- 1Department of Pain and Translational Symptom Science, School of Nursing, University of Maryland, Baltimore, MD, United States
- 2Center to Advance Chronic Pain Research (CACPR), University of Maryland, Baltimore, MD, United States
- 3College of Health Sciences, School of Nursing, University of Delaware, Newark, DE, United States
- 4Department of Organizational Systems and Adult Health, School of Nursing, University of Maryland, Baltimore, MD, United States
- 5Department of Family and Community Health, School of Nursing, University of Maryland, Baltimore, MD, United States
Chronic pain imposes a significant burden to the healthcare system and adversely affects patients' quality of life. Traditional subjective assessments, however, do not adequately capture the complex phenomenon of pain, which is influenced by a multitude of factors including environmental, developmental, genetic, and psychological. Quantitative sensory testing (QST), established as a protocol to examine thermal and mechanical sensory function, offers insight on potential mechanisms contributing to an individual's experience of pain, by assessing their perceived response to standardized delivery of stimuli. Although the use of QST as a research methodology has been described in the literature in reference to specific pain populations, this manuscript details application of QST across a variety of chronic pain conditions. Specific conditions include lower extremity chronic pain, knee osteoarthritis, chronic low back pain, temporomandibular joint disorder, and irritable bowel syndrome. Furthermore, we describe the use of QST in placebo/nocebo research, and discuss the use of QST in vulnerable populations such as those with dementia. We illustrate how the evaluation of peripheral sensory nerve function holds clinical promise in targeting interventions, and how using QST can enhance patient education regarding prognostic outcomes with particular treatments. Incorporation of QST methodology in research investigations may facilitate the identification of common mechanisms underlying chronic pain conditions, guide the development of non-pharmacological behavioral interventions to reduce pain and pain-related morbidity, and enhance our efforts toward reducing the burden of chronic pain.
Introduction
Pain is a complex phenomenon where the intensity, characteristics, extent, and duration varies from person to person, and reflects the complex biopsychosocial interactions between genetic, developmental, environmental, and psychological factors (1). While many studies rely on Patient-Reported Outcomes Measures (PROM) reports of pain such as the 11-point Numeric Rating Scale (a unidimensional measure of the magnitude of pain), or the Short-Form McGill Pain Questionnaire (measure of pain characteristics), adding measures that include a person's perceived response to a standardized somatosensory testing protocol offers an opportunity to further characterize the pain experience (2). Importantly, the ability to implement personalized treatment plans necessitates the careful identification of individual pain characteristics (3). Quantitative sensory testing (QST) provides a complementary assessment of an individual's response to stimuli and offers information about the potential underlying mechanisms contributing to pain. Thus, incorporating QST may help to address recognized challenges in pain assessment across patient populations, and guide the development of non-pharmacological interventions to reduce pain, as detailed below.
QST was established by the German Research Network on Neuropathic Pain as a protocol to examine thermal and mechanical sensory function (4). It refers to the use of a set of standardized testing procedures that allows the examiner to measure and quantify somatosensory function in large (Aβ-fibers) and small sensory nerve fibers (Aδ- and C-fibers) with an aim to detect sensory loss (i.e., hypoesthesia, hypoalgesia) or sensory gain (i.e., hyperesthesia, hyperalgesia, allodynia) (4) (see Table 1). Two key components of chronic pain are peripheral and central sensitization. Peripheral sensitization results from increased excitability of nociceptors, leading to the development of hyperalgesia (14). Using QST, peripheral sensitization is reflected as a decrease in thermal (hot and cold) pain thresholds (6). Central sensitization is a result of increased excitability of neurons in the central nervous system (CNS), which leads to the development of allodynia in the immediate area of pain, as well as hyperalgesia and allodynia in surrounding regions (6). Central sensitization is identified using the QST measure of temporal summation, where applying rapidly repeated mechanical or thermal stimuli results in increased pain perception. Of note, central sensitization as measured by QST is distinct from central sensitization-related symptoms and diagnoses, as measured by the Central Sensitization Inventory, or CSI (15).
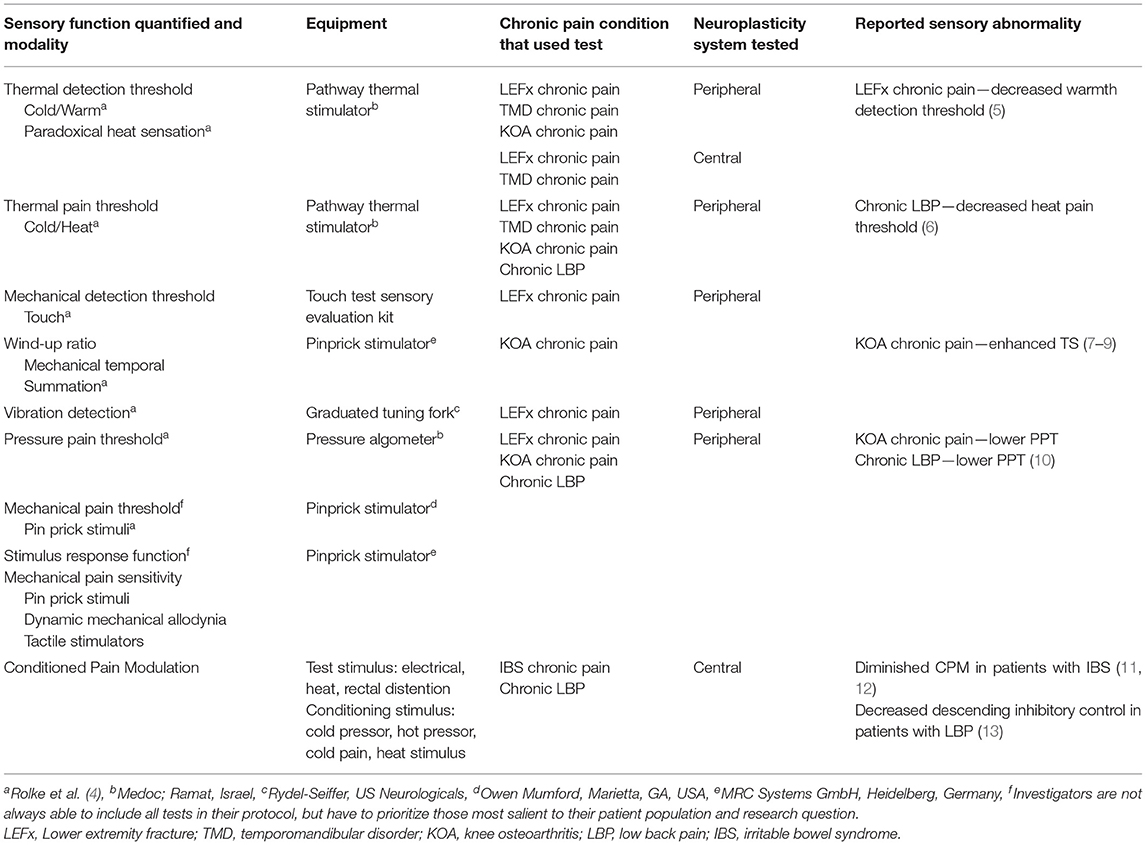
Table 1. Specific tests from the German Research Network on Neuropathic Pain QST protocol by chronic pain conditions of interest and Conditioned Pain Modulation.
Another component of chronic pain is a decrease in descending inhibitory control in the CNS. Changes in central inhibitory pain modulation can be identified by using the conditioned pain modulation test (13). CPM testing involves applying a noxious stimulus (thermal or mechanical) to a region remote from the painful site. If descending inhibitory control is functioning properly, the initial source of pain will be less intense during the application of the remote stimulus (16). QST measures reported pain based on standardized screens or via physical means to measure pain thresholds and accommodation to varying levels of stimuli, and can be used to better predict pain treatments and outcomes (17). For instance, QST has successfully been used to reveal decreased sensitivity to stimuli in persons with lower back pain and lower extremity fractures (5, 18) and increased sensitivity to stimuli in persons with knee osteoarthritis (19). Furthermore, QST can aid in characterizing peripheral sensory nerve function, which can then be correlated with a wide range of other factors such as genetic biomarkers, treatment efficacy, and biobehavioral activities to generate risk profiles and guide treatment. QST has also been shown to predict analgesic effects (20).
The objective of this manuscript is to review the application of tests from the QST protocol established by the German Research Network on Neuropathic Pain (DNFS) (4) across a variety of chronic pain conditions in our Omics Associated with Self-Management Interventions for Symptoms (OASIS) Center. Specific conditions include lower extremity (LE) chronic pain, knee osteoarthritis (KOA), chronic low back pain (LBP), temporomandibular joint disorder (TMD), and irritable bowel syndrome (IBS). Furthermore, we describe the use of QST in placebo/nocebo research, and discuss the use of QST in vulnerable populations such as those with Alzheimer's disease (AD). Relevant literature for each condition was retrieved by the co-author whose research surrounds that population of interest. As chronic pain imposes significant burden to the healthcare system and adversely affects patients' quality of life, utilizing QST methodology may offer insight on an individual's experience of pain, identify commonalities across chronic pain conditions, and direct tailored interventions, including non-pharmacological behavioral treatments, to reduce pain and pain-related morbidity.
Lower Extremity Chronic Pain/Knee Osteoarthritis
The etiology of lower extremity (LE) chronic pain is wide-ranging (i.e. arthritis, injuries, surgeries) and contributes significantly to negative physical, psychological, social, emotional, and economical consequences for adults across the life-span (1). While the biological mechanism associated with LE chronic pain is poorly understood, changes in peripheral sensory nerve function assessed with QST have been identified in persons with chronic pain following lower extremity fractures and arthritis. For instance, hypoesthesia, decreased warmth detection threshold, has been noted in persons with LE-fracture related chronic pain (5). This mirrors what has been reported in persons with traumatic partial nerve injuries (21) and may be due to peripheral nerve injuries that have not fully healed (22). Changes in LE sensation (23), whether hypoesthesia or hyperesthesia, is important information as poor peripheral sensory nerve function is associated with decreased LE function and mobility limitations, especially in older adults (24). Furthermore, LE chronic pain is associated with an increased fall risk and falls have catastrophic consequences with increased morbidity and mortality (25). Therefore, evaluating peripheral sensory nerve function in LE chronic pain has the potential for clinicians to target interventions on mobility and balance that may be affected by these changes.
One cause of LE chronic pain, knee osteoarthritis (KOA), affects approximately one third of adults in the United States (26, 27). Pain is the most prevalent and troublesome symptom of KOA (28) leading patients to seek medical interventions for relief. However, KOA pain type and intensity often does not correlate to visual damage noted by radiography (29). KOA is a degenerative locally inflammatory disease in which both peripheral and central sensitization contribute to pain (30, 31). KOA pain symptoms are typically mild and nociceptive to start and triggered by weight bearing or physical activity. KOA induces tissue injury and/or inflammation leading to peripheral sensitization. Chronic KOA pain is multifactorial and qualitative work has described two types of pain experience: a constant aching and intermittent severe pain (28). Moreover, many adults with KOA experience alterations in endogenous pain inhibitory capacity that have neurobiologic and central mechanisms. Those adults with centralized KOA pain tend to be resistant to traditional pain treatment and have substantial pain even after knee replacement surgery (32). Therefore, phenotyping KOA pain is imperative for personalized treatment.
Quantitative sensory testing has merit for phenotyping KOA pain. Most studies in KOA use pressure pain thresholds at the affected joint and often a distal site to quantify pain sensitization (7, 33). Heat and cold pain detection and threshold have also been used but less often. In addition, temporal summation (TS) is often assessed, but Moore et al. found only weak correlations between TS and clinical pain ratings (7). This may be due to racial differences as one study found TS associated with clinical pain scores among non-Hispanic White people but not among Black people (8). It may also be due to variation of specific phenotypes of KOA pain where women and Black people with KOA were more likely to ascribe to high TS (9).
Pressure pain thresholds (PPT) are the most reliable measure of peripheral sensitization with consistent test-retest correlations and are able to differentiate KOA from healthy controls (33–36). Wylde et al. (36) tested PPT at the affected knee, contralateral knee, and forearm sites of adults with KOA one week apart and report intraclass correlation coefficients (ICC) for heat ranging from 0.52 to 0.70 and cold detection of 0.31–0.70 and for PPT 0.77–0.86. PPT are also correlated with the manual tender point count clinical measure, an assessment of peripheral sensitization (7). Consistently, adults with KOA have lower pressure pain thresholds at the affected joint than healthy controls (33, 37). Similar to PPT, TS is associated with pain severity but not severity of radiographic evidence of KOA (37).
QST signs of central pain mechanisms in KOA have been defined as low PPT and/or enhanced TS and/or allodynia (19), whereas localized pain at the joint but not at distal sites reflect more peripheral pain mechanisms. Using these QST tools together, testing pain sensitivity in adults with KOA at both the affected knee and a distal site (ipsilateral non-painful hand) resulted in the identification of five distinct pain phenotypes: #1—low pressure pain threshold, #2—average pain sensitivity across most modalities (pressure pain, heat and cold pain, temporal summation heat/cold), #3—high temporal summation of punctate pain, #4—high cold pain sensitivity, and #5—high heat pain sensitivity/high temporal summation of heat pain (9). These findings reflect the influence of distinct central and peripheral mechanisms on KOA pain. For example, those who are sensitive to heat pain have more pain after total knee surgery and consume more morphine post-surgery. Additionally, Carlesso et al. (38) identified pain susceptibility phenotypes of adults with KOA but free of pain and found that those with low PPT and high TS had twice the odds (OR 1.98) of developing persistent knee pain.
Understanding the mechanisms of KOA pain type and sensitivity using QST can facilitate the development of targeted treatment options and better patient education as to prognostic outcomes with particular treatments. For example, presence of TS was predictive of poor response to pain treatment with non-steroidal anti-inflammatory (NSAIDS) drugs (39). Of those adults with KOA who undergo costly knee replacement surgery, up to 44% will continue to have chronic knee pain months and even years post-operatively (32, 40). QST testing can help predict surgical outcomes as patients with neuropathic-type pain and widespread hyperalgesia, as indicated by widespread pressure and cold hyperalgesia on QST, are reported to experience persistent pain a year after total knee arthroplasty (41). Therefore, incorporating QST methodology in patient assessment can help detail mechanisms that underlie their experience of pain, offer prognostic indicators, and help guide the development of individualized treatment plans.
Chronic Low Back Pain
Chronic low back pain (LBP) is among the most frequently diagnosed, most expensive to treat, and most debilitating pain condition in the United States (42, 43). It is estimated that approximately 40% of acute LBP patients will report having chronic LBP at 24 weeks after medical treatment for an acute episode of LBP (42). Furthermore, up to 90% of LBP patients have no identifiable etiology for their pain (44, 45). Being able to predict who will transition from an episode of acute LBP to developing chronic LBP will increase our ability to implement more aggressive therapies early in the treatment of the acute pain to prevent the transition to the chronic pain state. While many pain-related questionnaires provide insight into the patient's perception of LBP, unfortunately, the neurophysiological mechanisms underlying the development of chronic LBP remain unclear. The addition of QST measures to the assessment of LBP can provide valuable neurophysiological information that complements questionnaire data.
Research shows that patients with persistent LBP have evidence of peripheral and central sensitization, which may be key factors in their risk for chronic LBP (46, 47), and can be identified using the QST measures of thermal threshold, pressure pain threshold, temporal summation, as well as the conditioned pain modulation test. Changes in the QST measures during the acute LBP phase could be indicative of the potential for the acute LBP to transition into a chronic LBP state. Studies found peripheral sensitization was reflected as a decrease in the heat pain threshold (6), and pressure pain threshold (10). In patients with LBP (46, 47), central sensitization can be identified using temporal summation by applying rapidly repeated mechanical stimuli on the painful region resulting in increased pain perception. Conditioned pain modulation can be used to detect a decrease in CNS descending inhibitory control, which is another contributor to persistent LBP (13). Therefore, sensory alterations and increased pain sensitivity found in LBP patients can be measured using QST. Adding QST to clinical exams of LBP patients will allow clinicians to identify sensory function changes leading to increased pain, which will help enhance our understanding of the physiological mechanisms underlying this chronic pain condition.
Temporomandibular Disorders
There are numerous types of chronic orofacial pain conditions including temporomandibular disorders (TMD) (48), also referred to as temporomandibular joint disorders or TMJ. TMJ is the abbreviation for the joint itself, while TMD are the actual conditions and their accompanying pain and inflammation. TMD affects approximately 5–12% of the general population (49–51) and are associated with significant personal and societal burdens (48). QST is used in various ways to determine underlying causes and potential methods for analgesia in these conditions, and has been used in measurement of various aspects of TMD-related pain (52–55).
In a large prospective cohort study (Orofacial Pain: Prospective Evaluation and Risk Assessment, OPPERA), QST was used as a predictor to identify onset of TMD in individuals who were otherwise healthy when enrolled in the trial (56). Risk factors in four main areas were identified. Psychosocial factors examined concurrent and pre-existing mental health issues. Age, sex, and race were among those identified as sociodemographic factors. Standard instruments that delivered painful stimuli were used to measure elevated response to pain. Genetic factors included both psychological traits and pain sensitivity (56). Baseline QST measurements were taken for pressure pain thresholds (PPT), mechanical pain stimuli, and thermal heat pain stimuli. Additionally, self-report information was gathered using questionnaires, and blood draws were performed (53). Increased incidence of TMD was found to be associated with the psychosocial risk factors of anxiety, depression, perceived stress levels, and sensitivity to somatic issues. Likewise, incident TMD was found to occur in those with greater temporal summation of heat pain and clinical factors such as autonomic measurements including heart rate at rest. Sociodemographic factors of being younger, woman, and White, as well as genetic factors, are associated with increased incidence and development or worsening of chronic TMD (53, 56).
Greenspan and colleagues also used QST to measure sensitivity to three modalities of nociception in TMD patients: blunt pressure pain, mechanical pinprick pain, and thermal heat pain (54). These investigators reported pain sensitivity to vary according to the total number of pain conditions a participant with TMD experienced, suggesting that the combination of pain conditions influences each nociceptive modality and QST-related measurements (54). Recently, we assessed thermal heat pain threshold and thermal heat pain tolerance in TMD participants as a proxy for the QST procedure. We found that Afro-American Blacks had lower pain tolerance in both TMD participants and healthy control participants (57). Additionally, we found women in the TMD cohort to have lower levels of both thermal heat pain threshold and thermal heat pain tolerance than men (58). Both results are in line with the literature on racial and sex effects with regard to pain sensitivity, as in experimental pain studies, women tend to be more sensitive to pain than men in measures of heat, cold, and pressure pain tolerance (55, 59, 60). Furthermore, when compared to Whites, Afro-American Blacks and other minority races tend to experience greater sensitivity to pain stimuli and less efficient descending pain inhibition evaluated through conditioned pain modulation or CPM (61–66).
Irritable Bowel Syndrome
Irritable bowel syndrome (IBS) is a gastrointestinal disorder defined by chronic abdominal pain and alterations in bowel habits (67), that commonly co-occurs with other chronic pain conditions including headache and TMD (68). IBS is characterized by chronic visceral pain, which is poorly localized pain arising from the pelvic, thoracic, or abdominal organs (69), yet patients with IBS also suffer from abnormalities in gut-brain interactions (70). Patients with IBS display heightened pain sensitivity which may be attributed to a variety of mechanisms, including alterations in the processing of pain sensory information (both spinal and central), and afferent signaling of pain (71). As information surrounding endogenous pain modulation can be provided through QST paradigms, both pain facilitation and pain inhibition (72), quantifying sensory alterations in the IBS patient population offers a means whereby insight can be gained on pain modulatory systems.
Assessments of pain inhibition have most often been conducted in patients with IBS through the testing of conditioned pain modulation (CPM). Meta-analyses report patients with IBS display significantly diminished CPM in comparison with healthy controls, suggesting abnormalities with descending pain pathways (11, 12). Therefore, interventions which enhance deficiencies in pain inhibition, whether pharmacological, physiological, or electrical, may theoretically be helpful in the management of pain in patients with IBS (11). Importantly, this research also reports a high correlation between reduced CPM with psychological factors such as stress, anxiety, and pain catastrophizing, thus highlighting the complex relationship between mood, cognition, and CPM responses (12). Therefore, incorporating QST in the assessment of pain in patients with IBS, may lend insight on central vs. peripheral mechanisms and pain modulatory capabilities, which bears relevance when selecting therapeutic interventions.
QST in Placebo/Nocebo Research
The placebo effect represents the neurobiological response to inert treatments and interventions (73). A large study evaluated differences in levels of placebo effects, based on expectations and prior experiences, between participants with chronic orofacial pain and healthy controls (74). The intervention consisted of painful thermal stimuli, along with a sham electrode the participants were told would decrease the pain produced by the device. Results showed similar levels of placebo effects, with more responders in the control group, whereas pain relief expectations were higher in the chronic pain group. Interestingly, the occurrence of placebo effects in both groups was not attributed to participant expectations, but rather to their prior experiences of having received the painful stimuli (74). In a study conducted to explore racial differences in pain and placebo effects, when compared to Whites, Afro-American Blacks demonstrated less efficient placebo hypoalgesia (57) and lower thermal heat tolerance. Importantly, there was a significant interaction with the race of the experimenters and the race of TMD participants hinting to potential healthy inequities and not just mechanisms of nociception. Moreover, QST varies in Afro-American Blacks but this variation is not linked to placebo effects (57).
The nocebo effect refers to worsening in symptoms when an inert substance or treatment is administered (73). While the relationship between nocebo and catastrophizing has been posited (75), Taub et al. (76) demonstrated the presence of pain catastrophizing in women with chronic low back pain. Performance of QST pre- and post-induction of pain catastrophizing, demonstrated increased pain in women with higher levels of pain catastrophizing, as well as extension of pain to areas where it was previously non-existent. Again, QST facilitated an objective measurement of pain in a controlled setting to study nocebo effects.
QST in Special Populations/Alzheimer's Disease
Over 90% of individuals living with Alzheimer's disease (AD) are likely to experience pain at some point during the course of their dementia (77, 78), however, patient reported response of pain among individuals with AD has been inconsistent. A meta-analysis reports individuals with AD demonstrate higher pain sensitivity to experimental pain when using validated observational ratings of pain (79), and exhibit more pain behaviors when exposed to painful stimuli compared to individuals who are cognitively intact (80). Conversely, some studies note individuals with AD are less likely to report pain and use fewer analgesics compared with individuals without AD (81–85). Some individual items on observational instruments that measure pain are non-specific for pain exclusively and may be reflective of neuropsychiatric symptoms (restlessness, repetitive vocalizations) instead of pain. Several hypotheses have been proposed to explain differences in pain sensitivity and expression based on cognition. First, individuals with AD are unable to reliably report pain due to declining perceptual and communication abilities (86, 87). Second, individuals with AD experience fewer pain conditions, and third, these individuals experience alterations in nociceptive processing (e.g., peripheral sensory or central functioning) that impact their pain experience (84). The measurement of pain among individuals with AD may benefit from triangulation. The addition of QST measures to the assessment of pain among individuals with AD may provide neurophysiological data that complements the use of surveys and observational measures of pain.
To better understand whether altered nociceptive processing among individuals with AD contributes to altered pain sensitivity, some studies have utilized QST. Based on QST studies conducted in Italy and Australia, individuals with AD are reported to experience less pain sensitivity to temperature (88) and higher pressure pain thresholds (89) compared with controls. In contrast, groups in Denmark and the United States report diminished pain tolerance in individuals with AD compared with matched controls using QST (78, 80).
Use of QST among individuals with AD and related dementias has been limited. There are legitimate concerns regarding the reliability, safety, and ethical concerns of inducing discomfort in a vulnerable population. Additionally, challenges associated with obtaining informed consent in a population with impaired decision-making abilities contribute to its limited use. To best address such concerns, stimulus testing should be conducted over at least three trials, and false positive responses controlled for through application of a control or null stimulus (e.g. no filament or temperature applied). Safety strategies can also be put in place to minimize risks when working with older adults. Individuals with AD can be paired with a research team member during pain threshold testing who can assist with instructions and stimulus removal if the participant reports or exhibits signs of pain but does not withdraw. To help facilitate recruitment of individuals with AD, the proxy/legally authorized representative could be offered a trial experience of QST testing to better understand what the individual would experience.
Discussion
QST has been used primarily in the research setting to characterize peripheral and central mechanisms underlying pain. Our illustration of QST's relevance across chronic pain conditions and within special populations, as investigated in our OASIS Center, supports its clinical utility to guide the selection of appropriate therapies and optimize patient outcomes. For instance, detection of peripheral sensory nerve function in LE chronic pain can lead to targeted mobility interventions, recognition of abnormal pain inhibitory pathways in patients with IBS can direct prescription of neuromodulator therapy, and assessment of altered nociceptive processing among individuals with AD can guide adequate pain control. Therefore, QST offers insight not only regarding physiological underpinnings of pain from a research perspective, its incorporation among our clinical assessment methods can potentially enhance the efficacy of our therapeutic approach, including non-pharmacological interventions.
Alongside the promise of QST in improving the characterization of pain, and in turn, the prescription of individualized treatment interventions, limitations of this methodology must also be addressed. As highlighted in our descriptions of QST in TMD and placebo research, sex and racial effects regarding pain sensitivity, and racial effects regarding placebo efficiency have been observed, and thus need be accounted for when analyzing results (57, 58). In addition, effects of age must be considered when incorporating QST, as loss of sensory function has been observed in older adults for cold, warmth, mechanical, and vibratory detection thresholds (90). Moreover, a recent systematic review with meta-analysis surrounding the use of QST in individuals with joint pain, reports an association between depression, pain catastrophizing, and physical activity level with several QST measures; therefore, such variables need be acknowledged when evaluating the relationship between pain and somatosensory function (91). Despite the potential of such cofounding factors, the ability of QST to detect and discriminate peripheral vs. central contributors to pain, and its potential to help guide targeted therapies, including non-pharmacological interventions, argues for its greater incorporation in research and clinical initiatives. Moving forward, efforts to shorten and standardize QST protocols across chronic pain conditions, and increasing portability of QST machines, will facilitate greater application of this methodology in research investigations, ease its adaptation to the clinical arena, and enhance our efforts toward reducing the burden of chronic pain.
Author Contributions
KW, MG, NK, EG, AD, LC, and CR wrote sections of the manuscript. All authors contributed to the conception and design of the study, manuscript revision, read, and approved the submitted version.
Funding
The authors would like to acknowledge funding and support from the National Institutes of Health, National Institute of Nursing Research, P30 Omics Associated with Self-Management Interventions for Symptoms (OASIS) Center (P30NRO16579 to BR, SD, and CR), and National Institute of Dental Craniofacial Research (1R01DE025946 to LC).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Institute Institute of Medicine (US) Committee on Advancing Pain Research, Care, and Education. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. The National Academies Collection: Reports funded by National Institutes of Health. Washington, DC: National Academies Press (2011). doi: 10.17226/13172
2. Greenspan JD. Quantitative assessment of neuropathic pain. Curr Pain Headache Rep. (2001) 5:107–13. doi: 10.1007/s11916-001-0078-y
3. Edwards RR, Dworkin RH, Turk DC, Angst MS, Dionne R, Freeman R, et al. Patient phenotyping in clinical trials of chronic pain treatments: IMMPACT recommendations. Pain. (2016) 157:1851–71. doi: 10.1097/j.pain.0000000000000602
4. Rolke R, Baron R, Maier C, Tölle TR, Treede-Beyer A, Binder A, et al. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): standardized protocol and reference values. Pain. (2006) 123:231–43. doi: 10.1016/j.pain.2006.01.041
5. Griffioen MA, Greenspan JD, Johantgen M, Von Rueden K, O'Toole RV, Dorsey SG et al. Quantitative sensory testing and current perception threshold testing in patients with chronic pain following lower extremity fracture. Biol Res Nurs. (2018) 20:16–24. doi: 10.1177/1099800417720725
6. Granovsky Y, Yarnitsky D. Personalized pain medicine: the clinical value of psychophysical assessment of pain modulation profile. Rambam Maimonides Med J. (2013) 4:e0024. doi: 10.5041/RMMJ.10131
7. Moore RL, Clifford AM, Moloney N, Doody C, Smart KM, O'Leary H. The relationship between clinical and quantitative measures of pain sensitization in knee osteoarthritis. Clin J Pain. (2020) 36:336–43. doi: 10.1097/AJP.0000000000000798
8. Goodin BR, Bulls HW, Herbert MS, Schmidt J, King CD, Glover TL, et al. Temporal summation of pain as a prospective predictor of clinical pain severity in adults aged 45 years and older with knee osteoarthritis: ethnic differences. Psychosom Med. (2014) 76:302–10. doi: 10.1097/PSY.0000000000000058
9. Cardoso JS, Riley JL III, Glover T, Sibille KT, Bartley EJ, Goodin BR, et al. Experimental pain phenotyping in community-dwelling individuals with knee osteoarthritis. Pain. (2016) 157:2104–14. doi: 10.1097/j.pain.0000000000000625
10. den Brandt H, Paulis WD, Beckwee D, Ickmans K, Nijs J, Voogt L. Pain mechanisms in low back pain: A systematic review with meta-analysis of mechanical quantitative sensory testing outcomes in people with nonspecific low back pain. J Orthop Sports Phys Ther. (2019) 49:698–715. doi: 10.2519/jospt.2019.8876
11. Albusoda A, Ruffle JK, Friis KA, Gysan MR, Drewes AM, Aziz Q, et al. Systematic review with meta-analysis: conditioned pain modulation in patients with the irritable bowel syndrome. Aliment Pharmacol Ther. (2018) 48:797–806. doi: 10.1111/apt.14965
12. Marcuzzi, A, Chakiath RJ, Siddall PJ, Kellow JE, Hush JM, Jones MP, et al. Conditioned pain modulation (CPM) is reduced in irritable bowel syndrome: a systematic review and meta-analysis of CPM and the role of psychological factors. J Clin Gastroenterol. (2019) 53:399–408. doi: 10.1097/MCG.0000000000001181
13. O'Neill S, Manniche C, Graven-Nielsen T, Arendt-Nielsen L. Association between a composite score of pain sensitivity and clinical parameters in low-back pain. Clin J Pain. (2014) 30:831–8. doi: 10.1097/AJP.0000000000000042
14. Krumova EK, Geber C, Westermann A, Maier C. Neuropathic pain: is quantitative sensory testing helpful? Curr Diab Rep. (2012) 12:393–402. doi: 10.1007/s11892-012-0282-7
15. Neblett R. The central sensitization inventory: A user's manual. J Appl Biobehav Res. (2018) 23:e12123. doi: 10.1111/jabr.12123
16. Fernandes C, Pidal-Miranda M, Samartin-Veiga N, Carrillo-de-la-Peña MT. Conditioned pain modulation as a biomarker of chronic pain: a systematic review of its concurrent validity. Pain. (2019) 160:2679–90. doi: 10.1097/j.pain.0000000000001664
17. Davis KD. Imaging vs quantitative sensory testing to predict chronic pain treatment outcomes. Pain. (2019) 160:S59–65. doi: 10.1097/j.pain.0000000000001479
18. Starkweather AR, Lyon DE, Kinser P, Heineman A, Sturgill JL, Deng X, et al. Comparison of low back pain recovery and persistence: A descriptive study of characteristics at pain onset. Biol Res Nurs. (2016) 18:401–10. doi: 10.1177/1099800416631819
19. Hochman JR, Davis AM, Elkayam J, Gagliese L, Hawker GA. Neuropathic pain symptoms on the modified pain DETECT correlate with signs of central sensitization in knee osteoarthritis. Osteoarthritis Cartilage. (2013) 21:1236–42. doi: 10.1016/j.joca.2013.06.023
20. Peterson KK, Vaegter HB, Stubhaug A, Wolff A, Scammell BE, Arendt-Nielsen L, et al. The predictive value of quantitative sensory testing: a systematic review on chronic postoperative pain and the analgesic effect of pharmacological therapies in patients with chronic pain. Pain. (2021) 162:31–44. doi: 10.1097/j.pain.0000000000002019
21. Leffler AS, Hansson P. Painful traumatic peripheral partial nerve injury-sensory dysfunction profiles comparing outcomes of bedside examination and quantitative sensory testing. Eur J Pain (London, England). (2008) 12:397–402. doi: 10.1016/j.ejpain.2007.08.009
22. Grinsell D, Keating CP. Peripheral nerve reconstruction after injury: a review of clinical and experimental therapies. BioMed Res Int. (2014) 2014:698256. doi: 10.1155/2014/698256
23. Neogi T, Frey-Law L, Scholz J, Niu J, Arendt-Nielsen L, Woolf C, et al. Sensitivity and sensitisation in relation to pain severity in knee osteoarthritis: trait or state? Ann Rheum Dis. (2015) 74:682–8. doi: 10.1136/annrheumdis-2013-204191
24. Lange-Maia BS, Newman AB, Cauley JA, Boudreau RM, Jakicic JM, Caserotti P, et al. Sensorimotor peripheral nerve function and the longitudinal relationship with endurance walking in the health, aging and body composition study. Arch Phys Med Rehabil. (2016) 97:45–52. doi: 10.1016/j.apmr.2015.08.423
25. Stubbs B, Binnekade T, Eggermont L, Sepehry AA, Patchay S, Schofield P. Pain and the risk for falls in community-dwelling older adults: systematic review and meta-analysis. Archives of physical medicine and rehabilitation. (2014) 95:175–87.e9. doi: 10.1016/j.apmr.2013.08.241
26. Dillon CF, Rasch EK, Gu Q, Hirsch, R. Prevalence of knee osteoarthritis in the United States: arthritis data from the Third National Health and Nutrition Examination Survey 1991-94. J Rheumatol. (2006) 33:2271–9.
27. Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheumat. (2008) 58:26–35. doi: 10.1002/art.23176
28. Neogi T. The epidemiology and impact of pain in osteoarthritis. Osteoarthritis and Cartilage. (2013) 21:1145–53. doi: 10.1016/j.joca.2013.03.018
29. Finan PH, Buenaver LF, Bounds SC, Hussain S, Park RJ, Haque UJ, et al. Discordance between pain and radiographic severity in knee osteoarthritis: findings from quantitative sensory testing of central sensitization. Arthritis Rheum. (2013) 65:363–72. doi: 10.1002/art.34646
30. Neogi T, Guermazi A, Roemer F, Nevitt MC, Scholz J, Arendt-Nielsen L, et al. Association of joint inflammation with pain sensitization in knee osteoarthritis: The multicenter osteoarthritis study. Arthritis Rheumatol. (Hoboken, NJ). (2016) 68:654–61. doi: 10.1002/art.39488
31. Zhang RX, Ren K, Dubner R. Osteoarthritis pain mechanisms: basic studies in animal models. Osteoarthr Cartil. (2013) 21:1308–15. doi: 10.1016/j.joca.2013.06.013
32. Wylde V, Hewlett S, Learmonth ID, Dieppe P. Persistent pain after joint replacement: prevalence, sensory qualities, and postoperative determinants. Pain. (2011) 152:566–72. doi: 10.1016/j.pain.2010.11.023
33. Suokas AK, Walsh DA, McWilliams DF, Condon L, Moreton B, Wylde V, et al. Quantitative sensory testing in painful osteoarthritis: a systematic review and meta-analysis. Osteoarthr Cartil. (2012) 20:1075–85. doi: 10.1016/j.joca.2012.06.009
34. Cruz-Almeida Y, Fillingim RB. Can quantitative sensory testing move us closer to mechanism-based pain management? Pain Med. (2014) 15:61–72. doi: 10.1111/pme.12230
35. Wessel J. The reliability and validity of pain threshold measurements in osteoarthritis of the knee. Scand J Rheumatol. (1995) 24:238–42. doi: 10.3109/03009749509100881
36. Wylde V, Palmer S, Learmonth I, Dieppe P. Test–retest reliability of quantitative sensory testing in knee osteoarthritis and healthy participants. Osteoarthr Cartil. (2011) 19:655–8. doi: 10.1016/j.joca.2011.02.009
37. Arendt-Nielsen L, Nie H, Laursen MB, Laursen BS, Madeleine P, Simonsen OH, et al. Sensitization in patients with painful knee osteoarthritis. Pain. (2010) 149:573–81. doi: 10.1016/j.pain.2010.04.003
38. Carlesso LC, Segal NA, Frey-Law L, Zhang Y, Na L, Nevitt M, et al. Pain susceptibility phenotypes in those free of knee pain with or at risk of knee osteoarthritis: The multicenter osteoarthritis study. Arthritis Rheumatol. (2019) 71:542–9. doi: 10.1002/art.40752
39. Petersen KK, Olesen AE, Simonsen O, Arendt-Nielsen L. Mechanistic pain profiling as a tool to predict the efficacy of 3-week nonsteroidal anti-inflammatory drugs plus paracetamol in patients with painful knee osteoarthritis. Pain. (2019) 160:486–92. doi: 10.1097/j.pain.0000000000001427
40. Harden NR, Bruehl S, Stanos S, Brander V, Chung OY. Saltz, S, et al. Prospective examination of pain-related and psychological predictors of CRPS-like phenomena following total knee arthroplasty: a preliminary study. Pain. (2003) 106:393–400. doi: 10.1016/j.pain.2003.08.009
41. Wright A, Moss P, Sloan K, Beaver RJ, Pedersen JB, Vehof G. et al. Abnormal quantitative sensory testing is associated with persistent pain one year after TKA. Clin Orthop Relat Res. (2015) 473:246–54. doi: 10.1007/s11999-014-3990-2
42. Buchbinder R, Blyth FM, March LM, Brooks P, Woolf AD, Hoy DG. Placing the global burden of low back pain in context. Best Pract Res Clin Rheumatol. (2013) 27:575–89. doi: 10.1016/j.berh.2013.10.007
43. Hoy D, March L, Brooks P, Blyth F, Woolf A, Bain C, et al. The global burden of low back pain: estimates from the Global Burden of Disease 2010 study. Ann Rheum Dis. (2014) 73:968–974. doi: 10.1136/annrheumdis-2013-204428
44. Kleinstück F, Dvorak J, Mannion AF. Are “structural abnormalities” on magnetic resonance imaging a contraindication to the successful conservative treatment of chronic nonspecific low back pain? Spine. (2006) 31:2250–7. doi: 10.1097/01.brs.0000232802.95773.89
45. Chou R, Qaseem A, Snow V, Casey D, Cross JT Jr, Shekelle P, et al. Diagnosis and treatment of low back pain: a joint clinical practice guideline from the American College of Physicians and the American Pain Society. Ann Intern Med. (2007) 147:478–91. doi: 10.7326/0003-4819-147-7-200710020-00006
46. Clauw DJ, Williams D, Lauerman W, Dahlman M, Aslami A, Nachemson AL, et al. Pain sensitivity as a correlate of clinical status in individuals with chronic low back pain. Spine. (1999) 24:2035–41. doi: 10.1097/00007632-199910010-00013
47. Giesecke T, Gracely RH, Grant MA, Nachemson A, Petzke F, Williams DA, et al. Evidence of augmented central pain processing in idiopathic chronic low back pain. Arthritis Rheum. (2004) 50:613–23. doi: 10.1002/art.20063
48. National National Academies of Sciences, Engineering, and and Medicine; Health and Medicine Division; Board on Health Care Services; Board on Health Sciences Policy; Committee on Temporomandibular Disorders (TMDs): From Research Discoveries to Clinical Treatment. Yost O, Liverman CT, English R, Mackey S, Bond EC, editors. Temporomandibular Disorders: Priorities for Research and Care. US: National Academies Press. (2020).
49. Diatchenko L, Fillingim RB, Smith SB, Maixner W. The phenotypic and genetic signatures of common musculoskeletal pain conditions. Nat Rev Rheumatol. (2013) 9:340–50. doi: 10.1038/nrrheum.2013.43
50. Johansson A, Unell L, Carlsson GE, Söderfeldt B, Halling A. Gender difference in symptoms related to temporomandibular disorders in a population of 50-year-old subjects. J Orofac Pain. (2003) 17:29–35.
51. Ohrbach R, Slade GD, Bair E, Rathnayaka N, Diatchenko L, Greenspan JD, et al. Premorbid and concurrent predictors of TMD onset and persistence. Eur J Pain Suppl. (London, England). (2020) 24:145–58. doi: 10.1002/ejp.1472
52. Greenspan JD, Slade GD, Bair E, Dubner R, Fillingim RB, Ohrbach R, et al. Pain sensitivity risk factors for chronic TMD: descriptive data and empirically identified domains from the OPPERA case control study. J Pain. (2011) 12:T61–74. doi: 10.1016/j.jpain.2011.08.006
53. Greenspan JD, Slade GD, Bair E, Dubner R, Fillingim RB, Ohrbach R, et al. Pain sensitivity and autonomic factors associated with development of TMD: the OPPERA prospective cohort study. J Pain. (2013) 14:T63–74 e61-66. doi: 10.1016/j.jpain.2013.06.007
54. Greenspan JD, Slade GD, Rathnayaka N, Fillingim RB, Ohrbach R, Maixner W. Experimental pain sensitivity in subjects with temporomandibular disorders and multiple other chronic pain conditions: The OPPERA prospective cohort study. J Oral Facial Pain Headache. (2020) 34:s43–56. doi: 10.11607/ofph.2583
55. Ostrom C, Bair E, Maixner W, Dubner R, Fillingim RB, Ohrbach R, et al. Demographic predictors of pain sensitivity: Results from the OPPERA study. J Pain. (2017) 18:295–307. doi: 10.1016/j.jpain.2016.10.018
56. Maixner W, Diatchenko L, Dubner R, Fillingim RB, Greenspan JD, Knott C, et al. Orofacial pain prospective evaluation and risk assessment study–the OPPERA study. J Pain. (2011) 12:T4–11.e112. doi: 10.1016/j.jpain.2011.08.002
57. Okusogu C, Wang Y, Akintola T, Haycock NR, Raghuraman N, Greenspan JD, et al. Placebo hypoalgesia: racial differences. Pain. (2020) 161:1872–83. doi: 10.1097/j.pain.0000000000001876
58. Olson EM, Akintola T, Phillips J, Blasini M, Haycock NR, Martinez PE, et al. Effects of sex on placebo effects in chronic pain participants: a cross-sectional study. Pain. (2021) 162:531–42. doi: 10.1097/j.pain.0000000000002038
59. Greenspan JD, Traub RJ. Gender differences in pain and its relief. In: McMahon S, Klotzenburg M, Tracey I, Turk DC. Wall & Melzack's Textbook of Pain: Expert Consult - Online and Print. Amsterdam: Elsevier. (2013) p. 221–31
60. Mogil JS, Bailey AL. Sex and gender differences in pain and analgesia. Prog Brain Res. (2010) 186:141–57. doi: 10.1016/B978-0-444-53630-3.00009-9
61. Ahn H, Weaver M, Lyon DE, Kim J, Choi E, Staud R, et al. Differences in clinical pain and experimental pain sensitivity between Asian Americans and Whites with knee osteoarthritis. Clin J Pain. (2017) 33:174–80. doi: 10.1097/AJP.0000000000000378
62. Campbell CM, France CR, Robinson ME, Logan HL, Geffken GR, Fillingim RB. Ethnic differences in diffuse noxious inhibitory controls. J Pain. (2008) 9:759–66. doi: 10.1016/j.jpain.2008.03.010
63. Morris MC, Walker L, Bruehl S, Hellman N, Sherman AL, Rao U. Race effects on conditioned pain modulation in youth. J Pain. (2015) 16:873–80. doi: 10.1016/j.jpain.2015.06.001
64. Rahim-Williams FB, Riley JL III, Herrera D, Campbell CM, Hastie BA, Fillingim RB. Ethnic identity predicts experimental pain sensitivity in African Americans and Hispanics. Pain. (2007) 129:177–84. doi: 10.1016/j.pain.2006.12.016
65. Rahim-Williams B, Riley JL III, Williams AK, Fillingim RB. A quantitative review of ethnic group differences in experimental pain response: do biology, psychology, and culture matter? Pain Med. (Malden, Mass.). (2012) 13:522–40. doi: 10.1111/j.1526-4637.2012.01336.x
66. Rowell LN, Mechlin B, Ji E, Addamo M, Girdler SS. Asians differ from non-Hispanic Whites in experimental pain sensitivity. Eur J Pain (London, England). (2011) 15:764–71. doi: 10.1016/j.ejpain.2010.11.016
67. Mearin F, Lacy BE, Chang L, Chey WD, Lembo AJ, Simren M, et al. Bowel disorders. Gastroenterology. (2016). doi: 10.1053/j.gastro.2016.02.031
68. Maixner W, Fillingim RB, Williams DA, Smith SB, Slade GD. Overlapping chronic pain conditions: Implications for diagnosis and classification. J Pain. (2016) 17:T93–107. doi: 10.1016/j.jpain.2016.06.002
69. Greenwood-Van Meerveld B, Johnson AC. Stress-induced chronic visceral pain of gastrointestinal origin. Front Syst Neurosci. (2017) 11:86. doi: 10.3389/fnsys.2017.00086
70. Midenfjord I, Polster A, Sjövall H, Friberg P, Törnblom H, Simrén M. Associations among neurophysiology measures in irritable bowel syndrome (IBS) and their relevance for IBS symptoms. Scientific Rep. (2020) 10:9794. doi: 10.1038/s41598-020-66558-w
71. Jarrett ME, Han CJ, Cain KC, Burr RL, Shulman RJ, Barney PG, et al. Relationships of abdominal pain, reports to visceral and temperature pain sensitivity, conditioned pain modulation, and heart rate variability in irritable bowel syndrome. Neurogastroenterol Motil. (2016) 28:1094–103. doi: 10.1111/nmo.12812
72. Staud R. Abnormal endogenous pain modulation is a shared characteristic of many chronic pain conditions. Expert Rev Neurother. (2012) 12:577–85. doi: 10.1586/ern.12.41
73. Colloca L, Barsky AJ. Placebo and nocebo effects. N Engl J Med. (2020) 382:554–61. doi: 10.1056/NEJMra1907805
74. Colloca L, Akintola T, Haycock NR, Blasini M, Thomas S, Phillips J, et al. Prior therapeutic experiences, not expectation ratings, predict placebo effects: An experimental study in chronic pain and healthy participants. Psychother Psychosom. (2020) 89:371–8. doi: 10.1159/000507400
75. Darnall BD, Colloca L. Optimizing placebo and minimizing nocebo to reduce pain, catastrophizing, and opioid use: A review of the science and an evidence-informed clinical toolkit. Int Rev Neurobiol. (2018) 139:29–157. doi: 10.1016/bs.irn.2018.07.022
76. Taub CJ, Sturgeon JA, Johnson KA, Mackey SC, Darnall BD. Effects of a pain catastrophizing induction on sensory testing in women with chronic low back pain: A pilot study. Pain Res Manag. (2017) 2017:7892494. doi: 10.1155/2017/7892494
77. Abdulla A, Adams N, Bone M, Elliott A, Gaffin J, Jones D, et al. Evidence-based clinical practice guidelines on the management of pain in older people: executive summary. Br J Pain. (2013) 7:152–4. doi: 10.1177/2049463713495669
78. Jensen-Dahm C, Werner MU, Dahl JB, Jensen TS, Ballegaard M, Hejl AM, et al. Quantitative sensory testing and pain tolerance in patients with mild to moderate Alzheimer disease compared to healthy control subjects. Pain. (2014) 155:1439–45. doi: 10.1016/j.pain.2013.12.031
79. Stubbs B, Thompson T, Solmi M, Vancampfort D, Sergi G, Luchini C, et al. Is pain sensitivity altered in people with Alzheimer's disease? A systematic review and meta-analysis of experimental pain research. Exp Gerontol. (2016) 82:30–38. doi: 10.1016/j.exger.2016.05.016
80. Beach PA, Huck JT, Miranda MM, Bozoki AC. Autonomic, behavioral, and subjective pain responses in Alzheimer's Disease. Pain Med (Malden, Mass.). (2015) 16:1930–42. doi: 10.1111/pme.12769
81. Pickering G, Jourdan D, Dubray C Acute versus chronic pain treatment in Alzheimer's disease. Eur J Pain (London, England). (2006) 10:379–84. doi: 10.1016/j.ejpain.2005.06.010
82. Jensen-Dahm C, Vogel A, Waldorff FB, Waldemar G. Discrepancy between self- and proxy-rated pain in Alzheimer's disease: results from the Danish Alzheimer Intervention Study. Journal of the American Geriatrics Society. (2012) 60:1274–8. doi: 10.1111/j.1532-5415.2012.04036.x
83. Achterberg WP, Gambassi G, Finne-Soveri H, Liperoti R, Noro A, Frijters D, et al. Pain in European long-term care facilities: cross-national study in Finland, Italy and The Netherlands. Pain. (2010) 148:70–74. doi: 10.1016/j.pain.2009.10.008
84. Scherder EJ. Low use of analgesics in Alzheimer's disease: possible mechanisms. Psychiatry. (2000) 63:1–12. doi: 10.1080/00332747.2000.11024887
85. Horgas AL, Tsai, PF. Analgesic drug prescription and use in cognitively impaired nursing home residents. Nurs Res. (1998) 47:235–42. doi: 10.1097/00006199-199807000-00009
86. Fry M, Arendts G, Chenoweth L. Emergency nurses' evaluation of observational pain assessment tools for older people with cognitive impairment. J Clini Nurs. (2017) 26:1281–90. doi: 10.1111/jocn.13591
87. Ngu SS, Tan MP, Subramanian P, Abdul Rahman R, Kamaruzzaman S, Chin AV, et al. Pain assessment using self-reported, nurse-reported, and observational pain assessment tools among older individuals with cognitive impairment. Pain Manag Nurs. (2015) 16:595–601. doi: 10.1016/j.pmn.2014.12.002
88. Benedetti F, Vighetti S, Ricco C, Lagna E, Bergamasco B, Pinessi L, et al. Pain threshold and tolerance in Alzheimer's disease. Pain. (1999) 80:377–82. doi: 10.1016/s0304-3959(98)00228-0
89. Cole LJ, Farrell MJ, Duff EP, Barber JB, Egan GF, Gibson SJ. Pain sensitivity and fMRI pain-related brain activity in Alzheimer's disease. Brain. (2006) 129:2957–65. doi: 10.1093/brain/awl228
90. Johnson AJ, Wilson AT, Laffitte Nodarse C, Montesino-Goicolea S, Valdes-Hernandez PA, Somerville J, et al. Age differences in multimodal quantitative sensory testing and associations with brain volume. Innovat Aging. (2021) 5:1–14. doi: 10.1093/geroni/igab033
91. Othman R, Jayakaran P, Swain N, Dassanayake S, Tumilty S, Mani R. Relationships between psychological, sleep, and physical activity measures and somatosensory function in people with peripheral joint pain: A systematic review and meta-analysis. Pain Pract. (2021) 21:226–61. doi: 10.1111/papr.12943
Keywords: low back pain, temporomandibular joint disorder (TMD), irritable bowel syndrome (IBS), placebo, dementia, osteoarthritis
Citation: Weaver KR, Griffioen MA, Klinedinst NJ, Galik E, Duarte AC, Colloca L, Resnick B, Dorsey SG and Renn CL (2022) Quantitative Sensory Testing Across Chronic Pain Conditions and Use in Special Populations. Front. Pain Res. 2:779068. doi: 10.3389/fpain.2021.779068
Received: 17 September 2021; Accepted: 07 December 2021;
Published: 28 January 2022.
Edited by:
Jian Kong, Massachusetts General Hospital and Harvard Medical School, United StatesReviewed by:
Tatsunori Ikemoto, Aichi Medical University, JapanMartin Löffler, Balgrist University Hospital, Switzerland
Copyright © 2022 Weaver, Griffioen, Klinedinst, Galik, Duarte, Colloca, Resnick, Dorsey and Renn. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kristen R. Weaver, a3Jpc3Rlbi53ZWF2ZXJAdW1hcnlsYW5kLmVkdQ==
 Kristen R. Weaver
Kristen R. Weaver Mari A. Griffioen
Mari A. Griffioen N. Jennifer Klinedinst
N. Jennifer Klinedinst Elizabeth Galik
Elizabeth Galik Ana C. Duarte5
Ana C. Duarte5 Luana Colloca
Luana Colloca Susan G. Dorsey
Susan G. Dorsey Cynthia L. Renn
Cynthia L. Renn