- 1MedStar National Rehabilitation Hospital, Washington, DC, United States
- 2Department of Rehabilitation Medicine, Georgetown University, Washington, DC, United States
Assessment of cancer rehabilitation outcome measures is integral for patient assessment, symptom screening, and advancing scientific research. In the broad field of cancer rehabilitation, outcome measures can cross-cut across many different branches of oncologic care including clinician-reported, patient-reported, and objective measures. Specific outcome measures that apply to cancer rehabilitation include those pertinent to pain, function, quality of life, fatigue, and cognition. These outcome measures, when used in cancer rehabilitation, can be utilized to evaluate the effectiveness of an intervention and to triage to the appropriate supportive care service. This review article summarizes some of the commonly used outcome measures that can be applied in the cancer rehabilitation setting to support scholarly work and patient care.
Introduction
Living life post cancer diagnosis is becoming a reality in the United States and across the world for a growing number of patients. This is in large part due to the advancements in cancer disease, specific knowledge, screenings, and treatments. It is expected that by the year 2040, there will be more than 26 million cancer survivors in the United States (1). This growing population will result in an increase in the demand for specialists who will be tasked to address the increasing burden of the devastating complications associated with cancer. These not only include a variety of functional physical impairments but also extend to emotional, social, psychological, and cognitive stressors that can impact the overall quality of life of a patient. The current rate of cancer-related disabilities remains exceedingly high with the demands for even readily treatable physical conditions being met at a rate of 1–2% (2).
For these patients, alleviating the impact of physical, social, psychological, cognitive, and emotional burdens of the disease is paramount to improving their quality of life and function. Enabling the patient to achieve this is the goal of cancer rehabilitation. The field of cancer rehabilitation can be divided into four separate categories based on the temporal course of the disease (3), which includes preventative, restorative, supportive, and palliative rehabilitation. Preventative rehabilitation seeks to control the outcomes prior to diagnosis or cancer-related interventions to maximize functionality early in the treatment course. Restorative rehabilitation aims to maximize recovery in those undergoing treatments and having existing impairments. Supportive and palliative rehabilitation tend to focus on disease progression and declining function (3). These therapies are geared toward augmenting self-care ability and mobility and relieving distressing symptoms, such as pain, fatigue, and anorexia. Cancer rehabilitation can also be tailored to address system-specific, disease-specific, and symptom-specific problems. Specialists need to track the outcomes of the interventions used to address these problems. Data achieved through outcome measures is a primary vehicle in medicine to assess the quality of interventions. In a growing field such as cancer rehabilitation, a prudent understanding of these measures will create a foundation from which to develop.
In this review, we explore a variety of outcome measures used in cancer rehabilitation and the related fields. In the modern world of medicine and evidence-based treatments, every specialty needs to have focused assessments of the measures they use to analyze treatment effectiveness. Without a proper understanding of the appropriate outcome measures, it is impossible to gauge the effectiveness of the outcomes of treatments that are being validated by these measures. This is the critical first step and is consistent with a growing national trend on the use of defined values. Current research on outcome measures specific to cancer rehabilitation is limited. Creating a better understanding of the validity, scope, and action ability of these measures will allow providers to get a better sense of when to utilize specific treatments, an understanding of how effective they may be, and how they can fit into the overall patient-care goals. Creating this foundation increases the confidence of the providers and emphasizes the need for quality-based, evidence-based care. In this review article, we organize and assess the utility of specific outcome measures, commonly seen under the broader umbrella of cancer rehabilitation, such as function, quality of life, pain, fatigue, cognition, and objective measures. Please note that this review does not encompass all the pertinent and available outcome measures that can be used in cancer rehabilitation, but presents a starting point for commonly used measures.
An outcome measure is a tool, usually in the form of a questionnaire, used to reflect the impact of a healthcare service or intervention on the health status of a patient (29). Outcome measures may be used to determine the baseline function of a patient. Similarly, the same instrument can be used to determine the progress and efficacy after a certain intervention (30). Therefore, outcome measures are often used to assess the response to treatment.
Methods
This review discusses some of the more commonly used outcome measures in the field of cancer rehabilitation, specifically, those that pertain to general function, fatigue, pain, quality of life, cognition, and objective measures. We provide the following six key elements that help describe the properties of each measure:
• General description: includes the definition and purpose of the measure
• Psychometric properties: include a combination of validity, reliability, internal consistency, test-retest reliability, and ceiling/floor effects
• Burden: indicates the number of items in and the time taken to complete the questionnaire
• Scoring: outlines how the measure is scored
• Scope: includes any domain or subdomain that may be a part of the outcome measure (for example: if mobility is being assessed – are transfers, ambulation, and stairs part of this measure?)
• Clinical relevance: outlines how the measure can be most useful in a clinical setting.
The outcome measures selected in each section were chosen based on a careful review of the cancer rehabilitation literature, discussion amongst the authors of this paper, and discussion with cancer rehabilitation experts from other institutions.
Pain Outcome Measures
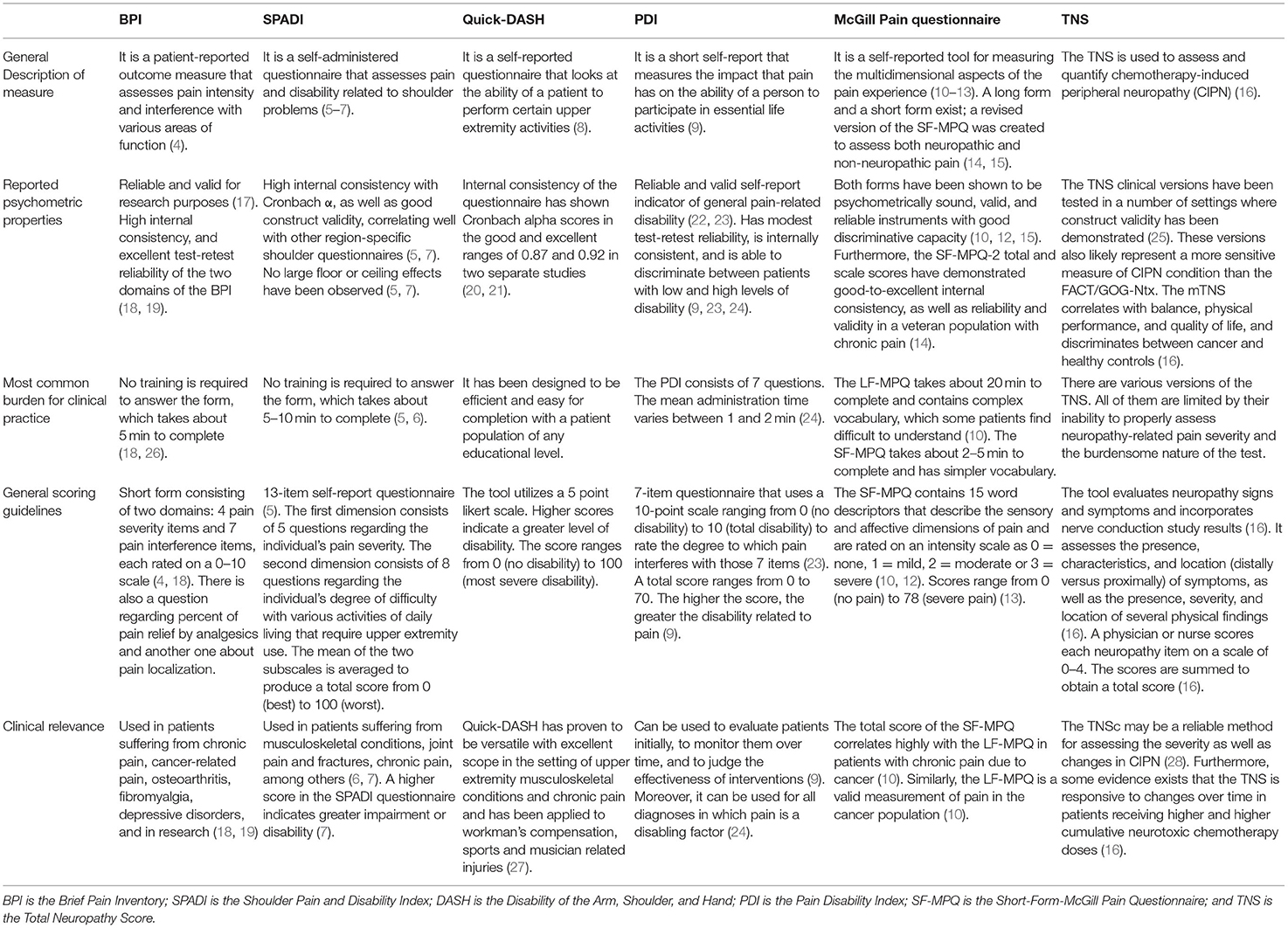
The frequency, severity, and impact which pain has on the quality of life of patients living with cancer are important factors to be considered by the clinician (17). Formal instruments have been developed to help describe and measure pain, thereby helping clinicians and patients track the progression of pain or response to treatment. We focus on five commonly used outcome measures in Table 1, which include the Brief Pain Inventory (BPI), the Shoulder Pain and Disability Index (SPADI), Quick-Disability of the Arm, Shoulder, and Hand (Quick-DASH), the Pain Disability Index (PDI), and the McGill Pain Questionnaire (MPQ).
General Functional Outcome Measures
Monitoring patient function prior to, during, and after cancer treatment is an essential function of cancer rehabilitation. Tracking function over time is an important way to assess how patients are progressing with rehabilitation. Functional outcome measures, such as the Functional Assessment of Cancer Therapy-General (FACT-G), Eastern Cooperative Oncology Group (ECOG), Karnofsky Performance Scale (KPS), and Common Terminology Criteria for Adverse Events (CTCAE) are several widely utilized outcome measures of general function that provide objective data that clinicians utilize before making treatment decisions and assessing the response to cancer and rehabilitation treatments. In Table 2, we break down each of these measures to better understand their utility and quality.
General Quality of Life Measures
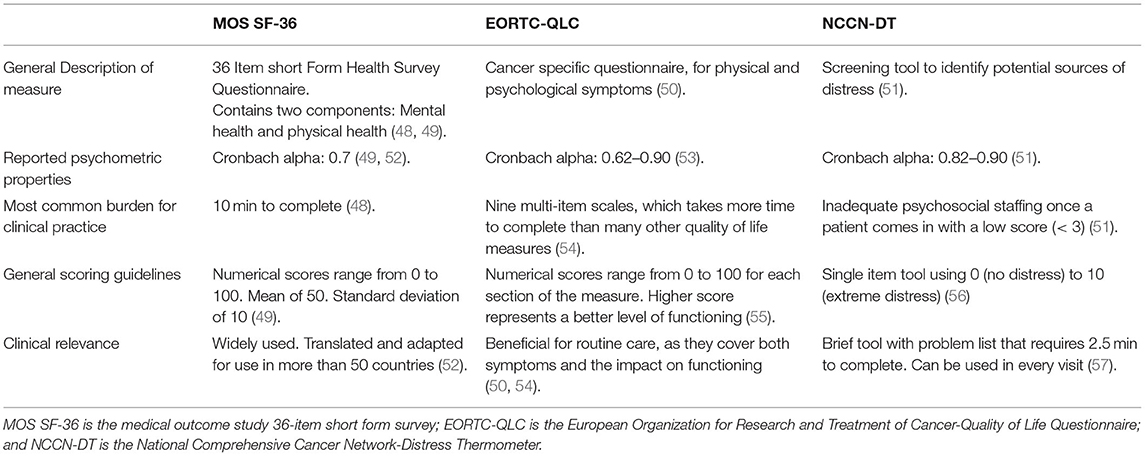
The assessment of the quality of life (QOL) has become one of the most critical parts of oncologic care. It is common that decisions to initiate, avoid, and cease treatment may be based on a discussion regarding QOL of the patient. In addition, QOL has become an important measure of the success (and failure) of the aspects of oncologic treatment. Therefore, familiarity with various QOL measurement tools is essential in oncology care. While different QOL measures exist, in Table 3, we review the Short Form-36 (SF-36), European Organization for Research and Treatment of Cancer (EORTC), and National Comprehensive Cancer Network -Distress Thermometer (NCCN-DT).
Fatigue Outcome Measures
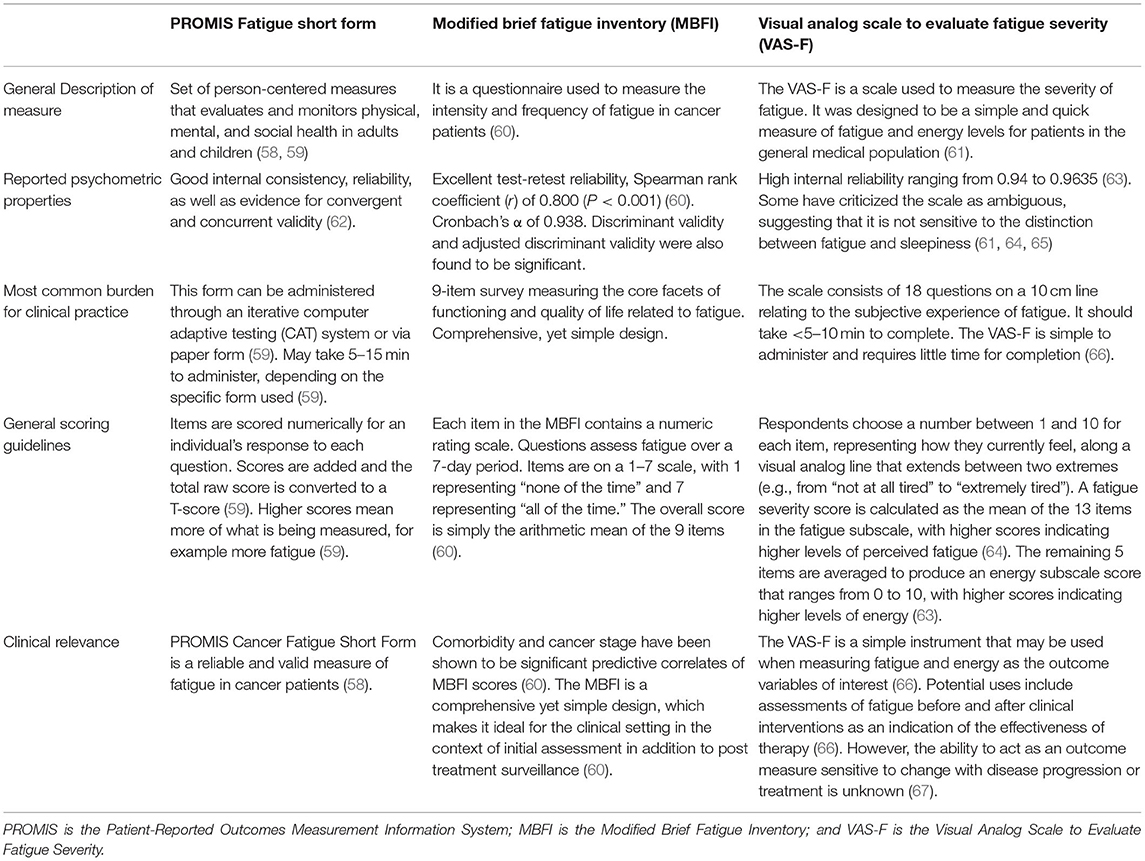
Cancer-related fatigue is a common experience among cancer survivors. It is estimated that the predominance of this symptom is close to 48% and may increase with disease burdens, such as metastasis, or treatment, such as chemotherapy (31). A significant variable driving the assessment and treatment of cancer-related fatigue has been the recognition of its negative effect on the quality of life (31). Various scales have been used to objectively measure fatigue in both the research and clinical settings. In Table 4, we present three outcome measures: the Patient-Reported Outcomes Measurement Information System (PROMIS) Fatigue Short Form, the Modified Brief Fatigue Inventory (MBFI), and the Visual Analog Scale to Evaluate Fatigue Severity (VAS-F).
Cognitive Outcome Measures
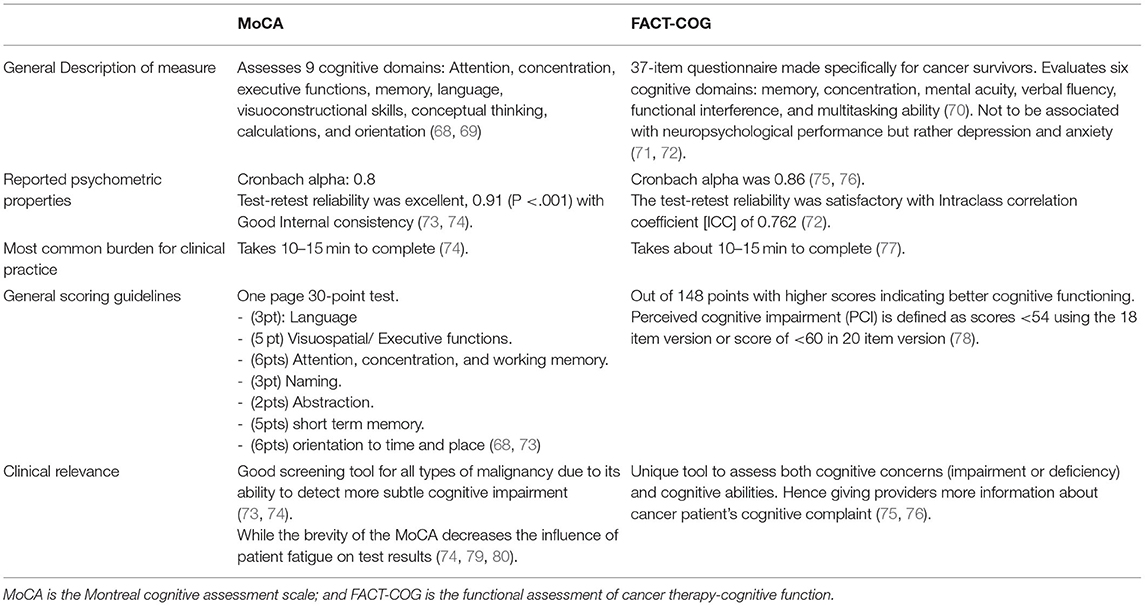
Impaired cognition is a common issue reported in patients undergoing cancer treatment as well as beyond treatment. Many factors have been proposed to impact cognition in cancer, including various cancer treatments, mood disorders, fatigue, and poor sleep. Given how pervasive these symptoms can be, it is important to assess and monitor cognitive function during and after cancer treatment. In Table 5, we review the Montreal cognitive assessment (MoCA) and the FACT-cognitive function (FACT-COG). While FACT-COG is designed specifically for cancer survivors, it should be noted that there is no gold standard cognitive assessment for the cancer population. Overall, it is important to consider that all cognitive screening measures carry a risk of false-positive errors, particularly when used with individuals whose education level and/or cultural and linguistic backgrounds differ from that of the normative sample (68, 73). In addition, they may also fail to detect more subtle cognitive deficits that can cause distress in many patients (73).
Objective Measures
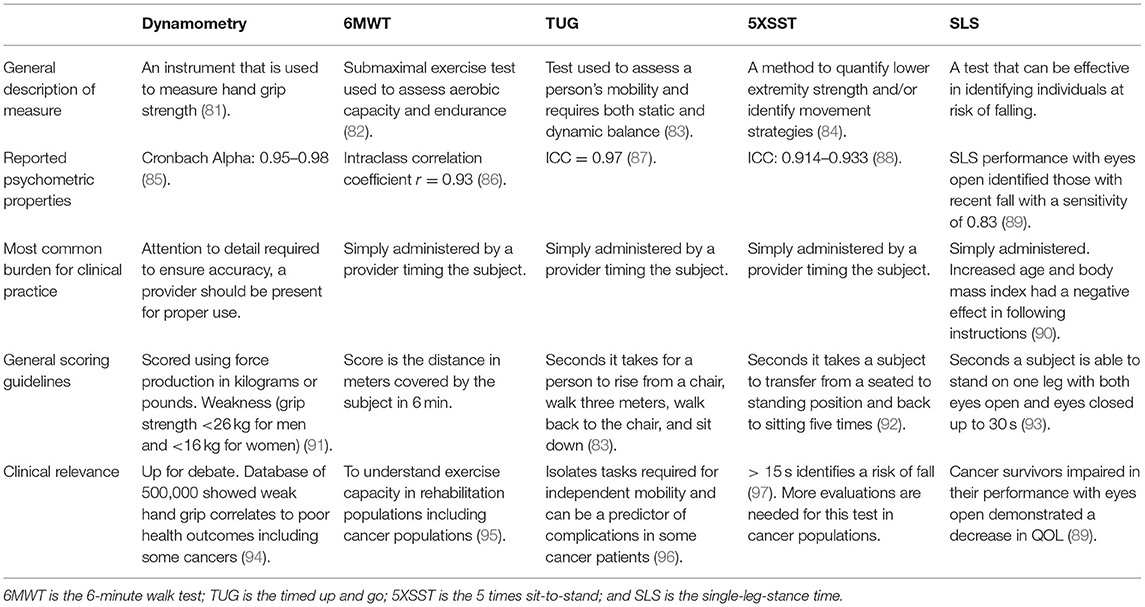
Strength, balance, mobility, and endurance are some of the important measures that rehabilitation providers look to assess carefully in their respective patient populations. Cancer rehabilitation specialists commonly need close assessments of these data points to better characterize functional capabilities, risk stratification, mortality prognostication, and QOL. Documentation of these data can vary greatly if done so on a subjective basis. However, special tests and instruments are described in Table 6, such as timed up and go (TUG) test, 5 times sit-to-stand (5XSST), and single-leg stance time (SLS) to create objective data points for providers to quantify and compare this data. In Table 6, we closely analyze the properties of common objective measures used in the cancer rehabilitation population and aim to individually assess the merit of each measure for continued use.
Conclusion
Outcome measures are a critical tool in assessing cancer patients before, during, and after cancer treatments. These assessments can include general function, QOL, pain, cognition, fatigue, and objective measures. These assessments not only monitor research outcomes but also assess a patient's positive and negative responses to interventions and safety to continue with cancer treatment. The outcome measures presented in this review are a small sampling of the available measures in the cancer rehabilitation setting. The author is optimistic that this review will provide the reader with a starting point in considering the useful outcome measures when starting a research project or focused patient assessment.
Author Contributions
All authors contributed equal parts of the literature review, writing, and reviewing of this manuscript. This was a collaborative effort and teamwork under the guidance of EW.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
2. Cheville AL, Mustian K, Winters-Stone K, Zucker DS, Gamble GL, Alfano CM. Cancer rehabilitation: an overview of current need, delivery models, and levels of care. Phys Med Rehabil Clin N Am.(2017) 28:1–17. doi: 10.1016/j.pmr.2016.08.001
3. Cuccurullo SJ. Physical Medicine and Rehabilitation Board Review, 4th Edn. Springer Publishing Company (2019).
4. Mathias SD, Crosby RD, Qian Y, Jiang Q, Dansey R, Chung K. Estimating minimally important differences for the worst pain rating of the brief pain inventory-short form. J Support Oncol. (2011) 9:72–8. doi: 10.1016/j.suponc.2010.12.004
5. Shoulder Pain and Disability Index. Available online at: https://www.sralab.org/rehabilitation-measures/shoulder-pain-and-disability-index (accessed: April 4, 2021).
6. Roach KE, Budiman-Mak E, Songsiridej N, Lertratanakul Y. Development of a shoulder pain and disability index. Arthritis Care Res. (1991) 4:143–9.
7. Breckenridge JD, McAuley JH. Shoulder Pain and Disability Index (SPADI). J Physiother. (2011) 57:197. doi: 10.1016/S1836-9553(11)70045-5
8. Quick Disabilities of Arm, Shoulder and Hand'. Available online at: https://www.sralab.org/rehabilitation-measures/quick-disabilities-arm-shoulder-hand (accessed: February 2, 2021).
9. Stubblefield J. The Pain Disability Index. Available online at: https://cme.dmu.edu/sites/default/files/The%20Pain%20Disability%20Index%20%28PDI%29.pdf (accessed: April 4, 2021).
10. Dudgeon D, Raubertas RF, Rosenthal SN. The short-form McGill Pain Questionnaire in chronic cancer pain. J Pain Symptom Manage. (1993) 8:191–5. doi: 10.1016/0885-3924(93)90126-g
11. Melzack R. The short-form McGill Pain Questionnaire. Pain. (1987) 30:191–7. doi: 10.1016/0304-3959(87)91074-8
12. Graham C, Bond SS, Gerkovich MM, Cook MR. Use of the McGill pain questionnaire in the assessment of cancer pain: replicability and consistency. Pain. (1980) 8:377–87.
13. McGill Pain Questionnaire. Available online at: https://www.sralab.org/rehabilitation-measures/mcgill-pain-questionnaire (accessed: April 4, 2021).
14. Lovejoy TI, Turk DC, Morasco BJ. Evaluation of the psychometric properties of the revised short-form McGill Pain Questionnaire. J Pain. (2012) 13:1250–7. doi: 10.1016/j.jpain.2012.09.011
16. Smith E, Lavoie M, Ellen M, Beck SL, Cohen J. The total neuropathy score: a tool for measuring chemotherapy-induced peripheral neuropathy. Oncol Nurs Forum. (2008) 35:96–102. doi: 10.1188/08.ONF.96-102
17. Daut RL, Cleeland CS, Flanery RC. Development of the Wisconsin brief pain questionnaire to assess pain in cancer and other diseases. Pain. (1983) 17:197–210. doi: 10.1016/0304-3959(83)90143-4
19. Brief Pain Inventory. Available online at: https://www.sralab.org/rehabilitation-measures/brief-pain-inventory (accessed: July 19, 2021).
20. Franchignoni F, Ferriero G, Giordano A, Sartorio F, Vercelli S, Brigatti E, et al. Psychometric properties of QuickDASH - a classical test theory and Rasch analysis study. Man Ther. (2011) 16:177–182. doi: 10.1016/j.math.2010.10.004
21. Gummesson C, Ward MM, Atroshi I. The shortened disabilities of the arm, shoulder and hand questionnaire (QuickDASH): validity and reliability based on responses within the full-length DASH. BMC Musculoskelet Disord. (2006) 7:44. doi: 10.1186/1471-2474-7-44
22. Mintken PE, Glynn P, Cleland JA. Psychometric properties of the shortened disabilities of the Arm, Shoulder, and Hand Questionnaire (QuickDASH) and Numeric Pain Rating Scale in patients with shoulder pain. J Shoulder Elbow Surg. (2009) 18:920–6. doi: 10.1016/j.jse.2008.12.015
23. Tait RC, Chibnall JT, Krause S. The Pain Disability Index: psychometric properties. Pain. (1990) 40:171–82. doi: 10.1016/0304-3959(90)90068-O
24. Soer R, Köke A, Vroomen P, Stegeman P, Rob S, Maarten C, et al. Extensive validation of the pain disability index in 3 groups of patients with musculoskeletal pain. Spine. (2013) 38:E562–8. doi: 10.1097/BRS.0b013e31828af21f
25. Griffith KA, Merkies I, Hill EE, Cornblath DR. Measures of chemotherapy-induced peripheral neuropathy: a systematic review of psychometric properties. J Peripher Nerv Syst. (2010) 15:314–25. doi: 10.1111/j.1529-8027.2010.00292.x
26. Brief Pain Inventory. Available online at: https://www.sralab.org/rehabilitation-measures/brief-pain-inventory (accessed: July 19, 2021).
27. DASH Outcome Measure. Available online at: https://www.physio-pedia.com/DASH_Outcome_Measure (accessed: February 2, 2021).
28. Molassiotis A, Cheng H, Lopez V, Au J, Chan A, Bandla A, et al. Are we mis-estimating chemotherapy-induced peripheral neuropathy? Analysis of assessment methodologies from a prospective, multinational, longitudinal cohort study of patients receiving neurotoxic chemotherapy. BMC Cancer. (2019) 19:132.
29. Types of Health Care Quality Measures. Content Last Reviewed July 2015. Agency for Healthcare Research and Quality, Rockville, MD. Available online at: https://www.ahrq.gov/talkingquality/measures/types.html (accessed: April 3, 2021).
30. The Harvard Clinical and Translational Research Center. Available online at: https://catalyst.harvard.edu/pdf/regulatory/CTR3_OutcomeMeasures.pdf (accessed: April 3, 2021).
31. Ebede CC, Jang Y, Escalante CP. Cancer-related fatigue in cancer survivorship. Med Clin N Am. (2017) 101:1085–97. doi: 10.1016/j.mcna.2017.06.007
32. Luckett T, King MT, Butow PN, Oguchi M, Rankin N, Price MA. Choosing between the EORTC QLQ-C30 and FACT-G for measuring health-related quality of life in cancer clinical research: issues, evidence and recommendations. Ann Oncol. (2011) 22:2179–90. doi: 10.1093/annonc/mdq721
33. ECOG Performance Status. Available online at: https://ecog-acrin.org/resources/ecog-performance-status (accessed: February 2, 2021).
34. Péus D, Newcomb N, Hofer S. Appraisal of the Karnofsky performance status and proposal of a simple algorithmic system for its evaluation. BMC Med Inform Decis Mak. (2013) 13:72. doi: 10.1186/1472-6947-13-72
35. CTCAE CTEP Cancer Protocol. Available online at: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf (accessed: April 3, 2021).
36. Anshabo A, Migbaru S, Mulu A, Negusu D, Asfaw AA, Awoke D, et al. Validation of the Amharic version of the M. D. anderson symptom inventory and assessment of symptoms in Ethiopian cancer patients. J Pain Symptom Manage. (2016) 51:947–53. doi: 10.1016/j.jpainsymman.2015.12.333
37. MD Anderson Symptom Inventory (MDASI)'. Available online at: https://www.mdanderson.org/research/departments-labs-institutes/departments-divisions/symptom-research/symptom-assessment-tools/md-anderson-symptom-inventory.html (accessed: April 4, 2021).
38. Fries JF, Bruce B, Cella D. The promise of PROMIS: using item response theory to improve assessment of patient-reported outcomes. Clin Exp Rheumatol. (2005). 23:S53–7.
39. Costet N, Lapierre V, Benhamou E, Le Galès C. Reliability and validity of the Functional Assessment of Cancer Therapy General (FACT-G) in French cancer patients. Qual Life Res. (2005) 14:1427–32. doi: 10.1016/0169-5002(95)00450-f
40. Piacentini P, Greco F, Mercanti A, Trolese A, Durante E, Moratello G, et al. Platinum doublets as first-line treatment for elderly patients with advanced non-small cell lung cancer. Tumori. (2013) 99:650–5. doi: 10.1700/1390.15451
41. Sørensen JB, Klee M, Palshof T, Hansen HH. Performance status assessment in cancer patients. An inter-observer variability study. Br J Cancer. (1993) 67:773–5.
42. Schag CC, Heinrich RL, Ganz PA. Karnofsky performance status revisited: reliability, validity, and guidelines. J Clin Oncol. (1984) 2:187–93.
43. Basch E, Reeve BB, Mitchell SA, Clauser SB, Minasian LM, Dueck AC, et al. Development of the National Cancer Institute's patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE). J Natl Cancer Inst. (2014) 106:dju244. doi: 10.1093/jnci/dju244
44. Webster K, Cella D, Yost K. The functional assessment of chronic illness therapy (FACIT) Measurement System: properties, applications, and interpretation. Health Qual Life Outcomes. (2003) 1:79. doi: 10.1186/1477-7525-1-79
45. Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, et al. Toxicity and response criteria of the Eastern cooperative oncology group. Am J Clin Oncol. (1982) 5:649–55.
46. Black N. Patient reported outcome measures could help transform healthcare. BMJ. (2013) 346:f167. doi: 10.1136/bmj.f167
47. Kelly CM, Shahrokni A. Moving beyond Karnofsky and ECOG performance status assessments with new technologies. J Oncol. (2016) 2016:6186543. doi: 10.1155/2016/6186543
48. Stam H, Grootenhuis MA, Caron HN, Last BF. Quality of life and current coping in young adult survivors of childhood cancer: positive expectations about the further course of the disease were correlated with better quality of life. Psychooncology. (2006) 15:31–43. doi: 10.1002/pon.920
49. Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. (1992) 30:473–83.
50. Wintner LM, Sztankay M, Aaronson N, Bottomley A, Giesinger JM, Groenvold M, et al. The use of EORTC measures in daily clinical practice-A synopsis of a newly developed manual. Eur J Cancer. (2016) 68:73–81. doi: 10.1016/j.ejca.2016.08.024
51. Cutillo A, O'Hea E, Person S, Lessard D, Harralson T, Boudreaux E. The distress thermometer: cutoff points and clinical use. Oncol Nurs Forum. (2017) 44:329–36. doi: 10.1188/17.ONF.329-336
52. Hayes V, Morris J, Wolfe C, Morgan M. The SF-36 health survey questionnaire: is it suitable for use with older adults? Age Ageing. (1995) 24:120–5.
53. Sztankay M, Aaronson NK, Arraras JI, Basso U, Bumbasirevic U, Efficace F, et al. International phase IV validation study of an EORTC quality of life questionnaire for testicular cancer patients: the EORTC QLQ-TC26. BMC Cancer. (2018) 18:1104. doi: 10.1186/s12885-018-5036-8
54. Petersen M, Aaronson NK, Arraras JI, Chie W, Conroy T, Costantini A, et al. The EORTC computer-adaptive tests measuring physical functioning and fatigue exhibited high levels of measurement precision and efficiency. J Clin Epidemiol. (2013) 66:330–9. doi: 10.1016/j.jclinepi.2012.09.010
55. Fisher MI, Davies CC, Colon G, Geyer, Pfalzer HL. Oncology section EDGE task force on prostate cancer outcomes: a systematic review of clinical measures of strength and muscular endurance. Rehabil Oncol. (2015) 33:37–44. doi: 10.1097/01893697-201533020-00006
56. Ownby KK. Use of the distress thermometer in clinical practice. J Adv Pract Oncol. (2019) 10:175–79. doi: 10.6004/jadpro.2019.10.2.7
57. Donovan KA, Deshields TL, Corbett C, Riba MB. Update on the implementation of NCCN guidelines for distress management by NCCN Member Institutions. J Natl Compr Cancer Netw. (2019) 17:1251–6. doi: 10.6004/jnccn.2019.7358
58. Cessna JM, Jim H, Sutton SK, Asvat Y, Small BJ, Salsman JM, et al. Evaluation of the psychometric properties of the PROMIS Cancer Fatigue Short Form with cancer patients. J Psychosom Res. (2016) 81:9–13. doi: 10.1016/j.jpsychores.2015.12.002
59. PROMIS Fatigue. Available online at: https://www.sralab.org/rehabilitation-measures/promis-fatigue (accessed: April 4, 2021).
60. Aynehchi BB, Obourn C, Sundaram K, Bentsianov BL, Rosenfeld RM. Validation of the modified brief fatigue inventory in head and neck cancer patients. Otolaryngol Head Neck Surg. (2013) 148:69–74. doi: 10.1177/0194599812460985
61. Dittner AJ, Wessely SC, Brown RG. The assessment of fatigue A practical guide for clinicians and researchers. J Psychosom Res. (2004) 56:157–70. doi: 10.1016/S0022-3999(03)00371-4
62. Cella D, Riley W, Stone A, Rothrock N, Reeve B, Yount S, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J Clin Epidemiol. (2010) 63:1179–94. doi: 10.1016/j.jclinepi.2010.04.011
63. Miaskowski C, Lee KA. Pain, fatigue, and sleep disturbances in oncology outpatients receiving radiation therapy for bone metastasis: a pilot study. J Pain Symptom Manage. (1999) 17:320–32.
64. Visual Analogue Scale to Evaluate Fatigue Severity (VAS-F). Available online at: https://www.med.upenn.edu/cbti/assets/user-content/documents/Visual%20Analogue%20Scale%20to%20Evaluate%20Fatigue%20Severity%20(VAS-F).pdf (accessed: April 4, 2021).
65. Fisher MI, Davies C, Lacy H, Doherty D. Oncology section EDGE task force on cancer: measures of cancer-related fatigue—a systematic review. Rehabil Oncol. (2018) 36:93. doi: 10.1097/01.REO.0000000000000124
66. Lee KA, Hicks G, Nino-Murcia G. Validity and reliability of a scale to assess fatigue. Psychiatry Res. (1991) 36:291–8.
67. Whitehead L. The measurement of fatigue in chronic illness: a systematic review of unidimensional and multidimensional fatigue measures. J Pain Symptom Manage. (2009) 37:107–28. doi: 10.1016/j.jpainsymman.2007.08.019
68. Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. (2005) 53:695–9. doi: 10.1111/j.1532-5415.2005.53221.x
69. MoCA Montreal - Cognitive Assessment'. Available online at: https://www.mocatest.org/ (accessed: January 30, 2021).
70. Jacobs SR, Jacobsen PB, Booth-Jones M, Wagner LI, Anasetti C. Evaluation of the functional assessment of cancer therapy cognitive scale with hematopoietic stem cell transplant patients. J Pain Symptom Manage. (2007) 33:13–23. doi: 10.1016/j.jpainsymman.2006.06.011
71. Wagner LI, Sweet J, Butt Z, Lai J, Cella D. Measuring patient self-reported cognitive function: development of the functional assessment of cancer therapy-cognitive function instrument. J Support Oncol. (2009) 7:W32–W39.
72. Cheung Y, Lim SR, Shwe M, Tan Y, Chan A. Psychometric Properties and measurement equivalence of the English and Chinese versions of the functional assessment of cancer therapy-cognitive in Asian patients with breast cancer. Value Health. (2013) 16:1001–13. doi: 10.1016/j.jval.2013.06.017
73. Roebuck-Spencer TM, Glen T, Puente AE, Denney RL, Ruff RM, Hostetter G, et al. Cognitive screening tests versus comprehensive neuropsychological test batteries: a national academy of neuropsychology education paper†. Arch Clin Neuropsychol. (2017) 32:491–8. doi: 10.1093/arclin/acx021
74. Smith T, Gildeh N, Holmes C. The Montreal Cognitive Assessment: validity and utility in a memory clinic setting'. Can J Psychiatry. (2007) 52:329–32. doi: 10.1177/070674370705200508
75. Von Ah, Tallman DEF. Perceived cognitive function in breast cancer survivors: evaluating relationships with objective cognitive performance and other symptoms using the functional assessment of cancer therapy-cognitive function instrument. J Pain Symptom Manage. (2015) 49:697–706. doi: 10.1016/j.jpainsymman.2014.08.012
76. Hajj A, Salameh P, Khoury R, Hachem R, Sacre H, Chahine G, et al. Psychometric Properties of the 37-Item Functional Assessment of Cancer Therapy-Cognitive Function (FACT-Cog) Scale in Cancer Patients. Research Square (2020). Available online at: https://www.researchsquare.com/article/rs-52087/latest.pdf.
77. FACT-Cog'. Available online at: https://www.facit.org/measures/FACT-Cog (accessed: February 1, 2021).
78. Van Dyk K, Crespi CM, Petersen L, Ganz PA. Identifying cancer-related cognitive impairment using the FACT-Cog perceived cognitive impairment. JNCI Cancer Spectrum. (2020) 4:kz099. doi: 10.1093/jncics/pkz099
79. Olson RA, Chhanabhai T, McKenzie M. Feasibility study of the Montreal Cognitive Assessment (MoCA) in patients with brain metastases. Supportive Care Cancer. (2008) 16:1273–8. doi: 10.1007/s00520-008-0431-3
80. Meyers, Brown CA. Role PD, and relevance of neurocognitive assessment in clinical trials of patients with CNS tumors. J Clin Oncol. (2006) 24:1305–9. doi: 10.1200/JCO.2005.04.6086
81. Hand-held Dynamometer/Grip Strength. Available online at: https://www.sralab.org/rehabilitation-measures/hand-held-dynamometergrip-strength (accessed: 18 February 2021).
82. Six Minute Walk Test/6 Minute Walk Test. Available online at: https://www.physio-pedia.com/Six_Minute_Walk_Test_/_6_Minute_Walk_Test (accessed: 18 February 2021).
83. Timed Up and Go Test (TUG). Available online at: https://www.physio-pedia.com/Timed_Up_and_Go_Test_(TUG) (accessed: 3 February 2021).
84. Five Times Sit to Stand Test'. Available online at: https://www.sralab.org/rehabilitation-measures/five-times-sit-stand-test (accessed: 3 February 2021).
85. Bellace JV, Healy D, Besser MP, Byron T, Hohman L. Validity of the Dexter Evaluation System's Jamar dynamometer attachment for assessment of hand grip strength in a normal population. J Hand Therapy. (2000) 13:46–51. doi: 10.1016/s0894-1130(00)80052-6
86. Schmidt K, Vogt L, Thiel C, Jäger E, Banzer W. Validity of the six-minute walk test in cancer patients. Int J Sports Med. (2013) 34:631–6.
87. Steffen TM, Hacker TA, Mollinger L. Age and gender-related test performance in community-dwelling elderly people: six-minute walk test, berg balance scale, timed up & go test, gait speeds. Phys Therapy. (2002) 82:128–37. doi: 10.1093/ptj/82.2.128
88. The repetitive five-times-sit-to stand: its reliability in older adults. Available online at: doi: 10.12968/ijtr.2013.20.3.122 (accessed: February 3, 2021).
89. Marker RJ, Kakar RS, Scorsone JJ, Peters JC, Purcell WT. Single-leg stance times in a diverse group of survivors of cancer and the relationship to history of recent falls. Rehabilitation Oncol. (2021) 39:23. doi: 10.1097/01.REO.0000000000000243
90. Springer BA, Marin R, Cyhan T, Roberts H, Gill NW. Normative values for the unipedal stance test with eyes open and closed. J Geriatr Phys Ther. (2007) 30:8–15. doi: 10.1519/00139143-200704000-00003
91. Hand-held Dynamometer/Grip Strength. Available online at: https://www.sralab.org/rehabilitation-measures/hand-held-dynamometergrip-strength (accessed: 18 February 2021).
92. Schaubert KL, Bohannon RW. Reliability and validity of three strength measures obtained from community-dwelling elderly persons. J. Strength Cond. Res. (2005) 19:717–20. doi: 10.1519/r-15954.1
93. Hurvitz EA, Richardson JK, Werner RA, Ruhl AM, Dixon MR. Unipedal stance testing as an indicator of fall risk among older outpatients. Arch Phys Med Rehabil. (2000) 81:587–91.
94. Celis-Morales CA, Welsh P, Lyall DM, Steell L. Associations of grip strength with cardiovascular, respiratory, and cancer outcomes and all cause mortality: prospective cohort study of half a million UK Biobank participants'. BMJ. (2018) 361:k1651. doi: 10.1136/bmj.k1651
95. Eden MM, Tompkins J, Verheijde JL. Reliability and a correlational analysis of the 6MWT, ten-meter walk test, thirty second sit to stand, and the linear analog scale of function in patients with head and neck cancer. Physiotherapy Theory Pract. (2018) 34:202–11. doi: 10.1080/09593985.2017.1390803
96. Huisman MG, Van Leeuwen BL, Ugolini G, Montroni I, Spiliotis J, Stabilini C, et al. “Timed Up & Go”: a screening tool for predicting 30-day morbidity in onco-geriatric surgical patients? A multicenter cohort study. PLoS ONE. (2014) 9:e86863. doi: 10.1371/journal.pone.0147993
Keywords: outcome measures, cancer rehabilitation, pain, function, rehabilitation
Citation: Maldonado E, Thalla N, Nepaul S and Wisotzky E (2021) Outcome Measures in Cancer Rehabilitation: Pain, Function, and Symptom Assessment. Front. Pain Res. 2:692237. doi: 10.3389/fpain.2021.692237
Received: 07 April 2021; Accepted: 05 August 2021;
Published: 29 September 2021.
Edited by:
Sara Parke, Mayo Clinic Arizona, United StatesReviewed by:
So Yeon Oh, Pusan National University Yangsan Hospital, South KoreaDenis Dupoiron, Institut de Cancérologie de l'Ouest (ICO), France
Copyright © 2021 Maldonado, Thalla, Nepaul and Wisotzky. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Eduardo Maldonado, ZWR1YXJkby5qLm1hbGRvbmFkb2NvbG9uQG1lZHN0YXIubmV0; Nirguna Thalla, bmlyZ3VuYS5yLnRoYWxsYUBtZWRzdGFyLm5ldA==; Sargoon Nepaul, c2FyZ29vbi5uZXBhdWxAbWVkc3Rhci5uZXQ=; Eric Wisotzky, ZXJpYy5tLndpc290emt5QG1lZHN0YXIubmV0
 Eduardo Maldonado
Eduardo Maldonado Nirguna Thalla1,2*
Nirguna Thalla1,2* Eric Wisotzky
Eric Wisotzky