
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pain Res. , 07 March 2024
Sec. Musculoskeletal Pain
Volume 5 - 2024 | https://doi.org/10.3389/fpain.2024.1362757
This article is part of the Research Topic Musculoskeletal Pain Phenotypes And Personalized Pain Medicine View all 5 articles
 Nidhi Sofat1,2*
Nidhi Sofat1,2* Andrew Lambarth1,2
Andrew Lambarth1,2
In the last few years there has been an increased appreciation that pain perception in rheumatic and musculoskeletal diseases (RMDs) has several mechanisms which include nociceptive, inflammatory, nociplastic and neuropathic components. Studies in specific patient groups have also demonstrated that the pain experienced by people with specific diagnoses can present with distinctive components over time. For example, the pain observed in rheumatoid arthritis has been widely accepted to be caused by the activation of nociceptors, potentiated by the release of inflammatory mediators, including prostaglandins, leukotrienes and cytokine networks in the joint environment. However, people with RA may also experience nociplastic and neuropathic pain components, particularly when treatments with disease modifying anti-rheumatic drugs (DMARDs) have been implemented and are insufficient to control pain symptoms. In other RMDs, the concept of pain sensitisation or nociplastic pain in driving ongoing pain symptoms e.g. osteoarthritis and fibromyalgia, is becoming increasingly recognised. In this review, we explore the hypothesis that pain has distinct modalities based on clinical, pathophysiological, imaging and genetic factors. The concept of pain stratification in RMD is explored and implications for future management are also discussed.
Chronic pain is a major health burden in the UK, estimated to affect almost one third to half the population at any time (1). Fayaz et al. (1) reported that up to 28 million UK adults are affected by chronic pain, with the most common conditions contributing to chronic pain including chronic widespread pain (CWP) (14.2%), chronic neuropathic pain (8.2%–8.9%) and fibromyalgia (5.4%). Factors influencing the development of chronic pain include age, sex, occupation, ethnicity, social background and psychological factors (2). The complex pathophysiology influencing chronic pain development is often termed as the biopsychosocial model of pain (3–5) (Figure 1). Some of the most common pain disorders include rheumatic and musculoskeletal diseases (RMD). In this review we will use RMD as an exemplar for discussing pain mechanisms. By gaining a deeper understanding of pain mechanisms, we might be able to improve diagnosis and treatments for people with chronic pain.
When considering the burden of conditions associated with chronic pain, RMD feature highly. The most common RMD contributing to the statistics of chronic pain include back pain, osteoarthritis (OA), fibromyalgia and autoimmune inflammatory disorders e.g., rheumatoid arthritis and psoriatic arthritis (6). Although the conditions described are considered to be their own entities with specific diagnostic criteria in many cases, symptoms of pain rate highly in all. Such observations have led to the consideration of what the underlying mechanisms of pain are in these conditions and have led researchers to consider if there may be distinct and also common shared mechanisms of pain in RMD. By gaining a deeper understanding of pain mechanisms in RMD, this would help to identify features of pain which may then be amenable to targeted therapies. We will focus on specific characteristics of pain, including inflammatory, nociceptive, nociplastic and neuropathic pain and explore how such descriptors are recognised in specific RMDs (Figure 2).
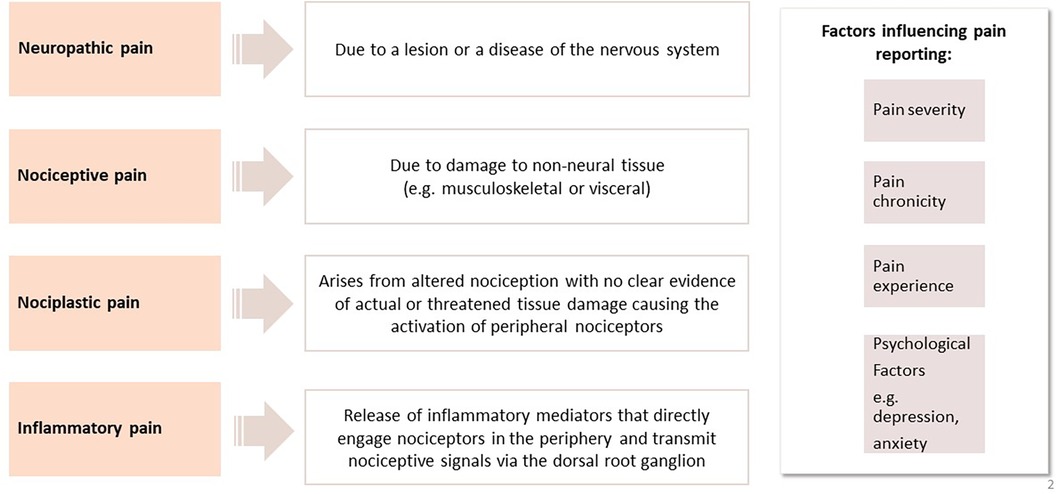
Figure 2. A summary of pain mechanisms in rheumatic and musculoskeletal diseases and factors influencing pain.
As a human species, we are designed to experience pain in response to noxious stimuli e.g., heat, cold, intense mechanical stimuli or chemical irritants (7). Nociceptive pain is an early warning system that has been maintained throughout evolution to protect us from noxious stimuli and as such is essential for maintaining the body's integrity. Our ability to respond to noxious painful stimuli is described as nociceptive pain. Sometimes analgesics may be used to reduce nociceptive pain e.g., post-surgery and can be very effective for short periods (8). However, it is important to be careful about not inhibiting nociceptive pain to such an extent that the protective role of nociceptive pain is lost (9).
Acute pain is often characterised by the release of inflammatory medicators, which leads to clinical features of redness, swelling, heat and ultimately pain (10). The underlying pathological processes driving acute inflammation include the release of inflammatory mediators, including cytokines, prostaglandins, leukotrienes and chemokines, coupled with an influx of inflammatory cells, including neutrophils, macrophages and mast cells (11). Release of inflammatory mediators can directly engage nociceptors to activate pain networks in the periphery e.g., a joint, with transmission of nociceptive stimulation to the neuronal cell bodies through activation of the dorsal root ganglion.
Cells and their released proteins work rapidly to resolve inflammation and the activation of nociceptors with it. Recent work has suggested that chronic pain may continue when inflammation e.g., mediated by neutrophils does not resolve rapidly, and may even persist after inflammation apparently resolves (12). In other people with no inflammatory stimulus, chronic pain may develop de novo and is described as pain that persists for more than 3 months (13). Many people with RMD may have persistent stimulation of inflammatory pathways that leads to ongoing activation of pain networks (14, 15). Genetic predisposition for the transition of acute pain to chronic pain e.g., back pain, has also become increasingly recognised as an important risk factor, and mechanistically may have inflammatory underpinnings (16).
In neuropathic pain, there is chronic activation of pain networks which results from abnormal functioning of the nervous system. This form of pain is considered maladaptive since it is not protective and can lead to significant burden of pain symptoms. Examples of neuropathic pain include conditions such as spinal cord injury, which lead to chronic pain activation (17). Low back pain and associated radiation into the legs, often termed sciatica, causing radicular pain is a neuropathic leg pain secondary to compressive lumbosacral nerve root pathology, which may be caused by degenerative changes and is a major cause of chronic pain worldwide.
The Terminology Task Force of the International Association for the Study of Pain (IASP) recently proposed a new term, called nociplastic pain (18). Nociplastic pain describes a category for pain in which the mechanisms are not fully understood. However, there is an appreciation that there is increased CNS pain and sensory processing with altered pain modulation. It has been proposed that this form of pain is mechanistically and clinically distinct from nociceptive and neuropathic pain (19). Often, in these conditions there is significant pain reported by the sufferer but no noxious stimulus can be identified. In addition, there is minimal or no local tissue inflammation at the site of pain. Conditions which have features of nociplastic pain include fibromyalgia, temporomandibular pain and back pain.
Pain sensitisation is a pain characteristic that is often observed in different categories of pain (20) and is divided into central and peripheral features (21). Central sensitisation is a term that describes overlapping and related pain mechanisms which are due to altered sensory processing in the brain (22). Central sensitisation has been described as “an amplification of neural signalling within the central nervous system that elicits pain hypersensitivity” (23). Peripheral sensitisation is described as heightened pain sensitivity in a peripheral nerve outside the brain (24). Central sensitisation is also typified by continual activation of central brain pain networks and lack of response of endogenous analgesia (25). Central sensitisation is a major mechanism of nociplastic pain. However, nociplastic pain covers a wider range of features in addition to central sensitisation and is often diagnosed clinically in the apparent absence of actual or threatened tissue damage. Central sensitisation is also an important mechanism of neuropathic pain and can be observed in subacute pain.
Pain perception is a complex measurable trait. A number of factors influence pain perception in RMD, including environmental exposures e.g., local injury, infection, smoking, increased body mass and multiple genetic factors (see discussion below). Several tools have been used in the clinic and in research studies to evaluate different forms of pain. These include a range of questionnaires to assess nociception and inflammatory pain. Self-reported pain intensity measures include the Visual Analogue Scale (VAS) (26) and the Numerical Rating Scale (NRS) (27). The impact of pain on physical function can be assessed using measures such as the Brief Pain Inventory BPI (28) and quality of life with measures such as Euroqol 5D (29). The impact of emotional factors e.g., anxiety and depression on pain can be measured using questionnaires such as the Hospital Anxiety and Depression Scale (HADS) (30). Neuropathic pain elements can be assessed using questionnaires (31) such as the Leeds Assessment of Neuropathic Symptoms and Signs (LANSS), Douleur Neuropathique en 4 questions (DN4), ID pain, Neuropathic Pain Scale (NPS), Brief Pain Inventory (BPI), painDETECT (32) and the Neuropathic Pain Questionnaire (NPQ) (33). In clinical practice, it would be more desirable to use questionnaires with a higher sensitivity and specificity (31). More recently, there has been interest in assessing nociplastic pain. Tools such as quantitative sensory testing (QST) have been used for evaluating central and peripheral pain sensitisation and are becoming more widely used but may be challenging clinically due to higher costs than using questionnaires (34). Neuroimaging tools e.g., functional magnetic resonance imaging (fMRI) (35) and spectroscopy (36) has also been deployed to evaluate brain regions involved in pain processing and to measure potential impacts of interventions e.g., drugs or behavioural changes (37). For back pain, the STarT Back screening tool has been validated to evaluate and stratify the likelihood of developing chronic pain after an acute episode and is used in clinical practice (38). The Oswestry Disability Index (39) and Roland Morris Disability Questionnaire (40) are also widely used to assess disability associated with back pain.
In the next section, we discuss how a range of assessment tools are being used to stratify pain in RMD.
The autoimmune inflammatory arthritides include disorders such as rheumatoid arthritis (RA), psoriatic arthritis (PsA) and axial spondyloarthritis (AxSpa). RA is common, affecting up to 1% of adult populations worldwide. Traditionally, pain mechanisms in RA, PsA and AxSpa have been shown to be mediated by nociceptive and inflammatory pathways (41). The local release of cytokines peripherally in the joint e.g., tumour necrosis factor alpha (TNF-α), interleukin-6 and other factors such as prostaglandins, leukotrienes and neuroptrophins such as Nerve Growth Factor (NGF), Substance P, engage with peripheral nociceptors in the joint and induce pain (42). Treatment of inflammatory arthritides has been transformed over the last few decades, with the introduction and use of both synthetic and biologic disease modifying anti-rheumatic drugs (DMARDs) which are targeted at suppression of pro-inflammatory pathways and activated cytokines (43). DMARD treatments for RA include targeted synthetic DMARDs e.g., methotrexate, sulfasalazine, hydroxychloroquine and in people where synthetic DMARDs are insufficient to control disease activity, biologic agents such as TNF-α inhibitors, rituximab, IL-6 receptor antagonists and Janus Kinase (JAK) Inhibitors may be prescribed. Although remission and treatment targets are met by many more patients than previously, there remains a high burden of disease in many patients, who have ongoing pain. People with inflammatory arthritides may well respond to analgesics e.g., NSAIDs which are prescribed to treat inflammatory pain (44). However, NSAIDs are usually prescribed at the lowest effective dose and for as short a duration as possible to minimise longer term side effects such as cardiovascular and renal morbidity (44). Despite control of inflammation, people with inflammatory arthritis may experience ongoing pain due to multiple factors, including pain sensitisation. Features of pain sensitisation can be evaluated using questionnaires, QST and neuroimaging (45, 46). A major question regarding pain sensitisation is whether neural networks can be “switched off” at some stage during treatment e.g., with early and tight control of inflammation, or whether development of pain sensitisation is an inevitable effect of the disease burden in certain individuals e.g., with risk factors such as anxiety, depression. It has been argued that there is some neuroplasticity in people with inflammatory conditions, which can be addressed using behavioural changes (47), pain management programmes (PMP) (37), antidepressants (48) or centrally-acting analgesics (49). Earlier recognition of pain sensitisation e.g., using QST, pain sensitisation questionnaires including LANSS, DN4, NPS, NPQ and painDETECT may be useful to recognise nociplastic pain and pain sensitisation early and consider methods to address pain management.
Osteoarthritis (OA) is the most common arthritis worldwide, with a prevalence of over 10 million in the UK alone (50). Pain is one of the most common symptoms of OA. Recent studies have interrogated the mechanisms of pain in OA (51, 52). Traditionally, OA has been described as a condition affecting cartilage, with denudation of the cartilage surface as the condition progresses, eventually leading to underlying bone changes including osteophytes, bone sclerosis and joint space narrowing (53). Since cartilage is avascular and aneural, other joint structures must be responsible for causing pain. Major structural components of the joint demonstrated as mediators of pain include synovium and underlying bone—in particular, bone marrow lesions (BMLs). How do these findings relate to clinical assessment of pain in OA? In recent years it has been recognised that, in addition to inflammatory pain, sensitisation is an important feature of pain in OA and is an important factor for pain chronicity (54). Recent work has suggested that addressing both inflammatory and nociplastic pain components in OA e.g., inflammatory pain (due to synovitis) with non-steroidal anti-inflammatory drugs (NSAIDs) and therapies to address nociplastic pain e.g., pain management programmes, exercise and physiotherapy and/or centrally acting analgesics are critical to optimal pain management in OA (55, 56).
There is currently a huge unmet need to address pain management in OA. Potential novel therapeutic targets have been identified e.g., monoclonal antibodies targeted against Nerve Growth Factor (NGF) (57, 58). However, in conditions such as OA, nociceptive pain may be protective. The trials of anti-NGF monoclonal antibodies demonstrated that there was a phenomenon of rapidly progressive OA identified in a subgroup of participants who were also taking NSAIDs (59). Results from this trial show that complete blockage of nociceptive pain in some people, which led to complete pain relief in the trial, can also accelerate joint destruction (60). Therefore, addressing pain management with disease-modifying interventions such as anti-NGF monoclonal biologic therapies can also be problematic in some settings.
Back pain is a complex condition with several clinical features including pain, stiffness and reduced range of movement. In RMD, back pain is mostly non-specific and mechanical in nature. For most people who experience back pain worldwide, a specific nociceptive cause is not identified (61). When assessing a patient, it is important to exclude specific causes of back pain e.g., cancer, infection, trauma or inflammatory disease such as spondyloarthritis. If non-specific back pain is confirmed, then guidelines recommend a multi-disciplinary approach, including weight loss, exercise, psychological treatments and regular monitoring of symptoms (62, 63). A large part of long-term care in back pain involves self-management, and several strategies to ensure good uptake of this are important e.g., adherence to a regular exercise programme (64, 65).
Specific causes of back pain are rarer and can include a wide spectrum of conditions that cause back pain, including inflammatory causes such as inflammatory axial spondyloarthritis (AxSpa), traumatic injury, infection, cancer and spinal degenerative disease (62). People with fibromyalgia may also experience chronic back pain as a prominent feature of their condition. Where an inflammatory cause is the underlying problem e.g in AxSpa, a complete assessment for inflammatory, neuropathic or nociceptive pain can be performed. Various treatment options can be considered for inflammatory back pain, including NSAIDs, and in cases of AxSpa where NSAIDs have been insufficient to control pain, a range of biologic therapies can be considered, including TNF-α inhibitors and JAK inhibitors (62).
Fibromyalgia has a high prevalence of 2%–4% in the general population, with a higher incidence in females (66). Fibromyalgia (FM) covers a range of symptoms, including widespread pain, sleep disturbance, poor concentration and memory. Studies of pain characteristics in FM have used a variety of tools to assess pain, including neuropathic pain questionnaires e.g., painDETECT and the neuropathic pain scale (67). Brain neuroimaging methods including functional brain neuroimaging have also demonstrated activation of central pain networks (66). There are genetic polymorphisms that have been reported more widely in FM, including the serotonin 5-HT2A receptor, serotonin transporter, dopamine 4 receptor and COMT polymorphisms (68–70). Currently it is not understood how specific genetic polymorphisms might be associated with specific pain endotypes and their response to potential therapeutic interventions is not understood. Considering the high burden of disease chronic pain places on populations worldwide, further work is urgently needed to identify the relation between genetic changes, their clinical endotypes and identification of selective therapeutics based on endotypes.
In RMD, patients may be in remission through suppression of inflammation, but chronic pain burden is high. Approaches to pain management are not optimised, may be considered late in an established diagnosis and access to care e.g., pain management programmes, is not universal.
The reasons for pain chronicity in RMD are multifactorial (71, 72). Chronic pain conditions can be associated with physical and psychological triggers. Both types of triggers can amplify pain and lead to ongoing psychological distress. Ongoing pain is a recognised issue observed in people treated for autoimmune conditions such as rheumatoid arthritis (71). Historically, physicians treating RMD may see evidence of good control of inflammatory markers such as Erythrocyte Sedimentation Rate (ESR) or C reactive protein (CRP). Suppression of inflammation in RMD assessed by ESR and CRP after treatment with disease-modifying anti-rheumatic drugs (DMARDs), including synthetic agents such as methotrexate and biologic agents including TNF-α modulators, IL-6 receptor antagonist and JAK-STAT pathway inhibitors e.g., baricitinib and tofacitinib (71) is a major objective of RMD treatments. In many autoimmune conditions such as rheumatoid arthritis, patients are provided with a management plan which aims to “treat-to-target”. For example, treatment to target in RA includes assessing tender and swollen joints, patient assessment of their disease control and inflammatory markers (ESR, CRP) and setting a treatment plan to control disease activity to a level using the parameters described for the Disease Activity Score (DAS28). Many patients treated with DMARDs nowadays achieve low levels of disease activity with low inflammatory markers (ESR, CRP), but may still report significant levels of pain (72). When inflammation is controlled but patients have ongoing pain, then fibromyalgia has been proposed as a potential mechanism for ongoing pain (14). Factors including ongoing activation of specific pro-inflammatory cytokines, depression and sleep disturbance can contribute to fibromyalgia in RA (14). Certain people may develop features of fibromyalgia early in the disease when chronic pain activation pathways may develop (73). There is increasing acceptance that fibromyalgia should be recognised and treated early in the context of existing RMD (74), which has recently been aided by a change in the classification criteria from a previous diagnosis of exclusion (75) to one that can exist in the presence of other painful conditions (76). Increased awareness and early monitoring by clinicians for ongoing pain is imperative so that patients can be offered early interventions (77).
There are some genetic polymorphisms which are linked with musculoskeletal pain, including several involved in serotonergic and adrenergic pathways (68–70). What is currently lacking is translation from genetic associations to the development of novel therapeutics and methods to assess pain, which could lead to more personalised treatment approaches for chronic pain in the future. Since there is now expanding evidence for the links between genetic risk factors for pain and specific pain disorders (see Table 1), researchers can aim to interrogate pathways which may have mechanisms with druggable therapeutic targets e.g., targeting COMT activity and/or elevated catecholamine levels can be treated with pharmacological agents that block both β2- and β3-adrenergic receptors (70). Although advances have been made in our understanding of the genetic risk factors and correlates for pain, further work is required to understand the clinical significance and therapeutic implications of research findings.
Many RMD have genetic risks with common and rare genetic variants which can contribute to specific endotypes. However, the causes of pain may differ between and within RMDs. Gaining a better understanding of how genetics influence musculoskeletal pain may yield new therapeutic avenues through prediction, prevention, and personalization. Producing reliable genetic association results that give meaningful mechanistic insights depends on appropriate specification of a trait or phenotype of interest (79). In common with other complex traits such as psychiatric disorders, genome-wide association studies (GWAS) that seek to understand the genetic determinants of pain can be hampered by aetiological and phenotypic heterogeneity (80). One approach to this is to group individuals into pain phenotypes based on a presumed shared disease mechanism. As described above, an example of mechanistic grouping of pain is into nociceptive, neuropathic, and nociplastic pain, though in practice these commonly overlap (81, 82). Accurate identification of these pain mechanisms also requires deep pain phenotyping, which is feasible in smaller scale genetic association studies (83), but is often not available in large-scale datasets required for conducting sensitive GWAS for complex polygenic diseases. A partial exception is neuropathic pain identified using questionnaires such as the Douleur Neuropathique 4 (84), though this does not characterise pain in as much depth as endotyping based on, for example, quantitative sensory testing (QST). In practice, the pain phenotype groupings used for genetics research are commonly based on anatomical site, duration, or primary pain syndrome. These may be more reliably captured using routinely collected health data or self-report; examples include chronic back pain (85), multi-site or widespread chronic pain (86–88), fibromyalgia (89), and sciatica (90). A potential limitation of site-based phenotypes is that they may be biased towards identifying variants associated with a primary pain-causing pathology (e.g., osteoarthritis) rather than pain itself (91).
Despite multifarious methodological challenges, a large number of variants have been identified which may be implicated in the development of musculoskeletal pain (Table 1). The weight of evidence to date would seem to suggest that genetics influence pain predominantly through the CNS (92–94). This may be particularly the case for chronic pain, with some recent work suggesting genetic variants associated with chronic, but not acute, back pain are brain-specific (93). The same study also found that acute back pain is less heritable than chronic pain. Similarly, pain sensitisation driven by active inflammation at a knee affected by OA may have different genetic drivers to distal sensitisation (83). These findings together can be seen to corroborate the idea that acute pain is almost exclusively nociceptive (95), secondary to actual or potential tissue damage caused by environmental factors rather than under direct genetic influence. However, the way acute pain is experienced may be modulated by pain sensitivity, the genetic underpinnings of which have been studied using subjective and objective measures of sensitivity and susceptibility (94). CNS-specific variants that have been implicated in pain include those that affect serotonergic, adrenergic, and glutamatergic pathways (96, 97).
Some more recent work uses a novel approach to move beyond grouping individuals based on a site-based phenotype (80). The approach uses principal component analysis to abstract a series of “genetically independent phenotypes” from GWAS summary statistics from different site-based chronic musculoskeletal pain phenotypes. This allows pooling of individuals reporting pain at different sites, and ostensibly observation of shared genetic determinants for the general tendency to chronic musculoskeletal pain. This work identified several variants in individuals of white European ancestry that are also reported by GWAS of chronic multisite pain (88), and further validated several of these variants in cohorts of other ethnicities. Of note, in common with other site-specific musculoskeletal pain studies, this still identifies loci that have known associations with osteoarthritis. Different approaches for looking into shared genetic associations across different pain phenotypes are also under exploration (98). While not without limitations, approaches such as these may complement research which uses deep, mechanism-based pain phenotyping within smaller cohorts, and large-scale studies using phenotypes identified with more typical methods.
There are several pharmacogenetic variants which may influence response to—or harm caused by—certain analgesic medicines. These include metabolic enzymes (e.g., CYP2D6, COMT), efflux pumps (e.g., ABCB1), and receptors (e.g., OPRM1) (99). While CYP2D6 is already considered to have actionable variants with implications for the selection of opioid type and dose, with further advancements and reduced costs of pharmacogenetic testing, more genes and variants may prove actionable in the future, providing further guidance for personalizing and optimizing medical pain management (100).
A pharmacological approach to chronic pain management can be formulated after a full clinical assessment based on patient needs. Assessing the individual patient's needs with a full history and clinical assessment, aided by relevant questionnaires, can be used to evaluate if pharmacological intervention is warranted based on a holistic assessment (Figure 3).
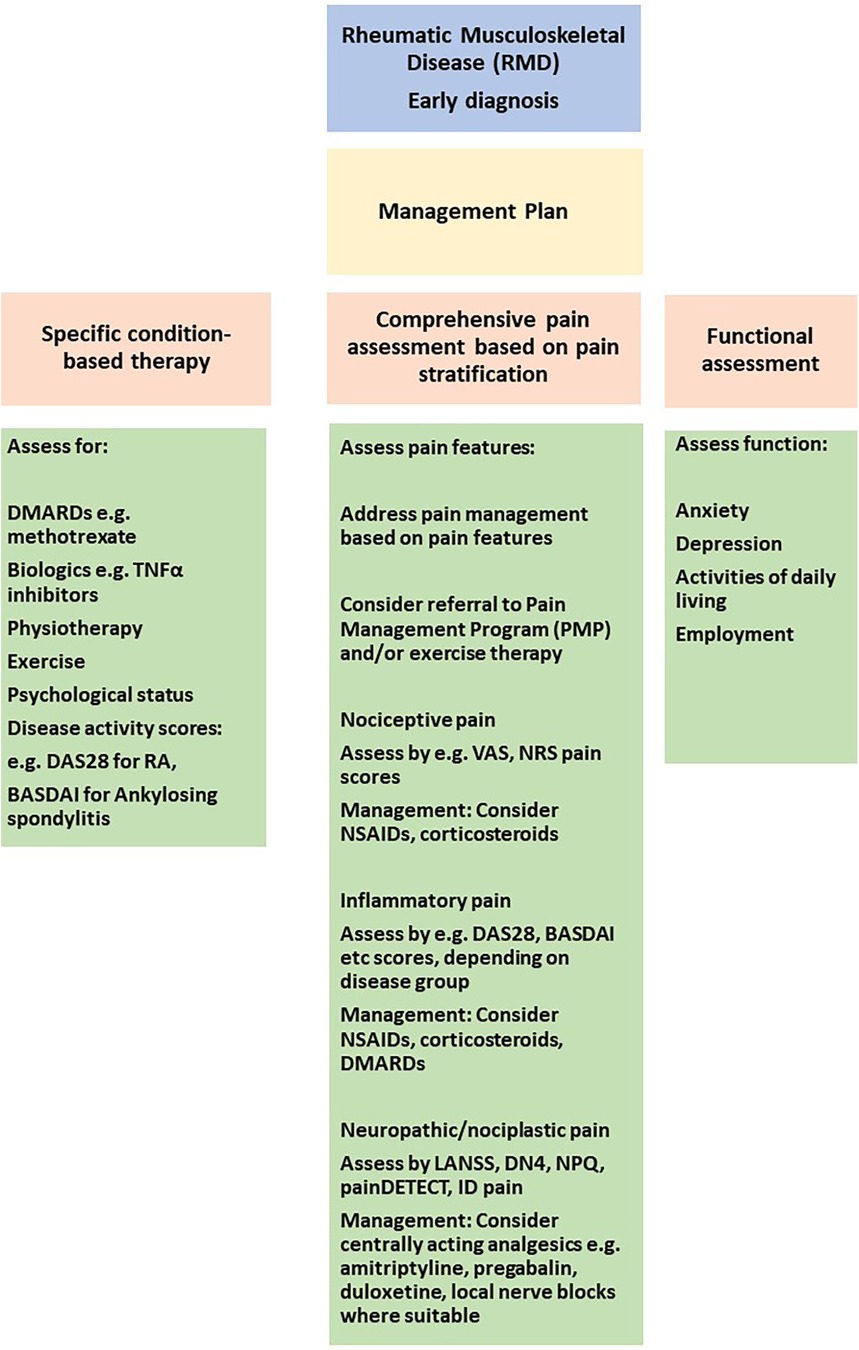
Figure 3. Approaches to pain stratification and management in the clinic. VAS, visual analogue scale; NRS, numerical rating scale; DAS28, disease activity score 28 for rheumatoid arthritis; BASDAI, bath ankylosing spondylitis disease activity index; LANNS, leeds assessment of neuropathic symptoms and signs, DN4, douleur neuropathique; NPQ, neuropathic pain questionnaire; TNF, tumor necrosis factor alpha.
For fibromyalgia and CWP, current NICE (National Institute for Health and Care Excellence) guidelines recommend that a multidisciplinary approach is desirable, including advice about exercise, talking therapies with pharmacological approaches to nociplastic pain management including amitriptyline, citalopram, duloxetine, fluoxetine, paroxetine or sertraline (101). Although NICE guidelines do not recommend opioids, NSAIDs, paracetamol or gabapentinoids for chronic primary pain (101), EULAR (European Alliance of Associations for Rheumatology) guidelines have included tramadol and the antiepileptic pregabalin, although the level of evidence for efficacy was low (102).
For the management of lower back pain, current NICE guidelines (63) recommend a multi-disciplinary approach that includes exercise, weight loss and advice about self-management, including diet, activity and symptom monitoring. Psychological support can also be beneficial such as cognitive behavioural therapy (CBT) and pain management programmes (PMP). In specific cases, NSAIDs and weak opioids may be of benefit. NICE guidelines do not recommend gabapentinoids, anti-epileptics, corticosteroids or benzodiazepines for back pain (63).
For OA, NICE guidelines (103) and EULAR guidelines (104) include NSAIDs (topical followed by oral if necessary) and potentially corticosteroid injections for pain management of nociceptive and nociplastic pain components. Typical doses of NSAIDs include ibuprofen up to 1,200 mg daily in divided doses or naproxen 1,000 mg daily in divided doses (103). NSAIDs should be taken at the lowest dose which is efficacious for the patient for as short a duration as possible e.g., due to flare-ups of symptoms and a proton pump inhibitor can also be prescribed concomitantly with NSAIDs to minimise gastrointestinal side-effects. Opioids are not recommended in OA due to low evidence.
In autoimmune-mediated inflammatory disorders including RA and axial spondyloarthritis, NICE guidelines recommend NSAIDs may be used for pain management, particularly when DMARDs are insufficient to control symptoms (105, 106). If people with inflammatory RMD have clinical and biochemical control of inflammation, but still have ongoing pain symptoms consistent with fibromyalgia or CWP, then it is suggested that pain management can follow recommended guidelines for fibromyalgia and CWP (71, 102, 103).
Regarding future treatments currently in trials, cannabinoids in fibromyalgia and other RMD are being considered, several of which are currently in progress (107, 108). While there are dozens of phytocannabinoids which may have future therapeutic value, recent clinical trial evidence would seem to suggest that cannabidiol, the most studied non-psychoactive cannabinoid, does not confer additional pain relief in knee OA (109). Drugs targeted at inhibiting inflammatory pathways in OA are also being conducted, including pentosan polysufate, targeting the NFKB pathway (110). A novel combination therapy trial is currently underway using a combination of LNA043, which targets cartilage damage and canakinumab, which targets inflammation using the IL-1-receptor antagonist canakinumab in OA (111).
In this review we have summarised current understanding of pain mechanisms in RMD, approaches to achieve pain stratification by defining endotypes, genetic risk factors and response to specific pharmacological approaches. A deeper understanding of pain mechanisms and management could be used to consider pain management pathways in clinical practice. Front and foremost is to make a definitive diagnosis of the RMD. Following this, a firm diagnosis will trigger a discussion with the patient about therapeutic interventions, including pharmacological and non-pharmacological approaches. It is important at the first patient encounter to conduct a comprehensive clinical assessment, including a physical examination and pain evaluation as summarised in Figure 3. By combining clinical assessments with questionnaires assessing specific pain components i.e., nociceptive, inflammatory, neuropathic and nociplastic, the patient can be stratified for their pain perception. Figure 3 demonstrates how distinct questionnaires can be used to stratify for specific pain components.
In the initial stages of diagnosis, it is important to control inflammation e.g., with corticosteroids, DMARDs as appropriate. Assessment of response to DMARDs can be made by disease-specific scores e.g., DAS28, BASDAI (see Figure 3). Pain management is often with NSAIDs in the initial stages. Once inflammation is better controlled, the requirement for NSAIDs/corticosteroids should reduce as the effect of DMARD therapy sets in. More long-term for chronic pain management, potential pharmacological interventions can be considered depending on the mechanisms mediating pain e.g., NSAIDs for inflammatory pain, opioids for neuropathic pain or centrally-acting analgesics for nociplastic pain. Non-pharmacological interventions, in particular, early exercise and physiotherapy-based interventions can assist is maintaining function, muscle mass and reducing pain. The information collected should assist in developing a pain management plan which is personalised for each patient. It is only through individual patient pain assessments that a personalised and tailored approach can be applied for long-term management and achieving sustained disease control.
NS: Writing – review & editing, Writing – original draft, Data curation, Conceptualization. AL: Writing – review & editing, Funding acquisition, Data curation.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article.
AL is an NIHR funded academic clinical fellow, award number ACF-2022-16-002. NS is supported by the Rosetrees' Trust (Grant number M11-F3), the Wellcome Trust Institutional Support Fund (ISSF) (Grant number 204809/16/Z) and the National Institute for Health Research (NIHR) Clinical Research Network. The views expressed are those of the author(s) and not necessarily those of the funders.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Fayaz A, Croft P, Langford RM, Donaldson LJ, Jones GT. Prevalence of chronic pain in the UK: a systematic review and meta-analysis of population studies. BMJ Open. (2016) 6(6):e010364. doi: 10.1136/bmjopen-2015-010364
2. Price C. Putting pain on the agenda: the report of the First English Pain Summit. UK (2012). Available online at: https://www.britishpainsociety.org/static/uploads/resources/files/members_articles_putting_pain_agenda.pdf (accessed December 4, 2023).
3. Raja SN, Carr DB, Cohen M, Finnerup NB, Flor H, Gibson S, et al. The revised IASP definition of pain: concepts, challenges, and compromises. Pain. (2020) 161(9):1976. doi: 10.1097/j.pain.0000000000001939
4. Nicholas M, Vlaeyen JW, Rief W, Barke A, Aziz Q, Benoliel R, et al. The IASP classification of chronic pain for ICD-11: chronic primary pain. Pain. (2019) 160(1):28–37. doi: 10.1097/j.pain.0000000000001390
5. Gatchel RJ, Peng YB, Peters ML, Fuchs PN, Turk DC. The biopsychosocial approach to chronic pain: scientific advances and future directions. Psychol Bull. (2007) 133(4):581. doi: 10.1037/0033-2909.133.4.581
6. Borenstein DG, Hassett AL, Pisetsky D. Pain management in rheumatology research, training, and practice. Clin Exp Rheumatol. (2017) 35(Suppl 107(5)):2–7. PMID: 28967362
7. Woolf CJ. What is this thing called pain. J Clin Invest. (2010) 120(11):3742–4. doi: 10.1172/JCI45178
8. Burgess G, Williams D. The discovery and development of analgesics: new mechanisms, new modalities. J Clin Invest. (2010) 120(11):3753–9. doi: 10.1172/JCI43195
9. Clark JD. The pitfalls of profoundly effective analgesic therapies. Clin J Pain. (2008) 24(9):825–31. doi: 10.1097/AJP.0b013e3181773b7f
10. Heidland A, Klassen A, Rutkowski P, Bahner U. The contribution of rudolf virchow to the concept of inflammation: what is still of importance? J Nephrol. (2006) 19(Suppl 10):S102–9. PMID: 16874721
11. Meyer RA, Ringkamp M, Campbell JN, Raja SN. Peripheral mechanisms of cutaneous nociception. Wall and Melzack’s Textbook of Pain. Philadelphia: Elsevier (2008). p. 3–34.
12. Parisien M, Lima LV, Dagostino C, El-Hachem N, Drury GL, Grant AV, et al. Acute inflammatory response via neutrophil activation protects against the development of chronic pain. Sci Translational Med. (2022) 14:eabj9954. doi: 10.1126/scitranslmed.abj9954
13. Barke A, Korwisi B, Jakob R, Konstanjsek N, Rief W, Treede R-D. Classification of chronic pain for the international classification of diseases (ICD-11): results of the 2017 international world health organization field testing. Pain. (2022) 163(2):e310–8. doi: 10.1097/j.pain.0000000000002287
14. Schaible HG. Nociceptive neurons detect cytokines in arthritis. Arthritis Res Ther. (2014) 16(5):470. doi: 10.1186/s13075-014-0470-8
15. Zhang L, Berta T, Xu ZZ, Liu T, Park JY, Ji RR. TNF-alpha contributes to spinal cord synaptic plasticity and inflammatory pain: distinct role of TNF receptor subtypes 1 and 2. Pain. (2011) 152(2):419–27. doi: 10.1016/j.pain.2010.11.014
16. Diatchenko L, Parisien M, Jahangiri Esfahani S, Mogil JS. Omics approaches to discover pathophysiological pathways contributing to human pain. Pain. (2022) 163(Suppl 1):S69–78. doi: 10.1097/j.pain.0000000000002726
17. Martinez-Lavin M. Fibromyalgia in women: somatisation or stress-evoked, sex-dimorphic neuropathic pain? Clin Exp Rheumatol. (2021) 39(2):422–5. doi: 10.55563/clinexprheumatol/0c7d6v
18. WHO. International Classification of Diseases 11th revision: the global standard for diagnostic health information. (2019). Available online at: https://icd.who.int/en (accessed December 4, 2023).
19. Fitzcharles M-A, Cohen SP, Clauw DJ, Littlejohn G, Usui C, Häuser W. Nociplastic pain: towards an understanding of prevalent pain conditions. Lancet. (2021) 397:2098–110. doi: 10.1016/S0140-6736(21)00392-5
20. Nijs J, George SZ, Clauw DJ, Fernandez de-Las-Penas C, Kosek E, Ickmans K, et al. Central sensitisation in chronic pain conditions: latest discoveries and their potential for precision medicine. Lancet Rheumatol. (2021) 3(5):e383–92. doi: 10.1016/S2665-9913(21)00032-1
21. Nijs J, Van Houdenhove B, Oostendorp RA. Recognition of central sensitization in patients with musculoskeletal pain: application of pain neurophysiology in manual therapy practice. Man Ther. (2010) 15(2):135–41. doi: 10.1016/j.math.2009.12.001
22. Kosek E, Clauw D, Nijs J, Baron R, Gilron I, Harris RE, et al. Chronic nociplastic pain affecting the musculoskeletal system: clinical criteria and grading system. Pain. (2021) 162(11):2629–34. doi: 10.1097/j.pain.0000000000002324
23. Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain. (2011)152(3 Suppl):S2–15. doi: 10.1016/j.pain.2010.09.030
24. Graven-Nielsen T, Arendt-Nielsen L. Peripheral and central sensitization in musculoskeletal pain disorders: an experimental approach. Curr Rheumatol Rep. (2002) 4(4):313–21. doi: 10.1007/s11926-002-0040-y
25. Yarnitsky D. Conditioned pain modulation (the diffuse noxious inhibitory control-like effect): its relevance for acute and chronic pain states. Curr Opin Anaesthesiol. (2010) 23(5):611–5. doi: 10.1097/ACO.0b013e32833c348b
26. Dworkin RH, Turk DC, Farrar JT, Haythornthwaite JA, Jensen MP, Katz NP. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. (2005) 113:9–19. doi: 10.1016/j.pain.2004.09.012
27. Turk DC, Dworkin RH, McDermott MP, Bellamy N, Burke LB, Chandler JM, et al. Analyzing multiple endpoints in clinical trials of pain treatments: IMMPACT recommendations. Pain. (2008) 139(3):485–93. doi: 10.1016/j.pain.2008.06.025
28. Cleeland CS, Ryan KM. Pain assessment: global use of the brief pain inventory. Annals, Academy of Medicine, Singap. (1994) 23(2):129–38. PMID: 8080219
29. Feng YS, Kohlmann T, Janssen MF, Buchholz I. Psychometric properties of the EQ-5D-5l: a systematic review of the literature. Qual Life Res. (2021) 30(3):647–73. doi: 10.1007/s11136-020-02688-y
30. Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the hospital anxiety and depression scale: an updated literature review. J Psychosom Res. (2002) 52:69–77. doi: 10.1016/S0022-3999(01)00296-3
31. May S, Serpell M. Diagnosis and assessment of neuropathic pain. F1000 Med Rep. (2009) 1:76. doi: 10.3410/M1-76
32. Ahmed S, Magan T, Vargas M, Harrison A, Sofat N. Use of the painDETECT tool in rheumatoid arthritis suggests neuropathic and sensitization components in pain reporting, 2014. J Pain Res. (2014) 7:579–88. doi: 10.2147/JPR.S69011
33. Cruccu G, Truini A. Tools for assessing neuropathic pain. PLoS Med. (2009) 6(4):e1000045. doi: 10.1371/journal.pmed.1000045
34. Wajed J, Ejindu V, Heron C, Hermansson M, Kiely P, Sofat N. Quantitative sensory testing in painful hand osteoarthritis demonstrates features of peripheral sensitisation. Int J Rheumatol (2012) 2012:703138. doi: 10.1155/2012/703138
35. Taylor P. Pain in the joints and beyond; the challenge of rheumatoid arthritis. The Lancet Rheumatology. (2023) 5(6):e351–60. doi: 10.1016/S2665-9913(23)00094-2
36. Emmer BJ, van der Bijl AE, Huizinga TW, Breedveld FC, Steens SC, Th Bosma GP, et al. Brain involvement in rheumatoid arthritis: a magnetic resonance spectroscopy study. Arthritis Rheum. (2009) 60(11):3190–5. doi: 10.1002/art.24932
37. Carmichael O, Schwarz AJ, Chatham CH, Scott D, Turner JA, Upadhyay J, et al. The role of fMRI in drug development. Drug Discov Today. (2018) 23(2):333–48. doi: 10.1016/j.drudis.2017.11.012
38. Available online at: https://startback.hfac.keele.ac.uk/ (accessed December 4, 2023).
39. Fairbank JC, Pynsent PB. The oswestry disability index. Spine. (2000) 25(22):2940–53. doi: 10.1097/00007632-200011150-00017
40. Roland M, Fairbank J. The roland-morris disability questionnaire and the oswestry disability questionnaire. Spine. (2000) 25(24):3115–24. doi: 10.1097/00007632-200012150-00006
41. Zhang A, Lee YC. Mechanisms for joint pain in rheumatoid arthritis (RA): from cytokines to central sensitisation. Curr Osteoporosis Rep (2018) 16(5): 603–10 doi: 10.1007/s11914-018-0473-5
42. Barthel C, Yeremenko N, Jacobs R, Schmidt RE, Bernateck M, Zeidler H, et al. Nerve growth factor and receptor expression in rheumatoid arthritis and spondyloarthritis. Arthritis Res Ther. (2009) 11(3):R82. doi: 10.1186/ar2716
43. Hazlewood GS, Whittle SL, Kamso MM, Akl EA, Wells GA, Tugwell P, et al. Disease-modifying anti-rheumatic drugs for rheumatoid arthritis: a systematic review and network meta-analysis. Cochrane Database Syst Rev. (2020) 2020(3):CD013562. doi: 10.1002/14651858.CD013562
44. Marks JL, Colebatch AN, Buchbinder R, Edwards CJ. Pain management for rheumatoid arthritis and cardiovascular or renal comorbidity. Cochrane Database Syst Rev. (2011) 10:CD008952. doi: 10.1002/14651858.CD008952.pub2
45. Wartolowska K, Hough MG, Jenkinson M, Anderssin J, Wordsworth BP, Tracey I. Structural changes of the brain in rheumatoid arthritis. Arthritis Rheum. (2012) 64(2):371–9. doi: 10.1002/art.33326
46. Arendt-Nielsen L, Morlion B, Perrot S, Dickenson A, Kress HG, Wells C, et al. Assessment and manifestation of central sensitisation across different chronic pain conditions. Eur J Pain. (2018) 22(2):216–41. doi: 10.1002/ejp.1140
47. Blundell A, Sofat N. Which biologic therapies to treat rheumatoid arthritis and when? Eur Med J. (2021) 6(3):101–10. doi: 10.33590/emj/21-00062
48. Kidd BL, Urban LA. Mechanisms of inflammatory pain. Br J Anaesth. (2001) 87:3–11. doi: 10.1093/bja/87.1.3
49. Richards BL, Whittle SL, Buchbinder R. Antidepressants for pain management in rheumatoid arthritis. Cochrane Database Syst Rev. (2011) 11:CD008920. doi: 10.1002/14651858.CD008920.pub2
50. Available online at: https://versusarthritis.org/about-arthritis/data-and-statistics/the-state-of-musculoskeletal-health/ (accessed December 4, 2023).
51. Eitner A, Hofmann GO, Schaible HG. Mechanisms of osteoarthritic pain. Studies in humans and experimental models. Front Mol Neurosci. (2017) 10:349. doi: 10.3389/fnmol.2017.00349
52. Neogi T, Frey-Law L, Scholz J, Niu J, Arendt-Nielsen L, Woolf C, et al. Multicenter osteoarthritis (MOST) study. Sensitivity and sensitisation in relation to pain severity in knee osteoarthritis: trait or state? Ann Rheum Dis. (2015) 74(4):682–8. doi: 10.1136/annrheumdis-2013-204191
53. Sofat N, Ejindu V, Heron C, Harrison A, Koushesh S, Assi L, Kuttapitiya A, Whitley GS, Howe FA. Biomarkers in painful symptomatic knee OA demonstrate that MRI assessed joint damage and type II collagen degradation products are linked to disease progression. Front Neurosci (2019) 13:1016. doi: 10.3389/fnins.2019.01016
54. Clauw DJ, Hassett AL. The role of centralised pain in osteoarthritis. Clin Exp Rheumatol. (2017) 35(Suppl 107(5)):79–84. PMID: 28967359
55. Bailly F, Cantagrel A, Bertin P, Perrot S, Thomas T, Lansaman T, et al. Part of pain labelled neuropathic in rheumatic disease might be rather nociplastic. RMD Open. (2020) 6(2):e001326. doi: 10.1136/rmdopen-2020-001326
56. Hurley MV, Walsh NE, Mitchell HL, Pimm TJ, Patel A, Williamson E, et al. Clinical effectiveness of a rehabilitation program integrating exercise, self-management, and active coping strategies for chronic knee pain: a cluster randomized trial. Arthritis Rheum. (2007) 57(7):1211–9. doi: 10.1002/art.22995
57. Lane NE, Schnitzer TJ, Birbara CA, Mokhtarani M, Shelton DL, Smith MD, et al. Tanezumab for the treatment of pain from osteoarthritis of the knee. N Engl J Med. (2010 Oct 14) 363(16):1521–31. doi: 10.1056/NEJMoa0901510
58. Schnitzer TJ, Easton R, Pang S, Levinson DJ, Pixton G, Viktrup L, et al. Effect of tanezumab on joint pain, physical function, and patient global assessment of osteoarthritis among patients with osteoarthritis of the hip or knee: a randomized clinical trial. JAMA. (2019) 322(1):37–48. doi: 10.1001/jama.2019.8044
59. Schmelz M, Mantyh P, Malfait A-M, Farrar J, Yaksh T, Tive L, et al. Nerve growth factor antibody for the treatment of osteoarthritis pain and chronic low-back pain: mechanism of action in the context of efficacy and safety. Pain. (2019) 160(10):2210–20. doi: 10.1097/j.pain.0000000000001625
60. Hochberg MC. Serious joint-related adverse events in randomized controlled trials of anti-nerve growth factor monoclonal antibodies. Osteoarthritis Cartilage. (2015) 23(suppl 1):S18–21. doi: 10.1016/j.joca.2014.10.005
61. Hartvigsen J, Hancock MJ, Kongsted A, Louw Q, Ferreira ML, Genevay S, et al. Underwood M; lancet low back pain series working group. What low back pain is and why we need to pay attention. Lancet. (2018) 391(10137):2356–67. doi: 10.1016/S0140-6736(18)30480-X
62. Allegri M, Montella S, Salici F, Valente A, Marchesini M, Compagnone C, et al. Mechanisms of low back pain: a guide for diagnosis and therapy. F1000Res. (2016) 5:1530. doi: 10.12688/f1000research.8105.2
63. Low back pain and sciatica in over 16s: assessment and management NICE guideline Published: 30 November 2016 Last updated: 11 December 2020. Available online at: www.nice.org.uk/guidance/ng59
64. Sveaas SH, Bilberg A, Berg IJ, Provan SA, Rollefstad S, Semb AG, et al. High intensity exercise for 3 months reduces disease activity in axial spondyloarthritis (axSpA): a multicentre randomised trial of 100 patients. Br J Sports Med. (2020) 54:292–7. doi: 10.1136/bjsports-2018-099943
65. Cooney JK, Law RJ, Matschke V, Lemmey AB, Moore JP, Ahmad Y, et al. Benefits of exercise in rheumatoid arthritis. J Aging Res. (2011) 2011:681640. doi: 10.4061/2011/681640
66. Häuser W, Fitzcharles MA. Facts and myths pertaining to fibromyalgia. Dialogues Clin Neurosci. (2018) 20(1):53–62. doi: 10.31887/DCNS.2018.20.1/whauser
67. Cheng CW, Wong CS, Hui GK, Chung EK, Wong SH. Fibromyalgia: is it a neuropathic pain? Pain Manag. (2018) 8(5):377–88. doi: 10.2217/pmt-2018-0024
68. Buskila D. Genetics of chronic pain states. Best Pract Res Clin Rheumatol. (2007) 21:535–47. doi: 10.1016/j.berh.2007.02.011
69. Zubeieta JK, Smith YR, Bueller JA, Xu Y, Kilbourn MR, Jewett DM, et al. Regional mu opioid receptor regulation of sensory and affective dimensions of pain. Science. (2011) 293:311–5. doi: 10.1126/science.1060952
70. Nackley AG, Tan KS, Fecho K, Flood P, Diatchenko L, Maixner W, et al. Catechol-O-methyltransferase inhibition increases pain sensitivity through activation of both beta-2 and beta-3 adrenergic receptors. Pain. (2007) 128:199–208. doi: 10.1016/j.pain.2006.09.022
71. Sarzi-Puttini P, Zen M, Arru F, Giorgi V, Choy EA. Residual pain in rheumatoid arthritis: is it a real problem? Autoimmun Rev. (2023) 22(11):103423. doi: 10.1016/j.autrev.2023.103423
72. Ishida M, Kuroiwa Y, Yoshida E, Sato M, Krupa D, Henry N, et al. Residual symptoms and disease burden among patients with rheumatoid arthritis in remission or low disease activity: a systematic literature review. Modern Rheumatology. (2018) 28(5):789–79. doi: 10.1080/14397595.2017.1416940
73. Minhas D, Murphy A, Clauw DJ. Fibromyalgia and centralized pain in the rheumatoid arthritis patient. Curr Opin Rheumatol. (2023) 35(3):170–4. doi: 10.1097/BOR.0000000000000929
74. Nagy G, Roodenrijs NM, Welsing PM, Kedves M, Hamar A, van der Goes MC, et al. EULAR Definition of difficult-to-treat rheumatoid arthritis. Ann Rheum Dis. (2020) 80(1):31–5. doi: 10.1136/annrheumdis-2020-217344
75. Macfarlane GJ, Kronisch C, Dean LE, Atzeni F, Hauser W, Fluß E, et al. EULAR Revised recommendations for the management of fibromyalgia. Ann Rheum Dis. (2017) 76:318–28. doi: 10.1136/annrheumdis-2016-209724
76. Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Hauser W, Katz RL. 2016 Revisions to the 2010/2011 fibromyalgia diagnostic criteria. Semin Arthritis Rheum. (2016) 46(3): 319–29 doi: 10.1016/j.semarthrit.2016.08.012
77. Kwiatek R. Treatment of fibromyalgia. Aust Prescr. (2017) 40(5):179–83. doi: 10.18773/austprescr.2017.056
78. Li S, Brimmers A, Van Boekel RLM, Vissers KCP, Coenen MJH. A systematic review of genome-wide association studies for pain, nociception, neuropathy, and pain treatment responses. Pain. (2023) 164:1891–911. doi: 10.1097/j.pain.0000000000002910
79. Craddock N, Kendler K, Neale M, Nurnberger J, Purcell S, Rietschel M, et al. Dissecting the phenotype in genome-wide association studies of psychiatric illness. Br J Psychiatry. (2009) 195(97):97–9. doi: 10.1192/BJP.BP.108.063156
80. Tsepilov YA, Freidin MB, Shadrina AS, Sharapov SZ, Elgaeva EE, van Zundert J, et al. Analysis of genetically independent phenotypes identifies shared genetic factors associated with chronic musculoskeletal pain conditions. Commun Biol. (2020) 3:329. doi: 10.1038/S42003-020-1051-9
81. Kosek E, Cohen M, Baron R, Gebhart GF, Mico JA, Rice ASC, et al. Do we need a third mechanistic descriptor for chronic pain states? Pain. (2016) 157:1382–6. doi: 10.1097/j.pain.0000000000000507
82. Freynhagen R, Parada HA, Calderon-Ospina CA, Chen J, Rakhmawati Emril D, Fernández-Villacorta FJ, et al. Current understanding of the mixed pain concept: a brief narrative review. Curr Med Res Opin. (2019) 35:1011–8. doi: 10.1080/03007995.2018.1552042
83. Kouraki A, Doherty M, Fernandes GS, Zhang W, Walsh DA, Kelly A, et al. Different genes may be involved in distal and local sensitization: a genome-wide gene-based association study and meta-analysis. Eur J Pain. (2022) 26:740. doi: 10.1002/ejp.1902
84. Veluchamy A, Hébert HL, Van Zuydam NR, Pearson ER, Campbell A, Hayward C, et al. Association of genetic variant at chromosome 12q23.1 with neuropathic pain susceptibility. JAMA Netw Open. (2021) 4(12):e2136560. doi: 10.1001/JAMANETWORKOPEN.2021.36560
85. Suri P, Palmer MR, Tsepilov YA, Freidin MB, Boer CG, Yau MS, et al. Genome-wide meta-analysis of 158,000 individuals of European ancestry identifies three loci associated with chronic back pain. PLoS Genet. (2018) 14(9):e1007601. doi: 10.1371/JOURNAL.PGEN.1007601
86. Johnston KJA, Ward J, Ray PR, Adams MJ, McIntosh AM, Smith BH, et al. Sex-stratified genome-wide association study of multisite chronic pain in UK Biobank. PLoS Genet. (2021) 17(4):e1009428. doi: 10.1371/JOURNAL.PGEN.1009428
87. Johnston KJA, Adams MJ, Nicholl BI, Ward J, Strawbridge RJ, Ferguson A, et al. Genome-wide association study of multisite chronic pain in UK Biobank. PLoS Genet. (2019) 15(6):e1008164. doi: 10.1371/JOURNAL.PGEN.1008164
88. Peters MJ, Broer L, Willemen HLDM, Eiriksdottir G, Hocking LJ, Holliday KL, et al. Genome-wide association study meta-analysis of chronic widespread pain: evidence for involvement of the 5p15.2 region. Ann Rheum Dis. (2013) 72:427–36. doi: 10.1136/ANNRHEUMDIS-2012-201742
89. Docampo E, Escaramís G, Gratacòs M, Villatoro S, Puig A, Kogevinas M, et al. Genome-wide analysis of single nucleotide polymorphisms and copy number variants in fibromyalgia suggest a role for the central nervous system. Pain. (2014) 155:1102–9. doi: 10.1016/J.PAIN.2014.02.016
90. Lemmelä S, Solovieva S, Shiri R, Benner C, Heliövaara M, Kettunen J, et al. Genome-Wide meta-analysis of sciatica in Finnish population. PLoS One. (2016) 11(10):e0163877. doi: 10.1371/JOURNAL.PONE.0163877
91. Meng W, Adams MJ, Palmer CNA, Agee M, Alipanahi B, Bell RK, et al. Genome-wide association study of knee pain identifies associations with GDF5 and COL27A1 in UK biobank. Commun Biol. (2019) 2:321. doi: 10.1038/S42003-019-0568-2
92. Chen M, Li S, Zhu Z, Dai C, Hao X. Investigating the shared genetic architecture and causal relationship between pain and neuropsychiatric disorders. Hum Genet. (2023) 142:431–43. doi: 10.1007/S00439-022-02507-Z
93. Bortsov A V, Parisien M, Khoury S, Martinsen AE, Lie MU, Heuch I, et al. Brain-specific genes contribute to chronic but not to acute back pain. Pain Rep. (2022) 7:E1018. doi: 10.1097/PR9.0000000000001018
94. Fontanillas P, Kless A, Bothmer J, Tung JY. Genome-wide association study of pain sensitivity assessed by questionnaire and the cold pressor test. Pain. (2022) 163:1763–76. doi: 10.1097/J.PAIN.000000000000256896
95. Dydyk AM, Grandhe S. Pain assessment. Fundam Pain Med. (2023):27–32. doi: 10.1007/978-3-319-64922-1_597
96. Diatchenko L, Fillingim RB, Smith SB, Maixner W. The phenotypic and genetic signatures of common musculoskeletal pain conditions. Nat Rev Rheumatol. (2013) 9:340–50. doi: 10.1038/NRRHEUM.2013.4398
97. Zorina-Lichtenwalter K, Meloto CB, Khoury S, Diatchenko L. Genetic predictors of human chronic pain conditions. Neuroscience. (2016) 338:36–62. doi: 10.1016/J.NEUROSCIENCE.2016.04.04199
98. Johnston KJA, Signer R, Huckins LM. Chronic overlapping pain conditions and nociplastic pain. medRxiv (2023) 2023.06.27.23291959. doi: 10.1101/2023.06.27.23291959100
99. Brandl E, Halford Z, Clark MD, Herndon C. Pharmacogenomics in pain management: a review of relevant gene-drug associations and clinical considerations. Ann Pharmacother. (2021) 55(12):1486–501. doi: 10.1177/10600280211003875
100. Crews KR, Monte AA, Huddart R, Caudle KE, Kharasch ED, Gaedigk A, et al. Clinical pharmacogenetics implementation consortium guideline for CYP2D6, OPRM1, and COMT genotypes and select opioid therapy. Clin Pharmacol Ther. (2021) 110(4):888–96. doi: 10.1002/cpt.2149
101. Chronic pain (primary and secondary) in over 16s: assessment of all chronic pain and management of chronic primary pain NICE guideline. Available online at: www.nice.org.uk/guidance/ng193 (published April 7, 2021).
102. Macfarlane GJ, Kronisch C, Dean LE, Atzeni F, Hauser W, FluB E, et al. EULAR Revised recommendations for the management of fibromyalgia. Ann Rheum Dis. (2017) 76:318–28. doi: 10.1136/annrheumdis-2016-209724
103. Osteoarthritis in over 16s: diagnosis and management NICE guideline. Available online at: www.nice.org.uk/guidance/ng226 (published October 19, 2022).
104. Kloppenburg M, Kroon FP, Blanco FJ, Doherty M, Dziedzic KS, Greibrokk E, et al. 2018 Update of the EULAR recommendations for the management of hand osteoarthritis. Ann Rheum Dis. (2019) 78(1):16–24. doi: 10.1136/annrheumdis-2018-213826
105. Rheumatoid arthritis in adults: management NICE guideline. Published: 11 July 2018. Last updated: 12 October 2020. Available online at: www.nice.org.uk/guidance/ng100
106. Spondyloarthritis in over 16s: diagnosis and management NICE guideline Published: 28 February 2017 Last updated: 2 June 2017. Available online at: www.nice.org.uk/guidance/ng65
107. Cameron EC, Hemingway SL. Cannabinoids for fibromyalgia pain: a critical review of recent studies (2015–2019). J Cannabis Res. (2020) 2:19. doi: 10.1186/s42238-020-00024-2
108. Bourke SL, Schlag AK, O’Sullivan SE, Nutt DJ, Finn DP. Cannabinoids and the endocannabinoid system in fibromyalgia: a review of preclinical and clinical research. Pharmacol Ther. (2022) 240:108216. doi: 10.1016/j.pharmthera.2022.108216
109. Pramhas S, Thalhammer T, Terner S, Pickelsberger D, Gleiss A, Sator S, et al. Oral cannabidiol (CBD) as add-on to paracetamol for painful chronic osteoarthritis of the knee: a randomized, double-blind, placebo-controlled clinical trial. The Lancet Regional Health—Europe. (2023) 35:100777. doi: 10.1016/j.lanepe.2023.100777
110. Liu X, Virk S, Fedorova T, Oo WM, Hunter DJ. The effect of pentosan polysulfate sodium for improving dyslipidaemia and knee pain in people with knee osteoarthritis: a pilot study. Osteoarthr Cartil Open. (2023) 5(2):100343. doi: 10.1016/j.ocarto.2023.100343
111. Available online at: https://clinicaltrials.gov/study/NCT04814368 (accessed December 4, 2023).
Keywords: rheumatic and musculoskeletal diseases, nociceptive pain, neuropathic pain, nociplastic pain, pain sensitisation
Citation: Sofat N and Lambarth A (2024) Can we achieve pain stratification in musculoskeletal conditions? Implications for clinical practice. Front. Pain Res. 5:1362757. doi: 10.3389/fpain.2024.1362757
Received: 28 December 2023; Accepted: 26 February 2024;
Published: 7 March 2024.
Edited by:
Maja R. Radojcic, The University of Manchester, United KingdomReviewed by:
Romain Perera, University College London, United Kingdom© 2024 Sofat and Lambarth. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nidhi Sofat bnNvZmF0QHNndWwuYWMudWs=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.