
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pain Res. , 19 August 2021
Sec. Neuropathic Pain
Volume 2 - 2021 | https://doi.org/10.3389/fpain.2021.725363
This article is part of the Research Topic Highlights in Neuropathic Pain 2021/22 View all 5 articles
 Ioannis N. Petropoulos1
Ioannis N. Petropoulos1 Gulfidan Bitirgen2
Gulfidan Bitirgen2 Maryam Ferdousi3
Maryam Ferdousi3 Alise Kalteniece3
Alise Kalteniece3 Shazli Azmi3,4
Shazli Azmi3,4 Luca D'Onofrio5
Luca D'Onofrio5 Sze Hway Lim6
Sze Hway Lim6 Georgios Ponirakis1
Georgios Ponirakis1 Adnan Khan1
Adnan Khan1 Hoda Gad1
Hoda Gad1 Ibrahim Mohammed1
Ibrahim Mohammed1 Yacob E. Mohammadi1
Yacob E. Mohammadi1 Ayesha Malik1
Ayesha Malik1 David Gosal6
David Gosal6 Christopher Kobylecki6
Christopher Kobylecki6 Monty Silverdale6
Monty Silverdale6 Handrean Soran3
Handrean Soran3 Uazman Alam7
Uazman Alam7 Rayaz A. Malik1,3*
Rayaz A. Malik1,3*Neuropathic pain has multiple etiologies, but a major feature is small fiber dysfunction or damage. Corneal confocal microscopy (CCM) is a rapid non-invasive ophthalmic imaging technique that can image small nerve fibers in the cornea and has been utilized to show small nerve fiber loss in patients with diabetic and other neuropathies. CCM has comparable diagnostic utility to intraepidermal nerve fiber density for diabetic neuropathy, fibromyalgia and amyloid neuropathy and predicts the development of diabetic neuropathy. Moreover, in clinical intervention trials of patients with diabetic and sarcoid neuropathy, corneal nerve regeneration occurs early and precedes an improvement in symptoms and neurophysiology. Corneal nerve fiber loss also occurs and is associated with disease progression in multiple sclerosis, Parkinson's disease and dementia. We conclude that corneal confocal microscopy has good diagnostic and prognostic capability and fulfills the FDA criteria as a surrogate end point for clinical trials in peripheral and central neurodegenerative diseases.
Minsky invented the first “double focusing stage scanning microscope” in 1955, which Petran evolved into a functional confocal microscope in 1960. The first clinical corneal confocal microscope was built by Dilly in 1988 and was used to image the corneal epithelium, endothelium and stromal keratocytes of conscious humans. The corneal confocal microscope (CCM) is currently used by ophthalmologists to assess epithelial and stromal abnormalities and acanthamoeba infection (1). We will highlight how this ophthalmic tool has been increasingly utilized to quantify C-fibers in peripheral and central neurodegenerative diseases.
The laser scanning CCM HRTIII (Heidelberg Retina Tomograph III Rostock Corneal Module, Heidelberg Engineering GmbH, Heidelberg, Germany) is the most commonly used instrument which utilizes a single wavelength (670 nm red) Helium-Neon Diode class 1 laser to generate high resolution images of the corneal epithelial cells, keratocytes, endothelial cells, sub-basal nerve plexus (Figure 1) and dendritic cells which are antigen-presenting cells that have been found to be increased in a number of autoimmune and inflammatory neuropathies. Other commercially available slit scanning in vivo CCM's are manufactured by Tomey Corporation (Cambridge, MA, USA), Nidek Technologies (Gamagori, Japan) and Helmut Hund (Wetzlar, Germany), but have limited image resolution for the sub-basal nerve plexus.
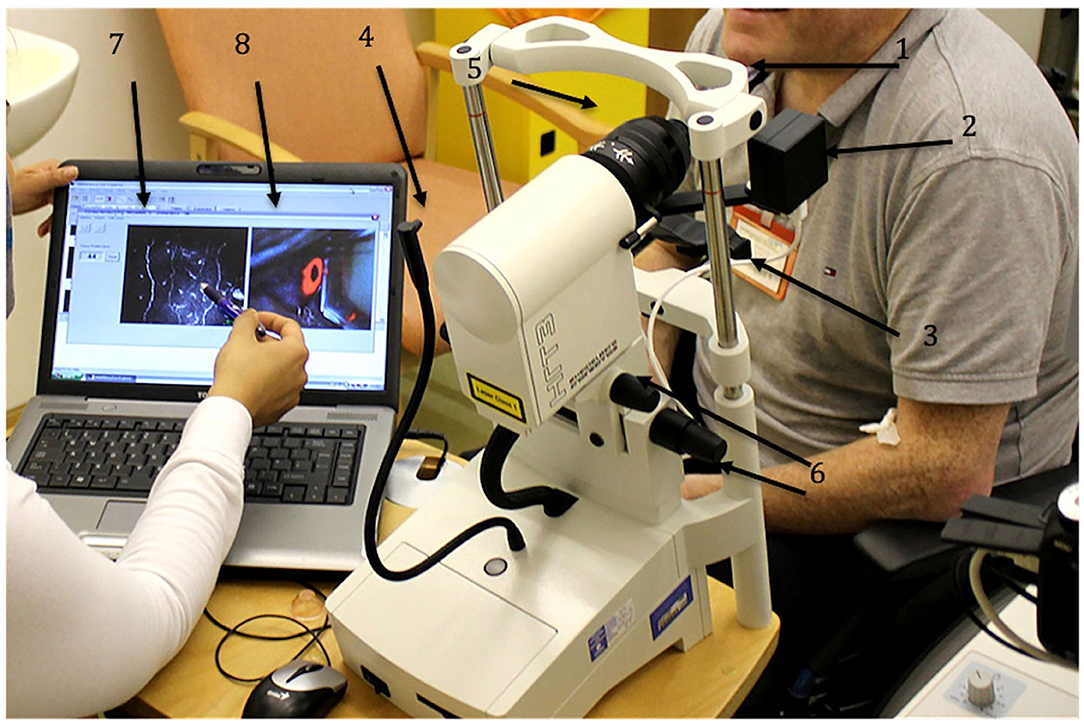
Figure 1. HRT III-RCM Corneal confocal microscope (CCM). 1 – forehead bar, 2 – CCD camera, 3 – chin rest, 4 – fixation target, 5 – corneal module, 6 – knobs to align the CCM, 7 – CCM live image, 8 – CCD camera live image.
Images can be captured using the section, volume or sequence modes. The section mode allows the examiner to manually focus the field of view on the area of interest and is more suitable for experienced users. The sequence and volume modes enable faster automated sequential image acquisition but have limited image quality. Most studies have analyzed 5–8 high-quality, non-overlapping images from the central cornea (2). A perceived limitation of CCM is the small field of view, therefore some centers have used wide field imaging to create maps of the subbasal nerve plexus (3).
The main corneal nerve morphological parameters quantified include corneal nerve fiber density (CNFD), branch density (CNBD), fiber length (CNFL) and inferior whorl length (IWL) (4). CNFD refers to the total number of main nerve fibers in a CCM image (fibers/mm2), CNBD is defined by the number of primary branches arising from the main nerve fibers (branches/mm2), CNFL is the total length of all nerve fibers and branches in a CCM image (mm/mm2) and IWL is the total length of nerves at the inferior whorl. Corneal nerve fractal dimension is a mathematical derivation of the pattern of corneal nerves and may allow the differentiation of neuropathies of different etiology (5). CCMetrics and ACCMetrics are freely available software for manual and automated quantification of sub-basal corneal nerves, respectively (6). CCMetrics also has been used to manually count the density of corneal dendritic cells (no./mm2). Novel Artificial Intelligence (AI) based algorithms have also been developed for fully automated corneal nerve quantification (7) and identification of patients with and without diabetic neuropathy (8).
We pioneered the use of CCM as a measure of neuropathy in 2003, by showing early and progressive loss of corneal nerve fibers in patients with increasing severity of diabetic neuropathy (9). Quattrini et al. (10) subsequently showed a comparable reduction in corneal nerve and intraepidermal nerve fiber density in DPN. Chen et al. (11) showed that CNFD had a superior diagnostic performance for DPN compared to intra epidermal nerve fiber density (IENFD) and this was confirmed in our subsequent study (12). The NIH consortium study (13) of 998 subjects with type 1 and type 2 diabetes reported a 0.88/0.88 sensitivity/specificity for corneal nerve fiber length in the diagnosis of DPN. Age-adjusted normative values for CCM show a small but progressive loss of corneal nerves with increasing age (14). More recently we have shown that the severity of and risk factors associated with corneal nerve loss differ between patients with type 1 and type 2 diabetes with an association between LDL cholesterol and triglycerides in type 1 and age, HbA1c and weight in type 2 diabetes (15). Corneal nerve loss also occurs in the early stages of diabetes in children (16, 17) and adults (18) with type 1 diabetes before the development of diabetic retinopathy and microalbuminuria and in subjects with impaired glucose tolerance (19, 20) and recently diagnosed type 2 diabetes (21). Patients with painful diabetic neuropathy show greater corneal nerve loss particularly at the inferior whorl (22, 23) (Figure 2). Studies from Italy (24), China (25), Japan (26), New Zealand (27) and the UK (28) show corneal nerve loss in patients with diabetic autonomic neuropathy and we have shown that corneal nerve loss occurs in patients with diabetes and obesity with erectile dysfunction (29, 30). Reduced corneal nerve fiber length predicts 4-year incident DPN (31, 32) and a more rapid decline in CNFL has been associated with the development of DPN (33) and foot ulceration (34).

Figure 2. CCM images of the central cornea and inferior whorl in a healthy control (A,D), patient with painless (B,E) and painful (C,F) diabetic neuropathy.
Chemotherapy-induced peripheral neuropathy (CIPN) is associated with pain, which may lead to dose reduction or discontinuation of chemotherapy. An early case-report showed that a patient with colorectal cancer treated with capecitabine had a reduction in corneal nerves with increased sprouting, indicative of concomitant nerve degeneration and regeneration (35). We have shown corneal nerve loss in a cohort of patients with gastro-esophageal cancer followed by an increase in corneal nerve fiber length after the 3rd cycle of platinum-based chemotherapy, indicative of nerve regeneration (36). Recently in patients with breast and colon cancer, 5 years after the initial development of CIPN, there was no change in corneal nerve morphology (37). However, a reduction in central and inferior whorl corneal nerve fiber length has been found in patients 3–24 months after treatment with paclitaxel or oxaliplatin (38). The association between changes in corneal nerve morphology and manifestations of CIPN are complex, appear to be temporally related and demand further study.
Behcet's disease is a chronic relapsing vascular inflammatory disease with a number of neurological manifestations, including peripheral neuropathy. We have recently shown corneal nerve loss and a significant increase in dendritic cell density (39). Schneider et al. (40) showed a significant reduction in subbasal nerve fiber density and length with an increase in dendritic cell density in patients with chronic inflammatory demyelinating polyneuropathy (CIDP). Stettner et al. (41) confirmed and extended these results by showing significant corneal nerve loss and an increase in dendritic cells in a large cohort of patients with CIDP, multifocal motor neuropathy (MMN) and monoclonal gammopathy of unknown significance (MGUS) which was associated with neurologic severity and the presence of pain. Moreover, whilst the extent of corneal nerve fiber loss was comparable, dendritic cell density in proximity to nerve fibers was found to be increased in patients with CIDP compared to diabetic neuropathy (42).
Neuropathic pain is a frequent and debilitating manifestation of Human Immunodeficiency Virus (HIV) infection. Kemp et al. (43) showed a reduction in corneal nerve fibers in patients with HIV-associated neuropathy and we also showed that corneal nerve fractal dimension differs between patients with HIV neuropathy and other peripheral neuropathies (44).
Idiopathic small fiber neuropathy is characterized by painful neuropathic symptoms and small fiber dysfunction/damage with preserved large nerve fiber function (45). In a cohort of patients with ISFN we previously showed a significant reduction in corneal nerve density and length and related it to sensory symptoms (46). In a case-control study, 86 patients with SFN underwent neurological examination, quantitative sensory testing, distal and proximal skin punch biopsy, and sub-group of 55 patients additionally underwent pain-related evoked potentials (PREP), corneal confocal microscopy (CCM), and a quantitative sudomotor axon reflex test (QSART). An abnormal distal intraepidermal nerve fiber density (IENFD) (60/86, 70%) and neurological examination (53/86, 62%) identified those with SFN and CCM and/or PREP further increased the proportion of patients identified with SFN to 85%, whilst QST, QSART, and proximal IENFD contributed minimally (47).
Corneal nerve loss has been reported in patients with Charcot Marie Tooth Disease Type 1A (48) and a hereditary neuropathy with a rare nerve growth factor-β mutation (49) where the severity of corneal nerve loss was related to the reported pain intensity. We have also demonstrated corneal nerve loss in patients with Friedreich's ataxia, a multi-system autosomal recessive disease caused by homozygous guanine-adenine-adenine (GAA) repeat expansions within intron 1 of the frataxin gene. The severity of corneal nerve loss has been related to the number of GAA repeats and clinical disability assessed using the Scale for the Assessment and Rating of Ataxia and Friedreich's Ataxia Rating Scale (50). In a cohort of 51 patients with neurofibromatosis type 1, 8% had abnormal nerve conduction studies, 13% had abnormal thermal thresholds, 22% had abnormal intraepidermal nerve fiber density, however, 52% had reduced corneal nerve fiber length (51).
Transthyretin familial amyloid polyneuropathy (TTR-FAP) is a fatal inherited disorder characterized by pain, numbness and weakness due to a progressive neuropathy (52). Small fiber neuropathy with loss of intra-epidermal nerve fibers (53) and altered thermal thresholds (54) are key features of TTR-FAP. CCM showed corneal nerve fiber loss in a patient with light chain amyloid neuropathy secondary to multiple myeloma (55). In a series of 15 patients with TTR-FAP a reduction in corneal nerve fiber length was related to the neuropathy impairment score of the lower limbs, autonomic dysfunction, sensory nerve action potential and IENFD (56). CNFL could be measured in all participants, whilst sural nerve amplitude and IENFD could only be measured in 73 and 27% of patients, respectively. This lack of a floor effect increases the utility of CNFL compared to IENFD in longitudinal and interventional studies of amyloid neuropathy. Recently, a study from China has confirmed and extended these findings by showing corneal nerve loss in the central and inferior whorl regions with an AUC for CNFL and IWL of 88.0 and 89.3%, respectively, for the diagnosis of familial amyloid neuropathy (57).
Painful neuropathy is a hallmark of Fabry's disease due to the accumulation of globotriaosylceramide (GI3) leading to nerve damage (58, 59). We were the first to report corneal nerve loss using a first generation Tomey ConfoScan in patients with Fabry disease (60). More recently using a HRT III CCM device we have extended these observations and confirmed corneal nerve loss and an increase in dendritic cells which correlated with the total Mainz severity score index (61).
In patients with primary hypothyroidism and in patients with hyperthyroidism undergoing radioiodine therapy, corneal nerve fiber density was reduced and improved after 12 months of treatment with levothyroxine (62).
Pain is a major feature of fibromyalgia and several studies have shown corneal nerve fiber loss in patients with fibromyalgia. In an early study from Mexico, stromal nerve thinning and a reduction in subbasal nerve fiber density was related to a variety of pain descriptors (63). In a subsequent very detailed phenotyping study from the Netherlands, corneal nerve loss was identified in 51% of patients with fibromyalgia and related to central sensitization (64). A study from Turkey reported a reduction in corneal nerve fiber length which correlated with the “widespread pain index” in patients with fibromyalgia (65). More recently in a large cohort of 117 women with fibromyalgia we have demonstrated multiple small fiber abnormalities including a comparable reduction in IENFD and corneal nerves (66).
In a small series of 4 patients who developed painful diabetic neuropathy during acute SARS-CoV 2 infection we recently showed evidence of altered taste and smell and increased thermal thresholds, suggestive of underlying small fiber neuropathy (67). There are small studies which indicate both large (68) and small (69) fiber neuropathy in patients following COVID-19, although this may reflect nerve damage associated with severe disease and critical illness (70, 71). However, we have recently shown corneal nerve loss and increased dendritic cells in a cohort of patients 12 weeks after relatively mild COVID-19, particularly those with neuropathic and fibromyalgia like symptoms who fulfilled the criteria for long-COVID (Figure 3) (72).
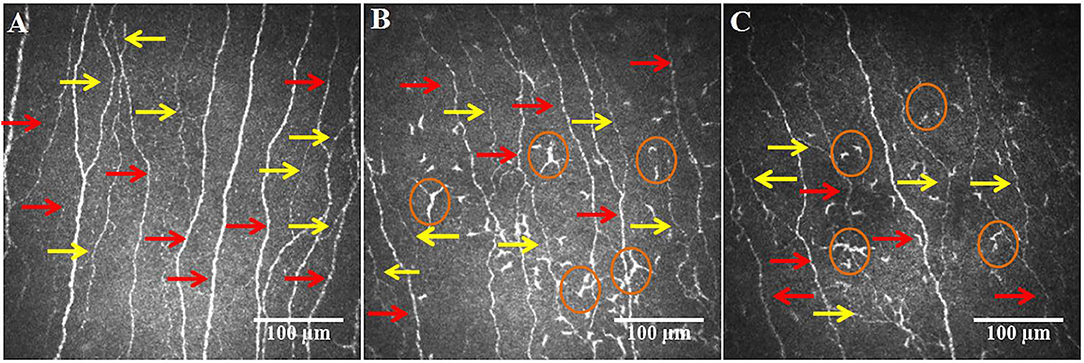
Figure 3. CCM images of the central cornea in a healthy control (A), post-COVID-19 patient without (B) and with (C) long-COVID, showing a loss of main nerves (red arrows) and branches (yellow arrows) in the patient with long-COVID and an increase in dendritic cells (circles) in patients with and without long-COVID.
An increasing number of centers have explored the utility of corneal nerve loss as a surrogate marker of neurodegeneration in central neurodegenerative diseases.
Pain is an increasingly recognized non-motor feature of PD (73). A study of 25 patients with PD showed reduced corneal sensitivity and a reduction in corneal nerve fiber density, branch density and length which was related to therapy with dopaminergic therapy (74). Kass-Iliyya et al. (75) showed corneal nerve loss in patients with PD which was related to the unified PD rating scale and autonomic dysfunction. Another study in 26 newly diagnosed patients with PD showed a reduction in corneal nerve parameters, with normal nerve conduction and IENFD (76). Corneal nerve loss has also been related to severity of cognitive dysfunction in patients with Parkinson's disease (77) and associated with altered white matter diffusion properties of the trigeminal nerve (78). Recently, a significant decrease in the directional anisotropy coefficient and an increase in the directional symmetry coefficient of corneal nerve fibers has been demonstrated in patients with PD (79). We have confirmed the loss of corneal nerve fibers in a large cohort of 98 participants with PD (80) (Figure 4). In a recent study from China, CNFD showed an excellent diagnostic performance with a AUC of 0.96 for PD and corneal nerve fiber parameters correlated with the severity of motor symptoms measured using the H-Y stage, UPDRS-III and UPDRS-Total (81). Furthermore, we have shown that a lower corneal nerve fiber length predicts progressive worsening of UPDRS-III over 12 months in patients with PD (82). CCM could therefore add to the diagnostic toolbox for pre-motor Parkinson's disease.
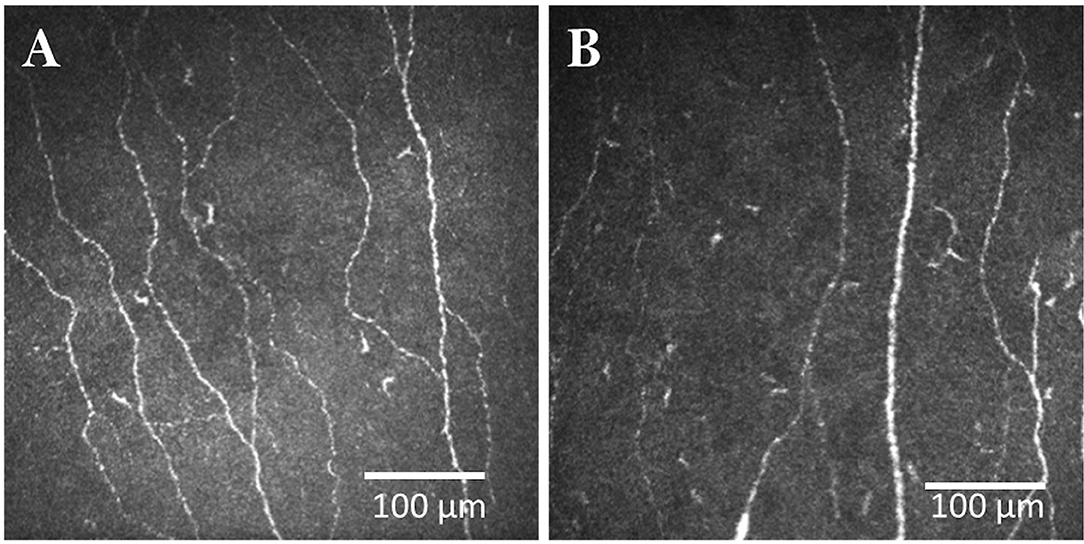
Figure 4. CCM images of the central cornea in a healthy control (A) and patient with Parkinson's disease (B).
Common initial symptoms of MS include hypoesthesia, dysesthesia, paraesthesia and mononuclear painful visual loss (83, 84) and of course Lhermitte's sign is characterized by a transient electric shock sensation extending down the spine and/or extremities upon flexion of the neck (85). The etiology of pain is heterogeneous but includes central neuropathic pain, which can occur in almost a third of patients with MS and has been associated with increased thermal thresholds (86). Trigeminal neuralgia is a troubling feature of multiple sclerosis, and a recent diffusion tensor imaging study has shown that patients with a poor response to treatment have lower fractional anisotropy and higher radial diffusivity of the pontine trigeminal fibers (87). Several studies (88–90) have demonstrated a significant reduction in subbasal corneal nerve density and a recent study has shown altered pupillary responses (91) in patients with multiple sclerosis. Corneal nerve loss correlates with disease severity and an increase in corneal immune cells (89, 92). More recently a longitudinal study over 2 years has shown that progressive corneal nerve loss is associated with worsening neurological disability (93). However, no study to date has assessed the relationship between pain and corneal nerve loss.
Pain is a neglected feature of ALS (94) but can arise in relation to cramps, spasticity and neuropathy and may even occur before the first motor symptom (95). Corneal nerve loss has been demonstrated in a small cohort of patients with ALS and associated with the bulbar function disability score (96).
Central post-stroke pain has been estimated to occur in 8–55% of stroke patients and may be constant or intermittent neuropathic pain accompanied by dysesthesia of temperature and/or pressure sensations within the area of the body corresponding to the stroke lesion (97). The etiology of this condition is poorly understood and it is often resistant to conventional pharmacological treatment options (98). Corneal nerve loss has been demonstrated in patients with Transient Ischemic Attack (TIA), minor stroke (99) and major stroke (100), with greater severity in patients with recurrent stroke (101) and it is associated with the presence of white matter hyperintensities, markers of small vessel disease (102). The relationship between corneal nerve loss and central post-stroke pain has not been explored.
Pain is highly prevalent in patients with dementia (103), however it is not routinely assessed and is poorly managed (104). There is a growing interest for the role of CCM as a biomarker of neurodegeneration in subjects with dementia (105). We have shown a significant reduction in corneal nerve fibers in patients with mild cognitive impairment and dementia (106, 107). We have also recently shown stromal nerve loss with preservation of sub-basal nerves in patients with front-temporal dementia (108).
Migraine is characterized by severe headache accompanied by nausea and sensitivity to light. In patients with migraine corneal nerve fiber density and length were reduced (109) and Shetty et al. demonstrated corneal nerve loss in patients with chronic migraine and photophobia, but not in patients without photophobia (110).
Trigeminal neuralgia manifests with severe often excruciating electric shock-like pain affecting the lower jaw and face. Interestingly, corneal nerve fiber density and length were reduced in both the ipsilateral and contralateral cornea of patients with trigeminal neuralgia and there was no difference between patients with and without nerve vessel conflict (111).
Burning mouth syndrome is a relatively rare condition which occurs more frequently in older women and is characterized by a burning feeling on the roof of the mouth, tongue and lips. In a study of 17 patients with BMS there was a significant reduction in corneal nerve fiber density and length and an increase in dendritic cell density (112).
Mehra et al. (113) showed that CCM can detect early nerve fiber repair with an increase in corneal nerve fiber density and length following normalization of glycaemia and renal function after simultaneous pancreas and kidney (SPK) transplantation. Tavakoli et al. (114) showed an improvement in CCM parameters 12 months after SPK transplantation with no change in symptoms and deficits, neurophysiology, quantitative sensory testing and skin biopsy. More recently we have shown an early (12 months) and continued improvement in corneal nerve fiber length which was associated with an improvement in neuropathic symptoms and neurophysiology at 36 months (115). We have also recently shown that bariatric surgery leads to corneal nerve regeneration (116). A novel first-in-class peptide (ARA290-Cibinetide) which reduces inflammation was associated with an increase in corneal nerve fiber density and length in patients with sarcoidosis-related neuropathy (117, 118) and T2DM (119) and was paralleled by an improvement in pain scores and functional outcomes. In a subsequent Phase 2b study, the improvement in corneal nerve morphology correlated with the expression of GAP-43+ intraepidermal nerve fibers, indicating nerve fiber repair and an improvement in pain intensity after 28 days (118). In a trial of seal oil omega-3 polyunsaturated fatty acid in patients with T1DM there was a significant 29% increase in CNFL, with no change in nerve conduction velocity and sensory function over 12 months (120) which was associated with higher baseline omega-3 levels (121). More recently, a randomized placebo controlled trial has confirmed that treatment with long chain omega-3 supplements over 6 months was associated with an increase in corneal nerve fiber length indicative of corneal nerve regeneration, without improvement in neurophysiology or thermal thresholds in patients with type 1 diabetes (122). Recently we have shown an improvement in corneal nerve parameters in a randomized clinical trial of once weekly GLP-1 or insulin in patients with poor glycemic control (123). Greater nerve regeneration was observed, especially in patients without insulin resistance (124) and those with painful diabetic neuropathy (125).
Regulatory agencies and pharmaceutical companies need to consider the compelling case for CCM as a marker of neurodegeneration and regeneration and as an end-point in clinical trials of new therapies in peripheral and central neurodegenerative diseases (1, 126).
HS has contributed significantly to the body of knowledge generated in this review by performing literature searches and reviewing the first draft. All authors contributed to the literature search and first draft of the review.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. There is no conflict of interest related to this work for any of the authors.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Petropoulos IN, Ponirakis G, Khan A, Gad H, Almuhannadi H, Brines M, et al. Corneal confocal microscopy: ready for prime time. Clin Exp Optomet. (2020) 103:265–77. doi: 10.1111/cxo.12887
2. Vagenas D, Pritchard N, Edwards K, Shahidi AM, Sampson GP, Russell AW, et al. Optimal image sample size for corneal nerve morphometry. Optomet Vis Sci. (2012) 89:812–7. doi: 10.1097/OPX.0b013e31824ee8c9
3. Kheirkhah A, Muller R, Mikolajczak J, Ren A, Kadas EM, Zimmermann H, et al. Comparison of standard versus wide-field composite images of the corneal subbasal layer by in vivo confocal microscopy. Investig Ophthalmol Vis Sci. (2015) 56:5801–7. doi: 10.1167/iovs.15-17434
4. Petropoulos IN, Ferdousi M, Marshall A, Alam U, Ponirakis G, Azmi S, et al. The inferior whorl for detecting diabetic peripheral neuropathy using corneal confocal microscopy. Investig Ophthalmol Vis Sci. (2015) 56:2498–504. doi: 10.1167/iovs.14-15919
5. Petropoulos IN, Al-Mohammedi A, Chen X, Ferdousi M, Ponirakis G, Kemp H, et al. The utility of corneal nerve fractal dimension analysis in peripheral neuropathies of different etiology. Transl Vis Sci Technol. (2020) 9:43. doi: 10.1167/tvst.9.9.43
6. Petropoulos IN, Alam U, Fadavi H, Marshall A, Asghar O, Dabbah MA, et al. Rapid automated diagnosis of diabetic peripheral neuropathy with in vivo corneal confocal microscopy. Investig Ophthalmol Vis Sci. (2014) 55:2071–8. doi: 10.1167/iovs.13-13787
7. Williams BM, Borroni D, Liu R, Zhao Y, Zhang J, Lim J, et al. An artificial intelligence-based deep learning algorithm for the diagnosis of diabetic neuropathy using corneal confocal microscopy: a development and validation study. Diabetologia. (2020) 63:419–30. doi: 10.1007/s00125-019-05023-4
8. Salahuddin T, Qidwai U. Computational methods for automated analysis of corneal nerve images: lessons learned from retinal fundus image analysis. Comp Biol Med. (2020) 119:103666. doi: 10.1016/j.compbiomed.2020.103666
9. Malik RA, Kallinikos P, Abbott C, van Schie CH, Morgan P, Efron N, et al. Corneal confocal microscopy: a non-invasive surrogate of nerve fibre damage and repair in diabetic patients. Diabetologia. (2003) 46:683–8. doi: 10.1007/s00125-003-1086-8
10. Quattrini C, Tavakoli M, Jeziorska M, Kallinikos P, Tesfaye S, Finnigan J, et al. Surrogate markers of small fiber damage in human diabetic neuropathy. Diabetes. (2007) 56:2148–54. doi: 10.2337/db07-0285
11. Chen X, Graham J, Dabbah MA, Petropoulos IN, Ponirakis G, Asghar O, et al. Small nerve fiber quantification in the diagnosis of diabetic sensorimotor polyneuropathy: comparing corneal confocal microscopy with intraepidermal nerve fiber density. Diabetes Care. (2015) 38:dc142422. doi: 10.2337/dc14-2422
12. Alam U, Jeziorska M, Petropoulos IN, Asghar O, Fadavi H, Ponirakis G, et al. Diagnostic utility of corneal confocal microscopy and intra-epidermal nerve fibre density in diabetic neuropathy. PLoS ONE. (2017) 12:e0180175. doi: 10.1371/journal.pone.0180175
13. Perkins BA, Lovblom LE, Bril V, Scarr D, Ostrovski I, Orszag A, et al. Corneal confocal microscopy for identification of diabetic sensorimotor polyneuropathy: a pooled multinational consortium study. Diabetologia. (2018) 61:1–6. doi: 10.1007/s00125-018-4653-8
14. Tavakoli M, Ferdousi M, Petropoulos IN, Morris J, Pritchard N, Zhivov A, et al. Normative values for corneal nerve morphology assessed using corneal confocal microscopy: a multinational normative data set. Diabetes Care. (2015) 38:838–43. doi: 10.2337/dc14-2311
15. Ferdousi M, Kalteniece A, Azmi S, Petropoulos IN, Ponirakis G, Alam U, et al. Diagnosis of neuropathy and risk factors for corneal nerve loss in type 1 and type 2 diabetes: a corneal confocal microscopy study. Diabetes Care. (2020) 44:150–6. doi: 10.2337/figshare.13020023.v1
16. Ferdousi M, Romanchuk K, Mah JK, Virtanen H, Millar C, Malik RA, et al. Early corneal nerve fibre damage and increased Langerhans cell density in children with type 1 diabetes mellitus. Sci Rep. (2019) 9:8758. doi: 10.1038/s41598-019-45116-z
17. Gad H, Al-Jarrah B, Saraswathi S, Petropoulos IN, Ponirakis G, Khan A, et al. Corneal nerve loss in children with type 1 diabetes mellitus without retinopathy or microalbuminuria. J Diabetes Investig. (2020) 11:1594–601. doi: 10.1111/jdi.13313
18. Petropoulos IN, Green P, Chan AW, Alam U, Fadavi H, Marshall A, et al. Corneal confocal microscopy detects neuropathy in patients with type 1 diabetes without retinopathy or microalbuminuria. PLoS ONE. (2015) 10:e0123517. doi: 10.1371/journal.pone.0123517
19. Asghar O, Petropoulos IN, Alam U, Jones W, Jeziorska M, Marshall A, et al. Corneal confocal microscopy detects neuropathy in subjects with impaired glucose tolerance. Diabetes Care. (2014) 37:2643–6. doi: 10.2337/dc14-0279
20. De Clerck EEB, Schouten J, Berendschot T, Koolschijn RS, Nuijts R, Schram MT, et al. Reduced corneal nerve fibre length in prediabetes and type 2 diabetes: The Maastricht Study. Acta Ophthalmol. (2020) 98:485–91. doi: 10.1111/aos.14359
21. Ziegler D, Papanas N, Zhivov A, Allgeier S, Winter K, Ziegler I, et al. Early detection of nerve fiber loss by corneal confocal microscopy and skin biopsy in recently diagnosed type 2 diabetes. Diabetes. (2014) 63:2454–63. doi: 10.2337/db13-1819
22. Kalteniece A, Ferdousi M, Azmi S, Mubita WM, Marshall A, Lauria G, et al. Corneal confocal microscopy detects small nerve fibre damage in patients with painful diabetic neuropathy. Sci Rep. (2020) 10:3371. doi: 10.1038/s41598-020-60422-7
23. Kalteniece A, Ferdousi M, Petropoulos I, Azmi S, Adam S, Fadavi H, et al. Greater corneal nerve loss at the inferior whorl is related to the presence of diabetic neuropathy and painful diabetic neuropathy. Sci Rep. (2018) 8:3283. doi: 10.1038/s41598-018-21643-z
24. Maddaloni E, Sabatino F, Del Toro R, Crugliano S, Grande S, Lauria Pantano A, et al. In vivo corneal confocal microscopy as a novel non-invasive tool to investigate cardiac autonomic neuropathy in Type 1 diabetes. Diabet Med. (2015) 32:262–6. doi: 10.1111/dme.12583
25. Wang H, Fan D, Wang W, Zhang S, Wang X. [Early diagnosis of diabetic autonomic neuropathy by corneal confocal microscopy]. Zhonghua Yi Xue Za zhi. (2015) 95:2851–6.
26. Ishibashi F, Kojima R. The preferential impairment of pupil constriction stimulated by blue light in patients with type 2 diabetes without autonomic neuropathy. J Diabetes Res. (2017) 2017:6069730. doi: 10.1155/2017/6069730
27. Misra SL, Craig JP, Patel DV, McGhee CN, Pradhan M, Ellyett K, et al. In vivo confocal microscopy of corneal nerves: an ocular biomarker for peripheral and cardiac autonomic neuropathy in type 1 diabetes mellitus. Invest Ophthalmol Vis Sci. (2015) 56:5060–5. doi: 10.1167/iovs.15-16711
28. Tavakoli M, Begum P, McLaughlin J, Malik RA. Corneal confocal microscopy for the diagnosis of diabetic autonomic neuropathy. Muscle Nerve. (2015) 52:363–70. doi: 10.1002/mus.24553
29. Azmi S, Ferdousi M, Alam U, Petropoulos IN, Ponirakis G, Marshall A, et al. Small-fibre neuropathy in men with type 1 diabetes and erectile dysfunction: a cross-sectional study. Diabetologia. (2017) 60:1094–101. doi: 10.1007/s00125-017-4245-z
30. Dhage S, Ho JH, Ferdousi M, Kalteniece A, Azmi S, Adam S, et al. Small fibre pathology is associated with erectile dysfunction in men with type 2 diabetes. Diabetes Metab Res Rev. (2020) 36:e3263. doi: 10.1002/dmrr.3263
31. Pritchard N, Edwards K, Russell AW, Perkins BA, Malik RA, Efron N. Corneal confocal microscopy predicts 4-year incident peripheral neuropathy in type 1 diabetes. Diabetes Care. (2015) 38:dc142114. doi: 10.2337/dc14-2114
32. Lovblom LE, Halpern EM, Wu T, Kelly D, Ahmed A, Boulet G, et al. In vivo corneal confocal microscopy and prediction of future-incident neuropathy in type 1 diabetes: a preliminary longitudinal analysis. Can J Diabetes. (2015) 39:390–7. doi: 10.1016/j.jcjd.2015.02.006
33. Lewis EJH, Lovblom LE, Ferdousi M, Halpern EM, Jeziorska M, Pacaud D, et al. Rapid corneal nerve fiber loss: a marker of diabetic neuropathy onset and progression. Diabetes Care. (2020) 43:1829–35. doi: 10.2337/dc19-0951
34. Dehghani C, Russell AW, Perkins BA, Malik RA, Pritchard N, Edwards K, et al. A rapid decline in corneal small fibers and occurrence of foot ulceration and Charcot foot. J Diabetes Comp. (2016) 30:1437–9. doi: 10.1016/j.jdiacomp.2016.07.004
35. Ferrari G, Gemignani F, Macaluso C. Chemotherapy-associated peripheral sensory neuropathy assessed using in vivo corneal confocal microscopy. Arch Neurol. (2010) 67:364–5. doi: 10.1001/archneurol.2010.17
36. Ferdousi M, Azmi S, Petropoulos IN, Fadavi H, Ponirakis G, Marshall A, et al. Corneal confocal microscopy detects small fibre neuropathy in patients with upper gastrointestinal cancer and nerve regeneration in chemotherapy induced peripheral neuropathy. PLoS ONE. (2015) 10:e0139394. doi: 10.1371/journal.pone.0139394
37. Bennedsgaard K, Ventzel L, Andersen NT, Themistocleous AC, Bennett DL, Jensen TS, et al. Oxaliplatin- and docetaxel-induced polyneuropathy: clinical and neurophysiological characteristics. J Periph Nerv Syst. (2020) 25:377–87. doi: 10.1111/jns.12413
38. Chiang JCB, Goldstein D, Trinh T, Au K, Mizrahi D, Muhlmann M, et al. A cross-sectional study of sub-basal corneal nerve reduction following neurotoxic chemotherapy. Transl Vis Sci Technol. (2021) 10:24. doi: 10.1167/tvst.10.1.24
39. Bitirgen G, Tinkir Kayitmazbatir E, Satirtav G, Malik RA, Ozkagnici A. In vivo confocal microscopic evaluation of corneal nerve fibers and dendritic cells in patients with Behcet's disease. Front Neurol. (2018) 9:204. doi: 10.3389/fneur.2018.00204
40. Schneider C, Bucher F, Cursiefen C, Fink GR, Heindl LM, Lehmann HC. Corneal confocal microscopy detects small fiber damage in chronic inflammatory demyelinating polyneuropathy (CIDP). J Periph Nerv Syst. (2014) 19:322–7. doi: 10.1111/jns.12098
41. Stettner M, Hinrichs L, Guthoff R, Bairov S, Petropoulos IN, Warnke C, et al. Corneal confocal microscopy in chronic inflammatory demyelinating polyneuropathy. Ann Clin Transl Neurol. (2016) 3:88–100. doi: 10.1002/acn3.275
42. Fleischer M, Lee I, Erdlenbruch F, Hinrichs L, Petropoulos IN, Malik RA, et al. Corneal confocal microscopy differentiates inflammatory from diabetic neuropathy. J Neuroinflamm. (2021) 18:89. doi: 10.1186/s12974-021-02130-1
43. Kemp HI, Petropoulos IN, Rice AS, Vollert J, Maier C, Sturm D, et al. Use of corneal confocal microscopy to evaluate small nerve fibers in patients with human immunodeficiency virus. JAMA Ophthalmol. (2017) 135:795–800. doi: 10.1001/jamaophthalmol.2017.1703
44. Chen X, Graham J, Petropoulos IN, Ponirakis G, Asghar O, Alam U, et al. Corneal nerve fractal dimension: a novel corneal nerve metric for the diagnosis of diabetic sensorimotor polyneuropathy. Investig Ophthalmol Vis Sci. (2018) 59:1113–8. doi: 10.1167/iovs.17-23342
45. Freeman R, Gewandter JS, Faber CG, Gibbons C, Haroutounian S, Lauria G, et al. Idiopathic distal sensory polyneuropathy: ACTTION diagnostic criteria. Neurology. (2020) 95:1005–14. doi: 10.1212/WNL.0000000000010988
46. Tavakoli M, Marshall A, Pitceathly R, Fadavi H, Gow D, Roberts ME, et al. Corneal confocal microscopy: a novel means to detect nerve fibre damage in idiopathic small fibre neuropathy. Exp Neurol. (2010) 223:245–50. doi: 10.1016/j.expneurol.2009.08.033
47. Egenolf N, Altenschildesche CM zu, Kreß L, Eggermann K, Namer B, Gross F, et al. Diagnosing small fiber neuropathy in clinical practice: a deep phenotyping study. Ther Adv Neurol Disord. (2021) 14:1–13. doi: 10.1177/17562864211004318
48. Tavakoli M, Marshall A, Banka S, Petropoulos IN, Fadavi H, Kingston H, et al. Corneal confocal microscopy detects small-fiber neuropathy in Charcot–Marie–Tooth disease type 1A patients. Muscle Nerve. (2012) 46:698–704. doi: 10.1002/mus.23377
49. Perini I, Tavakoli M, Marshall A, Minde J, Morrison I. Rare human nerve growth factor-beta mutation reveals relationship between C-afferent density and acute pain evaluation. J Neurophysiol. (2016) 116:425–30. doi: 10.1152/jn.00667.2015
50. Pagovich OE, Vo ML, Zhao ZZ, Petropoulos IN, Yuan M, Lertsuwanroj B, et al. Corneal confocal microscopy: neurologic disease biomarker in Friedreich ataxia. Ann Neurol. (2018) 84:893–904. doi: 10.1002/ana.25355
51. Barnett C, Alon T, Abraham A, Kim RH, McCuaig JM, Kongkham P, et al. Evidence of small-fiber neuropathy in neurofibromatosis type 1. Muscle Nerve. (2019) 60:673–8. doi: 10.1002/mus.26687
52. Plante-Bordeneuve V. Transthyretin familial amyloid polyneuropathy: an update. J Neurol. (2018) 265:976–83. doi: 10.1007/s00415-017-8708-4
53. Masuda T, Ueda M, Suenaga G, Misumi Y, Tasaki M, Izaki A, et al. Early skin denervation in hereditary and iatrogenic transthyretin amyloid neuropathy. Neurology. (2017) 88:2192–7. doi: 10.1212/WNL.0000000000004016
54. Pinto MV, Dyck PJB, Gove LE, McCauley BM, Ackermann EJ, Hughes SG, et al. Kind and distribution of cutaneous sensation loss in hereditary transthyretin amyloidosis with polyneuropathy. J Neurol Sci. (2018) 394:78–83. doi: 10.1016/j.jns.2018.08.031
55. Sturm D, Schmidt-Wilcke T, Greiner T, Maier C, Schargus M, Tegenthoff M, et al. Confocal cornea microscopy detects involvement of corneal nerve fibers in a patient with light-chain amyloid neuropathy caused by multiple myeloma: a case report. Case Rep Neurol. (2016) 8:134–9. doi: 10.1159/000446538
56. Rousseau A, Cauquil C, Dupas B, Labbe A, Baudouin C, Barreau E, et al. Potential role of in vivo confocal microscopy for imaging corneal nerves in transthyretin familial amyloid polyneuropathy. JAMA Ophthalmol. (2016) 134:983–9. doi: 10.1001/jamaophthalmol.2016.1889
57. Zhang Y, Liu Z, Zhang Y, Wang H, Liu X, Zhang S, et al. Corneal sub-basal whorl-like nerve plexus: a landmark for early and follow-up evaluation in transthyretin familial amyloid polyneuropathy. Eur J Neurol. (2020) 28:630–8. doi: 10.1111/ene.14563
58. Politei JM, Durand C, Schenone AB. Small fiber neuropathy in Fabry disease: a review of pathophysiology and treatment. J Inborn Errors Metab Screen. (2016) 4:2326409816661351. doi: 10.1177/2326409816661351
59. Burand AJ Jr., Stucky CL. Fabry disease pain: patient and preclinical parallels. Pain. (2021) 162:1305–21. doi: 10.1097/j.pain.0000000000002152
60. Tavakoli M, Marshall A, Thompson L, Kenny M, Waldek S, Efron N, et al. Corneal confocal microscopy: a novel noninvasive means to diagnose neuropathy in patients with Fabry disease. Muscle Nerve. (2009) 40:976–84. doi: 10.1002/mus.21383
61. Bitirgen G, Turkmen K, Malik RA, Ozkagnici A, Zengin N. Corneal confocal microscopy detects corneal nerve damage and increased dendritic cells in Fabry disease. Sci Rep. (2018) 8:12244. doi: 10.1038/s41598-018-30688-z
62. Sharma S, Tobin V, Vas PRJ, Rayman G. The LDIFLARE and CCM methods demonstrate early nerve fibre abnormalities in untreated hypothyroidism: a prospective study. J Clin Endocrinol Metab. (2018) 103:3094–102. doi: 10.1210/jc.2018-00671
63. Ramirez M, Martinez-Martinez LA, Hernandez-Quintela E, Velazco-Casapia J, Vargas A, Martinez-Lavin M. Small fiber neuropathy in women with fibromyalgia. An in vivo assessment using corneal confocal bio-microscopy. Semin Arthrit Rheumat. (2015) 45:214–9. doi: 10.1016/j.semarthrit.2015.03.003
64. Oudejans L, He X, Niesters M, Dahan A, Brines M, van Velzen M. Cornea nerve fiber quantification and construction of phenotypes in patients with fibromyalgia. Sci Rep. (2016) 6:23573. doi: 10.1038/srep23573
65. Turan KE, Kocabeyoglu S, Unal-Cevik I, Bezci F, Akinci A, Irkec M. Ocular surface alterations in the context of corneal in vivo confocal microscopic characteristics in patients with fibromyalgia. Cornea. (2018) 37:205–10. doi: 10.1097/ICO.0000000000001447
66. Evdokimov D, Frank J, Klitsch A, Unterecker S, Warrings B, Serra J, et al. Reduction of skin innervation is associated with a severe fibromyalgia phenotype. Ann Neurol. (2019) 86:504–16. doi: 10.1002/ana.25565
67. Odriozola A, Ortega L, Martinez L, Odriozola S, Torrens A, Corroleu D, et al. Widespread sensory neuropathy in diabetic patients hospitalized with severe COVID-19 infection. Diabetes Res Clin Pract. (2020) 172:108631. doi: 10.1016/j.diabres.2020.108631
68. Daia C, Scheau C, Neagu G, Andone I, Spanu A, Popescu C, et al. Nerve conduction study and electromyography findings in patients recovering from Covid-19 - case report. Int J Infect Dis. (2021) 103:420–2. doi: 10.1016/j.ijid.2020.11.146
69. Hinduja A, Moutairou A, Calvet JH. Sudomotor dysfunction in patients recovered from COVID-19. Clin Neurophysiol. (2021) 51:193–6. doi: 10.1016/j.neucli.2021.01.003
70. Bureau BL, Obeidat A, Dhariwal MS, Jha P. Peripheral neuropathy as a complication of SARS-Cov-2. Cureus. (2020) 12:e11452. doi: 10.7759/cureus.11452
71. Cabañes-Martínez L, Villadóniga M, González-Rodríguez L, Araque L, Díaz-Cid A, Ruz-Caracuel I, et al. Neuromuscular involvement in COVID-19 critically ill patients. Clin Neurophysiol. (2020) 131:2809–16. doi: 10.1016/j.clinph.2020.09.017
72. Bitirgen G, Korkmaz C, Zamani A, Ozkagnici A, Zengin N, Ponirakis G, et al. Corneal confocal microscopy identifies corneal nerve fibre loss and increased dendritic cells in patients with long COVID. Br J Ophthalmol. (2021). doi: 10.1136/bjophthalmol-2021-319450. [Epub ahead of print].
73. Marques A, Brefel-Courbon C. Chronic pain in Parkinson's disease: clinical and pathophysiological aspects. Rev Neurol. (2020) 177:394–9. doi: 10.1016/j.neurol.2020.06.015
74. Anjos R, Vieira L, Sousa A, Maduro V, Alves N, Candelaria P. Peripheral neuropathy in Parkinson disease: an in vivo confocal microscopy study. Acta Ophthalmol. (2014) 92. doi: 10.1111/j.1755-3768.2014.2433.x
75. Kass-Iliyya L, Javed S, Gosal D, Kobylecki C, Marshall A, Petropoulos IN, et al. Small fiber neuropathy in Parkinson's disease: a clinical, pathological and corneal confocal microscopy study. Parkinson Relat Disord. (2015) 21:1454–60. doi: 10.1016/j.parkreldis.2015.10.019
76. Podgorny PJ, Suchowersky O, Romanchuk KG, Feasby TE. Evidence for small fiber neuropathy in early Parkinson's disease. Parkinson Relat Disord. (2016) 28:94–9. doi: 10.1016/j.parkreldis.2016.04.033
77. Misra SL, Kersten HM, Roxburgh RH, Danesh-Meyer HV, McGhee CN. Corneal nerve microstructure in Parkinson's disease. J Clin Neurosci. (2017) 39:53–8. doi: 10.1016/j.jocn.2017.02.033
78. Arrigo A, Rania L, Calamuneri A, Postorino EI, Mormina E, Gaeta M, et al. Early corneal innervation and trigeminal alterations in Parkinson disease: a pilot study. Cornea. (2018) 37:448–54. doi: 10.1097/ICO.0000000000001517
79. Avetisov SE, Karabanov AV, Surnina ZV, Gamidov AA. [Changes in corneal nerves fibers in the early stages of Parkinson's disease according to in vivo confocal microscopy (preliminary report)]. Vestnik Oftalmol. (2020) 136:191–6. doi: 10.17116/oftalma2020136052191
80. Lim SH, Ferdousi M, Kalteniece A, Kass-Iliyya L, Petropoulos IN, Malik RA, et al. Corneal confocal microscopy detects small fibre neurodegeneration in Parkinson's disease using automated analysis. Sci Rep. (2020) 10:20147. doi: 10.1038/s41598-020-76768-x
81. Che NN, Ding GX, Chen SY, Li DS, Li X, Ma JJ, et al. [Measurement of corneal nerve fiber parameters in patients with Parkinson's disease]. Zhonghua yi xue za zhi. (2021) 101:498–503. doi: 10.3760/cma.j.cn112137-20200614-01851
82. Lim SH, Ferdousi M, Kalteniece A, Mahfoud ZR, Petropoulos IN, Malik RA, et al. Corneal confocal microscopy identifies Parkinson's disease with more rapid motor progression. Mov Disord. (2021). doi: 10.1002/mds.28602. [Epub ahead of print].
83. Knowles LM, Phillips KM, Herring TE, Alschuler KN, Jensen MP, Turner AP, et al. Pain intensity and pain interference in people with progressive multiple sclerosis compared to people with relapsing-remitting multiple sclerosis. Arch Phys Med Rehabil. (2021). doi: 10.1016/j.apmr.2021.05.003. [Epub ahead of print].
84. Pellkofer HL, Kümpfel T. [Pain in multiple sclerosis and neuromyelitis optica spectrum disorders]. Schmerz. (2021) 35:211–22. doi: 10.1007/s00482-021-00554-5
85. Teoli D, Rocha Cabrero F, Ghassemzadeh S. Lhermitte Sign. StatPearls. Treasure Island, FL: StatPearls Publishing Copyright © 2021, StatPearls Publishing LLC (2021).
86. Rivel M, Achiron A, Dolev M, Stern Y, Zeilig G, Defrin R. Central neuropathic pain in multiple sclerosis is associated with impaired innocuous thermal pathways and neuronal hyperexcitability. Pain Med. (2021). doi: 10.1093/pm/pnab103. [Epub ahead of print].
87. Tohyama S, Walker MR, Zhang JY, Cheng JC, Hodaie M. Brainstem trigeminal fiber microstructural abnormalities are associated with treatment response across subtypes of trigeminal neuralgia. Pain. (2021) 162:1790–9. doi: 10.1097/j.pain.0000000000002164
88. Mikolajczak J, Zimmermann H, Kheirkhah A, Kadas EM, Oberwahrenbrock T, Muller R, et al. Patients with multiple sclerosis demonstrate reduced subbasal corneal nerve fibre density. Multiple Scler J. (2017) 23:1847–53. doi: 10.1177/1352458516677590
89. Bitirgen G, Akpinar Z, Malik RA, Ozkagnici A. Use of corneal confocal microscopy to detect corneal nerve loss and increased dendritic cells in patients with multiple sclerosis. JAMA Ophthalmol. (2017) 135:777–82. doi: 10.1001/jamaophthalmol.2017.1590
90. Petropoulos IN, Kamran S, Li Y, Khan A, Ponirakis G, Akhtar N, et al. Corneal confocal microscopy: an imaging endpoint for axonal degeneration in multiple sclerosis. Investig Ophthalmol Vis Sci. (2017) 58:3677–81. doi: 10.1167/iovs.17-22050
91. Bitirgen G, Akpinar Z, Turk HB, Malik RA. Abnormal dynamic pupillometry relates to neurologic disability and retinal axonal loss in patients with multiple sclerosis. Transl Vis Sci Technol. (2021) 10:30. doi: 10.1167/tvst.10.4.30
92. Khan A, Li Y, Ponirakis G, Akhtar N, Gad H, George P, et al. Corneal immune cells are increased in patients with multiple sclerosis. Transl Vis Sci Technol. (2021) 10:19. doi: 10.1167/tvst.10.4.19
93. Bitirgen G, Akpinar Z, Uca AU, Ozkagnici A, Petropoulos IN, Malik RA. Progressive loss of corneal and retinal nerve fibers in patients with multiple sclerosis: a 2-year follow-up study. Transl Vis Sci Technol. (2020) 9:37. doi: 10.1167/tvst.9.13.37
94. Kong Z, Chen P, Jiang J, Wang X, Wang Y, Shi Y, et al. Pain characteristics in amyotrophic lateral sclerosis patients and its impact on quality of life: a prospective observational study in a northern city of China. Ann Palliat Med. (2021) 10:1668–74. doi: 10.21037/apm-20-864
95. Delpont B, Beauvais K, Jacquin-Piques A, Alavoine V, Rault P, Blanc-Labarre C, et al. Clinical features of pain in amyotrophic lateral sclerosis: a clinical challenge. Rev Neurol. (2019) 175:11–5. doi: 10.1016/j.neurol.2017.11.009
96. Ferrari G, Grisan E, Scarpa F, Fazio R, Comola M, Quattrini A, et al. Corneal confocal microscopy reveals trigeminal small sensory fiber neuropathy in amyotrophic lateral sclerosis. Front Aging Neurosci. (2014) 6:278. doi: 10.3389/fnagi.2014.00278
97. Choi HR, Aktas A, Bottros MM. Pharmacotherapy to manage central post-stroke pain. CNS Drugs. (2021) 35:151–60. doi: 10.1007/s40263-021-00791-3
98. Xu XM, Luo H, Rong BB, Zheng XM, Wang FT, Zhang SJ, et al. Nonpharmacological therapies for central poststroke pain: a systematic review. Medicine. (2020) 99:e22611. doi: 10.1097/MD.0000000000022611
99. Gad H, Khan A, Akhtar N, Kamran S, El-Sotouhy A, Dargham SR, et al. Corneal nerve and endothelial cell damage in patients with transient ischemic attack and minor ischemic stroke. PLoS ONE. (2019) 14:e0213319. doi: 10.1371/journal.pone.0213319
100. Khan A, Kamran S, Akhtar N, Ponirakis G, Al-Muhannadi H, Petropoulos IN, et al. Corneal confocal microscopy detects a reduction in corneal endothelial cells and nerve fibres in patients with acute ischemic stroke. Sci Rep. (2018) 8:17333. doi: 10.1038/s41598-018-35298-3
101. Khan A, Akhtar N, Kamran S, Almuhannadi H, Ponirakis G, Petropoulos IN, et al. Corneal confocal microscopy identifies greater corneal nerve damage in patients with a recurrent compared to first ischemic stroke. PLoS ONE. (2020) 15:e0231987. doi: 10.1371/journal.pone.0231987
102. Kamran S, Khan A, Salam A, Akhtar N, Petropoulos I, Ponirakis G, et al. Cornea: a window to white matter changes in stroke; corneal confocal microscopy a surrogate marker for the presence and severity of white matter hyperintensities in ischemic stroke. J Stroke Cerebrovasc Dis. (2020) 29:104543. doi: 10.1016/j.jstrokecerebrovasdis.2019.104543
103. van Kooten J, Smalbrugge M, van der Wouden JC, Stek ML, Hertogh C. Prevalence of pain in nursing home residents: the role of dementia stage and dementia subtypes. J Am Med Direct Assoc. (2017) 18:522–7. doi: 10.1016/j.jamda.2016.12.078
104. Husebo BS, Achterberg W, Flo E. Identifying and managing pain in people with Alzheimer's disease and other types of dementia: a systematic review. CNS Drugs. (2016) 30:481–97. doi: 10.1007/s40263-016-0342-7
105. Dehghani C, Frost S, Jayasena R, Masters CL, Kanagasingam Y. Ocular biomarkers of Alzheimer's disease: the role of anterior eye and potential future directions. Investig Ophthalmol Vis Sci. (2018) 59:3554–63. doi: 10.1167/iovs.18-24694
106. Ponirakis G, Al Hamad H, Sankaranarayanan A, Khan A, Chandran M, Ramadan M, et al. Association of corneal nerve fiber measures with cognitive function in dementia. Ann Clin Transl Neurol. (2019) 6:689–97. doi: 10.1002/acn3.746
107. Al-Janahi E, Ponirakis G, Al Hamad H, Vattoth S, Elsotouhy A, Petropoulos IN, et al. Corneal nerve and brain imaging in mild cognitive impairment and dementia. J Alzheimers Dis. (2020) 77:1533–43. doi: 10.3233/JAD-200678
108. Marquez A, Guernsey LS, Frizzi KE, Cundiff M, Constantino I, Muttalib N, et al. Tau associated peripheral and central neurodegeneration: identification of an early imaging marker for tauopathy. Neurobiol Dis. (2021) 151:105273. doi: 10.1016/j.nbd.2021.105273
109. Kinard KI, Smith AG, Singleton JR, Lessard MK, Katz BJ, Warner JE, et al. Chronic migraine is associated with reduced corneal nerve fiber density and symptoms of dry eye. Headache. (2015) 55:543–9. doi: 10.1111/head.12547
110. Shetty R, Deshmukh R, Shroff R, Dedhiya C, Jayadev C. Subbasal nerve plexus changes in chronic migraine. Cornea. (2018) 37:72–5. doi: 10.1097/ICO.0000000000001403
111. Lee JI, Böcking T, Holle-Lee D, Malik RA, Kieseier BC, Hartung HP, et al. Corneal confocal microscopy demonstrates corneal nerve loss in patients with trigeminal neuralgia. Front Neurol. (2020) 11:661. doi: 10.3389/fneur.2020.00661
112. O'Neill F, Marshall A, Ferdousi M, Malik RA. Corneal confocal microscopy detects small-fiber neuropathy in burning mouth syndrome: a cross-sectional study. J Oral Fac Pain Headache. (2019) 33:337–41. doi: 10.11607/ofph.2338
113. Mehra S, Tavakoli M, Kallinikos PA, Efron N, Boulton AJ, Augustine T, et al. Corneal confocal microscopy detects early nerve regeneration after pancreas transplantation in patients with type 1 diabetes. Diabetes Care. (2007) 30:2608–12. doi: 10.2337/dc07-0870
114. Tavakoli M, Mitu-Pretorian M, Petropoulos IN, Fadavi H, Asghar O, Alam U, et al. Corneal confocal microscopy detects early nerve regeneration in diabetic neuropathy after simultaneous pancreas and kidney transplantation. Diabetes. (2013) 62:254–60. doi: 10.2337/db12-0574
115. Azmi S, Jeziorska M, Ferdousi M, Petropoulos IN, Ponirakis G, Marshall A, et al. Early nerve fibre regeneration in individuals with type 1 diabetes after simultaneous pancreas and kidney transplantation. Diabetologia. (2019) 62:1478–87. doi: 10.1007/s00125-019-4897-y
116. Adam S, Azmi S, Ho JH, Liu Y, Ferdousi M, Siahmansur T, et al. Improvements in diabetic neuropathy and nephropathy after bariatric surgery: a prospective cohort study. Obesity Surg. (2020) 31:554–63. doi: 10.1007/s11695-020-05052-8
117. Dahan A, Dunne A, Swartjes M, Proto PL, Heij L, Vogels O, et al. ARA 290 improves symptoms in patients with sarcoidosis-associated small nerve fiber loss and increases corneal nerve fiber density. Mol Med. (2013) 19:334. doi: 10.2119/molmed.2013.00122
118. Culver DA, Dahan A, Bajorunas D, Jeziorska M, van Velzen M, Aarts LP, et al. Cibinetide improves corneal nerve fiber abundance in patients with sarcoidosis-associated small nerve fiber loss and neuropathic pain. Investig Ophthalmol Vis Sci. (2017) 58:BIO52–60. doi: 10.1167/iovs.16-21291
119. Brines M, Dunne AN, van Velzen M, Proto PL, Ostenson C-G, Kirk RI, et al. ARA 290, a nonerythropoietic peptide engineered from erythropoietin, improves metabolic control and neuropathic symptoms in patients with type 2 diabetes. Mol Med. (2014) 20:658. doi: 10.2119/molmed.2014.00215
120. Lewis EJ, Perkins BA, Lovblom LE, Bazinet RP, Wolever TM, Bril V. Effect of omega-3 supplementation on neuropathy in type 1 diabetes: a 12-month pilot trial. Neurology. (2017) 88:2294–301. doi: 10.1212/WNL.0000000000004033
121. Lewis EJH, Lovblom LE, Cisbani G, Chen DK, Bazinet RP, Wolever TMS, et al. Baseline omega-3 level is associated with nerve regeneration following 12-months of omega-3 nutrition therapy in patients with type 1 diabetes. J Diabetes Comp. (2021) 35:107798. doi: 10.1016/j.jdiacomp.2020.107798
122. Britten-Jones AC, Kamel JT, Roberts LJ, Braat S, Craig JP, MacIsaac RJ, et al. Investigating the neuroprotective effect of oral omega-3 fatty acid supplementation in type 1 diabetes (nPROOFS1): a randomized placebo-controlled trial. Diabetes. (2021) db210136. doi: 10.2337/db21-0136
123. Ponirakis G, Abdul-Ghani MA, Jayyousi A, Almuhannadi H, Petropoulos IN, Khan A, et al. Effect of treatment with exenatide and pioglitazone or basal-bolus insulin on diabetic neuropathy: a substudy of the Qatar Study. BMJ Open Diabetes Res Care. (2020) 8:e001420. doi: 10.1136/bmjdrc-2020-001420
124. Ponirakis G, Abdul-Ghani MA, Jayyousi A, Zirie MA, Al-Mohannadi S, Almuhannadi H, et al. Insulin resistance limits corneal nerve regeneration in patients with Type 2 diabetes undergoing intensive glycemic control. J Diabetes Investig. (2021). doi: 10.1111/jdi.13582. [Epub ahead of print].
125. Ponirakis G, Abdul-Ghani MA, Jayyousi A, Zirie MA, Qazi M, Almuhannadi H, et al. Painful diabetic neuropathy is associated with increased nerve regeneration in patients with type 2 diabetes undergoing intensive glycemic control. J Diabetes Investig. (2021) 127:287–317. doi: 10.1111/jdi.13544
Keywords: corneal confocal microscopy, neurodegeneration, painful neuropathy, diabetes, biomarker
Citation: Petropoulos IN, Bitirgen G, Ferdousi M, Kalteniece A, Azmi S, D'Onofrio L, Lim SH, Ponirakis G, Khan A, Gad H, Mohammed I, Mohammadi YE, Malik A, Gosal D, Kobylecki C, Silverdale M, Soran H, Alam U and Malik RA (2021) Corneal Confocal Microscopy to Image Small Nerve Fiber Degeneration: Ophthalmology Meets Neurology. Front. Pain Res. 2:725363. doi: 10.3389/fpain.2021.725363
Received: 15 June 2021; Accepted: 26 July 2021;
Published: 19 August 2021.
Edited by:
Monique Van Velzen, Leiden University Medical Center, NetherlandsReviewed by:
Jeremy Chung Bo Chiang, University of New South Wales, AustraliaCopyright © 2021 Petropoulos, Bitirgen, Ferdousi, Kalteniece, Azmi, D'Onofrio, Lim, Ponirakis, Khan, Gad, Mohammed, Mohammadi, Malik, Gosal, Kobylecki, Silverdale, Soran, Alam and Malik. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rayaz A. Malik, cmFtMjA0NUBxYXRhci1tZWQuY29ybmVsbC5lZHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.