
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pain Res., 11 October 2021
Sec. Pain Research Methods
Volume 2 - 2021 | https://doi.org/10.3389/fpain.2021.756771
This article is part of the Research TopicMechanisms and Effectiveness of Complementary and Alternative Medicine for Pain ManagementView all 8 articles
Introduction: Clumsiness has been described as a symptom associated with neck pain and injury. However, the actuality of this symptom in clinical practice is unclear. The aim of this investigation was to collect definitions and frequency of reports of clumsiness in clinical studies of neck pain/injury, identify objective measures of clumsiness and investigate the association between the neck and objective measures of clumsiness.
Methods: Six electronic databases were systematically searched, records identified and assessed including a risk of bias. Heterogeneity in designs of studies prevented pooling of data, so qualitative analysis was undertaken.
Results: Eighteen studies were retrieved and assessed; the overall quality of evidence was moderate to high. Eight were prospective cross-sectional studies comparing upper limb sensorimotor task performance and ten were case series involving a healthy cohort only. Clumsiness was defined as a deficit in coordination or impairment of upper limb kinesthesia. All but one of 18 studies found a deterioration in performing upper limb kinesthetic tasks including a healthy cohort where participants were exposed to a natural neck intervention that required the neck to function toward extreme limits.
Conclusion: Alterations in neck sensory input occurring as a result of requiring the neck to operate near the end of its functional range in healthy people and in patients with neck pain/injury are associated with reductions in acuity of upper limb kinesthetic sense and deterioration in sensorimotor performance. Understanding the association between the neck and decreased accuracy of upper limb kinesthetic tasks provide pathways for treatment and rehabilitation strategies in managing clumsiness.
Neck pain is common (1), and moderately to severely limits activity in 17–19% of the population (2). Complications from neck pain and injury, including Whiplash Associated Disorders (WAD), are frequently reported in the literature. These include the development of chronic pain (3), dizziness (often referred to as cervical vertigo) (4, 5) and disturbances in balance (6). Bring and Westman (7) made the first clinical reference to the symptom of “fumbling” as a late symptom of those suffering from traumatic neck pain. They noted that this symptom was usually described as a tendency to drop things or an insecurity or difficulty in gripping. As recognized by Bring and Westman (7) the clinical picture presented by traumatic neck pain patients is complex yet objective findings are weak. Later, Treleaven et al. (5) reported that 30% of whiplash patients described feeling “clumsy” as a symptom, exacerbating feature, or concurrent symptom associated with their dizziness or unsteadiness. The authors also found that these patients had greater cervical joint position errors when returning their head to its natural head posture after actively extending or rotating their head. These larger errors, most frequently an overshoot in estimating the position of their natural head position, were attributed to deficits in the proprioceptive information available from agonist neck muscles.
Subsequent reports referred to disturbances in sensorimotor control as a likely consequence of damage to neck structures as a result of neck injury (8–10) and included symptoms such as deficits in coordination of upper limb movement (11) fumbling (7, 12) or clumsiness (12, 13).
Knox et al. (13) reported that rotation of the neck just prior to the point of pain reproduction, was associated with an increased elbow joint position error in a clinical population of people with whiplash injury. A further study reported that decreased elbow joint position sense accuracy occurred even in healthy people at end range of neck rotation (14). The authors of this latter study (14) suggested that the processing of neck proprioceptive information at the extreme of neck range of motion (ROM) might be responsible for the decreased acuity of upper limb position sense. The result of the Knox et al. study (13) that demonstrated increased elbow joint position error when the head-neck was rotated to a point just before participants reported an increase in neck pain or discomfort was interpreted as those suffering neck pain nearing the end point of their functional neck range of motion. The results of the Knox et al. study therefore may be considered to mirror the results of the Knox and Hodges study (14).
These studies point to the role that the neck plays in the brain's understanding of where its body parts are positioned in relation to each other. The central nervous system must be able to differentiate between: the whole body moving; the body changing position relative to the head; and movement of just the head. Roll et al. (15) described the contribution of whole-body proprioceptive inputs, including those from the neck, in the construction of an internal representation of body segments in relation to each other and in extrapersonal space. Therefore, proprioceptive signals from the neck play a pivotal role in both the construction and continual updating of the CNS' internal body representation. This requires the integration of vestibular, neck and trunk proprioceptive signals and of these, the neck proprioceptive signals provide critical information regarding head-neck information relative to the trunk (16).
So, the question that arises is, what evidence do we have that neck pain is associated with the symptom of clumsiness? As noted above Bring and Westman (7) made the first clinical reference to “fumbling” which was then given an operational definition by Sandlund et al. (11) and Knox et al. (13) the latter who described fumbling or clumsiness as a deficit in coordination of the upper limb. Therefore, the purpose of this systematic review was to review evidence of an association between the neck and clumsiness, when clumsiness was defined as upper limb position and movement sense and sensorimotor task performance. This was investigated in both neck pain and/or injury and healthy cohorts. In particular, the aims were to:
1. Summarize frequency of reports of clumsiness from the clinical literature where clumsiness is associated with neck pain and/or injury and how clumsiness was defined in this context;
2. Review how clumsiness, a deterioration in performance of an upper limb sensorimotor task as this symptom is operationally defined from clinical studies, is investigated in cohorts experiencing neck pain and/or injury; and in a healthy cohort where studies used upper limb sensorimotor task performance as the outcome and non-artificial neck exposures; and
3. Review the evidence that there is an impairment in performance of upper limb sensorimotor tasks in the presence of neck pain or injury or, in a healthy group when a natural intervention is applied.
This review was carried out in line with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (17). The PRISMA 2009 27-item checklist has been provided as Supplementary Material A. A review protocol was established and included search strategies, inclusion and exclusion criteria and methods of analysis. An outline of this protocol has been listed below. A full copy of the protocol is available upon request.
To be included, the studies must have met all the selection criteria.
1. human and between the ages of 18–65 years;
2. acute, sub-acute or chronic non-specific neck pain and/or neck injury and/or whiplash;
3. and/or healthy participants where as part of the testing protocol there was either an induced change in the neck position of the participant or fatigue of their neck muscles;
4. excluded if participants had a history of cancer, fracture, infections, rheumatological disorders, neurological disorders, or surgical procedures for spinal or extremity disorders.
5. excluded if there was insufficient documentation or information on participant demographics or if data extraction was not possible.
The type of study designs included case control, cohort and randomized control trials. Articles were included if they were published as a full paper or an abstract with sufficient detail to extract the main attributes of the study.
Studies were limited to those published in peer-reviewed journals, without language restriction. Publications were excluded if they were duplicate studies or reviews.
To be included, studies had to report one of the following outcome measures: upper limb joint position sense; upper limb joint position error; upper limb movement task; clumsiness and/or fumbling; upper limb proprioception; or coordination in the extremity/limb. Results were excluded from analyses if methods involved the use of artificial stimulation of senses (such as galvanic vestibular stimulation or vibration) or microgravity as these were considered beyond the scope of natural interventions.
In the initial design of this review the lower limb was included in the search terms. No papers pertinent to lower limb and clumsiness were found, therefore this review only reported on clumsiness associated with the upper limb.
Searches (initial and updated) were conducted using PubMed, EMBASE, CINAHL, Index to Chiropractic Literature, Cochrane Library and Scopus from date of inception to 3/3/21. This was conducted by the first author and checked by another author (JK).
A comprehensive search strategy was developed by identifying and listing all potentially relevant search terms, categorizing these into specific search phrases and combining them using Boolean terms. Terms included keywords and phrases “neck pain,” “neck injury,” “whiplash,” “healthy,” “position sense,” “kinesthesis,” “clumsiness,” “proprioception,” “joint position sense,” “upper extremity.” PubMed was searched using MeSH terms. A sample of the PubMed search strategy is provided in Supplementary Material B. The detailed search strategy is available upon contacting the corresponding author.
The reference lists of selected articles retrieved in the original online search were also screened for relevant studies not identified through electronic searches by the lead and last author. Citation searches of the identified relevant studies were conducted using PubMed and Scopus databases.
The first and last author (SH and BP) screened the title and abstracts of the articles based on the inclusion/exclusion criteria and full reports were obtained of all the studies identified as potentially eligible. If any title or abstract did not provide enough information to decide whether the inclusion criteria were met, then the full text was obtained. All of the full-text studies were then independently evaluated. Discrepancies in judgement were first resolved by discussion with two reviewers, however, if consensus was not reached, a third reviewer was used to arrive at a decision.
An assessment of the risk of bias and precision was conducted by the lead and last author using the risk of bias assessment tool of Viswanathan and Berkman (18). As reported by these authors the tool has been developed to evaluate “…the degree to which the effects reported by the study represent the ‘true' causal relationship between exposure and outcome.” This assessment tool has an item bank with a series of items applicable for observational studies from which relevant items may be selected and defined in order to assess their internal validity. Individual items from this bank identified by the authors as relevant to this review were then selected, defined and applied to each of the studies retrieved for analysis, with the subsequent risk of bias findings tabulated (see Table 1). To support this quality assessment, a numerical value was assigned to each criterion. If an assessment was determined as high, it received a numerical score of 1. Any lower assessment received a score of 0. We then classified the overall score of quality as low (0–2), moderate (3, 4) or high (5, 6) after Zhang et al. (19). The sum of all values provided the basis to quantitatively assess overall quality.
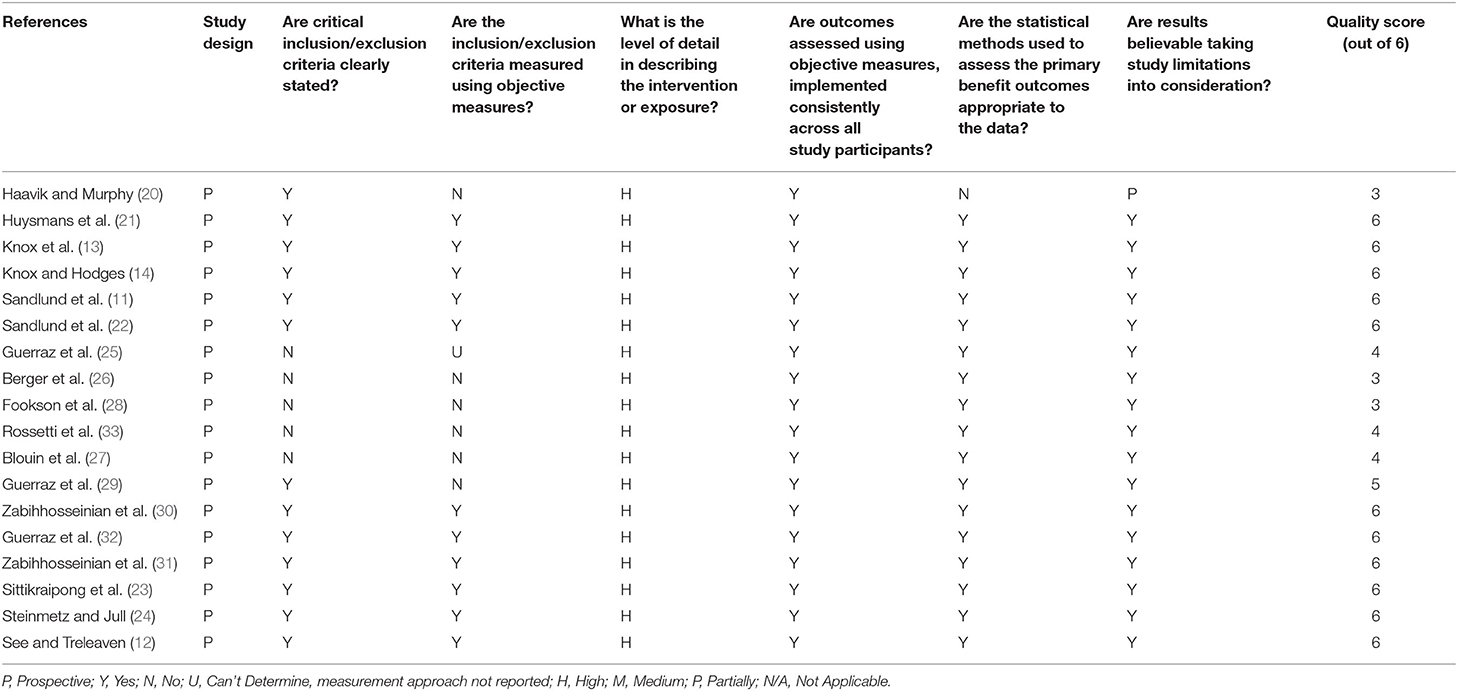
Table 1. Risk of bias [adapted from Viswanathan and Berkman (18)].
General information regarding participants' characteristics and demographics (see Table 2) was obtained by the lead and last author, including the number of participants, age, gender, criteria for inclusion/exclusion, health status (i.e., healthy, idiopathic neck pain, whiplash), questionnaires used to measure pain and/or disability, function and mental status, and pain duration and intensity. In order to standardize the data extraction process between the reviewers, detailed data extraction sheets were devised and used to acquire information concerned with research questions about the role of the neck in joint position and movement sense and motor performance of the upper extremity. These results are shown in Tables 3–5. In particular information was sought to determine (i) whether clumsiness was mentioned and how it was operationally defined, (ii) if clumsiness was not mentioned, an explanation for why joint position sense and/or motor performance were used as outcome measures, (iii) the association between neck position or movement and joint position sense or motor performance of the upper limb, (iv) the association between neck pain/injury and joint position sense or motor performance of the upper limb, (v) previous pain intensity and duration, (vi) neck and body position of participants during the tests, (vii) visual condition (eyes open/closed) and (viii) the method used to measure joint position sense/motor performance (e.g., electrogoniometer, electromagnetic tracking).
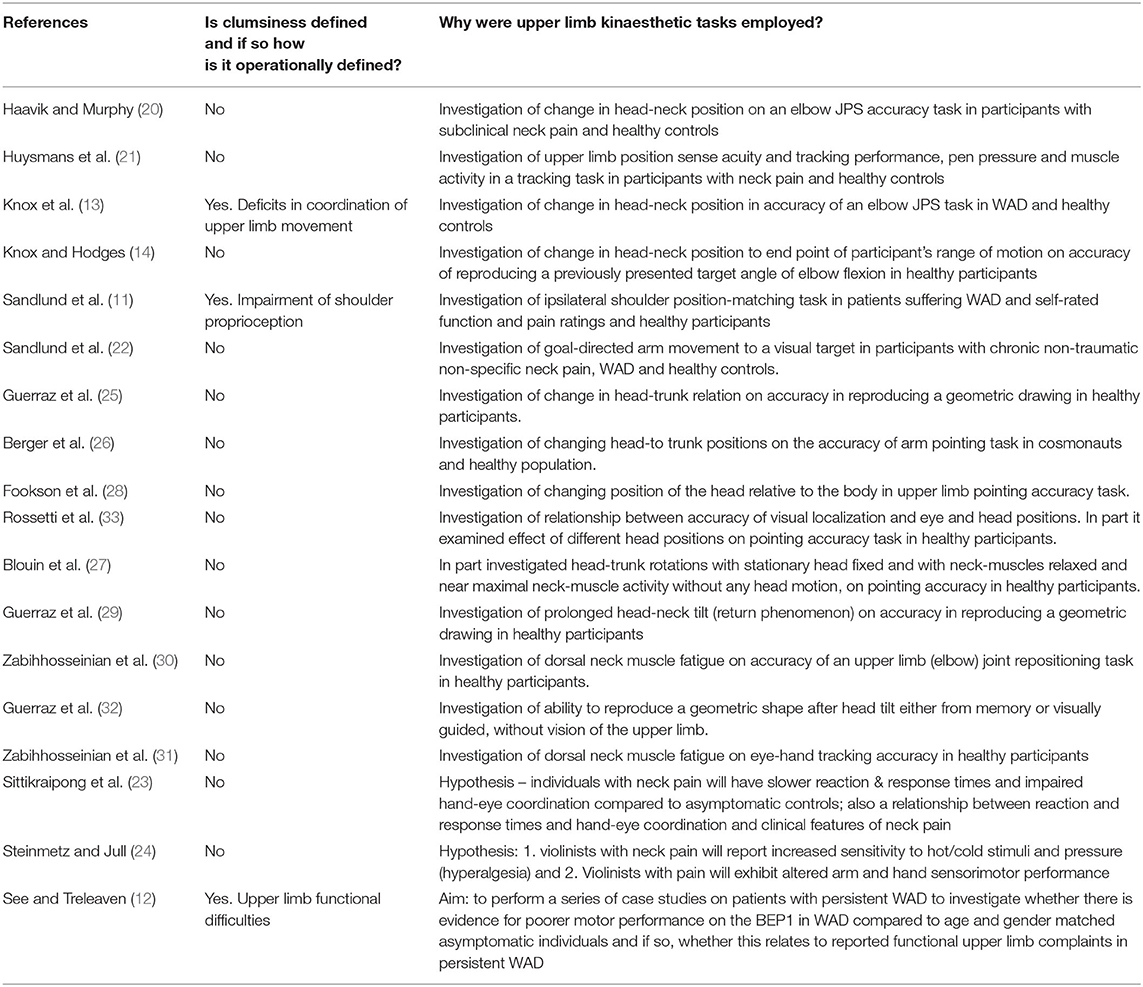
Table 3. Definitions of clumsiness and investigations of performance of upper limb kinesthestic tasks.

Table 4. Association between the neck and changes in accuracy in completion of upper limb sensorimotor tasks.

Table 5. Association between pain and self-rated function and upper limb sensorimotor task performance.
Finally, in order to investigate evidence in support of the role of pain and/or functional limitations in neck neuromusculoskeletal performance in contributing to an impairment in performance of upper limb sensorimotor tasks, additional data were extracted to determine whether there was a relationship between measures of disability or self-rated functioning and performance or pain and the results of sensorimotor tasks (see Tables 4, 5).
The online search strategy identified 2006 studies. Additional records identified through other sources numbered 29. Duplicates were removed leaving 1,239 studies. Of these, abstracts were screened by both reviewers based on the inclusion/exclusion criteria. From this process 42 studies were selected for full-text retrieval and assessed for eligibility. Consensus was reached to include 18 studies in the review. The most common reasons for rejecting articles were that they: were animal studies; didn't have sufficient detail; were not relevant (e.g., investigated head-trunk position sense without reference to the upper limb); or used artificial stimulation (e.g., vibration or galvanic stimulation). Search results and the selection process are summarized in Figure 1. Heterogeneity in designs of studies prevented pooling of data, so qualitative analysis was undertaken.
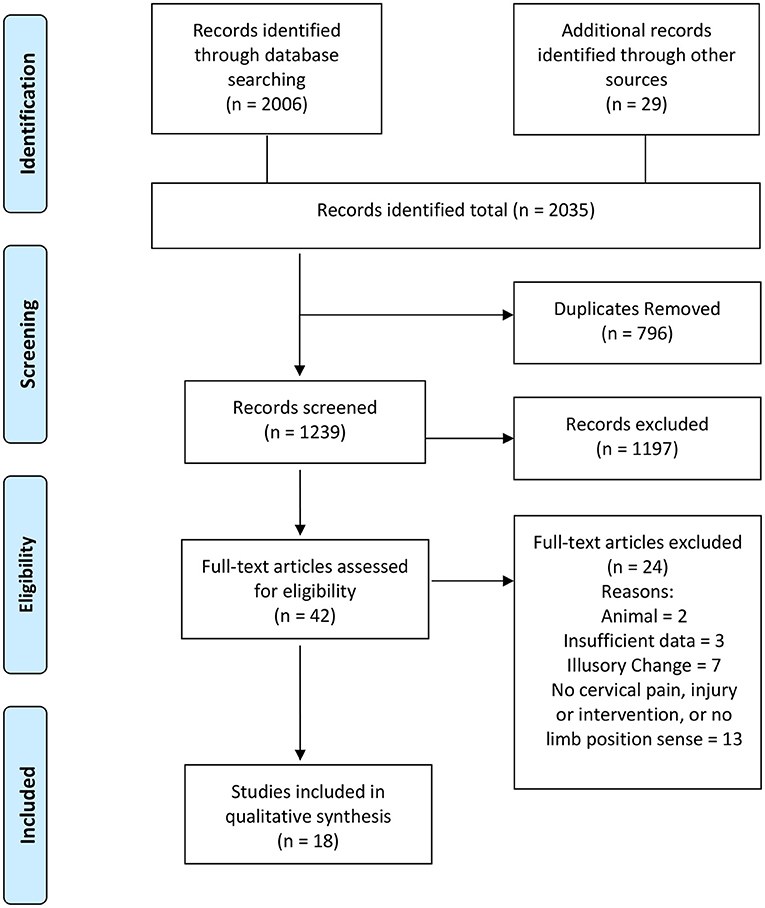
Figure 1. PRISMA flow diagram. Adapted from Moher et al. (17).
From the studies included in the review, all were prospective. Eight were cross-sectional studies and ten were case series. The cross-sectional studies compared healthy participants and participants with neck pain and/or injury (11–13, 20–24); and the case series assessed a healthy cohort in pre-test/post-test studies (14, 25–33).
Participant demographics and health status were described in appropriate detail in 17/18 (94%) studies and included: the inclusion/exclusion criteria; the number of participants; participant gender, age and health condition; and questionnaires used to evaluate pain, disability, function and mental status in clinical cohorts (see Table 2) (11–14, 20–27, 29–33). Of the eight cross-sectional studies, participant groups included healthy 8/8 (100%), subclinical neck pain (1/8) (13%) (20), neck pain 4/8 (50%) (21–24) and those suffering WAD in 4/8 50%) (11–13, 22) studies. Three of four of the WAD studies (11, 13, 22) graded the severity of WAD as II or III, in accordance with the Quebec Task Force classification on whiplash-associated disorders (34). This classification is a widely accepted system for describing the different levels of dysfunction and symptomatology for whiplash. Participants for the majority of studies were predominantly aged 18–45 years (15/18, 83 %) (11, 13, 14, 20, 22–27, 29–33) with 3/18 (20%) including participants who were over 45 years old (12, 21, 28). Information was also presented on gender in 15/18 studies (83%) (11–13, 20–25, 28–33), handedness was reported in 14/18 (78%) (11, 13, 20–22, 25–33) studies and dominant upper limb reported as used in performance of upper limb kinesthetic tasks by all participants in 13/18 studies (72%) (11, 21–23, 25–33). One study assessed motor performance of dominant and non-dominant hands (12).
A high level of detail of inclusion/exclusion criteria was used to recruit participants and was reported in 13/18 (72%) studies (11–14, 20–24, 29–32). Questionnaires were used to measure characteristics of participants including pain, disability, function and/or mental status in relevant studies (see below).
In those studies that included participants experiencing pain, outcomes of pain duration and intensity were recorded in 6/8 (75%) (11–13, 21–23) and 6/8 (75%) (12, 13, 21–24) studies, respectively. Outcome measures used to evaluate the various dimensions of neck pain/injury included the Neck Disability Index questionnaire and pain visual analog scales used in 7/8 (88%) studies (11–13, 21–24) and the Quality of life, self-efficacy, Disability arm/shoulder/hand and TAMPA scale of kinesiophobia questionnaires variously used in 5/8 (63%) studies (11–13, 21, 22). Out of the remaining 10/18 (56%) studies which used a healthy cohort only, one study (31) confirmed the absence of neck pain using the Neck Disability Index.
The risk of bias analysis for all studies included in this review is presented in Table 1.
The overall quality of all studies was moderate to high with 17/18 (94%) (11–14, 21–33) studies having believable results as indicated by Viswanathan and Berkman (18). Fifteen of the 18 studies (83%) (11–14, 21–25, 27, 29–33) achieved a score of 4/6 or greater resulting in a quality of score of high. The remaining studies scored more than 2/6 (20, 26, 28) indicating a quality score of moderate.
All studies were prospective, used objective measures and had a high level of detail in describing the exposure used. Inclusion/exclusion criteria were clearly stated in 13/18 (72%) studies (see above), with 11/18 (61%) measuring inclusion/exclusion criteria using objective measures (11–14, 21–24, 30–32) and all used appropriate statistical methods. One study did not accurately report statistical analysis (20).
Details of definitions of clumsiness, and/or investigations of the effect of head-neck position and/or pain on upper limb kinesthesia are shown in Table 3. Clumsiness was defined in 3/18 (17%) studies (11–13) where each reported it as a clinical manifestation associated with neck pain and/or injury. Two studies operationally defined this as a deficit in coordination of upper limb movement or an impairment in upper limb proprioception (11, 13). Both Sandlund et al. (11) and Knox et al. (13) made reference to Bring and Westman (7) who reported fumbling as a clinical sign/symptom of whiplash. Knox et al. ( 13) used a cross-sectional study (WAD and healthy participants) to examine accuracy of reproducing a target elbow angle after head and neck movement to a point before pain was experienced. The change in head-neck position was, therefore, less than full range of neck motion. The aim of their study was to investigate the effect of changes in head and neck position on the perception of elbow position in people with chronic and disabling neck pain after a whiplash injury. The group suffering WAD recorded reduced proprioceptive acuity compared to their healthy counterparts when the head-neck of the healthy group was moved to the same average degree of rotation as the WAD group away from midline (neutral) position. Sandlund et al. (11) also used a cross-sectional study design (WAD and healthy groups) but examined shoulder joint movement to previously defined target positions with no change in head position. They hypothesized that people with WAD have impaired shoulder proprioception.
Clumsiness was not explicitly referred to in the remaining studies. However, all studies made reference to the role of the neck in interpreting the position of body segments relative to each other and/or in extra-personal space.
The position/s and/or movement/s of the neck and the form of the upper limb task are detailed in Table 4. In particular this table identified: the head-neck movement performed; the position of the body during upper limb task performance; the upper limb kinesthetic task performed, including the visual condition of the participant during the performance of the upper limb task; the instruments used for measurement of upper limb performance; the outcome measures reported; and the results of the studies.
Ten of 18 (59%) studies investigated a change in head position on upper limb task performance with 6/10 (60%) of these studies passively changing head position (13, 14, 20, 25, 29, 32). One study (27) induced neck rotation by passive movement of the trunk against a fixed head while neck muscles were relaxed or when neck muscles were actively contracted and a further two studies (28, 33) required participants to actively move their head-neck to different angles of rotation. Fookson et al. (28) stated that the neck was moved to its end ROM while participants of the Rosetti et al. (33) study were required to move their head-neck incrementally to 80 degrees. Berger et al. (26) did not indicate whether a change in head-trunk position was achieved actively or passively. Six of 18 (33%) studies maintained the head in a straight-ahead position with each study investigating participants with neck pain and/or those having a WAD (11, 12, 21–24). Two studies induced dorsal neck muscle fatigue prior to measurement of the upper limb kinesthetic task (30, 31).
Studies investigated the role of neck pain/injury, or changes in head-trunk position, or muscle fatigue on: (i) an active upper limb goal directed movement such as the participant pointing at a target 7/18 (39%) (21, 22, 26–28, 31, 33); (ii) upper limb joint position sense (JPS) 5/18 studies (28%) (11, 13, 14, 20, 30); (iii) drawing tasks 3/18 (17%) (25, 29, 32); (iv) reaction and response times 3/18 (17%) (12, 23, 24) and (v) upper limb tracking task 3/18 (17%) (21, 23, 31). Experimental protocol descriptions for upper limb tasks and head-neck position/s or movement/s were detailed in all but one study (26) as was the visual condition of participants 18/18 (100%). The test position of the participant was stated in the majority of studies, 6/18 (33%) supine (13, 14, 20, 25, 29, 32), 2/18 (11%) (21, 30) standing and 9/18 (50%) seated (11, 21, 22, 25–28, 31, 33). Three studies did not explicitly indicate participants' posture during testing although it may be presumed that participants were seated (12, 23, 24).
All studies used objective means of measurement for upper limb task performance. The types of instrumentation used included electromagnetic trackers used in 6/18 (33%) studies (11, 22, 25, 27, 29, 32); an electrogoniometer used in 3/18 (17%) studies (13, 14, 20); Infra-Red LEDs (IR-LEDs) used in 4/18 (22%) studies (26, 28, 30, 33) and a digitized tablet or touchscreen used in 3/18 (17%) studies (21, 23, 31). One study (23) measured response and reaction time using a hand-held electronic timer with modified computer mouse and two studies used the Human Performance Measurement/Basic Elements of Performance (HPM/BEP) device (12, 24).
Only eight of the 18 studies (44%) included in this review involved a clinical cohort where participants suffered a neck complaint. These complaints included subclinical neck pain (20), chronic neck pain (21–24) and WAD (11–13, 22). All studies (100%) used various forms of an upper limb sensorimotor task (that is a task involving a sensory stimulus and an active upper limb movement in response to the sensory stimulus). Two of the eight studies (25%) involved a passive head movement prior to assessment of task performance (13, 20). Of these, one involved movement of the head and neck “almost to the end of their range of motion” (20) while the other passively moved the head and neck to a point that avoided increasing each participant's pain or discomfort (13). In three studies (38%), the head-neck remained in a head-neck straight ahead position (11, 21, 22) while a further three studies (12, 23, 24) (38%) did not indicate head position although given the type of task performed, it would be expected that the head was maintained in a neutral position. All but one of the cross-sectional studies (88%) demonstrated a deterioration in performance of the sensorimotor task when compared to a healthy control group. The study not reporting a reduction in performance of an upper limb sensorimotor task was conducted with a cohort of professional violinists/violists (24).
Ten of the 18 studies (56%) investigated a healthy cohort only. The majority of these studies, 7/10 (70%) involved passive head-neck or neck-trunk movement approximating the extreme of head neck range of motion (14, 25–28, 32, 33). Two of ten studies (20%) induced dorsal neck muscle fatigue (30, 31), while one study used the return phenomenon which consisted of passively tilting the head-neck to one side and maintaining that position for 15 min prior to the head-neck being returned to the head straight ahead position (29). In all studies, these natural maneuvers resulted in a deterioration in performance of upper limb sensorimotor tasks.
Of the eight studies included in this review that conducted prospective, cross-sectional research all broadly included a neck pain group and a healthy group as previously reported. We were interested in exploring whether any relationship existed between upper limb sensorimotor task performance and measures of pain or self-rated function and disability. The results of this analysis are provided in Table 5. Six of the eight studies specifically reported on this relationship (11–13, 21–23). Of these, 3/6 studies reported a significant association between measures of pain and upper limb sensorimotor task performance (12, 13, 22); that is, the more intense the pain, the more impaired the upper limb task performance. Four of six studies reported an association between disability, self-rated functioning and sensorimotor task performance (11, 21–23) and a similar relationship applied to the intensity of pain. Further, 3/6 of these studies reported an increased variability of errors in task performance (11, 21, 22).
This systematic review found a small body of literature of moderate to high quality, with all but one study, demonstrating that in the presence of neck pain or injury or when a natural intervention is applied to the head-neck that provokes the neck to function close to extreme limits in a healthy cohort, this is associated with a deterioration in the accuracy of performance of upper limb sensorimotor tasks, or the accurate perception of upper limb joint position. As far as we are aware, this is the first study to link experimental and clinical studies that demonstrate disordered sensorimotor integration to altered neck sensory input. While only three studies explicitly referred to clumsiness/fumbling (11–13) and two of these referred to this clinical phenomenon as a deficit in coordination of upper limb movement or impairment of upper limb proprioception (11, 13), all studies except one in this review arrived at a consistent result. The result of this review highlights the importance to clinicians to specifically investigate whether their patient experiences clumsiness or fumbling associated with their neck pain or injury and to implement rehabilitative strategies to address this issue.
This review also uncovered a pattern of evidence that supports the proposal that pain itself, or decreased levels of self-rated functioning in the presence of neck pain/or injury is associated with a decrease in the performance of various upper limb sensorimotor tasks.
As noted, analysis of the details of these studies was restricted to a qualitative analysis principally due to the variability in study designs as well as outcome measures used to measure upper limb sensorimotor task performance. Nevertheless, the overall quality of the studies was considered moderate to high. We conclude that the strong consistency of the results of this review overrides any deficiency in the assessment of the quality of the studies specifically as this relates to the reporting of inclusion and exclusion criteria, the reporting of statistical analyses or some lack of detail in describing exposures.
All the studies included in this review can be broadly divided into one of two categories. The first category (5/18 studies) includes those studies that examined the performance of a sensorimotor task in intra-personal space in the presence of neck pain only (20), neck pain associated with injury (11, 13) or in a healthy cohort when a natural intervention was applied (14, 30). That is, sensorimotor tasks involved on-going assessment of segment-to-segment movement and position. The second category included studies (13/18) where sensorimotor performance involved the assessment of the position and/or movement of an object in extra-personal space as well as knowledge of the position and movement of the upper limb (12, 21–29, 31–33). In the first category the conclusion reached by all studies was that changes in head-to-trunk orientation, extensor neck muscle fatigue or in the presence of neck pain or injury is associated with a decrease in accuracy in estimating a previously presented upper limb joint position. This inaccuracy was interpreted as a disruption in the updating of the internal body schema as a consequence of altered neck afferent input.
The second category of studies is more complex to analyze because these studies examined upper limb task performance on multiple levels. On the first level, the CNS must identify the position and movement of the upper limb, that is, it makes reference to an internal representation of the body as described above. The CNS must do this while, on the next level, the upper limb moves and positions itself in relation to an object in external space. Therefore, the CNS must also encode an external target position. Blouin et al. (27) concluded that this is achieved by the CNS using a trunk-centered reference system allowing the external target to be located relative to changing body position. This is achieved partly by using proprioceptive input from the neck and integrating this information with that of vision although there is evidence that proprioception may play a dominant sensory input in motor planning (32). Indeed as articulated by Guerraz et al. (32), regardless of the sensorimotor task, changes in the positional relationship between the head-trunk, results in an internal bias in the representation of the head-trunk which is used to accurately navigate the position and movement of the self's body segments to the location of the external target.
In this regard it is worth noting the study of Guerraz et al. (29) who used the “return phenomenon” as the intervention to change head-on-neck orientation. The return phenomenon occurs as a result of a change in the perception of position of the head-to-trunk position rather than the actual head-to-trunk position, the perception of which changes over time. This was first demonstrated by Gurfinkel and Levik [as cited by Levik (35)]. In the Guerraz et al. study (29) participants were asked to repeatedly draw a line “aligned with their trunk.” The magnitude of angular deviation of the line drawing task gradually diminished during head tilt, but never entirely. When the head was returned to be aligned with the trunk, participants reported the perception that their head tilted in the opposite direction with respect to the initial direction of tilt. Interestingly so too did the angular deviation of the drawn straight line with the degree of deviation decreasing as the perception of head tilt reduced.
Guerraz et al. (29) argued that both the position of the head during the initial stages of head tilt and the resultant deviation in line drawing represented a bias in the trunk-centered reference system of the internal body schema provided by neck proprioception alone. Over time, as participants perceived that their head slowly returned to a neutral position so too did the extent of line orientation of the drawing task although there was no significant correlation between them. The authors argued that the return phenomenon was responsible for participants' perception of their head position rather than the actual head position and this was reflected in the motor task of line drawing. This study highlighted the depth of the concept of the system of internal representations of body constructs both within intrapersonal space as well as the body's relationship to external objects in the environment as described by Levik (35). Therefore, the results of the study during the initial stages of head tilt are consistent with the finding that neck proprioceptors contribute to the continual updating of the internal body schema.
In summary, all the studies in this second category, except one (24), arrived at the same conclusion, that, there is a deterioration in the performance of sensorimotor tasks in the presence of neck pain, and/or an injury to the neck or when the head-neck functions close to the endpoint of its range.
Eight of the 18 studies of this review investigated upper limb sensorimotor task performance in the presence of neck pain. All but one of these studies determined that in the presence of neck pain, the accuracy in performance of sensorimotor tasks was reduced. Furthermore, six of these studies reported a positive association between the severity of reduction in performance of the upper limb task and levels of pain, physical functioning or levels of reported self-efficacy. While the number of studies that reported these findings is small and the variety of testing procedures varied, the results of this review point to a relationship between neck pain where an individual experiences a higher level of reduced physical functioning or self-efficacy and clumsiness.
It is of interest to note here the findings of (24) the only study that did not report the association. Their study investigated sensorimotor function in violin and viola players with and without neck pain. They found no difference in the performance of motor tasks between violinists and violists with and without neck pain. As concluded by these authors it may be that fine motor tasks associated with the playing of their instruments may be a more appropriate way to assess whether a deterioration in function is actually occurring. Further, to our knowledge, it is not yet established whether the types of finely developed motor skills of these musicians might mask the effects of a deterioration in sensorimotor function as measured by standard upper limb sensorimotor tests.
The results of our review also demonstrate that when the neck is required to function near the limits of its normal range, that here too, a deterioration in the performance of upper limb sensorimotor tasks does occur. All in all, the results of this review provide strong evidence that disturbances in and of the neck are associated with clumsiness.
How do we know the position of our limb in relation to the rest of our body or where it has moved if we cannot see it? How do we perceive where our different body segments are positioned in relation to the external world? Scientists refer to this knowing as the “body schema” where the brain maps and continually updates the body's “shape and posture” (36). It is well-known that the concept of the internal body schema is reliant on the collective proprioceptive inputs to both construct and update the internal body schema (37) including those of the neck (16). It is also well-known that neck muscles have a high density of proprioceptors, notably muscle spindles (38–40) and that projections from these receptors enter the CNS reaching multiple areas of the brain including the vestibular system - an important integration center for sensorimotor control (8, 16). Proprioceptive information also reaches the cerebral cortex and “tunes motor commands for spatially oriented movements” as stated by Palliard (41).
In a recent review by Pettorossi and Schiepatti (16), they reported there is strong evidence that proprioception from neck muscles is significantly involved in the construction and updating of the position and movement of the body and its segments in order to inform reference frames for movement. Haavik et al. too (42), identified that the function of the deep (small) paraspinal muscles and their proprioceptive input are capable of altering (or maintaining) an accurate record of the brain's internal body schema.
Therefore, it seems reasonable that given the central role of neck proprioceptors in the construction and updating of the internal body schema that, in the face of a disturbance in neck function such as occurs in the presence of pain or injury, there is the potential to disrupt the transmission of proprioceptive signals or to distort the processing of proprioceptive input which, in turn, alters the internal body schema.
This review also provided support for the contribution of pain in disrupting the body schema. While the studies included in this review did not use quantitative sensory testing of pain to examine the presence of central sensitization except for the article of Steinmetz and Jull (24), we did find evidence for an association between the extent of deterioration in performance of upper limb sensorimotor tasks and self-reported measures of pain, physical functioning and levels of reported self- efficacy. There is evidence, that central sensitization is responsible for ongoing reports of pain and disability in people suffering chronic WAD (43) and altered central pain processing in those with non-traumatic neck pain (44). Future studies need to examine if disturbed body schema is part of central sensitization, and partly responsible for deteriorated quality of life and disability associated with chronic musculoskeletal pain.
This is the first review that has attempted to systematically investigate whether there is an association between clumsiness and neck pain injury. Strengths of this study include that it followed the Cochrane protocol (17) with a comprehensive search conducted among key databases and independent reviewers selecting studies and extracting the data.
Overall, the quality of evidence was moderate to high; and importantly, the results, regardless of whether studies involved a clinical sample or healthy volunteers, consistently showed that when the neck's neuromusculoskeletal elements were compromised due to pain [cf. (24)], injury, fatigue or extreme range of movement, that this was associated with increased errors in upper limb performance. Another strength of this review is that the authors came from both neuroscience and clinical disciplines. The experience from these different backgrounds allows for dual perspectives to ensure the findings of neurophysiological studies remained clinically relevant.
The main weakness of this review is the relatively low number of studies found, 18 in total, with only three studies (11–13) describing clumsiness as a clinical manifestation associated with neck pain and injury and one (13) operationally defining clumsiness. This issue was identified during our initial search and addressed by developing a range of terms to represent clumsiness to broaden the literature search. In addition, the sample size of each study was small, ranging from 6 to 120 participants. The outcome measures applied were also diverse. All together it was not possible to pool all data to conduct a meta-analysis. Nevertheless, all studies provided sufficient details of their sample, interventions and outcomes used to measure performance of upper limb sensorimotor tasks to enable us to conduct a qualitative review and assess the association between the neck and upper limb performance.
Given the findings of this review the major question that arises is what do we know about current prevalence of clumsiness in patients who present to our clinics? We were able to identify only one study from 2003 (5) reporting 30% of their sample (N = 102) of people suffering persistent WAD with an accompanying symptom of dizziness (N = 76) who also complained of clumsiness. Yet, the results of this review indicate that disturbances in upper limb sensorimotor task performance might be expected to occur more frequently in clinical populations presenting with conditions other than WAD given that a deterioration in performance of upper limb tasks can occur even when the neck is required to function at more extreme limits. Examples of such neck function may include the operation of machinery that requires the head-neck to be turned to near end range of motion, or fatigue of neck muscles when the head-neck is required to assume a static posture for a prolonged period.
A further area of much needed research is in older people with neck pain. Vogt et al. (45) found a prevalence of between 11 and 19% (male/female) musculoskeletal pain involving the neck and shoulder in 70–79 year-old people living in the community in a study that collected data between 1997 and 1998. A more recent study by Quek et al. (46) found higher levels of dizziness handicap in older people with neck pain when compared to those without neck pain. This latter study used the dizziness handicap inventory as part of a battery of tests to examine characteristics of older people with neck pain who also presented with impaired postural stability. These authors concluded that neck-pain induced deficits in indices of postural instability including dizziness may have occurred as a result of deficits in neck proprioception as a consequence of neck pain. It is well-established that dizziness in older people is highly prevalent and that prevalence increases with age, while causes of dizziness remain unknown in 20–40% of patients reporting to medical practice physicians according to Dros et al. (47). Given the finding of Treleaven (5) that a substantial proportion of WAD sufferers also described symptoms of clumsiness, there is a need for further investigation of clumsiness and neck pain in older people to enable non-pharmacological treatment and rehabilitation strategies for this age group.
We contend that there is a need to specifically address the clinical issue of clumsiness including investigations of clumsiness in older people and in the workplace, the latter to discover whether tasks that require the head-neck to function at extreme limits are associated with a deterioration in upper limb sensorimotor task performance.
This review found limited research reporting on the specific symptom of clumsiness despite reports of a prevalence of 30% of WAD sufferers reporting symptoms of clumsiness as a consequence of neck injury (5). Disturbances in neck sensory input in both healthy people and those with neck pain and/or injury are associated with reductions in the acuity of upper limb kinesthetic sense and a deterioration in sensorimotor performance. Further research should investigate the specific role that a disruption in neck muscle proprioceptive input plays in alterations to the internal body schema in both healthy people and those with musculoskeletal conditions. This will enable the development of evidence-based manual therapy treatment and rehabilitation strategies for restoring the distorted body schema associated with neck pain/injury.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
SH: concept development, study selection, literature search, data extraction, data analysis, data interpretation, and manuscript draft. ZZ: concept development, study selection, data analysis, data interpretation, manuscript draft, and manuscript revision. JK: literature search, data interpretation, and manuscript draft. DV: concept development, data interpretation, and manuscript draft. BP: concept development, study selection, literature search, data extraction, data analysis, data interpretation, manuscript draft, and manuscript revision. All authors contributed to the article and approved the submitted version.
SH was supported by a scholarship from the Australian Chiropractors' Association and an RMIT University postgraduate scholarship.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpain.2021.756771/full#supplementary-material
1. Hoy D, Protani M, De R, Buchbinder R. The epidemiology of neck pain. Best Pract Res Clin Rheumatol. (2010) 24:783–92. doi: 10.1016/j.berh.2011.01.019
2. Fejer R, Kyvik KO, Hartvigsen J. The prevalence of neck pain in the world population: a systematic critical review of the literature. Eur Spine J. (2006) 15:834–48. doi: 10.1007/s00586-004-0864-4
3. Carroll L, Holm L, Hogg-Johnson S. Results of the bone and joint decade 2000–2010 task force on neck pain and its associated disorders. Spine. (2008) 33:S83–S92. doi: 10.1097/BRS.0b013e3181643eb8
4. Devaraja K. Approach to cervicogenic dizziness: a comprehensive review of its aetiopathology and management. Eur Arch Oto Rhino Laryngol. (2018) 275:2421–33. doi: 10.1007/s00405-018-5088-z
5. Treleaven J, Jull G, Sterling M. Dizziness and unsteadiness following whiplash injury: characteristic features and relationship with cervical joint position error. J Rehabil Med. (2003) 35:36–43. doi: 10.1080/16501970306109
6. Stokell R, Yu A, Williams K, Treleaven J. Dynamic and functional balance tasks in subjects with persistent whiplash: a pilot trial. Man Ther. (2011) 16:394–8. doi: 10.1016/j.math.2011.01.012
7. Bring G, Westman G. Chronic posttraumatic syndrome after whiplash injury: a pilot study of 22 patients. Scand J Prim Health Care. (1991) 9:135–41. doi: 10.3109/02813439109026597
8. Treleaven J. Sensorimotor disturbances in neck disorders affecting postural stability, head and eye movement control. Man Ther. (2008) 13:2–11. doi: 10.1016/j.math.2007.06.003
9. Montfoort I, Kelders WP, Van Der Geest JN, Schipper IB, Feenstra L, de Zeeuw CI, et al. Interaction between ocular stabilization reflexes in patients with whiplash injury. Invest Ophthalmol Vis Sci. (2006) 47:2881–4. doi: 10.1167/iovs.05-1561
10. Treleaven J. Dizziness, unsteadiness, visual disturbances, and sensorimotor control in traumatic neck pain. J Orthop Sports Phys Ther. (2017) 47:492–502. doi: 10.2519/jospt.2017.7052
11. Sandlund J, Djupsjöbacka M, Ryhed B, Hamberg J, Björklund M. Predictive and discriminative value of shoulder proprioception tests for patients with whiplash-associated disorders. J Rehabil Med. (2006) 38:44–9. doi: 10.1080/16501970510042847
12. See KS, Treleaven J. Identifying upper limb disability in patients with persistent whiplash. Man Ther. (2015) 20:487–93. doi: 10.1016/j.math.2014.12.001
13. Knox JJ, Beilstein DJ, Charles SD, Aarseth GA, Rayar S, Treleaven J, et al. Changes in head and neck position have a greater effect on elbow joint position sense in people with whiplash-associated disorders. Clin J Pain. (2006) 22:512–8. doi: 10.1097/01.ajp.0000210997.53082.c9
14. Knox JJ, Hodges PW. Changes in head and neck position affect elbow joint position sense. Exp Brain Res. (2005) 165:107–13. doi: 10.1007/s00221-005-2293-y
15. Roll JP, Roll R, Velay J-L. Proprioception as a link between body space and extra-personal space. In: Paillard J, editor. Brain and Space. Oxford: Oxford University Press (1991). p. 112–32.
16. Pettorossi VE, Schieppati M. Neck proprioception shapes body orientation and perception of motion. Front Hum Neurosci. (2014) 8:895. doi: 10.3389/fnhum.2014.00895
17. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
18. Viswanathan M, Berkman ND. Development of the RTI item bank on risk of bias and precision of observational studies. J Clin Epidemiol. (2012) 65:163–78. doi: 10.1016/j.jclinepi.2011.05.008
19. Zhang NM, Vesty G, Zheng Z. Healthcare professionals' attitudes to integration of acupuncture in western medicine: a mixed-method systematic review. Pain Manage Nurs. (2021). doi: 10.1016/j.pmn.2021.03.010. [Epub ahead of print].
20. Haavik H, Murphy B. Subclinical neck pain and the effects of cervical manipulation on elbow joint position sense. J Manipulative Physiol Ther. (2011) 34:88–97. doi: 10.1016/j.jmpt.2010.12.009
21. Huysmans MA, Hoozemans MJ, van der Beek AJ, de Looze MP, van Dieën JH. Position sense acuity of the upper extremity and tracking performance in subjects with non-specific neck and upper extremity pain and healthy controls. J Rehabil Med. (2010) 42:876–83. doi: 10.2340/16501977-0585
22. Sandlund J, Röijezon U, Björklund M, Djupsjöbacka M. Acuity of goal-directed arm movements to visible targets in chronic neck pain. J Rehabil Med. (2008) 40:366–74. doi: 10.2340/16501977-0175
23. Sittikraipong K, Silsupadol P, Uthaikhup S. Slower reaction and response times and impaired hand-eye coordination in individuals with neck pain. Musculoskeletal Sci Pract. (2020) 50:102273. doi: 10.1016/j.msksp.2020.102273
24. Steinmetz A, Jull GA. Sensory and sensorimotor features in violinists and violists with neck pain. Arch Phys Med Rehabil. (2013) 94:2523–8. doi: 10.1016/j.apmr.2013.04.019
25. Guerraz M, Blouin J, Vercher J-L. From head orientation to hand control: evidence of both neck and vestibular involvement in hand drawing. Exp Brain Res. (2003) 150:40–9. doi: 10.1007/s00221-003-1411-y
26. Berger M, Lechner-Steinleitner S, Kozlovskaya I, Holzmüller G, Mescheriakov S, Sokolov A, et al. The effect of head-to-trunk position on the direction of arm movements before, during, and after space flight. J Vestib Res. (1998) 8:341–54. doi: 10.3233/VES-1998-8501
27. Blouin J, Okada T, Wolsley C, Bronstein A. Encoding target-trunk relative position: cervical versus vestibular contribution. Exp Brain Res. (1998) 122:101–7. doi: 10.1007/s002210050496
28. Fookson O, Smetanin B, Berkinblit M, Adamovich S, Feldman G, Poizner H. Azimuth errors in pointing to remembered targets under extreme head rotations. Neuroreport. (1994) 5:885–8. doi: 10.1097/00001756-199404000-00008
29. Guerraz M, Navarro J, Ferrero F, Cremieux J, Blouin J. Perceived versus actual head-on-trunk orientation during arm movement control. Exp Brain Res. (2006) 172:221–9. doi: 10.1007/s00221-005-0316-3
30. Zabihhosseinian M, Holmes MW, Murphy B. Neck muscle fatigue alters upper limb proprioception. Exp Brain Res. (2015) 233:1663–75. doi: 10.1007/s00221-015-4240-x
31. Zabihhosseinian M, Yielder P, Holmes MW, Murphy B. Neck muscle fatigue affects performance of an eye-hand tracking task. J Electromyogr Kinesiol. (2019) 47:1–9. doi: 10.1016/j.jelekin.2019.04.001
32. Guerraz M, Caudron S, Thomassin N, Blouin J. Influence of head orientation on visually and memory-guided arm movements. Acta Psychol. (2011) 136:390–8. doi: 10.1016/j.actpsy.2011.01.004
33. Rossetti Y, Tadary B, Prablanc C. Optimal contributions of head and eye positions to spatial accuracy in man tested by visually directed pointing. Exp Brain Res. (1994) 97:487–96. doi: 10.1007/BF00241543
34. Spitzer WO. Scientific monograph of the Quebec Task Force on Whiplash-Associated Disorders: redefining “whiplash” and its management. Spine. (1995) 20:1S−73S. doi: 10.1097/00007632-199504151-00004
35. Levik YS. Motor control based on the internal representation system on the earth and in space. Hum Physiol. (2021) 47:335–51. doi: 10.1134/S0362119721030099
36. Morasso P, Casadio M, Mohan V, Rea F, Zenzeri J. Revisiting the body-schema concept in the context of whole-body postural-focal dynamics. Front Hum Neurosci. (2015) 9:83. doi: 10.3389/fnhum.2015.00083
37. Proske U, Gandevia SC. The proprioceptive senses: their roles in signaling body shape, body position and movement, and muscle force. Physiol Rev. (2012) 92:1651–97. doi: 10.1152/physrev.00048.2011
38. Kulkarni V, Chandy MJ, Babu KS. Quantitative study of muscle spindles in suboccipital muscles of human foetuses. Neurol India. (2001) 49:355. Available online at: https://www.neurologyindia.com/text.asp?2001/49/4/355/1221 (accessed September 16, 2021).
39. Boyd-Clark L, Briggs C, Galea M. Muscle spindle distribution, morphology, and density in longus colli and multifidus muscles of the cervical spine. Spine. (2002) 27:694–701. doi: 10.1097/00007632-200204010-00005
40. Liu J-X, Thornell L-E, Pedrosa-Domellöf F. Muscle spindles in the deep muscles of the human neck: a morphological and immunocytochemical study. J Histochem Cytochem. (2003) 51:175–86. doi: 10.1177/002215540305100206
41. Paillard J. Body schema and body image: a double dissociation in deafferented subjects. In: Gantchev G,Mori S,Massion J, editors. Motor Control Today and Tomorrow. Sofia: Academic Press (1999). p. 197–214.
42. Haavik H, Kumari N, Holt K, Niazi IK, Amjad I, Pujari AN, et al. The contemporary model of vertebral column joint dysfunction and impact of high-velocity, low-amplitude controlled vertebral thrusts on neuromuscular function. Eur J Appl Physiol. (2021) 121:2675–720. doi: 10.1007/s00421-021-04727-z
43. Stone AM, Vicenzino B, Lim EC, Sterling M. Measures of central hyperexcitability in chronic whiplash associated disorder–a systematic review and meta-analysis. Man Ther. (2013) 18:111–7. doi: 10.1016/j.math.2012.07.009
44. Xie Y, Jun D, Thomas L, Coombes BK, Johnston V. Comparing central pain processing in individuals with non-traumatic neck pain and healthy individuals: a systematic review and meta-analysis. J Pain. (2020) 21:1101–24. doi: 10.1016/j.jpain.2020.02.007
45. Vogt MT, Simonsick EM, Harris TB, Nevitt MC, Kang JD, Rubin SM, et al. Neck and shoulder pain in 70-to 79-year-old men and women: findings from the health, aging and body composition study. Spine J. (2003) 3:435–41. doi: 10.1016/S1529-9430(03)00150-5
46. Quek J, Treleaven J, Clark RA, Brauer SG. An exploratory study examining factors underpinning postural instability in older adults with idiopathic neck pain. Gait Posture. (2018) 60:93–8. doi: 10.1016/j.gaitpost.2017.11.016
Keywords: internal body schema, upper limb (UL), neck (MeSH), chronic neck pain, whiplash associated disorder (WAD), clumsiness, kinesthesia, proprioception (MeSH)
Citation: Harman SC, Zheng Z, Kendall JC, Vindigni D and Polus BI (2021) Does My Neck Make Me Clumsy? A Systematic Review of Clinical and Neurophysiological Studies in Humans. Front. Pain Res. 2:756771. doi: 10.3389/fpain.2021.756771
Received: 11 August 2021; Accepted: 06 September 2021;
Published: 11 October 2021.
Edited by:
Mathieu Piché, Université du Québec à Trois-Rivières, CanadaReviewed by:
Bernadette Ann Murphy, Ontario Tech University, CanadaCopyright © 2021 Harman, Zheng, Kendall, Vindigni and Polus. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Barbara I. Polus, YmFyYmFyYS5wb2x1c0BybWl0LmVkdS5hdQ==
†ORCID: Samantha C. Harman orcid.org/0000-0003-4323-1143
Zhen Zheng orcid.org/0000-0001-6777-1166
Julie C. Kendall orcid.org/0000-0002-7670-8692
Dein Vindigni orcid.org/0000-0002-3236-3725
Barbara I. Polus orcid.org/0000-0003-2227-2659
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.