- 1Department of Community and Population Health, University of Tennessee Health Science Center, College of Nursing, Memphis, TN, United States
- 2Department of Biobehavioral Nursing Science, University of Florida, College of Nursing, Gainesville, FL, United States
- 3Department of Pathology and Laboratory Medicine, Childrens Hospital of Los Angeles, Los Angeles, CA, United States
- 4Department of Molecular Genetics and Microbiology, University of Florida, College of Medicine, Gainesville, FL, United States
- 5University of Florida Genetics Institute, Gainesville, FL, United States
- 6College of Dentistry, University of Florida, Gainesville, FL, United States
- 7Pain Research and Intervention Center of Excellence (PRICE), University of Florida, Gainesville, FL, United States
- 8Department of Medicine, University of Illinois at Chicago, College of Medicine, Chicago, IL, United States
- 9Department of Pharmaceutical Sciences, Neurology and Bioengineering, University of Illinois College of Pharmacy, Chicago, IL, United States
- 10Medical Service, Jesse Brown VA Medical Center, Chicago, IL, United States
Aim: In patients with sickle cell disease (SCD), negative physical and emotional experiences result from intense chronic and acute pain episodes, but factors underlying these, and their interactions, are not well understood. The arginine vasopressin receptor 1a gene (AVPR1A) single nucleotide polymorphism rs10877969 has been previously associated with aspects of acute pain and stress related pain. In this study, we tested for associations between this SNP, thermal and pressure pain thresholds, clinical pain, and stress in people with SCD.
Methods: 150 adults enrolled with SCD completed pain intensity measures (Average Pain Intensity, API) and the Perceived Stress Questionnaire (PSQ). Thermal and pressure pain threshold data were available from quantitative sensory testing (QST), and rs10877969 genotypes were obtained.
Results: In models adjusted for age and gender, between rs10877969 genotypes, we observed no significant differences in thermal (cold, p = 0.66; heat, p = 0.91) and mechanical (pressure, p = 0.33) pain thresholds. The association of rs10877969 with API (p = 0.09) was borderline, but non-significant with PSQ (p = 0.51). The correlation between clinical pain and environmental stress was significant, r = 0.18, p = 0.024, however, the interaction of genotype and PSQ was not significant (p = 0.63).
Conclusion: Clinical and experimental pain were not significantly associated with the rs10877969 genotype. The rs10877969 genotype did not moderate the correlation between environmental stress and clinical pain in this population. However, a trend toward a protective T allele effect on average pain rating in SCD warrants future exploration of this SNP/gene in SCD.
1. Introduction
Biopsychosocial factors known to contribute to multiple pain conditions have not yet been studied in sickle cell disease (SCD), a genetic condition leading to microvasculature occlusion with resulting chronic and acute pain episodes (1–3). This absence of information on mechanisms that exacerbate the unpredictability of the potency and types of SCD-related pain is one of the major barriers impacting the ability to adequately address pain in this population. Pain results from multiple biological and psychological factors that have been investigated in other pain-related conditions. This includes genetic polymorphisms. The arginine vasopressin receptor (AVPR1A) single nucleotide polymorphism (SNP, rs10877969, C > T) was implicated in previous studies of pain not related to SCD. The purpose of this study was to determine whether pain and stress in adults with SCD is moderated by the rs10877969 genotype.
Pain phenotyping using quantitative sensory testing (QST) had been reported in many previous studies and has been shown to be safe for use in individuals with SCD with evidence of sensitization (4–7). However, no one has examined QST pain thresholds in association with the AVPR1A rs10877969 SNP in SCD.
In healthy individuals, previous work found a male-specific interaction of rs10877969 with stress, and observed that an endogenous analgesia mechanism can be activated by vasopressin if it has not already been activated by stress (8, 9). A moderate genetic (AVPR1A SNPs, including rs10877969) and psychological interaction was identified in studies of a model of exercise induced muscle injury (10). This SNP has also been found associated with autism and is theorized to affect gene expression because of its position in the AVPR1A promoter (11).
In patients living with SCD, harmful psychological effects from emotional stress have been shown to trigger or increase pain (12, 13). Recent studies have shown that activities such as relaxation and stress relieving modalities such as hypnosis (13), attention control with music, and guided relaxation in patients with SCD correlate significantly with reduced pain and stress (14–16). It has been shown in patients with SCD that anticipation of painful experiences enhances microvascular responses in blood flow, parasympathetic withdrawal, and sympathetic activation (13, 14, 17–19).
In our pilot study of the relationship between rs10877967 and self-reported pain in adults with SCD, we found that acute care utilization events and spontaneous reports of stress cited as an aggravator of pain were associated with this genotype (p = .04 and p = .002, respectively) (20). In particular, the CC genotype was associated with not spontaneously reporting stress as a pain aggravator (20). In this study, we extended those findings in a new sample of adults with SCD by examining QST pain thresholds and their psychosocial interactions on variables such as stress and pain. We hypothesized that thermal and mechanical pain thresholds would differ by rs10877969 genotype, and that it would influence the correlation between environmental stress and SCD clinical pain.
2. Methods and procedures
The dataset analyzed was generated from a SCD study whose detailed measures were published in 2020 (7, 21), briefly summarized here. Inclusion criteria: age ≥18 years; African ancestry; confirmed SCD diagnosis; SCD-related pain (≥3 on 1–10 scale) within previous 12 months; chronic SCD pain (>0 on scale 1–10 for at least half of the days over the previous 3 months); ability to speak and read standard English; ability to understand and sign the consent form. Exclusion criteria: legally blind; physically unable to complete the study measures; and/or had a confirmed diagnosis of diabetes mellitus or polyneuropathy. Patients were not excluded if they were using pain medications (opioid, non-opioid, or adjuvant).
Demographic data included: age, gender, ethnicity, race, marital status, education, income, partner, and type of sickle hemoglobin (HbS, HbC, or other). Participants provided blood samples and completed two questionnaires: PAINReportIt, providing information about recent and current pain [and its derivative Average Pain Intensity (API)] (22), and the Perceived Stress Questionnaire (PSQ) (23). In addition, using the left and right anterior forearm, somatosensory threshold data were obtained from Quantitative Sensory Testing (QST, thermal and mechanical pressure modalities). Methods were previously reported QST, body sites, and questionnaires (7, 21).
2.1. Measures
2.1.1. Quantitative sensory testing
Quantitative sensory testing is a well validated battery of tests used at assess the function of the somatosensory system (24). Responses to sensations are tested and include, but are not limited to cold, hot, and pressure (25). QST was used to measure thermal and mechanical pain thresholds and was consistent with the European Federation of Neurological Sciences (EFNS) protocol (25). For the purposes of this study, the 30 × 30 mm thermode was placed on the skin and used to deliver standardized stimuli (26). The anterior forearm was used as the primary reference site (non-painful site). In the case where the anterior forearm was a painful, we selected the contralateral side for the non-painful site, if possible. The non-painful, reference site, was also used at the practice trial site (7).
Thermal pain thresholds (Hot and cold) were measured using the Medoc TSA -II sensory testing system. This is a computer-controlled device that generates and documents repeatable thermal stimuli that allows for assessment of sensory nerve function via pain thresholds, heat pain threshold (HPTh) and cold pain threshold (CPTh) (26, 27). The limits protocol was employed to avoid tissue damage. The cutoff temperature was 0°C for hold and 50°C for heat for all trials. The baseline temperature was 32°C. The temperature increased for heat and decreased for cold at a rate of 0.5°C per second until the participant pressed the button to indicate when pain was first felt. Thermal testing was stopped when the participant reported pain, at which time the participants verbally indicated the intensity of the pain they experienced. At that time, the participant was asked to assign the pain sensation a number on a 0–10 pain intensity scale.
Mechanical pain threshold was measured using standardized, calibrated von Frey filaments. Seven filaments were used and the sizes (thicknesses): 3.84 (0.6 g), 4.17 (1.4 g), 4.56 (4.0 g), 4.74 (6.0 g), 5.07 (26 g), and 5.88 (60 g), these were consistent with the EFNS protocol. The filament was perpendicularly touched to the skin until it obtained a s-shaped bend. The contact time to the skin was approximately 3 s. Each trial was approximately 2 inches from the previous trial up the forearm. With each trial, the filament thickness was increased. Testing was stopped when the participant reported feeling pain. At that time, the participant was asked to assign the pain sensation a number on a 0–10 pain intensity scale.
2.1.2. PAINReportIt
PAINReportIt®, a valid and reliable self-reported pain assessment tool, is an internet-based version of the McGill Pain Questionnaire (MPQ) (28–30) and has been validated in patients living with SCD (31). This tool examines pain outcome measures with limited burden to the participant (32). The device is an interactive program that has a touch screen interface. It is used for pain assessment that may be self-administered by the patient or by a trained care provider or an assistant. The process takes approximately 15 min to complete. In addition to pain information, demographic variables are captured by the program (29). These variables included: diagnosis, age, gender, ethnicity, religion, education, occupation, income, substance use history, concurrent illnesses, 9-item medical history checklist related to SCD, and items examining previous pain experiences, which include worst toothache, headache and stomachache. Recalling worst pain experiences allows the participant recall the magnitude of common pain experiences and compare them to pain from the QST testing.
2.1.3. Average pain intensity
Average Pain intensity (API) is taken as the mean of PAINReportIt's three 0 to 10 Pain Intensity Number Scales (PINS) (33, 34): (1) current pain, (2) least in the past 24 h, and (3) worst pain in the past 24 h. The PINS measured the patient's perception of the level of pain now (34) and it provides ratio level data (31, 33, 34). The patient designates the pain as a number between 0 and 10, where 0 is “no pain” and 10 is “pain as bad as it could be” (34, 35). Concurrent (r = 0.80 to 0.89) and construct validity (34, 36) had been reported for this tool and standardized instructions (33, 34) are available.
2.1.4. Perceived stress questionnaire
The Perceived Stress Questionnaire (PSQ) is a validated 30-question instrument used to assess stressful life events (37). This tool takes about 15 min to complete. Internal consistency (α = 0.90 to 0.92) and test re-test reliability (r = 0.82) have been evaluated for this scale (37). This scale evaluates how frequently the participant experiences stress-related feelings (37). Responses range from “almost never” (1) to “usually” (4). Higher scores represent greater stress (37). The PSQ Index is generated by (raw score-30)/90, resulting in final scores ranging from 0 to 1 (37).
Participant leukocyte DNA genotype data were generated using the 800,000-SNP Axiom Precision Medicine Research Array. Because the SNP of interest, rs10877969, is not on the PMRA array, we imputed this genotype with high confidence using the Michigan Imputation Server (https://imputationserver.sph.umich.edu) with imputation score R2 > 0.8. Under an additive genetic model with a sample size of 150, we have 84% power to detect a significant association between SNP and pain score(s) with effect size of 0.06 defined by Cohen (1988) at alpha 0.05 level (38).
We used the statistical package R to interrogate the association of demographic variables and SNP genotype with thermal and pressure pain thresholds, clinical pain, and environmental stress. The rs10877969 genotype was coded based on the number of C alleles based on previous reports (e.g., CC = 2, CT = 1, TT = 0). To better represent the data by normal distribution, we log transformed thermal and pressure pain thresholds, clinical pain, and environmental pain variables. We used robust linear regression to attenuate the impact of model fitting by high influential points. The statistical significance cut-off was p < 0.05.
3. Results
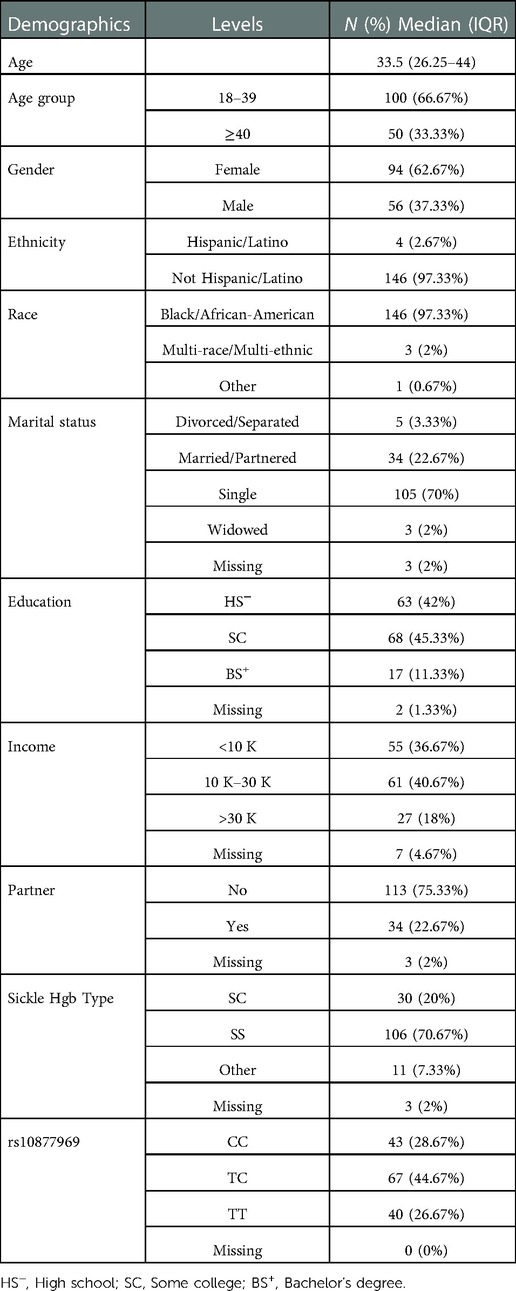
A total of 186 evaluable SCD patients met inclusion criteria for the parent study, and of those, 150 participants completed the PSQ. The participants were mainly Black or African American (97.3%), single (70%), with some college or high school education (87%), income ≤30 K (77%), age 18–39 (66.7%) and female (62.7%). The majority of participants had SS Hgb type (70.7%), with others being heterozygous with hemoglobin S and C (SC Hgb). Demographics along with sickle cell types are also provided in Table 1. In this cohort, the rs10877969 genotype frequency distributions were: CC n = 43 (28%), CT n = 67 (44%), TT n = 40 (26%) (Table 1). The analytic sample included 150 participants with completed genotype and PSQ data and 148 participants from whom we also had QST data.
3.1. Rs10877969 association with thermal pain thresholds
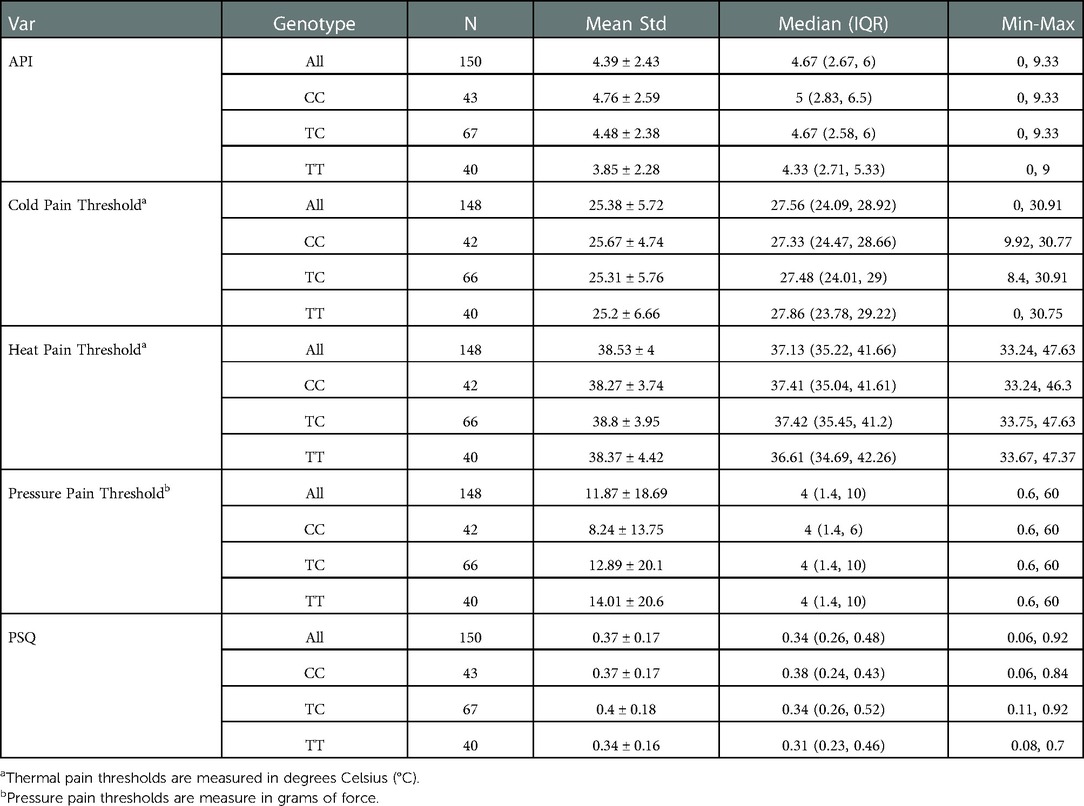
We examined the association of demographic variables and rs10877969 SNP genotype with thermal pain thresholds. The mean, standard deviation, max and min for thermal pain thresholds for the 148 participants are as follows: cold pain threshold 25.38 (5.72), [0–30.91], heat pain threshold 38.53 (4.00), [33.24–47.63] (Table 2). Robust linear regressions adjusting for age and gender indicated that neither cold nor heat pain thresholds were significantly associated with rs10877969 genotype (p = 0.66, and p = 0.91, respectively) (Table 3).
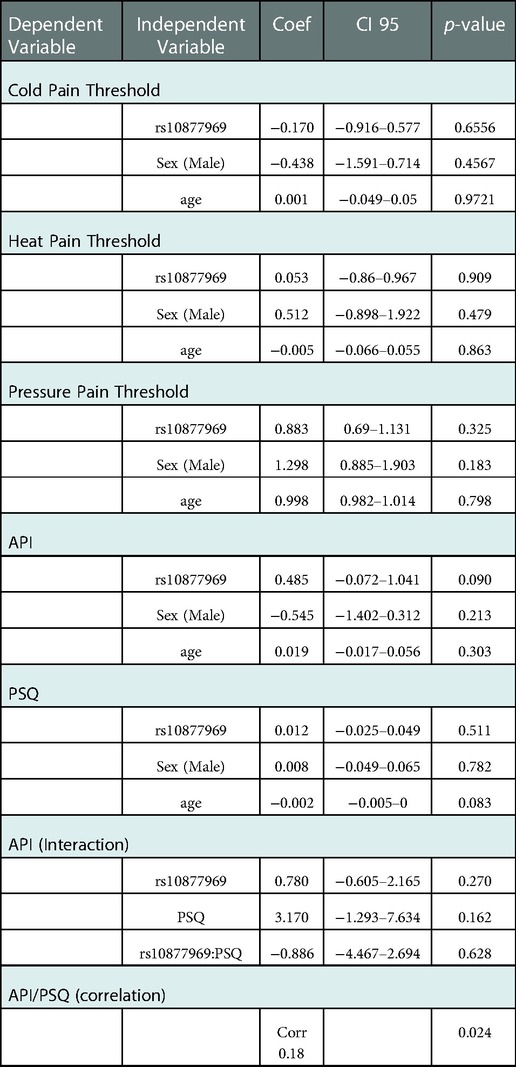
Table 3. Experimental and clinical pain associations with rs10877969 adjusting for relevant variables.
3.2. Rs10877969 association with mechanical pain thresholds
The mean, standard deviation, max and min for mechanical pain thresholds are as follows: N = 148, 11.87 (18.69), [0.6–60] (Table 2). In robust linear regression models adjusted for age and gender, we observed no significant association between mechanical pain thresholds and rs10877969 genotype (p = 0.33) (Table 3).
3.3. Rs10877969 association with clinical pain and environmental stress
The mean, standard deviation, max and min for clinical pain and environmental stress in the full cohort of 150 are as follows: clinical pain, 4.39 (2.43), [0–9.33] and environmental stress, 0.37(0.17), [0.06–0.92] (Table 2). The Median (Inter Quartile Range, IQR) for API was: CC 5 (2.83, 6.5), CT 4.67 (2.58, 6), TT 4.33 (2.71, 5.33); and PSQ: CC 0.38 (0.24, 0.43), CT 0.34 (0.26, 0.52), TT 0.31 (0.23, 0.46) (Table 2). In robust linear regression models adjusting for age and sex, environmental stress (coef = 0.012; p = 0.51) was not significantly associated with rs10877969 (Table 3). However, there was a trend toward association of clinical pain (API) and genotype (coef 0.485, p = 0.09) (Table 3). We fitted a robust linear regression model of API with rs10877969, PSQ and the interaction term. We did not observe significant interaction of rs10877969 and PSQ in association with API (−0.886, p = 0.63) (Table 3). However, the correlation between clinical pain and environmental stress was significant, r = 0.18, p = 0.024.
Additionally, an exploratory analysis was done examining a male specific interaction. In our sample we examined the interaction of sex, SNP genotype and PSQ; sex, SNP genotype and API. No significant interactions were found.
4. Discussion
In this analysis of data from 150 adults with SCD, we evaluated association of clinical and experimental pain and environmental stress with the rs10877969 SNP of the AVPR1A gene. We found that experimental pain as indicated by thermal and mechanical pain thresholds, clinical pain, and environmental stress were not significantly associated with the rs10877969 genotype, although the p-value for genotype vs. API was 0.09. The rs10877969 SNP did not moderate the association between clinical pain (API) and stress (PSQ) in this population.
There have been variable conclusions in reports of stress related pain in patients with SCD, such as pain resulting from the stress of perceived injustice (23), and mental stress being associated with vasoconstrictions in patients with SCD and healthy controls (19). Here we found a correlation between clinical pain (API) and the PSQ measure of stress in SCD. We previously reported that individuals with SCD and rs10877969 CC genotype were less like to cite stress as aggravating their pain compared to the other two genotypes (20). Utilization and composite pain index were proxies for acute pain and chronic pain (respectively). We did not measure API in the previous study. CT was higher for acute pain (6.1); (CC = 3.9, TT 3.3). The genotype level was measured in the previous study, and we did not control for age and sex. In the current study CC is higher for API, and allele level was measured (TT = 0, CT = 1, CC = 2). Chronic clinical pain is associated with the CC allele whereas a proxy for acute pain is associated with the CT allele, and TT has the lowest score in both samples.Currently, there is inconclusive evidence for the contribution of the rs10877969 CC genotype to average pain intensity. The positive association between C allele and API, though statistically not significant in this sample, warrants further study. The current findings in environmental stress are in contrast to that of our previous study (20). It is important to note, however, that the PSQ is a different measure than spontaneously citing stress as a pain aggravator. In our pilot study we examined the relationship between the SNP, self-reported pain, stress, and acute pain. In both studies, the C allele is associated with more pain. Previous studies showed that rs10877969 was related to indicators of environmental stress and acute pain (8, 9, 20), however, our findings in this cohort differed from those other studies. Individuals in this study appeared not to be very stressed. PSQ queries stressful feelings over the past month (37) on a 0 to 1 scale. At the twenty-fifth percentile, the average stress score was 0.26 and at the seventy-fifty percentile, the average stress score was 0.48. It is also possible that a different stress scale may be better suited for this population whose disease process is variable. We found that rs10877969 did not influence the weak but significant association between pain and stress in this sample of patients with SCD.
4.1. Study limitations
Since reports of stress are not consistently associated with the rs10877969 genotype, the precise measurement tool may be important to properly interrogate this relationship. Thus, PSQ may not be the appropriate tool to measure stress in this population or investigators may need to sample in ways to include individuals with higher stress levels in future studies. Additionally, use of antidepressants was not an exclusion criterion in this study. We cannot rule out if PSQ scores were impacted. Lastly, a larger sample size testing multiple measurements is needed for future studies, to fully answer the question about this SNP's role in SCD.
5. Conclusion
In this study of AVPR1A rs1087796 in a cohort of individuals with SCD, we examined a direct measure of stress and measures of experimental and clinical pain. Although none of the associations were significant, the trend toward a protective T allele effect on pain in SCD warrants future exploration of this SNP/gene in SCD, perhaps by analyzing more individuals, perhaps starting with the ends of the SCD pain spectrum. As this SNP is located in the promoter region of the gene, further research is warranted to examine the functional impact of this polymorphism on gene transcription and protein production, to understand underlying biological impact. Analysis of additional AVPR1A SNPs, particularly in linkage disequilibrium with rs10877969, in a larger SCD cohort, could also shed light on the involvement of this gene in SCD pain.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by IRBs of the following institutions: University of Illinois at Chicago, Chicago, IL; University of Florida, Gainesville, FL; University of Tennessee Health Science Center, Memphis TN. The patients/participants provided their written informed consent to participate in this study.
Author contributions
KLP-R, DJW, MRW, YC-A, and YY contributed to conception and design of the study. YY, and SC organized the database. YY, SC, and XC performed the statistical analysis. KLP-R wrote the first draft of the manuscript. KLP-R, DJW, MRW, SC, and XC wrote sections of the manuscript. All authors including REM and ZJW contributed to manuscript revision, read, and approved the submitted version. All authors contributed to the article and approved the submitted version.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: National Institutes of Health, National Heart Lung & Blood Institute (NHLBI) and the National Institute on Aging (NIA) grant numbers. The data collection was supported by R01HL124945, 1R01HL124945S1. Keesha Roach was supported by T32AG049673 Integrative and Multidisciplinary Pain and K01HL153210. DW is supported by U54CA233444 from the National Cancer Institute (NCI). The manuscript contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH, NHLBI, NIA, NCI, or Veteran's Administration. The authors declare no conflict of interest.
Acknowledgments
We thank the patients of the University of Illinois Chicago Adult Sickle Cell Clinic. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH, the NHLBI, or the NCI. The final peer-review manuscript is subject to the NIH Public Access Policy.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Roach KL, Hershberger PE, Rutherford JN, Molokie RE, Wang ZJ, Wilkie DJ. The AVPR1A gene and its single nucleotide polymorphism rs10877969: a literature review of associations with health conditions and pain. Pain Manag Nurs. (2018) 19(4):430–44. doi: 10.1016/j.pmn.2018.01.003
2. Ballas SK, Darbari DS. Review/overview of pain in sickle cell disease. Complement Ther Med. (2020) 49:102327. doi: 10.1016/j.ctim.2020.102327
3. Gupta K, Jahagirdar O, Gupta K. Targeting pain at its source in sickle cell disease. Am J Physiol Regul Integr Comp Physiol. (2018) 315(1):R104–r12. doi: 10.1152/ajpregu.00021.2018
4. Campbell CM, Carroll CP, Kiley K, Han D, Haywood C Jr, Lanzkron S, et al. Quantitative sensory testing and pain-evoked cytokine reactivity: comparison of patients with sickle cell disease to healthy matched controls. Pain. (2015) 1:949–56. doi: 10.1097/j.pain.0000000000000473
5. Campbell CM, Moscou-Jackson G, Carroll CP, Kiley K, Haywood C Jr, Lanzkron S, et al. An evaluation of central sensitization in patients with sickle cell disease. J Pain. (2016) 17(5):617–27. doi: 10.1016/j.jpain.2016.01.475
6. Ezenwa MO, Molokie RE, Wang ZJ, Yao Y, Suarez ML, Pullum C, et al. Safety and utility of quantitative sensory testing among adults with sickle cell disease: indicators of neuropathic pain? Pain Pract. (2016) 16(3):282–93. doi: 10.1111/papr.12279
7. Molokie RE, Wang ZJ, Yao Y, Powell-Roach KL, Schlaeger JM, Suarez ML, et al. Sensitivities to thermal and mechanical stimuli: adults with sickle cell disease compared to healthy, pain-free African American controls. J Painety. (2020) 21(9–10):957–67. doi: 10.1016/j.jpain.2019.11.002
8. Mogil JS, Sorge RE, LaCroix-Fralish ML, Smith SB, Fortin A, Sotocinal SG, et al. Pain sensitivity and vasopressin analgesia are mediated by a gene-sex-environment interaction. Nat Neurosci. (2011) 14(12):1569–73. doi: 10.1038/nn.2941
9. Wiltshire T, Maixner W, Diatchenko L. Relax, you won’t feel the pain. Nat Neurosci. (2011) 14(12):1496–7. doi: 10.1038/nn.2987
10. George SZ, Parr JJ, Wallace MR, Wu SS, Borsa PA, Dai Y, et al. Biopsychosocial influence on exercise-induced injury: genetic and psychological combinations are predictive of shoulder pain phenotypes. J Pain. (2014) 15(1):68–80. doi: 10.1016/j.jpain.2013.09.012
11. Yang SY, Cho SC, Yoo HJ, Cho IH, Park M, Kim BN, et al. Association study between single nucleotide polymorphisms in promoter region of AVPR1A and Korean autism spectrum disorders. Neurosci Lett. (2010) 479(3):197–200. doi: 10.1016/j.neulet.2010.05.050
12. Gil KM, Carson JW, Porter LS, Scipio C, Bediako SM, Orringer E. Daily mood and stress predict pain, health care use, and work activity in African American adults with sickle-cell disease. Health psychology: official journal of the Division of Health Psychology. Am Psychol Assoc. (2004) 23(3):267–74. doi: 10.1037/0278-6133.23.3.267
13. Martin SR, Shah P, Denton C, Zeltzer LK, Veluswamy S, Khoo MCK, et al. Autonomically-mediated decrease in microvascular blood flow due to mental stress and pain in sickle cell disease: a target for neuromodulatory interventions. Complement Ther Med. (2020) 49:102334. doi: 10.1016/j.ctim.2020.102334
14. Thomas LS, Stephenson N, Swanson M, Jesse DE, Brown S. A pilot study: the effect of healing touch on anxiety, stress, pain, pain medication usage, and physiological measures in hospitalized sickle cell disease adults experiencing a vaso-occlusive pain episode. J Holist Nurs. (2013) 31(4):234–47. doi: 10.1177/0898010113491631
15. Ezenwa MO, Molokie RE, Wang ZJ, Suarez ML, Yao Y, Wilkie DJ. Satisfied or not satisfied: pain experiences of patients with sickle cell disease. J Adv Nurs. (2016) 72(6):1398–408. doi: 10.1111/jan.12678
16. Mu PF, Chen YC, Cheng SC. The effectiveness of non-pharmacological pain management in relieving chronic pain for children and adolescents. JBI Libr Syst Rev. (2009) 7(34):1489–543. doi: 10.11124/jbisrir-2009-215
17. Coates TD, Chalacheva P, Zeltzer L, Khoo MCK. Autonomic nervous system involvement in sickle cell disease. Clin Hemorheol Microcirc. (2018) 68(2–3):251–62. doi: 10.3233/CH-189011
18. Khaleel M, Puliyel M, Shah P, Sunwoo J, Kato RM, Chalacheva P, et al. Individuals with sickle cell disease have a significantly greater vasoconstriction response to thermal pain than controls and have significant vasoconstriction in response to anticipation of pain. Am J Hematol. (2017) 92(11):1137–45. doi: 10.1002/ajh.24858
19. Shah P, Khaleel M, Thuptimdang W, Sunwoo J, Veluswamy S, Chalacheva P, et al. Mental stress causes vasoconstriction in sickle cell disease and normal controls. Haematologica. (2020) 105(1):83–90. doi: 10.3324/haematol.2018.211391
20. Powell-Roach KL, Yao Y, Jhun EH, He Y, Suarez ML, Ezenwa MO, et al. Vasopressin SNP pain factors and stress in sickle cell disease. PLoS One. (2019) 14(11):e0224886. doi: 10.1371/journal.pone.0224886
21. Powell-Roach KL, Yao Y, Rutherford JN, Schlaeger JM, Patil CL, Suarez ML, et al. Thermal and mechanical quantitative sensorytesting values among healthy African Americanadults. J Pain Res. (2019) 12:2511–27. doi: 10.2147/JPR.S211855
22. Powell-Roach K, Yao Y, Ezenwa MO, Schlaeger JM, Suarez ML, Molokie RE, et al. Neuropathic pain screening: construct validity in patients with sickle cell disease. West J Nurs Res. (2020) 42(2):125–30. doi: 10.1177/0193945919836446
23. Ezenwa MO, Molokie RE, Wilkie DJ, Suarez ML, Yao Y. Perceived injustice predicts stress and pain in adults with sickle cell disease. Pain Manag Nurs. (2015) 16(3):294–306. doi: 10.1016/j.pmn.2014.08.004
24. Campbell CM, Edwards RR, Fillingim RB. Ethnic differences in responses to multiple experimental pain stimuli. Pain. (2005) 113(1–2):20–6. doi: 10.1016/j.pain.2004.08.013
25. Cruccu G, Anand P, Attal N, Garcia-Larrea L, Haanpaa M, Jorum E, et al. EFNS Guidelines on neuropathic pain assessment. Eur J Neurol. (2004) 11(3):153–62. doi: 10.1111/j.1468-1331.2004.00791.x
26. Mücke M, Cuhls H, Radbruch L, Baron R, Maier C, Tölle T, et al. Quantitative sensory testing. Schmerz (Berlin, Germany) U6 - ctx_ver=Z3988-2004&ctx_enc=info%3Aofi%2Fenc%3AUTF-8&rfr_id=info:sid/summonserialssolutionscom&rft_val_fmt=info:ofi/fmt:kev:mtx:journal&rftgenre=article&rftatitle=Quantitative+sensory+testing&rftjtitle=Schmerz+%28Berlin%2C+Germany%29&rftau=M%C3%BCcke%2C+M&rftau=Cuhls%2C+H&rftau=Radbruch%2C+L&rftau=Baron%2C+R&rftdate=2014-12-01&rfteissn=1432-2129&rftvolume=28&rftissue=6&rftspage=635&rft_id=info:pmid/25403802&rftexternalDocID=25403802¶mdict=en-US U7 - Journal Article. (2014) 28(6):635.
27. Rolke R, Baron R, Maier C, Tölle TR, Treede RD, Beyer A, et al. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): standardized protocol and reference values. Pain. (2006) 123(3):231–43. doi: 10.1016/j.pain.2006.01.041
28. Melzack R. The McGill pain questionnaire: major properties and scoring methods. Pain. (1975) 1(3):277–99. doi: 10.1016/0304-3959(75)90044-5
29. Wilkie DJ, Judge MK, Berry DL, Dell J, Zong S, Gilespie R. Usability of a computerized PAINReportIt in the general public with pain and people with cancer pain. J Pain Symptom Manage. (2003) 25(3):213–24. doi: 10.1016/S0885-3924(02)00638-3
30. Wilkie DJ, Savedra MC, Holzemer WL, Tesler MD, Paul SM. Use of the McGill pain questionnaire to measure pain: a meta-analysis. Nurs Res. (1990) 39(1):36–41. doi: 10.1097/00006199-199001000-00008
31. Jha A, Suarez ML, Ferrans CE, Molokie R, Kim YO, Wilkie DJ. Cognitive testing of PAINReportIt in adult African Americans with sickle cell disease. Comput Inform Nurs. (2010) 28(3):141–50. doi: 10.1097/NCN.0b013e3181d7820b
32. Ngamkham S, Vincent C, Finnegan L, Holden JE, Wang ZJ, Wilkie DJ. The McGill Pain Questionnaire as a multidimensional measure in people with cancer: an integrative review. Pain Manag Nurs. (2012) 13(1):27–51. doi: 10.1016/j.pmn.2010.12.003
33. Murphy DF, McDonald A, Power C, Unwin A, MacSullivan R. Measurement of pain: a comparison of the visual analogue with a nonvisual analogue scale. Clin J Pain. (1987) 3(4):197–200. doi: 10.1097/00002508-198712000-00003
34. Wilkie D, Lovejoy N, Dodd M, Tesler M. Cancer pain intensity measurement: concurrent validity of three tools–finger dynamometer, pain intensity number scale, visual analogue scale. Hosp J. (1990) 6(1):1–13. doi: 10.1080/0742-969X.1990.11882662
35. Jensen MP, Karoly P, Huger R. The development and preliminary validation of an instrument to assess patients’ attitudes toward pain. J Psychosom Res. (1987) 31(3):393–400. doi: 10.1016/0022-3999(87)90060-2
36. Jacob E, Chan VW, Hodge C, Zeltzer L, Zurakowski D, Sethna NF. Sensory and thermal quantitative testing in children with sickle cell disease. J Pediatr Hematol Oncol. (2015) 37(3):185–9. doi: 10.1097/MPH.0000000000000214
Keywords: sickle cell disease, pain, AVPR1A, genotype, quantitative sensory testing (QST), stress
Citation: Powell-Roach KL, Yao Y, Cao X, Chamala S, Wallace MR, Cruz-Almeida Y, Molokie RE, Wang ZJ and Wilkie DJ (2023) Analysis of AVPR1A, thermal and pressure pain thresholds, and stress in sickle cell disease. Front. Pain Res. 3:1060245. doi: 10.3389/fpain.2022.1060245
Received: 3 October 2022; Accepted: 6 December 2022;
Published: 4 January 2023.
Edited by:
Ericka N. Merriwether, New York University, United StatesReviewed by:
Inna E. Tchivileva, University of North Carolina at Chapel Hill, United StatesChristopher Michael Peters, Wake Forest University, United States
© 2023 Powell-Roach, Yao, Cao, Chamala, Wallace, Cruz-Almeida, Molokie, Wang and Wilkie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Keesha L. Powell-Roach a3JvYWNoMTBAdXRoc2MuZWR1
†ORCID Keesha L. Powell-Roach orcid.org/0000-001-8117-3445 Yingwei Yao orcid.org/0000-0001-5389-2717 Xueyuan Cao orcid.org/0000-0002-4396-7460 Srikar Chamala orcid.org/0000-0002-4396-7460 Margaret R. Wallace orcid.org/0000-0002-5202-8895 Yenisel Cruz-Almeida orcid.org/0000-0002-0065-0236 Robert E. Molokie orcid.org/0000-0003-3623-7395 Zaijie Jim Wang orcid.org/0000-0002-3496-4655 Diana J. Wilkie orcid.org/0000-0002-3954-8933
Specialty Section: This article was submitted to Pain Research Methods, a section of the journal Frontiers in Pain Research
 Keesha L. Powell-Roach
Keesha L. Powell-Roach Yingwei Yao2,†
Yingwei Yao2,† Xueyuan Cao
Xueyuan Cao Srikar Chamala
Srikar Chamala Margaret R. Wallace
Margaret R. Wallace Yenisel Cruz-Almeida
Yenisel Cruz-Almeida Robert E. Molokie
Robert E. Molokie Zaijie Jim Wang
Zaijie Jim Wang Diana J. Wilkie
Diana J. Wilkie