
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CLINICAL TRIAL article
Front. Pain Res. , 11 July 2024
Sec. Clinical Trials, Methods, and Evidence Synthesis
Volume 5 - 2024 | https://doi.org/10.3389/fpain.2024.1376608
Objective: The World Health Organization (WHO) recommended addition of local anesthetic to reduce the intense pain of intramuscular injection of 50% Magnesium Sulphate (MgSO4) salt solution has been found to be ineffective. We tested whether giving the local anesthetic 5 min before the MgSO4 injection would reduce pain.
Methods: We conducted a prospective cross-over trial where each participant with pre-eclampsia or eclampsia received sequential and mixed injection methods in random sequence during sequential MgSO4 administrations. Pain and preference were assessed using descriptive words, a numeric pain scale and direct comparison between the two injection methods. Differences were measured using the Wilcoxon signed rank test, risk ratios with 95% confidence intervals and the Chi squared or Fisher's test. The administration techniques were refined based on an initial pilot of 8 participants.
Results: We enrolled 49 consented participants and analysed data from 41 post-pilot participants The sequential injection method had a non-significantly lower mean pain score than the mixed injection method (3.1 vs. 3.3, p = 0.44). Severe pain was reported for 3/41 vs. 9/41, p = 0.12. The sequential injection method was perceived to be more painful by 13 (37%) vs. 22 (63%) participants (p = 0.03). The sequential injection was preferred by 21(60%) vs. 14 participants (40%) (p = 0.1).
Conclusion: Our results consistently favoured the novel sequential injection method. The lack of statistical significance for most results is not surprising given the small sample size. Given the potential for clinically important benefits to women, a larger study to confirm these results is justified.
Clinical Trial Registration: https://pactr.samrc.ac.za/, Identifier (PACTR202201521544765).
Magnesium sulphate (MgSO4) is the international standard-of-care anticonvulsant for the management of eclampsia and preeclampsia (1, 2). MgSO4 can be administered by intravenous (IV) and intramuscular (IM) routes, the Zuspan (3) and Prichard (4) regimens respectively. In low and middle-income countries like Botswana, use of the Zuspan (IV) regimen is not common because infusion pumps are not available and continuous monitoring required for manually administered IV administration is not feasible. IM administration of MgSO4 is the preferred method given these safety limitations, however, it is given in a large volume (10 ml, 5 g of 50% MgSO4) of highly concentrated salt solution and is exceptionally painful (5). Severe and very severe pain with IM injection of MgSO4 has been reported by 55% of women, which results in lower compliance for IM MgSO4 administration in comparison to IV infusion (6).
The World Health Organisation (WHO) guidelines recommend addition of lignocaine to IM injections of MgSO4 (7), yet there are few data to support this practice. One randomized trial found no benefit with the addition of lignocaine to MgSO4 injection in reducing pain at the site of injection (mean pain score 5.23 vs. 5.26 for MGSO4 alone) (5). Other methods of reducing pain with IM injections which have been studied include lavender inhalations (8), manual acupressure (9) and cryotherapy (10).
This limited size, preliminary proof of concept study aimed to evaluate whether a novel method of giving local analgesia with 2% lignocaine five minutes prior to intramuscular injection of MgSO4 (sequential injection method) shows potential to reduce pain at the injection site compared to the current standard administration of mixed 2% lignocaine and MgSO4 (mixed injection method), and to assess participant's preference for the injection method.
This randomised crossover trial was conducted in Princess Marina Hospital, Gaborone, the largest referral hospital in Botswana, serving the southern part of the country.
Participants were recruited from the labour ward, antenatal clinic, antenatal ward and postnatal ward from 4th May 2022 till 23rd July 2022. The investigator was informed about potential study participants by colleagues when admitting patients with preeclampsia or eclampsia who were to receive or receiving MgSO4. The researcher assessed patients for eligibility. Eligibility criteria were: 18–50 years and, diagnosed with preeclampsia or eclampsia, receiving magnesium sulphate, conscious, willing and able to give consent and provide answers to questions about pain and preference. Eligible participants were informed about the study, and both the sequential injection and mixed injection methods and the pain rating scale were explained in their preferred language (either English or Setswana). All questions were answered by the investigator and written informed consent obtained from those willing to participate. After signing the consent form the participants were educated on the numeric pain scale of 0–10 using the participants preferred language (Setswana or English) and each given a numeric pain scale to circle the pain experienced immediately after the injection.
A sample size of 41 was chosen to detect a reduction in the primary outcome (severe pain) from 75% to 45% with 95% certainty and 80% power (Epi Info software).
Participant's demographic information, diagnosis and history of receiving MgSO4 was obtained and entered on the case record form. Computer generated randomization was used by a researcher not involved in the clinical work to prepare numbered sealed opaque envelopes indicating which method of injection the participant should receive first. The participant's name was entered on a numbered register and the participant opened the matching numbered sealed envelope.
The procedure for the sequential injection method was refined in a pilot group of the first 8 participants. Initially, for the sequential injection method, different size needles were used for the lignocaine injection and the MgSO4 injection and it was found to be difficult to ensure that the MgSO4 injection was placed at the precisely the same site as the lignocaine injection. The method was modified to use the same needle, left in position between the lignocaine and the MgSO4 injections.
For the sequential injection 1 ml of 2% lignocaine was administered in the upper outer quadrant of either the left or right buttock, using a 21 gauge needle and the needle was left in position. After 5 min, 10 ml of 50% MgSO4 (5 g MgSO4) was administered through the same needle. For the mixed injection method, 1 ml of 2% lignocaine was mixed with 10 ml of MgSO4 in one syringe and the administered as a single injection using a 21 gauge needle on the upper outer quadrant of the buttock. The primary investigator prepared and administered all injections to the participant. The participant then received the alternative injection method when due for the next MgSO4 injection (immediately for loading doses, or after 4 h for maintenance doses). This allowed participants to serve as their own controls. In Princess Marina Hospital MgSO4 is given as 14 g loading dose, 4 g in 200 ml normal saline IV and 10 g IM as 5 g to each buttock, followed by maintenance 5 g IM every 4 h for 24 h.
Participants were asked to rate the pain at the injection site by indicating the number correlating with the perceived pain on a numeric pain rating scale of 0–10 immediately after receiving the injections. Participants were also asked to choose the word best describing their pain: no pain, mild pain, moderate pain and severe pain. After both administrations, participants were asked to judge which was more painful, and which method of injection they would prefer to receive in the future if they were to receive IM MgSO4 again.
The study data was entered onto paper case record forms. The data was entered from the case record form into an Excel spreadsheet and checked for errors. Statistical analysis was conducted using Epi Info 7 and Stata version 17. Mean, standard deviation, minimum and maximum pain scores were computed and compared with the Wilcoxon matched–pairs signed–rank test with 95% confidence intervals. Proportions were compared using the Chi Square test or the Fisher's test for small numbers less than 5. P-value less than 0.05 was considered statistically significant.
Institutional review board (IRB) approval was obtained from the University of Botswana (reference UBR/RES/IRB/BIO/GRAD/160, 6 October 2021), Ministry of Health and Wellness Research Unit, and Princess Marina Hospital IRB Committee [reference PMH 2/2A(7)/153, 10 January 2022]. The study was registered with the Pan African Clinical Trials Registry (PACTR) on 13th March 2021, (CLINICAL TRIAL REG Number PACTR202201521544765) assessable on https://pactr.samrc.ac.za/TrialDisplay.aspx?TrialID=15746. The actual recruitment started on 4th May 2022, ended 23rd July 2022 and the clinical trial completion was 23rd July 2023 after the sample size was met.
During the study period from 2 May to 29 July 2022, a total of 125 patients were admitted to Princess Marina Hospital with a diagnosis of preeclampsia (118) and eclampsia (7). A total of 49 participants scheduled to receive MgSO4 gave written informed consent and were enrolled in the study (see CONSORT flow diagram Figure 1). The first 8 participants constituted the pilot group to refine the sequential method procedure, thus a total of 41 participants were included in the study.
The mean age of the study participants was 31 (range 18–43) years, the mean gravidity was 2.6 (1–5) and mean parity 1.4 (0–4). Most of the study participants (78.1%) had secondary education, 39 (95%) were diagnosed with preeclampsia, and 2 (5%) with eclampsia and 3 (7.32%) had a history of receiving magnesium sulphate in their previous pregnancy. Most of the study participants received the loading dose of MgSO4 28 (68.3%) and 13 (31.7%) received the maintenance doses of MgSO4, as shown in Table 1.
The pain scores are shown in Table 2. The mean pain score for the sequential injection method was 3.1 (0–9) and for the mixed injection method was 3.3 (0–10). The difference was not statistically significant (p = 0.44).
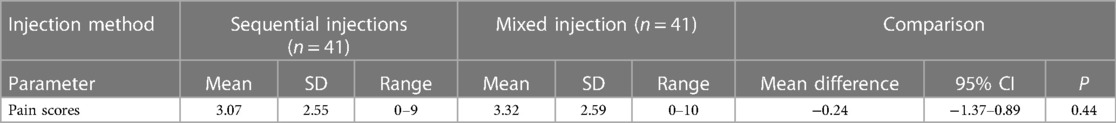
Table 2 Pain scores expressed as mean values and standard deviations (SD) and compared as mean difference with 95% confidence interval (CI) and p value (Wilcoxon matched–pairs signed–rank test).
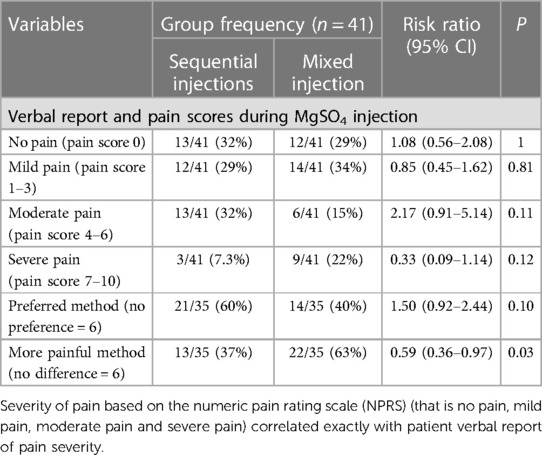
Table 3 Reported pain and preferences between the sequential and mixed injection methods, expressed as proportions (%) and compared as risk ratios with 95% confidence intervals (CI), and the Chi square test (Mantel Heinszel 2-tailed) or *Fisher's exact test.
Severe pain was reported by 3 (7%) for the sequential injection method compared with 9 (22%) for the mixed injection method (p = 0.12). Six participants (15%) indicated that the perceived pain for both injection methods were similar. Of 35 who noted a difference, 13 (37%) participants rated the sequential injection method more painful whereas 22 (63%) participants rated the mixed injection method to be the more painful method (RR 0.59, 95%CI 0.36–0.97; p = 0.03).
Six (15%) participants did not have a preference regarding the method for future injections. Of the 35 who had a preference, 21 (60%) preferred the sequential injection method, whereas 14 (40%) preferred the mixed injection method (RR 1.50, 95% CI 0.92–2.44; p = 0.10).
This preliminary randomised controlled crossover trial aimed to evaluate the potential of a novel method of administering local anaesthesia prior to IM MgSO4 injection to reduce pain at the injection site compared to the current standard mixed lignocaine and MgSO4 method, and the participants' preference for the injection method. The findings of the study consistently favour the novel sequential injection method though the differences for most outcomes were not statistically significant in the numbers studied.
Given the widespread use of IM MgSO4 and the severe pain associated with this injection, it is surprising how little robust research there is on IM MgSO4 pain reduction with lignocaine. A randomised trial by Swathi and colleagues found that addition of 1 ml of 2% lignocaine with 10 ml of MgSO4 does not reduce pain at the IM injection site (5). In the same study (5), the mean pain score within five minutes of injection were somewhat higher than in our study: 5.23 for MgSO4 mixed with lignocaine and 5.26 for MgSO4 alone. It is not surprising that there is little data supporting a combined injection. Lignocaine as a local anaesthetic blocks voltage-gated sodium channels leading to a reversible block of action potential propagation. Lignocaine binds preferably to the open or inactivated state of voltage-gated sodium channels and has a rapid onset of action of 3–5 min (11, 12). In the single injection method there is presumably pain with the initial administration of the large amount of MgSO4 because the lignocaine has not had time to take effect. In the sequential injection method, administration of lignocaine prior to MgSO4 injection would presumably ease the pain of the IM injection and have sustained anaesthetic effect. The findings of the current small exploratory study suggest the potential for clinically meaningful benefit from the novel method investigated and justify further larger trials to determine the effectiveness of this method with greater precision.
The importance of attention to pain experienced by pregnant women has been highlighted by the 2018 WHO guidelines for intrapartum care for a positive birth experience, supported by an explicit requirement to document attention to pain relief measures in the new WHO labour care guide (13).
The strengths of the study are the use of a randomized crossover design with participants acting as their own controls. The limitations of the study are the small sample size and the lack of blinding. While the study could have been blinded by using a placebo initial injection in the mixed injection group, it was felt that this would obscure a potential benefit of the mixed method (only one injection) and bias the responses against the mixed injection method. Leaving the needle in situ for the sequential injection method may have impacted the participants' anxiety level and perception of pain. Because of the small sample pilot study, it was not possible to assess whether the amount of time between the quick succession for the loading dose participants and having four hours between the maintenance dose injections for the maintenance dose participants impacted the perception of pain, but this could be evaluated in a larger study. Additionally a larger future study may also offer the opportunity to blind using a placebo injection first in the mixed injection group, stratify the results by those receiving immediate MGSO4 loading dose injection vs. maintenance dosing, using blinded adjudicators to administer pain questions and ensuring accurate pain reporting training.
To our knowledge this is the first study to investigate whether pre-emptive local anaesthetic injection has the potential to reduce the pain experienced by participants receiving intramuscular MgSO4, compared with mixing the local analgesic with the MgSO4. The results consistently favoured the sequential injection method. While most differences were not statistically significant in the numbers studied, the point estimate differences between groups were sufficiently large to justify a larger study to definitively determine the effectiveness of pre-emptive analgesia for IM MgSO4 with greater precision.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
The studies involving humans were approved by University of Botswana, Office of Research and Development Ministry of Health, Botswana, Health Research and Development Division Princess Marina Hospital, Institute Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
MJ: Investigation, Project administration, Writing – original draft, Writing – review & editing. RL: Formal Analysis, Methodology, Supervision, Writing – review & editing. GH: Conceptualization, Formal Analysis, Methodology, Supervision, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
This study would not have been possible without huge support and understanding from my colleagues, friends, and family. I would like to thank the study participants for their contribution to this research.
GJH has a conflict of interest with respect to the MaternaWell Tray for blood loss monitoring after birth and receives royalties from UpToDate.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpain.2024.1376608/full#supplementary-material
1. WHO. WHO Recommendations for Prevention and Treatment Preeclampsia and Eclampsia. Geneva: World Health Organization (2011). Available online at: https://www.who.int/publications/i/item/9789241548335 (accessed March 2, 2023)
2. American College of Obstetrician and Gynaecologist. Gestational hypertension and preeclampsia. ACOG Pract Bull. (2020) 135(6):246–7.
3. Tukur J. The use of magnesium sulphate for the treatment of severe preeclampsia and eclampsia. Ann Afr Med. (2009) 8(2):78. doi: 10.4103/1596-3519.56232
4. Gordon R, Magee LA, Payne B, Firoz T, Sawchuck D, Tu D, et al. Magnesium sulphate for the management of preeclampsia and eclampsia in low and middle income countries: a systemic review of tested dosing regimens. J Obstet Gynaecol Can. (2014) 36(2):153. doi: 10.1016/S1701-2163(15)30662-9
5. Swathi AR, Bellad M, Ganachari MS, Hema D, Hema P. Addition of lignocaine in intramuscular injection of magnesium sulfate: does it reduce pain in women with severe preeeclampsia and conscious eclamptic women-a randomised controlled trial. J South Asian Fed Obstet Gynecol. (2017) 9(4):391–4. doi: 10.5005/jp-journals-10006-1535
6. Uzma K, Anjali K. A comparative study of intramuscular sulfate versus intravenous magnesium injection among eclampsia patients in eastern Uttar Pradesh, India. Int J Reprod Contracept Obstet Gynecol. (2020) 9(6):2475. doi: 10.18203/2320-1770.ijrcog20202331
7. WHO Department of Reproductive Health and Research. Managing Complications in Pregnancy and Childbirth: A Guide to Midwives and Doctors. Geneva: WHO Press (2017). p. S-59.
8. Vaziri F, Taheri M, Tavana Z. Lavender inhalation on intramuscular injection pain of magnesium sulphate in Pre-eclamptic mothers: a randomized control trial. Womens Health Bull. (2018) 5(2):62448–9. doi: 10.5812/whb.62449
9. Najaf S, Nazaribin S, Yoosefinejad M. The effects of manual acupressure (point BL32) on pain associated with intramuscular injections of magnesium sulfate. J Acupunct Meridian Stud. (2019) 12(2):68–70. doi: 10.1016/j.jams.2018.07.002
10. Largo DG, Ligtas C, Oppus K, Palileo KM, Saurez J, Villegas E, et al. Cryotherapy as a comfort for magnesium sulphate administration. Asian J Health. (2013) 3:26–35. doi: 10.7828/ajoh.v3i1.408
11. Craig CR, Stitzel RE. Modern Pharmacology with Clinical Application. 5th ed. New York: Lippincott Williams & Wilkins (1997). p. 335.
12. Hermanns H, Hollmann MW, Stevens MF, Lirk P, Brandenburger T, Piegeler T, et al. Molecular mechanisms of action of systemic lidocaine in acute and chronic pain: a narrative review. Br J Anaesth. (2019) 123(3):335–49. doi: 10.1016/j.bja.2019.06.014
Keywords: intramuscular injection, lignocaine, local anaesthesia, magnesium sulphate, pain
Citation: Jamieson M, Luckett R and Hofmeyr G.J (2024) Novel use of local analgesia prior to intramuscular magnesium sulphate injection compared to mixed local analgesia with magnesium sulphate to reduce pain: a randomised crossover study in patients being managed for eclampsia and preeclampsia. Front. Pain Res. 5:1376608. doi: 10.3389/fpain.2024.1376608
Received: 5 March 2024; Accepted: 19 June 2024;
Published: 11 July 2024.
Edited by:
William K. Schmidt, NorthStar Consulting, LLC, United StatesReviewed by:
Amy Rachfal, Stage Gate Partners, LLC, United States© 2024 Jamieson, Luckett and Hofmeyr. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Modimowame Jamieson, amFtaWVzb253YW1lQGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.