
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pain Res. , 25 October 2023
Sec. Headache
Volume 4 - 2023 | https://doi.org/10.3389/fpain.2023.1231054
This article is part of the Research Topic Insight in Headache – 2023 View all 5 articles
 Keturah R. Faurot1*
Keturah R. Faurot1* Jinyoung Park1
Jinyoung Park1 Vanessa Miller1
Vanessa Miller1 Gilson Honvoh1
Gilson Honvoh1 Anthony Domeniciello2
Anthony Domeniciello2 J. Douglas Mann3
J. Douglas Mann3 Susan A. Gaylord1
Susan A. Gaylord1 Chanee E. Lynch1
Chanee E. Lynch1 Olafur Palsson4
Olafur Palsson4 Christopher E. Ramsden2,5
Christopher E. Ramsden2,5 Beth A. MacIntosh6
Beth A. MacIntosh6 Mark Horowitz2,†
Mark Horowitz2,† Daisy Zamora1,2,7,†
Daisy Zamora1,2,7,†
Background: Migraine is a prevalent disabling condition often associated with comorbid physical and psychological symptoms that contribute to impaired quality of life and disability. Studies suggest that increasing dietary omega-3 fatty acid is associated with headache reduction, but less is known about the effects on quality of life in migraine.
Methods: After a 4-week run-in, 182 adults with 5–20 migraine days per month were randomized to one of the 3 arms for sixteen weeks. Dietary arms included: H3L6 (a high omega-3, low omega-6 diet), H3 (a high omega-3, an average omega-6 diet), or a control diet (average intakes of omega-3 and omega-6 fatty acids). Prespecified secondary endpoints included daily diary measures (stress perception, sleep quality, and perceived health), Patient-Reported Outcome Measurement Information System Version 1.0 ([PROMIS©) measures and the Migraine Disability Assessment (MIDAS). Analyses used linear mixed effects models to control for repeated measures.
Results: The H3L6 diet was associated with significant improvements in stress perception [adjusted mean difference (aMD): −1.5 (95% confidence interval: −1.7 to −1.2)], sleep quality [aMD: 0.2 (95% CI:0.1–0.2)], and perceived health [aMD: 0.2 (0.2–0.3)] compared to the control. Similarly, the H3 diet was associated with significant improvements in stress perception [aMD: −0.8 (−1.1 to −0.5)], sleep quality [aMD: 0.2 (0.1, 0.3)], and perceived health [aMD: 0.3 (0.2, 0.3)] compared to the control. MIDAS scores improved substantially in the intervention groups compared with the control (H3L6 aMD: −11.8 [−25.1, 1.5] and H3 aMD: −10.7 [−24.0, 2.7]). Among the PROMIS-29 assessments, the biggest impact was on pain interference [H3L6 MD: −1.8 (−4.4, 0.7) and H3 aMD: −3.2 (−5.9, −0.5)] and pain intensity [H3L6 MD: −0.6 (−1.3, 0.1) and H3 aMD: −0.6 (−1.4, 0.1)].
Discussion: The diary measures, with their increased power, supported our hypothesis that symptoms associated with migraine attacks could be responsive to specific dietary fatty acid manipulations. Changes in the PROMIS© measures reflected improvements in non-headache pain as well as physical and psychological function, largely in the expected directions. These findings suggest that increasing omega-3 with or without decreasing omega-6 in the diet may represent a reasonable adjunctive approach to reducing symptoms associated with migraine attacks.
Trial Registration: ClinicalTrials.gov NCT02012790.
Migraine is a common, painful disorder, second only to low back pain as a disabling condition in the United States (US) (1). Comorbid physical and psychological symptoms—including stress, perceived health, insomnia, anxiety, and depression—are prevalent and reduce quality of life among patients with migraine (1, 2). Many strategies for treating migraine improve the frequency and/or duration of attacks but may be associated with side effects that have a negative overall impact on quality of life. Hence, it is critical to consider all symptoms related to quality of life when judging the therapeutic effectiveness of strategies for reducing migraine.
Targeting dietary intakes of omega-3 and omega-6 fatty acid to address migraine-related pain is supported in previous studies. Lowering dietary omega-6 polyunsaturated fatty acids (PUFA), especially linoleic acid (LA) in the diet, has been shown to result in lower levels of circulating omega-6 PUFA derived lipid mediators with pro-nociceptive properties (3–5). In addition, increasing dietary omega-3 eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) increases circulating omega-3 derived lipid mediators, such as omega-3 monoepoxides (6, 7) and resolvins (8), with anti-nociceptive effects. Increasing omega-3 in the diet and reducing omega-6 over 12 weeks (compared with reducing omega-6 alone) resulted in reductions in headache frequency in chronic daily headache (9).
In a three-arm, 16-week dietary intervention for chronic and episodic migraine, compared with a diet with average intakes of omega-3 and omega-6, increasing omega-3 in the diet with or without decreasing omega-6 resulted in fewer and shorter headaches (10). Based on the migraine literature, we expected that the burden of comorbid non-headache pain, disability, and psychological symptoms among the study participants with migraine would be high. We hypothesized that the two active intervention diets would result in an improvement in comorbid symptoms. Furthermore, we expected that symptom improvements would be related to changes in plasma omega-3 and omega-6 levels.
This 3-arm parallel-group, 16-week randomized, controlled trial sought to evaluate the biochemical and clinical effects of dietary interventions that manipulated omega-3 and omega-6 PUFA in a sample of individuals with chronic migraine and frequent episodic migraine. The interventions included a control diet consistent with Dietary Guidelines for Americans as well as a diet that increased omega-3 PUFA intakes and a diet that both increased omega-3 PUFA intakes and decreased omega-6 PUFA intakes. The trial was registered with ClinicalTrials.gov (NCT02012790) prior to recruitment of participants. Please see the protocol, diet methods, and primary outcomes papers for additional details regarding the composition of the diets and specific study procedures (10–12). In brief, the control diet recommendations included low saturated fat, but levels of omega-6 PUFA and omega-3 PUFA consistent with average intakes in the United States (11). The H3 diet increased omega-3 PUFA intakes but did not decrease omega-6 PUFA intakes compared with the control diet. The H3L6 diet increased omega-3 PUFA intakes and sought to decrease omega-6 intakes compared to the control diet. All three dietary interventions included provision of key foods to alter omega-3 and omega-6 intake, diet education and diet adherence counseling delivered by trained research dietitians every 2–3 weeks at randomization and at intervention weeks 2, 4, 7, 10, 13, and 16.
Most of the participants were recruited from headache specialty clinics. At the baseline visit, after participants reviewed, discussed, and signed the informed consent documents, they reviewed eligibility criteria with the study physician. Eligible participants were adults (18 years of age and older) of any gender or ethnicity with documented migraine under the care of a physician who had at least 5 migraine days per month and no more than 20 migraine days per month. Participants were excluded if they were pregnant or breast-feeding or if they had changed their hormone medication intakes within the past 6 months. Other exclusions included the following: (1) serious psychiatric illness or substance abuse; (2) major medical illness; (3) recent head/neck trauma or surgery; (4) cognitive impairment; (5) food allergy in adulthood; (6) aversion to fish; (7) regular exposure to fish oil supplements; (8) recent or intended weight loss or prior bariatric surgery; (9) earlier participation in a dietary intervention or recent participation in any migraine intervention trial.
After a 4–6-week run-in period, participants who met the criteria for participation (5–20 migraine days per month) were randomized 1:1:1 to one of the three diets by the dietitian using an uneditable computer interface with randomized blocks to ensure concealed allocation. The computer sequence was generated by a co-investigator who maintained the diary but was otherwise not involved in the trial. Initially, recruitment was limited to 5–14 migraine days per month (with up to 20 headache days), but was liberalized (5–20 migraine days per month) to meet recruitment targets, thereby including individuals with chronic migraine, as defined in ICHD-3 (13, 14) (headaches 15 days per month for at least 3 months with migraine features on at least 8 days per month). Only the dietitian and participant were aware of diet assignment. Participants were unaware of trial hypotheses related to PUFA intakes and were introduced to the diets as potentially equally efficacious for reduction of headaches. All other study personnel, including research assistants and analysts were blinded to intervention assignment.
Participants met with the research dietitian in person at the clinical research center 7 times during the active intervention, receiving enough food for 2 meals and 2 snacks per day for 2–3 weeks, extensive dietary counseling, and access to a website that detailed grocery and restaurant guides, recipes, and other dietary education materials. Diets were designed to be as alike as possible, containing the same proportions of fat, carbohydrates, and protein across the three, differing only in fatty acid content and protein sources (11). For example, the high omega-3 diets contained fatty fish and the control diet contained low fat fish and chicken breast. Diets aimed to provide enough calories to maintain a participant's current weight. Weight was recorded at each study visit.
Participants completed electronic headache diaries throughout the study, supplied blood samples and completed questionnaires. The study was approved by the Institutional Review Board of the University of North Carolina at Chapel Hill in accordance with the Declaration of the World Medical Association.
The safety of participants in the trial was both actively and passively monitored. At each intervention visit, the dietitian inquired about potential adverse events related to the diets including gastrointestinal symptoms, allergy symptoms, and weight changes. Adverse events were also monitored through the comments section of the electronic headache diary. Adverse event reports were made biannually to the Data and Safety Monitoring Board at the University of North Carolina at Chapel Hill. The relationship of an adverse event to the intervention was judged by a neurologist blind to intervention assignment.
At baseline, characteristics that may have affected either level of participation or benefit from the diet were collected. These include age, sex, Body Mass Index (BMI), blood pressure, education, race/ethnicity, income level, and relationship status. We also collected the baseline values of the Headache Impact Test (HIT-6) as well as measures of headache frequency and severity and preventive medication use. These include potentially psychoactive medications such as anti-depressants and anti-anxiety drugs as well as other medications such as gabapentinoids and muscle relaxers which can have an impact on sleep, fatigue, and physical function.
Although participants were not made aware of the hypotheses associated with diet interventions, they were aware of the details of their assigned diet. In this situation, it is recommended that investigators assess expectations associated with the interventions. This study used a credibility measure in common use in integrative health studies (15, 16). The measure was adapted for use in headache populations and administered after participants received diet instruction, but before any food intakes. It measured (0–9 scale, 9 most credible) how likely participants would be to recommend the diet to others, how important it would be to make the treatment available, and how successful the treatment seemed for treating associated symptoms, e.g., tension, anxiety, or insomnia (17).
Key clinical outcomes have been reported in a previous publication (10). These include the Headache Impact Test (HIT-6) as well as headache frequency, duration, and severity as measured in a daily headache diary. Here we report the additional prespecified clinical and biochemical endpoints.
Participants completed an electronic daily diary for at least 4 weeks before randomization and during the 16 weeks of the intervention. The password-protected diary could be accessed from a computer or smartphone and, to reduce bias, was limited to entries for the current and previous day. To promote adherence, if participants did not complete a diary entry for the day before by 6 PM, they received a text message prompt.
In addition to headache frequency, severity, and duration, the diary included a question about the participant's stress level during the previous day on a 0–10 scale with 10 representing the greatest level of stress. Two 4-level Likert-style questions were also included: a rating of overall health and a rating of sleep quality, both with responses of poor (1), fair (2), good (3), and excellent (4). The diary was developed by our group and tested in prior headache studies (18).
The National Institutes of Health (NIH) commissioned the PROMIS measures to enable flexible cross-study comparisons of health-related quality-of-life with a first wave of testing from 2005 to 2008. The PROMIS-29 covers physical (physical function, pain intensity, pain interference, fatigue), emotional (anxiety, depression, social functioning), and sleep disturbance. The PROMIS measures have been validated in multiple populations, including the general public and chronic disease populations (19–21). Analysis involves a translation to T scores that are normed to the general population, enabling ready interpretation. T scores of 50 correspond to the population mean and the standard deviation is set at 10 for all measures except the pain intensity score. The pain intensity score is consistent with the numeric rating scale for average pain over the past 7 days, measured on a 0–10 scale. Higher scores on the physical function and social functioning scores reflect improvements and lower scores on the pain intensity, pain interference, fatigue, sleep disturbance, anxiety, and depression measures represent improvements. Minimal clinically important differences (MCID) have been reported provisionally as 3.0–3.5 T-score points for depression (22), 2–3 T-score points for pain interference (in a sample of individuals with chronic pain or osteoarthritis) (22, 23), 2.3–3.4 T-score points for anxiety (osteoarthritic population) (22), and 1.9–2.2 T-score points for physical function (osteoarthritis population) (22).
The Center for Medicare and Medicaid Services assigns G-codes to levels of functional impairment for the purposes of billing for therapy services. A few of the PROMIS measures have been mapped to G-codes (24), including physical function, pain interference, and fatigue. For example, a physical function score of 50 corresponds to 1%–19% impairment for physical function and pain interference, but to 20%–39% impairment for fatigue.
The MIDAS assesses the number of days over a 3-month period that a migraine sufferer is either unable to or limited in their ability to participate in work or social activities (44, 45). The MIDAS score consists of 5 summed scores consisting of (1) the number of days of missed school or work, (2) the number of days of halved productivity at school or work, (3) the number of days of missed household work, (4) the number of days of halved productivity in the household and (5) the number of days missed for social activities (25). A MIDAS score of 11–20 indicates moderate disability and a score >20 indicates severe (26). The MIDAS has been found to be reliable (test-retest r = 0.8) and highly correlated with a paper headache diary and physician assessments (25). Clinically meaningful changes in the MIDAS have been defined as a 5-point decrease (27).
Participants evaluate how their headache symptoms and overall health has changed over the course of the intervention using a Likert scale with responses ranging from much worse (5) to much better (1). Similarly, they provide assessments of their satisfaction with their clinical care with responses ranging from very dissatisfied (1) to very satisfied (5). The questions were designed to measure the overall perceptions of benefit of the interventions.
The Whole-Body Pain Scale was developed by co-author Olafur Palsson for this study to assess pain in addition to headache as experienced in the previous 7 days. The scale measures pain intensity as mild (1), moderate (2), and severe (3) across multiple body parts and asks about the total percentage of time a person experiences pain (0%–100% of the time). For the purposes of this analysis, we are examining the number of total body parts impacted by pain without considering the severity of the pain. A second analysis addresses the percentage of time people have pain.
In the H3 and the H3L6 diet groups, omega-3 fatty acids were expected to increase in plasma substantially. In addition, the H3L6 group was expected to decrease L6. These fatty acid measures were expected to be associated with clinical outcomes. Fatty acids were extracted from plasma and quantified by gas chromatography coupled to a flame ionization detector as previously described (28). Reported here are the model-predicted values at baseline and 16 weeks, the pre-post difference, and the between-group differences (controlling for multiple comparisons). We also present the association between changes in plasma fatty acids (EPA, DHA, arachidonic acid, and linoleic acid) and the diary-based outcomes (daily stress, perceived health, and sleep).
Sample size estimates were calculated for the primary biochemical endpoint, 17-hydroxy-DHA and the primary clinical endpoint, the HIT-6, as reported previously (10). No adjustments are made for multiple outcomes or multiple comparisons.
We addressed missing data using longitudinal mixed effects models and, where appropriate, multiple imputation retaining all randomized individuals in their original groups. Initial examinations indicated that we could assume data were missing at random and that missingness could be predicted. We used within-group chained equations with predicted mean matching and 30 imputed datasets, using Rubin's rules to combine them. Imputation models included demographic and clinical characteristics, headache variables, sleep quality, stress, overall health, medication use, expectation of benefit, and recruitment site. For the fatty acid measures, we used a simple imputation, consisting of the last value carried forward for all participants with at least one post-randomization measurement.
We used analysis of covariance (ANCOVA) for analyses with two time points and mixed effects models for more than two time points, with model choices based on the distribution of the outcome data. For example, the PROMIS measures, using T-scores, were analyzed using linear models. The MIDAS, with a right skew, was transformed to achieve normality. Because the transformed data did not change the interpretation, the untransformed medians are reported along with untransformed differences in means with 95% confidence intervals. Mixed effects models included a random intercept for individual participants, a time variable, indicator variables for group assignment, and time-by-group interactions. All models controlled for recruitment site and the baseline level of the variable examined as prescribed in the protocol. Associations between changes in sleep quality, perceived health, stress and plasma fatty acids are reported in exploratory mixed effects models.
A sensitivity analysis examined the effects of the interventions across episodic vs. chronic migraine and migraine with and without aura.
One hundred eighty-two individuals with episodic (n = 60) or chronic (n = 122) migraine were randomized into the three diet groups with 61 participants each in the intervention groups and 60 participants in the control group (Figure 1). The sample was comparable at baseline. Participants were 18–70 years old. The mean age across the groups ranged from 36.9 in the control group to 39.4 in the H3L6 group with an overall mean of 38.3. Mean body mass index was 29.4. Over 88% of the sample were women and 76% were White. Most (66%) were living with a partner. Over 77% had a college education and the majority had household incomes of more than $40,000 per year. The mean HIT-6 at baseline was in the severely affected range (>60) and the mean MIDAS score was consistent with severe disability in all three groups. Participants had a mean of 5.4 headache hours per day with an average of 16.2 headache days per month along with an average number of severe headache days per month of 2.0. At baseline, participants were taking several preventative and adjunctive medications, including Botulinum toxin, anticonvulsants, muscle relaxers, antidepressants, anxiolytics, and beta blockers. The credibility of the interventions was somewhat higher for the diets that increased omega-3 compared to the control (Table 1).
Perceived stress, based on daily estimates, was lower in the intervention groups at the end of the trial compared with the control group. On a 0–10 scale, the post-intervention adjusted mean was 3.9 [95% Confidence Interval (CI): 3.7–4.1] in the control group compared with 2.5 (95% CI: 2.3–2.7) in the H3L6 group and 3.1 (95% CI: 2.9–3.3) in the H3 group representing large effect sizes as measured by Cohen's d. Sleep quality improved significantly in the intervention groups compared with the control group with moderate effect sizes. At baseline, the overall sleep quality was 2.5 (SD 0.49) on a 1–4 scale. Adjusted means at intervention end were greater for the H3L6 and H3 intervention groups: H3L6 2.7 (2.6–2.7) and H3 2.7 (2.6–2.8). Perceived overall health also improved significantly in the intervention groups compared with the control group with large effect sizes (Table 2 and Figure 2).
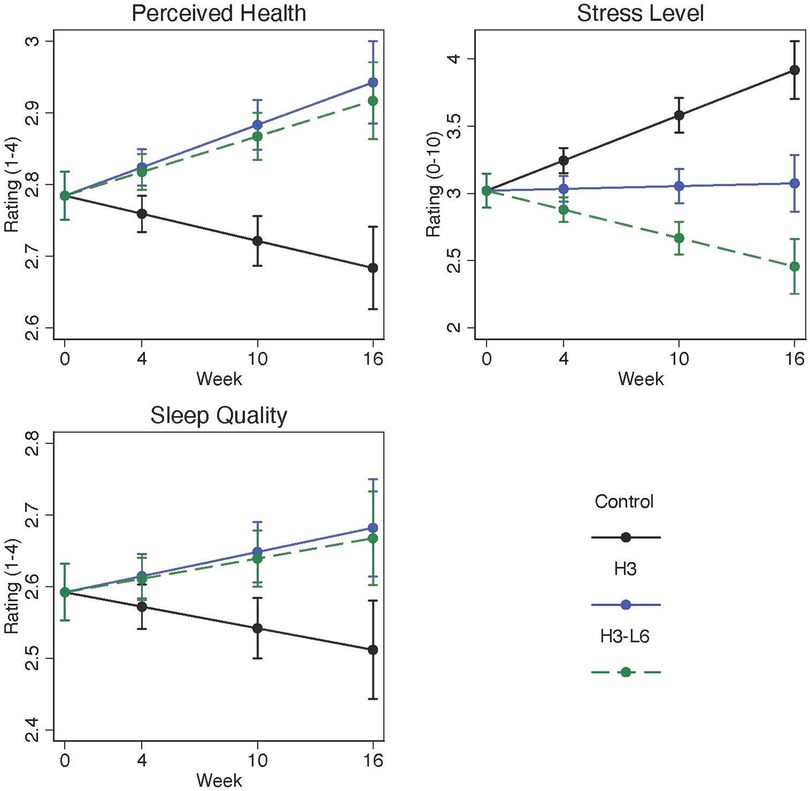
Figure 2. Change in daily perceived health, stress, and sleep quality over 16 weeks. Plots are model-predicted means and 95% confidence intervals for each group at each of the study visits. Linear population-averaged mixed effects models were constructed using an autoregressive correlation using daily diary measurements of each endpoint regressed on group-by-time interaction, time, and recruitment site.
Of the PROMIS-29 measures, only pain interference, with a mean of 59.0 [Standard Deviation (SD) 6.6] was substantially elevated at baseline (Table 1: Baseline Characteristics). The pain interference score at baseline corresponded to a G-code severity assessment of 40%–59% impaired (24). The baseline physical function score of 47.3 (SD 9.0) and the PROMIS fatigue score of 54.3 (SD 9.6) corresponded to a G-code impairment of 20%–39%. The mean pain intensity was 4.4 (SD 2.0) on a 0–10 scale. Scores for mean anxiety, depression, and sleep disturbance were not elevated at baseline. Measures of social role function were not meaningfully different from population norms.
Self-reported pain interference improved within both diet intervention groups (Figure 3). The H3 diet resulted in a pain interference score at 16 weeks 3.2 points lower in the H3 compared with the control group (95% CI: −5.9, −0.5) and 1.8 points lower in the H3L6 diet group compared with the control group (95% CI: −4.4, 0.7). Pain intensity improved by 19% and 22% in the two intervention groups and by 9% in the Control group. The post-intervention differences in pain intensity favored the intervention groups with a change in the post-intervention (0–10) pain scale of −0.6 (−1.4, 0.1) in the H3 group compared with the control and −0.6 (−1.3, 0.1) in the H3L6 group.
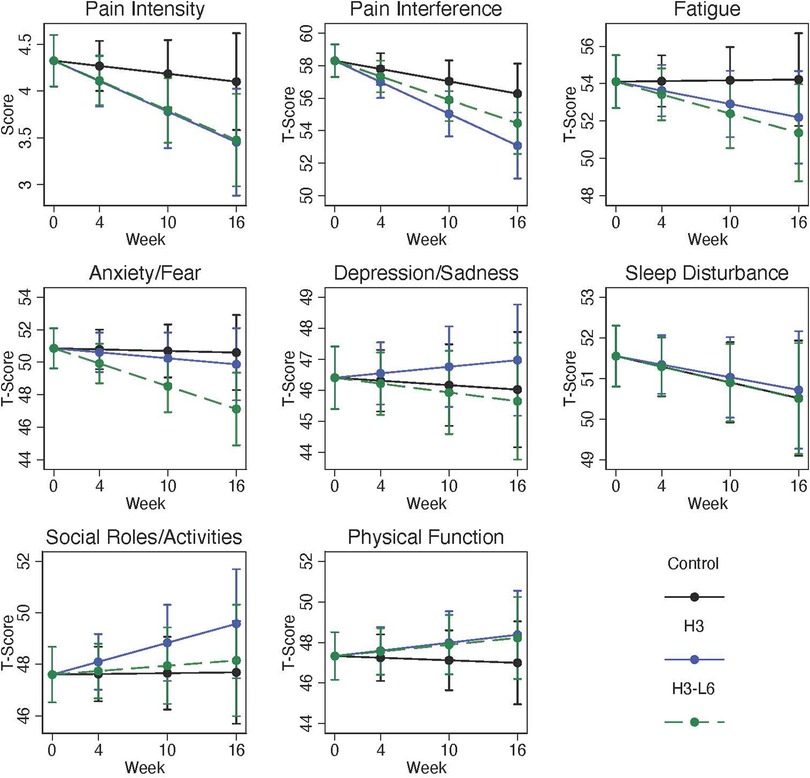
Figure 3. Change in PROMIS-29 domains over 16 weeks. Plots are model-predicted means and 95% confidence intervals based on generalized linear models with each outcome regressed on the group-by-time interaction, time, and recruitment site.
Small increases in depression scores occurred in all three groups; these were not significantly different between groups. Similarly, anxiety and social role function levels were close to the population average (50) at baseline and changed little over the course of the trial. Based on the PROMIS measures, sleep disturbance was slightly higher than the general population at baseline and changed very little over the course of the 16-week intervention.
MIDAS scores dropped significantly within each group (Table 2), with greater reductions in the intervention groups. At 16 weeks, the median score dropped from 28 to 12, 31 to 12, and 22 to 17 in the H3, H3L6, and control groups, respectively. The between-group differences in the means at 16 weeks, were not statistically significant (p = 0.1 and 0.08 for the H3-vs.-control and H3L6-vs.-control comparisons, respectively).
Perceived benefits of the diets for overall health were reported in all three groups, without significant between-group differences. Similarly, satisfaction with care improved in all three groups. Consistent with the change in headache hours per day, perceived benefit to the headache condition was greater in the intervention groups than in the control group.
At baseline, the average number of painful body sites at baseline across the sample was 7.1 (SD 4.7). The average percentage of time participants said they had at least some pain was 47.1% (SD 28.8). The number of painful body sites was comparable across the three groups and changed little over time, but the percentage of time that individuals experienced pain changed by the end of the trial by −42% in the H3 diet group, −14% in the H3L6 diet group, and 3% in the Control group. The differences between groups favored the intervention groups but were not statistically significant.
In the sensitivity analysis, no clear pattern emerged in the intervention effects comparing chronic migraine (n = 122) to episodic migraine (n = 60) (Supplementary Table S1). The MIDAS disability score changed more in the intervention groups relative to the control for participants with chronic migraine relative to those with episodic migraine but the differences were not statistically significant.
Participants were classified as having migraine with aura if at least some of their migraine attacks included an aura. Comparing migraine with aura (n = 51) to migraine without aura (n = 131) revealed greater improvements in the diary measures (perceived health, sleep quality, stress) for the intervention groups compared with the control among participants with migraine with aura (Supplementary Table S2). For example, perceived stress was 2.1 points lower (95% CI: 1.6, 2.7) comparing the H3 group (n = 14) to the control (n = 18) among participants with aura whereas a reduction of 0.3 (95% CI: 0.01, 0.7) was seen comparing the H3 group (n = 47) to the control (n = 42) among participants without aura. Similarly, in the H3L6 group (n = 19) relative to the control (n = 18), a reduction of 2.3 points (1.8, 2.8) was seen among patients with aura and a reduction of 1.1 points (95% CI: 0.8, 1.4) comparing the H3L6 group (n = 42) to the control (n = 42) was seen among participants without aura.
As expected, the average increase in EPA and DHA in plasma was 84% and 90% respectively in the H3 diet group. Increases in EPA and DHA in the H3L6 group were 63% and 56%; both groups exhibited substantial increases as compared with the control group. The H3L6 diet was also designed to decrease LA in plasma. Decreases in LA were modest at 8% with a 10% reduction in AA (Table 3).
Increases in omega-3 EPA were associated with improved sleep quality (t = 2.31, p = 0.02) and a trend in improved perceived health at the end of the trial (t = 1.64, p = 0.10). Increases in omega-3 DHA were associated with improved perceived health (t = 2.23, n = 0.03) and a trend in improved sleep quality (t = 1.88, p = 0.06). Changes in omega-6 LA were not significantly associated with sleep quality, perceived stress, or perceived health. Perceived stress increased with increasing omega-6 AA (t = 2.03, p = 0.04). (Figure 4).
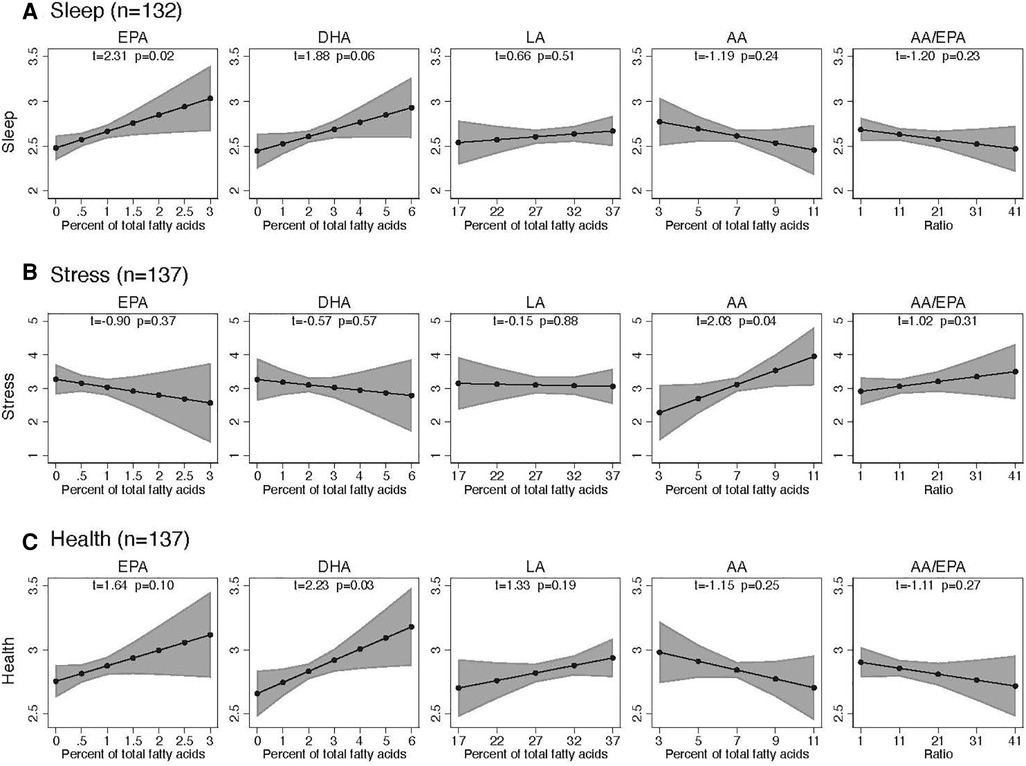
Figure 4. Association of sleep, stress, and health with precursor fatty acids in plasma over 16 weeks. Each plot stands for coefficients and 95% confidence intervals from a linear regression model adjusted for the following baseline variables: respective endpoint, respective fatty acid, age, BMI, sex, headache days per month, HIT-6, and chronic-vs.-episodic migraine.
Adverse event rates overall did not differ among the intervention groups: 47% in the control diet group, 38% in the H3 diet group, and 49% in the H3L6 diet group. Most (93%) were mild and most (81%) were not related or possibly related to any aspect of the interventions (10). Phlebotomy-related events occurred in all three groups (bruising, vasovagal reaction). Of the remaining six adverse events reported in the H3L6 diet group, three were associated with gastrointestinal complaints (nausea, dyspepsia), two with a rash or itching, two with weight loss, and one with a worsening headache. In the H3 diet group, one participant reported a gastrointestinal symptom, and one reported a rash. In the control diet group, one participant reported a worsening headache.
In this 3-arm trial that randomized 182 adults with chronic/episodic migraine to an H3 diet, H3L6 diet or a control diet, at baseline, participants had over 16 mean headache days per month and a HIT-6 mean score in the severe impact range. This analysis focused on the prespecified secondary trial endpoints including daily diary measures (stress, perceived health, and sleep quality), non-headache pain and disability measures (PROMIS pain intensity and pain interference, MIDAS), and other symptoms that co-occur with headache (PROMIS anxiety, depression, sleep quality, fatigue, social role satisfaction). Briefly, at the end of the trial, stress, perceived health, and sleep quality improved significantly in the intervention diet groups relative to the control diet group and these changes were stronger among participants who reported migraines with aura. Pain interference, non-headache pain, and pain-related disability also improved meaningfully in the intervention diet groups relative the control, but the difference was not statistically significant. We saw a meaningful and statistically significant decrease in anxiety in the H3L6 diet group relative to the control but not in the H3 diet group. Marked increases in omega-3 fatty acids in the intervention diet groups is consistent with increased intakes. Linoleic acid in plasma was modestly reduced.
At baseline, as measured in the diary, perceived health and sleep quality were less than 3 (good) on a scale ranging from 1 (poor) to 4 (excellent) and perceived stress was not elevated. Over the course of the trial, all three daily measures improved significantly more in the H3 and H3L6 intervention groups compared with the control intervention, with large effect sizes. Changes in omega-3 plasma fatty acid concentrations were associated with improvements in sleep and perceived health. Changes in arachidonic acid were associated with changes in stress.
These results suggest that a dietary intervention increasing dietary omega-3 fatty acids with and without reduction in omega-6 linoleic acid can improve sleep, stress, and perceived health among people with chronic/episodic migraine. While it is not clear whether the diets have a direct effect on these comorbid symptoms, or whether they improve due to pain reductions, the association between higher omega-3 fatty acid levels and improved sleep and perceived health provide a mechanistic clue.
In the literature, little data are available examining the effect of omega-3 intakes on sleep in adults. A cross-sectional study examined the association between omega-3 intakes and diagnosed sleep disorders in the National Health and Nutrition Examination Survey (NHANES) 2007–2014 (29). Compared with the lowest tertile, the highest tertile of omega-3 intakes was associated with a lower prevalence of sleep disorders [adjusted odds ratio (aOR) 0.85; CI 0.70–1.03] and the highest omega-6-to-omega-3 ratio was associated with a higher prevalence of sleep disorders (aOR 1.36; CI 1.08–1.70) (29). Another study based on NHANES 2011–2012 examined the association between serum fatty acid levels and sleep parameters (30). In multivariable models, very short (<5 h) and short (5–6 h) sleep durations were associate with lower mean omega-3% of fatty acid levels (adjusted mean difference: −34%; 95% CI −0.58, −0.11 for very short and −0.10%; −0.28, 0.08 for short) (30).
Data on the association between perceived stress and arachidonic acid (AA) levels is also sparse. In a study of motor vehicle accident survivors, higher baseline levels of both AA and EPA were associated with a lower risk of posttraumatic stress disorder) (31).
Baseline levels of (PROMIS) pain interference were consistent with 40%–59% impairment and the average pain intensity was in the moderate range. Baseline MIDAS scores were also consistent with a high degree of disability. In this analysis, we showed that pain interference improved to a greater extent in the intervention groups. Similarly, average pain intensity was lower in the intervention groups at the end of the trial. Controlling for baseline levels, MIDAS disability scores were also clinically meaningfully improved in the intervention groups compared to the control group (although not statistically significant). These findings support the hypotheses that those with high pain and pain-related disability at baseline improved when assigned to dietary omega-3 fatty acid diets. Moreover, these findings are consistent with our previous findings that increases in omega-3 fatty acids are associated with improvements in pain (10).
Surprisingly, mean anxiety, sleep disturbance, and mean depression scores were at or below population norms despite high baseline levels of disability as evidenced by the MIDAS and Headache Impact Test. This may be explained in part by the high proportion of individuals taking antidepressants at baseline (33%). Unsurprisingly, these measures changed little over the course of the intervention and between-group differences were small. In addition, baseline levels of fatigue, physical function, and social role functioning were not meaningfully abnormal. These findings contradict those in the literature—depression, anxiety, and sleep disorders often co-occur with migraine (32–34) and, based on the baseline headache history, sleep disturbance was diagnosed in 30% of participants.
One possible explanation for the lack of abnormality in these symptoms is the measurement instrument. PROMIS measures were developed and validated in a general US population and may not be sensitive enough to phenotype chronic pain populations. In a large cross-sectional study in an orthopedic population with chronic pain, depression, anxiety, fatigue, and sleep disturbance were measured at levels similar to our study (35). In another large (n = 750) study of chronic low back pain patients, PROMIS anxiety and depression levels were measured at <50 (36). In two large studies of orthopedic patients, the PROMIS depression score showed a strong floor effect, bringing the validity of the measurement into question (37, 38) Another possible explanation is that individuals with chronic pain did not consider their depression/anxiety levels to be abnormal.
Unlike the sparse data on the association between sleep and fatty acids, the impact of essential dietary fatty acids on depression/anxiety has been an active area of research with 17 ongoing studies of omega-3 intakes vs. placebo for depression reported in a recent Cochrane review (39). Despite the interest, definitive conclusions have been elusive. For example, a meta-analysis of 33 studies of omega-3 intakes in varying doses (from food or supplements) reported a small reduction in depressive symptomatology (−0.40, 95% CI: −0.64, −0.16) albeit with a high degree of heterogeneity in sample populations, omega-3 sources, trial durations, and outcome measures. In addition, risk of bias was high in some studies and omega-6 intakes were not reported. A second 2021 systematic review and meta-analysis investigated the effects of high vs. lower omega-3 intakes, mostly from supplements, on the development of anxiety or depression (40). Intakes of omega-6 were not considered although one trial examined total PUFA intakes. The authors concluded that higher omega-3 intakes are not associated with a lower risk of depression [Risk Ratio (RR) 1.01; 95% CI: 0.92, 1.10]. Fewer studies examined anxiety outcomes. Results suggested that higher omega-3 slightly increased anxiety symptoms (standardized mean difference: 0.15; 95% CI 0.05–0.26), but the quality of included studies was poor.
Well-documented challenges of conducting dietary intervention trials include difficulties with blinding, high attrition, and limited compliance (41, 42). In this three-arm trial in a free-living migraine population, blinding was achieved using a credible control diet. Attrition was higher than desired, at 23%, but 85% remained in the trial for at least 10 weeks. Dietary adherence was acceptable: omega-3 intakes increased by greater than 2900% in both intervention diets and omega-6 decreased by 49% in the H3L6 diet (24). With these dietary adjustments, we saw significant and meaningful changes in daily assessments of sleep quality and perceived health, but fewer changes in recalled self-reported measures compared to the Chronic Daily Headache study.
A strength of the study is its use of an electronic headache diary that captured details of the headache experience, including associated symptoms. For example, the diary assessed not only pain at each hour of the day, but also sleep quality, perceived health, stress levels, and acute medication taken for pain. The diary was completed daily, with individualized prompts for non-completion leading to an overall completion rate of >80% throughout the active intervention. Participants had to complete the diary within 48 h which vastly reduced the potential for recall bias common to intermittent questionnaires and paper diary measures. In fact, in a comparison of the diary with recalled measures of headache frequency and severity, more headaches were reported in the diary (43). Other studies also suggest that an electronic headache diary may be superior to recall measures (44).
The value of omega-3 and omega-6 manipulations in the diet for health outcomes clearly is still an open question. The importance of precision nutrition in the heterogeneity of responses to dietary interventions has been recognized in recent years with a steady increase in precision nutrition studies (45). Unfortunately, changes in the tissue levels of fatty acids and their derivatives that could be responsible for changes in clinical outcomes take weeks to months rather than days, making short-term feeding studies untenable. We continue to feel that this line of research is critical due to the burden of migraine, incomplete responses to migraine medications, side effects associated with medication use, and quality-of-life deficits associated with migraine. We plan to continue this line of research to understand the impact of omega-3 and omega-6 metabolites on migraine and their psychological correlates.
In this study of a whole-foods dietary intervention for the prevention of migraine headaches, the H3 and H3L6 intervention diets resulted in greater improvements in perceived stress, perceived health, and sleep quality compared to the control diet. Further research is needed to prove the value of these interventions for the management of chronic pain and comorbid symptoms in the overall population of individuals with chronic pain.
The data analyzed in this study is subject to the following licenses/restrictions: De-identified data may be requested from the corresponding author subject to a data use agreement with the University of North Carolina at Chapel Hill. Requests to access these datasets should be directed toZmF1cm90QG1lZC51bmMuZWR1.
The studies involving humans were approved by University of North Carolina at Chapel Hill Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
CR, BM, DM, SG, OP, and KF contributed to the conception and design of the study. DM, KF, BM, and CL conducted the study. KF and DZ directed the statistical analyses and MH, VM, GH, and JP conducted the statistical analyses. AD conducted biochemical analyses. KF prepared the first draft of the manuscript. All authors contributed to the article and approved the submitted version.
The National Center for Complementary and Integrative Health (NCCIH), National Institutes of Health (NIH) funded the study (# 1R01AT007813–01A1). The study received added support from the intramural programs of the National Institute on Aging and the National Institute on Alcohol Abuse and Alcoholism, NCCIH T32 Research Fellowship Program (T32 AT003378), the Mayday Fund, and the University of North Carolina Nutrition Obesity Research Center, CHAI Core (grant DK056350, National Institute of Diabetes and Digestive and Kidney Diseases). We acknowledge the database support of the NC Translational and Clinical Sciences (NC TraCS) Institute, which is supported by the National Center for Advancing Translational Sciences (NCATS), NIH, through Grant Award Number UL1TR002489. Support for JP and DZ was provided through a gift to the Department of Physical Medicine and Rehabilitation for nutrition-related research. The funding entities did not have a role in the design of the study, analysis, or interpretation of the results.
The following individuals contributed to the conduct of the trial and deserve our heartfelt thanks: Angela Johnston, Paula Anderson, Michele Bean, Jennifer Wills, Gregory Keyes, Zhi-Xin Yuan, Russell Levy, Sharon Majchrzak-Hong, Joseph R. Hibbeln, David Barrow, James Loewke, Andrew Mannes, and John M. Davis.
The National Institutes on Aging claims intellectual property related to stable analogs of oxidized lipid mediators (PCT/US2018/041086) with CR and Gregory Keyes as inventors. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpain.2023.1231054/full#supplementary-material
1. Buse DC, Reed ML, Fanning KM, Bostic R, Dodick DW, Schwedt TJ, et al. Comorbid and co-occurring conditions in migraine and associated risk of increasing headache pain intensity and headache frequency: results of the migraine in America symptoms and treatment (MAST) study. J Headache Pain. (2020) 21(1):23. doi: 10.1186/s10194-020-1084-y
2. Buse DC, Rupnow MFT, Lipton RB. Assessing and managing all aspects of migraine: migraine attacks, migraine-related functional impairment, common comorbidities, and quality of life. Mayo Clin Proc. (2009) 84(5):422–35. doi: 10.1016/S0025-6196(11)60561-2
3. Patwardhan AM, Scotland PE, Akopian AN, Hargreaves KM. Activation of TRPV1 in the spinal cord by oxidized linoleic acid metabolites contributes to inflammatory hyperalgesia. Proc Natl Acad Sci USA. (2009) 106(44):18820–4. doi: 10.1073/pnas.0905415106
4. Ramsden CE, Ringel A, Feldstein AE, Taha AY, MacIntosh BA, Hibbeln JR, et al. Lowering dietary linoleic acid reduces bioactive oxidized linoleic acid metabolites in humans. Prostaglandins Leukot Essent Fatty Acids. (2012) 87(4–5):135–41. doi: 10.1016/j.plefa.2012.08.004
5. Ramsden CE, Domenichiello AF, Yuan Z-X, Sapio MR, Keyes GS, Mishra SK, et al. A systems approach for discovering linoleic acid derivatives that potentially mediate pain and itch. Sci Signal. (2017) 10(493):eaal5241. doi: 10.1126/scisignal.aal5241
6. Morisseau C, Inceoglu B, Schmelzer K, Tsai H-J, Jinks SL, Hegedus CM, et al. Naturally occurring monoepoxides of eicosapentaenoic acid and docosahexaenoic acid are bioactive antihyperalgesic lipids. J Lipid Res. (2010) 51(12):3481–90. doi: 10.1194/jlr.M006007
7. Domenichiello AF, Wilhite BC, Nara P, Pitcher MH, Keyes GS, Mannes AJ, et al. Biochemical and behavioral effects of decreasing dietary linoleic acid and increasing eicosapentaenoic acid and docosahexaenoic acid in a rat chronic monoarthrits model. Prostaglandins Leukot Essent Fatty Acids. (2022) 187:102512. doi: 10.1016/j.plefa.2022.102512
8. Park C-K, Xu Z-Z, Liu T, Lü N, Serhan CN, Ji R-R. Resolvin D2 is a potent endogenous inhibitor for transient receptor potential subtype V1/A1, inflammatory pain, and spinal cord synaptic plasticity in mice: distinct roles of resolvin D1, D2, and E1. J Neurosci. (2011) 31(50):18433–8. doi: 10.1523/JNEUROSCI.4192-11.2011
9. Ramsden CE, Faurot KR, Zamora D, Suchindran CM, MacIntosh BA, Gaylord S, et al. Targeted alteration of dietary n-3 and n-6 fatty acids for the treatment of chronic headaches: a randomized trial. Pain. (2013) 154(11):2441–51. doi: 10.1016/j.pain.2013.07.028
10. Ramsden CE, Zamora D, Faurot KR, MacIntosh B, Horowitz M, Keyes GS, et al. Dietary alteration of n-3 and n-6 fatty acids for headache reduction in adults with migraine: randomized controlled trial. Br Med J. (2021) 374:n1448. doi: 10.1136/bmj.n1448
11. MacIntosh BA, Ramsden CE, Honvoh G, Faurot KR, Palsson OS, Johnston AD, et al. Methodology for altering omega-3 EPA + DHA and omega-6 linoleic acid as controlled variables in a dietary trial. Clin Nutr. (2021) 40(6):3859–67. doi: 10.1016/j.clnu.2021.04.050
12. Mann JD, Faurot KR, MacIntosh B, Palsson OS, Suchindran CM, Gaylord SA, et al. A sixteen-week three-armed, randomized, controlled trial investigating clinical and biochemical effects of targeted alterations in dietary linoleic acid and n-3 EPA + DHA in adults with episodic migraine: study protocol. Prostaglandins Leukot Essent Fatty Acids. (2018) 128:41–52. doi: 10.1016/j.plefa.2017.11.002
13. The International Classification of Headache Disorders—ICHD-3. Available at: https://ichd-3.org/ (Cited August 8, 2023).
14. ICHD-3. The international classification of headache disorders, 3rd edition. Cephalalgia. 2018;38(1):1–211. doi: 10.1177/0333102417738202
15. Borkovec TD, Nau SD. Credibility of analogue therapy rationales. J Behav Therap & Exp Psychiat. (1972) 3:257–60. doi: 10.1016/0005-7916(72)90045-6
16. Devilly GJ, Borkovec TD. Psychometric properties of the credibility/expectancy questionnaire. J Behav Ther Exper Psychiat. (2000) 31:73–86. doi: 10.1016/S0005-7916(00)00012-4
17. Ramsden CE, Mann JD, Faurot KR, Lynch C, Imam ST, MacIntosh BA, et al. Low omega-6 vs. Low omega-6 plus high omega-3 dietary intervention for chronic daily headache: protocol for a randomized clinical trial. Trials. (2011) 12:97. doi: 10.1186/1745-6215-12-97
18. Mann JD, Faurot KR, Wilkinson L, Curtis P, Coeytaux RR, Suchindran C, et al. Craniosacral therapy for migraine: protocol development for an exploratory controlled clinical trial. BMC Complement Altern Med. (2008) 8:28. doi: 10.1186/1472-6882-8-28
19. Hays RD, Spritzer KL, Sherbourne CD, Ryan GW, Coulter ID. Group and individual-level change on health-related quality of life in chiropractic patients with chronic low back or neck pain. Spine. (2019) 44(9):647–51. doi: 10.1097/BRS.0000000000002902
20. Herman PM, Edelen MO, Rodriguez A, Hilton LG, Hays RD. A protocol for chronic pain outcome measurement enhancement by linking PROMIS-29 scale to legacy measures and improving chronic pain stratification. BMC Musculoskelet Disord. (2020) 21(1):671. doi: 10.1186/s12891-020-03696-2
21. Tang E, Ekundayo O, Peipert JD, Edwards N, Bansal A, Richardson C, et al. Validation of the patient-reported outcomes measurement information system (PROMIS)-57 and -29 item short forms among kidney transplant recipients. Qual Life Res. (2019) 28(3):815–27. doi: 10.1007/s11136-018-2058-2
22. Lee AC, Driban JB, Price LL, Harvey WF, Rodday AM, Wang C. Responsiveness and minimally important differences for 4 patient-reported outcomes measurement information system short forms: physical function, pain interference, depression, and anxiety in knee osteoarthritis. J Pain. (2017) 18(9):1096–110. doi: 10.1016/j.jpain.2017.05.001
23. Chen CX, Kroenke K, Stump TE, Kean J, Carpenter JS, Krebs EE, et al. Estimating minimally important differences for the PROMIS pain interference scales: results from 3 randomized clinical trials. Pain. (2018) 159(4):775–82. doi: 10.1097/j.pain.0000000000001121
24. Northwestern University. G-code SeverityModifiers for PROMIS. HealthMeasures–Transforming how health is measured. (2022). Available at: https://www.healthmeasures.net/score-and-interpret/interpret-scores/promis/g-code-severity-modifiers (Cited October 11, 2022).
25. Stewart WF, Lipton RB, Dowson AJ, Sawyer J. Development and testing of the migraine disability assessment (MIDAS) questionnaire to assess headache-related disability. Neurology. (2001) 56(6 Suppl 1):S20–8. doi: 10.1212/WNL.56.suppl_1.S20
26. Stewart WF, Lipton RB, Kolodner K. Migraine disability assessment (MIDAS) score: relation to headache frequency, pain intensity, and headache symptoms. Headache. (2003) 43(3):258–65. doi: 10.1046/j.1526-4610.2003.03050.x
27. Lipton RB, Lombard L, Ruff DD, Krege JH, Loo LS, Buchanan A, et al. Trajectory of migraine-related disability following long-term treatment with lasmiditan: results of the GLADIATOR study. J Headache Pain. (2020) 21(1):20. doi: 10.1186/s10194-020-01088-4
28. Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. (1957) 226(1):497–509. doi: 10.1016/S0021-9258(18)64849-5
29. Luo J, Ge H, Sun J, Hao K, Yao W, Zhang D. Associations of dietary ω-3, ω-6 fatty acids consumption with sleep disorders and sleep duration among adults. Nutrients. (2021) 13(5):1475. doi: 10.3390/nu13051475
30. Murphy RA, Devarshi PP, Mun JG, Marshall K, Mitmesser SH. Association of omega-3 levels and sleep in US adults, national health and nutrition examination survey, 2011-2012. Sleep Health. (2022) 8(3):294–7. doi: 10.1016/j.sleh.2021.12.003
31. Matsuoka Y, Nishi D, Hamazaki K. Serum levels of polyunsaturated fatty acids and the risk of posttraumatic stress disorder. Psychother Psychosom. (2013) 82(6):408–10. doi: 10.1159/000351993
32. Buse DC, Silberstein SD, Manack AN, Papapetropoulos S, Lipton RB. Psychiatric comorbidities of episodic and chronic migraine. J Neurol. (2013) 260(8):1960–9. doi: 10.1007/s00415-012-6725-x
33. Lipton RB, Seng EK, Chu MK, Reed ML, Fanning KM, Adams AM, et al. The effect of psychiatric comorbidities on headache-related disability in migraine: results from the chronic migraine epidemiology and outcomes (CaMEO) study. Headache. (2020) 60(8):1683–96. doi: 10.1111/head.13914
34. Sancisi E, Cevoli S, Vignatelli L, Nicodemo M, Pierangeli G, Zanigni S, et al. Increased prevalence of sleep disorders in chronic headache: a case-control study. Headache. (2010) 50(9):1464–72. doi: 10.1111/j.1526-4610.2010.01711.x
35. Horn ME, Reinke EK, Yan X, Luo S, Bolognesi M, Reeve BB, et al. Use of patient-reported outcomes measurement information system (PROMIS) measures to characterise health status for patients seeking care from an orthopaedic provider: a retrospective cohort study. BMJ Open. (2021) 11(9):e047156. doi: 10.1136/bmjopen-2020-047156
36. Vining R, Long CR, Minkalis A, Gudavalli MR, Xia T, Walter J, et al. Effects of chiropractic care on strength, balance, and endurance in active-duty U.S. Military personnel with low back pain: a randomized controlled trial. J Altern Complement Med. (2020) 26(7):592–601. doi: 10.1089/acm.2020.0107
37. Bernstein DN, Atkinson J, Fear K, Baumhauer JF, Mesfin A, Rubery PT, et al. Determining the generalizability of the PROMIS depression domain’s floor effect and completion time in patients undergoing orthopaedic surgery. Clin Orthop Relat Res. (2019) 477(10):2215–25. doi: 10.1097/CORR.0000000000000782
38. Guattery JM, Dardas AZ, Kelly M, Chamberlain A, McAndrew C, Calfee RP. Floor effect of PROMIS depression CAT associated with hasty completion in orthopaedic surgery patients. Clin Orthop Relat Res. (2018) 476(4):696–703. doi: 10.1007/s11999.0000000000000076
39. Appleton KM, Voyias PD, Sallis HM, Dawson S, Ness AR, Churchill R, et al. Omega-3 fatty acids for depression in adults. Cochrane Database Syst Rev. (2021) 11(11):CD004692. doi: 10.1002/14651858.CD004692.pub5
40. Deane KHO, Jimoh OF, Biswas P, O’Brien A, Hanson S, Abdelhamid AS, et al. Omega-3 and polyunsaturated fat for prevention of depression and anxiety symptoms: systematic review and meta-analysis of randomised trials. Br J Psychiatry. (2021) 218(3):135–42. doi: 10.1192/bjp.2019.234
41. Crichton GE, Howe PRC, Buckley JD, Coates AM, Murphy KJ, Bryan J. Long-term dietary intervention trials: critical issues and challenges. Trials. (2012) 13:111. doi: 10.1186/1745-6215-13-111
42. Staudacher HM, Irving PM, Lomer MCE, Whelans K. The challenges of control groups, placebos and blinding in clinical trials of dietary interventions. Proc Nutr Soc. (2017) 76(4):628. doi: 10.1017/S0029665117002816
43. Miller VE, Faurot KR, Palssson OS, MacIntosh BA, Suchindran C, Honvoh G, et al. Comparing prospective headache diary and retrospective four-week headache questionnaire over 20 weeks: secondary data analysis from a randomized controlled trial. Cephalalgia. (2020) 40(13):1523–31. doi: 10.1177/0333102420949180
44. van Casteren DS, Verhagen IE, de Boer I, de Vries Lentsch S, Fronczek R, van Zwet EW, et al. E-diary use in clinical headache practice: a prospective observational study. Cephalalgia. (2021) 41(11–12):1161–71. doi: 10.1177/03331024211010306
45. National Institutes of Health. NIH NIH awards $170 million for precision nutrition study$170 million for precision nutrition study. News REleases. 2022. Available at: https://www.nih.gov/news-events/news-releases/nih-awards-170-million-precision-nutrition-study (Cited May 23, 2022).
Keywords: migraine, dietary intervention, fatty acids, sleep quality, stress, perceived health, quality of life
Citation: Faurot KR, Park J, Miller V, Honvoh G, Domeniciello A, Mann JD, Gaylord SA, Lynch CE, Palsson O, Ramsden CE, MacIntosh BA, Horowitz M and Zamora D (2023) Dietary fatty acids improve perceived sleep quality, stress, and health in migraine: a secondary analysis of a randomized controlled trial. Front. Pain Res. 4:1231054. doi: 10.3389/fpain.2023.1231054
Received: 30 May 2023; Accepted: 6 October 2023;
Published: 25 October 2023.
Edited by:
Csaba Ertsey, Semmelweis University, HungaryReviewed by:
Parisa Gazerani, Oslo Metropolitan University, Norway© 2023 Faurot, Park, Miller, Honvoh, Domenichiello, Mann, Gaylord, Lynch, Palsson, Ramsden, MacIntosh, Horowitz and Zamora. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Keturah R. Faurot ZmF1cm90QG1lZC51bmMuZWR1
†These authors have contributed equally to this work and share senior authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.