
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pain Res., 14 April 2022
Sec. Neuropathic Pain
Volume 3 - 2022 | https://doi.org/10.3389/fpain.2022.865032
Signs and symptoms of optic neuritis (ON), an autoimmune disorder of the central nervous system (CNS), differ between patients. Pain, which is commonly reported by ON patients, may be the major reason for some patients to visit the clinic. This article reviews the presence of pain related to ON with respect to underlying disorders, including multiple sclerosis (MS), neuromyelitis optica spectrum disorder (NMOSD), and myelin oligodendrocyte glycoprotein associated disease (MOGAD). The aim of this review is to provide an overview of pain symptoms in accordance with the context of various pathophysiological explanations, assist in differential diagnosis of ON patients, especially at the onset of disease, and make recommendations to aid physicians make decisions for follow up diagnostic examinations.
Optic neuritis (ON) is an optic neuropathy caused by inflammation. ON can arise from several etiologies, including multiple sclerosis (MS), neuromyelitis optica spectrum disorder (NMOSD), myelin oligodendrocyte glycoprotein antibody associated disease (MOGAD), acute disseminated encephalomyelitis (ADEM), and some systemic autoimmune diseases. Usually, ON presents as a symptom of an episode of one of several inflammatory diseases occurring in the central nervous system (CNS). It may, however, emerge as an isolated disease unity. Prompt and intensive therapy is required to control the development of disease, restore the comfort of the patient, and reduce relapses of disease based on accurate and rapid diagnosis of background diseases. Optic nerve atrophy may lead to a permanent visual loss in severe cases. This poor prognosis of ON in some cases highlights the importance of correct diagnosis of ON. Baseline data to consider for diagnosis include age, gender, visual loss, absence or presence of pain, and auxiliary tests. Consideration of follow-up tests should be guided by a detailed evaluation of symptoms, especially for patients that otherwise appear healthy.
Phenotypes of ON associated with various etiologies overlap and, thus, careful assessment of their difference is required, since typical ON responds well to steroid therapy, whilst atypical ON is resistant to steroid therapy and tends to relapse. Although a published review has provided an introduction of prevalence and the underlying mechanism of pain in NMOSD and MOGAD without focusing on pain related to other etiologies, they were concerned primarily with systemic pain, especially neuropathic pain, which was highly relevant to the quality of life and paid less attention to ON related pain. The major purpose was to analyze how to improve the quality of life of patients (1). There have been limited reports concentrating on the symptoms of pain in ON and there is a lack of an extensive clinical database of prevalence of pain in different types of ON. This review focuses on the pain experienced by ON patients according to different disease etiologies, explores the mechanism of pain in ON, and summarizes the prevalence of different pain manifestations, including ocular pain, pain with eye movement, and headache. Treatments for pain for ON patients are also suggested.
Relevant articles were identified by a search of of PubMed, by inputting the terms “optic neuritis” AND “pain”, “optic neuritis” AND “classification”, “multiple sclerosis” AND “pain”, “neuromyelitis optica” AND “pain”, “myelin oligodendrocyte glycoprotein AND “pain”, “pain with eye movement” and “treatment” AND “pain related to ON”. Only English language publications were included in the review. Titles and abstracts were reviewed to determine which articles were relevant. The full content of studies deemed relevant was subjected to detailed review. The references of each article reviewed were carefully examined to identify additional related publications within this field.
A total of over 47 articles, including 21 original studies and 26 reviews were identified and were included in this article. The overview of the cited articles related to each sections below was summarized in Table 1.
ON is commonly recognized to be related to MS. Magnetic resonance imaging (MRI) plays a paramount role in diagnosis of MS. If an ON patient manifests a normal MRI, the conversion rate to MS is only 25%. In contrast, if hyperintense lesions are observed on the T2 sequence MRI, the conversion rate to MS is 75%. The typical lesions of MS are multifocal, sporadic, and demarcated (52). Over half of the ON patients involved in the Optic Neuritis Treatment Trial (ONTT) were affected by MS (52). Isolated ON and MS-associated ON (MS-ON) are usually grouped together as typical ON, since they share concurrent characteristics, including age ranging from 18 to 50, unilaterality, pain with eye movement, rapid vision loss, and the tendency to reach the lowest level of visual acuity within two weeks of onset. Clinically isolated syndrome (CIS) refers to that the patient underwent a single or multiple phases of neurologic symptoms that might progressed to MS but at that moment these symptoms cannot fulfill the diagnostic criteria of MS (Karussis, 2014). White matter lesions observed in MRI are mostly associated with MS (5). The recovery of visual acuity is always very good for MS patients, even without corticosteroid medication. Visual acuity of at least one-line was restored within three weeks for almost all patients without any treatment (53). Another study reported that 72% of impaired eyes achieved a complete recovery of visual acuity, while 92% recovered visual acuity better than 20/40 (7). The visual impairment of ON in older subjects (onset of ON later than 45 years old) is worse than that of their younger counterparts (8), often lasting for 8 weeks for a full recovery of the lesions of ON with frequent residual impairment (6). Atypical ON, especially the neuromyelitis optica spectrum disorder (NMOSD) associated ON (NMOSD-ON), is more commonly bilateral (around 20%) than MS-ON (9). In NMOSD, lesions in the spinal cord appear to be long and extensive, whilst the few lesions in the brain always lie in the hypothalamus and brain stem (10). For differentiated diagnosis, an oligoclonal band (OCB) is more frequently discovered in the cerebrospinal fluid (CSF) of MS patients (85%) than NMOSD patients (15-30%) (11). The presence of aquaporin protein 4 antibodies (AQP4-IgG), which are found in more than 90% of NMOSD-ON patients in conjunction with the clinical features, helps to diagnose NMOSD with high sensitivity (99%) and specificity (90%) (12, 54). Visual acuity in NMOSD-ON is relatively poor at the early stage of disease, with 80% of patients having visual acuity worse than 20/200. The recovery of vision is also reported to be very poor, with the visual acuity of ~30% of patients remaining at 20/200, with no further improvement (9). Of patients testing seronegative for AQP4-IgG, 20% tested positive for myelin oligodendrocyte glycoprotein (MOG) antibodies (MOG-IgG). Due to its unique clinical characteristics, including dominance of male gender, a single attack, better recovery of visual acuity, presentation as ON at onset is usually in adults, but presentation as acute demyelinating encephalomyelitis (ADEM) at onset is mostly in children. MOG associated disorder (MOGAD) is increasingly considered as a disease entity distinct from NMOSD. Unlike MS-ON, presentation with NMOSD-ON requires prompt therapy with glucocorticoids to relieve the damage to the optic nerve axons or immunomodulators to reduce the relapse rate (13). Recurrence of inflammation confined to the optic nerve in ON patients without fulfilling the criteria of MS-ON or NMOSD-ON is highly suggestive of a diagnosis of chronic relapsing inflammatory optic neuropathy (CRION). CRION responds well to both corticosteroid and immunomodulatory agents, but cessation of medication often results in relapse of disease (14). The patterns of visual field defect in these patients are variable and include scotomas, arcuate defects, and nasal step defects. Scotomas were the most common defects reported if eyes were examined by perimetry with a large field (2, 15, 16). Relative afferent pupillary defect (RAPD) is often present in patients with a monocular deficit. Optic swelling arises in only one third of patients (7).
Table 2 generalize several disease entities related to ON and summarize the clinical features of them.
MS and NMOSD are both sporadic diseases and the familiar segregations of them are compatible with non-Mendelian inheritance. It suggested that rare relatives of MS or NMOSD were affected by the same condition. However, the susceptibility of individuals with MS or NMOSD family members is higher than that of the general population. (17, 18) Previous screening of human leukocyte antigens (HLA) class II DR genes found that HLA-DRB1*15 (Odds ratio value = 15.89) and DRB5 alleles were more likely to be represented in MS patients. On the other hand, HLA-DRB1*03 (Odds ratios value = 3.23) and DRB3 alleles were associated with the emergence of NMOSD (19). In another study investigating non-major histocompatibility complex (non-MHC) genes, it screened the susceptibility of 35 MS-related loci among 110 NMOSD patients, but no significant higher risk of developing NMOSD in patients with these predisposing loci can be found compared with 332 normal subjects (20).
One genotyping research conducted its investigation on HLA-DRB1 and DPB1 alleles in 211 Japanese NMOSD patients compared with 1,919 normal healthy subjects as controls. A single nucleotide polymorphism (SNP) rs1964995 located in MHC region was reported to be related to occurrence of NMOSD (OR value = 2.33). Two risk alleles (HLA-DRB1*08:02 and HLA-DRB1*16:02) were also detected by the same research while HLA-DRB1*9:01 was found to be a protective factor. One more to mention in this study was that a SNP rs1516512 in gene potassium calcium-activated channel subfamily M alpha 1 (KCNMA1) correlated with disability and transverse myelitis. Immunohistochemistry also found KCNMA1 with the area of endfeet of astrocytes where most NMOSD lesions located (74). In a Chinese Han cohort, rs117026326 was screened in 144 NMOSD patients, 168 MS patients and 1,403 healthy controls due to that NMOSD always coexists with some systemic autoimmune diseases Sjogren's syndrome, systemic lupus erythematous (SLE) and rheumatoid arthritis (RA) associated with overrepresentation of rs117026326. The result revealed that rs117026326 in the upstream of general transcription factor II-I (GTF-2I) gene could also be the risk factor of developing NMOSD (OR value = 2.535) (21). However, no significant association between MS and rs117026326 was detected compared with controls. Overall, from mentioned above, MS and NMOSD are supposed to be two entities of disease with distinct genetic background.
Due to complex associated ON diseases, pain presents with multiple characteristics. Pain can be the symptom indicating ON especially when this pain experience is following eye movements. Besides pain related to ON, NMOSD-ON and MS-ON patients also share similar systemic pain symptoms according to NMOSD and MS. Table 3 shows and summarizes the pain arising from non-ocular courses. Typical ON patients are more likely to experience mild periocular pain and pain following eye movement. Experience of such pain is relieved only after several days. Pain can either precede or occur after the visual loss (5), although pain usually occurs 2–3 days before the impairment of visual function (22). The duration of pain with eye movement usually persists for less than one week (6). The ONTT reported a high rate of ocular and periocular pain (92%) and pain following eye movement (87%) (7). Pain was worsened by eye movement (90%), while the orbital segment is enhanced on MRI after gadolinium injection (23). Lack of pain following eye movement is always related to the lesion being located in the intracranial part of the optic nerve (79). Fazzone et al. (23) also reported that pain with eye movement is presumably absent if the lesion is confined to the intracranial and canalicular part of the optic nerve. Pain arises from different mechanisms when different portions of the optic nerve are involved.
Sometimes, patients may mistakenly indicate the site of their lesions because ocular pain can radiate to the periocular area, since the whole orbital structure shares the same innervation of the first division of the trigeminal nerve. It may be postulated that the pain sensation radiates from the eye to the periocular area or other regions of the orbital structure (24). It is very common for ON patients to complain that they suffer from ocular, periocular, and retroocular pain. Table 4 summarize the incidence of retrobulbar pain and pain following eye movement in several studies.
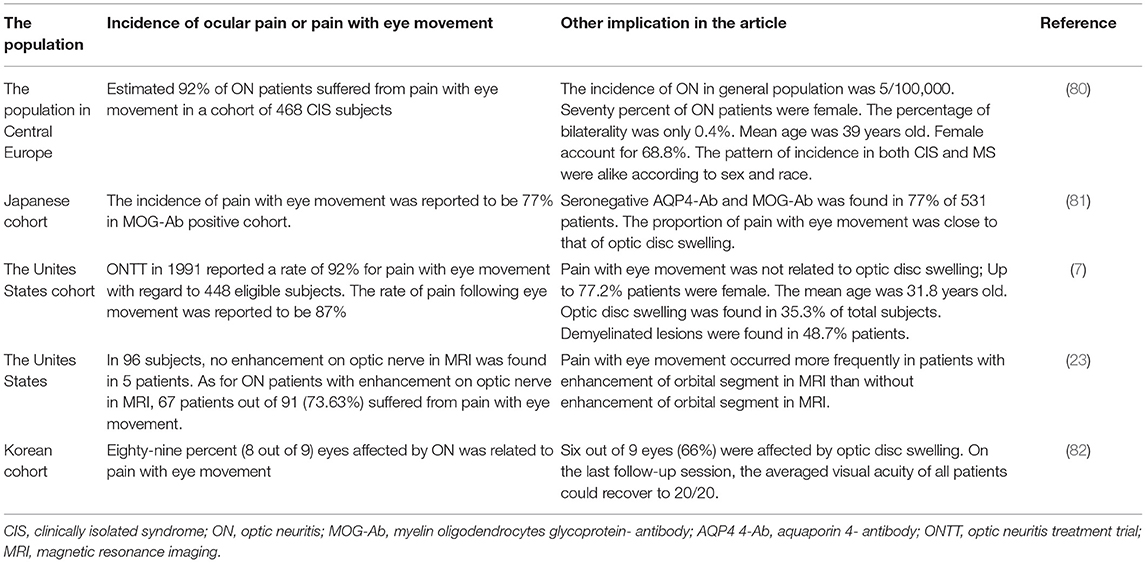
Table 4. Overview of researches with respect to the incidence of ocular pain and pain with eye movement in ON patients.
Pain sensation worsening after movement of the eye ball is specific to typical ON. At the site of the apex, the optic nerve sheath is proximal to the superior and medial rectus. Consequently, when the patient moves the eye ball, contraction of the ocular muscles irritates the inflamed optic nerve sheath to cause the pain (25) (Figure 1). It is less likely for patients with anterior ON to develop pain with eye movement, because the optic nerve sheath of the anterior nerve is not pulled by the ocular muscles. In a study involving 91 patients, the frequency of ocular pain and pain with eye movement was higher for patients with enhanced orbital segment in MRI, whilst patients without an enhanced orbital segment were more likely not to experience any painful sensation (23) (Table 4).
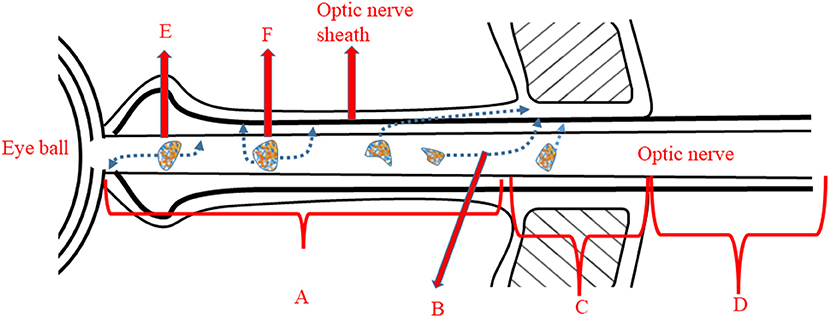
Figure 1. Illustration of origin of pain with eye movement and headache. A, Orbital segment of optic nerve: lesions lying in this segment often trigger pain with eye movement due to proximity to site B; B, The optic nerve sheath attaches to the ocular muscles at this site. Movement of the eye induces the pain experience. C, Intracanalicular portion of the optic nerve. D, Intracranial segment of the optic nerve. E, A mild lesion within the orbital segment which is unlikely to infiltrate to the optic nerve sheath. F, A severe lesion within the orbital segment, which will penetrate the optic nerve sheath and lead to headache.
A recent study showed that MOGAD related ON (MOGAD-ON) predominantly affects the anterior segment of the optic nerve, especially the optic nerve head (ONH), whereas the posterior segment of the optic nerve is most commonly involved in patients with seropositive AQP4-IgG (26). Consistent results were reported by other studies, which observed that ON with positive AQP4-IgG mostly involved the optic chiasm and optic tract (27, 28). This may explain why the frequency of eye pain is relatively lower in NMOSD-ON patients (67%) than in MOGAD-ON (1). A prospective study could analyze whether patients with MOGAD-ON and ON with positive AQP4-IgG manifest a lower proportion of pain with eye movement because the original lesions of both ON reside distally to the mobile portion of the orbital apex (Figure 1).
Apart from ocular pain and pain following eye movement, headache is another common symptom related to ON, especially if the optic nerve sheath is severely involved (22). Headache can be related to both an ocular or a non-ocular cause, the latter varying in both MS and NMOSD patients. The pain may arise from a cervicogenic source, while the lesions remain at the cervical segment of the spinal cord. Alternatively, sensitization of the trigeminal nerve system may produce spontaneous pain in some cases. Therefore, it is evident that MRI scanning to locate the lesions in MS or NMOSD is necessary to differentiate distinct etiologies of headache. The current section only concerns headache related to ON.
The lesions of MOGAD-ON are more likely to be located at the orbital segment of the optic nerve, inducing the swelling of the optic nerve sheath seen using MRI (27–29). However, the relationship between the extent of oedema in the optic nerve sheath and severity of pain remains controversial. A recent retrospective study of headache related to ON, reported that 50.5% of MOGAD-ON patients had symptoms of headache as opposed to only 14% of MS patients presenting with a headache (1). Visual impairment following headache is profound for MOGAD-ON patients. Fifteen of the 64 patients who underwent MRI investigation were found to have lesions involving the anterior segment of the optic nerve, of whom nine had lesions extending to the intracanalicular segment. The researchers concluded that headache and ensuing visual impairment could be prodromal symptoms for MOGAD-ON hence, stressing the necessity of an MOG-IgG test. They also proposed that inflammation involving the anterior segment of the optic nerve in MOGAD-ON is likely to be the cause of the high rate of headache and visual loss observed in this condition: the more severe the inflammatory attack on the optic nerve sheath surrounding the optic nerve, the higher the possibility that the patient will suffer from headache (1) (Figure 1). This retrospective study, however, may have underestimated the proportion of headache with MOGAD-ON, since some physicians did not ask whether the patient had a headache. A longitudinal study is essential to investigate the relation of types of headaches, such as migraine-like headache, pulsive headache, or mild headache, to locations of affected segments of the optic nerve. Table 5 listed all headaches symptoms due to brain or spinal cord lesions for physician to differentiate pain related to ON from pain due to various etiologies.
Pentraxin-3 and interleukin-6 (IL-6) have already been recognized as biomarkers for headache syndromes (22). Pentraxin-3, measured in serum, is related to immune reaction in autoimmune diseases and has been reported to increase to a high level in patients with headache. IL-6 in CSF has been shown to be another pro-inflammatory factor able to predict the inflammatory status in NMOSD. IL-6 is also suggested to be linked with headache, especially migraine attacks (22).
The sensory pain components, nociceptive pain and neuropathic pain, are generated via different pathways. For the nociceptive pain, action potential is produced upon activation of nociceptors and transmitted along the sensory pathway to the CNS. Influxes of sodium and calcium cause depolarization of action potential to initiate neural firing. The opening of sodium and calcium gates is mediated by transducer proteins, including G-protein-coupled channels, ion linked channels, and tyrosine kinase linked channels. To partially regulate the transmission of action potential, hyperpolarization of the nerve is caused by potassium channels that respond to overload of voltage, calcium, and adenosine triphosphate (ATP). In contrast, neuropathic pain is caused by sensitization of transmission. This condition is attributed to dysfunction or turbulence of the nervous system. Spontaneous pain and hyperalgesia reflect features of neuropathic pain (30).
With regards to pain associated with ON, the optic nerve itself is not able to convey pain information. Thus, the origin of this pain is always from the meninges covering the optic nerve. Sporadic nociceptors within the meninges respond to noxious stimuli and deliver impulses to surrounding axons.
Initially, nervi nervorum, a group of unmyelinated fibers, innervate the territories of the optic nerve sheath and receive the nociceptive stimuli from nociceptors in the peripheral tissues. Peripheral nervi nervorum also mediate chronic pain if the axons are involved (36). Polymodal nociceptors can respond to different stimuli due to diverse expression of ion channels of the trigeminal ganglion (TG). The ion channels of Piezo 2, the transient receptor potential vanilloid 1 (TRPV1), the transient receptor potential ankyrin 1 (TRPA1), and the transient receptor potential melastatin 8 (TRPM8) correspond to sensations of mechanical forces, heat, chemical agents, and coldness, respectively. The sensitivity and excitability increase, while the terminals of axons within the TG are involved by lesions. Subsequently, these ion channels are modulated to reduce the pain threshold of nerve membranes, which cause persistent pain (96). Central sensitization processed in nociceptors also facilitates hyperalgesia of the eye due to the continuous input of pain, leading to the frequent combination of glutamate and its receptors (31). The trigeminal nerve can be sensitized and activated to cause neuropathic pain and pain enhancement, by which it becomes susceptible to noxious stimuli (32). Subsequently, the axons of the TG are stimulated by noxious stimuli to transmit the pain information.
Finally, the TG sends the stimulus to the axons to end at the bodies of the trigeminal brainstem complex (33). Thus, the pain associated with ON arising from nociceptors in the meninges of the optic nerve constitute nociceptive pain (29). During the process of ON, mediators, including prostaglandin E2 (PGE2), tumor necrosis factor α (TNF-α), and interleukin-6 (IL-6), are released from the lesions of ON stimulated nociceptors within the orbital region (34).
However, it is unlikely that mediators of inflammation in ON infiltrate deeply and directly to stimulate the nervi nervorum. The less likely possibility of involvement of the nervous system, which produce neuropathic pain, has been described in an earlier review (35). Therefore, the trigeminal ganglion would be spared and central sensitization would be absent in ON. This may also explain why ON patients commonly complain of transient pain.
It has previously been reported that neuropathic pain rarely occurred in ON patients (1, 24, 27, 28). However, most of the ON patients in these studies experienced pain associated with MS, only a few having ambiguous etiologies. In contrast, NMOSD-ON pain seems to be more severe (36). NMOSD is mediated by the lymphocytes T helper (Th1 and Th17) (37). They may induce deaths of neurons, oligodendrocytes and astrocytes. Microglia cells phagocytose those cell debris and release chemokines, cytokines and reactive oxygen species (ROS) which is followed by recruitment of adhesion molecules. The speed of bloodstream is slowed down and cause high concentration of chemokines to facilitate production of macrophages. By presenting their components to T lymphocytes, macrophages initial the inflammation within the tissue (38). Following the initiation of inflammation in peripheral organs, mast cells are considered to be able to cross over the blood brain barrier (BBB) and release chemokines and tumor necrosis factor (TNF). Mast cells also generate proteinases Matrix metalloproteinases (MMP) 2 and MMP 9 to increase the permeability of the BBB and recruit more inflammatory cells into CNS system. TNF has already been demonstrated to be a strong pro-inflammatory factor that is capable of sensitization of nociceptors in meninges (39) Moreover, it is well known that NMOSD preferentially affects the astrocytes around the trigeminal nerve. Astrocyte re-uptake glutamate from the synaptic pool and convert it to glutamine by the excitatory amino acid transporters-2 (EAAT-2). In order to lower the concentration of glutamate within the cleft of synaptic contact, EAAT-2 was activated when suffering from an overdose of glutamate. Disorders of astrocytes will thus lead to a build-up of glutamate due to loss of EAAT-2. This excessive glutamate depolarizes the postsynaptic contact and, in turn, intensifies the pain experience (40). It remains uncertain whether NMOSD-ON or MOGAD-ON patients experience neuropathic pain. Some neuropathic pain may be neglected by physicians. Further studies are needed to investigate the long-erm pain symptoms of NMOSD-ON.
No studies have explored the function of potent analgesia and recommended their use for relief of pain, since this pain arises from the pathogenesis of ON and it is tolerable for the majority of patients. As mentioned previously, steroids are the first line therapy for ON, although the necessity for pain relief in typical ON still remains uncertain (41). Up to 500–1,000 mg intravenous administration of methylprednisolone (IVMP) is prescribed to inhibit inflammation of optic nerve. If this is not successful or the visual acuity does not recover after 3 days of IVMP, an additional 2 days are recommended to enhance the effect. For resistant cases, a double dose of IVMP is suggested. An alternative strategy for resistant cases is use of plasma exchange (PLEX) which aims to filter out autoantibodies throughout the circulation system. Five continuous cycles have been recommended to treat relapsing or resistant cases. Each cycle involves exchanging of one volume of plasma by 5% albumin (42).
Immunomodulatory agents have been shown to reduce the incidence of relapses (43). As described previously, IL-6 presumably gives rise to headaches in NMOSD and disrupts the CNS system, since it facilitates the activation of TH17 cells. Tocilizumab has been noted to be an antibody against the IL-6 receptor and its administration ameliorates the progression and pathological changes of NMOSD (44). It also relieves pain by turning down the glutamate transporter 1 (GLT1), which is associated with the depolarization of the post-synaptic contact (45).
Mycophenolate mofetil (MMF), which is suggested to be useful in the treatment of NMOSD and MOGAD, has been shown to inhibit the proliferation of B cells and T cells (46). It is acknowledged that patients with MOGAD rely strongly on a combination of steroid and MMF to relieve their pain (46).
Rituximab depletes B-cells by anchoring at the CD 20 epitope located on the surface of B cells and is effective in controlling the CNS damage in NMOSD (97). However, it is estimated that one third of MOGAD patients are refractory to rituximab. This population of patients relapse even after the administration of rituximab (98).
The endocannabinoid system is effective in modulating ocular pain and suppressing the inflammation with safer administration. It aims to bind to the cannabinoids-2 receptor (CB2R) and have an anti-inflammatory action. Current treatment with endocannabinoids is mainly confined to injuries of the cornea, such as alkali burns and dry eye. There is a lack of a detailed investigation into the effect on the pain related to optic neuritis. In addition, it has been noted that the pain modulation of endocannabinoids may be associated with inhibition of sensitization of TG. However, further research is needed to determine whether the biomarker L-type voltage gated Ca2+ channels are significantly inhibited in TG by endocannabinoids. Topical formulations of endocannabinoid are preferred to reduce systemic side effects (47).
MS and NMOSD are always comorbid with emotional problems or disabilities that may cause some influence on their quality of life. In one previous study by Barzegar in 2021, 35 (52.2%) patients were affected by 44 comorbidities including 29 somatic, 13 psychiatric and 2 autoimmune disorders. Anxiety, migraine and depression disorder were all listed as commonest symptoms. With respect to those patients with AQP4-Ab (+), the frequency of suffering from psychiatric disorders is significant higher (48). Furthermore, the depression NMOSD patients suffered from was correlated with pain experience. Pain and depression coexisted to impose the influence on the quality of life (49). Activation of glutamatergic system and inactivation of GABAergic system were both considered to be mechanisms of developing depression in NMOSD (50, 51).
Pain is a frequently reported symptom associated with ON. Pain symptoms differ between the various associated diseases and can be a useful diagnostic tool for identifying the type of disease. Bilateral severe orbital pain and headache with mild vision loss may indicate the diagnosis of MOGAD-ON. Pain followed by severe and irreversible visual loss as well as bilaterality suggests NMOSD-ON, while classical pain with eye movement, unilaterality, and reversible visual impairment can be an indicator of MS-ON. Pain of ON is initially triggered by activation of nociceptors in peripheral tissues. Through the progression of TG and the thalamus, the action potential caused by pain is projected to the cortex of the brain. Although pain can be aggravated by sensitization of the central nervous system, it remains uncertain whether the overreaction of the nervous system is evoked in ON patients. The transient and tolerable pain induced by ON does not require any analgesia to relieve. Pain can be resolved over a short period of time and by steroid treatment.
ST and HC: concept and design. XY, XL, ML, JW, ST, and HC: acquisition, analysis, and interpretation of data. XY: drafting of the manuscript and statistical analysis. ST and HC: revision of the manuscript. All authors had full access to all the data in the study and were responsible for the integrity and accuracy of data.
This work was supported by the National Natural Science Foundation of China (Project Code: 81800822 to ST) and the Internal Research Grant from the Hong Kong Polytechnic University 2020-23 (Project Code: ZVS7 to ST). The funding organization had no role in the design or conduct of this research.
ST and HC are employed by Centre for Eye and Vision Research.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We are grateful to all participants in the preparation of this review article. We express our sincere gratitude to Dr. Maureen Boost from the School of Optometry in the Hong Kong Polytechnic University for editing the language in this article. We would like to thank the research students and staff at the School of Optometry in the Hong Kong Polytechnic University for their assistance.
1. Asseyer S, Hamblin J, Messina S, Mariano R, Siebert N, Everett R, et al. Prodromal headache in MOG-antibody positive optic neuritis. Mult Scler Relat Disord. (2020) 40:101965. doi: 10.1016/j.msard.2020.101965
2. Nevalainen J, Krapp E, Paetzold J, Mildenberger I, Besch D, Vonthein R, et al. Visual field defects in acute optic neuritis—distribution of different types of defect pattern, assessed with threshold-related supraliminal perimetry, ensuring high spatial resolution. Graefes Arch Clin Exp Ophthalmol. (2008) 246:599–607. doi: 10.1007/s00417-007-0722-2
3. Karussis D. The diagnosis of multiple sclerosis and the various related demyelinating syndromes: a critical review. J Autoimmun. (2014) 48-49:134–42. doi: 10.1016/j.jaut.2014.01.022
4. Chan JW. Early diagnosis, monitoring, and treatment of optic neuritis. Neurologist (2012) 18:23–31. doi: 10.1097/NRL.0b013e31823d7acd
5. Toosy AT, Mason DF, Miller DH. Optic neuritis. Lancet Neurol. (2014) 13:83–99. doi: 10.1016/S1474-4422(13)70259-X
6. Beck RW, Cleary PA, Backlund JC. The course of visual recovery after optic neuritis. Exp Optic Neuritis Treat Trial Ophthalmol. (1994) 101:1771–8. doi: 10.1016/S0161-6420(94)31103-1
7. Optic Neuritis Study Group. The clinical profile of optic neuritis. Experience of the Optic Neuritis Treatment Trial. Optic Neuritis Study Group. Arch Ophthalmol. (1991) 109:1673–8. doi: 10.1001/archopht.1991.01080120057025
8. Zhou H, Zhao S, Yin D, Chen X, Xu Q, Chen T, et al. Optic neuritis: a 5-year follow-up study of Chinese patients based on aquaporin-4 antibody status and ages. J Neurol. (2016) 263:1382–9. doi: 10.1007/s00415-016-8155-7
9. Galetta SL, Cornblath WT. Should most patients with optic neuritis be tested for neuromyelitis optica antibodies and should this affect their treatment? J Neuroophthalmol. (2010) 30:376–8. doi: 10.1097/WNO.0b013e3181f68c19
10. Sellner J, Boggild M, Clanet M, Hintzen RQ, Illes Z, Montalban X, et al. EFNS guidelines on diagnosis and management of neuromyelitis optica. Eur J Neurol. (2010) 17:1019–32. doi: 10.1111/j.1468-1331.2010.03066.x
11. Wingerchuk DM, Lennon VA, Lucchinetti CF, Pittock SJ, Weinshenker BG. The spectrum of neuromyelitis optica. Lancet Neurol. (2007) 6:805–15. doi: 10.1016/S1474-4422(07)70216-8
12. Takahashi T, Fujihara K, Nakashima I, Misu T, Miyazawa I, Nakamura M, et al. Anti-aquaporin-4 antibody is involved in the pathogenesis of NMO: a study on antibody titre. Brain. (2007) 130:1235–43. doi: 10.1093/brain/awm062
13. Chen JJ, Bhatti MT. Clinical phenotype, radiological features, and treatment of myelin oligodendrocyte glycoprotein-immunoglobulin G (MOG-IgG) optic neuritis. Curr Opin Neurol. (2020) 33:47–54. doi: 10.1097/WCO.0000000000000766
14. Kidd D, Burton B, Plant GT, Graham EM. Chronic relapsing inflammatory optic neuropathy (CRION). Brain. (2003) 126:276–84. doi: 10.1093/brain/awg045
15. Gerling J, Meyer JH, Kommerell G. Visual field defects in optic neuritis and anterior ischemic optic neuropathy: distinctive features. Graefes Arch Clin Exp Ophthalmol. (1998) 236:188–92. doi: 10.1007/s004170050062
16. Keltner JL, Johnson CA, Spurr JO, Beck RW. Comparison of central and peripheral visual field properties in the optic neuritis treatment trial. Am J Ophthalmol. (1999) 128:543–53. doi: 10.1016/S0002-9394(99)00304-9
17. Matiello M, Kim HJ, Kim W, Brum DG, Barreira AA, Kingsbury DJ, et al. Familial neuromyelitis optica. Neurology (2010) 75:310–5. doi: 10.1212/WNL.0b013e3181ea9f15
18. Oksenberg JR, Baranzini SE, Barcellos LF, Hauser SL. Multiple sclerosis: genomic rewards. J Neuroimmunol. (2001) 113:171–84. doi: 10.1016/s0165-5728(00)00444-6
19. Brum DG, Barreira AA, Dos Santos AC, Kaimen-Maciel DR, Matiello M, Costa RM, et al. HLA-DRB association in neuromyelitis optica is different from that observed in multiple sclerosis. Mult Scler. (2010) 16:21–9. doi: 10.1177/1352458509350741
20. Liu QB, Li ZX, Zhao GX, Yu H, Wu ZY. No association between identified multiple sclerosis non-MHC risk loci and neuromyelitis optica. Neurosci Bull. (2014) 30:1036–44. doi: 10.1007/s12264-013-1457-1
21. Liang H, Gao W, Liu X, Liu J, Mao X, Yang M, et al. The GTF2I rs117026326 polymorphism is associated with neuromyelitis optica spectrum disorder but not with multiple sclerosis in a Northern Han Chinese population. J Neuroimmunol. (2019) 337:577045. doi: 10.1016/j.jneuroim.2019.577045
22. Marzoli SB, Criscuoli A. Pain in optic neuropathies. Neurol Sci. (2018) 39:25–31. doi: 10.1007/s10072-018-3334-1
23. Fazzone HE, Lefton DR, Kupersmith MJ. Optic neuritis: correlation of pain and magnetic resonance imaging. Ophthalmology. (2003) 110:1646–9. doi: 10.1016/S0161-6420(03)00477-9
24. Agostoni E, Frigerio R, Protti A. Controversies in optic neuritis pain diagnosis. Neurol Sci. (2005) 26 Suppl 2:s75–78. doi: 10.1007/s10072-005-0413-y
25. Lepore FE. The origin of pain in optic neuritis. Determinants of pain in 101 eyes with optic neuritis. Arch Neurol. (1991) 48:748–9. doi: 10.1001/archneur.1991.00530190096021
26. Ojha PT, Aglave VB, Soni G, Jagiasi KA, Singh RK, Singh RK, et al. Myelin Oligodendrocyte Glycoprotein (MOG) Antibody-Associated CNS Demyelination: Clinical Spectrum and Comparison with Aquaporin-4 Antibody Positive Neuromyelitis Optica Spectrum Disorder. Neurol India. (2020) 68:1106–14. doi: 10.4103/0028-3886.294831
27. Ramanathan S, Prelog K, Barnes EH, Tantsis EM, Reddel SW, Henderson AP, et al. Radiological differentiation of optic neuritis with myelin oligodendrocyte glycoprotein antibodies, aquaporin-4 antibodies, and multiple sclerosis. Mult Scler. (2016) 22:470–82. doi: 10.1177/1352458515593406
28. Srikajon J, Siritho S, Ngamsombat C, Prayoonwiwat N, Chirapapaisan N. Differences in clinical features between optic neuritis in neuromyelitis optica spectrum disorders and in multiple sclerosis. Mult Scler J Exp Transl Clin. (2018) 4:2055217318791196. doi: 10.1177/2055217318791196
29. Jarius S, Ruprecht K, Kleiter I, Borisow N, Asgari N, Pitarokoili K, et al. MOG-IgG in NMO and related disorders: a multicenter study of 50 patients. Part 2: Epidemiology. Clinical presentation, radiological and laboratory features, treatment responses, and long-term outcome. J Neuroinflammation. (2016) 13:280. doi: 10.1186/s12974-016-0718-0
30. Sandkühler J. Models and mechanisms of hyperalgesia and allodynia. Physiol Rev. (2009) 89:707–58. doi: 10.1152/physrev.00025.2008
31. Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain. (2011) 152:S2–s15. doi: 10.1016/j.pain.2010.09.030
32. Belmonte C, Acosta MC, Merayo-Lloves J, Gallar J. What Causes Eye Pain? Curr Ophthalmol Rep. (2015) 3:111–21. doi: 10.1007/s40135-015-0073-9
33. Hirata H, Okamoto K, Tashiro A, Bereiter DA. A novel class of neurons at the trigeminal subnucleus interpolaris/caudalis transition region monitors ocular surface fluid status and modulates tear production. J Neurosci. (2004) 24:4224–32. doi: 10.1523/JNEUROSCI.0381-04.2004
34. Bista P, Imlach WL. Pathological mechanisms and therapeutic targets for trigeminal neuropathic pain. Medicines (Basel). (2019) 6:91. doi: 10.3390/medicines6030091
35. Truini A, Barbanti P, Pozzilli C, Cruccu G. A mechanism-based classification of pain in multiple sclerosis. J Neurol. (2013) 260:351–67. doi: 10.1007/s00415-012-6579-2
36. Bove GM, Light AR. The nervi nervorum. Pain Forum. (1997) 6:181–90. doi: 10.1016/S1082-3174(97)70011-4
37. Sagan SA, Cruz-Herranz A, Spencer CM, Ho PP, Steinman L, Green AJ, et al. Induction of paralysis and visual system injury in mice by t cells specific for neuromyelitis optica autoantigen aquaporin-4. J Vis Exp. (2017) 56185. doi: 10.3791/56185
38. Lyman M, Lloyd DG, Ji X, Vizcaychipi MP, Ma D. Neuroinflammation: the role and consequences. Neurosci Res. (2014) 79:1–12. doi: 10.1016/j.neures.2013.10.004
39. Medeiros WLGJ, Bandeira IP, Franzoi AEA, Brandāo WN, Santos Durāo A, Gonçalves MVM. Mast cells: A key component in the pathogenesis of Neuromyelitis Optica Spectrum Disorder? Immunobiology (2019) 224:706–9. doi: 10.1016/j.imbio.2019.05.010
40. Da Silva AP, Souza DG, Souza DO, Machado DC, Sato DK. Role of glutamatergic excitotoxicity in neuromyelitis optica spectrum disorders. Front Cell Neurosci. (2019) 13:142. doi: 10.3389/fncel.2019.00142
41. Gal RL, Vedula SS, Beck R. Corticosteroids for treating optic neuritis. Cochrane Database Syst Rev. (2015) 2015:Cd001430. doi: 10.1002/14651858.CD001430.pub4
42. Bonnan M, Valentino R, Olindo S, Mehdaoui H, Smadja D, Cabre P. Plasma exchange in severe spinal attacks associated with neuromyelitis optica spectrum disorder. Mult Scler. (2009) 15:487–92. doi: 10.1177/1352458508100837
43. Evers S. Facial pain: Overlapping syndromes. Cephalalgia. (2017) 37:705–13. doi: 10.1177/0333102417703761
44. Agasing AM, Wu Q, Khatri B, Borisow N, Ruprecht K, Brandt AU, et al. Transcriptomics and proteomics reveal a cooperation between interferon and T-helper 17 cells in neuromyelitis optica. Nat Commun. (2020) 11:2856. doi: 10.1038/s41467-020-16625-7
45. Guptarak J, Wanchoo S, Durham-Lee J, Wu Y, Zivadinovic D, Paulucci-Holthauzen A, et al. Inhibition of IL-6 signaling: a novel therapeutic approach to treating spinal cord injury pain. Pain. (2013) 154:1115–28. doi: 10.1016/j.pain.2013.03.026
46. Huang Q, Wang J, Zhou Y, Yang H, Wang Z, Yan Z, et al. Low-dose mycophenolate mofetil for treatment of neuromyelitis optica spectrum disorders: a prospective multicenter study in South China. Front Immunol. (2018) 9:2066. doi: 10.3389/fimmu.2018.02066
47. Scuteri D, Rombola L, Hamamura K, Sakurada T, Watanabe C, Sakurada S, et al. (2021). Is there a rational basis for cannabinoids research and development in ocular pain therapy? A systematic review of preclinical evidence. Biomed Pharmacother. (2021) 146:112505. doi: 10.1016/j.biopha.2021.112505
48. Barzegar M, Mirmosayyeb O, Nehzat N, Vaheb S, Shaygannejad V, Asgari N. Frequency of comorbidities in Neuromyelitis Optica spectrum disorder. Mult Scler Relat Disord. (2021) 48:102685. doi: 10.1016/j.msard.2020.102685
49. Chavarro VS, Mealy MA, Simpson A, Lacheta A, Pache F, Ruprecht K, et al. Insufficient treatment of severe depression in neuromyelitis optica spectrum disorder. Neurol Neuroimmunol Neuroinflamm. (2016) 3:e286. doi: 10.1212/NXI.0000000000000286
50. Mitchell ND, Baker GB. An update on the role of glutamate in the pathophysiology of depression. Acta Psychiatr Scand. (2010) 122:192–210. doi: 10.1111/j.1600-0447.2009.01529.x
51. Sałat K, Podkowa A, Malikowska N, Kern F, Pabel J, Wojcieszak E, et al. Novel, highly potent and in vivo active inhibitor of GABA transporter subtype 1 with anticonvulsant, anxiolytic, antidepressant and antinociceptive properties. Neuropharmacology (2017) 113:331–42.
52. Optic Neuritis Study Group. Multiple sclerosis risk after optic neuritis: final optic neuritis treatment trial follow-up. Arch Neurol. (2008) 65:727–32. doi: 10.1001/archneur.65.6.727
53. Beck RW, Cleary PA, Anderson MM, Keltner JL, Shults WT, et al. A randomized, controlled trial of corticosteroids in the treatment of acute optic neuritis. The Optic Neuritis Study Group. N Engl J Med. (1992) 326:581–8. doi: 10.1056/NEJM199202273260901
54. Wingerchuk DM, Lennon VA, Pittock SJ, Lucchinetti CF, Weinshenker BG. Revised diagnostic criteria for neuromyelitis optica. Neurology. (2006) 66:1485–9. doi: 10.1212/01.wnl.0000216139.44259.74
55. Annus Á, Bencsik K, Obál I, Kincses ZT, Tiszlavicz L, Höftberger R, et al. Paraneoplastic neuromyelitis optica spectrum disorder: A case report and review of the literature. J Clin Neurosci. (2018) 48:7–10. doi: 10.1016/j.jocn.2017.10.030
56. Beck RW, Cleary PA, Backlund JC. The course of visual recovery after optic neuritis: experience of the optic neuritis treatment trial. Ophthalmology (2020) 127:S174–81. doi: 10.1016/s0161-6420(94)31103-1
57. Barnett MH, Prineas JW, Buckland ME, Parratt JD, Pollard JD. Massive astrocyte destruction in neuromyelitis optica despite natalizumab therapy. Mult Scler. (2012) 18:108–12. doi: 10.1177/1352458511421185
58. Soelberg K, Jarius S, Skejoe H, Engberg H, Mehlsen JJ, Nilsson AC, et al. A population-based prospective study of optic neuritis. Mult Scler. (2017) 23:1893–901. doi: 10.1177/1352458517734070
59. Baik KW, Kim SH, Shin HY. Paraneoplastic Neuromyelitis Optica Associated with Lung Adenocarcinoma in a Young Woman. J Clin Neurol. (2018) 14:246–7. doi: 10.3988/jcn.2018.14.2.246
60. Jarius S, Ruprecht K, Wildemann B, Kuempfel T, Ringelstein M, Geis C, et al. Contrasting disease patterns in seropositive and seronegative neuromyelitis optica: a multicentre study of 175 patients. J Neuroinflammation. (2012) 9:14. doi: 10.1186/1742-2094-9-14
61. Pereira WL, Reiche EM, Kallaur AP, Kaimen-Maciel DR. Epidemiological, clinical, and immunological characteristics of neuromyelitis optica: A review. J Neurol Sci. (2015) 355:7–17. doi: 10.1016/j.jns.2015.05.034
62. Wingerchuk DM, Banwell B, Bennett JL, Cabre P, Carroll W, Chitnis T, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. (2015) 85:177–89. doi: 10.1212/WNL.0000000000001729
63. Shahmohammadi S, Doosti R, Shahmohammadi A, Mohammadianinejad SE, Sahraian MA, Azimi AR, et al. Autoimmune diseases associated with neuromyelitis optica spectrum disorders: a literature review. Mult Scler Relat Disord. (2019) 27:350–63. doi: 10.1016/j.msard.2018.11.008
64. Chen JJ, Flanagan EP, Jitprapaikulsan J, López-Chiriboga ASS, Fryer JP, Leavitt JA, et al. Myelin oligodendrocyte glycoprotein antibody-positive optic neuritis: clinical characteristics, radiologic clues, and outcome. Am J Ophthalmol. (2018) 195:8–15. doi: 10.1016/j.ajo.2018.07.020
65. Chen JJ, Tobin WO, Majed M, Jitprapaikulsan J, Fryer JP, Leavitt JA, et al. Prevalence of Myelin Oligodendrocyte Glycoprotein and Aquaporin-4-IgG in Patients in the Optic Neuritis Treatment Trial. JAMA Ophthalmol. (2018) 136:419–22. doi: 10.1001/jamaophthalmol.2017.6757
66. Jitprapaikulsan J, Chen JJ, Flanagan EP, Tobin WO, Fryer JP, Weinshenker BG, et al. Aquaporin-4 and Myelin Oligodendrocyte Glycoprotein Autoantibody Status Predict Outcome of Recurrent Optic Neuritis. Ophthalmology. (2018) 125:1628–37. doi: 10.1016/j.ophtha.2018.03.041
67. Ramanathan S, Mohammad S, Tantsis E, Nguyen TK, Merheb V, Fung VSC, et al. Clinical course, therapeutic responses and outcomes in relapsing MOG antibody-associated demyelination. J Neurol Neurosurg Psychiatry. (2018) 89:127–37. doi: 10.1136/jnnp-2017-316880
68. Sudhakar P, Kedar S, Berger J. A Retrospective Study of Chronic Relapsing Inflammatory Optic Neuropathy (CRION)(P6. 293). (2014). AAN Enterprises.
69. Webb LM, Chen JJ, Aksamit AJ, Bhattacharyya S, Chwalisz BK, Balaban D, et al. A multi-center case series of sarcoid optic neuropathy. J Neurol Sci. (2021) 420:117282. doi: 10.1016/j.jns.2020.117282
70. Boomer JA, Siatkowski RM. Optic neuritis in adults and children. Semin Ophthalmol. (2003) 18:174–80. doi: 10.1080/08820530390895172
71. Mikaeloff Y, Suissa S, Vallée L, Lubetzki C, Ponsot G, Confavreux C, et al. First episode of acute CNS inflammatory demyelination in childhood: prognostic factors for multiple sclerosis and disability. J Pediatr. (2004) 144:246–52. doi: 10.1016/j.jpeds.2003.10.056
72. Young NP, Weinshenker BG, Lucchinetti CF. Acute disseminated encephalomyelitis: current understanding and controversies. Semin Neurol. (2008) 28:84–94. doi: 10.1055/s-2007-1019130
73. Cakmakli G, Kurne A, Güven A, Serdaroglu A, Topaloglu H, Teber S, et al. Childhood optic neuritis: the pediatric neurologist's perspective. Eur J Paediatr Neurol. (2009) 13:452–7. doi: 10.1016/j.ejpn.2008.09.003
74. Matsushita T, Masaki K, Isobe N, Sato S, Yamamoto K, Nakamura Y, et al. Genetic factors for susceptibility to and manifestations of neuromyelitis optica. Ann Clin Transl Neurol. (2020) 7:2082–93. doi: 10.1002/acn3.51147
75. Solaro C, Trabucco E, Messmer Uccelli M. Pain and multiple sclerosis: pathophysiology and treatment. Curr Neurol Neurosci Rep. (2013) 13:320. doi: 10.1007/s11910-012-0320-5
76. Heitmann H, Biberacher V, Tiemann L, Buck D, Loleit V, Selter RC, et al. Prevalence of neuropathic pain in early multiple sclerosis. Mult Scler. (2016) 22:1224–30. doi: 10.1177/1352458515613643
77. Shiao R, Lee-Kubli CA. Neuropathic Pain After Spinal Cord Injury: Challenges and Research Perspectives. Neurotherapeutics. (2018) 15:635–53. doi: 10.1007/s13311-018-0633-4
78. Zhao S, Mutch K, Elsone L, Nurmikko T, Jacob A. Neuropathic pain in neuromyelitis optica affects activities of daily living and quality of life. Mult Scler. (2014) 20:1658–61. doi: 10.1177/1352458514522103
79. Wilhelm H, Schabet M. The diagnosis and treatment of optic neuritis. Dtsch Arztebl Int. (2015) 112:616–25. doi: 10.3238/arztebl.2015.0616
80. Langer-Gould A, Brara SM, Beaber BE, Zhang JL. The incidence of clinically isolated syndrome in a multi-ethnic cohort. J Neurol. (2014) 261:1349–55. doi: 10.1007/s00415-014-7349-0
81. Ishikawa H, Kezuka T, Shikishima K, Yamagami A, Hiraoka M, Chuman H, et al. Epidemiologic and Clinical Characteristics of Optic Neuritis in Japan. Ophthalmology. (2019) 126:1385–98. doi: 10.1016/j.ophtha.2019.04.042
82. Choi J, Kim SJ, Chang JW, Kim JH, Yu YS. Clinical characteristics of optic neuritis in Koreans greater than 50 years of age. Korean J Ophthalmol. (2012) 26:111–5. doi: 10.3341/kjo.2012.26.2.111
83. D'Amico D, La Mantia L, Rigamonti A, Usai S, Mascoli N, Milanese C, et al. Prevalence of primary headaches in people with multiple sclerosis. Cephalalgia. (2004) 24:980–4. doi: 10.1111/j.1468-2982.2004.00790.x
84. Gee JR, Chang J, Dublin AB, Vijayan N. The association of brainstem lesions with migraineΓÇÉlike headache: an imaging study of multiple sclerosis. Headache. 45:670–7. doi: 10.1111/j.1526-4610.2005.05136.x
85. Tortorella P, Rocca MA, Colombo B, Annovazzi P, Comi G, Filippi M. Assessment of MRI abnormalities of the brainstem from patients with migraine and multiple sclerosis. (2006) 244:137–41. doi: 10.1016/j.jns.2006.01.015
86. Villani V, Prosperini L, Ciuffoli A, Pizzolato R, Salvetti M, Pozzilli C, et al. Primary headache and multiple sclerosis: preliminary results of a prospective study. 29(1). (2008) 146–8. doi: 10.1007/s10072-008-0908-3
87. Carnero Contentti E, Leguizamón F, Hryb JP, Celso J, Pace JL, Ferrari J, et al. Neuromyelitis optica: association with paroxysmal painful tonic spasms. Neurologia. (2016) 31:511–5. doi: 10.1016/j.nrleng.2014.12.015
88. Kanamori Y, Nakashima I, Takai Y, Nishiyama S, Kuroda H, Takahashi T, et al. Pain in neuromyelitis optica and its effect on quality of life: a cross-sectional study. (2011) 77:652–8. doi: 10.1212/WNL.0b013e318229e694
89. Qian P, Lancia S, Alvarez E, Klawiter EC, Cross AH, Naismith RTJ A. o. n. (2012). Association of neuromyelitis optica with severe and intractable pain. Arch Neurol. 69:1482–7. doi: 10.1001/archneurol.2012.768
90. Kremer L, Mealy M, Jacob A, Nakashima I, Cabre P, Bigi S, et al. Brainstem manifestations in neuromyelitis optica: a multicenter study of 258 patients. Mult Scler. (2014) 20:843–7. doi: 10.1177/1352458513507822
91. Choi SI, Lee YJ, Do Wan Kim J. A case of neuromyelitis optica misdiagnosed as cervicogenic headache. Korean J Pain. (2014) 27:77. doi: 10.3344/kjp.2014.27.1.77
92. Rubio-Ochoa J, Benítez-Martínez J, Lluch E, Santacruz-Zaragozá S, Gómez-Contreras P, et al. Physical examination tests for screening and diagnosis of cervicogenic headache: A systematic review. Man Ther. (2016) 21:35-40. doi: 10.1016/j.math.2015.09.008
93. Mathew T, Nadimpally US, Sarma G, Nadig RJMS, Disorders R. Trigeminal autonomic cephalalgia as a presenting feature of Neuromyelitis Optica: “A rare combination of two uncommon disorders”. Mult Scler Relat Disord. (2016) 6:73–4. doi: 10.1016/j.msard.2016.01.006
94. Olesen J. The international classification of headache disorders. Headache. (2008). 48:691–3. doi: 10.1111/j.1526-4610.2008.01121.x
95. Fujikura M, Yokokawa K, Shizukawa H, Shimohama S. A case of neuromyelitis optica presenting marked pleocytosis and hypoglycorrhachia. Rinsho Shinkeigaku. (2016) 56:569–72. doi: 10.5692/clinicalneurol.cn-000753
96. Dib-Hajj SD, Cummins TR, Black JA, Waxman SG. Sodium channels in normal and pathological pain. Annu Rev Neurosci. (2010) 33:325–47. doi: 10.1146/annurev-neuro-060909-153234
97. Evangelopoulos ME, Andreadou E, Koutsis G, Koutoulidis V, Anagnostouli M, Katsika P, et al. Treatment of neuromyelitis optica and neuromyelitis optica spectrum disorders with rituximab using a maintenance treatment regimen and close CD19 B cell monitoring. A six-year follow-up J Neurol Sci. (2017) 372:92–6. doi: 10.1016/j.jns.2016.11.016
Keywords: pain, optic neuritis, mechanism, treatment, multiple sclerosis, neuromyelitis optica spectrum disorder, myelin oligodendrocyte glycoprotein associated disease
Citation: Yang X, Li X, Lai M, Wang J, Tan S and Chan HH-l (2022) Pain Symptoms in Optic Neuritis. Front. Pain Res. 3:865032. doi: 10.3389/fpain.2022.865032
Received: 29 January 2022; Accepted: 21 March 2022;
Published: 14 April 2022.
Edited by:
Tingjun Chen, Mayo Clinic, United StatesReviewed by:
Qian Wang, Sichuan University, ChinaCopyright © 2022 Yang, Li, Lai, Wang, Tan and Chan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shaoying Tan, c2hhb3lpbmcudGFuQHBvbHl1LmVkdS5oaw==; Henry Ho-lung Chan, aGVucnlobC5jaGFuQHBvbHl1LmVkdS5oaw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.