- 1MJHS Institute for Innovation in Palliative Care, New York, NY, United States
- 2Department of Family & Social Medicine, Albert Einstein College of Medicine, Bronx, NY, United States
- 3Division of Geriatrics and Palliative Medicine, Weill Cornell Medicine, New York, NY, United States
- 4Department of Psychology, Cornell University, Ithaca, NY, United States
- 5Department of Neurology, Albert Einstein College of Medicine, Bronx, NY, United States
- 6Rogosin Institute, Weill Cornell Medicine, New York, NY, United States
- 7Division of Nephrology and Hypertension, Weill Cornell Medicine, New York, NY, United States
Background: Poorly controlled pain remains a problem for many patients with end-stage kidney disease requiring hemodialysis (ESKD/HD) and customary approaches to pain management (e.g., opioids, non-steroidals) confer substantial risk. Accordingly, non-pharmacologic therapies are needed for use in this population. Non-invasive transcranial Direct Current Simulation (tDCS) constitutes a promising nonpharmacologic method for pain management in affected individuals.
Aims: This study seeks to: 1) determine the effects of an 8-week course of at-home tDCS vs. sham tDCS on pain intensity, pain interference, medication usage, quality of life, and mood; 2) determine if tDCS effects vary by race/ethnicity; and 3) ascertain patient satisfaction with device use.
Methods: This double-blind, randomized, sham-controlled clinical trial will enroll 100 ESKD/HD patients with moderate-to-severe (≥4 on 0–10 scale) chronic pain. The active study intervention consists of 20 min of tDCS delivered over the primary motor cortex 5 days/week for 8 weeks. The comparator is a sham procedure that provides no effective stimulation. The primary outcome analysis will evaluate efficacy of tDCS for pain reduction after two months of stimulation. We will also assess the effects of treatment on analgesic consumption, pain interference, depressed mood, and quality of life. The statistical plan will include fixed classification factors for treatment (vs. sham), clinic sites, and assessment time, and the interaction of these factors adjusting for covariates (e.g., race/ethnicity, pain level).
Conclusion: At-home tDCS constitutes a promising nonpharmacologic treatment for pain mitigation in persons with ESKD/HD. This unique RCT could transform the way pain is managed in this vulnerable population.
Trial Registration: NCT05311956.
Background
The prevalence of end-stage kidney disease requiring chronic hemodialysis (ESKD/HD) is rising and racial/ethnic minorities are disproportionately affected (1–3). More than 30% of ESKD/HD are Black patients, and during the past two decades, the prevalence of ESKD/HD has increased by more than 70% in the Hispanic population (4–6). Chronic pain is highly prevalent among those with ESKD/HD and pain intensity is associated with mortality, particularly among racial/ethnic minorities (7). Patients develop ESKD as a consequence of many different conditions, and suffer from disparate comorbidities; pain syndromes are therefore heterogeneous in this population, including pain stemming from neuropathic, musculoskeletal, orthopedic, and rheumatologic disorders, as well as the discomfort arising from ESKD and dialysis itself. Thus, many patients with ESKD present with mixed pain disorders that include nociceptive, neuropathic as well as nociplastic components. Conventional treatment for chronic pain relies on systemic drug therapy with opioid drugs or adjuvant analgesics, but evidence of efficacy is limited and the potential for drug-disease interactions confer significant risk of adverse outcomes (8–10). There is a compelling need for novel analgesic treatment approaches that pose less risk in this medically vulnerable population.
Transcranial direct current stimulation (tDCS) is a non-invasive neuromodulatory intervention, designated by the United States. Food and Drug Administration as having minimal risk, that may reduce pain and analgesic consumption in patients with diverse types of chronic pain (11–18). tDCS delivers low intensity (1 or 2 milliamperes) electrical current through the skull to selected areas of the brain and induces changes in excitability and activation of brain neurons and neuronal circuits. The primary mechanism of tDCS is a subthreshold modulation of neuronal resting membrane potential. Stimulation for a few minutes results in neuroplasticity of glutamatergic synapses that may be associated with longer-term effects (19–21). In addition, recent evidence suggests that tDCS interacts with various neurotransmitters in the brain, such as dopamine, acetylcholine, serotonin or GABA, and can also trigger changes in brain-derived neurotrophic factor (BDNF) that is associated with pain processing (22–25). These effects may up-regulate or down-regulate functional connectivity within brain networks, such as those important for pain processing (26).
The most common anatomical target for tDCS in pain management is the primary motor cortex (M1). Research suggests that the analgesic effects of M1 stimulation involve multiple neural circuits and are at least partially attributed to modulation of thalamic activity, motor-cortex-driven inhibition of the somatosensory cortex, and modulation of endogenous opioid release (27–29).
Numerous studies of various populations with acute pain as well as those with various chronic pain disorders conclude that tDCS can reduce pain and opioid consumption, and improve QoL, without the risk of serious adverse events. A recent meta-analysis of 27 randomized controlled trials (RCTs) using various tDCS stimulation protocols in patients with chronic pain demonstrated analgesic efficacy of tDCS as compared to sham, complemented by improved QoL (30). However, this evidence has important gaps. Previous RCTs, including an ongoing study of tDCS for pain in ESKD/HD (31), have been limited by small sample sizes and brief stimulation protocols, have not employed an at-home stimulation component, and assessed short-term outcomes only. To our knowledge, no tDCS analgesic trial has evaluated longer-term treatment effects or determined whether treatment effects vary by race/ethnicity.
RCTs of tDCS that include larger and heterogeneous samples and assess short-term and longer-term outcomes are needed to establish whether tDCS could transform the way pain is managed in the growing and ethnically diverse population of ESKD/HD patients. We are conducting a randomized trial evaluating 8-weeks of at-home tDCS in 100 adults with moderate-to-severe chronic pain due to ESKD/HD (score of ≥4 on a 0–10 scale). Double-blind assignment to either active stimulation or sham ensues for 8 treatment weeks. Change in pain intensity after 8 weeks is the primary outcome. Change in pain intensity after 2, 12, 16, and 26 weeks constitute secondary outcomes. Additional secondary outcomes include changes in analgesic drug use, pain interference, mood, and quality of life (Aim 1) after 8 and 26 weeks. The study also examines racial/ethnic differences in these tDCS effects (Aim 2) and ascertains the tolerability of tDCS and satisfaction with the device and procedure (Aim 3).
Hypotheses
We hypothesize that the active tDCS stimulation applied over M1 for 20 min per day, 5 days per week for 8 weeks, at the intensity of 2 mA, will significantly reduce pain, lessen analgesic consumption, and improve QoL; and that the analgesic effects of tDCS will extend into the follow-up period. We also hypothesize that no significant treatment differences will be found across the three primary race/ethnicity groups targeted in this study and that tolerability of and satisfaction with the intervention will be high.
Methods
Study design
This trial employs a double-blind, sham controlled, randomized, 2-parallel arm design. ESKD/HD patients are screened for eligibility criteria at participating dialysis centers. Eligible patients who provide informed consent are stratified to ensure that roughly equal numbers of Hispanic or Latino(a), Black or African American, and non-Hispanic White participants, assigned to the active and sham treatment arms, respectively. Each patient undergoes 8 study visits over the 26-week study period, including consenting and screening (V1); tDCS familiarization/training (V2); baseline assessment and tDCS refresh training, device deployment and first tDCS application under supervision by study personnel (V3); outcome assessment at 2 weeks (V4); outcome assessment at the 8-week conclusion of the study intervention (V5); and finally, outcome assessments at 12 weeks (V6), 16 weeks (V7), and 26 weeks (V8) from baseline. This study has institutional review board approval from Ethical & Independent Review Services (22,048).
Screening, recruitment, randomization
Potential participants are identified by staff at the dialysis centers. Staff ascertain patient interest and those who agree to be contacted by study personnel complete an “agree-to-contact” sheet. Study personnel discuss consent in person or remotely, reviewing each section of the consent in detail. The process is designed to allow enough time for the patient and family to obtain sufficient information about the study in the manner that is not overwhelming and answers all questions before the patient decides whether to participate and then sign the secure e-consent (DocuSign) form.
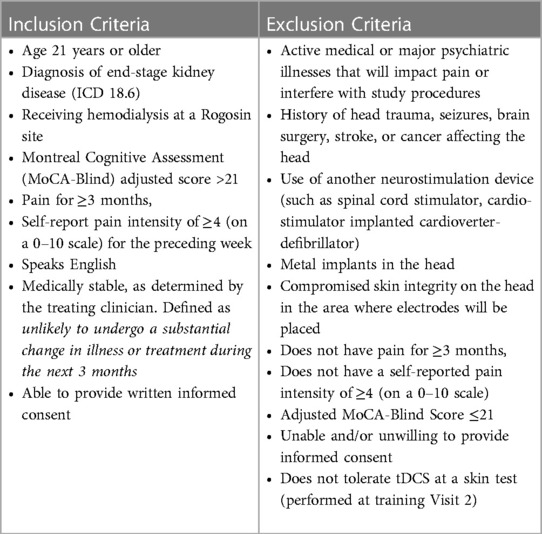
Patients who provide consent undergo confirmation of eligibility for the study (Table 1). Eligible patients receive training in the use of the tDCS device and undergo random assignment to active tDCS or sham. Random assignment is performed in double-blind fashion. Information about the patient is provided to an unblinded study team member who determines assignment from a computer-generated list. The unblinded team member provides the blinded study team member with the identification number of the device that will be delivered to the participant. At Visit 3, the patient completes baseline measures and the team member who is blinded to the programming of the tDCS device provides refresher training about stimulation procedures and observes the patient performing the first stimulation. The patient interacts with the blinded study team member throughout the 8-week period of daily treatments, and during the assessment period that follows. The tDCS device is returned after the 8 weeks of study treatments.
Study measures
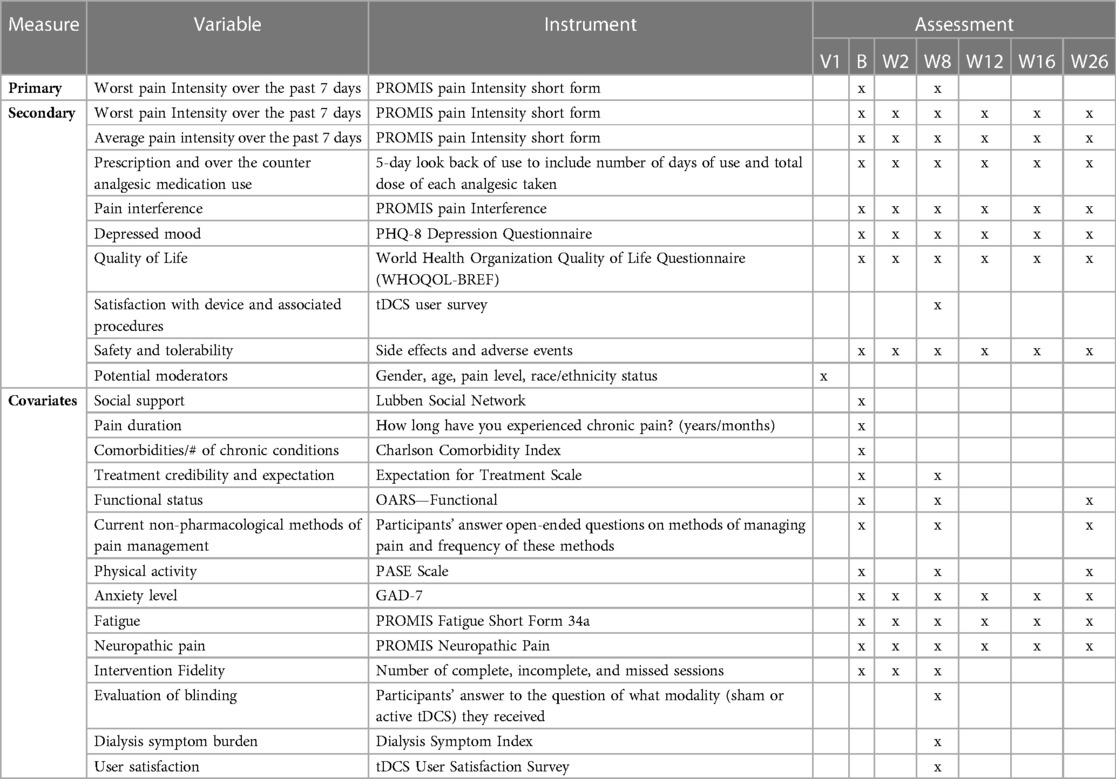
A complete list of all variables obtained at baseline and follow-up visits is shown in Table 2 below.
Primary and secondary outcomes
The primary outcome for Aim 1 is change in worst pain intensity over the past seven days measured by the PROMIS pain Intensity short form after 8 weeks of the study intervention. Secondary outcomes include change in worst pain intensity after 2, 8, 12, 16, and 26 weeks after baseline as well as analgesic consumption, pain interference with function, depressed mood, and QoL. In addition, we are assessing the tolerability of the treatment in terms of side effects and adverse events reported through the study. Quality of blinding is being assessed by participants' guess at the end of the 8-week intervention of what treatment modality (sham or active tDCS) they received, and fidelity to the treatment is evaluated by the number of incomplete stimulation sessions during the 8-week intervention.
The tDCS intervention
Participants randomized to the active tDCS group receive 20 min of direct current at the intensity of 2 mA once a day 5 days/week for 8 weeks, delivered via 2 sponge electrodes of size 5 × 5 cm presoaked by the manufacturer with normal saline and placed on the head using an EasyStrap headband for accurate electrode placement. The electrodes are inserted into a size-fitted headgear that allows for accurate and replicable positioning of the electrodes by patients at home (32). In the case of unilateral pain, the electrodes are placed with the anode over M1 contralateral to the pain-affected side of the body, and the cathode is placed over the supraorbital region on the hemisphere contralateral to the anode placement. In case of bilateral pain, the anode is placed over M1 of the left hemisphere and the cathode over the supraorbital region on the right. Devices programmed to sham produce 1 min of direct current that is ramped up to 2 mA over 30 s, ramped down over 30 s, and stay at 0 current for the remaining time. This model of sham mimics the sensory sensation of real stimulation without inducing neuroplasticity changes and has been successfully employed in numerous tDCS studies (33–35).
The tDCS device used to deliver the study intervention is a Soterix Mini-CT (Soterix Medical Inc.), programmed either to active tDCS or sham. The device has built-in dose control only allowing the user to apply the pre-determined dose each day, suitable for use at home. The device is paired with a tablet equipped with cellular connection, which enables video connection providing a real-time linkage to study personnel who can supervise and provide remote assistance. Participants do not need to have Internet connection at home to participate.
Participants and caregivers are trained in the use of the device at study Visit 2 and Visit 3. The first treatment using the device programmed to either active tDCS or sham is performed under the observation of the study team member. Subsequently, the team member contacts the patient prior to each application to provide the electronic code that unlocks the daily stimulation dose. This ensures that the device is being used correctly in compliance with the protocol, and allows for tracking of any adverse events. Refamiliarization on tDCS equipment is offered as needed.
To promote adherence and retention, patients are assigned to one staff member for the intervention and receive reminders prior to all study visits. They also receive materials on tDCS usage. Co-participation of each participant's informal caregiver is encouraged. Participants who do not have access to an informal caregiver and decide to participate alone are helped via video-connection and technical-assistance visits by the field study personnel. Patients who participate in the trial receive compensation for their time.
Statistical plan
The analysis evaluates outcomes using models that adjust for covariates, including race/ethnicity and other patient characteristics such as gender, age, body mass index, cognitive status, and baseline pain level. The core model for evaluation of the tDCS intervention includes fixed classification factors for treatment (active vs. sham), clinic sites, and time of assessment (baseline, and 2, 8, 12, 16, and 26 weeks after baseline, giving us 6 assessment points for each participant); the interaction of these factors; and individuals as levels of a random classification factor. All variables as described in Table 1 will be examined for inclusion in the evaluation models, specifically race/ethnicity and all additional health and sociodemographic patient variables (e.g., age, education, gender, mental health, time on hemodialysis, pain level reported, PHQ-8 score, and comorbid conditions) as classification factors or covariates. The primary and secondary outcomes will be analyzed in the same core model.
There will be a focus on interactions of the other independent variables with treatment and time. Interactions are examined under Aim 1 as to obtain correctly specified models; potential moderators are examined in greater detail in the analysis for Aim 2 to determine whether treatment effects hold only for or are stronger for certain model subgroups or for a certain time points to determine anticipated reduction in treatment effects over time. Analysis for Aim 3, examining the tolerability and patient satisfaction with device and procedure will be examined in models of the same type as for Aims 1 and 2 to estimate levels of satisfaction and tolerability overall and whether these differ by factors such as race/ethnicity, gender, age, and pain level. Using data collected during each of the total of 40 stimulation sessions, we will examine the relationship between completed/successful sessions and better outcomes, i.e., if higher number of completed sessions results in better pain relief.
Finally, we will undertake a responder analysis to determine the proportion of participants in each group that achieve a pre-defined level of improvement in their pain levels. We consider a change score of −2.0 on our primary outcome measure or a percent change of >30% on our primary outcome measure relative to baseline to constitute a clinically meaningful change. We will also conduct analyses to assess whether treatment effects vary as a function of pain site with respect to our primary outcome (pain reduction) and our secondary outcomes including quality of life and analgesic consumption.
Sample size
To achieve the planned sample size of 100 intent-to-treat participants, we are identifying through prescreening activities as many as 500 patients to produce 125 consenting and potentially eligible patients, i.e., endorse the presence of a pain problem and speak English. We estimate that approximately 80% of the 125 patients who pass the initial screen will be found eligible to participate after undergoing the full screening assessment, yielding 100 consented participants that will be randomized to active (or sham) treatment. With an estimated attrition rate of 20%, we are conducting follow-up assessments on approximately 80 patients at the scheduled 26-week assessment, with greater numbers at earlier assessments. Participants are recruited by race/ethnicity status to ensure roughly equal numbers of non-Hispanic White, Black or African American, and Hispanic or Latino(a) participants are enrolled. No participant is excluded based on race/ethnicity.
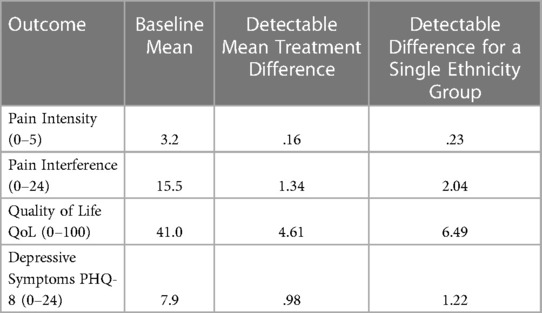
Power calculations for main outcome variables are given under the assumptions of a Type I error of.05, a Type II error of.20 (power of.80), 10 percent of the variance accounted for by other fixed terms in the model, a ratio of patient variance to error variance of 1.3, and a sample of 95 patients at the 8-week assessment. Based on means and standard deviations from previous studies for the primary and secondary outcome variables, detectable effect sizes for each variable are computed in a mixed model as described in the preceding section. Table 3 shows for selected outcomes the detectable treatment mean difference (the smallest detectable change resulting from the intervention) for treatment differences (baseline to 8 weeks) and for differences limited to a single level of a second variable such as race/ethnicity. With the target n = 100, we have adequate power to detect clinically meaningful differences for each of the main outcomes for 2-way interactions.
Discussion
To our knowledge, this is the first RCT assessing the efficacy of at-home tDCS for chronic pain in patients with ESKD/HD. tDCS is a cutting-edge, nonpharmacological analgesic approach, and at-home tDCS is a new approach that may facilitate long-term treatment of chronic conditions and overcome the limitations of previous short-term research-center-based tDCS interventions. Our at-home device has a remote-supervision element that also allows for enhanced outreach and better communication and interaction among patients, caregivers, and research staff.
Unlike previous studies of tDCS for pain, this trial includes a large sample, allowing meaningful evaluation of characteristics that could potentially influence outcomes. These include variation in the pain and patient characteristics, such as race/ethnicity. The study evaluates a longer treatment period than most tDCS studies and assesses both short-term and longer-term outcomes.
The unique features of this trial will enhance the understanding of short- and long-term analgesic effects of tDCS and determine whether treatment of chronic pain in an ESKD/HD population is affected by race/ethnicity or other patient characteristics. At-home tDCS is a promising nonpharmacologic treatment for pain in ESKD/HD. Establishing its long-term effects could transform the way pain is managed in this ethnically diverse growing population of patients.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by Ethical and Independent Review Services. The patients/participants provided their written informed consent to participate in this study.
Author contributions
JVZ: Writing-Original Draft, Writing-Review & Editing, Visualization, Supervision; HK: Conceptualization, Methodology, Writing-Original Draft, Writing-Review & Editing, Funding Acquisition; PK: Writing-Review & Editing, Visualization, Supervision, Project Administration; CH: Conceptualization, Methodology, Writing-Review & Editing; RP: Conceptualization, Methodology, Writing-Review & Editing; NB: Conceptualization, Methodology, Writing-Review & Editing; MF: Writing-Review & Editing, Supervision; MCR: Conceptualization, Methodology, Reviewing-Editing, Funding Acquisition. All authors contributed to the article and approved the submitted version.
Funding
This study is supported by the National Institutes of Health [grant numbers 1R01DK131050, 5P30AG022845, 2K24AG053462, 5UL1TR002384]; the R01 funds the study, the P30 & K24 provide Reid with protected research time, and the UL1 supports the data management (REDCap) system.
Acknowledgments
We are extremely grateful to Nicole Dagen, Phoenixia Rene, Elizabeth Carr, Erica Sluys, Isel Vazquez-Carbajal, and Rebecca Wartels for their assistance operationalizing the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bock F, Stewart TG, Robinson-Cohen C, Morse J, Kabagambe EK, Cavanaugh KL, et al. Racial disparities in end-stage renal disease in a high-risk population: the southern community cohort study. BMC Nephrol. (2019) 20(1):308. doi: 10.1186/s12882-019-1502-z
2. Albertus P, Morgenstern H, Robinson B, Saran R. Risk of ESRD in the United States. Am J Kidney Dis. (2016) 68(6):862–72. doi: 10.1053/j.ajkd.2016.05.030
3. Desai N, Lora CM, Lash JP, Ricardo AC. CKD And ESRD in US hispanics. Am J Kidney Dis. (2019) 73(1):102–11. doi: 10.1053/j.ajkd.2018.02.354
4. United States Renal Data System. 2020 USRDS Annual Data Report: Epidemiology of kidney disease in the United States. Updated, 2020. Accessed February 3, 2021. https://adr.usrds.org/2020
5. Lora CM, Daviglus ML, Kusek JW, Porter A, Ricardo AC, Go AS, et al. Chronic kidney disease in United States hispanics: a growing public health problem. Ethn Dis. (2009) 19(4):466–72. PMID: 20073150.20073150
6. Lora CM, Gordon EJ, Sharp LK, Fischer MJ, Gerber BS, Lash JP. Progression of CKD in hispanics: potential roles of health literacy, acculturation, and social support. Am J Kidney Dis. (2011) 58(2):282–90. doi: 10.1053/j.ajkd.2011.05.004
7. Kalantar SS, You AS, Norris KC, Nakata T, Novoa A, Juarez K, et al. The impact of race and ethnicity upon health-related quality of life and mortality in dialysis patients. Kidney Med. (2019) 1(5):253–62. doi: 10.1016/j.xkme.2019.07.005
8. Lai KM, Chen TL, Chang CC, Chen HH, Lee YW. Association between NSAID use and mortality risk in patients with end-stage renal disease: a population-based cohort study. Clin Epidemiol. (2019) 11:429–41. doi: 10.2147/CLEP.S204322
9. Ishida JH, McCulloch CE, Steinman MA, Grimes BA, Johansen KL. Opioid analgesics and adverse outcomes among hemodialysis patients. Clin J Am Soc Nephrol. (2018) 13(5):746–53. doi: 10.2215/CJN.09910917
10. Ishida JH, McCulloch CE, Steinman MA, Grimes BA, Johansen KL. Gabapentin and pregabalin use and association with adverse outcomes among hemodialysis patients. J Am Soc Nephrol. (2018) 29(7):1970–8. doi: 10.1681/ASN.2018010096
11. Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J. Physiol. (2000) 527:633–9. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x
12. Borckardt JJ, Romagnuolo J, Reeves ST, Madan A, Frohman H, Beam W, et al. Feasibility, safety, and effectiveness of transcranial direct current stimulation for decreasing post-ERCP pain: a randomized, sham-controlled, pilot study. Gastrointest Endosc. (2011) 73(6):1158–64. doi: 10.1016/j.gie.2011.01.050
13. Borckardt JJ, Reeves ST, Robinson SM, May JT, Epperson TI, Gunselman RJ, et al. Transcranial direct current stimulation (tDCS) reduces postsurgical opioid consumption in total knee arthroplasty (TKA). Clin J Pain. (2013) 29(11):925–8. doi: 10.1097/AJP.0b013e31827e32be
14. Glaser J, Reeves ST, Stoll WD, Epperson TI, Hilbert M, Madan A, et al. Motor/prefrontal transcranial direct current stimulation (tDCS) following lumbar surgery reduces postoperative analgesia use. Spine (Phila Pa 1976). (2016) 41(10):835–9. doi: 10.1097/BRS.0000000000001525
15. Knotkova H, Soto E, Leuschner Z. Transcranial direct current stimulation (tDCS) for the treatment of chronic pain. J Pain. (2013) 14(4):S64–S64. doi: 10.1016/j.jpain.2013.01.592
16. Knotkova H, Borckardt J, Riggs A, DaSilva A. Transcranial direct current stimulation (tDCS) potential for pain management. In: Knotkova H, Nitsche M, Bikson M, Woods A, eds. Practical guide to the transcranial direct stimulation. New York, NY: Springer Nature; (2019):541–68. doi: 10.1007/978-3-319-95948-1
17. Baptista AF, Fernandes AMBL, Sá KN, Okano AH, Brunoni AR, Lara-Solares A, et al. Latin American and Caribbean consensus on noninvasive central nervous system neuromodulation for chronic pain management (LAC2-NIN-CP). Pain Rep. (2019) 4(1):e692. doi: 10.1097/PR9.0000000000000692
18. Fregni F, El-Hagrassy MM, Pacheco-Barrios K, Carvalho S, Leite J, Simis M, et al. Evidence-based guidelines and secondary meta-analysis for the use of transcranial direct current stimulation (tDCS) in neurological and psychiatric disorders. Int J Neuropsychopharmacol. (2021) 24(4):256–313. doi: 10.1093/ijnp/pyaa051
19. Nitsche MA, Liebetanz D, Antal A, Lang N, Tergau F, Paulus W. Modulation of cortical excitability by weak direct current stimulation–technical, safety and functional aspects. Suppl Clin Neurophysiol. (2003) 56:255–76. doi: 10.1016/s1567-424x(09)70230-2
20. Nitsche MA, Cohen LG, Wassermann EM, Priori A, Lang N, Antal A, et al. Transcranial direct current stimulation: state of the art. Brain Stimul. (2008) 1:206–23. doi: 10.1016/j.brs.2008.06.004
21. Antal A, Paulus W, Nitsche MA. Principle and mechanisms of transcranial direct current stimulation (tDCS). In: Knotkova H, Cruciani R, Merrick J eds. Pain brain stimulation in the treatment of pain. New York: Nova; (2010);129–42.
22. Nitsche MA, Grundey J, Liebetanz D, Lang N, Tergau F, Paulus W. Catecholaminergic consolidation of motor cortical neuroplasticity in humans. Cereb. Cortex. (2004a) 14:1240–5. doi: 10.1093/cercor/bhh085
23. Nitsche MA, Jaussi W, Liebetanz D, Lang N, Tergau F, Paulus W. Consolidation of human motor cortical neuroplasticity by D- cycloserine. Neuropsychopharmacology. (2004b) 29:1573–8. doi: 10.1038/sj.npp.1300517
24. Nitsche MA, Liebetanz D, Schlitterlau A, Henschke U, Fricke K, Frommann K. GABAergic modulation of DC stimulation-induced motor cortex excitability shifts in humans. Eur. J. Neurosci. (2004c) 19:2720–6. doi: 10.1111/j.0953-816X.2004.03398.x
25. Stefani LC, Torres IL, de Souza IC, Rozisky JR, Fregni F, Caumo W. BDNF As an effect modifier for gender effects on pain thresholds in healthy subjects. Neurosci Lett. (2012) 514(1):62–6. doi: 10.1016/j.neulet.2012.02.057
26. Stagg C, Lin R, Mezue M, Segerdahl A. Widespread modulation of cerebral perfusion induced during and after transcranial direct current stimulation applied to the left dorsolateral prefrontal Cortex. J Neurosci. (2013) 33(28):11425–31. doi: 10.1523/JNEUROSCI.3887-12.2013
27. Polania R, Paulus W, Nitsche MA. Modulating cortico-striatal and thalamo-cortical functional connectivity with transcranial direct current stimulation. Hum Brain Mapp. (2011) 33:2499–508. doi: 10.1002/hbm.21380
28. DosSantos MF, Love TM, Martikainen IK, Nascimento TD, Fregni F, Cummiford C, et al. Immediate effects of tDCS on the mu-opioid system of a chronic patient. Front. Psychiatry. (2012) 3:93. doi: 10.3389/fpsyt.2012.00093
29. Knotkova H, Nitsche MA, Cruciani RA. Putative physiological mechanisms underlying tDCS analgesic effects. Front Hum Neurosci. (2013) 7:628. doi: 10.3389/fnhum.2013.00628
30. O'Connell NE, Marston L, Spencer S, DeSouza LH, Wand BM. Non-invasive brain stimulation techniques for chronic pain. Cochrane Database Syst Rev. (2018) 4:CD008208. doi: 10.1002/14651858.CD008208.pub5
31. Quintiliano A, Oehmen T, Kirsztajn GM, Pegado R. Transcranial direct current stimulation in management of pain, mood, functionality, and quality of life in patients undergoing hemodialysis: a study protocol for a double-blind controlled randomized trial. Trials. (2019) 20(1):805. doi: 10.1186/s13063-019-3769-6
32. Knotkova H, Riggs A, Berisha D, Borges H, Bernstein H, Patel V, et al. Automatic M1-SO montage headgear for transcranial direct current stimulation (TDCS) suitable for home and high-throughput in-clinic applications. Neuromodulation J Int Neuromodulation Soci. (2019) 22(8):904–10. doi: 10.1111/ner.12786
33. Im JJ, Jeong H, Bikson M, Woods AJ, Unal G, Oh JK, et al. Effects of 6-month at-home transcranial direct current stimulation on cognition and cerebral glucose metabolism in Alzheimer's Disease. Brain Stimul. (2019) 12(5):1222–8. doi: 10.1016/j.brs.2019.06.003
34. Woods AJ, Antal A, Bikson M, Boggio PS, Brunoni AR, Celnik P, et al. A technical guide to tDCS, and related non-invasive brain stimulation tools. Clin Neurophysiol. (2016) 127(2):1031–48. doi: 10.1016/j.clinph.2015.11.012
Keywords: transcranial direct current stimulation (tDCS), non-invasive neurostimulation, end-stage kidney disease (ESKD), hemodialysis (HD), chronic pain, clinical trial protocol
Citation: Van Zyl J, Knotkova H, Kim P, Henderson Jr CR, Portenoy RK, Berman N, Frederic MW and Reid M.C (2023) Delivery of an at-home transcranial direct current stimulation intervention to mitigate pain in patients with end-stage kidney disease receiving hemodialysis (ESKD/HD). Front. Pain Res. 4:1132625. doi: 10.3389/fpain.2023.1132625
Received: 27 December 2022; Accepted: 20 March 2023;
Published: 5 April 2023.
Edited by:
Fusao Kato, Jikei University School of Medicine, JapanReviewed by:
Koichi Hosomi, Osaka University, JapanKazuaki Nagasaka, Niigata University of Health and Welfare, Japan
© 2023 Van Zyl, Knotkova, Kim, Henderson Jr., Portenoy, Berman, Frederic and Reid. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Helena Knotkova aGtub3Rrb3ZAbWpocy5vcmc= M. Carrington Reid bWNyMjAwNEBtZWQuY29ybmVsbC5lZHU=
Specialty Section: This article was submitted to Pain Research Methods, a section of the journal Frontiers in Pain Research
 Jordan Van Zyl1
Jordan Van Zyl1 Helena Knotkova
Helena Knotkova Patricia Kim
Patricia Kim M. Carrington Reid
M. Carrington Reid