
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
POLICY AND PRACTICE REVIEWS article
Front. Pain Res., 04 August 2022
Sec. Pain Research Methods
Volume 3 - 2022 | https://doi.org/10.3389/fpain.2022.884674
A correction has been applied to this article in:
Corrigendum: Overdose, opioid treatment admissions and prescription opioid pain reliever relationships: United States, 2010–2019
 Larry Aubry1
Larry Aubry1 B. Thomas Carr2*
B. Thomas Carr2*Background: “As part of the U.S. government's urgent response to the epidemic of overdose deaths (1)” the United States Centers for Disease Control and Prevention (CDC) issued the “CDC Guideline for Prescribing Opioids for Chronic Pain-United States, 2016 (2)” (guideline) followed by the “CDC Clinical Practice Guideline for Prescribing Opioids–United States, 2022 (3) (guideline update). ” The guideline and guideline update cite a direct correlation between prescription opioids sales (POS) and opioid treatment admissions (OTA) and prescription opioid deaths (POD), which was based on data from 1999 to 2010. This paper updates those relationships and includes the correlations between prescription opioid sales (POS) and any opioid deaths (AOD) and total overdose deaths (TOD) from 2010 to 2019.
Methods: Linear regression models were fit to each response separately. Opioid sales (measured as MME (morphine milligram equivalent) per capita) was the independent variable. Total overdose deaths (TOD), any opioid overdose deaths (AOD), prescription opioid overdose deaths (POD) and opioid treatment admissions (OTA) were the dependent, response variables. The models were assessed using three criteria: the statistical significance of the model (Overall P-Value), the quality of the fit (R2), and the sign of the slope coefficient (positive or negative).
Results: The analyses revealed that the direct correlations (i.e., significant, positive slopes) reported by the CDC based on data from 1999 to 2010 no longer exist. Based on data from 2010 to 2019, the relationships either have reversed (i.e., significant, negative slopes) or are non-existent (i.e., no significant model).
Conclusions: The guideline, guideline update, CDC's public, medical profession, and intergovernmental communications should be corrected/updated to state no direct correlation has existed between POS to OTA, POD, AOD, and TOD since 2010. Individualized patient care and public health policy should be amended accordingly.
Direct correlations that existed between Prescription Opioid Deaths (POD), Opioid Treatment Admissions/addiction (OTA) and Prescription Opioid Sales (POS) from 1999 to 2010 (4) (see Figure 1) led the CDC to conclude that POS are the determinant for POD, any opioid overdose deaths (AOD), Total overdose deaths (TOD), and OTA (1–14).
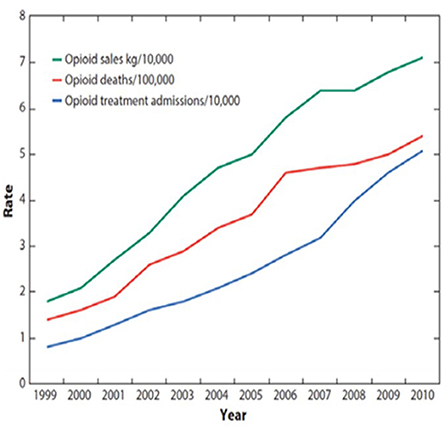
Figure 1. CDC chart 1999–2010, February 28, 2018, Congressional testimony “Combatting the Opioid Crisis,” made before the Committee on Energy and Commerce, Subcommittee on Health U.S. House of Representatives (5): “The CDC has shown that a sharp increase in prescriptions for opioids resulted in a corresponding rise in addiction and overdose deaths. This is a CDC graph. The green line represents opioid prescribing, the red line represents opioid deaths, and the blue line represents opioid addiction. The green line went up as opioid prescriptions started to soar, it led to parallel increases in addiction and overdose deaths (6)”.
The U.S. Department of Health and Human Services (HHS) declared in 2015 “There is a clear correlation between opioid prescribing rates and overdose death rates in the United States (7).” With the guideline's release, then CDC Director Tom Frieden stated, “Overprescribing opioids—largely for chronic pain—is a key driver of America's drug-overdose epidemic (1).”
Cutting POS has been CDC's, DEA's, legislative policy makers', healthcare system providers and practitioners' solution to cut overdose deaths and OTA (1–18).
The impact of the CDC guideline has been systemic. Long term opioid therapy patients are not accepted as new patients by over 40% of primary pain clinics (18). 2021 MME per capita use declined to 309 (19), a level last seen in 2000, while the over 55 population with its age-related health conditions increased by 40 million since then and COVID care has required “high demand.” “Forty-seven states and the District of Columbia have laws that set time or dosage limits for controlled substances (20).” “All 50 states have established prescription drug monitoring programs (PDMPs) (21)” to collect and surveil doctor, patient, and dispensed medication information. Since 2009, U.S. morphine milligram equivalents per 1,000 inhabitants per day (MID) declined by 48% from second in the world (22) to third in 2019 (23).
The American Medical Association (AMA) reports “72% of pain medicine specialists said that they—or their patients—have been required to reduce the quantity or dose of medication they have prescribed (24)” as a result of the guideline.
The objective of the guideline was to cut opioid addiction and overdose deaths while ensuring to first do no harm. Considering “The epidemic of overdose deaths in the USA has been growing, inexorably and exponentially, for four decades” per the U.S. Drug Enforcement Administration (DEA) (25), an increase in U.S. overdose deaths of nearly 70% from 2016 to 2021, and an annual overdose cost of $1 trillion in the United States (26),” it is critical that public health policy and individual patient care not be based on out-of-date or misleading information.
The 2022 guideline update revises and expands upon the recommendations of the 2016 guideline considering a substantial amount of more recent data. However, it continues to cite the positive relationship between opioid prescribing rates and overdose deaths between 1999 and 2010 but makes no mention of the fact that those relationships have not existed for more than a decade. It is important that both clinical practice and regulatory policy be based on as much valid data as is readily available. This paper is intended to augment the new information contained in the guideline update to address the current relationships between POS and OTA, POD, AOD, and TOD.
The direct correlation of POS with OTA, POD, AOD and TOD has been cited in communications of public health policy, individual patient care and doctor conduct by HHS and CDC, referenced in congressional testimony, intergovernmental communications, and legal proceedings, thereby making these correlations a critical material fact. The analyses presented in this paper covering the period from 2010 to 2019 updates these material facts to avoid misrepresentation or omission of relevant evidence.
Data limitations have the potential for over or underestimating overdose deaths. The authors of a 2018 report “Quantifying the Epidemic of Prescription Opioid Overdose Deaths,” with the CDC, acknowledged that systemic errors and omissions in the source data along with the CDC's methodology for compiling drug-related mortality data “could significantly inflate (27)” prescription opioid overdose death estimates (27, 28). In 2018, the CDC cut their estimates of prescription opioid deaths from 1999 to 2016 by 48,000 or 19.5%, with the 2016 estimates cut by more than 15,000 or 47.3% (27, 28).
Confounding factors impacting the accuracy of overdose deaths are that “multiple drugs are often involved” (27), the source of opioids detected in postmortem blood toxicity screens is not known (e.g., legally prescribed vs. illicitly obtained), among other issues (27, 28). With this occurrence and/or when multiple conditions resulted in an overdose death a single sequence/cause will be documented based on the physician's “best medical opinion (29).”
The same data sources that the CDC guideline appears to be based upon were used for this paper. As such, the results of analyses presented here are at least as reliable and subject to the same limitations as what the CDC obtained from their own analyses of 1999–2010 and if they chose to undertake them for the most recent decade of 2010–2019. Thus, the following sources have been applied.
Drug Overdose Deaths (National); Total Overdose Deaths, Any Opioid Overdose Deaths and Prescription Opioid Overdose Deaths (30): 1999–2019 data accessed from Drugabuse.gov., Published 2021. Deaths are classified according to the International Classification of Diseases, 10th Revision. Drug overdose deaths are identified with underlying cause-of-death codes X40–X44, X60–X64, X85, and Y10–Y14. The following multiple cause-of-death codes were used to identify specific drug types: T40.2 for natural and semisynthetic opioid analgesics, T40.3 for methadone, and T40.4 for synthetic opioid analgesics other than methadone. Accessed January 10, 2021 https://www.drugabuse.gov/sites/default/files/Overdose_data_1999-2019.xlsx.
Opioid Overdose Death Crude Rates (U.S. States) (31): 1999–2019 data accessed from CDC, National Center for Health Statistics. Underlying Cause of Death, 1999–2019 were sourced from CDC WONDER Online Database, released in 2020. Data are from the Multiple Cause of Death Files, 1999–2019, as compiled from data provided by 57 vital statistics jurisdictions through the Vital Statistics Cooperative Program. Identified using underlying cause-of-death codes X40–X44, X60–X64, X85, and Y10–Y14. Accessed Feb 7, 2021, 12:01:39 PM from http://wonder.cdc.gov/ucd-icd10.html.
Opiate/Opioid Treatment Admissions (National) (32): 2006–2008 data accessed from Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality, “Treatment Episode Data Set (TEDS): 2000–2010”. National Admissions to Substance Abuse Treatment Services. DASIS Series S-61, HHS Publication No. (SMA) 12-4701. Rockville, MD: Substance Abuse and Mental Health Services Administration (samhsa.gov), 2012. P. 43. Accessed April 18, 2021 from Treatment Episode Data Set (TEDS) 2000–2010 (samhsa.gov).
Opiate/Opioid Treatment Admissions (National) (33): 2008–2018 data accessed from Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality, “Treatment Episode Data Set (TEDS): 2018.” Admissions to and Discharges from Publicly Funded Substance Use Treatment. Rockville, MD: 2018 TEDS Annual Report. Substance Abuse and Mental Health Services Administration (samhsa.gov), 2020. Table 1.1a. Accessed April 18, 2021 from https://www.samhsa.gov/data/sites/default/files/reports/rpt31097/2018_TEDS/2018_TEDS.html#PSU. 2018 TEDS Annual Report (samhsa.gov).
Opioid Prescribing; MME per Capita (National) (34): 2006–2013 data accessed from CDC,” Annual Surveillance Report of Drug-Related Risks and Outcomes—United States Surveillance Special Report” 0.2019 CDC, U.S. Department of Health and Human Services. Published November 1, 2019. P. 115. Accessed January 10, 2021 from https://www.cdc.gov/drugoverdose/pdf/pubs/2019-cdc-drug-surveillance-report.pdf.
Opioid Prescribing; MME per Capita (National) (35): 2014–2018 data accessed from Statista,” Annual morphine milligram equivalents (MME) dispensed per capita in the U.S. from 2014 to 2018”, MME per capita U.S. 2014–2018. Statista. May 28, 2021. Accessed July 8, 2021 from https://www.statista.com/statistics/753284/number-of-mme-dispensed-per-capita-in-us/.
Opioid Prescribing; MME per Capita (National) (36): 2019 data accessed from The IQVIA Institute, “Prescription Opioid Trends in the United States,” Institute Report, Dec 16, 2020. P.4. Accessed January 10, 2021 from https://www.iqvia.com/insights/the-iqvia-institute/reports/prescription-opioid-trends-in-the-united-states.
Opioid Prescribing; Opioid Dispensing Rates per 100 (U.S. States) (37): 2006–2019 data accessed from CDC, “U.S. Opioid Dispensing Rate Maps, Drug Overdose,” CDC Injury Center. Accessed February 9, 2021 from https://www.cdc.gov/drugoverdose/rxrate-maps/index.html.
Opioid Sales kg/10,000 (National): For the period from 2006 through 2018/2019, these data were not known to be publicly available. We instead examined Opioid Prescribing by separately computing MME per Capita.
The CDC used Annual Prescription Opioid Sales to support the guideline (Figure 1). Data on Annual Prescription Opioid Sales are not readily available since 2010. However, MME per Capita data are available from 2006 to 2019 and offer a reasonable surrogate. Annual Sales data from the CDC chart were visually extracted and correlated with MME per Capita data, using simple linear regression analysis. The goal of the analysis was to evaluate MME per Capita as a legitimate alternative measure of Annual Prescription Opioid Sales.
Consistent with the methods used by the CDC, simple linear regression models were fit to the data. Separate models were fit to each of the four dependent variables (TOD, AOD, POD, and OTA) using Annual Opioid Sales (i.e., MME per Capita) as the independent variable. Two models were fit to each dependent variable. One model covered the years presented in the original CDC chart (for which MME per Capita data were available) (2006–2010) and the second model covered the years since the published CDC chart (2010–2019).
For both objectives, the strength and nature of relationships in all the regression models were assessed using three criteria:
1) significance of the regression model (overall P-Value),
2) the quality of the model's fit (R2), and
3) the sign of the linear slope coefficient (+ or –).
All models were fit using PROC REG from SAS/STAT software Version 9.4.
Data from CDC's original chart was reconstructed using graphical analysis. The reconstructed Annual Prescription Opioid Sales values from the original CDC chart are highly correlated with publicly available MME per Capita values (R2 = 94%). MME per Capita, which are available for more recent years than the data originally used by the CDC, is thus used in place of Annual Prescription Opioid Sales for all subsequent analyses.
For the years covered in the CDC's original chart (for which MME per Capita data are available, i.e., 2006–2010), the CDC's claim of positive/direct relationships between TOD, AOD, POD, and OTA and Annual Prescription Opioid Sales (i.e., MME per Capita) were validated (91% < R2 <97%), with statistically significant, positive slopes.
For more recent years (i.e., 2010–2019), however, the CDC's assertion of continued direct relationships is not valid. The relationships between TOD, AOD, POD, and OTA and Annual Prescription Opioid Sales (i.e., MME per Capita) are either non-existent or significantly negative/inverse (Figures 2, 3).
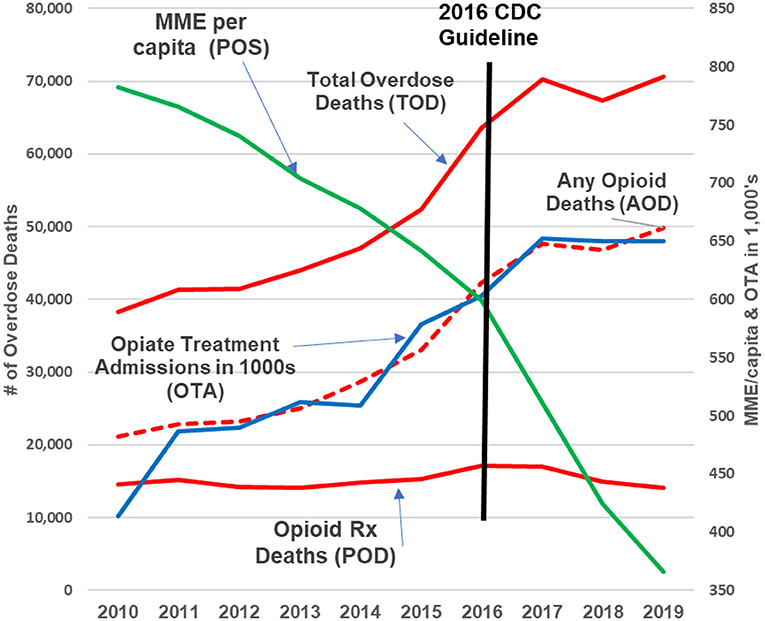
Figure 2. 2010–2019 update. The green line represents opioid prescribing (POS, MME/capita); the red lines are opioid deaths (POD, AOD, and TOD); the blue line represents opioid addiction (OTA). Over the past decade, as the green line (prescription opioids) declined by +50%, prescription opioid deaths remained flat while opioid addiction, any opioid and total overdose deaths continued increasing “exponentially (9)”.
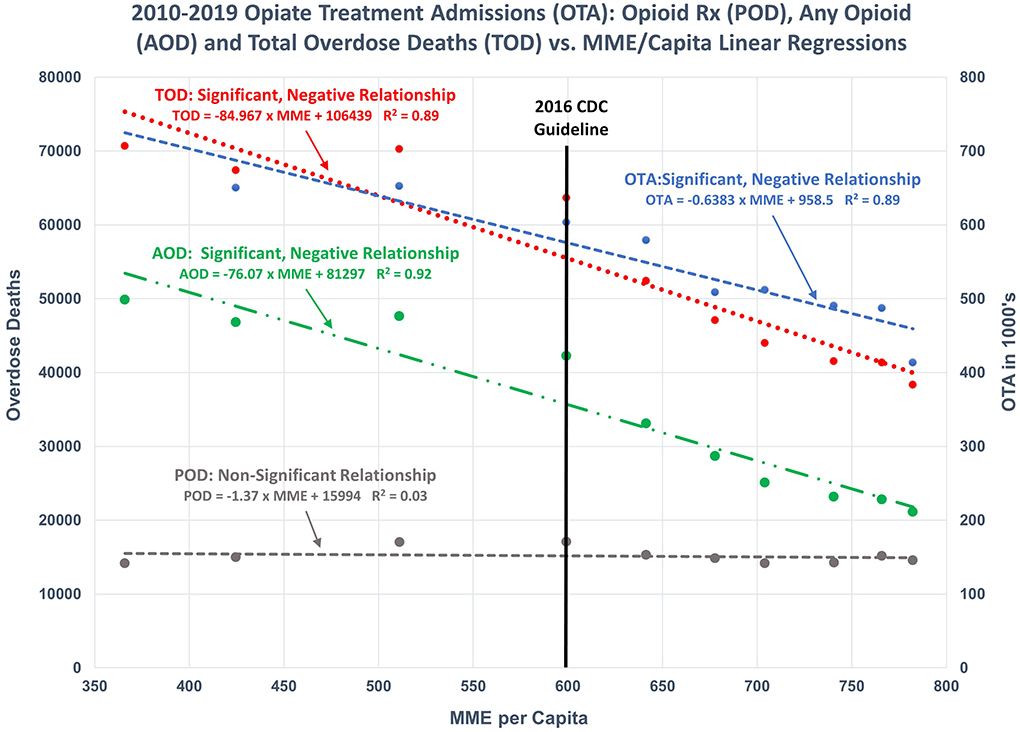
Figure 3. 2010–2019 regression models: Illustrates the regression of OTA, POD, AOD, and TOD as functions of POS. Significant, negative relationships were found for OTA, AOD, and TOD. No significant relationship exists between POD and POS.
Results for all regression models are presented in Table 1.
National trends since 2010 are paralleled in a strong majority of states. Between 2010 and 2019 inclusive, there was a statistically significant negative correlation (95% confidence level) between AOD and Annual Prescription Opioid Sales in 38 states, with significant positive correlations occurring in only 2 states. Ten (5) states did not exhibit a significant (95% confidence level) relationships between overdose deaths and prescription opioid sales during the 2010–2019 time period (Table 2).
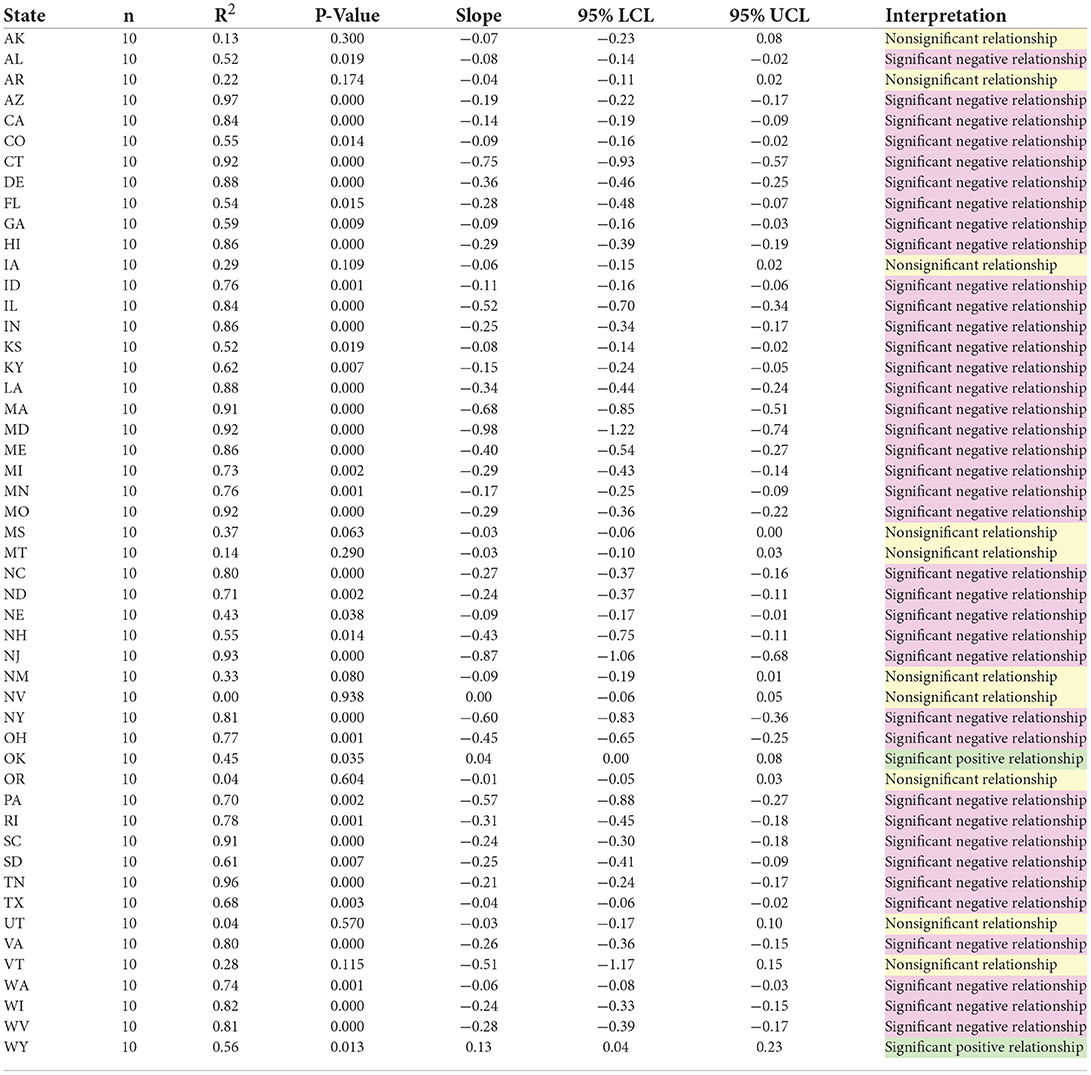
Table 2. Summary of regression models by state, any opioid overdose death by opioid prescribing rate/100 people.
The guideline emphasized to clinicians that opioid dosages should be limited to no more than 90 MME/day based on the “evidence regarding the association of opioid dosage and overdose risk” in that “overdose risk is increased at higher opioid dosages” (2).
This recommendation is not supported by the available data. Regression analyses of TOD, AOD, and OTA on POS from 2010 to 2019 among patients receiving doses of at least 90 MME/day show significant negative relationships, indicating that lower POS in this high-dosage cohort do not correspond to lower death rates. As with the national results, the relationship between POD and POS in this cohort is not significant (Table 3).
The direct correlations used to justify the CDC guideline and guideline update that existed from 1999 to 2010 are no longer present. Starting in 2010, opioid MME per Capita (POS) does not have a “clear correlation” (7) or move “in parallel” (2) or “in lockstep” (8) with OTA, POD, AOD or TOD. The relationships changed from direct to inverse in 2010. These results hold on a national level, in a large majority of states, and even among patients receiving opioid dosages greater than the recommended maximum dosage in the guideline (much less the reduced maximum dosage recommended in the guideline update). Based on the results presented in this paper and the current trends in opioid deaths, the policies of cutting POS to reduce TOD, AOD, POD, and OTA as presented in the guideline and the guideline update are unfounded and ineffective.
In 2019, the DEA concluded “Without effective new interventions, this overall pattern of predictable exponential growth is likely to continue into the future” (25). Government resources should be allocated to identify the root cause of drug addiction and overdose mortality and then applied to an effective approach that will consistently reduce addiction and overdose deaths.
Reasonable judgment would dictate tracking and reporting of chronic pain patient outcomes (deaths, suicides, returns in benefits, reported pain, function, etc.) for individuals since the guideline or the guideline update. However, there appears to be no publicly available evidence that a monitoring process is required or is planned to measure and confirm outcomes. PDMP records may provide a basis for contact and to survey a random sample of long-term opioid therapy patients to confirm consent, check their status and to evaluate the effectiveness of policy to date.
The results of the analyses presented here help to inform the public, legislators, and the medical community that since 2010 there has been no direct correlation of POS to OTA, POD, AOD, and TOD. The basis for the guideline, the guideline update, communications of public health policy, individual patient care, doctor conduct, congressional testimony, and intergovernmental communication that state and/or imply a direct correlation of POS to OTA, POD, AOD, and TOD are not valid. Based on the current relationships that have existed for a decade the guideline, guideline update and public health policy should be corrected/updated along with an acknowledgment of this material information to avoid misrepresentation or omission of relevant evidence.
LA obtained all of the data used in the manuscript. BC analyzed the data using regression analysis. Both authors contributed equally to the drafting of the manuscript.
Author BC is employed by Carr Consulting LLC, United States. Author LA is an independent researcher with no current professional affiliations. This study was independent research based on publicly available data. No outside funding was involved in the study design, data acquisition and analysis, decision to publish, or preparation of the manuscript. Both authors declare no other competing interests.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. CDC Press Releases. CDC (2016). Available online at: https://www.cdc.gov/media/releases/2016/p0315-prescribing-opioids-guidelines.html
2. CDC Guideline for Prescribing Opioids for Chronic Pain -United States 2016 Morbidity Mortality Weekly Report. (2016). p. 2, 18. Available online at: https://www.cdc.gov/mmwr/volumes/65/rr/pdfs/rr6501e1.pdf doi: 10.15585/mmwr.rr6501e1 (accessed May 11, 2022).
3. Federal, Register:: Request Access,.. unblock.federalregister.gov. p. 10–11, 108. Available online at: https://www.federalregister.gov/documents/2022/02/10/2022-02802/proposed-2022-cdc-clinical-practice-guideline-for-prescribing-opioids (accessed May 11, 2022).
4. Vital Vital Signs: Overdoses of Prescription Opioid Pain Relievers — United States, 1999–2008 (2019). Available online at: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6043a4.htm
5. Kolodny A. (n.d.). Statement by 2018. p. 1, 2. Available online at: https://docs.house.gov/meetings/IF/IF14/20180228/106915/HHRG-115-IF14-Wstate-KolodnyA-20180228.pdf (accessed May 21, 2022).
6. NCIPC Board of Scientific Counselors. “Draft Meeting Minutes” Department of Health and Human Services Public Health Service Board of Scientific Counselors (BSC), Centers for Disease Control and Prevention (CDC), National Center for Injury Prevention and Control (NCIPC) Seventeenth Meeting: January 7, 2016 (2016). p. 12. Available online at: cdc.gov (accessed May 21, 2022).
7. ASPE ISSUE BRIEF (2015),. p. 1–2, 5. Available online at: https://aspe.hhs.gov/sites/default/files/migrated_legacy_files/56406/ib_OpioidInitiative.pdf (accessed January 25, 2022).
8. NCIPC Board of Scientific Counselors. “Draft Meeting Minutes” Department of Health and Human Services Public Health Service Board of Scientific Counselors (BSC), Centers for Disease Control and Prevention (CDC), National Center for Injury Prevention and Control (NCIPC) Eighteenth Meeting: Thursday, January 28, 2016 (2016). p. 9. Available online at: https://www.cdc.gov/injury/pdfs/bsc/NCIPC_BSC_01_28_2016_Minutes_Final_3_15_2016.pdf. (accessed May 21, 2022).
9. Haegerich T,. The Opioid Overdose Epidemic in the United States NCIPC/CDC Research Priorities. (2018). p. 34 Available online at: https://www.cdc.gov/injury/pdfs/bsc/Opioid-Research-Priorities_BSCJune2018_Haegerich-a.pdf (accessed May 21, 2022).
10. Noonan R,. Understanding Addressing the Opioid Crisis Nevada's Prescription Drug Abuse Summit CDC PERSPECTIVE. (2016). p. 8, 42. Available online at: https://dpbh.nv.gov/uploadedFiles/dpbh.nv.gov/content/Programs/ClinicalSAPTA/Meetings/5%20%20Rise%20of%20Heroin%20Rita%20Noonan.pdf (accessed May 21, 2022).
11. Kolodny A,. Statement for the Record. (2018). Available online at: https://www.hsgac.senate.gov/imo/media/doc/Testimony-Kolodny-2018-01-17-REVISED.pdf (accessed November 2, 2021).
12. Bacon S,. CDC's Approach to Addressing the Opioid Overdose Epidemic. (2016). p. 7. Available online at: https://www.ncsl.org/portals/1/documents/health/InjuryMtg2016_SBacon.pdf (accessed May 21, 2022).
13. Kolodny A, Courtwright DT, Hwang CS, Kreiner P, Eadie JL, Clark TW, et al. The prescription opioid and heroin crisis: a public health approach to an epidemic of addiction. Annu Rev Public Health. (2015) 36:559–74. doi: 10.1146/annurev-publhealth-031914-122957
14. CDC Press Releases. CDC (2016). Available online at: https://www.cdc.gov/media/releases/2016/t0315-prescribing-opioids-guidelines.html
15. Nadeau SE, Wu JK, Lawhern RA. Opioids and chronic pain: an analytic review of the clinical evidence. Front Pain Res. (2021) 17:2. doi: 10.3389/fpain.2021.721357
16. Dasgupta N, Beletsky L, Ciccarone D. Opioid crisis: no easy fix to its social and economic determinants. Am J Public Health. (2018) 108:182–6. doi: 10.2105/AJPH.2017.304187
17. Chen Q, Larochelle MR, Weaver DT, Lietz AP, Mueller PP, Mercaldo S, et al. Prevention of prescription opioid misuse and projected overdose deaths in the United States. JAMA Network Open. (2019) 2:e187621. doi: 10.1001/jamanetworkopen.2018.7621
18. Lagisetty PA, Healy N, Garpestad C, Jannausch M, Tipirneni R, Bohnert ASB. Access to primary care clinics for patients with chronic pain receiving opioids. JAMA Network Open. (2019) 2:e196928. doi: 10.1001/jamanetworkopen.2019.6928
19. The Use of Medicines in the U.S. 2022. www.iqvia.com. p. 19. Available online at: https://www.iqvia.com/insights/the-iqvia-institute/reports/the-use-of-medicines-in-the-us-2022 (accessed May 22, 2022).
20. Prescription Drug Time Dosage Limit Laws (2015). p. 2. Available online at: https://www.cdc.gov/phlp/docs/menu_prescriptionlimits.pdf (accessed May 21, 2022).
21. Rutkow L, Vernick JS. Emergency legal authority and the opioid crisis. N Engl J Med. (2017) 377:2512–4. doi: 10.1056/NEJMp1710862
22. Jayawardana S, Forman R, Johnston-Webber C, Campbell A, Berterame S, de Joncheere C, et al. Global consumption of prescription opioid analgesics between 2009-2019: a country-level observational study. eClinicalMedicine. (2021) 42:101198. doi: 10.1016/j.eclinm.2021.101198
23. Ju C, Wei L, Man KKC, Wang Z, Ma T-T, Chan AYL, et al. Global, regional, and national trends in opioid analgesic consumption from 2015 to 2019: a longitudinal study. Lancet Glob Health. (2022) 7:e335–46. doi: 10.1016/S2468-2667(22)00013-5
24. Majara JL. Docket No. CDC-2020-0029 Letter Dowell D, Dowell. (2020). p. 5, 7. Available online at: https://searchlf.ama-assn.org/undefined/documentDownload?uri=%2Funstructured%2Fbinary%2Fletter%2FLETTERS%2F2020-6-16-Letter-to-Dowell-re-Opioid-Rx-Guideline.pdf
25. Guzman, A,. The Opioid Epidemic and the Practice of Medicine. p. 12–13. Available online at: https://www.deadiversion.usdoj.gov/mtgs/pract_awareness/conf_2019/dec_2019/guzman.pdf (accessed May 11, 2022).
26. Commission on Combating Synthetic Opioid Trafficking. Commission on Combating Synthetic Opioid Trafficking: Final Report. Reports from the Commission on Combating Synthetic Opioid Trafficking (2022). p. ix. Available online at: https://www.rand.org/pubs/external_publications/EP68838.html
27. Seth P, Rudd RA, Noonan RK, Haegerich TM. Quantifying the epidemic of prescription opioid overdose deaths. Am J Public Health. (2018) 108:500–2. doi: 10.2105/AJPH.2017.304265
28. Peppin JF, Coleman JJ. CDC's efforts to quantify prescription opioid overdose deaths fall short. Pain and Therapy. (2021) 10:25–38. doi: 10.1007/s40122-021-00254-z
29. Instructions for Completing the Cause-of-Death Section of the Death Certificate. Available online at: https://www.cdc.gov/nchs/data/dvs/blue_form.pdf
30. Drugabuse.gov. 2021. Available online at: https://www.drugabuse.gov/sites/default/files/Overdose_data_1999-2019.xlsx
31. Centers for Disease Control and Prevention National Center for Health Statistics. Underlying Cause of Death 1999-2019 on CDC WONDER Online Database, released in 2020. Data are from the Multiple Cause of Death Files, 1999-2019, as compiled from data provided by the 57 vital statistics jurisdictions through the Vital Statistics Cooperative Program. Available online at http://wonder.cdc.gov/ucd-icd10.html (accessed February 7, 2021).
32. Treatment Episode Data Set (TEDS) 2000-2010 National Admissions to Substance Abuse Treatment Services. Available online at: https://www.samhsa.gov/data/sites/default/files/2010_Treatment_Episode_Data_Set_National/2010_Treatment_Episode_Data_Set_National.pdf (accessed December 17, 2021).
33. TEDS Annual Report. www.samhsa.gov. Available online at: https://www.samhsa.gov/data/sites/default/files/reports/rpt31097/2018_TEDS/2018_TEDS.html#PSU (accessed December 17, 2021).
34. Annual Surveillance Report of Drug-Related Risks Outcomes. (2019). Available online at: https://www.cdc.gov/drugoverdose/pdf/pubs/2019-cdc-drug-surveillance-report.pdf
35. MME, per capita U,.S. 2014–2018. Statista. Available online at: https://www.statista.com/statistics/753284/number-of-mme-dispensed-per-capita-in-us/
36. Prescription Opioid Trends in the United States. www.iqvia.com. Available online at: https://www.iqvia.com/insights/the-iqvia-institute/reports/prescription-opioid-trends-in-the-united-states
37. CDC. U.S. Opioid Dispensing Rate Maps | Drug Overdose | CDC Injury Center. www.cdc.gov. (2021). Available online at: https://www.cdc.gov/drugoverdose/rxrate-maps/index.html
Keywords: prescription opioid sales, overdose deaths, opioid treatment admissions, CDC guideline, statistical analysis, analysis of historical data
Citation: Aubry L and Carr BT (2022) Overdose, opioid treatment admissions and prescription opioid pain reliever relationships: United States, 2010–2019. Front. Pain Res. 3:884674. doi: 10.3389/fpain.2022.884674
Received: 26 February 2022; Accepted: 11 July 2022;
Published: 04 August 2022.
Edited by:
Tony L. Yaksh, University of California, San Diego, United StatesReviewed by:
S. Stevens Negus, Virginia Commonwealth University, United StatesCopyright © 2022 Aubry and Carr. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: B. Thomas Carr, dG9tLmNhcnJAY2FycmNvbnN1bHRpbmcubmV0
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.