
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Pain Res. , 10 November 2022
Sec. Neuromodulatory Interventions
Volume 3 - 2022 | https://doi.org/10.3389/fpain.2022.959609
This article is part of the Research Topic Advancements in Deep Brain Stimulation for Chronic Pain Control View all 7 articles
 Cory A. Alcon1,2*
Cory A. Alcon1,2* Sharon Wang-Price2
Sharon Wang-Price2
Chronic low back pain (CLBP) is among the leading causes of disability worldwide. Beyond the physical and functional limitations, people's beliefs, cognitions, and perceptions of their pain can negatively influence their prognosis. Altered cognitive and affective behaviors, such as pain catastrophizing and kinesiophobia, are correlated with changes in the brain and share a dynamic and bidirectional relationship. Similarly, in the presence of persistent pain, attentional control mechanisms, which serve to organize relevant task information are impaired. These deficits demonstrate that pain may be a predominant focus of attentional resources, leaving limited reserve for other cognitively demanding tasks. Cognitive dysfunction may limit one's capacity to evaluate, interpret, and revise the maladaptive thoughts and behaviors associated with catastrophizing and fear. As such, interventions targeting the brain and resultant behaviors are compelling. Pain neuroscience education (PNE), a cognitive intervention used to reconceptualize a person's pain experiences, has been shown to reduce the effects of pain catastrophizing and kinesiophobia. However, cognitive deficits associated with chronic pain may impact the efficacy of such interventions. Non-invasive brain stimulation (NIBS), such as transcranial direct current stimulation (tDCS) or repetitive transcranial magnetic stimulation (rTMS) has been shown to be effective in the treatment of anxiety, depression, and pain. In addition, as with the treatment of most physical and psychological diagnoses, an active multimodal approach is considered to be optimal. Therefore, combining the neuromodulatory effects of NIBS with a cognitive intervention such as PNE could be promising. This review highlights the cognitive-affective deficits associated with CLBP while focusing on current evidence for cognition-based therapies and NIBS.
Chronic low back pain (CLBP) is among the leading causes of disability worldwide (1). Nearly one-third of the world's population lives with some form of ongoing pain, with low back and neck pain contributing the most to years lived with disability (2). This high rate of disability is associated with significant individual, social, and financial impact along with high rates of recurrence (3, 4). Despite its prevalence, CLBP often lacks a specific, identifiable cause, making it difficult to treat (5). Besides physical and functional impairments, many other factors influence the prognosis of CLBP, including a person's beliefs, cognitions, and perceptions of their pain. Those suffering from persistent pain often demonstrate altered cognitive and affective behaviors, such as pain catastrophizing, kinesiophobia, and executive control deficits (6–8). These alterations are believed to have arisen from maladaptive reorganization of brain networks, including cognitive-evaluative and affective cortical networks, and often are predictive of poorer recovery and development of chronic pain (9, 10). Elements of pain catastrophizing, fear of movement, and executive function deficits can be observed clinically through behaviors, such as magnification of pain, rumination, avoidance, withdrawal, and other adverse responses. These variables share a dynamic relationship with subjective reports of pain severity in that these behaviors can be both a consequence of pain and a predictor of chronicity. Structural and functional changes, including alterations of brain matter volume and network activation (11), also occur throughout the nervous system and correlate with the presence of pain behaviors (12). Interestingly, these cortical changes have been shown to reverse when pain is successfully treated (13). Historically, conservative management of LBP has focused on pain reduction and function improvement, with interventions targeting injured tissues taking priority (Figure 1). However, this approach could be further enhanced by also addressing coexisting psychosocial deficits (14).
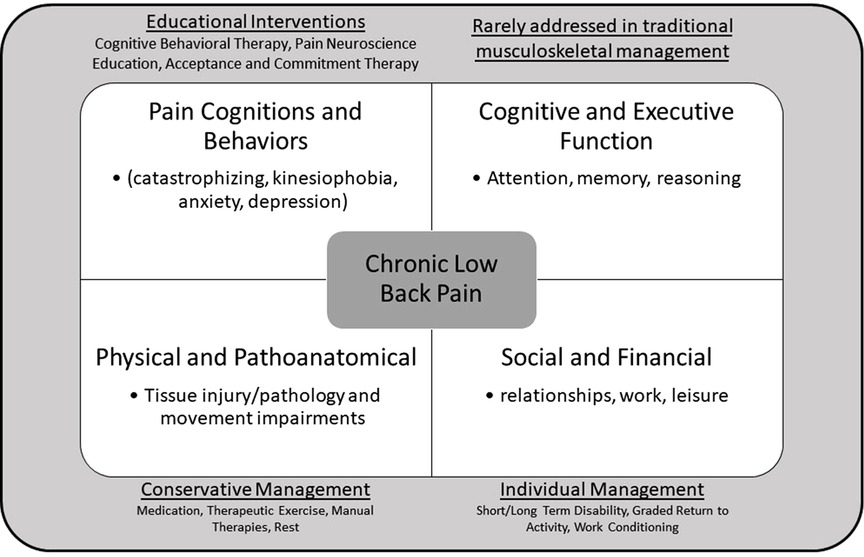
Figure 1. Multidimensional nature of chronic low back pain and it's traditional management approaches.
As the high prevalence of CLBP continues and the evidence for the psychological contributors has grown, the effort to develop evidence-based interventions that address these behaviors has expanded. Many treatment methods are now utilized by clinicians to reduce the deleterious effects that maladaptive beliefs and behaviors can cause. Approaches such as pain neuroscience education (PNE) aim to reconceptualize a person's pain experience away from a biomedical model of pain and towards a biopsychosocial model that incorporates all facets of the pain experience. PNE consists of patient education about neurophysiology, typical pain processing, neuroplasticity, and psychosocial factors associated with acute and chronic pain. This helps patients develop more effective strategies to cope with and recover from the various dimensions of pain (15).
Considering that alterations in the cortical structure and subsequent behavioral changes are influenced by the amount of excitability within the brain regions responsible for processing the experience of pain, non-invasive brain stimulation (NIBS) techniques, such as transcranial direction current stimulation (tDCS) or repetitive transcranial magnetic stimulation (rTMS), could potentially modulate pain perception and subsequent pain behaviors (16, 17). As is the case with the treatment of most physical and psychological diagnoses, an active multimodal approach is considered optimal. Therefore, in theory, combining the neuromodulatory effects of NIBS with an active, cognitive intervention such as PNE could be promising. This review highlights cognitive-affective deficits in chronic pain, specifically CLPB, and focuses on current evidence and future directions for the therapeutic combination of cognitive therapies and NIBS for CLBP.
Because pain is a biopsychosocial experience, the cognitive and emotional components, such as pain catastrophizing and kinesiophobia, cannot be ignored when assessing and treating patients with pain, particularly those with chronic pain. Pain catastrophizing is a maladaptive pain response characterized by rumination, helplessness and magnification regarding one's pain experience (18, 19). A person with high levels of pain catastrophizing may report feeling that their pain will continue only to worsen, progress until they are unable to function, or be caused by sinister pathology. They often have difficulty shifting their focus from painful or potentially painful stimuli and report higher threat values to non-painful stimuli (20, 21). Kinesiophobia or fear of movement is an excessive, irrational, and debilitating fear to carry out a physical movement due to a feeling of vulnerability to a painful injury or re-injury (22). Catastrophizing and kinesiophobia often coexist with an increased attentional awareness of one's pain leading to avoidance of activity based on the belief that movement will lead to further harm (23). Pain catastrophizing and kinesiophobia involve the persistence of distressing cognitive and emotional responses to pain or in anticipation of future pain, suggesting that although these behaviors can be a consequence of pain, they may also be a precursor of chronic pain (24). Evidence has shown that catastrophic thoughts and behaviors could predict the development and persistence of chronic pain (25–31). Catastrophizing also has been shown to increase attentional interference, a form of cognitive deficit, in those with chronic pain, likely a result of hypervigilance towards one's pain or in avoidance of pain that diminishes cognitive resources (32, 33).
The experience of pain is a complex cognitive process by which a person must evaluate their situation, make comparisons to previous experiences, choose a reaction, and ultimately form a mental representation of the event (34). Cognition can be defined as the procurement, processing, storage, and retrieval of information by the brain and is comprised of many factors, such as attention, perception, memory, reasoning, psychomotor skill, and executive function (35). Strong evidence has shown a close relationship between chronic pain and deficits in cognitive function, as approximately one-third of patients with chronic pain have cognitive dysfunction including difficulty with attention, learning, memory, and decision making (34, 36). Pain can be demanding of one's attention as the nervous system upregulates the amount of information needed for protection. In the presence of persistent pain, attentional control mechanisms are impaired (37). Deficits in performing tasks that require attentional shifting and the ability to selectively inhibit extraneous stimuli indicate that pain may be a predominant focus of attentional resources, leaving limited reserve for other cognitively demanding tasks (38). Similar deficits in attentional control are found in those with high pain catastrophizing (39). Impaired cognitive flexibility, attentional inhibition, attentional interference, learning, and memory are also associated with high levels of pain catastrophizing (40, 41). Cognitive dysfunction may limit one's capacity to evaluate, interpret, and revise the maladaptive thoughts and behaviors associated with catastrophizing and fear. Therefore, better understanding the cognitive profile of patients in pain can improve intervention selections and outcomes.
Considerable neuroanatomical and neurophysiological overlap exists between pain, emotion, and cognition. Although not clinically apparent, structural and functional changes in the brain are associated with altered cognitive and emotional processing in patients with CLBP (42). In individuals with CLBP, changes occur in areas and networks involved in the cognitive-emotional processes rather than those characteristically related to the sensory processing of pain. Specifically, the dorsolateral prefrontal cortex (DLPFC) is primarily involved in cognitive and affective processing in addition to pain processing (11). Decreased gray matter in the DLPFC has been observed in those suffering from chronic musculoskeletal pain, including LBP (43–45). Studies also have demonstrated that as levels of pain catastrophizing increase, gray matter density in the DLPFC decreases (46–48).
Moreover, the DLPFC has been shown to have a role in top-down modulation of appropriate behavioral responses (49), cognition (50), decision-making (51), and emotional control (52). Functional abnormalities involving the DLPFC are associated with the above-mentioned dysfunction in patients with depression (53), a common clinical manifestation of catastrophizing (54). Therefore, researchers proposed the abnormal functional connectivity of the DLPFC evident in patients with depression and post-traumatic disorders to be the underlying mechanism of chronic pain behaviors (i.e., catastrophizing and kinesiophobia) and cognitive deficits (42). The DLPFC is thought to serve as an interface between the three major brain networks, the resting-state default mode network (DMN), the salience network (SN) responsible for detecting behavior-relevant stimuli and allocating cognitive resources to those stimuli, and the fronto-parietal network (FPN), which coordinates behavior in a rapid, accurate and flexible goal-driven manner (11). Pain, being a relevant stimulus, typically results in the SN reducing activity of the DMN and increasing FPN activity in order to attend to the situation at hand (55, 56).
Increased DLPFC connectivity to the DMN has been observed in patients with increased levels of pain catastrophizing (45). This relationship may be partially explained by the coupling of pain and the DMN. As pain persists, it becomes part of one's identity, promotes sustained worry and fear, and progresses to functional and structural changes as the DLPFC remains activated to sustain cognitive engagement with the pain (57, 58). Further aberrant connectivity of the DLPFC to the DMN and the FPN has been shown to influence one's ability to balance cognitive demands and attention to new salient information (59, 60). For a simple task in which cognitive demand is low, patients with CLBP and high pain catastrophizing have elevated DLPFC activation, whereas healthy controls have relative deactivation (13, 47, 59). Therefore, interventions that aim at altering DLPFC activation may change pain behaviors.
PNE is a cognitive-behavioral therapy (CBT) strategy, which aims to restructure a person's perception of pain and to promote a positive impact on the multidimensional experience of pain. When compared with other conservative strategies of pacing and self-management, participants with chronic pain who received PNE demonstrated superior knowledge of pain physiology and a significant reduction in pain catastrophizing (61). These findings have been replicated by providing participants with a booklet containing PNE metaphors and stories. Interestingly, these positive responses occurred without significant improvement in pain or self-reported disability (62, 63). PNE has also been found to reduce worry and improve physical function, mental health, and health perceptions in those diagnosed with fibromyalgia (FM) (64). When comparing PNE to biomedically focused education, small-to-moderate effect sizes were found in favor of PNE in patients with chronic spinal pain who had improved catastrophizing, kinesiophobia, and illness perceptions. However, no significance was found for perceived disability (65).
Systematic reviews support the use of PNE for musculoskeletal disorders to improve pain catastrophizing, fear-avoidance, unhealthy attitudes and behaviors, and healthcare utilization (66). While demonstrating limited short-term efficacy for measures of pain, PNE shows consistent ability to modulate the cognitive-affective domains of pain, many of which contribute to the chronicity and severity of chronic pain (67, 68). The literature on PNE contains several limitations that make results difficult to generalize, including heterogenous study designs, participant populations, outcomes measures, and PNE delivery approaches.
Neuroimaging has been used to study the underlying mechanism of CBT effects on cortical changes (71, 72). An fMRI study in FM showed that CBT normalized activation of several cortical regions related to cognitive and emotional regulations, including the DLPFC, with a concurrent reduction in depression, and anxiety symptoms (73). These fMRI results suggest that CBT could change the brain's processing of pain through increased access to executive centers for the reappraisal of pain behaviors such as catastrophizing and fear (73). The study results indicate a strong top-down control of pain, enhanced cognitive function, and altered perception of stimuli generated by CBT. To date, only two, single-subject fMRI reports have investigated the effects of PNE on brain function. Both studies showed marked differences between pre- and post-treatment fMRI scans, indicating that PNE appears to have neuromodulating effects on frontal, cingulate, and insular cortices (69, 70). Despite limited evidence, PNE, a type of CBT, could influence structural and functional connectivity changes via reduction of pain catastrophizing and kinesiophobia that may be occupying cognitive reserves.
tDCS and rTMS are two common NIBS techniques which have been advocated for chronic pain management although its use for chronic pain is still in the investigative phase. tDCS uses a low-intensity current that passes between two electrodes on the head, whereas rTMS uses an electromagnetic field that directs an electric current to modulate neuronal activity in targeted areas of the brain. These techniques are widely used to treat various impairments associated with depression, anxiety, stroke, spinal cord injury, Parkinson's disease, and chronic pain. Evidence also supports the use of these techniques for improving memory, attention, and learning in cognitively-impaired, and pain-suffering participants when the DLPFC was targeted (74–76). While the therapeutic mechanisms are not entirely understood, the techniques appear to be able to modulate cortical excitability and to facilitate neuroplastic changes (77–79). The effects of NIBS are shown to be related to long-term potentiation (LTP) and long-term depression (LTD)-like results depending on the direction of the tDCS current or frequency of the rTMS pulses (80, 81). Anodal tDCS leads to depolarization of the neuronal membranes that increases cortical excitability while cathodal stimulation induces hyperpolarization that decreases excitability (82). rTMS produces LTP or LTD based on pulse frequency at high (≥5 Hz) or low (≤1 Hz) frequencies respectively (78, 83–86).
Meta-analyses have demonstrated that NIBS has significant effect on pain reduction for FM, migraine, CLBP, and spinal cord injury-related pain (87–92). Studies also demonstrated that NIBS targeting the primary motor cortex (M1) had greater pain reduction than NIBS targeting the DLPFC (83–86). However, considering the cognitive-evaluative and motivational-affective domains of pain, the overlap in symptoms between those with anxiety or depression and those of chronic pain makes the DLPFC a promising therapeutic target. When targeting the left DLPFC, both tDCS and rTMS have been found to consistently and positively affect measures of depression, anxiety, and cognitive dysfunction in patients with depression (93, 94). Furthermore, NIBS targeting the DLPFC has been found to reduce depression and anxiety in patients with FM, likely as a result of targeting the two conditions that share neurological substrates. For example, a RCT showed that when tDCS targeted the DLPFC, improvements in measures of cognition and depression were superior to the intervention targeting M1 (95). Two studies investigated the influence of home-based tDCS targeting the DLPFC and showed significant improvement in pain catastrophizing, depressive symptoms, and sleep quality for FM (96, 97). Similar results have been shown following rTMS targeting the DLPFC on the affective domain of pain, including short-term improvement of depression symptoms and pain catastrophizing (98–100).
It has been speculated that the analgesic effects derived from NIBS targeting the DLPFC are the result of modulation of cognitive function (11), as DLPFC stimulation has been shown to reduce response time during working memory tasks (101) and improve sustained attention. An RCT compared effects of active vs. sham rTMS targeting the DLPFC on participants with experimentally induced elbow pain and found that participants who received active rTMS showed a trend toward improved cognitive task performance (102). In another RCT, patients with FM also demonstrated an increase of orienting and executive attentional performance following rTMS (75, 103, 104). Imaging studies suggest that tDCS to DLPFC modulates the connectivity to other areas involved in the emotional and motivational aspects of pain such as the cingulate cortex, insula, amygdala, and thalamus (105). Significant changes, including normalization of DMN and FPN connectivity have been found after anodal tDCS to the DLPFC compared to sham stimulation (106). rTMS to the DLPFC also has been shown to activate inhibitory circuits involved in pain reduction in healthy participants (107). Furthermore, higher pain thresholds and functional connectivity changes have been demonstrated with tDCS and rTMS targeting the DLPFC (108–110). These findings support that targeting the DLPFC modulates both sensory and affective networks, confirming the role of the DLPFC in pain modulation both specifically and beyond that of pain processing.
Few studies have investigated the augmentative effect of combining NIBS with another non-pharmacological therapy. Due to its ability to alter cortical excitability, tDCS and rTMS are thought to produce a priming effect on subsequent interventions (111, 112). To date, studies have combined tDCS or rTMS to the M1 with exercise, visual illusion, and peripheral electrical stimulation (113–117). Most of these studies have shown a greater effect on pain reduction with combined interventions than isolated interventions alone (115, 116, 118, 119). Few studies (120, 121) have assessed the effects of combined NIBS with CBT for pain. However, these studies investigated either a sample of healthy participants (97) or a heterogeneous sample (98). In addition, these studies targeted the M1 for NIBS. Furthermore, these studies did not use outcome measures that can capture change of pain behaviors. A single-subject case report demonstrated that rTMS combined with CBT is a feasible intervention that significantly reduced depression (122). To date, no study has yet examined the combined effects of NIBS to the DLPFC and PNE, using outcome measures that were designed to detect changes of pain behaviors and cognition. Considering the influence of pain catastrophizing and kinesiophobia have on various domains of cognition, CBT techniques such as PNE could benefit from a precursory intervention such as tDCS or rTMS that normalize the brain function of subsequent CBT.
Many conservative approaches exist for the treatment of CLBP such as exercise, manual therapy, electrotherapeutic modalities, and medications. These interventions primarily focus on the injured tissues. The effects of these interventions often are small and likely due to the poorly understood mechanisms that underlie CLBP itself and typically neglect the complex cognitive and emotional factors facilitating symptom progression. However, specific assessment and determination of central nervous system mediators of pain, such as cognition/executive function, pain catastrophizing, and kinesiophobia provides insight into matching interventions with mechanisms (123, 124). Patients with CLBP who exhibit pain catastrophizing and kinesiophobia appear to be less responsive to standard, conservative interventions due to these central barriers. Therefore, approaches aimed at modulating involved brain regions, such as tDCS or rTMS, could potentially allow subsequent behavioral therapies (e.g., PNE) targeting the same regions to be more effective. Despite supporting evidence for these individual approaches, the combined effects of these two interventions have not been investigated. It remains unclear if priming the cognitive-affective circuitry that is conceptualized to support PNE with NIBS will augment the behavioral effect of PNE. However, more rigorously designed clinical trials may elucidate a novel approach to treatment of the cognitive-affective domains of pain and result in improved management of persistent pain that has grown to become one of the largest public health issues of our time.
CA completed literature review and appraisal and wrote the manuscript while working on PhD at Texas Woman's University. SW-P provided editorial and writing assistance as PhD mentor and advisor. Both authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. James SL, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet. (2018) 392(10159):1789–858. doi: 10.1016/S0140-6736(18)32279-7
2. Institute of Medicine (US) Committee on Advancing Pain Research, Care, and Education. Relieving pain in America: A blueprint for transforming prevention, care, education, and research. Washington, DC: National Academies Press (2011).
3. Gore M, Sadosky A, Stacey BR, Tai KS, Leslie D. The burden of chronic low back pain: clinical comorbidities, treatment patterns, and health care costs in usual care settings. Spine. (2012) 37:11. doi: 10.1097/BRS.0b013e318241e5de
4. Maher C, Underwood M, Buchbinder R. Non-specific low back pain. Lancet. (2017) 89(10070):736–47. doi: 10.1016/S0140-6736(16)30970-9
5. Brinjikji W, Luetmer PH, Comstock B, Bresnahan BW, Chen LE, Deyo RA, et al. Systematic literature review of imaging features of spinal degeneration in asymptomatic populations. Am. J Neuroradiol. (2015) 36(4):811–6. doi: 10.3174/ajnr.A4173
6. Huysmans E, Ickmans K, van Dyck D, Nijs J, Gidron Y, Roussel N, et al. Association between symptoms of central sensitization and cognitive behavioral factors in people with chronic nonspecific low back pain: a cross-sectional study. J Manipulative Physiol Ther. (2018) 41(2):92–101. doi: 10.1016/j.jmpt.2017.08.007
7. Marshall PWM, Schabrun S, Knox MF. Physical activity and the mediating effect of fear, depression, anxiety, and catastrophizing on pain related disability in people with chronic low back pain. PLoS One. (2017) 12(7):1–15. doi: 10.1371/journal.pone.0180788
8. Oosterman JM, Derksen LC, Van Wijck A, Kessels RP, Veldhuijzen DS. Executive and attentional functions in chronic pain: does performance decrease with increasing task load? Pain Res Manag. (2012) 17(3):159–65. doi: 10.1155/2012/962786
9. Smeets RJEM, Maher CG, Nicholas MK, Refshauge KM, Herbert RD. Do psychological characteristics predict response to exercise and advice for subacute low back pain? Arthritis Care Res. (2009) 61(9):1202–9. doi: 10.1002/art.24731
10. Kraemer HC, Wilson GT, Fairburn CG, Agras WS. Mediators and moderators of treatment effects in randomized clinical trials. Arch Gen Psychiatry. (2002) 59(10):877–83. doi: 10.1001/archpsyc.59.10.877
11. Seminowicz DA, Moayedi M. The dorsolateral prefrontal cortex in acute and chronic pain. J Pain. (2017) 18(9):1027–35. doi: 10.1016/j.jpain.2017.03.008
12. Gracely RH, Geisser ME, Giesecke T, Grant MAB, Petzke F, Williams DA, et al. Pain catastrophizing and neural responses to pain among persons with fibromyalgia. Brain. (2004) 127(4):835–43. doi: 10.1093/brain/awh098
13. Seminowicz DA, Wideman TH, Naso L, Hatami-Khoroushahi Z, Fallatah S, Ware MA, et al. Effective treatment of chronic low back pain in humans reverses abnormal brain anatomy and function. J Neurosci. (2011) 31(20):7540–50. doi: 10.1523/JNEUROSCI.5280-10.2011
14. Thomas EN, Pers YM, Mercier G, Cambiere JP, Frasson N, Ster F, et al. The importance of fear, beliefs, catastrophizing and kinesiophobia in chronic low back pain rehabilitation. Ann Phys Rehabil Med. (2010) 53(1):3–14. doi: 10.1016/j.rehab.2009.11.002
15. King R, Robinson V, Ryan CG, Martin DJ. An exploration of the extent and nature of reconceptualization of pain following pain neurophysiology education: a qualitative study of experiences of people with chronic musculoskeletal pain. Patient Educ Couns. (2016) 99(8):1389–93. doi: 10.1016/j.pec.2016.03.008
16. Volz MS, Medeiros L, da Graca Tarrago M, Vidor LP, Agnol D, Deitos A, et al. The relationship between cortical excitability and pain catastrophizing in myofascial pain. J Pain. (2013) 14(10):1140–7. doi: 10.1016/j.jpain.2013.04.013
17. Harris-Love M. Transcranial magnetic stimulation for the prediction and enhancement of rehabilitation treatment effects. J Neurol Phys Ther. (2012) 36(2):87–93. doi: 10.1097/NPT.0b013e3182564d26
18. Rosenstiel AK, Keefe FJ. The use of coping strategies in chronic low back pain patients: relationship to patient characteristics and current adjustment. Pain. (1983) 17(1):33–44. doi: 10.1016/0304-3959(83)90125-2
19. Sullivan MJL, Bishop SR, Pivik J. The pain catastrophizing scale: development and validation. Psychol Assess. (1995) 7(4):524–32. doi: 10.1037/1040-3590.7.4.524
20. Crombez G, Eccleston C, Van Damme S, Vlaeyen JW, Karoly P. Fear-avoidance model of chronic pain: the next generation. Clin J Pain. (2012) 28(6):475–83. doi: 10.1097/AJP.0b013e3182385392
21. Crombez G, Eccleston C, Baeyens F, Eelen P. When somatic information threatens, catastrophic thinking enhances attentional interference. Pain. (1998) 75(2–3):187–98. doi: 10.1016/S0304-3959(97)00219-4
22. Luque-Suarez A, Martinez-Calderon J, Falla D. Role of kinesiophobia on pain, disability and quality of life in people suffering from chronic musculoskeletal pain: a systematic review. Br J Sports Med. (2019) 53:554–9. doi: 10.1136/bjsports-2017-098673
23. Peters ML, Vlaeyen JWS, van Drunen C. Do fibromyalgia patients display hypervigilance for innocuous somatosensory stimuli? Application of a body scanning reaction time paradigm. Pain. (2000) 86(3):283–92. doi: 10.1016/S0304-3959(00)00259-1
24. Darnall BD, Colloca L. Optimizing placebo and minimizing nocebo to reduce pain, catastrophizing, and opioid use: a review of the science and an evidence-informed clinical toolkit. Int Rev Neurobiol. (2018) 139:129–57. doi: 10.1016/bs.irn.2018.07.022
25. Severeijns R, Vlaeyen JW, van den Hout MA, Picavet HSJ. Pain catastrophizing and consequences of musculoskeletal pain: a prospective study in the Dutch community. J Pain. (2005) 6(2):125–32. doi: 10.1016/j.jpain.2004.11.006
26. Picavet HSJ, Vlaeyen JW, Schouten JS. Pain catastrophizing and kinesiophobia: predictors of chronic low back pain. Am J Epidemiol. (2002) 156(11):1028–34. doi: 10.1093/aje/kwf136
27. Comachio J, Magalhães MO, Silva APDMCC, Marques AP. A cross-sectional study of associations between kinesiophobia, pain, disability, and quality of life in patients with chronic low back pain. Adv Rheumatol. (2018) 58(1):8. doi: 10.1186/s42358-018-0011-2
28. Sullivan MJ, Thorn B, Haythornthwaite JA, Keefe F, Martin M, Bradley LA, et al. Theoretical perspectives on the relation between catastrophizing and pain. Clin J Pain. (2001) 17(1):52–64. doi: 10.1097/00002508-200103000-00008
29. Edwards RR, Cahalan C, Mensing G, Smith M, Haythornthwaite JA. Pain, catastrophizing, and depression in the rheumatic diseases. Nat Rev Rheumatol. (2011) 7(4):216–24. doi: 10.1038/nrrheum.2011.2
30. Lazaridou A, Franceschelli O, Buliteanu A, Cornelius M, Edwards RR, Jamison RN. Influence of catastrophizing on pain intensity, disability, side effects, and opioid misuse among pain patients in primary care. J Appl Biobehav Res. (2017) 22(1):e12081. doi: 10.1111/jabr.12081
31. De Boer MJ, Struys MMRF, Versteegen GJ. Pain-related catastrophizing in pain patients and people with pain in the general population. Eur J Pain. (2012) 16(7):1044–52. doi: 10.1002/j.1532-2149.2012.00136.x
32. Vancleef LMG, Peters ML. Pain catastrophizing, but not injury/illness sensitivity or anxiety sensitivity, enhances attentional interference by pain. J Pain. (2006) 7(1):23–30. doi: 10.1016/j.jpain.2005.04.003
33. Crombez G, Eccleston C, Van den Broeck A, Van Houdenhove B, Goubert L. The effects of catastrophic thinking about pain on attentional interference by pain: no mediation of negative affectivity in healthy volunteers and in patients with low back pain. Pain Res Manage. (2002) 7(1):31–9. doi: 10.1155/2002/576792
34. Moriarty O, McGuire BE, Finn DP. The effect of pain on cognitive function: a review of clinical and preclinical research. Prog Neurobiol. (2011) 93:385–404. doi: 10.1016/j.pneurobio.2011.01.002
35. Lawlor PG, Med MS. The panorama of opioid-related cognitive dysfunction in patients with cancer. Cancer. (2002) 94(6):1836–53. doi: 10.1002/cncr.10389
36. Moriarty O, Ruane N, O’Gorman D, Maharaj CH, Mitchell C, Sarma KM, et al. Cognitive impairment in patients with chronic neuropathic or radicular pain: an interaction of pain and age. Front Behav Neurosci. (2017) 13:11. doi: 10.3389/fnbeh.2017.00100
37. Legrain V, van Damme S, Eccleston C, Davis KD, Seminowicz DA, Crombez G. A neurocognitive model of attention to pain: behavioral and neuroimaging evidence. Pain. (2009) 114:230–2. doi: 10.1016/j.pain.2009.03.020
38. Moore DJ, Meints SM, Lazaridou A, Johnson D, Franceschelli O, Cornelius M, et al. The effect of induced and chronic pain on attention. J Pain. (2019) 20(11):1353–61. doi: 10.1016/j.jpain.2019.05.004
39. Ellingson LD, Stegner AJ, Schwabacher IJ, Lindheimer JB, Cook DB, Middleton Memorial WS. Catastrophizing interferes with cognitive modulation of pain in women with fibromyalgia. Pain Med. (2018) 19:2408–22. doi: 10.1093/pm/pny008
40. Legarreta M, Bueler E, DiMuzio JM, McGlade E, Yurgelun-Todd D. Pain catastrophizing, perceived pain disability, and pain descriptors in veterans: the association with neuropsychological performance. Prof Psychol Res Pr. (2016) 47(6):418–26. doi: 10.1037/pro0000104
41. Bell T, Mirman JH, Stavrinos D. Pain, pain catastrophizing, and individual differences in executive function in adolescence. Child Health Care. (2018) 48(1):18–37. doi: 10.1080/02739615.2018.1441028
42. de Ridder D, Adhia D, Vanneste S. The anatomy of pain and suffering in the brain and its clinical implications. Neurosci Biobehav Rev. (2021) 130:125–46. doi: 10.1016/j.neubiorev.2021.08.013
43. Apkarian AV, Sosa Y, Sonty S, Levy RM, Harden RN, Parrish TB, et al. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. J Neurosci. (2004) 24(46):10410–5. doi: 10.1523/JNEUROSCI.2541-04.2004
44. Schmidt-Wilcke T, Leinisch E, Gänßbauer S, Draganski B, Bogdahn U, Altmeppen J, et al. Affective components and intensity of pain correlate with structural differences in gray matter in chronic back pain patients. Pain. (2006) 125(1–2):89–97. doi: 10.1016/j.pain.2006.05.004
45. Hubbard CS, Khan SA, Keaser ML, Seminowicz DA, Mathur VA, Goyal M. Altered brain structure and function correlate with disease severity and pain catastrophizing in migraine patients. eNeuro. (2014) 1(1). doi: 10.1523/ENEURO.0006-14.2014
46. Ceko M, Bushnell MC, Fitzcharles MA, Schweinhardt P. Fibromyalgia interacts with age to change the brain. Neuroimage Clin. (2013) 3:249–60. doi: 10.1016/j.nicl.2013.08.015
47. Seminowicz DA, Shpaner M, Keaser ML, Krauthamer GM, Mantegna J, Dumas JA, et al. Cognitive-behavioral therapy increases prefrontal cortex gray matter in patients with chronic pain. J Pain. (2013) 14(12):1573–84. doi: 10.1016/j.jpain.2013.07.020
48. Malfliet A, Coppieters I, van Wilgen P, Kregel J, de Pauw R, Dolphens M, et al. Brain changes associated with cognitive and emotional factors in chronic pain: a systematic review. Eur J Pain. (2017) 21(5):769–86. doi: 10.1002/ejp.1003
49. O’Reilly RC. The what and how of prefrontal cortical organization. Trends Neurosci. (2010) 33(8):355–61. doi: 10.1016/j.tins.2010.05.002
50. Sallet J, Mars RB, Noonan MP, Neubert FX, Jbabdi S, O'Reilly JX, et al. The organization of dorsal frontal cortex in humans and macaques. J Neurosci. (2013) 33(30):12255–74. doi: 10.1523/JNEUROSCI.5108-12.2013
51. Rahnev D, Nee DE, Riddle J, Larson AS, D’Esposito M. Causal evidence for frontal cortex organization for perceptual decision making. Proc Natl Acad. (2016) 113(21):6059–64. doi: 10.1073/pnas.1522551113
52. Buhle JT, Silvers JA, Wager TD, Lopez R, Onyemekwu C, Kober H, et al. Cognitive reappraisal of emotion: a meta-analysis of human neuroimaging studies. Cereb Cortex. (2014) 24(11):2981–90. doi: 10.1093/cercor/bht154
53. Salehinejad MA, Ghanavai E, Rostami R, Nejati V. Cognitive control dysfunction in emotion dysregulation and psychopathology of major depression (MD): evidence from transcranial brain stimulation of the dorsolateral prefrontal cortex (DLPFC). J Affect Disord. (2017) 210:241–8. doi: 10.1016/j.jad.2016.12.036
54. Geisser ME, Casey KL, Brucksch CB, Ribbens CM, Appleton BB, Crofford LJ. Perception of noxious and innocuous heat stimulation among healthy women and women with fibromyalgia: association with mood, somatic focus, and catastrophizing. Pain. (2003) 102(3):243–50. doi: 10.1016/S0304-3959(02)00417-7
55. Rolston JD, Thomas M, Ramos-Zúñiga R, de Ridder D, Vanneste S, Smith M, et al. Pain and the triple network model. Front Neurol. (2022) 1:757241. doi: 10.3389/fneur.2022.757241
56. Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. (2007) 27(9):2349–56. doi: 10.1523/JNEUROSCI.5587-06.2007
57. Pei Y, Zhang Y, Zhu Y, Zhao Y, Zhou F, Huang M, et al. Hyperconnectivity and high temporal variability of the primary somatosensory cortex in low-back-related leg pain: an fMRI study of static and dynamic functional connectivity. J Pain Res. (2020) 13:1665–75. doi: 10.2147/JPR.S242807
58. Baliki MN, Mansour AR, Baria AT, Apkarian AV. Functional reorganization of the default mode network across chronic pain conditions. PLoS One. (2014) 9(9):e106133. doi: 10.1371/journal.pone.0106133
59. Čeko M, Shir Y, Ouellet JA, Ware MA, Stone LS, Seminowicz DA. Partial recovery of abnormal insula and dorsolateral prefrontal connectivity to cognitive networks in chronic low back pain after treatment. Hum Brain Mapp. (2015) 36(6):2075–92. doi: 10.1002/hbm.22757
60. Ellingsen DM, Beissner F, Moher Alsady T, Lazaridou A, Paschali M, Berry M, et al. A picture is worth a thousand words: linking fibromyalgia pain widespreadness from digital pain drawings with pain catastrophizing and brain cross-network connectivity. Pain. (2021) 162(5):1352–63. doi: 10.1097/j.pain.0000000000002134
61. Meeus M, Nijs J, van Oosterwijck J, van Alsenoy V, Truijen S. Pain physiology education improves pain beliefs in patients with chronic fatigue syndrome compared with pacing and self-management education: a double-blind randomized controlled trial. Arch Phys Med Rehabil. (2010) 91(8):1153–9. doi: 10.1016/j.apmr.2010.04.020
62. Gallagher L, McAuley J, Moseley GL. A randomized-controlled trial of using a book of metaphors to reconceptualize pain and decrease catastrophizing in people with chronic pain. Clin J Pain. (2013) 29(1):20–5. doi: 10.1097/AJP.0b013e3182465cf7
63. van Oosterwijck J, Nijs J, Meeus M, Truijen S, Craps J, van den Keybus N, et al. Pain neurophysiology education improves cognitions, pain thresholds, and movement performance in people with chronic whiplash: a pilot study. J Rehabil Res Dev. (2011) 48(1):43–58. doi: 10.1682/JRRD.2009.12.0206
64. van Oosterwijck J, Meeus M, Paul L, de Schryver M, Pascal A, Lambrecht L, et al. Pain physiology education improves health status and endogenous pain inhibition in fibromyalgia: a double-blind randomized controlled trial. Clin J Pain. (2013) 29(10):873–82. doi: 10.1097/AJP.0b013e31827c7a7d
65. Malfliet A, Kregel J, Meeus M, Roussel N, Danneels L, Cagnie B, et al. Blended-learning pain neuroscience education for people with chronic spinal pain: randomized controlled multicenter trial anneleen. Phys Ther. (2018) 98(5):357–68. doi: 10.1093/ptj/pzx092
66. Louw A, Zimney K, Puentedura EJ, Diener I. The efficacy of pain neuroscience education on musculoskeletal pain: a systematic review of the literature. Physiother Theory Pract. (2016) 32(5):332–55. doi: 10.1080/09593985.2016.1194646
67. Watson JA, Ryan CG, Cooper L, Ellington D, Whittle R, Lavender M, et al. Pain neuroscience education for adults with chronic musculoskeletal pain: a mixed-methods systematic review and meta-analysis. J Pain. (2019) 20(10):1140.e1–1140.e22. doi: 10.1016/j.jpain.2019.02.011
68. Schütze R, Rees C, Smith A, Slater H, Campbell JM, O’Sullivan P. How can we best reduce pain catastrophizing in adults with chronic noncancer pain? A systematic review and meta-analysis. J Pain. (2018) 19(3):233–56. doi: 10.1016/j.jpain.2017.09.010
69. Louw A, Puentedura EJ, Diener I, Peoples RR. Preoperative therapeutic neuroscience education for lumbar radiculopathy: a single-case fMRI report. Physiother Theory Pract. (2015) 31(7):496–508. doi: 10.3109/09593985.2015.1038374
70. Moseley GL. Widespread brain activity during an abdominal task markedly reduced after pain physiology education: fMRI evaluation of a single patient with chronic low back pain. Aust J Physiother. (2005) 51(1):49–52. doi: 10.1016/S0004-9514(05)70053-2
71. Bao S, Qiao M, Lu Y, Jiang Y. Neuroimaging mechanism of cognitive behavioral therapy in pain management. Pain Res Manag. (2022) 2022:6266619. doi: 10.1155/2022/6266619
72. Lazaridou A, Kim J, Cahalan CM, Loggia ML, Franceschelli O, Berna C, et al. Effects of cognitive-behavioral therapy (CBT) on brain connectivity supporting catastrophizing in fibromyalgia. Clin J Pain. (2017) 33(3):215–21. doi: 10.1097/AJP.0000000000000422
73. Jensen KB, Kosek E, Wicksell R, Kemani M, Olsson G, Merle JV, et al. Cognitive behavioral therapy increases pain-evoked activation of the prefrontal cortex in patients with fibromyalgia. Pain. (2012) 153(7):1495–503. doi: 10.1016/j.pain.2012.04.010
74. Brasil-Neto JP, Fregni F. Learning, memory, and transcranial direct current stimulation. Front Psychiatry. (2012) 3:80. doi: 10.3389/fpsyt.2012.00080
75. Ferreira Silva A, Zortea M, Carvalho S, Leite J, Lucena da Silva Torres I, Fregni F, et al. Anodal transcranial direct current stimulation over the left dorsolateral prefrontal cortex modulates attention and pain in fibromyalgia: randomized clinical trial open. Sci Rep. (2017) 7(1):135. doi: 10.1038/s41598-017-00185-w
76. Soltaninejad Z, Nejati V, Ekhtiari H. Effect of anodal and cathodal transcranial direct current stimulation on DLPFC on modulation of inhibitory control in ADHD. J Atten Disord. (2019) 23(4):325–32. doi: 10.1177/1087054715618792
77. Lefaucheur JP, Wendling F. Mechanisms of action of tDCS: a brief and practical overview. Neurophysiol Clin. (2019) 49:269–75. doi: 10.1016/j.neucli.2019.07.013
78. Galhardoni R, Correia GS, Araujo H, Yeng LT, Fernandes DT, Kaziyama HH, et al. Repetitive transcranial magnetic stimulation in chronic pain: a review of the literature. Arch Phys Med Rehabil. (2015) 96(4):S156–72. doi: 10.1016/j.apmr.2014.11.010
79. Moisset X, de Andrade DC, Bouhassira D. From pulses to pain relief: an update on the mechanisms of rTMS-induced analgesic effects. Eur J Pain. (2016) 20(5):689–700. doi: 10.1002/ejp.811
80. Lømo T. The discovery of long-term potentiation. Philos Trans R Soc Lond B Biol Sci. (2003) 358(1432):617. doi: 10.1098/rstb.2002.1226
81. Esser SK, Huber R, Massimini M, Peterson MJ, Ferrarelli F, Tononi G. A direct demonstration of cortical LTP in humans: a combined TMS/EEG study. Brain Res Bull. (2006) 69(1):86–94. doi: 10.1016/j.brainresbull.2005.11.003
82. Jacobson L, Koslowsky M, Lavidor M. MINI-REVIEW tDCS polarity effects in motor and cognitive domains: a meta-analytical review. Exp Brain Res. (2012) 216(1):1–10. doi: 10.1007/s00221-011-2891-9
83. Yang S, Chang MC. Effect of repetitive transcranial magnetic stimulation on pain management: a systematic narrative review. Front Neurol. (2020) 11:114. doi: 10.3389/fneur.2020.00114
84. Lima MC, Fregni F. Motor cortex stimulation for chronic pain. Neurology. (2008) 70(24):2329–37. doi: 10.1212/01.wnl.0000314649.38527.93
85. Lefaucheur JP, Drouot X, Keravel Y, Nguyen JP. Pain relief induced by repetitive transcranial magnetic stimulation of precentral cortex. Neuroreport. (2001) 12(13):2963–5. doi: 10.1097/00001756-200109170-00041
86. Lefaucheur JP, André-Obadia N, Antal A, Ayache SS, Baeken C, Benninger DH, et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS). Clin Neurophysiol. (2014) 125(11):2150–206. doi: 10.1016/j.clinph.2014.05.021
87. Pinto CB, Costa BT, Duarte D, Fregni F. Transcranial direct current stimulation as a therapeutic tool for chronic pain. J Ect. (2018) 34(3):e36–350. doi: 10.1097/YCT.0000000000000518
88. Mehta S, McIntyre A, Guy S, Teasell RW, Loh E. Effectiveness of transcranial direct current stimulation for the management of neuropathic pain after spinal cord injury: a meta-analysis. Spinal Cord. (2015) 53(11):780–5. doi: 10.1038/sc.2015.118
89. Zhu CE, Yu B, Zhang W, Chen WH, Qi Q, Miao Y. Effectiveness and safety of transcranial direct current stimulation in fibromyalgia: a systematic review and meta-analysis. J Rehabil Med. (2017) 49(1):2–9. doi: 10.2340/16501977-2179
90. Shirahige L, Melo L, Nogueira F, Rocha S, Monte-Silva K. Efficacy of noninvasive brain stimulation on pain control in migraine patients: a systematic review and meta-analysis. Headache. (2016) 56(10):1565–96. doi: 10.1111/head.12981
91. Hou WH, Wang TY, Kang JH. The effects of add-on non-invasive brain stimulation in fibromyalgia: a meta-analysis and meta-regression of randomized controlled trials. Rheumatology. (2016) 55(8):1507–17. doi: 10.1093/rheumatology/kew205
92. Marlow NM, Bonilha HS, Short EB. Efficacy of transcranial direct current stimulation and repetitive transcranial magnetic stimulation for treating fibromyalgia syndrome: a systematic review. Pain Pract. (2013) 13(2):131–45. doi: 10.1111/j.1533-2500.2012.00562.x
93. Lefaucheur JP, Antal A, Ayache SS, Benninger DH, Brunelin J, Cogiamanian F, et al. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clin Neurophysiol. (2017) 128(1):56–92. doi: 10.1016/j.clinph.2016.10.087
94. Serafini G, Pompili M, Belvederi Murri M, Respino M, Ghio L, Girardi P, et al. The effects of repetitive transcranial magnetic stimulation on cognitive performance in treatment-resistant depression. A systematic review. Neuropsychobiology. (2015) 71(3):125–39. doi: 10.1159/000381351
95. Fregni F, Gimenes R, Valle AC, Ferreira MJL, Rocha RR, Natalle L, et al. A randomized, sham-controlled, proof of principle study of transcranial direct current stimulation for the treatment of pain in fibromyalgia. Arthritis Rheum. (2006) 54(12):3988–98. doi: 10.1002/art.22195
96. Caumo W, Lopes Alves R, Vicu P, Fernanda C, Alves S, Ramalho L, et al. Impact of bifrontal home-based transcranial direct current stimulation in pain catastrophizing and disability due to pain in fibromyalgia: a randomized, double-blind sham-controlled study. J Pain. (2022) 23(4):641–56. doi: 10.1016/j.jpain.2021.11.002
97. Brietzke AP, Zortea M, Carvalho F, Sanches PR, Danton P Jr, da Silva Torres IL, et al. Large treatment effect with extended home-based transcranial direct current stimulation over dorsolateral prefrontal cortex in fibromyalgia: a proof of concept sham-randomized clinical study. J Pain. (2020) 21(1–2):212–24. doi: 10.1016/j.jpain.2019.06.013
98. Short EB, Borckardt JJ, Anderson BS, Frohman H, Beam W, Reeves ST, et al. Ten sessions of adjunctive left prefrontal rTMS significantly reduces fibromyalgia pain: a randomized, controlled pilot study. Pain. (2011) 152(11):2477–84. doi: 10.1016/j.pain.2011.05.033
99. Lee SJ, Kim DY, Chun MH, Kim YG. The effect of repetitive transcranial magnetic stimulation on fibromyalgia: a randomized sham-controlled trial with 1-mo follow-up. Am J Phys Med Rehabil. (2012) 91(12):1077–85. doi: 10.1097/PHM.0b013e3182745a04
100. Mhalla A, Baudic S, de Andrade DC, Gautron M, Perrot S, Teixeira MJ, et al. Long-term maintenance of the analgesic effects of transcranial magnetic stimulation in fibromyalgia. Pain. (2011) 152(7):1478–85. doi: 10.1016/j.pain.2011.01.034
101. Deldar Z, Rustamov N, Blanchette I, Piché M. Improving working memory and pain inhibition in older persons using transcranial direct current stimulation. Neurosci Res. (2019) 148:19–27. doi: 10.1016/j.neures.2018.12.007
102. Seminowicz DA, de Martino E, Schabrun SM, Graven-Nielsen T. Left dorsolateral prefrontal cortex repetitive transcranial magnetic stimulation reduces the development of long-term muscle pain. Pain. (2018) 159(12):2486–92. doi: 10.1097/j.pain.0000000000001350
103. Miniussi C, Cappa SF, Cohen LG, Floel A, Fregni F, Nitsche MA, et al. Efficacy of repetitive transcranial magnetic stimulation/transcranial direct current stimulation in cognitive neurorehabilitation. Brain Stimul. (2008) 1:326–36. doi: 10.1016/j.brs.2008.07.002
104. Elder GJ, Taylor JP. Transcranial magnetic stimulation and transcranial direct current stimulation: treatments for cognitive and neuropsychiatric symptoms in the neurodegenerative dementias? Alzheimers Res Ther. (2014) 6(9):74. doi: 10.1186/s13195-014-0074-1
105. Boggio PS, Zaghi S, Fregni F. Modulation of emotions associated with images of human pain using anodal transcranial direct current stimulation (tDCS). Neuropsychologia. (2009) 47(1):212–7. doi: 10.1016/j.neuropsychologia.2008.07.022
106. Keeser D, Meindl T, Bor J, Palm U, Pogarell O, Mulert C, et al. Prefrontal transcranial direct current stimulation changes connectivity of resting-state networks during fMRI. J Neurosci. (2011) 31(43):15284–93. doi: 10.1523/JNEUROSCI.0542-11.2011
107. Martin L, Borckardt JJ, Reeves ST, Frohman H, Beam W, Nahas Z, et al. Psychology, psychiatry & brain neuroscience section original research articles A pilot functional MRI study of the effects of prefrontal rTMS on pain perception. Pain Med. (2013) 14(7):999–1009. doi: 10.1111/pme.12129
108. Sankarasubramanian V, Cunningham DA, Potter-Baker KA, Beall EB, Roelle SM, Varnerin NM, et al. Transcranial direct current stimulation targeting primary motor versus dorsolateral prefrontal cortices: proof-of-concept study investigating functional connectivity of thalamocortical networks specific to sensory-affective information processing. Brain Connect. (2017) 7(3):182–96. doi: 10.1089/brain.2016.0440
109. Borckardt JJ, Smith AR, Reeves ST, Weinstein M, Kozel FA, Nahas Z, et al. Fifteen minutes of left prefrontal repetitive transcranial magnetic stimulation acutely increases thermal pain thresholds in healthy adults. Pain Res Manag. (2007) 12(4):287–90. doi: 10.1155/2007/741897
110. Tanwar S, Mattoo B, Kumar U, Bhatia R. Repetitive transcranial magnetic stimulation of the prefrontal cortex for fibromyalgia syndrome: a randomised controlled trial with 6-months follow up. Adv Rheumatol. (2020) 60(1):34. doi: 10.1186/s42358-020-00135-7
111. Schabrun SM, Chipchase LS. Priming the brain to learn: the future of therapy? Man Ther. (2011) 17(2):184–6. doi: 10.1016/j.math.2011.12.001
112. Malavera A, Vasquez A, Fregni F. Novel methods to optimize the effects of transcranial direct current stimulation: a systematic review of transcranial direct current stimulation patents. Expert Med Rev Devices. (2015) 12(6):679–88. doi: 10.1586/17434440.2015.1090308
113. Pinto CB, Ghassan F, Velez S, Bolognini N, Crandell D. Optimizing rehabilitation for phantom limb pain using mirror therapy and transcranial direct current stimulation : a randomized, double – blind clinical trial study protocol. JMIR Res Protoc. (2016) 5(3):e138. doi: 10.2196/resprot.5645
114. Mercier C, Le G, Laroche S, Tousignant-laflamme Y. The effectiveness of transcranial direct current stimulation as an add-on modality to graded motor imagery for treatment of complex regional pain syndrome A randomized proof of concept study. Clin J Pain. (2018) 34(2):145–54. doi: 10.1097/AJP.0000000000000522
115. Schabrun SM, Jones E, Elgueta Cancino EL, Hodges PW. Targeting chronic recurrent low back pain from the top-down and the bottom-up: a combined transcranial direct current stimulation and peripheral electrical stimulation intervention. Brain Stimul. (2014) 7(3):451–9. doi: 10.1016/j.brs.2014.01.058
116. Chipchase LS, Schabrun SM, Hodges PW. Peripheral electrical stimulation to induce cortical plasticity: a systematic review of stimulus parameters. Clin Neurophysiol. (2011) 122(3):456–63. doi: 10.1016/j.clinph.2010.07.025
117. Zhou P, Zhang Y, Xu D, Shan C, Xie Q, Pan W, et al. The effects of combined low frequency repetitive transcranial magnetic stimulation and motor imagery on upper extremity motor recovery following stroke. Front Neurol. (2019) 10:96. doi: 10.3389/fneur.2019.00096
118. Hazime FA, Baptista AF, de Freitas DG, Monteiro RL, Maretto RL, Hasue RH, et al. Treating low back pain with combined cerebral and peripheral electrical stimulation: a randomized, double-blind, factorial clinical trial. Eur J Pain. (2017) 21(7):1132–43. doi: 10.1002/ejp.1037
119. Mendonca ME, Simis M, Grecco LC, Battistella LR, Baptista AF, Fregni F. Transcranial direct current stimulation combined with aerobic exercise to optimize analgesic responses in fibromyalgia: a randomized placebo-controlled clinical trial. Front Hum Neurosci. (2016) 10:68. doi: 10.3389/fnhum.2016.00068
120. Powers A, Madan A, Hilbert M, Reeves ST, George M, Nash MR, et al. Effects of combining a brief cognitive intervention with transcranial direct current stimulation on pain tolerance: a randomized controlled pilot study. Pain Med. (2018) 19(4):677–85. doi: 10.1093/pm/pnx098
121. Luedtke K, Rushton A, Wright C, Jürgens T, Polzer A, Mueller G, et al. Effectiveness of transcranial direct current stimulation preceding cognitive behavioural management for chronic low back pain: sham controlled double blinded randomised controlled trial. Br Med J. (2015) 350:1–9. doi: 10.1136/bmj.h1640
122. Vedeniapin A, Cheng L, George MS, Johnson RH. Feasibility of simultaneous cognitive behavioral therapy and left prefrontal rTMS for treatment resistant depression. Brain Stimul. (2010) 3(4):207–10. doi: 10.1016/j.brs.2010.03.005
123. Phillips K, Clauw DJ. Central pain mechanisms in chronic pain states - maybe it is all in their head. Best Pract Res Clin Rheumatol. (2011) 25(2):141–54. doi: 10.1016/j.berh.2011.02.005
Keywords: pain catastophizing, kinesiophobia, transcranial direct current simulation, transcranial magenetic stimulation, cognition
Citation: Alcon CA and Wang-Price S (2022) Non-invasive brain stimulation and pain neuroscience education in the cognitive-affective treatment of chronic low back pain: Evidence and future directions. Front. Pain Res. 3:959609. doi: 10.3389/fpain.2022.959609
Received: 1 June 2022; Accepted: 24 October 2022;
Published: 10 November 2022.
Edited by:
Ausaf Bari, University of California, United StatesReviewed by:
Daniel Lu, University of California, United States© 2022 Alcon and Wang-Price. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cory A. Alcon Y2FsY29uQGhpZ2hwb2ludC5lZHU=
Specialty Section: This article was submitted to Neuromodulatory Interventions, a section of the journal Frontiers in Pain Research
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.