patient populations (37–39, 42, 43, 45).
Over recent years, various clinical trials have assessed the efficacy of different medications in treating chronic low back pain (CLBP), which has led to several promising discoveries. Urquhart et al. (2016) and Donna et al. (2018) conducted randomized control trials on the use of low-dose amitriptyline in CLBP patients, showing that it may help reduce disability and pain intensity (52, 58). In 2016, Konno et al. (51) and Schukro et al. (50) studied the effectiveness of duloxetine and found significant pain reduction and improvement in secondary outcome measures. Skljarevski et al. (2009) and Williamson et al. (2014) further explored the impacts of duloxetine on pain reduction and found that patients with only a 10% reduction in pain after four weeks had a slim chance of achieving moderate pain relief after 12 weeks (48, 49). Atkinson et al. (2016) found that gabapentin had no statistically significant impact on pain relief for CLBP patients (47). Schliessbach et al. (2018) found imipramine had no overall effect on CLBP, but patients more sensitive to cold or heat pain felt better with its use (54). Atkinson et al. (2007) reported significant pain reduction and improved daily functioning in chronic back pain patients with low- and high-concentration desipramine and all-concentration fluoxetine (53). In a study conducted by Horne AW et al. (2021), gabapentin was measured in women with chronic pelvic pain and no apparent pelvic pathology (57). The study found that gabapentin did not significantly improve pain scores compared to a placebo. Lastly, Kalita et al. (2014) compared the efficacy of amitriptyline and pregabalin, finding both reduced pain and disability, but amitriptyline performed significantly better (46).
4 Results
4.1 Primary analysis
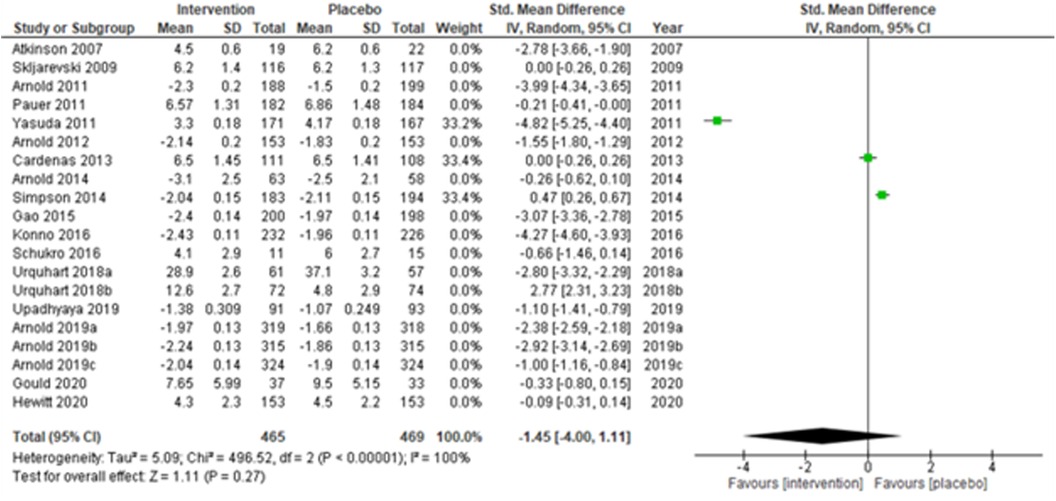
The overall summary measure (SMD (95% CI) −1.45 (−2.15, −0.75)) across all twenty studies for the difference in pain scores was statistically significant (z = 4.05, p-value < 0.001) in the intervention group when compared with the placebo. Still, there was a staggering amount of heterogeneity among the included trials (I2 = 99%). Due to their small-sized trials (n < 30 in each study arm), Atkinson et al. (2007) (53) and Schukro et al. (2016) (50) exhibited broader confidence intervals of the effect size. However, the intervals were narrow in large trials (Figure 1B).
4.2 Sensitivity analyses
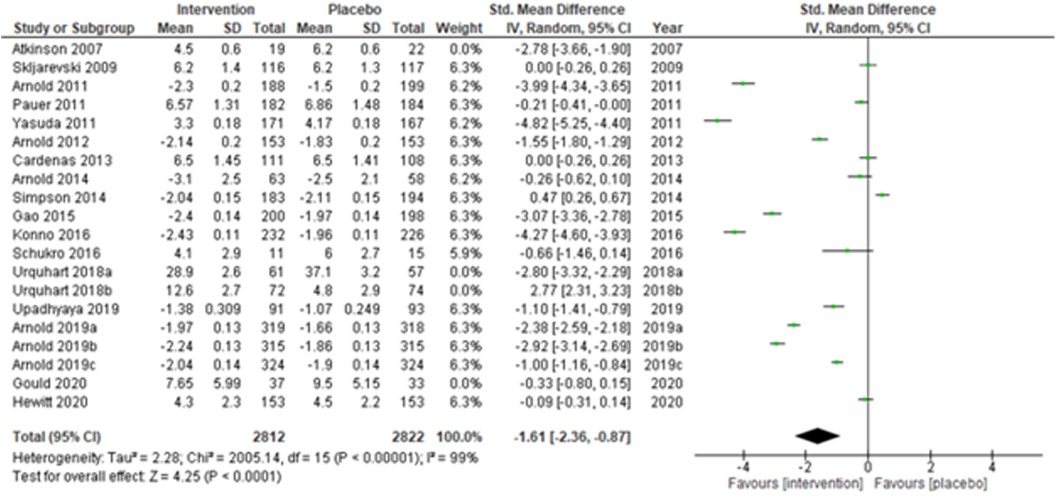
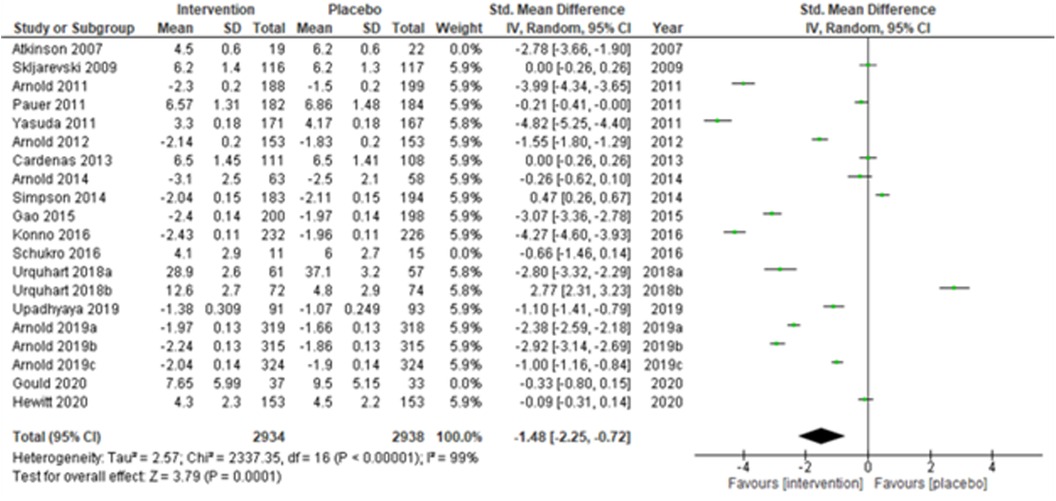
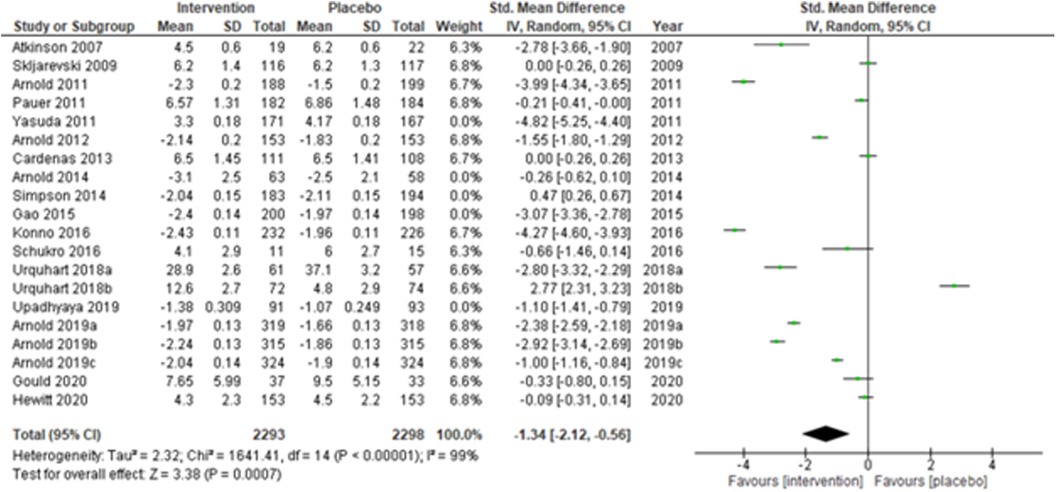
We performed two to three sensitivity analyses by excluding (a) studies with active placebo, such as benztropine (52, 53) (Atkinson et al., 2007; Urquhart et al., 2018a; and Urquhart et al., 2018b); (b) studies with sample sizes < 50 in each study arm (50, 53, 55) (Atkinson et al., 2007; and Schukro et al., 2016; Gould 2020), and (c) studies with a high risk of bias (30, 34, 36, 43, 44) (Yasuda et al., 2011; Arnold et al., 2014; Simpson et al., 2014; Gao et al., 2015; and Upadhyaya et al., 2019). On excluding the effects of active placebo (Figure 2), the intervention effects (SMD (95% CI) −1.61 (−2.36, −0.87)) remained significant (z = 4.25, p-value < 0.001). Likewise, on excluding the effects of small sample-sized trials (Figure 3), the intervention effects (SMD (95% CI) −1.48 (−2.25, −0.72)) remained statistically significant (z = 3.79, p-value < 0.001) as well. Furthermore, the intervention effects (SMD (95% CI) −1.34 (−2.12, −0.56)) also remained statistically significant (z = 3.38, p-value < 0.001) on excluding the effects of high-risk biased studies (Figure 4). The impact of heterogeneity (I2 = 99%) did not change in any of the sensitivity analyses.
4.3 Sub-group analyses
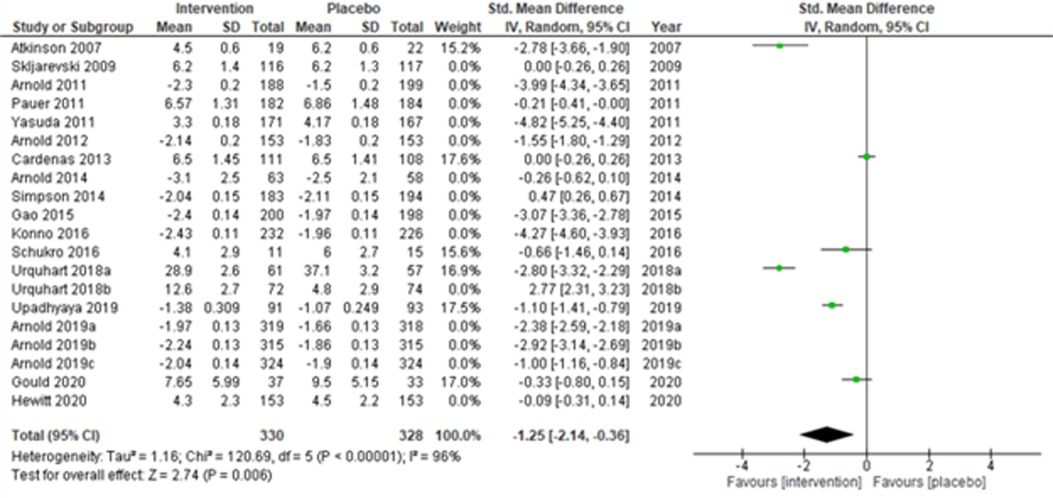
Fourteen multi-centered studies (28, 30, 31, 33, 36, 43, 44, 48, 51, 52, 56) (Skljarevski et al., 2009; Arnold et al., 2011; Pauer et al., 2011; Yasuda et al., 2011; Arnold et al., 2012; Arnold et al., 2014; Simpson et al., 2014; Gao et al., 2015; Konno et al., 2016; Urquhart et al., 2018b; Arnold et al., 2019a; Arnold et al., 2019b; Arnold et al., 2019c; and Hewitt et al., 2020) showed significant intervention effects (SMD (95% CI) −1.52 (−2.40, −0.64); z = 3.40; p-value < 0.001; I2 = 99%) when compared with the placebo group (Figure 5). Similarly, the remaining six single-centered studies (34, 38, 50, 52, 53, 56) (Atkinson et al., 2007; Cardenas et al., 2013; Schukro et al., 2016; Urquhart et al., 2018a; Upadhyaya et al., 2019; and Hewitt et al., 2020) also showed significant intervention effects (SMD (95% CI) −1.25 (−2.14, −0.36); z = 2.74; p-value = 0.006; I2 = 96%) (Figure 6).
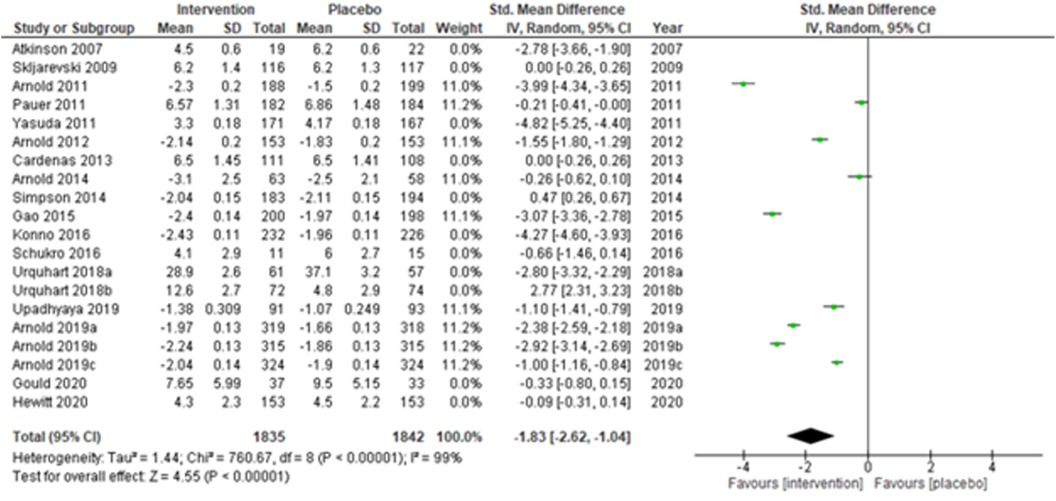
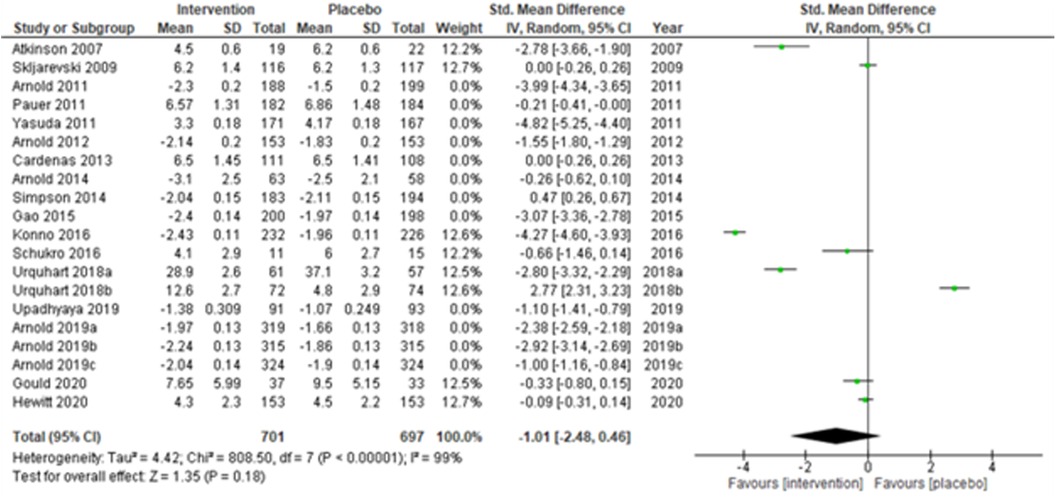
Studies were also grouped according to the type of chronic pain, such as neuropathic pain (Figure 7), fibromyalgia (Figure 8), and chronic low back pain (Figure 9). Fibromyalgia studies (28, 30–33, 44) (Arnold et al., 2011; Pauer et al., 2011; Arnold et al., 2012; Arnold et al., 2014; Gao et al., 2015; Arnold et al., 2019a; Arnold et al., 2019b; and Arnold et al., 2019c) showed significant intervention effects (SMD (95% CI) −1.83 (−2.62, −1.04); z = 4.55; p-value < 0.001; I2 = 99%) when compared with placebo. Interestingly, intervention effects were found to be statistically insignificant in studies with neuropathic pain and chronic low back pain.
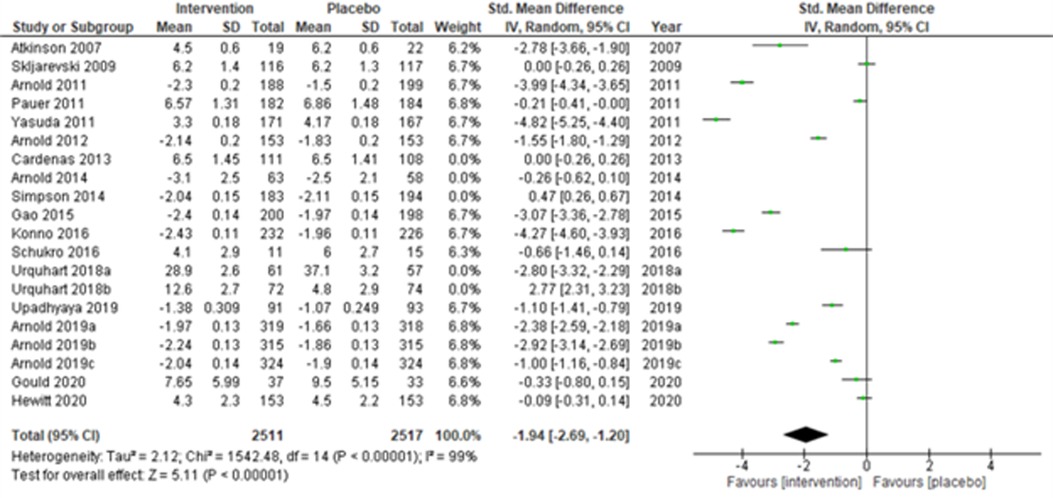
Lastly, when the studies were grouped according to short-term (≤14 weeks) and long-term (>14 weeks) study duration, the present meta-analyses showed that the intervention effects in the short-term studies (28, 31–34, 43, 44, 48, 50, 51, 53, 55, 56) (Atkinson et al., 2007; Skljarevski et al., 2009; Arnold et al., 2011; Pauer et al., 2011; Yasuda et al., 2011; Arnold et al., 2012; Gao et al., 2015; Konno et al., 2016; Schukro et al., 2016; Upadhyaya et al., 2019; Arnold et al., 2019a; Arnold et al., 2019b; Arnold et al., 2019c; Gould et al., 2020; and Hewitt et al., 2020) were more statistically significant (SMD (95% CI) −1.94 (−2.69, −1.20); z = 5.11; p-value < 0.001; I2 = 99%) (Figure 10) than the long-term studies (Figure 11).
4.4 Publication bias
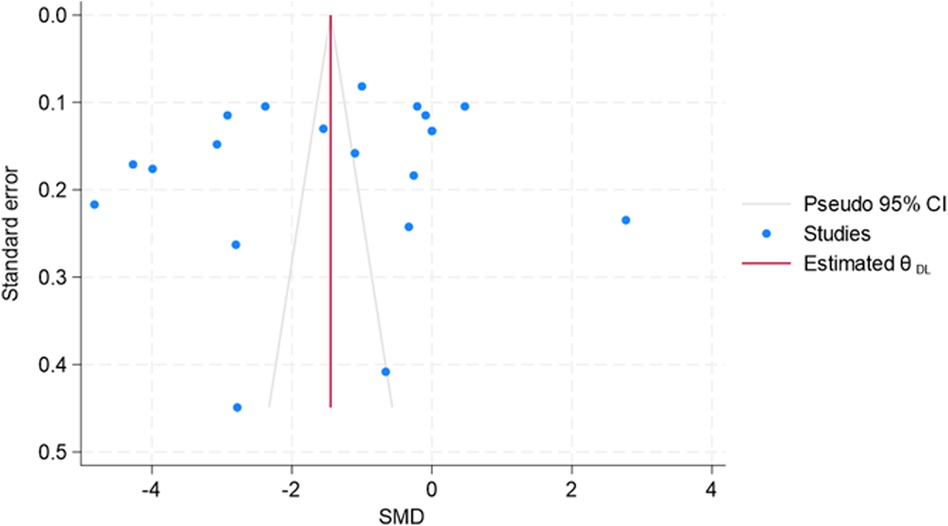
Since small-study effects are associated with publication bias, a funnel plot was used to explore the presence of small-study effects (Figure 12). Here, the study-specific effect sizes (SMD) are plotted on the x-axis and their standard errors (S.E.s) on the y-axis. The estimated was the summary (or pooled) treatment effect. The diagonal lines are referred to as the “pseudo 95% confidence interval” of , which was calculated for each S.E.s and indicated the expected distribution of studies without selection bias. However, Egger's test results suggest no small-study effects (p = 0.442). However, due to considerable heterogeneity across the studies or outliers, the decision related to publication bias cannot be substantiated.
4.5 Risk of bias assessments
The findings derived from the Cochrane Risk of Bias assessment tool were reported as low risk, high risk, and unclear risk (Figure 13). The majority of the studies adopted random sequence generation techniques and allocation concealment. However, most studies failed to use appropriate statistical models to accommodate missingness in the study outcomes. The risk of bias assessment indicated that most studies carried a low risk of selection, performance, and detection bias, as well as a high risk of attrition and reporting bias. Other biases were unknown.
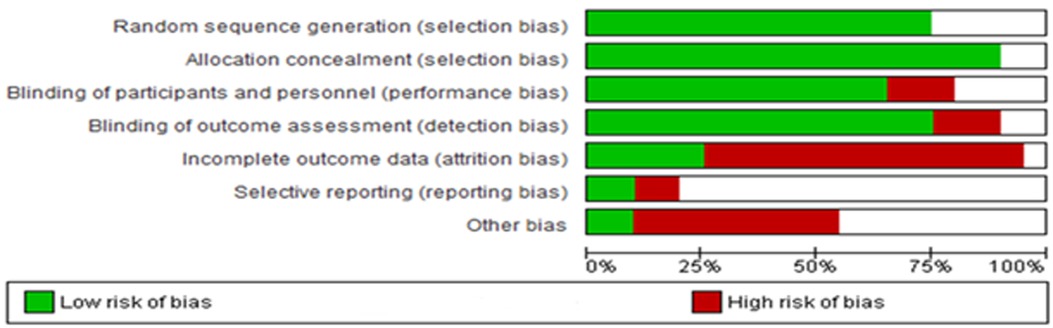
Figure 13. Risk of bias across all included studies. The white area suggests an unclear risk of bias.
We adopted an intention-to-treat analysis, wherein the total sample size was randomized for the intervention, and the placebo groups at the beginning of the study were considered for the present meta-analysis instead of completers. Thus, our findings minimized the effect of attrition bias.
5 Discussion
In our systematic review and meta-analysis, which included 20 eligible studies, we categorized these studies into three groups based on the type of chronic pain they addressed: neuropathic pain (3 studies), fibromyalgia (9 studies), and chronic low back pain (8 studies). We conducted sub-group analyses for these specific categories. Our review of 29 RCTs and meta-analysis of 20 RCTs demonstrated a significantly greater intervention effect than placebo in the fibromyalgia subgroup. However, in studies focusing on neuropathic pain and chronic low back pain, the intervention effects were statistically insignificant. This suggests that while certain psychiatric medications, such as duloxetine and mirogabalin, reduced pain in the short term (≤14 weeks), particularly for fibromyalgia, their specific effectiveness was not established for neuropathic pain and chronic low back pain. The distinction between short-term and long-term study outcomes is important for understanding the sustained impact of psychiatric medications on chronic pain. Our findings indicate that while these medications are effective in the short term, there is a need for further research into their long-term benefits and risks. This could involve studying how factors such as dosage adjustments, patient adherence, and the development of tolerance affect long-term outcomes. By addressing these factors, future guidelines could be developed to optimize the use of psychiatric medications for chronic pain management over extended periods. The long-term (>14 weeks) effects were generally insignificant. Our observation of limited long-term benefits suggests that differences in study designs, patient populations, and types of chronic pain conditions may have influenced these results.
While psychiatric medications like antidepressants and anticonvulsants are often viewed as safer alternatives to opioids, these medications have some risks as well, especially when used over the long term. Patients might experience side effects such as weight gain, sexual dysfunction, or cognitive issues. One of the long-term side effects is also the development of metabolic problems. Over time, some individuals may need higher doses to get the same pain relief, which can increase the chances of higher side effects. Additionally, stopping these medications after long-term use can sometimes cause withdrawal symptoms, which can make managing chronic pain even more challenging. Given these potential risks, it's essential for doctors to carefully consider the benefits and downsides when prescribing these medications for chronic pain. Regular check-ins, adjusting dosages as needed, and thinking about other treatment options can help minimize these side effects and ensure that patients maintain a good quality of life.
A critical factor in clinical practice is how well patients remain compliant with their prescribed psychiatric medications. Medication compliance plays a critical role in the effectiveness of these treatments, especially when managing chronic pain, where it is vital to take the medication regularly over an extended period to keep the pain controlled. In the studies we reviewed, adherence rates weren't always clearly reported, but differences in how well patients followed their treatment plans could have influenced the results. If patients are not compliant with their medication schedule, the actual effectiveness of psychiatric drugs might be underestimated since they are not getting the full benefit. On the flip side, when adherence is high, patients might see better results, but this could also lead to more side effects, which might cause some to drop out of long-term studies. Research should look at ways to boost adherence, like better patient education, simpler dosing schedules, and regular check-ins, to ensure patients get the most out of these treatments.
Several published studies investigated the use of non-opioid psychiatric medications for managing chronic pain. McDonagh et al. (2020) performed a comparative review to explore the effectiveness of non-opioid treatments for chronic pain across various durations (short-term 3 to <6 months, intermediate-term 6 to <12 months, and long-term ≥ 12 months) (20). McDonagh et al. (2020) reported minor improvements in pain and function with duloxetine for chronic low back pain, where our study did not find a statistically significant effect for this condition. The high heterogeneity in our study, particularly in the chronic low back pain subgroup, may explain this discrepancy. Both studies, however, emphasize the limited evidence for long-term efficacy of these medications. As McDonagh et al. highlighted the need for careful interpretation of non-opioid treatments for chronic pain (20), our findings similarly underscore the importance of further research, particularly in identifying specific subgroups that might benefit more from psychiatric medications.
Ferreira et al. (2021) conducted a systematic review and meta-analysis examining the efficacy and safety of antidepressants in treating back pain and osteoarthritis pain (24). The authors found that selective serotonin-norepinephrine reuptake inhibitors (SNRIs) such as duloxetine and milnacipran may offer short-term pain reduction for these conditions within 3–13 weeks. However, they noted that the effect on back pain was clinically insignificant, though a clinically important effect could not be excluded for osteoarthritis. Regarding sciatica pain, they observed a very low certainty of evidence for SNRIs reducing pain within the first two weeks, with no sustained effect beyond this period. For tricyclic antidepressants (TCAs), their study found that while TCAs were not effective for back pain, they showed some efficacy in reducing sciatica pain in the short and medium term (3–12 months). In contrast, our research specifically examined the efficacy of TCAs (Amitriptyline and Desipramine) for chronic low back pain and found no statistically significant effects. For Amitriptyline, the effect size was almost zero with an extensive confidence interval. Similarly, Desipramine's effect size also suggested no clear benefit for chronic low back pain, with a wide confidence interval that included the possibility of no effect. Although Ferreira et al. focused on back pain and osteoarthritis, Caruso et al. (2019) evaluated the role of antidepressants in treating psychiatric symptoms like depression and anxiety in patients with neuropathic pain (25). These conditions are often comorbid with chronic pain. The authors found that antidepressants were more effective than placebo in reducing depressive symptoms (SMD = −0.11; NNT = 24), though their effect on anxiety was insignificant. Their study shows the improvement in quality of life, which suggests that managing psychiatric symptoms may play an important role in the overall treatment of neuropathic pain. This highlights the broader utility of antidepressants in chronic pain management, especially for patients who experience comorbid psychiatric conditions.
Regarding TCAs, Urquhart et al. 2008 (62) found no clear evidence of their effectiveness in chronic low-back pain management. However, In a later study, Urquhart et al. 2018 suggested that low-dose amitriptyline could reduce disability at three months, although there were no significant improvements in pain reduction or disability at six months (52). Schliessbach et al. (2018) evaluated the effect of the tricyclic antidepressant imipramine on chronic low-back pain and found no significant immediate analgesic effect (54). However, they noted that anti-nociceptive effects might depend on the CYP2D6 genotype, suggesting that metabolizer status should be considered in future studies with tricyclic antidepressants. These findings resonate with our observations, underscoring the complexity of treatment responses and the potential influence of genetic factors on the efficacy of TCAs in chronic low back pain. The systematic review and meta-analysis conducted by Finnerup et al. in 2015 investigated the efficacy of various pharmacotherapies for treating neuropathic pain, including medications from the gabapentinoids family (23). This study found moderate effectiveness for drugs like SNRIs such as duloxetine and venlafaxine (NNT 6.4, 95% CI of 5.2–8.4), pregabalin (NNT 7.7, 95% CI of 6.5–9.4), and gabapentin (NNT 6.3, 95% CI of 5.0–8.3). The study also noted that tricyclic antidepressants had lower NNTs compared to SNRIs and gabapentinoids, making them a potentially more effective first-line treatment for neuropathic pain (23). Additionally, some recent trials conducted in Japan and the U.S. with 40 and 60 mg of once-daily duloxetine further strengthen duloxetine as an effective agent for diabetic neuropathic pain (43–45).
Our study aimed to assess the therapeutic efficacy of gabapentinoids for treating fibromyalgia and various types of neuropathic pain. Our results provide new insights into the role of these drugs in managing chronic pain conditions. Our findings on pregabalin showed a statistically significant improvement in pain scores for fibromyalgia, which aligns with previous research on its use in chronic pain conditions. However, as noted in our meta-analysis, the effect size was modest, suggesting that while statistically significant. The clinical impact such finding may be limited, particularly when weighing potential side effects and patient preferences. Our study investigated the impact of Mirogabalin, and it emerges as a viable treatment option for fibromyalgia. The important effect size, represented by an SMD of −2.10, which suggests its clinical significance, potentially leading to a noticeable improvement in patients’ symptoms. Our research observed a small to moderate average effect size for pregabalin in treating neuropathic pain. However, the overall effect was not statistically significant. As for duloxetine, only one study was available for analysis, making a meta-analysis inapplicable. Thus, pregabalin may have limited clinical significance in treating neuropathic pain, while the effectiveness of duloxetine remains unclear.
The findings of this systematic review highlight a noticeable short-term benefit of certain psychiatric medications, particularly Duloxetine and Mirogabalin for the treatment of fibromyalgia. However, the lack of consistent evidence supporting the long-term efficacy of these drugs requires a cautious approach to their use in chronic pain management. The marked heterogeneity across studies warrants careful consideration of individual patient factors, including psychiatric comorbidities, while assessing the appropriateness of these treatments. It also highlights the need for future research to better understand the complexities of chronic pain and its treatment, with the goal of improving patient selection and optimizing treatment durations for better outcomes. While the current analysis does not endorse the widespread application of these medications across all forms of chronic pain, the substantial short-term efficacy observed in fibromyalgia patients offers a viable non-opioid alternative that may be particularly beneficial in contexts where opioid use is contraindicated or poses a significant risk.
6 Limitation
Our study has several limitations that should be considered. our systematic review only included randomized controlled trials (RCTs). While this decision enhances the quality of the included studies, it may lead to the exclusion of quality clinical trials that do not employ an RCT design, potentially introducing bias to our findings. This meta-analysis was conducted across different disease categories, such as fibromyalgia, neuropathic pain, and chronic back pain. This approach allowed us to assess the overall effectiveness of non-opioid psychiatric pharmacotherapies across different pain domains, which was the primary objective of our study. However, our analysis does not offer a detailed comparison of the effectiveness of specific medication types or dosages. Our study focused solely on psychoactive non-opioid pharmacotherapies, such as SNRIs and TCAs, and excluded non-psychoactive treatments like NSAIDs, lidocaine patches, or capsaicin. While these non-psychoactive options could provide valuable insights into pain management, they were not within the scope of our analysis.
7 Conclusion
This study evaluates the efficacy of various psychiatric medications in pain management. We found that duloxetine and mirogabalin were significantly effective in the short-term treatment of fibromyalgia. However, the effectiveness of these medications, as well as others such as amitriptyline, desipramine, and gabapentin, remains uncertain for mixed neuropathic conditions and chronic low back pain remains uncertain due to inconsistent results and limited data, as indicated by published literature. While some evidence supports the use of these medications in specific types of neuropathic pain, such as diabetic neuropathy or postherpetic neuralgia, our analysis did not find consistent long-term benefits for the treatment of chronic pain conditions. The lack of consistent long-term benefits for these treatments highlights the need for further research into both the short- and long-term effects of psychiatric medications in chronic pain management. Clinicians should interpret these findings with caution, considering the individual characteristics and clinical contexts of their patients. A comprehensive, interdisciplinary approach that integrates both pharmacological and non-pharmacological strategies remains vital in managing chronic pain effectively.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
ShA: Investigation, Writing – original draft, Writing – review & editing, Conceptualization. AB: Conceptualization, Data curation, Formal Analysis, Resources, Validation, Writing – review & editing, Investigation, Methodology. LJ: Writing – review & editing, Data curation, Formal Analysis, Investigation, Resources, Supervision, Writing – original draft, Conceptualization, Methodology. ShP: Writing – original draft, Data curation, Resources. SB: Writing – original draft, Writing – review & editing, Conceptualization, Data curation, Investigation. RA: Writing – original draft. JK: Writing – original draft, Writing – review & editing. SK: Writing – review & editing, Data curation. SaP: Data curation, Writing – review & editing. HK: Writing – original draft, Data curation. OA: Writing – original draft, Data curation. SM: Writing – original draft, Data curation. BG: Writing – review & editing, Data curation. SS: Writing – original draft. SaA: Data curation, Writing – original draft. BP: Writing – review & editing. PM: Writing – review & editing, Writing – original draft, Data curation, Formal Analysis, Investigation, Validation. SS: Writing – review & editing. MH: Writing – original draft, Writing – review & editing. DD: Resources, Validation, Writing – review & editing. SaeA: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Vowles KE, McEntee ML, Julnes PS, Frohe T, Ney JP, van der Goes DN. Rates of opioid misuse, abuse, and addiction in chronic pain: a systematic review and data synthesis. Pain. (2015) 156(4):569–76. doi: 10.1097/01.j.pain.0000460357.01998.f1
2. Muhuri PK, Gfroerer YC, Davies MC. Associations concerning Nonmedical Pain Reliefs Use and Initiation of Heroin Usage in the United Country).
3. Burke DS. Forecasting the opioid epidemic. Science. (2016) 354(6312):529. doi: 10.1126/science.aal2943
4. Chen Q, Larochelle MR, Weaver DT, Lietz AP, Mueller PP, Mercaldo S, et al. Prevention of prescription opioid misuse and projected overdose deaths in the United States. JAMA Netw Open. (2019) 2(2):e187621. doi: 10.1001/jamanetworkopen.2018.7621
5. Mattson CL, O'Donnell J, Kariisa M, Seth P, Scholl L, Gladden RM. Opportunities to prevent overdose deaths involving prescription and illicit opioids, 11 states, July 2016–June 2017. MMWR Morb Mortal Wkly Rep. (2018) 67(34):945–51. doi: 10.15585/mmwr.mm6734a2
6. O'Donnell J, Tanz LJ, Gladden RM, Davis NL, Bitting J. Trends in and characteristics of drug overdose deaths involving illicitly manufactured fentanyls - United States, 2019–2020. MMWR Morb Mortal Wkly Rep. 2021;70(50):1740–6. doi: 10.15585/mmwr.mm7050e3
7. Ballreich J, Mansour O, Hu E, Chingcuanco F, Pollack HA, Dowdy DW, et al. Modeling mitigation strategies to reduce opioid-related morbidity and mortality in the U.S. JAMA Netw Open. (2020) 3(11):e2023677. doi: 10.1001/jamanetworkopen.2020.23677
8. Stephenson J. CDC Warns of surge in drug overdose deaths during COVID-19. JAMA Health Forum. (2021) 2(1):e210001-e. doi: 10.1001/jamahealthforum.2021.0001
9. Appa A, Rodda LN, Cawley C, Zevin B, Coffin PO, Gandhi M, et al. Drug overdose deaths before and after shelter-in-place orders during the COVID-19 pandemic in San Francisco. JAMA Netw Open. (2021) 4(5):e2110452-e. doi: 10.1001/jamanetworkopen.2021.10452
10. Mann B. Overdose Deaths Surged In Pandemic, As More Drugs Were Laced With Fentanyl. NPR (2021). Available online at: https://www.delawarepublic.org/, https://www.delawarepublic.org/national-headlines/2021-04-22/overdose-deaths-surged-in-pandemic-as-more-drugs-were-laced-with-fentanyl (accessed October 10, 2023).
11. Koehl JL, Zimmerman DE, Bridgeman PJ. Medications for management of opioid use disorder. Am J Health Syst Pharm. (2019) 76(15):1097–103. doi: 10.1093/ajhp/zxz105
12. New AMA Substance Use & Pain Care Task Force urges action to help patients [press release]. AMA (2021). Available online at: https://www.ama-assn.org/ (Accessed February 12, 2023).
13. Swift A. Non-opioid analgesia is as effective as opioid management in acute pain and supports a change in prescribing practice to help address the ‘opioid epidemic’. Evid Based Nurs. (2018) 21(2):50. doi: 10.1136/eb-2018-102877
14. Chang AK, Bijur PE, Esses D, Barnaby DP, Baer J. Effect of a single dose of oral opioid and nonopioid analgesics on acute extremity pain in the emergency department: a randomized clinical trial. JAMA. (2017) 318(17):1661–7. doi: 10.1001/jama.2017.16190
15. Center for Substance Abuse T. SAMHSA/CSAT Treatment Improvement Protocols. Managing Chronic Pain in Adults With or in Recovery From Substance Use Disorders. Rockville, MD: Substance Abuse and Mental Health Services Administration (U.S.) (2012).
16. Fenske JN, Berland DW, Chandran S, Van Harrison R, Schneiderhan J, Hilliard PE, et al. Medicine M, editor. Pain Management. Ann Arbor, MI: University of Michigan; (2021). p. 9–11.
17. Ferraro MC, Bagg MK, Wewege MA, Cashin AG, Leake HB, Rizzo RRN, et al. Efficacy, acceptability, and safety of antidepressants for low back pain: a systematic review and meta-analysis. Syst Rev. (2021) 10(1):62. doi: 10.1186/s13643-021-01599-4
18. Chou R, Deyo R, Friedly J, Skelly A, Weimer M, Fu R, et al. Systemic pharmacologic therapies for low back pain: a systematic review for an American college of physicians clinical practice guideline. Ann Intern Med. (2017) 166(7):480–92. doi: 10.7326/M16-2458
19. Qaseem A, Wilt TJ, McLean RM, Forciea MA, Denberg TD, Barry MJ, et al. Noninvasive treatments for acute, subacute, and chronic low back pain: a clinical practice guideline from the American college of physicians. Ann Intern Med. (2017) 166(7):514–30. doi: 10.7326/M16-2367
20. McDonagh MS, Selph SS, Buckley DI, Holmes RS, Mauer K, Ramirez S, et al. AHRQ Comparative Effectiveness Reviews. Nonopioid Pharmacologic Treatments for Chronic Pain. Rockville, MD: Agency for Healthcare Research and Quality (U.S.) (2020).
21. Walitt B, Urrútia G, Nishishinya MB, Cantrell SE, Häuser W. Selective serotonin reuptake inhibitors for fibromyalgia syndrome. Cochrane Database Syst Rev. (2015) 2015(6):Cd011735. doi: 10.1002/14651858.CD011735
22. Wang L, Tobe J, Au E, Tran C, Jomy J, Oparin Y, et al. Selective serotonin reuptake inhibitors and serotonin-norepinephrine reuptake inhibitors as adjuncts for postoperative pain management: systematic review and meta-analysis of randomised controlled trials. Br J Anaesth. (2022) 128(1):118–34. doi: 10.1016/j.bja.2021.08.032
23. Finnerup NB, Attal N, Haroutounian S, McNicol E, Baron R, Dworkin RH, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. (2015) 14(2):162–73. doi: 10.1016/S1474-4422(14)70251-0
24. Ferreira GE, McLachlan AJ, Lin CC, Zadro JR, Abdel-Shaheed C, O'Keeffe M, et al. Efficacy and safety of antidepressants for the treatment of back pain and osteoarthritis: systematic review and meta-analysis. Br Med J. (2021) 372:m4825. doi: 10.1136/bmj.m4825
25. Caruso R, Ostuzzi G, Turrini G, Ballette F, Recla E, Dall'Olio R, et al. Beyond pain: can antidepressants improve depressive symptoms and quality of life in patients with neuropathic pain? A systematic review and meta-analysis. Pain. (2019) 160(10):2186–98. doi: 10.1097/j.pain.0000000000001622
26. Tort S, Urrútia G, Nishishinya MB, Walitt B. Monoamine oxidase inhibitors (MAOIs) for fibromyalgia syndrome. Cochrane Database Syst Rev. (2012) 4:Cd009807. doi: 10.1002/14651858.CD009807
27. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Br Med J. (2021) 372:n71. doi: 10.1136/bmj.n71
28. Pauer L, Winkelmann A, Arsenault P, Jespersen A, Whelan L, Atkinson G, et al. An international, randomized, double-blind, placebo-controlled, phase III trial of pregabalin monotherapy in treatment of patients with fibromyalgia. J Rheumatol. (2011) 38(12):2643–52. doi: 10.3899/jrheum.110569
29. Ohta H, Oka H, Usui C, Ohkura M, Suzuki M, Nishioka K. A randomized, double-blind, multicenter, placebo-controlled phase III trial to evaluate the efficacy and safety of pregabalin in Japanese patients with fibromyalgia. Arthritis Res Ther. (2012) 14(5):R217. doi: 10.1186/ar4056
30. Arnold LM, Arsenault P, Huffman C, Patrick JL, Messig M, Chew ML, et al. Once daily controlled-release pregabalin in the treatment of patients with fibromyalgia: a phase III, double-blind, randomized withdrawal, placebo-controlled study. Curr Med Res Opin. (2014) 30(10):2069–83. doi: 10.1185/03007995.2014.928275
31. Arnold LM, Whitaker S, Hsu C, Jacobs D, Merante D. Efficacy and safety of mirogabalin for the treatment of fibromyalgia: results from three 13-week randomized, double-blind, placebo- and active-controlled, parallel-group studies and a 52-week open-label extension study. Curr Med Res Opin. (2019) 35(10):1825–35. doi: 10.1080/03007995.2019.1629757
32. Arnold LM, Zhang S, Pangallo BA. Efficacy and safety of duloxetine 30 mg/d in patients with fibromyalgia: a randomized, double-blind, placebo-controlled study. Clin J Pain. (2012) 28(9):775–81. doi: 10.1097/AJP.0b013e3182510295
33. Arnold LM, Wang F, Ahl J, Gaynor PJ, Wohlreich MM. Improvement in multiple dimensions of fatigue in patients with fibromyalgia treated with duloxetine: secondary analysis of a randomized, placebo-controlled trial. Arthritis Res Ther. (2011) 13(3):R86. doi: 10.1186/ar3359
34. Upadhyaya HP, Arnold LM, Alaka K, Qiao M, Williams D, Mehta R. Efficacy and safety of duloxetine versus placebo in adolescents with juvenile fibromyalgia: results from a randomized controlled trial. Pediatr Rheumatol Online J. (2019) 17(1):27. doi: 10.1186/s12969-019-0325-6
35. Chappell AS, Littlejohn G, Kajdasz DK, Scheinberg M, D’Souza DN, Moldofsky H. A 1-year safety and efficacy study of duloxetine in patients with fibromyalgia. Clin J Pain. (2009) 25(5):365–75. doi: 10.1097/AJP.0b013e31819be587
36. Simpson DM, Rice AS, Emir B, Landen J, Semel D, Chew ML, et al. A randomized, double-blind, placebo-controlled trial and open-label extension study to evaluate the efficacy and safety of pregabalin in the treatment of neuropathic pain associated with human immunodeficiency virus neuropathy. Pain. (2014) 155(10):1943–54. doi: 10.1016/j.pain.2014.05.027
37. Kim JS, Bashford G, Murphy KT, Martin A, Dror V, Cheung R. Safety and efficacy of pregabalin in patients with central post-stroke pain. Pain. (2011) 152(5):1018–23. doi: 10.1016/j.pain.2010.12.023
38. Cardenas DD, Nieshoff EC, Suda K, Goto S, Sanin L, Kaneko T, et al. A randomized trial of pregabalin in patients with neuropathic pain due to spinal cord injury. Neurology. (2013) 80(6):533–9. doi: 10.1212/WNL.0b013e318281546b
39. Satoh J, Yagihashi S, Baba M, Suzuki M, Arakawa A, Yoshiyama T, et al. Efficacy and safety of pregabalin for treating neuropathic pain associated with diabetic peripheral neuropathy: a 14 week, randomized, double-blind, placebo-controlled trial. Diabet Med. (2011) 28(1):109–16. doi: 10.1111/j.1464-5491.2010.03152.x
40. Boyle J, Eriksson ME, Gribble L, Gouni R, Johnsen S, Coppini DV, et al. Randomized, placebo-controlled comparison of amitriptyline, duloxetine, and pregabalin in patients with chronic diabetic peripheral neuropathic pain: impact on pain, polysomnographic sleep, daytime functioning, and quality of life. Diabetes Care. (2012) 35(12):2451–8. doi: 10.2337/dc12-0656
41. Devi P, Madhu K, Ganapathy B, Sarma G, John L, Kulkarni C. Evaluation of efficacy and safety of gabapentin, duloxetine, and pregabalin in patients with painful diabetic peripheral neuropathy. Indian J Pharmacol. (2012) 44(1):51–6. doi: 10.4103/0253-7613.91867
42. Rauck R, Makumi CW, Schwartz S, Graff O, Meno-Tetang G, Bell CF, et al. A randomized, controlled trial of gabapentin enacarbil in subjects with neuropathic pain associated with diabetic peripheral neuropathy. Pain Pract. (2013) 13(6):485–96. doi: 10.1111/papr.12014
43. Yasuda H, Hotta N, Nakao K, Kasuga M, Kashiwagi A, Kawamori R. Superiority of duloxetine to placebo in improving diabetic neuropathic pain: results of a randomized controlled trial in Japan. J Diabetes Investig. (2011) 2(2):132–9. doi: 10.1111/j.2040-1124.2010.00073.x
44. Gao Y, Guo X, Han P, Li Q, Yang G, Qu S, et al. Treatment of patients with diabetic peripheral neuropathic pain in China: a double-blind randomised trial of duloxetine vs. Placebo. Int J Clin Pract. (2015) 69(9):957–66. doi: 10.1111/ijcp.12641
45. Yasuda H, Hotta N, Kasuga M, Kashiwagi A, Kawamori R, Yamada T, et al. Efficacy and safety of 40 mg or 60 mg duloxetine in Japanese adults with diabetic neuropathic pain: results from a randomized, 52-week, open-label study. J Diabetes Investig. (2016) 7(1):100–8. doi: 10.1111/jdi.12361
46. Kalita J, Kohat AK, Misra UK, Bhoi SK. An open labeled randomized controlled trial of pregabalin versus amitriptyline in chronic low backache. J Neurol Sci. (2014) 342(1-2):127–32. doi: 10.1016/j.jns.2014.05.002
47. Atkinson JH, Slater MA, Capparelli EV, Patel SM, Wolfson T, Gamst A, et al. A randomized controlled trial of gabapentin for chronic low back pain with and without a radiating component. Pain. (2016) 157(7):1499–507. doi: 10.1097/j.pain.0000000000000554
48. Skljarevski V, Ossanna M, Liu-Seifert H, Zhang Q, Chappell A, Iyengar S, et al. A double-blind, randomized trial of duloxetine versus placebo in the management of chronic low back pain. Eur J Neurol. (2009) 16(9):1041–8. doi: 10.1111/j.1468-1331.2009.02648.x
49. Williamson OD, Schroer M, Ruff DD, Ahl J, Margherita A, Sagman D, et al. Onset of response with duloxetine treatment in patients with osteoarthritis knee pain and chronic low back pain: a post hoc analysis of placebo-controlled trials. Clin Ther. (2014) 36(4):544–51. doi: 10.1016/j.clinthera.2014.02.009
50. Schukro RP, Oehmke MJ, Geroldinger A, Heinze G, Kress HG, Pramhas S. Efficacy of duloxetine in chronic low back pain with a neuropathic component: a randomized, double-blind, placebo-controlled crossover trial. Anesthesiology. (2016) 124(1):150–8. doi: 10.1097/ALN.0000000000000902
51. Konno S, Oda N, Ochiai T, Alev L. Randomized, double-blind, placebo-controlled phase III trial of duloxetine monotherapy in Japanese patients with chronic low back pain. Spine (Phila Pa 1976). (2016) 41(22):1709–17. doi: 10.1097/BRS.0000000000001707
52. Urquhart DM, Wluka AE, van Tulder M, Heritier S, Forbes A, Fong C, et al. Efficacy of low-dose amitriptyline for chronic low back pain: a randomized clinical trial. JAMA Intern Med. (2018) 178(11):1474–81. doi: 10.1001/jamainternmed.2018.4222
53. Atkinson JH, Slater MA, Capparelli EV, Wallace MS, Zisook S, Abramson I, et al. Efficacy of noradrenergic and serotonergic antidepressants in chronic back pain: a preliminary concentration-controlled trial. J Clin Psychopharmacol. (2007) 27(2):135–42. doi: 10.1097/jcp.0b013e3180333ed5
54. Schliessbach J, Siegenthaler A, Bütikofer L, Limacher A, Juni P, Vuilleumier PH, et al. Effect of single-dose imipramine on chronic low-back and experimental pain. A randomized controlled trial. PLoS One. (2018) 13(5):e0195776. doi: 10.1371/journal.pone.0195776
55. Gould HM, Atkinson JH, Chircop-Rollick T, D'Andrea J, Garfin S, Patel SM, et al. A randomized placebo-controlled trial of desipramine, cognitive behavioral therapy, and active placebo therapy for low back pain. Pain. (2020) 161(6):1341–9. doi: 10.1097/j.pain.0000000000001834
56. Hewitt CA, Vincent K, Middleton LJ, Romaniuk L, Koscielniak M, Doust AM, et al. Efficacy and Mechanism Evaluation. Gabapentin to Reduce Pain in Women Aged Between 18 and 50 Years with Chronic Pelvic Pain: The GaPP2 RCT. Southampton, UK: NIHR Journals Library (2020. Copyright © Queen’s Printer and Controller of HMSO 2020. This work was produced by Hewitt et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
57. Horne AW, Vincent K, Hewitt CA, Middleton LJ, Koscielniak M, Szubert W, et al. Gabapentin for chronic pelvic pain in women (GaPP2): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet. (2020) 396(10255):909–17. doi: 10.1016/S0140-6736(20)31693-7
58. Urquhart DM, Wluka AE, Sim MR, van Tulder M, Forbes A, Gibson SJ, et al. Is low-dose amitriptyline effective in the management of chronic low back pain? Study protocol for a randomised controlled trial. Trials. (2016) 17(1):514. doi: 10.1186/s13063-016-1637-1
59. Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. Rob 2: a revised tool for assessing risk of bias in randomised trials. Br Med J. (2019) 366:l4898. doi: 10.1136/bmj.l4898
60. Clauw DJ. Fibromyalgia: a clinical review. Jama. (2014) 311(15):1547–55. doi: 10.1001/jama.2014.3266
61. Häuser W, Ablin J, Fitzcharles MA, Littlejohn G, Luciano JV, Usui C, et al. Fibromyalgia. Nat Rev Dis Primers. (2015) 1:15022. doi: 10.1038/nrdp.2015.22
Keywords: chronic pain & fibromyalgia, non-opioid analgesics, pain management (MeSH), SNRI (serotonin-norepinephrine reuptake inhibitors), tricyclic antidepressant drug, gabapentinoids, back pain, psychiatric medication use
Citation: Ayub S, Bachu AK, Jain L, Parnia S, Bhivandkar S, Ahmed R, Kaur J, Karlapati S, Prasad S, Kochhar H, Ayisire OE, Mitra S, Ghosh B, Srinivas S, Ashraf S, Papudesi BN, Malo PK, Sheikh S, Hsu M, De Berardis D and Ahmed S (2024) Non-opioid psychiatric medications for chronic pain: systematic review and meta-analysis. Front. Pain Res. 5:1398442. doi: 10.3389/fpain.2024.1398442
Received: 12 March 2024; Accepted: 9 September 2024;
Published: 10 October 2024.
Edited by:
Bamidele Victor Owoyele, University of Ilorin, NigeriaReviewed by:
Filippo Drago, University of Catania, ItalyMohsin Raza, HCA Healthcare North Florida Division, United States
Copyright: © 2024 Ayub, Bachu, Jain, Parnia, Bhivandkar, Ahmed, Kaur, Karlapati, Prasad, Kochhar, Ayisire, Mitra, Ghosh, Srinivas, Ashraf, Papudesi, Malo, Sheikh, Hsu, De Berardis and Ahmed. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Domenico De Berardis, ZG9tZW5pY28uZGViZXJhcmRpc0Bhc2x0ZXJhbW8uaXQ=
†These authors have contributed equally to this work and share first authorship
 Shahana Ayub
Shahana Ayub Anil Krishna Bachu
Anil Krishna Bachu Lakshit Jain
Lakshit Jain Shanli Parnia4
Shanli Parnia4 Siddhi Bhivandkar
Siddhi Bhivandkar Rizwan Ahmed
Rizwan Ahmed Jasleen Kaur
Jasleen Kaur Surya Karlapati
Surya Karlapati Sakshi Prasad
Sakshi Prasad Bikona Ghosh
Bikona Ghosh Palash Kumar Malo
Palash Kumar Malo Shoib Sheikh
Shoib Sheikh Michael Hsu
Michael Hsu Domenico De Berardis
Domenico De Berardis Saeed Ahmed
Saeed Ahmed