- 1Department of Physical Therapy, Faculty of Medical Rehabilitation Sciences, King Abdulaziz University, Jeddah, Saudi Arabia
- 2Department of Neurology, University of Munich, Munich, Germany
Background: The Pain Sensitivity Questionnaire (PSQ) is a reliable and valid self-reported tool for the assessment of pain sensitivity in clinical practice. The PSQ has been translated, validated, and cross-culturally adapted into multiple languages. However, a validated Arabic version of the PSQ is not available. Thus, this study aims to translate, validate, and cross-culturally adapt the English version of the PSQ into the Arabic language.
Methods and materials: The English version of the PSQ was translated and culturally adapted into Arabic following international guidelines. The psychometric properties of the final version of the PSQ-Arabic (PSQ-A) were tested among 119 patients with different persistent musculoskeletal (MSK) pain.
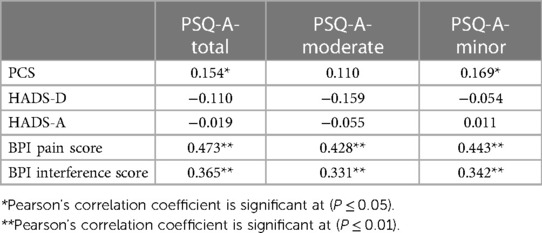
Findings: The Cronbach’s α for the PSQ-A-total, PSQ-A-moderate, and PSQ-C-minor were 0.81, 0.79, and 0.76, respectively. The means for the PSQ-A-total, PSQ-A-moderate, and PSQ-C-minor scores were 5.07 (±1.28), 5.64 (±2.07), and 4.50 (±0.50). The test-retest reliability measured with the interclass correlation coefficient for 68 subjects was 0.80 for the PSQ-A-total, 0.74 for the PSQ-A-moderate, and 0.77 for the PSQ-A-minor. The PSQ-A-total and the PSQ-A-minor showed positive significant correlations with pain catastrophizing scale (PCS) (r = 0.15, 0.17); P ≤ 0.05), respectively. The PSQ-A-total, PSQ-A-moderate, and PSQ-A-minor showed positive significant correlations with the Brief Pain Inventory (BPI)-pain scores (r = 0.47, 0.43, 0.45; P ≤ 0.01), respectively and with the BPI-pain interference scores (r = 0.37, 0.33, 0.34; P ≤ 0.01), respectively.
Conclusions: This study shows that the PSQ-A is a reliable and valid tool to assess individuals with pain sensitivity in Arabic populations. Further studies are recommended to examine the concurrent validity of the PSQ-A against experimental pain sensitivity measures.
1 Introduction
Persistent pain is a global burden, affecting up to a quarter of the global population, and has a massive effect on economic and healthcare systems (1, 2). In particular, persistent musculoskeletal (MSK) pain is considered one of the most common causes of years lived with disability (3–5). Persistent MSK pain refers to “pain in muscles, tendons, joints, and ligaments for more than three months (6, 7). Persistent MSK pain is largely affected by the central nervous system including peripheral and central sensitization, reduced anti-nociception, increased pro-nociception, and alteration of cortical pain processing (8–10). The International Association for the Study of Pain defines central sensitization as “increased responsiveness of nociceptive neurons in the central nervous system to either normal or subthreshold afferent input” (11). Several persistent MSK pain conditions showed evidence of central sensitization/elevated pain sensitivity, such as low back pain (12), neck pain (13), knee osteoarthritis (14), shoulder pain (15, 16). In an attempt to assess altered pain sensitivity, multiple test procedures have been proposed in the literature, such as quantitative sensory testing (QST) procedures.
The QST test procedures are psychophysical experimental tests designed to measure the pain threshold to controlled sensory stimuli (17). However, the QST test procedures are time-consuming, require a battery of specialized expensive tools, and standardized protocols (18). Therefore, there is a need for an alternative simple, easy to administer, and less time-consuming testing measure for pain sensitivity. The Pain Sensitivity Questionnaire (PSQ) has a potential advantage in clinical settings for assessing pain sensitivity (19). The PSQ is a reliable and valid self-reported questionnaire, which was developed to assess a patient's perception various imagined physical stimuli occurring in daily life (20).
The English version of the PSQ has shown associations with a variety of QST, including pain threshold and suprathreshold responses, in healthy individuals and patients with chronic pain conditions (20–23). The PSQ has been translated, validated, and cross-culturally adapted into multiple languages, such as English (22), Norwegian (23), Polish (24), French (25), Dutch (26), Mandarin Chinese (27), Iranian (28), and Turkish (29). The previous studies indicated that the PSQ could be utilized in research and clinical sitting.
To date, the PSQ has not been translated into the Arabic language. Hence, translation and adaptation of the PSQ into Arabic will assist in assessing many patients with chronic pain in Saudi Arabia and other countries using Arabic as a spoken language for providing better assessment and management strategies. Therefore, this study aims to translate, validate, and cross-culturally adapt the English version of the PSQ into the Arabic language.
2 Materials and methods
2.1 Translation and cross-cultural adaptation
The English version of the PSQ was translated into Arabic according to Wild et al. (30) and Beaton (31) recommendations as follows; permission was sought from the original author of the PSQ (20) to translate the English version of the PSQ into Arabic. This was followed by a forward translation of the English version of the PSQ into Arabic by two native Arabic (a medical practitioner, and a non-medical practitioner) who are fluent in both English and Arabic. Both translators independently translated the English version of the PSQ into Arabic. One of the translators was aware of the purpose of the PSQ translation, while the other was not. Then, the two translators and the research team combined the two Arabic versions into a single Arabic version. Backward translation was conducted by two professional translators (One with a medical background and one who has no experience in using medical terminologies) who were fluent in both English and Arabic languages. Both translators were not aware of the purpose of translation and were not aware of the English version of the scale. An expert committee [previous four translators involved in the process, the developer (Ruscheweyh) of the English version of the PSQ, and an Arabic translation expert] discussed the two back-translated versions of the PSQ and the English version. Then, the committee evaluated the semantic, idiomatic, experiential, and conceptual equivalence of all items and answered until a consensus was achieved on the pre-final version of PSQ. The pre-final version was piloted among 30 participants for cognitive debriefing/face validity. Participants were asked for opinions about the understanding of the wording and clarity of the pre-final version. The committee approved the pre-final version without amendments.
2.2 Validation of the PSQ-Arabic
2.2.1 Study design
Cross-sectional observational study. The study was approved by the Centre of Excellence in Genomic Medical Research, King Abdul-Aziz University, Jeddah, (Reference: 10-CEGMR-Bioeth-2020).
2.2.2 Participants and setting
This study was conducted at the outpatient clinic of the department of physical therapy at the faculty of applied medical sciences, King Abdulaziz University. The inclusion criteria for participants were a native Arabic who speaks and reads the Arabic language, an adult (aged ≥18 years) with persistent MSK pain (>3months), and has no cognitive impairments. Participants were excluded if they have a fever or infectious disease (e.g., Covid-19) at the time of participation in the study, psychiatric disorders or neurological diseases (e.g., stroke, hemiparesis, or epilepsy), or used any painkillers within the past 24 h. Informed consent was obtained from the subjects at recruitment. Participants completed the Brief Pain Inventory-Arabic (BPI-Arabic) (32), Hospital Anxiety and Depression Scale-Arabic (HADS-Arabic) (33), Pain Catastrophizing Scale-Arabic (PCS-Arabic) (33), and the PSQ-Arabic.
2.2.3 Pain sensitivity questionnaire (PSQ)
The PSQ is based on an individual’s rating of pain intensity in response to 17 imagined daily life painful situations (20). Respondents score their pain intensity on a numerical rating scale (NRS) of 0 to 10, with (0) indicating no pain at all and (10) indicating the worst pain imaginable. The PSQ-total has two subscales (PSQ-moderate and PSQ-minor) of seven items. The PSQ-total is the average rating of items (1–4, 6–8, 10–12, 14–17). The PSQ-moderate subscale score is the average rating of items (1–3, 8, 15–17) indicating moderate pain, while PSQ-minor is the average rating of items (3, 6, 7, 10–12, and 14) indicating minor pain. Three items (5, 9, and 13) are not included in the scores as these items are directed to normally non-painful situations.
2.2.4 Brief pain inventory-Arabic (BPI-Arabic)
The Brief Pain Inventory (BPI) is designed to assess patients’ pain intensity and pain interference (34). Pain severity is measured with four items: worst pain in the last 24 h, least pain in the last 24 h, pain on average, and pain right now. The intensity of pain is rated using a 0–10 rating scale anchored at zero (no pain) to 10 (pain as bad as you can imagine). Pain interference is measured with seven domains of functioning including general activities, mood, walking ability, normal work, relations with others, sleep, and enjoyment of life. Patients rated from 0 (does not interfere) to 10 (completely interferes). This study adopted the Arabic version of the BPI. An Arabic version of the BPI was available and Cronbach's alpha coefficients were reported as 0.82 and 0.92 for the severity and interference items, respectively. Factor analysis yielded two factors and the correlations between the severity and interference items ranged between 0.25 and 0.57 (P < 0.05) (32).
2.2.5 Hospital anxiety and depression scale—Arabic
Zigmond and Snaith (35) identified the original HADS for measuring anxiety and depression disorders among patients in general clinics. The HADS consists of 14 items: anxiety (7-item) and depression (7-item). These items are rated on a 4-point scale (0 = absence of symptoms, 3 = maximum symptoms). The scores for each subscale range from 0 to 21, with a score of 0–7 is considered normal, 8–10 (mild), 11–14 (moderate), and 15–21 (severe). The entire scale ranges from 0 to 42, with higher scores indicating a higher level of emotional distress. This study adopted the Arabic version of HADS. The Cronbach’s αs for the HADS anxiety subscale were 0.83 (95%) confidence interval (0.79–0.88), and for the HADS depression subscale were 0.77 (0.7–0.83). HADS anxiety score was strongly correlated (r = 0.67) with generalized anxiety disorder 7-item scale, and the HADS depression score was strongly associated (r = 0.66) with the major depression inventory (36).
2.2.5 Catastrophizing scale—Arabic
Sullivan et al. (37) developed the PCS, which contains 13 items assessing the thoughts and feelings associated with pain. The PCS includes three subscales: Rumination, magnification, and helplessness. The PCS items are rated on 5-points scale (0 = not at all, 1 = to a slight degree, 2 = to a moderate degree, 3 = to a great degree, 4 = all the time). The higher PCS score indicates a higher tendency to pain catastrophizing. Arabic version of the PCS was available and a Cronbach's alpha of 0.94 was reported, test-retest reliability (r = 0.84). This study adopted the Arabic version of PCS (36).
2.3 Statistical analysis
2.3.1 Sample size
A power analysis was conducted using G Power software (version 3.1.2; Kiel, Germany) to determine the number of participants included in the study. Assuming correlations for PSQ and Pain Catastrophizing scale to be 0.3 (moderate reference), power of 95 and ∝ error as 0.05, resulted in a minimum sample size of 111.
2.4 Descriptive analysis
All statistical analyses were performed using SPSS 20.0 statistics package (SPSS, Inc., Chicago, IL, U.S.A.). Demographic and clinical characteristics of the sample were analyzed using frequencies, means, and standard deviations (SDs). Questionnaires with missing items in any scales were excluded from the analysis.
2.5 Inferential analysis
2.5.1 Reliability
2.5.1.1 Internal consistency
The Cronbach’s alpha was used with a value of 0.60 indicating acceptable internal consistency and more than 0.70 indicating good internal consistency.
2.5.1.2 Test-retest reliability
The final PSQ—Arabic version was assessed on two occasions, which were separated by two weeks. Reliability was analyzed using the intraclass correlation coefficient (ICC). The values of ICC were indicated as excellent at 0.8, moderate from 0.6 to 0.79, and poor at 0.61.
2.5.2 Validity
2.5.2.1 Floor and ceiling effects
This was measured as the per cent of patients who reported a minimum or maximum score of PSQ-minor, PSQ-moderate, and PSQ-total. The desired value for the floor/ceiling effect is less than 15% to 20%.
2.5.2.2 Convergent validity
Pearson's correlation was utilized according to the normality of the data for comparing the results of the PSQ-A with the results of the validated Arabic version of the PCS-A, the Brief Pain Inventory-A, and the Hospital Anxiety and Depression Scale-(HADS)-Arabic. The correlation coefficient was considered strong if it was greater than 0.50, and moderate between 0.30 and 0.50.
2.5.2.3 Construct validity
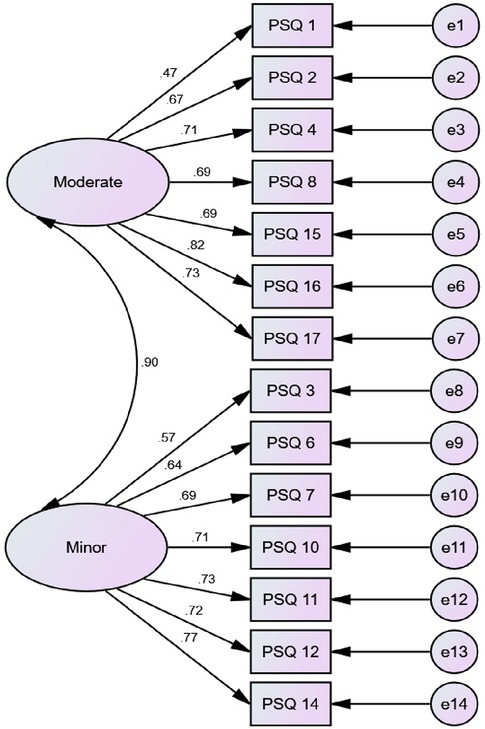
A confirmatory factor analysis (CFA) was conducted using AMOS software. This analysis showed the correlation between PSQ-A items and the PSQ-A subscales (minor and moderate). The model-fit indices included chi-square (χ2), comparative fit index (CFI), and root mean square error of approximation (RMSEA). For RMSEA, values of 0.08 or below indicate a close fit, and values in the range from 0.08 to 0.10 indicate an acceptable fit.
3 Results
3.1 Participants
A total of 119 patients with different persistent MSK pain (neck (n = 22), back (n = 60), shoulder (n = 26), knee (n = 8), and ankle (n = 3)) participated in this study. Of this sample, 67% (n = 80) were female, and the mean age of participants was 39.5 years (Table 1). Among the participants, 58% were not working. The mean for the PSQ-A-total score was 5.07 (±1.28), 5.64 (±2.07) for the PSQ-A-moderate, and 4.50 (±0.50) for PSQ-A-minor.
3.2 Floor and ceiling effects
For the PSQ-A-total and the minor scores, the percentage of participants who scored the minimum and the maximum was 0.84%. For the PSQ-A-moderate score, the percentage of participants who scored the minimum was 0.84%, and the maximum was 3.36%.
3.3 Internal consistency
The Cronbach's α for the PSQ-A-total was 0.81, 0.76 for PSQ-A-minor, and 0.79 for PSQ-A-moderate.
3.4 Test-retest reliability
The first 68 subjects participated in a retest assessment after two weeks. The intraclass correlation coefficient was 0.80 for the PSQ-A-total, 0.74 for the PSQ-A-moderate, and 0.77 for the PSQ-A-minor.
3.5 Convergent validity
The PSQ-A-total and the PSQ-A-minor showed positive significant correlations with the pain-specific measure PCS at (P ≤ 0.05). In addition, the PSQ-A-total and the two PSQ-A subscales showed positive significant correlations with the BPI-pain score and BPI-pain interference score at (P ≤ 0.01). However, there was no significant correlation with the HADS-D or HADS-A (Table 2).
3.6 Construct validity
According to Ruscheweyh et al. (20), a 2-factor model for the PSQ-A was built: the PSQ-A-moderate (7-item factor) and the PSQ-A-minor (7-item factor). An acceptable model fit was achieved: Chi-Square/Degree of Freedom (CMIN/DF) = 3.33, Goodness of fit Root (GFI) = 0.85, Comparative Fit Index (CFI) = 0.87, Root Mean Square Error of Approximation (RMSEA) = 0.11. Correlations between items within the same factor were shown in Figure 1.
4 Discussion
This study aimed at cross-cultural adaptation, reliability, and validity of the PSQ-A. The finding from this study indicated that the PSQ-A is an easy to understand, administered, reliable, and valid measure of pain sensitivity in individuals with persistent MSK pain. The internal consistency of the PSQ-A-total, PSQ-A-moderate, and PSQ-A-minor was good; the Cronbach's α were 0.81, 0.76, and 0.79, respectively. These were similar to Cronbach's alphas of the Chinese version (0.90, 0.86, and 0.81) (27), the German PSQ version (0.92, 0.91, and 0.81) (20), the Korean version (0.93, 0.88, and 0.87) (38), the Iranian version (0.81, 0.82, and 0.82) (28), the Dutch version (0.90, 0.86, and 0.82) (26), the Norwegian version (0.92, 0.90, and 0.85) (23), and the Spanish version (0.95, 0.91, and 0.92) (39). Confirmatory factor analysis confirmed the two-factor structure of the PSQ-A with the two subscores PSQ-A minor and PSQ-A moderate to be consistent with the original description (20).
The test-retest reliability measured with the interclass correlation coefficient (ICC) was 0.80 for the PSQ-A-total, 0.74 for the PSQ-A-moderate, and 0.77 for the PSQ-A-minor. These values were similar to the values for the Korean version, which were 0.78, 0.79, and 0.75 (38), the Chinese (0.73, 0.74, and 0.68) (27), but slightly lower than those for the German PSQ version (0.83, 0.79, and 0.86) (20), the Polish version [(0.93, 0.87, 0.91) (24)], the Iranian version (0.84, 0.84, and 0.85) (28), and the Spanish (0.84, 0.82, and 0.84) (39).
The PSQ-A-total and the PSQ-A-minor showed weak positive significant correlations with the pain-specific measure PCS (r = 0.15, 0.17), respectively. This may due to the large proportion of female participants (67%, n = 80) in this study who reported higher level of catastrophizing related pain than male. This is not surprising, as it has been reported in the literature that female would show higher levels of catastrophizing than male (37). In addition, altered pain sensitivity linked with increased catastrophizing. Meints et al. (40), reported that there is association between catastrophizing and sensitization which resulted in an increase of clinical pain among individuals with chronic LBP. Our results were similar in the magnitude of correlation for the previous translated versions including the Chinese (r = 0.27, 0.27) (27), German (r = 0.45, 0.38) (20), English (r = 0.32, 0.33) (22), and Korean (r = 0.38, 0.37) (38). The Spanish version of PSQ-total and PSQ-minor showed a stronger positive correlation with the PCS at (0.58, 0.60) (39) and Iranian version for the PSQ-total score (r = 0.81) (28). On the contrary, the Turkish version of the PSQ-total and subscales did not correlate with the PCS (29). The PSQ-A-moderate did not correlate with the PCS; the other translated versions were positively correlated. Moreover, the PSQ-A-total and both subscales did not significantly correlate with the HADS-D or the HADS-A. These findings were similar to the English and Turkish versions of the PSQ (22, 29). This may due to the fact that the PSQ is based on an individual's rating of pain intensity in response to imagining situations, which more directly measure the sensory facilitation involved in CS (20), however the degree to which PSQ reflects the top–down pain mechanisms related to psychological factors remain open (41). Recent systematic review and meta-analysis indicated that the psychological measures of depression and anxiety including the HADS-D and the HADS-A showed a weak correlation with the PSQ (r = 0.11, 0.16, respectively), while pain catastrophizing showed a moderate correlation with the PSQ (r = 0.32) (41). Accordingly, correlations between PSQ-A and the pain-specific measure PCS were higher than correlations between PSQ-A and HADS in the present study.
The PSQ-A-total, PSQ-A-moderate, and PSQ-A-minor showed positive significant correlations with the BPI-pain scores (r = 0.47, 0.43, 0.45, respectively) and with the BPI-pain interference scores (r = 0.37, 0.33, 0.34, respectively). The previous findings were similar to the Turkish version (29), which showed a similar magnitude of correlations for the BPI-pain scores (r = 0.28, 0.31, 0.24, respectively) and with the BPI-pain interference scores of the total and minor subscale (r = 0.31, 0.34, respectively). On the other hand, the PSQ-total score of the English version was the only score significantly correlated with the BPI-pain score (r = 0.25) (22), while the correlations with the BPI interference score did not reach significance for the PSQ-E-total and both subscales.
The PSQ-A scores were similar to those found in previous studies. The mean of PSQ-A-total scores was 5.07, while for the Korean version was (5.93) (38), Chinese (4.7) (27), and Norwegian (4.5) (23). However, the PSQ-A-total was higher than those reported in the original study (4.0) (20), the Dutch version (4.1) (26), and the English version (3.6) (22). The mean of the PSQ-A-moderate score was 5.64, which was similar to other versions, which range from 4.7 to 6.5 (20, 22, 23, 26, 27, 38). The mean of the PSQ-A-minor score was 4.4, which is higher than the English version (2.5) (22) and other European versions, such as the German version (2.5) (20), Dutch (2.8) (26), and the Norwegian version (3.1) (23). In the contrary, the PSQ-A-minor score was similar to the Asian versions, such as the Chinese version (3.9) (27) and the Korean version (5.4) (38). The discrepancies in scoring the PSQ-minor may be related to the ethnicity or cultural influence of reporting pain sensitivity (42). In an experimental pain sensitivity, Asians demonstrated significantly lower pain threshold and tolerance levels than Whites (43). In addition, Asians report a higher widespread musculoskeletal pain than Whites (44, 45).
5 Limitations
This study had some limitations as it included a higher number of female participants, which might inflate the PSQ-A scores. Evidence from the literature revealed that females report higher pain sensitivity than males (46). Another possible limitation of the PSQ-A is that one question asks participants about snow (item 12), which makes it less applicable to countries with a warmer climate. Furthermore, the Arabic version of the PSQ has translated the English version of the same construct rather than the original German version.
6 Conclusion
This study demonstrated that the Arabic version of the PSQ is a reliable and valid tool to assess pain sensitivity in individuals with persistent MSK pain. Therefore, this tool can be used to assess pain sensitivity in clinical practice. Further studies are needed to examine the concurrent validity of the Arabic version of the PSQ against experimental pain sensitivity measures, such as QST procedures.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethical approval of this study (10-CEGMR-Bioeth-2020) was obtained from the EEGMR Bioethic Committee, Center of Excellence in Genomic Medicine Research, King Abdulaziz University, Jeddah, Saudi Arabia. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
AA: Writing – original draft, Writing – review & editing. FK: Writing – review & editing. UA: Writing – review & editing. RR: Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
This project was funded by the Deanship of Scientific Research (DSR) at King Abdulaziz University, Jeddah, under grant number (G-128-883-1442). Therefore, the authors acknowledge with thanks, DSR, for technical and financial support. The funding agency had no involvement in the study.
Acknowledgment
The authors would like to thank all participants for their participation in this research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Vos T, Abajobir AA, Abate KH, Abbafati C, Abbas KM, Abd-Allah F, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the global burden of disease study 2016. Lancet. (2017) 390(10100):1211–59. doi: 10.1016/S0140-6736(17)32154-2
2. Breivik H, Eisenberg E, O’Brien T. The individual and societal burden of chronic pain in Europe: the case for strategic prioritisation and action to improve knowledge and availability of appropriate care. BMC public Health. (2013) 13:1–14. doi: 10.1186/1471-2458-13-1229
3. Lang JJ, Alam S, Cahill LE, Drucker AM, Gotay C, Kayibanda JF, et al. Global burden of disease study trends for Canada from 1990 to 2016. CMAJ. (2018) 190(44):E1296–304. doi: 10.1503/cmaj.180698
4. Johannes CB, Le TK, Zhou X, Johnston JA, Dworkin RH. The prevalence of chronic pain in United States adults: results of an internet-based survey. J Pain. (2010) 11(11):1230–9. doi: 10.1016/j.jpain.2010.07.002
5. Martinez-Calderon J, Flores-Cortes M, Morales-Asencio JM, Luque-Suarez A. Which psychological factors are involved in the onset and/or persistence of musculoskeletal pain? An umbrella review of systematic reviews and meta-analyses of prospective cohort studies. Clin J Pain. (2020) 36(8):626–37. doi: 10.1097/AJP.0000000000000838
6. Cimmino MA, Ferrone C, Cutolo M. Epidemiology of chronic musculoskeletal pain. Best Pract Res Clin Rheumatol. (2011) 25(2):173–83. doi: 10.1016/j.berh.2010.01.012
7. Coppieters I, Meeus M, Kregel J, Caeyenberghs K, De Pauw R, Goubert D, et al. Relations between brain alterations and clinical pain measures in chronic musculoskeletal pain: a systematic review. J Pain. (2016) 17(9):949–62. doi: 10.1016/j.jpain.2016.04.005
8. Apkarian AV, Hashmi JA, Baliki MN. Pain and the brain: specificity and plasticity of the brain in clinical chronic pain. Pain. (2011) 152(3):S49–64. doi: 10.1016/j.pain.2010.11.010
9. Seminowicz DA, Moayedi M. The dorsolateral prefrontal cortex in acute and chronic pain. J Pain. (2017) 18(9):1027–35. doi: 10.1016/j.jpain.2017.03.008
10. Kohns DJ, Urbanik CP, Geisser ME, Schubiner H, Lumley MA. The effects of a pain psychology and neuroscience self-evaluation internet intervention: a randomized controlled trial. Clin J Pain. (2020) 36(9):683–92. doi: 10.1097/AJP.0000000000000857
11. International Association for the Study of Pain (IASP). IASP Terminology. (2017). Available online at: https://www.iasp-pain.org/temionlogy (accessed July 20, 2023).
12. Neziri AY, Curatolo M, Limacher A, Nüesch E, Radanov B, Andersen OK, et al. Ranking of parameters of pain hypersensitivity according to their discriminative ability in chronic low back pain. Pain. (2012) 153(10):2083–91. doi: 10.1016/j.pain.2012.06.025
13. Van Oosterwijck J, Nijs J, Meeus M, Paul L. Evidence for central sensitization in chronic whiplash: a systematic literature review. Eur J Pain. (2013) 17(3):299–312. doi: 10.1002/j.1532-2149.2012.00193.x
14. Lluch E, Torres R, Nijs J, Van Oosterwijck J. Evidence for central sensitization in patients with osteoarthritis pain: a systematic literature review. Eur J Pain. (2014) 18(10):1367–75. doi: 10.1002/j.1532-2149.2014.499.x
15. Sanchis MN, Lluch E, Nijs J, Struyf F, Kangasperko M. The role of central sensitization in shoulder pain: a systematic literature review. Semin Arthritis Rheum. (2015) 44(6):710–6. doi: 10.1016/j.semarthrit.2014.11.002
16. Noten S, Struyf F, Lluch E, D'Hoore M, Van Looveren E, Meeus M. Central pain processing in patients with shoulder pain: a review of the literature. Pain Pract. (2017) 17(2):267–80. doi: 10.1111/papr.12502
17. Rolke R, Baron R, Maier CA, Tölle TR, Treede RD, Beyer A, et al. Quantitative sensory testing in the German research network on neuropathic pain (DFNS): standardized protocol and reference values. Pain. (2006) 123(3):231–43. doi: 10.1016/j.pain.2006.01.041
18. Cruz-Almeida Y, Fillingim RB. Can quantitative sensory testing move US closer to mechanism-based pain management? Pain Med. (2014) 15(1):61–72. doi: 10.1111/pme.12230
19. Coronado RA, George SZ. The central sensitization inventory and pain sensitivity questionnaire: an exploration of construct validity and associations with widespread pain sensitivity among individuals with shoulder pain. Musculoskelet Sci Pract. (2018) 36:61–7. doi: 10.1016/j.msksp.2018.04.009
20. Ruscheweyh R, Marziniak M, Stumpenhorst F, Reinholz J, Knecht S. Pain sensitivity can be assessed by self-rating: development and validation of the pain sensitivity questionnaire. Pain. (2009) 146(1–2):65–74. doi: 10.1016/j.pain.2009.06.020
21. Ruscheweyh R, Verneuer B, Dany K, Marziniak M, Wolowski A, Çolak-Ekici R, et al. Validation of the pain sensitivity questionnaire in chronic pain patients. Pain. (2012) 153(6):1210–8. doi: 10.1016/j.pain.2012.02.025
22. Sellers AB, Ruscheweyh R, Kelley BJ, Ness TJ, Vetter TR. Validation of the English language pain sensitivity questionnaire. Reg Anesth Pain Med. (2013) 38(6):508–14. doi: 10.1097/AAP.0000000000000007
23. Valeberg BT, Pedersen LM, Girotto V, Christensen VL, Stubhaug A. Validation of the Norwegian pain sensitivity questionnaire. J Pain Res. (2017) 10:1137–42. doi: 10.2147/JPR.S129540
24. Latka D, Miekisiak G, Kozlowska K, Olbrycht T, Chowaniec J, Latka K, et al. Translation, validation, and cross-cultural adaptation of the Polish version of the pain sensitivity questionnaire. J Pain Res. (2019) 12:969–73. doi: 10.2147/JPR.S189427
25. Dualé C, Bauer U, Storme B, Eljezi V, Ruscheweyh R, Eschalier S, et al. Transcultural adaptation and French validation of the pain sensitivity questionnaire. Can J Anaesth. (2019) 66:1202–12. doi: 10.1007/s12630-019-01377-w
26. Van Boekel RL, Timmerman H, Bronkhorst EM, Ruscheweyh R, Vissers KC, Steegers MA. Translation, cross-cultural adaptation, and validation of the pain sensitivity questionnaire in Dutch healthy volunteers. Pain Res Manag. (2020) 2020:1050935. doi: 10.1155/2020/1050935
27. Quan X, Fong DYT, Leung AYM, Liao Q, Ruscheweyh R, Chau PH. Validation of the Mandarin Chinese Version of the Pain Sensitivity Questionnaire. Pain Pract. (2018) 18(2):180–93. doi: 10.1111/papr.12587
28. Azimi P, Azhari S, Shahzadi S, Aghaei HN, Mohammadi HR, Montazeri A. Outcome measure of pain in patients with lumbar disc herniation: validation study of the Iranian version of pain sensitivity questionnaire. Asian Spine J. (2016) 10(3):480. doi: 10.4184/asj.2016.10.3.480
29. Inal FY, Gul K, Yilmaz Camgoz Y, Daskaya H, Kocoglu H. Validation of the Turkish version of the pain sensitivity questionnaire in patients with chronic pain. J Int Med Res. (2021) 49(12):03000605211060158. doi: 10.1177/03000605211060158
30. Wild D, Grove A, Martin M, Eremenco S, McElroy S, Verjee-Lorenz A, et al. Principles of good practice for the translation and cultural adaptation process for patient-reported outcomes (PRO) measures: report of the ISPOR task force for translation and cultural adaptation. Value Health. (2005) 8(2):94–104. doi: 10.1111/j.1524-4733.2005.04054.x
31. Beaton DE, Bombardier C, Guillemin F, Ferraz MB. Guidelines for the process of cross-cultural adaptation of self-report measures. Spine. (2000) 25(24):3186–91. doi: 10.1097/00007632-200012150-00014
32. Ballout S, Noureddine S, Huijer HAS, Kanazi G. Psychometric evaluation of the Arabic brief pain inventory in a sample of Lebanese cancer patients. J Pain Symptom Manage. (2011) 42(1):147–54. doi: 10.1016/j.jpainsymman.2010.09.019
33. Terkawi AS, Sullivan M, Abolkhair A, Al-Zhahrani T, Terkawi RS, Alasfar EM, et al. Development and validation of Arabic version of the pain catastrophizing scale. Saudi J Anaesth. (2017) 11(Suppl 1):S63. doi: 10.4103/sja.SJA_130_17
35. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. (1983) 67(6):361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x
36. Terkawi AS, Tsang S, AlKahtani GJ, Al-Mousa SH, Al Musaed S, AlZoraigi US, et al. Development and validation of Arabic version of the hospital anxiety and depression scale. Saudi J Anaesth. (2017) 11(Suppl 1):S11. doi: 10.4103/sja.SJA_43_17
37. Sullivan MJ, Bishop SR, Pivik J. The pain catastrophizing scale: development and validation. Psychol Assess. (1995) 7(4):524. doi: 10.1037/1040-3590.7.4.524
38. Kim HJ, Ruscheweyh R, Yeo JH, Cho HG, Yi JM, Chang BS, et al. Translation, cross-cultural adaptation, and validity of the Korean version of the pain sensitivity questionnaire in chronic pain patients. Pain Pract. (2014) 14(8):745–51. doi: 10.1111/papr.12123
39. Ibancos-Losada MDR, Osuna-Pérez MC, Cortés-Pérez I, Montoro-Cárdenas D, Díaz-Fernández Á. Validation and cross-cultural adaptation of the Spanish version of the pain sensitivity questionnaire (PSQ-S). J Clin Med. (2021) 11(1):151. doi: 10.3390/jcm11010151
40. Meints SM, Mawla I, Napadow V, Kong J, Gerber J, Chan ST, et al. The relationship between catastrophizing and altered pain sensitivity in patients with chronic low back pain. Pain. (2019) 160(4):833. doi: 10.1097/j.pain.0000000000001461
41. Adams GR, Gandhi W, Harrison R, Van Reekum CM, Wood-Anderson D, Gilron I, et al. Do “central sensitisation” questionnaires reflect measures of nociceptive sensitisation or psychological constructs? a systematic review and meta-analyses. Pain. (2023) 164(6):1222–39. 36729810
42. Quan X, Fong DYT, Leung AYM, Liao Q, Ruscheweyh R, Chau PH. Validation of the mandarin Chinese version of the pain sensitivity questionnaire. Pain Pract. (2018) 18(2):180–93. doi: 10.1111/papr.12587
43. Rowell LN, Mechlin B, Ji E, Addamo M, Girdler SS. Asians differ from non-hispanic whites in experimental pain sensitivity. Eur J Pain. (2011) 15(7):764–71. doi: 10.1016/j.ejpain.2010.11.016
44. Palmer B, Macfarlane G, Afzal C, Esmail A, Silman A, Lunt M. Acculturation and the prevalence of pain amongst south Asian minority ethnic groups in the UK. Rheumatology (Oxford, England). (2007) 46(6):1009–14. doi: 10.1093/rheumatology/kem037
45. Allison TR, Symmons DPM, Brammah T, Haynes P, Rogers A, Roxby M, et al. Musculoskeletal pain is more generalised among people from ethnic minorities than among white people in greater Manchester. Ann Rheum Dis. (2002) 61(2):151–6. doi: 10.1136/ard.61.2.151
Keywords: central sensitization, pain sensitivity questionnaire, Arabic translation, cultural adaptation, psychometric properties
Citation: Alqarni A, Khan F, Alabasi U and Ruscheweyh R (2024) Translation, cross-cultural adaptation, and measurement properties of the Arabic version of the pain sensitivity questionnaire. Front. Pain Res. 5:1339449. doi: 10.3389/fpain.2024.1339449
Received: 16 November 2023; Accepted: 23 January 2024;
Published: 6 February 2024.
Edited by:
Karin N. Westlund, University of New Mexico Health Sciences Center, United StatesReviewed by:
Guilherme Lucas, University of São Paulo, BrazilFerda Yilmaz Inal, Istanbul Medeniyet University, Türkiye
© 2024 Alqarni, Khan, Alabasi and Ruscheweyh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Abdullah Alqarni YW1hbGdhcm5pQGthdS5lZHUuc2E=
 Abdullah Alqarni
Abdullah Alqarni Fayaz Khan
Fayaz Khan Umar Alabasi
Umar Alabasi Ruth Ruscheweyh
Ruth Ruscheweyh