- 1Gastroenterology, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, United States
- 2Pediatrics, University of Cincinnati, Cincinnati, OH, United States
- 3Biostatistics, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, United States
Introduction: Standard medical therapy (SMT) in children with functional abdominal pain disorders (FAPD) includes cyproheptadine and amitriptyline. While percutaneous electrical nerve field stimulation (PENFS) has shown benefit, no study has compared outcomes of PENFS to SMT. We aimed to examine changes in abdominal pain, nausea and disability before and after treatment and compare outcomes between treatments.
Methods: The records of FAPD patients ages 11–21 years, treated with 4 weeks of PENFS, cyproheptadine or amitriptyline were reviewed. Outcomes were evaluated using validated questionnaires [Abdominal Pain Index (API), Nausea Severity Scale (NSS), and the Functional Disability Inventory (FDI)] at baseline and follow-up within 3 months (FU).
Result: Of 101 patients, 48% received PENFS, 31% cyproheptadine and 21% received amitriptyline. Median ages were 17 (15–19), 16 (15–18) and 15 (11–16) years respectively and the majority were females (75%, 90% and 52% respectively). In the PENFS group, API (p = 0.001), NSS (p = 0.059) and FDI (p = 0.048) were significantly lower at FU. API (p = 0.034) but not NSS and FDI (p > 0.05) decreased significantly at FU in the amitriptyline group. API, NSS and FDI did not change significantly with cyproheptadine at FU (p > 0.05). FU API scores were lower in PENFS vs. cyproheptadine (p = 0.04) but not vs. amitriptyline (p = 0.64). The FDI scores were significantly lower in the amitriptyline vs. cyproheptadine group (p = 0.03).
Conclusion: Therapy with PENFS showed improvements in abdominal pain, nausea and disability while amitriptyline showed improvements in abdominal pain within 3 months of treatment. PENFS was more effective than cyproheptadine in improving abdominal pain. Amitriptyline improved disability scores more than cyproheptadine and showed promise for treatment. PENFS may be a good non-pharmacologic alternative for FAPD.
Introduction
Functional abdominal pain disorders (FAPD), namely irritable bowel syndrome (IBS), functional dyspepsia (FD), abdominal migraine, and functional abdominal pain—not otherwise specified (FAP-NOS) have increased disability and an extensive health care cost burden (1–3). Chronic nausea and abdominal pain are debilitating symptoms that often co-exist in children with FAPD and often associated with high physical and psychosocial distress. Standard medical therapy (SMT) in children with FAPD have traditionally been sub-optimal and frequently includes off-label medications such as cyproheptadine and amitriptyline (4–6).
There has been a recent shift towards non-pharmacologic management, particularly in children, where chronic medication use can be problematic. Percutaneous electrical nerve field stimulation (PENFS) is an emerging minimally invasive approach to treat patients with chronic abdominal pain. It modulates central pain pathways through stimulation of the auricular branches of cranial nerves after just 4-weeks of treatment, with sustained efficacy (7). Several pediatric studies have demonstrated the benefits of PENFS in children with FAPD (7–9). However, no study has compared outcomes of PENFS to SMT. We aimed to compare improvements in abdominal pain, nausea and disability using validated measures between these treatment options. We hypothesized greater improvements in abdominal pain, nausea and disability with use of PENFS compared with SMT.
Methods
After obtaining institutional review board approval, the electronic medical records of patients ages 11–21 years who met the Rome 4 criteria for a FAPD and had been treated with 4 weeks of PENFS, cyproheptadine or amitriptyline between January 2019 and December 2021 were retrospectively reviewed. Patients with organic gastrointestinal conditions known to cause abdominal pain were excluded. Demographic data and medical history were obtained from the medical record. Outcomes were evaluated using validated questionnaires that were prospectively collected routinely as part of clinical care at baseline and follow-up visit within 3 months (FU). These included:
Abdominal Pain Index (API): The API is validated for children to determine the frequency, severity, intensity and duration of abdominal pain over 2 weeks (10). We used a modified version with the duration over one week. A composite score was obtained summing individual items.
Nausea Severity Scale (NSS): The NSS, is a validated tool to determine the number of days, daily episodes and duration and intensity of nausea over 2 weeks (11). This measure normally assesses nausea severity over 2 weeks. We used a modified version with the duration over one week.
Functional Disability Inventory (FDI): The FDI is a 15 item validated instrument to measure daily physical and psychosocial functioning (12). It determines the degree of impairment caused by patients' symptoms. This measure normally assesses functioning over 2 weeks. While this measure has not been validated for shorter periods, we used a modified version with the duration over one week.
Statistical analysis
Categorical variables were presented as frequency counts and percentages while continuous data were presented as mean (95% CI) or median (IQR). Outcomes were assessed for each group using Chi Square test. Outcomes were evaluated from baseline to FU in each group, as a three-way comparison between groups as well as pairwise comparisons between groups.
Outcomes were compared between groups at 3 months via linear mixed modeling with subject as random effect. Baseline demographic differences between groups were accounted for during linear mixed modeling. Results were presented as Least Squares Mean (LS Mean) and 95% confidence interval. All analyses were conducted as two-sided test with p ≤ 0.05 to be statistically significant using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Demographic data
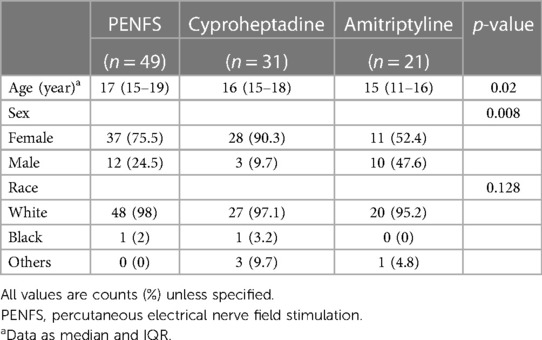
Demographic details are presented in Table 1. Of the 101 patients, 35% had FD, 35% IBS and 30% had FAP-NOS. Of these, 49 (48%) were treated with PENFS, 31 (31%) with cyproheptadine and 21 (21%) with amitriptyline. Doses for medications ranged from 10 to 50 mg nightly for amitriptyline and 2–4 mg daily to TID for cyproheptadine. In the PENFS group, 29 (59%) patients had been on medications but failed treatment and hence, received PENFS. These patients remained on a stable medication dose for the duration of treatment with PENFS. Median (IQR) ages in these groups were 17 years (15–19), 16 years (15–18) and 15 years (11–16) respectively. In all three groups, the majority were females (75%, 90% and 52% respectively) and Caucasian (98%, 87% and 95% respectively). Age and gender differed at baseline (p = 0.02 and p = 0.008 respectively) but no significant differences were noted in racial distribution between the groups.
Changes in measures in each group
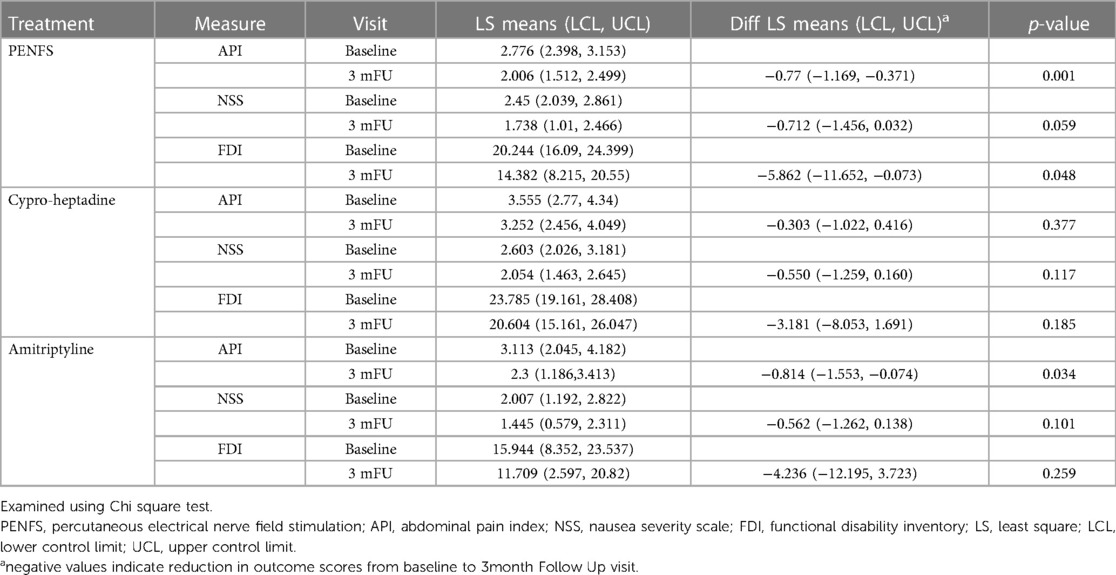
Table 2 presents changes measures in each group. In the PENFS group, API (p = 0.001), NSS (p = 0.059) and FDI (p = 0.048) were significantly lower at 3-month FU compared with baseline. API scores decreased significantly at FU in the amitriptyline group (p = 0.034). However, NSS and FDI scores did not change significantly at FU in the amitriptyline group. All scores decreased but were not significant in the cyproheptadine group. Examining each outcome longitudinally, the API and NSS scores were lowest in the PENFS group. The FDI scores, however, were lowest in the amitriptyline group.
Comparison of outcomes between groups
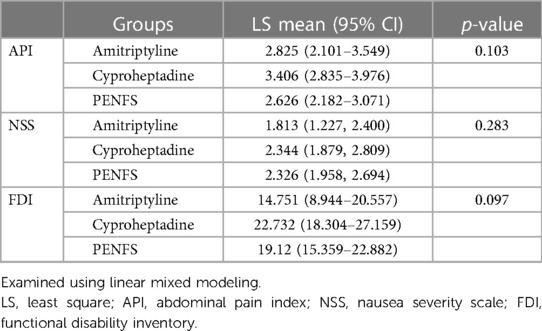
Table 3 represents a three-way comparison of outcomes between groups. Changes in abdominal pain and nausea were not significant between the three groups (p > 0.05). There was a trend for decrease in FDI in the amitriptyline group compared with both PENFS and cyproheptadine (p = 0.097). Figure 1 denotes pairwise comparison of outcomes between groups. API scores were significantly lower in PENFS vs. cyproheptadine (p = 0.04) but not between PENFS and amitriptyline (p = 0.64). The NSS scores did not differ between the groups (p > 0.05). The FDI scores were only lower in the amitriptyline vs. cyproheptadine group (p = 0.03).
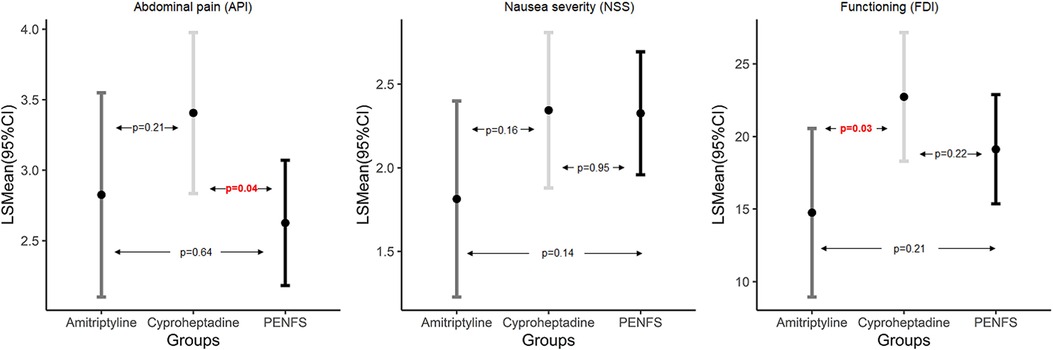
Figure 1. Pairwise comparison of outcomes between PENFS and SMT examined using linear mixed modeling; data presented as Least Square (LS) means and 95% Confidence Interval (CI) and p-values indicate pairwise group difference; PENFS, percutaneous electrical nerve field stimulation; SMT, standard medical therapy, API, abdominal pain index; NSS, nausea severity scale; FDI, functional disability inventory, n = 101 patients.
Discussion
This is the first study to compare outcomes of PENFS with standard medical therapy (amitriptyline or cyproheptadine) in adolescents with FAPD. Compared to pre-treatment,we found that PENFS significantly improved both abdominal pain and disability, with a trend for improvement in nausea, while amitriptyline significantly improved abdominal pain. PENFS was more effective than cyproheptadine in improving abdominal pain scores but did not differ from amitriptyline. Amitriptyline improved disability scores more than cyproheptadine.
Multiple biopsychosocial factors affect outcomes in FAPD. In this study, we chose to measure changes not just in abdominal pain, but also disability and nausea. This is important because FAPD are heterogenous conditions that include comorbidities once not considered as important (13). In fact, we know now that patients with co-existing nausea, for example, have worse disability (14, 15). This can result in decreased functioning which further worsens GI symptoms. Also, studies in patients with chronic pain have demonstrated the importance of functioning in terms of improving long-term outcomes, despite the presence of pain (16, 17). Thus, we chose to measure changes in disability, abdominal pain and nausea with different treatment strategies and compare outcomes.
There is a paucity of high-quality randomized placebo-controlled trials of standard medical therapy in FAPD. Several systematic reviews and meta-analysis have shown low quality of evidence to support routine use of medical therapy for FAPD and functional nausea (5, 18–20). Amitriptyline, a tricyclic antidepressant (TCA), has been commonly used in children with FAPD for years despite questionable efficacy data (21–23). The underlying mechanism for the anti-nociceptive effects of amitriptyline is not well known, but like many other drugs used to treat FAPDs, it does have strong anticholinergic properties (24). Usual doses to treat abdominal pain include 10–50 mg daily at bedtime. In addition to abdominal pain, it is commonly used to treat headaches and improve sleep at similar doses. It may also improve anxiety even at the lower doses (24). In a multicenter randomized-controlled trial including 83 children ages with FAPD, amitriptyline showed 63% improvement in pain relief and sense of overall improvement (21). However, this did not differ from placebo (58% improvement in the placebo group, p = 0.63). Interestingly, while amitriptyline was not better than placebo in improving pain scores in that study, it was better for reducing anxiety scores than placebo, even at the lower doses (p < 0.05). In contrast, a smaller double blind RCT (22) of adolescents 12–18 years with IBS (n = 33) showed overall improvement and reductions in abdominal pain as well as diarrhea in the amitriptyline group compared to placebo (p < 0.05 for each). In another study, 61% of children with FAP receiving TCA demonstrated decreased pain or improved daily functioning (23). However, this response was lesser compared to SSRIs (61% vs. 75% improvement, p = 0.003). TCAs, like many other antidepressants, have an FDA black box warning and can be associated with side effects such drowsiness, constipation, and worsened mood including suicidal ideation (25). Abruptly stopping the drug could lead to sleep disturbances and nightmares and overdose can be lethal (26). It is usually imperative to ask about a family history of sudden cardiac deaths suggestive of familial arrythmias prior to starting amitriptyline. While TCAs are associated with a higher risk of cardiac arrhythmias (27), studies have shown no higher risk for QTc interval prolongation in children (28, 29). It should also be noted that the long-term effects of these medications on the developing brain are unknown, and more studies are needed to determine the effects on memory and cognition in children. In our study, we noted significant improvements in abdominal pain with the use of amitriptyline in children with FAPD and the doses that were used were equivalent to what is routinely used in clinical practice and used in other studies (24, 25). However, compared to baseline, it did not significantly improve nausea or disability at 3 months post treatment. Nonetheless, changes in disability were better when compared to cyproheptadine and it can be speculated that perhaps this is due to not only improvements in pain, but the anxiolytic effects as well.
Cyproheptadine is a serotonin (5HT3) receptor antagonist with antihistaminic and anticholinergic effects, commonly used to treat FAPD in children. Doses in the range of 0.2–0.6 mg/kg/day or 4 mg up once or twice daily have been used (24). It can also improve headaches and sleep disturbances. In a small Iranian double blinded randomized controlled trial of children with FAP (30), cyproheptadine was superior to placebo in improving global symptoms (p = 0.005), pain intensity (p = 0.001) and frequency (p = 0.002). Similarly, a retrospective review of 80 children with dyspepsia treated with cyproheptadine showed a 55% response rate and 30% adverse event rate (31). In contrast, another retrospective study of 300 children with FAPD ages 1–18 years showed a 73% response rate and 32% adverse event rate with the use of cyproheptadine (32). Common adverse effects included sleepiness, weight gain and mood changes. While lower seizure threshold has been reported in animal models (33), this adverse effect has not been described in prior studies including pediatric DGBI patients. Surprisingly, cyproheptadine performed the poorest in the current study compared to PENFS and amitriptyline. One plausible explanation is tachyphylaxis to the drug which would require cycling to improve its sustained efficacy. Also, previous studies have reported benefits in children younger than 12 years (31) and it is possible that cyproheptadine may work better in younger children. However, more studies are needed to determine the most likely responders.
Auricular PENFS is the only FDA cleared treatment for IBS and associated abdominal pain conditions. It accesses central pain pathways and has been shown to modulate the limbic system in an animal model of IBS (34). More specifically, it decreases firing of neurons in the amygdala by greater than 50% (34). Studies have suggested that the amygdala is involved in the pathophysiology of IBS in adults (35, 36). In a randomized sham-controlled clinical trial in children with FAPD, decrease in worst pain and composite pain scores and improved disability and well-being were noted in patients receiving the active device vs. sham (7). A sub-analysis of just IBS patients showed significant improvements in abdominal pain and global symptoms after 4 weeks of PENFS treatment compared to sham (37). Interestingly, another study showed that those who responded to treatment were patients with vagal nerve insufficiency, suggesting a vagally mediated pathway (38). Improvements in resting as well as induced abdominal pain and nausea, sleep disturbances, pain catastrophizing, somatization, anxiety and disability are sustained at 6–12 months in adolescents with FAPD (8). PENFS also improved these outcomes at 3 weeks and 3 months in children with functional dyspepsia (9). Another proposed mechanism for PENFS includes microbiome changes after treatment, which may also reflect changes in vagal anti-inflammatory pathways (39). In a recent study, patients with IBS, post-PENFS treatment, were found to have decreased Clostridial species and long chain fatty acid microbial pathways that have been implicated in the pathophysiology of IBS (39). In that study, improvements in abdominal pain, functioning, and catastrophizing were noted as well. Similar to previous studies, our study confirms the benefits of PENFS in improving abdominal pain and disability. Unlike SMT, it demonstrated a trend for improvement in nausea. Compared to side effects associated with medications, no serious adverse events have previously been reported with PENFS therapy (40).
In our study, 59% of the patients in the PENFS group had failed prior SMT. It is possible that the PENFS group may have more severe, refractory symptoms compared to SMT groups which could have affected treatment outcomes. Similarly, the higher ratio of males to females in the PENFS group could also impact the results of our study.
It is interesting to consider how PENFS differs mechanistically from medications like amitriptyline that are considered “neuromodulators”. While the exact mechanism for the therapeutic effect of these drugs is not known, some have suggested different central pathways for PENFS and SMT. A recent study investigated connectivity differences between PENFS and standard medical therapy in adults with fibromyalgia (41). In that study, PENFS increased connectivity post-treatment from the right posterior insula to the right middle occipital gyrus, left midbrain, left anterior insula, and right lobule IX of the cerebellum that was associated with decreased pain scores. Conversely, those treated with standard medical therapy without PENFS, which included tricyclic antidepressants, were found to have decreased connectivity from the right posterior insula to the other brain regions. These changes were also associated with decreased pain scores. This was an interesting finding and suggests that PENFS may promote neuromodulation across brain areas and networks that are different from SMT.
This is the first study to compare treatment outcomes of PENFS with SMT. We had a moderate sample size in each group allowing for meaningful comparisons. We used validated, pediatric questionnaires that provided objective assessments however, the modified versions over 1 week were not validated compared to the standard questionnaires assessing symptoms over 2 weeks. Unfortunately, the study design did not allow us to evaluate baseline psychological comorbidities that could theoretically impact treatment outcomes. Similarly, the retrospective study design precluded assessment of other biopsychosocial factors that could contribute to symptoms. Also, data were assessed at 3 months, and it would have been ideal to have a longer follow-up with the entire cohort. Only a prospective head-to-head trial would allow for this longer follow-up since medications are typically discontinued if there are no benefits after proper dose adjustments. Future trials should also include prospective analysis of adverse event comparisons between PENFS and SMT.
In conclusion, PENFS improved abdominal pain and disability in adolescents with FAPD with a trend for improvement in nausea when assessed three months after treatment. Amitriptyline also improved abdominal pain compared to baseline. PENFS showed a trend for greater improvement in disability compared to SMT. PENFS was superior in decreasing abdominal pain compared with cyproheptadine while amitriptyline was superior to cyproheptadine in improving disability. These findings may help guide provider choices when considering pharmacotherapy in children with FAPD, particularly in cases involving patients that have side-effects to medications or drug interactions. Several studies now support PENFS as an effective treatment option to pharmacotherapy with a relatively safe side-effect profile.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Cincinnati Children's Hospital Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The Ethics Committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because of the retrospective study design and chart review.
Author contributions
NS designed the study, supervised data collection as well as analysis and wrote the manuscript. MW and MV did data collection and contributed to the manuscript. KE-C and KG provided expertise in PENFS and FAPD, the study design, interpretation of data analysis and edited each iteration of the manuscript. JH organized the data and RS and LF organized the data as well as performed data analyses for the study. All authors contributed to the article and approved the submitted version.
Funding
NS was supported by the NIH NIDDK K23 grant (1K23DK135797-01) and the CCHMC Procter Scholar Award.
Acknowledgments
Peter Farrell for assistance with electronic database management.
Conflict of interest
KE is a consultant for Neuraxis.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Hyams JS, Di Lorenzo C, Saps M, Shulman RJ, Staiano A, van Tilburg M. Functional disorders: children and adolescents. Gastroenterology. (2016) S0016–5085(16):00181–5. doi: 10.1053/j.gastro.2016.02.015
2. Cunningham NR, Jagpal A, Tran ST, Kashikar-Zuck S, Goldschneider KR, Coghill RC, et al. Anxiety adversely impacts response to cognitive behavioral therapy in children with chronic pain. J Pediatr. (2016) 171:227–33. doi: 10.1016/j.jpeds.2016.01.018
3. Dhroove G, Chogle A, Saps M. A million-dollar work-up for abdominal pain: is it worth it? J Pediatr Gastroenterol Nutr. (2010) 51(5):579–83. doi: 10.1097/MPG.0b013e3181de0639
4. Santucci NR, Saps M, van Tilburg MA. New advances in the treatment of paediatric functional abdominal pain disorders. Lancet Gastroenterol Hepatol. (2020) 5(3):316–28. doi: 10.1016/S2468-1253(19)30256-0
5. Rexwinkel R, de Bruijn C, Gordon M, Benninga MA, Tabbers MM. Pharmacologic treatment in functional abdominal pain disorders in children: a systematic review. Pediatrics. (2021) 147(6):e2020042101. doi: 10.1542/peds.2020-042101
6. Saps M, Biring HS, Pusatcioglu CK, Mintjens S, Rzeznikiewiz D. A comprehensive review of randomized placebo-controlled pharmacological clinical trials in children with functional abdominal pain disorders. J Pediatr Gastroenterol Nutr. (2015) 60(5):645–53. doi: 10.1097/MPG.0000000000000718
7. Kovacic K, Hainsworth K, Sood M, Chelimsky G, Unteutsch R, Nugent M, et al. Neurostimulation for abdominal pain-related functional gastrointestinal disorders in adolescents: a randomised, double-blind, sham-controlled trial. Lancet Gastroenterol Hepatol. (2017) 2(10):727–37. doi: 10.1016/S2468-1253(17)30253-4
8. Santucci NR, King C, El-Chammas KI, Wongteerasut A, Damrongmanee A, Graham K, et al. Effect of percutaneous electrical nerve field stimulation on mechanosensitivity, sleep, and psychological comorbidities in adolescents with functional abdominal pain disorders. Neurogastroenterol Motil. (2022) 34(8):e14358. doi: 10.1111/nmo.14358
9. Santucci NR, Beigarten AJ, Khalid F, El-Chammas KI, Graham K, Sahay R, et al. Percutaneous electrical nerve field stimulation in children and adolescents with functional dyspepsia-integrating a behavioral intervention. Neuromodulation. (2023) :S1094–7159(23)00707-9. doi: 10.1016/j.neurom.2023.07.005. [published online ahead of print]37589640
10. Laird KT, Sherman AL, Smith CA, Walker LS. Validation of the abdominal pain index using a revised scoring method. J Pediatr Psychol. (2015) 40(5):517–25. doi: 10.1093/jpepsy/jsu118
11. Russell AC, Stone AL, Wang A, Walker LS. Development and validation of a nausea severity scale for assessment of nausea in children with abdominal pain-related functional gastrointestinal disorders. Children. (2018) 5(6):68. doi: 10.3390/children5060068
12. Walker LS, Greene JW. The functional disability inventory: measuring a neglected dimension of child health status. J Pediatr Psychol. (1991) 16(1):39–58. doi: 10.1093/jpepsy/16.1.39
13. Walker LS, Sherman AL, Bruehl S, Garber J, Smith CA. Functional abdominal pain patient subtypes in childhood predict functional gastrointestinal disorders with chronic pain and psychiatric comorbidities in adolescence and adulthood. Pain. (2012) 153(9):1798–806. doi: 10.1016/j.pain.2012.03.026
14. Kovacic K, Kapavarapu PK, Sood MR, Li BU, Nugent M, Simpson P, et al. Nausea exacerbates symptom burden, quality of life, and functioning in adolescents with functional abdominal pain disorders. Neurogastroenterol Motil. (2019) 31(7):e13595. doi: 10.1111/nmo.13595
15. Russell AC, Stone AL, Walker LS. Nausea in children with functional abdominal pain predicts poor health outcomes in young adulthood. Clin Gastroenterol Hepatol. (2017) 15(5):706–11. doi: 10.1016/j.cgh.2016.07.006
16. Beinvogl B, Burch E, Snyder J, Schechter N, Hale A, Okazaki Y, et al. Multidisciplinary treatment reduces pain and increases function in children with functional gastrointestinal disorders. Clin Gastroenterol Hepatol. (2019) 17(5):994–6. doi: 10.1016/j.cgh.2018.07.025
17. Lynch-Jordan AM, Sil S, Peugh J, Cunningham N, Kashikar-Zuck S, Goldschneider KR. Differential changes in functional disability and pain intensity over the course of psychological treatment for children with chronic pain. Pain. (2014) 155(10):1955–61. doi: 10.1016/j.pain.2014.06.008
18. Korterink JJ, Rutten JM, Venmans L, Benninga MA, Tabbers MM. Pharmacologic treatment in pediatric functional abdominal pain disorders: a systematic review. J Pediatr. (2015) 166(2):424–31. doi: 10.1016/j.jpeds.2014.09.067
19. Browne PD, Nagelkerke SC, van Etten-Jamaludin FS, Benninga MA, Tabbers MM. Pharmacological treatments for functional nausea and functional dyspepsia in children: a systematic review. Expert Rev Clin Pharmacol. (2018) 11(12):1195–208. doi: 10.1080/17512433.2018.1540298
20. de Bruijn CM, Rexwinkel R, Gordon M, Benninga MA, Tabbers MM. Antidepressants for functional abdominal pain disorders in children and adolescents. Cochrane Database Syst Rev. (2021) 2(2):CD008013. doi: 10.1002/14651858.CD008013.pub3
21. Saps M, Youssef N, Miranda A, Nurko S, Hyman P, Cocjin J, et al. Multicenter, randomized, placebo-controlled trial of amitriptyline in children with functional gastrointestinal disorders. Gastroenterology. (2009) 137(4):1261–9. doi: 10.1053/j.gastro.2009.06.060
22. Bahar RJ, Collins BS, Steinmetz B, Ament ME. Double-blind placebo-controlled trial of amitriptyline for the treatment of irritable bowel syndrome in adolescents. J Pediatr. (2008) 152(5):685–9. doi: 10.1016/j.jpeds.2007.10.012
23. Zar-Kessler CA, Belkind-Gerson J, Bender S, Kuo BM. Treatment of functional abdominal pain with antidepressants: benefits, adverse effects, and the gastroenterologist’s role. J Pediatr Gastroenterol Nutr. (2017) 65(1):16–21. doi: 10.1097/MPG.0000000000001416
24. Saps M, Miranda A. Gastrointestinal pharmacology. Handb Exp Pharmacol. (2017) 239:147–76. doi: 10.1007/164_2016_119
25. Hussain SZ, Hyman PE. Psychotropic medications for pediatric functional gastrointestinal disorders. J Pediatr Gastroenterol Nutr. (2014) 59(3):280–7. doi: 10.1097/MPG.0000000000000445
26. Hyman PE, Santucci NR. Approach to the child with a functional gastrointestinal disorder. In: Wyllie R, Hyams JS, Kay M, editors. Pediatric gastrointestinal and liver disease. 6th ed. Elsevier (2021). p. 61–69.e3. https://www.sciencedirect.com/science/article/pii/B9780323672931000074
27. Blair J, Taggart B, Martin A. Electrocardiographic safety profile and monitoring guidelines in pediatric psychopharmacology. J Neural Transm (Vienna). (2004) 111(7):791–815. doi: 10.1007/s00702-004-0153-8
28. Klein LJ, Chamberlain RC, Bonello K, Milazzo AS, Noel RJ. Electrocardiogram before tricyclic antidepressant use: minimal impact in pediatric functional gastrointestinal disorders. J Pediatr Gastroenterol Nutr. (2021) 73(4):523–8. doi: 10.1097/MPG.0000000000003226
29. Chogle A, Saps M. Electrocardiograms changes in children with functional gastrointestinal disorders on low dose amitriptyline. World J Gastroenterol. (2014) 20(32):11321. doi: 10.3748/wjg.v20.i32.11321
30. Sadeghian M, Farahmand F, Fallahi GH, Abbasi A. Cyproheptadine for the treatment of functional abdominal pain in childhood: a double-blinded randomized placebo-controlled trial. Minerva Pediatr. (2008) 60(6):1367–74.18971897
31. Rodriguez L, Diaz J, Nurko S. Safety and efficacy of cyproheptadine for treating dyspeptic symptoms in children. J Pediatr. (2013) 163(1):261–7. doi: 10.1016/j.jpeds.2012.12.096
32. Madani S, Cortes O, Thomas R. Cyproheptadine use in children with functional gastrointestinal disorders. J Pediatr Gastroenterol Nutr. (2016) 62(3):409–13. doi: 10.1097/MPG.0000000000000964
33. Singh D, Goel RK. Proconvulsant potential of cyproheptadine in experimental animal models. Fundam Clin Pharmacol. (2010) 24(4):451–5. doi: 10.1111/j.1472-8206.2009.00797.x
34. Babygirija R, Sood M, Kannampalli P, Sengupta JN, Miranda A. Percutaneous electrical nerve field stimulation modulates central pain pathways and attenuates post-inflammatory visceral and somatic hyperalgesia in rats. Neuroscience. (2017) 356:11–21. doi: 10.1016/j.neuroscience.2017.05.012
35. Labus JS, Dinov ID, Jiang Z, Ashe-McNalley C, Zamanyan A, Shi Y, et al. Irritable bowel syndrome in female patients is associated with alterations in structural brain networks. Pain. (2014) 155(1):137–49. doi: 10.1016/j.pain.2013.09.020
36. Qi R, Liu C, Ke J, Xu Q, Ye Y, Jia L, et al. Abnormal amygdala resting-state functional connectivity in irritable bowel syndrome. AJNR Am J Neuroradiol. (2016) 37(6):1139–45. doi: 10.3174/ajnr.A4655
37. Krasaelap A, Sood MR, Li BU, Unteutsch R, Yan K, Nugent M, et al. Efficacy of auricular neurostimulation in adolescents with irritable bowel syndrome in a randomized, double-blind trial. Clin Gastroenterol Hepatol. (2020) 18(9):1987–94. doi: 10.1016/j.cgh.2019.10.012
38. Kovacic K, Kolacz J, Lewis GF, Porges SW. Impaired vagal efficiency predicts auricular neurostimulation response in adolescent functional abdominal pain disorders. Am J Gastroenterol. (2020) 115(9):1534–8. doi: 10.14309/ajg.0000000000000753
39. Castillo DF, Denson LA, Haslam DB, Hommel KA, Ollberding NJ, Sahay R, et al. The microbiome in adolescents with irritable bowel syndrome and changes with percutaneous electrical nerve field stimulation. Neurogastroenterol Motil. (2023) 35(7):e14573. doi: 10.1111/nmo.14573
40. Roberts A, Sithole A, Sedghi M, Walker CA, Quinn TM. Minimal adverse effects profile following implantation of periauricular percutaneous electrical nerve field stimulators: a retrospective cohort study. Medical Devices: Evidence and Research. (2016) 9:389–93. doi: 10.2147/MDER.S107426
Keywords: neurostimulation, amitriptyline, cyproheptadine, pediatrics, chronic abdominal pain
Citation: Santucci NR, Sahay R, El-Chammas KI, Graham K, Wheatley M, Vandenbrink M, Hardy J and Fei L (2023) Percutaneous electrical nerve field stimulation compared to standard medical therapy in adolescents with functional abdominal pain disorders. Front. Pain Res. 4:1251932. doi: 10.3389/fpain.2023.1251932
Received: 4 July 2023; Accepted: 6 September 2023;
Published: 19 September 2023.
Edited by:
Anna Woodbury, Emory University, United StatesReviewed by:
Jamie Kitzman, Emory University, United StatesGeetanjali Bora, George Washington University, United States
Richard Noel, Duke University, United States
© 2023 Santucci, Sahay, El-Chammas, Graham, Wheatley, Vandenbrink, Hardy and Fei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Neha R. Santucci bmVoYS5zYW50dWNjaUBjY2htYy5vcmc=
 Neha R. Santucci
Neha R. Santucci Rashmi Sahay3
Rashmi Sahay3 Lin Fei
Lin Fei