- 1Department of Anesthesia, Stark Neurosciences Research Institute, Indiana University School of Medicine, Indianapolis, IN, United States
- 2Division of Hematology/Oncology, Department of Medicine, Indiana University School of Medicine, Indianapolis, IN, United States
- 3Center for Integrative Health, The Ohio State University, Columbus, OH, United States
- 4Indiana University Simon Cancer Center, Indianapolis, IN, United States
- 5Department of Anesthesiology, Chronic Pain and Fatigue Research Center, University of Michigan Medical School, Ann Arbor, MI, United States
- 6Department of Anesthesiology and Perioperative Care, School of Medicine, Susan Samueli Integrative Health Institute, University of California, Irvine, Irvine, CA, United States
Background: Pain is a common, debilitating, and poorly understood complication of sickle cell disease (SCD). The need for clinical pain management of SCD is largely unmet and relies on opioids as the main therapeutic option, which leads to a decreased quality of life (QoL). According to the literature, acupuncture has shown certain therapeutic effects for pain management in SCD. However, these clinical studies lack the guidance of Traditional Chinese Medicine (TCM) Syndrome Differentiation principles for treatment.
Aim: To characterize differences in clinical presentation amongst TCM diagnosed Syndromes in SCD patients.
Method: Fifty-two patients with SCD and 28 age- and sex-matched healthy controls (HCs) were enrolled in an ongoing trial of acupuncture. Each participant completed a series of questionnaires on pain, physical function, fatigue, sleep, anxiety, depression and QoL and underwent cold- and pressure-based quantitative sensory testing at baseline. Data on prescription opioid use over the 12 months prior to study enrollment was used to calculate mean daily morphine milligram equivalents (MME). Differences among the three TCM Syndromes were analyzed by one-way ANOVA followed by Tukey post hoc testing. Two-sample t-tests were used to compare SCD and HC groups.
Results: TCM diagnosis criteria classified SCD patients into one of three TCM Syndromes: (a) Equal; (b) Deficiency; and (c) Stagnation. The Stagnation group exhibited higher pain interference, physical dysfunction, nociplastic pain, fatigue, anxiety, depression, MME consumption and lower sleep quality and QoL compared to the Equal group. Few differences were observed between HCs and the Equal SCD group across outcomes. Deficiency and Stagnation groups were differentiated with observed- and patient-reported clinical manifestations.
Conclusion: These findings suggest that TCM diagnosed Syndromes in SCD can be differentially characterized using validated objective and patient-reported outcomes. Because characteristics of pain and co-morbidities in each SCD patient are unique, targeting specific TCM “Syndromes” may facilitate treatment effectiveness with a Syndrome-based personalized treatment plan that conforms to TCM principles. These findings lay the foundation for the development of tailored acupuncture interventions based on TCM Syndromes for managing pain in SCD. Larger samples are required to further refine and validate TCM diagnostic criteria for SCD.
1 Introduction
Pain in sickle cell disease (SCD) is both common and debilitating. While the pain can be chronic in nature, severe acute vaso-occlusive pain crises (VOCs) are also present in many of these individuals, resulting in recurrent pain episodes that are unpredictable, and frequently require hospitalization. As such VOCs are a leading factor lowering the quality of life (QoL) in SCD (1). While opioids are the main therapeutic option for managing these events, there are limited alternative and effective treatments beyond opioids for these patients (2).
Acupuncture, a mainline component of traditional Chinese medicine (TCM), has been used for over four-thousand years and has been recognized as an effective treatment approach for chronic and acute pain conditions (3, 4). Emerging data suggests that acupuncture may alleviate pain in both adult and pediatric patients with SCD (5–8). However, a key principle of TCM practice is “treatment based on differentiation of Syndrome” wherein patients are evaluated on a set of diagnostic criteria which can allow classification into a specific TCM “Syndrome” for customized treatment. This classification, along with consideration of additional individual specific symptom patterns, guides the tailored selection of acupuncture points to treat a particular patient (9, 10).
Two recent studies categorized different TCM patterns in women with pelvic pain (11) or fibromyalgia (12). These studies showed that there were 6 TCM patterns in female patients with pelvic pain whereas 3 TCM patterns in women with fibromyalgia. There have been no studies on TCM Syndrome differentiation in SCD. The small sample size and failure to apply TCM principles in previous studies of SCD have made it difficult to assess the full potential benefit of acupuncture for SCD. For example, it is not known if subgroups of SCD patients exhibit different pathogenic features when classified by TCM principles. Identification of different subtypes may also be relevant for other therapies in SCD.
The classic pathophysiology of SCD is polymerization of deoxy sickle hemoglobin which leads to a complex interplay of disrupted red blood cell rheology, hemolysis, inflammation, and vasculopathy. However, pain in SCD is not well understood, and is characterized by substantial heterogeneity in clinical manifestation and underlying pathophysiology (13–21). Notably, clinical pain varies in many aspects including quality, intensity, location, and duration under both chronic and VOC stages. The onset of VOCs can happen anywhere in the body, with the most painful sites being consistent or fixed, and ranging from mild to severe intensity during a specific VOC. The frequency of VOCs also varies widely in SCD with a recent systematic review reporting a range of 0–18.2 per year (22), and variable distribution of VOCs throughout the year between individuals. Finally, occurrence of VOCs can also be triggered by various multidimensional factor(s), such as temperature, mental stress, dehydration, and/or fatigue. These individual differences add significant challenges in the currently unmet management of clinical pain in SCD and thus strongly warrants the need for individualized diagnosis and personalized treatment. Establishing TCM Syndrome differentiation in SCD to help guide acupuncture treatment is the current priority.
Here we report preliminary baseline data from our ongoing randomized sham-controlled trial of acupuncture for SCD (ClinicalTrials.gov Identifier: NCT05045820). Patients with SCD were classified into three different TCM Syndrome groups prior to treatment according to TCM principles. Our objective was to investigate differences in patient reported outcome measures (PROMs), quantitative sensory testing (QST), and blood-based assessments across these TCM Syndrome groups.
2 Methods
2.1 Participants
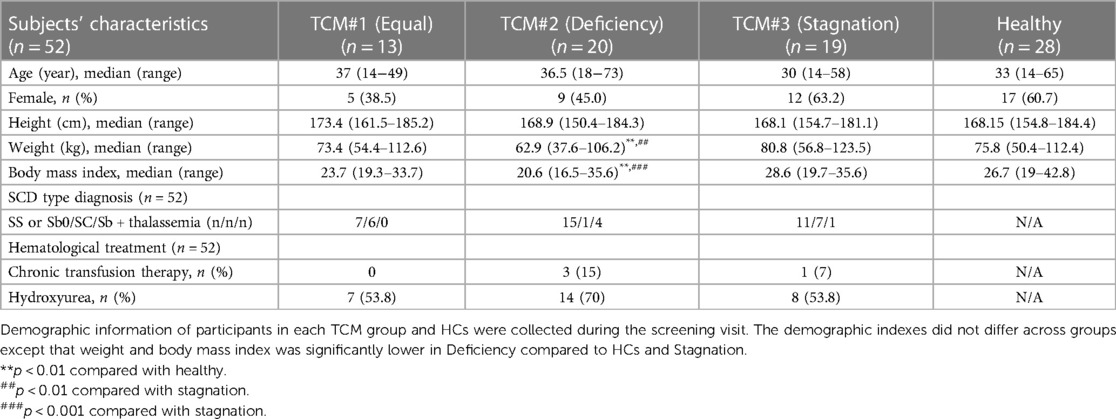
Fifty-two patients with SCD (25 male and 27 female), aged 14–73, were enrolled. The main inclusion eligibility criteria included: (1) experiencing chronic pain in the past 6 months or at least one VOC in the past 12 months, (2) willing to maintain current treatments, and (3) willing to not introduce any new medications or treatment modalities for control of pain symptoms during the study. Detailed inclusion and exclusion criteria can be found in Supplementary Table 1. Participants’ demographics can be found in Table 1. Twenty-eight pain-free ethnicity-, age- and gender-matched participants without SCD were enrolled as healthy controls (HCs). A subset of SCD and HCs were matched on age and gender for comparisons of PROMs (n = 25–27 each) and QST (n = 21–28 each).
Patients underwent a TCM diagnostic examination and phenotyping with validated PROMs, QST, and blood tests. HCs completed PROMs, QST, and blood tests. The study was approved by the Institutional Review Board at Indiana University. All patients provided written informed consent before the study.
2.2 TCM diagnosis
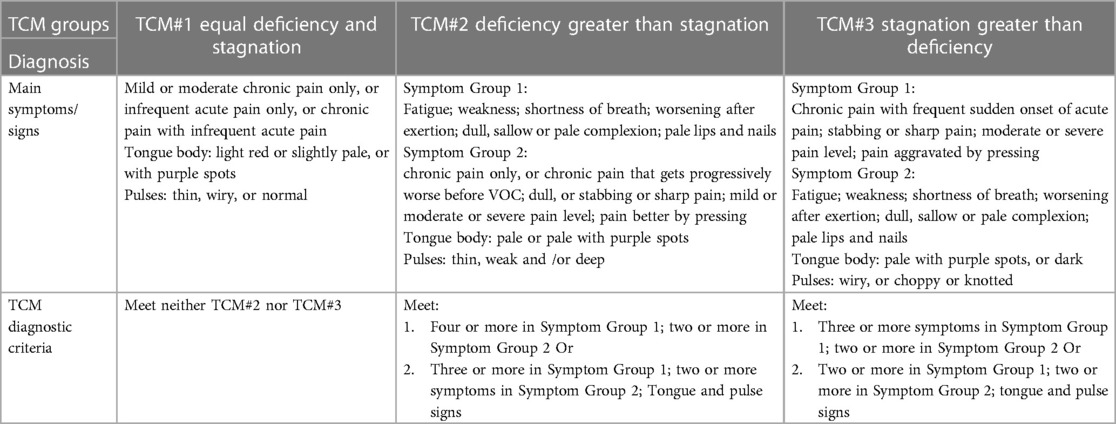
Participants who completed the baseline stage of data collection were then randomized to either verum or sham acupuncture for 5 weeks. All data presented here are cross-sectional at baseline. Longitudinal results related with acupuncture intervention will be reported elsewhere. TCM diagnosis was performed before the treatment by one practitioner with over 15 years of clinical experience. This practitioner utilized a standardized procedure with TCM diagnostic methods such as “Observation,” “Listening and Smelling,” “Inquiry,” and “Pulse-feeling and palpation” (see Table 2 for a full description of diagnostic criteria) (23). Following differentiation of Syndrome, patients were classified into one of three TCM Syndrome groups: (a) “Stagnation greater than Deficiency” (abbreviated as “Stagnation”), (b) “Deficiency greater than Stagnation” (abbreviated as “Deficiency”), and (c) “Equal Stagnation and Deficiency” (abbreviated as “Equal”). Diagnoses were confirmed in a blinded manner by a second practitioner with more than 20 years of experience.
2.3 Patient reported outcome measures (PROMs)
Pain (pain intensity and interference), physical function, and satisfaction with one's social role were assessed with the PROMIS-29 (24). Neuropathic pain symptoms were evaluated using the PainDETECT (25, 26). Nociplastic pain is a mechanistic term describing pain that arises or is sustained by augmented and/or dysregulated central nervous system processing (27–30). Nociplastic pain is distinguished from nociceptive pain (due to peripheral inflammation/tissue damage) and neuropathic pain (due to damage or dysfunction of nerves). While nociplastic pain has a distinct pathophysiology from nociceptive and neuropathic pain, these pain mechanisms often overlap within individuals contributing to the intractability of this type of chronic pain. Fibromyalgia is the prototypical nociplastic pain condition and key symptoms include pain in multiple body regions, hypersensitivity to a variety of external stimuli (e.g., mechanical/tactile, thermal, and visual, auditory), fatigue, sleep disturbances, cognitive dysfunction, and poor mood (i.e., depression and anxiety)—all of which are also observed in many SCD individuals (14, 17, 31–34). The Fibromyalgia Survey Questionnaire (FSQ), which consists of the Widespread Pain Index and the Symptom Severity scale (35, 36), was utilized as a surrogate measure of nociplastic pain. Fatigue and quality of sleep were assessed using the Multi-dimensional Fatigue Inventory (MFI) (37) and the Pittsburgh Sleep Quality Index (PSQI) (14), respectively. Anxiety and depression were evaluated using Hospital Anxiety and Depression Scale (HADS) (14).
2.4 Quantitative sensory testing (QST)
QST is an established experimental protocol that examines ascending excitatory and descending inhibitory aspects of pain processing by assessing an individual's perceptual response to applied stimuli. QST was conducted at up to three different body sites: the primary testing site was the most painful area of the body as reported by each patient; the dominant-side ventral forearm, and/or the dominant-side upper trapezius muscle as in previous SCD studies (38, 39). For non-SCD controls, primary testing sites matched those identified in SCD patients in addition to testing at the dominant forearm and/or trapezius. The QST battery was performed only at the baseline visit in the following order from least painful to most painful/aversive test, following standardized methods: mechanical detection threshold → mechanical pain threshold → thermal detection threshold → thermal pain threshold → pressure pain threshold → pressure pain tolerance → conditioned pain modulation test. To prevent peripheral sensitization: each testing in this study was alternatively performed between different body areas, each trial within each test has a 30-second to 1-min interval, with additional minimum of 5–10 min break between each test.
Mechanical Detection Threshold / Mechanical Pain Threshold was examined using von Frey monofilaments (Stoelting Co., USA) and calibrated pinprick stimuli (MRC Systems GmbH, Germany). Each von Frey monofilament was applied three times in ascending sequence until the stimulus was detected in at least two of the three trials. Then, the next lower von Frey monofilament was applied, and the lowest filament to be detected at least twice was determined as the mechanical detection threshold. The mechanical pain threshold was determined using different pinprick probes applied to the skin surface of each site. Testing started with a stimulation intensity of 8 mN and in each case, the next higher pinprick stimulator was applied until the perception of “touch” changed its quality towards an additional “sharp”, “pricking” or “stinging” impression. The corresponding intensity represented the first suprathreshold value. Once the first painful stimulus was perceived, the testing direction was changed towards lower stimulus intensities until the first stimulus, when applied to the skin, that was perceived as “blunt” and no longer as being “sharp”, “pricking” or “stinging” (subthreshold value). Again, a directional change towards higher intensities occurred and the cycle was repeated until all five supra- and five subthreshold values were found that represent the inflection point to determine the mechanical pain threshold.
Thermal (heat/cold) Detection/Pain Threshold was determined at each testing site using a TCA11 (QST-Lab, France) with a thermal probe in contact with the subject's skin surface. The thermode temperature was increased/decreased from a baseline temperature by adapting to the individual's own body temperature at a rate of 0.5–1°C/s. Subjects indicated the thermal detection threshold (when they first felt the thermal stimuli) and the pain threshold (when they first felt pain from the thermal stimuli). The average of three trials of each test was used for analysis.
Temporal Summation of Pain (TSP) was assessed using single stimulus in triplicate with a 256 mN pinprick (MRC Systems GmbH) applied to the skin surface of the selected sites, followed by a series of 10 identical stimuli (1 Hz—metronome-guided). TSP was calculated as the average pain rating from the series of 10 stimuli minus the average pain rating from the three trials with the single stimulus.
Pressure Pain Threshold (PPT)/Pressure Pain Tolerance (PPTol) was assessed using a digital, handheld, clinical grade pressure algometer (Algometer II, Somedic SenseLab AB, Sweden). The pressure was manually increased at a rate of 50 kPa/s (1,000 kPa max) until participants indicated that the sensation of pressure becomes one of faint pain (PPT) and the maximum pressure pain that the participant can tolerate (PPTol), respectively. The average of 3 trials per site was used for analysis.
Conditioned Pain Modulation (CPM): A sustained pressure pain stimulus was used as the conditioning stimulus delivered using a cuff inflator on the gastrocnemius muscle of the non-dominant leg to elicit moderate pain (pain rating at 40–60 on a scale of 100) (40, 41). The PPT was then measured 3 times at the primary site and at the dominant trapezius muscle prior to and during the cuff stimulation. Pain ratings were obtained every 15 s prior to and during cuff conditioned stimuli. CPM magnitude was calculated as PPT during cuff pressure minus PPT at baseline.
2.5 SCD specific outcomes and morphine milligram equivalent (MME)
The severity and frequency of pain crises were assessed by the Adult Sickle Cell Quality-of-Life Measurement Information System (ASCQ-ME) (42), and the number of patient-reported pain crises in the preceding 12 months was documented, a method which is commonly used in pain research for SCD (15, 43). Pain-related QoL was evaluated using the Pediatric Quality of Life Inventory (PedsQL) (44). The average MME in the preceding 12 months was calculated using prescription data for opioid use before study enrollment. These data were retrieved from patients’ medical records and used to calculate the mean daily MME for each opioid, following the Centers for Disease Control recommended MME dose calculation formula (45): {strength per unit × number of units × number of prescriptions × MME conversion factor}/number of days = MME/Day.
2.6 Hematological analysis
Laboratory blood testing, including complete blood cell count, reticulocytes, and hemoglobin electrophoresis, was conducted on the day of TCM diagnosis (Supplementary Table 4).
2.7 Statistical analysis
Assessment of differences between TCM Syndrome groups was performed using one-way analysis of variance (ANOVA) followed by Tukey post hoc testing. Two-sample t-tests (two tailed) were used to compare a subset of SCD patents and matched HCs at baseline. P-values less than 0.05 were considered significant. All analyses were conducted using GraphPad Prism software. The study was appropriately powered at greater than 0.90 with the current sample size of n = 80. Using the Kolmogorov-Smirnov test, the data was found to be distributed normally.
3 Results
3.1 Demographics
Demographic information is shown in Table 1. TCM groups did not differ in age (p = 0.373), although the Stagnation group presented with a lower median age as compared to other groups. The Deficiency group showed significantly lower weight and body mass index (BMI) as compared with both the HCs and Stagnation group (p = 0.0032; p = 0.0002), respectively. The Stagnation group also had more participants numerically with chronic transfusion compared to the Deficiency and Equal groups. The distribution of hydroxyurea users was also numerically higher in the Deficiency group compared to the other two groups, but this was not statistically significant. Each SCD genotype was equally distributed across the three TCM groups.
3.2 Clinical characteristics
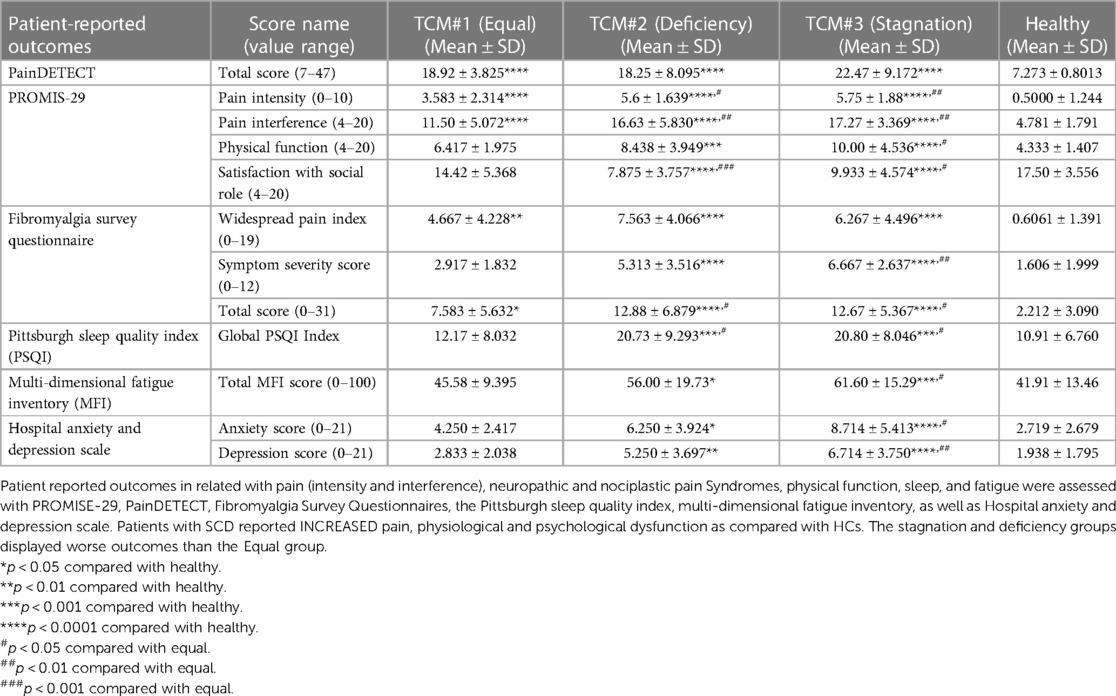
All three TCM Syndrome groups showed increased pain intensity and interference, as well as symptoms of neuropathic pain, compared to the control group (Table 3). However, only the Stagnation and Deficiency groups reported worse sleep quality, increased levels of fatigue, anxiety, and depression relative to HCs. In addition, it is worth noting that patients with SCD also reported pain in more body areas on the Widespread Pain Index and higher overall fibromyalgia severity. As seen in Table 2, these Syndromes were primarily differentiated on the characteristics of pain (e.g., quality, intensity, onset, time of duration) and physical dysfunction (e.g., fatigue/weakness) followed by other symptoms (e.g., ease of sweating, facial complexion, voice, lips or nails, characteristics of “Tongue” and “Pulses”). The clinical manifestations of the Stagnation group featured severe pain, sudden onset, stabbing or sharp pain that is exacerbated by pressure, irritability, fatigue, and weakness. The clinical manifestations of Deficiency group featured weakness, fatigue, pale lips and nails, sallow complexion, chronic pain that gradually worsens. The clinical manifestations of Equal featured fatigue and pain symptoms, with no clear distinction between Deficiency of qi and blood and Stagnation. The clinical manifestations within each category provide insights into the specific symptoms and characteristics associated with different TCM Syndrome types in SCD.
Compared to the Equal group, both the Stagnation (p = 0.0013) and Deficiency (p = 0.0046) groups reported increased multi-dimensional pain interference. Both groups also showed higher total scores for the FSQ (p = 0.0328 vs. Deficiency and p = 0.0477 vs. Stagnation) compared to the Equal group, while the Stagnation group displayed significantly higher Symptom Severity Scores (p = 0.0013). Additionally, both the Deficiency and Stagnation groups exhibited worse sleep quality than the Equal group (p = 0.0293 vs. Deficiency and p = 0.0277 vs. Stagnation). Furthermore, compared to the Equal group, the Stagnation group showed significantly reduced physical function (p = 0.0131) and elevated fatigue (p = 0.0342), anxiety (p = 0.0119), and depression (p = 0.0035).
Notably, the Stagnation (n = 6.526 ± 4.718) and Deficiency (n = 5.19 ± 3.855) groups exhibited a higher frequency of pain crises compared to the Equal group (n = 1.714 ± 1.684) (Figure 1A) along with higher combined pain crisis recency/severity as assessed by the ASCQ-ME (Figure 1C), and lowest SCD pain-related QoL (Figure 1E), despite no difference in pain severity during crisis between groups (Figure 1D). Additionally, both Stagnation (p = 0.0011) and Deficiency (p = 0.0029) groups had significantly higher opioid usage over the past 12 months compared to the Equal group (Figure 1B).
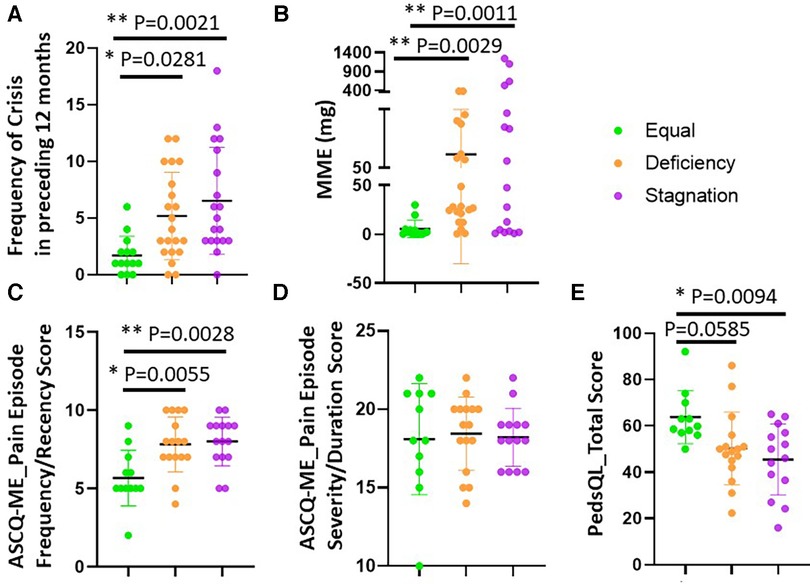
Figure 1. SCD patients differed on VOC, MME and QoL across TCM groups. The severity and frequency of pain crises and SCD Pain-related QoL were assessed by the ASCQ-ME (C) and PedsQL (E), respectively while the number of patient-reported pain crises in the preceding 12 months was also shown (A) The average MME in the preceding 12 months was calculated using: {strength per unit × number of units × number of prescriptions × MME conversion factor}/number of days = MME/Day. Stagnation and Deficiency groups exhibited a higher frequency of pain crises compared to the Equal group (n = 1.714 ± 1.684) (A,C) and lowest SCD pain-related QoL (E) No difference in pain severity during crisis between groups (D) Importantly, both Stagnation and Deficiency groups had significantly higher opioid usage over the past 12 months compared to the Equal group (B).
In a subset of SCD patients and matched HCs, the patients reported comprehensive physiological and psychological dysfunction (Supplementary Table 2), consistent with previous studies (14, 17, 25, 42).
3.3 QST
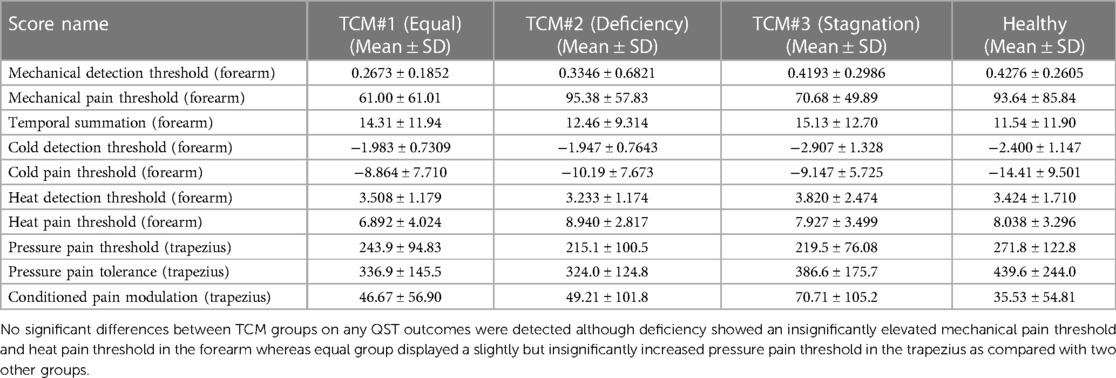
Interestingly, no significant differences between TCM groups on any QST outcomes were detected (Table 4), although Deficiency showed an insignificantly elevated MPT (95.38 ± 57.83) and HPT (8.940 ± 2.817) in the forearm whereas Equal group displayed a slightly increased PPT (243.9 ± 94.83) in the trapezius as compared with two other groups for each test. Consistent with previous studies, the differences between SCD and their matched healthy controls exhibited robust changes across mechanical and thermal sensitivity indexes (Supplementary Table 3). In a separate matched subset of SCD patients (n = 28) and HCs (n = 28), the patients exhibited decreased mechanical detection thresholds (p = 0.0261 at the primary painful site), increased mechanical pain thresholds (primary painful site: p = 0.0238; forearm: p = 0.0358), increased mechanical temporal summation (primary painful site: p = 0.0158), pressure pain thresholds (primary painful site: p = 0.0003; trapezius: p = 0.0107), pressure pain tolerance (primary painful site: p = 0.0009; trapezius: p = 0.0186), and cold pain thresholds (primary painful site: p = 0.0332; forearm: p = 0.0250), indicating an overall pattern of increased mechanical and thermal sensitivity in SCD.
3.4 Hematological indexes
Fresh blood samples from all patients were analyzed for complete blood count (CBC) with differential and hemoglobin electrophoresis (refer to Supplementary Table 4). Most hematological indexes did not present any differences across the three different TCM Syndrome groups. However, the Deficiency and Stagnation groups, but not the Equal group, exhibited significantly higher white blood cell (WBC) levels compared to HCs.
4 Discussion
Pain and other symptoms are heterogeneously expressed across SCD patients (46). Indeed, the existing literature supports the notion that not all SCD patients have the same underlying pathophysiology, similar to other chronic pain disorders (47). The present work explores Syndrome classification and describes the characteristics of different Syndromes of SCD from the perspective of TCM theory. The aim is to better guide the use of acupuncture for more effective relief of pain in patients with SCD.
TCM Syndromes, also known as pattern differentiation or “Zheng,” refer to a diagnostic classification system used in TCM to understand and describe the complex manifestations of a patient's illness. These Syndromes are derived from the comprehensive analysis of various clinical information, including signs and symptoms, tongue and pulse diagnosis, medical history, and lifestyle factors. Different TCM Syndromes reflect distinct patterns of disharmony or imbalance within the body, Organs, and Qi and Blood. TCM practitioners (including acupuncturists) identify these Syndromes to tailor treatment plans and herbal prescriptions for each patient.
Chronic anemia, recurrent acute VOCs, and chronic pain are common manifestations of SCD caused by complex pathophysiology from hemoglobin S polymerization, hemolysis, endothelial dysfunction, and vaso-occlusion (48). According to TCM theory, symptoms associated with chronic anemia, like low energy, excessive fatigue, sallow complexion, and pale lips and nails, are regarded as clinical manifestations of deficiency of both Qi and Blood (9). Recurrent pain in the chest, waist, back, and limbs, including severe pain and swelling of hands and feet due to capillary microthrombosis in SCD, are considered clinical manifestations of blood Stagnation blocking meridians (49). In TCM, Qi is often described as the “vital energy” or simply “energy.” Qi Deficiency can manifest as fatigue, shortness of breath, weakness, pale complexion, lack of appetite, weak voice, etc. Blood is considered an essential substance that nourishes and sustains the body. It is one of the fundamental substances along with Qi that forms the basis of the body's vital life force. Blood has both physical and energetic aspects, and its function in TCM extends beyond the Western biomedical understanding of blood. Imbalances in the quality or quantity of Blood are thought to contribute to various health conditions. For example, Blood Deficiency may manifest as pale complexion, dizziness, and fatigue, while stagnant or blocked Blood may result in conditions like pain, menstrual irregularities, or even emotional issues. A meridian refers to a pathway or channel in the body through which vital energy (Qi) and Blood flow (50).
From a TCM perspective, the pathogenesis of SCD primarily manifests as a Deficiency of both Qi and Blood, accompanied by Blood Stagnation obstructing the meridians. We found that in patients with SCD, some individuals primarily exhibit a characteristic Deficiency of both Qi and Blood, followed by the blockage of meridians due to Blood Stagnation. In contrast, others may primarily manifest blockage of meridians by Blood Stagnation, followed by Qi and Blood Deficiency. Many individuals across our three TCM Syndrome groups consistently displayed anemic manifestations such as fatigue, pale lips and nails. The features of tongue observed in the TCM Syndrome groups mainly reflected blood stasis-related manifestations, such as purple/dark spots in the tongue body. Many individuals across all TCM Syndrome groups also presented with weak or thin wiry pulse, with more individuals in the Stagnation group who exhibited knotted pulse features indicating Stagnation Syndrome. Those confirm that Qi and Blood Deficiency and Blood Stagnation are the basic pathogenesis of SCD in TCM.
There are also cases where both Deficiency of Qi and Blood as well as Blood Stagnation are at the same level. Therefore, we have categorized SCD into three main TCM Syndrome types as shown in Table 2: (1) “Deficiency,” characterized by general weakness, fatigue, pale lips and nails, sallow complexion, and chronic pain that gradually worsens; (2) “Stagnation” which is characterized by severe pain, sudden onset pain, stabbing or sharp pain that is exacerbated by pressure, irritability, fatigue, and weakness; and (3) “Equal,” which features the symptoms of Deficiency (Qi and Blood) and Stagnation (Blood) equally. The deficiency in the Equal group generally manifests as fatigue alongside Stagnation symptoms such as pain. However, there is no clear distinction regarding which is greater between Deficiency and Stagnation within the symptom profile.
The differentiation of three TCM Syndrome types is critical for TCM diagnosis-guided treatment and is also reflected in patient-reported outcomes that were related to pain and somatic symptoms, such as fatigue, physical function, sleep disturbance, as well as SCD related outcomes, including VOC specific pain and frequency and opioid consumption. Noticeably, both the Stagnation and Deficiency groups also had more intense pain-related clinical symptoms (Table 3) but differed in the duration and character of pain at the chronic stage of SCD and during acute SCD episodes (Figure 1). The Stagnation and Deficiency groups also showed greater nociplastic pain features and worse sleep compared to the Equal group. The Stagnation group also exhibited the lowest QOL scores compared to the Equal group. Additionally, the Stagnation group also displayed worse physical dysfunction, and higher levels of anxiety and depression as compared to the other two groups. These results suggest that the Stagnation group may have more nociplastic pain symptoms and other characteristics (sleep/fatigue/mood) that are commonly amplified in fibromyalgia, the canonical nociplastic pain condition. With respect to SCD pathology, both Stagnation and Deficiency groups presented with higher frequency of VOCs (Figures 1A,C) and opioid usage (MME) compared to Equal group (Figure 1B). This increase in opioid requirement in the Stagnation group is consistent with nociplastic pain patients having reduced endogenous opioid efficacy (51). Importantly, the Stagnation group also reported the most frequent, intense, and sudden onset of VOCs as their main symptoms/signs, whereas the chief complaint of Deficiency group was more reflected in fatigue, weakness and chronic pain as summarized in Table 2. The symptoms that may reflect a higher prevalence of “blood stasis” were more prominent in the Stagnation group whereas symptoms of more “blood and Qi Deficiency” were found in the Deficiency group from the TCM perspective. This may explain the difference in general overall pain interference reported across the TCM Syndrome groups, with the Stagnation group reporting the highest pain interference.
We also observed differences between the Deficiency and other two groups based on pain presentation. Noticeably, chronic pain was not always present and was sometimes absent in individuals of both Equal and Stagnation groups. The Deficiency group experienced more profound chronic pain along with Qi and Blood Deficiency Syndrome as reported in Table 2. The higher incidence of chronic pain in the Deficiency group can be explained by the inadequate supply of Qi and Blood, which deprives the meridians of essential nourishment, subsequently causing blockages and the initiation of pain. Moreover, the insufficiency of Qi and Blood may contribute to the dysfunction of visceral organs, further contributing to the prevalence of chronic pain within this group. This long-term persistent pain with recurrent VOCs in the Deficiency group could be related to the significantly lower weight and BMI (Table 1) and RBC (Supplementary Table 4) as compared with Stagnation, which may further explain other co-existing symptoms such as intense fatigue, weakness, and profuse sweating that are also associated with “Deficiency” in TCM (Table 2).
Interestingly, the SS type of SCD seems to be equally distributed across the three TCM Syndrome groups. The SCD subtype HbSS is known to be a more severe form of the disease on average compared to the HbSC genotype (52, 53), and it is positively correlated with the degree of psychological symptoms experienced by the patient (53, 54). More importantly, even though the WBC % was lower in the Equal group, this group presented with comparable levels of all pathological hematological indexes as compared to the two other groups (Supplementary Table 4). This finding may suggest that the less severe clinical symptoms presented in the individuals of Equal group are not related to the SCD genotype but may be associated with other underlying pathophysiological mechanisms in these patients.
In an effort to examine participant differences in touch and pain sensitivity we also conducted a battery of QST using methods that have been widely applied across various chronic pain conditions. Existing literature documenting altered QST thresholds using fixed testing sites in SCD has identified increased sensitivity in response to thermal/mechanical painful stimuli compared to non-SCD pain-free HCs (14, 38, 39, 55). However, pain in SCD can occur anywhere in the body or sometimes throughout the entire body during an active crisis, and the features and locations of pain can vary significantly among individuals. In our study, patients reported a variety of painful locations including: the lower- and mid- to upper-back, hips, shoulders, thighs, knees, and lower extremities. This occurred during both the steady phase and active crises. Moreover, the painful locations during acute pain episodes under VOC could also be exaggerated or replaced by more painful regions elsewhere (e.g., chest, large joints) or a combination of locations. We did not detect any significant differences in QST outcomes across the three TCM patterns. This suggests that our TCM Syndromes were not different across nociceptive experimental pain stimuli. Of note, the 3 TCM groups also did not differ in neuropathic pain symptoms assessed with the PainDETECT questionnaire. As mentioned above, the Stagnation and Deficiency groups displayed higher nociplastic pain symptoms than the Equal groups which was driven mostly by the Symptom Severity sub-scale for the Stagnation group. Overall, these data may suggest involvement of nociplastic pain mechanisms in SCD more so in the Stagnation and Deficiency groups than the Equal group.
Our study has limitations. This is a cross-sectional study; therefore, causation cannot be determined. For example, the two TCM patterns (“Stagnation” and “Deficiency”) had significantly higher opioid consumption (MME) than “Equal” pattern before enrollment, as such we could not determine if there was a relationship between the level of opioid consumption and TCM pattern. Moreover, we could not gauge the potential effects of this medication usage on TCM patterns. The consistent performance of accurate diagnosis relies on the clinical experience of the practitioner and requires training. Our diagnosis criteria were also established based on the enrolled SCD patients in this study and therefore may not be a definitive reference for these results. In addition, the 3 TCM Syndromes did not differentiate in other objective parameters such as laboratory exams or QST but primarily in patient-reported outcomes. Thus, this study lacks objective markers associated with different TCM patterns. Future studies should consider a more comprehensive diagnosis regimen at different stages of the disease (e.g., patients at active VOC stages can have different subtypes of Stagnation). The diagnosis criteria should be further validated and standardized in a larger sample size.
To our knowledge, this is the first and largest clinical trial investigating TCM Syndrome diagnoses in SCD. Optimal therapeutic effects could be achieved by administering the TCM-guided treatment protocol with the appropriate acupuncture points and needling manipulations (50). The implementation of TCM Syndrome diagnosis can not only guide acupuncture treatment but also other alternative treatment such as herbology to facilitate consistent and personalized integrative care. The present study provides a novel and objective clinical relevance for TCM diagnosis and lays the foundation for assessing the outcomes of TCM-guided interventions for managing pain not only in SCD but also other clinical population with complex pathophysiology and large heterogeneity needing individualized treatment for optimal clinical outcomes. More in-depth investigations are warranted to enhance evidence-based integrative and complementary pain management in challenging clinical populations such as SCD.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Institutional Review Board at Indiana University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
YW, DDW, and AQP analyzed, validated, and interpreted the data; YW drafted the manuscript; DDW, SEH, and REH revised the manuscript and helped with data interpretation; YW and ARWO directed patient recruitment; YW designed the study, supervised the overall study performance, and edited the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by NIH K99/R00 award (Grant # 5R00AT010012) and Indiana University Health—Indiana University School of Medicine Strategic Research Initiative funding to YW.
Acknowledgments
The authors would like to thank Brandon Alec Reyes, Tyler James Barret, Nayana Dutt, Payton Mittman, and Bea Paras for assisting with data collection; Candice Debats and Justin Smith for assisting with chart info retrieval and data summarization; and Siddhi Gandhi, Charanjit Kaur, and Veena Vijayan for data management and validation, as well as all the providers affiliated with Indiana University Health and Indiana Hemophilia & Thrombosis Center for patient referral to this study. The authors also thank Indiana University Clinical Research Center and Indiana University Research MRI Center for resource support.
Conflict of interest
SEH has consulted for Aptinyx, Memorial Sloan Kettering Cancer Institute, University of North Carolina-Chapel Hill, and University of Glasgow and received grant funding from NIH, Arbor Medical Innovations, and Aptinyx. REH has consulted for Pfizer, Aptinyx Inc. and has received grant funding from Pfizer, Aptinyx, Cerephex. and National Institutes of Health (NIH). ARWO has served on advisory boards for Pfizer.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpain.2023.1233293/full#supplementary-material
References
1. Osunkwo I, Andemariam B, Minniti CP, Inusa BPD, El Rassi F, Francis-Gibson B, et al. Impact of sickle cell disease on patients’ daily lives, symptoms reported, and disease management strategies: results from the international sickle cell world assessment survey (SWAY). Am J Hematol. (2021) 96(4):404–17. doi: 10.1002/ajh.26063
2. Sagi V, Mittal A, Tran H, Gupta K. Pain in sickle cell disease: current and potential translational therapies. Transl Res. (2021) 234:141–58. doi: 10.1016/j.trsl.2021.03.007
3. Vickers AJ, Cronin AM, Maschino AC, Lewith G, MacPherson H, Foster NE, et al. Acupuncture for chronic pain: individual patient data meta-analysis. Arch Intern Med. (2012) 172(19):1444–53. doi: 10.1001/archinternmed.2012.3654
4. Xiang A, Cheng K, Shen X, Xu P, Liu S. The immediate analgesic effect of acupuncture for pain: a systematic review and meta-analysis. Evid Based Complement Alternat Med. (2017) 2017:3837194. doi: 10.1155/2017/3837194
5. Williams H, Tanabe P. Sickle cell disease: a review of nonpharmacological approaches for pain. J Pain Symptom Manage. (2016) 51(2):163–77. doi: 10.1016/j.jpainsymman.2015.10.017
6. Lu K, Cheng MC, Ge X, Berger A, Xu D, Kato GJ, et al. A retrospective review of acupuncture use for the treatment of pain in sickle cell disease patients: descriptive analysis from a single institution. Clin J Pain. (2014) 30(9):825–30. doi: 10.1097/AJP.0000000000000036
7. Tsai SL, McDaniel D, Taromina K, Lee MT. Acupuncture for sickle cell pain management in a pediatric emergency department, hematology clinic, and inpatient unit. Med Acupunct. (2015) 27(6):510–4. doi: 10.1089/acu.2015.1146
8. Li H, Patil CL, Molokie RE, Njoku F, Steffen AD, Doorenbos AZ, et al. Acupuncture for chronic pain in adults with sickle cell disease: a mixed-methods pilot study. Acupunct Med. (2021) 39(6):612–8. doi: 10.1177/09645284211017303
10. Flaws B, Sionneau P. The treatment of modern western medical diseases with Chinese medicine. Boulder, CO: Blue Poppy Press (2002).
11. Arentz S, Smith C, Redmond R, Abbott J, Armour M. A cross-sectional study of traditional Chinese medicine practitioner’s knowledge, treatment strategies and integration of practice of chronic pelvic pain in women. BMC Complement Med Ther. (2021) 21(1):174. doi: 10.1186/s12906-021-03355-6
12. Mist SD, Wright CL, Jones KD, Carson JW. Traditional Chinese medicine diagnoses in a sample of women with fibromyalgia. Acupunct Med. (2011) 29(4):266–9. doi: 10.1136/acupmed-2011-010052
13. Campbell CM, Carroll CP, Kiley K, Han D, Haywood C Jr., Lanzkron S, et al. Quantitative sensory testing and pain-evoked cytokine reactivity: comparison of patients with sickle cell disease to healthy matched controls. Pain. (2016) 157(4):949–56. doi: 10.1097/j.pain.0000000000000473
14. Campbell CM, Moscou-Jackson G, Carroll CP, Kiley K, Haywood C Jr., Lanzkron S, et al. An evaluation of central sensitization in patients with sickle cell disease. J Pain. (2016) 17(5):617–27. doi: 10.1016/j.jpain.2016.01.475
15. Darbari DS, Hampson JP, Ichesco E, Kadom N, Vezina G, Evangelou I, et al. Frequency of hospitalizations for pain and association with altered brain network connectivity in sickle cell disease. J Pain. (2015) 16(11):1077–86. doi: 10.1016/j.jpain.2015.07.005
16. Belfer I, Youngblood V, Darbari DS, Wang Z, Diaw L, Freeman L, et al. A GCH1 haplotype confers sex-specific susceptibility to pain crises and altered endothelial function in adults with sickle cell anemia. Am J Hematol. (2014) 89(2):187–93. doi: 10.1002/ajh.23613
17. Brandow AM, Stucky CL, Hillery CA, Hoffmann RG, Panepinto JA. Patients with sickle cell disease have increased sensitivity to cold and heat. Am J Hematol. (2013) 88(1):37–43. doi: 10.1002/ajh.23341
18. Cataldo G, Rajput S, Gupta K, Simone DA. Sensitization of nociceptive spinal neurons contributes to pain in a transgenic model of sickle cell disease. Pain. (2015) 156(4):722–30. doi: 10.1097/j.pain.0000000000000104
19. Hillery CA, Kerstein PC, Vilceanu D, Barabas ME, Retherford D, Brandow AM, et al. Transient receptor potential vanilloid 1 mediates pain in mice with severe sickle cell disease. Blood. (2011) 118(12):3376–83. doi: 10.1182/blood-2010-12-327429
20. Vincent L, Vang D, Nguyen J, Gupta M, Luk K, Ericson ME, et al. Mast cell activation contributes to sickle cell pathobiology and pain in mice. Blood. (2013) 122(11):1853–62. doi: 10.1182/blood-2013-04-498105
21. Bogdanova A, Kaestner L, Simionato G, Wickrema A, Makhro A. Heterogeneity of red blood cells: causes and consequences. Front Physiol. (2020) 11:392. doi: 10.3389/fphys.2020.00392
22. Zaidi AU, Glaros AK, Lee S, Wang T, Bhojwani R, Morris E, et al. A systematic literature review of frequency of vaso-occlusive crises in sickle cell disease. Orphanet J Rare Dis. (2021) 16(1):460. doi: 10.1186/s13023-021-02096-6
23. Lufen W. Diagnosis of traditional Chinese medicine. Shanghai: College of Traditional Chinese Medicine (2002).
24. Curtis S, Brandow AM. Responsiveness of patient-reported outcome measurement information system (PROMIS) pain domains and disease-specific patient-reported outcome measures in children and adults with sickle cell disease. Hematology Am Soc Hematol Educ Program. (2017) 2017(1):542–5. doi: 10.1182/asheducation-2017.1.542
25. Brandow AM, Farley RA, Panepinto JA. Neuropathic pain in patients with sickle cell disease. Pediatr Blood Cancer. (2014) 61(3):512–7. doi: 10.1002/pbc.24838
26. Freynhagen R, Baron R, Gockel U, Tolle TR. painDETECT: a new screening questionnaire to identify neuropathic components in patients with back pain. Curr Med Res Opin. (2006) 22(10):1911–20. doi: 10.1185/030079906X132488
27. Harte SE, Harris RE, Clauw DJ. The neurobiology of central sensitization. J Appl Biobehav Res. (2018) 23(2):e12137. doi: 10.1111/jabr.12137
28. Fitzcharles MA, Cohen SP, Clauw DJ, Littlejohn G, Usui C, Hauser W. Nociplastic pain: towards an understanding of prevalent pain conditions. Lancet. (2021) 397(10289):2098–110. doi: 10.1016/S0140-6736(21)00392-5
29. Martinez-Lavin M. Centralized nociplastic pain causing fibromyalgia: an emperor with no cloths? Clin Rheumatol. (2022) 41(12):3915–7. doi: 10.1007/s10067-022-06407-5
30. Sluka KA, Clauw DJ. Neurobiology of fibromyalgia and chronic widespread pain. Neuroscience. (2016) 338:114–29. doi: 10.1016/j.neuroscience.2016.06.006
31. Al-Marzouki AF, Alrefaie NI, Aljohani NA, Alandanusi RA, Alghamdi AA, Radhwi OO. The prevalence of depression and anxiety among sickle cell disease patients in king abdulaziz university hospital. Cureus. (2021) 13(9):e18374. doi: 10.7759/cureus.18374
32. Doglioni D O, Chabasseur V, Barbot F, Galacteros F, Gay MC. Depression in adults with sickle cell disease: a systematic review of the methodological issues in assessing prevalence of depression. BMC Psychol. (2021) 9(1):54. doi: 10.1186/s40359-021-00543-4
33. Barrett TJ, Pucka A, Reyes B, Jacob SA, O'Brien AR, Harris R, et al. Acupuncture alleviates pain and improves quality of life in patients with sickle cell disease. Blood. (2022) 140(Supplement 1):5444–5. doi: 10.1182/blood-2022-169013
34. Ojinnaka U, Ahmed Z, Kannan A, Quadir H, Hakobyan K, Gaddam M, et al. A traditional review of sickle cell disease and the associated onset of dementia: hematological and neurocognitive crossroads. Cureus. (2021) 13(10):e18906. doi: 10.7759/cureus.18906
35. Clauw DJ. Fibromyalgia: a clinical review. JAMA. (2014) 311(15):1547–55. doi: 10.1001/jama.2014.3266
36. Dudeney J, Law EF, Meyyappan A, Palermo TM, Rabbitts JA. Evaluating the psychometric properties of the widespread pain Index and the symptom severity scale in youth with painful conditions. Can J Pain. (2019) 3(1):137–47. doi: 10.1080/24740527.2019.1620097
37. Lin JM, Brimmer DJ, Maloney EM, Nyarko E, Belue R, Reeves WC. Further validation of the multidimensional fatigue inventory in a US adult population sample. Popul Health Metr. (2009) 7:18. doi: 10.1186/1478-7954-7-18
38. Darbari DS, Vaughan KJ, Roskom K, Seamon C, Diaw L, Quinn M, et al. Central sensitization associated with low fetal hemoglobin levels in adults with sickle cell anemia. Scand J Pain. (2017) 17:279–86. doi: 10.1016/j.sjpain.2017.08.001
39. Miller RE, Brown DS, Keith SW, Hegarty SE, Setty Y, Campbell CM, et al. Quantitative sensory testing in children with sickle cell disease: additional insights and future possibilities. Br J Haematol. (2019) 185(5):925–34. doi: 10.1111/bjh.15876
40. Nahman-Averbuch H, Yarnitsky D, Granovsky Y, Gerber E, Dagul P, Granot M. The role of stimulation parameters on the conditioned pain modulation response. Scand J Pain. (2013) 4(1):10–4. doi: 10.1016/j.sjpain.2012.08.001
41. Schoen CJ, Ablin JN, Ichesco E, Bhavsar RJ, Kochlefl L, Harris RE, et al. A novel paradigm to evaluate conditioned pain modulation in fibromyalgia. J Pain Res. (2016) 9:711–9. doi: 10.2147/JPR.S115193
42. Treadwell MJ, Hassell K, Levine R, Keller S. Adult sickle cell quality-of-life measurement information system (ASCQ-me): conceptual model based on review of the literature and formative research. Clin J Pain. (2014) 30(10):902–14. doi: 10.1097/AJP.0000000000000054
43. Wang Y, Hardy SJ, Ichesco E, Zhang P, Harris RE, Darbari DS. Alteration of grey matter volume is associated with pain and quality of life in children with sickle cell disease. Transl Res. (2022) 240:17–25. doi: 10.1016/j.trsl.2021.08.004
44. Panepinto JA, Torres S, Bendo CB, McCavit TL, Dinu B, Sherman-Bien S, et al. PedsQL sickle cell disease module: feasibility, reliability, and validity. Pediatr Blood Cancer. (2013) 60(8):1338–44. doi: 10.1002/pbc.24491
45. Dowell D RK, Jones CM, Baldwin GT, Chou R. CDC clinical practice guideline for prescribing opioids for pain — united States, 2022. MMWR Recomm Rep. (2022) 71(3):1–95. doi: 10.15585/mmwr.rr7103a1
46. McClish DK, Smith WR, Dahman BA, Levenson JL, Roberts JD, Penberthy LT, et al. Pain site frequency and location in sickle cell disease: the PiSCES project. Pain. (2009) 145(1-2):246–51. doi: 10.1016/j.pain.2009.06.029
47. Fillingim RB. Individual differences in pain: understanding the mosaic that makes pain personal. Pain. (2017) 158(Suppl 1):S11–S8. doi: 10.1097/j.pain.0000000000000775
48. Sundd P, Gladwin MT, Novelli EM. Pathophysiology of sickle cell disease. Annu Rev Pathol. (2019) 14:263–92. doi: 10.1146/annurev-pathmechdis-012418-012838
49. Maciocia G. The foundations of Chinese medicine. Singapore: Longman Singapore Publishers (Pte) Ltd (1994).
50. Xinnong C. Chinese Acupuncture and moxibustion. 4th edn. Beijing, China: Foreign Languages Press (2019).
51. Schrepf A, Harper DE, Harte SE, Wang H, Ichesco E, Hampson JP, et al. Endogenous opioidergic dysregulation of pain in fibromyalgia: a PET and fMRI study. Pain. (2016) 157(10):2217–25. doi: 10.1097/j.pain.0000000000000633
52. Konotey-Ahulu FID. The sickle cell disease patient: natural history from a clinico-epidemiological study of the first 1550 patients of korle bu hospital sickle cell clinic. London: Macmillan (1991). 643.
53. Platt OS, Brambilla DJ, Rosse WF, Milner PF, Castro O, Steinberg MH, et al. Mortality in sickle cell disease. Life expectancy and risk factors for early death. N Engl J Med. (1994) 330(23):1639–44. doi: 10.1056/NEJM199406093302303
54. Scott KD, Scott AA. Cultural therapeutic awareness and sickle cell anemia. Journal of Black Psychology. (1999) 25(3):316–35. doi: 10.1177/0095798499025003004
Keywords: sickle cell disease, pain, traditional Chinese medicine, syndrome differentiation, acupuncture, patient-reported outcomes, quantitative sensory testing, morphine milligram equivalents
Citation: Wang Y, Wang DD, Pucka AQ, O’Brien ARW, Harte SE and Harris RE (2024) Differential clinical characteristics across traditional Chinese medicine (TCM) Syndromes in patients with sickle cell disease. Front. Pain Res. 4:1233293. doi: 10.3389/fpain.2023.1233293
Received: 1 June 2023; Accepted: 11 December 2023;
Published: 5 January 2024.
Edited by:
Keesha Roach, University of Tennessee Health Science Center (UTHSC), United StatesReviewed by:
Girindra Raval, Augusta University, United StatesCristian Acosta, Conicet Mendoza, Argentina
© 2024 Wang, Wang, Pucka, O'Brien, Harte and Harris. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ying Wang eXdhMTJAaXUuZWR1
 Ying Wang
Ying Wang David D. Wang
David D. Wang Andrew Q. Pucka
Andrew Q. Pucka Andrew R. W. O’Brien
Andrew R. W. O’Brien Steven E. Harte
Steven E. Harte Richard E. Harris5,6
Richard E. Harris5,6