- 1Department of Physical Therapy and Rehabilitation Science, University of Iowa, Iowa City, IA, United States
- 2College of Nursing, University of Iowa, Iowa City, IA, United States
- 3Department of Physical Therapy, St. Ambrose University, Davenport, IA, United States
- 4College of Public Health, University of Iowa, Iowa City, IA, United States
- 5Department of Medicine/Rheumatology & Immunology, Vanderbilt University, Nashville, TN, United States
Background: Nonrestorative sleep is commonly reported by individuals with fibromyalgia, but there is limited information on the reliability and responsiveness of self-reported sleep measures in this population.
Objectives: (1) Examine the reliability and validity of the Patient-Reported Outcomes Measurement Information System (PROMIS) sleep measures in women with fibromyalgia, and (2) Determine the responsiveness of the PROMIS sleep measures to a daily transcutaneous electrical nerve stimulation (TENS) intervention in women with fibromyalgia over 4 weeks compared with other measures of restorative sleep.
Methods: In a double-blinded, dual-site clinical trial, 301 women with fibromyalgia were randomly assigned to utilize either Active-TENS, Placebo-TENS, or No-TENS at home. Measures were collected at baseline and after 4 weeks of treatment. To assess self-reported sleep, the participants completed three PROMIS short forms: Sleep Disturbance, Sleep-Related Impairment, Fatigue, and the Pittsburgh Sleep Quality Index (PSQI). To assess device-measured sleep, actigraphy was used to quantify total sleep time, wake after sleep onset, and sleep efficiency. Linear mixed models were used to examine the effects of treatment, time, and treatment*time interactions.
Results: The PROMIS short forms had moderate test–retest reliability (ICC 0.62 to 0.71) and high internal consistency (Cronbach's alpha 0.89 to 0.92). The PROMIS sleep measures [mean change over 4 weeks, 95% confidence interval (CI)], Sleep Disturbance: −1.9 (−3.6 to −0.3), Sleep-Related Impairment: −3 (−4.6 to −1.4), and Fatigue: −2.4 (−3.9 to −0.9) were responsive to improvement in restorative sleep and specific to the Active-TENS group but not in the Placebo-TENS [Sleep Disturbance: −1.3 (−3 to 0.3), Sleep-Related Impairment: −1.2 (−2.8 to 0.4), Fatigue: −1.1 (−2.7 to 0.9)] or No-TENS [Sleep Disturbance: −0.1 (−1.6 to 1.5), Sleep-Related Impairment: −0.2 (−1.7 to 1.4), Fatigue: –.3 (−1.8 to 1.2)] groups. The PSQI was responsive but not specific with improvement detected in both the Active-TENS: −0.9 (−1.7 to −0.1) and Placebo-TENS: −0.9 (−1.7 to 0) groups but not in the No-TENS group: −0.3 (−1.1 to 0.5). Actigraphy was not sensitive to any changes in restorative sleep with Active-TENS [Sleep Efficiency: −1 (−2.8 to 0.9), Total Sleep Time: 3.3 (−19.8 to 26.4)].
Conclusion: The PROMIS sleep measures are reliable, valid, and responsive to improvement in restorative sleep in women with fibromyalgia.
Clinical Trial Registration: www.ClinicalTrials.gov, identifier: NCT01888640.
Introduction
Individuals with fibromyalgia commonly report widespread musculoskeletal pain, fatigue, and nonrestorative sleep contributing to limited participation in work and recreational activities (1, 2). There are conflicting findings with nonrestorative sleep detected on self-report questionnaires yet an absence of impairment with device-measured sleep assessment (e.g., accelerometer, polysomnography) in this population (2–5). Conflicting results could be explained in that self-reported and device-measured sleep are different constructs or by limitations in reliability or validity. The Pittsburgh Sleep Quality Index (PSQI) has long been considered a gold standard for self-reported sleep (6). The PSQI has a total score that can distinguish between “good” and “poor” sleepers (7). While the PSQI recognizes multiple dimensions of sleep with seven component scores, these components have not been subsequently validated and are inconsistent with factor analysis (8). Moreover, there may be a floor effect, where the PSQI may not be responsive to treatment in the population with fibromyalgia, where 96% is categorized as “poor” sleepers (9). Together, this underscores the importance of examining the reliability and responsiveness of the PSQI in women with fibromyalgia and other measures that may have superior psychometric properties.
Both the Patient-Reported Outcomes Measurement Information System (PROMIS) sleep disturbance and sleep-related impairment short forms measure a single component of sleep (6, 10). PROMIS questionnaires were developed using item response theory, which could provide greater reliability and validity than legacy questionnaires (10). The reliability and validity of the PROMIS sleep short-forms are established with quantitative and qualitative measures (6, 10–14). However, there remains a lack of information on reliability and responsiveness over a sufficiently long time period when the effectiveness of an intervention may be assessed. Information on reliability, when no treatment is provided, and responsiveness to detect change, when treatment is provided, are needed to determine the interpretation of improvement in sleep measures for individuals with fibromyalgia.
A reciprocal relationship between sleep and pain has been reported by individuals with fibromyalgia and supported by cross-sectional studies (15, 16), yet prospective study designs either fail to detect sleep quality to predict pain (17, 18) or detect small effect sizes (beta = 0.11 to 0.25) (9, 19, 20). We have recently shown that active transcutaneous electrical nerve stimulation (TENS) has reduced resting pain by −1.2 points (0 to 10-point scale, 95% CI: −0.4 to −2.1) compared with Placebo-TENS and by −1.4 points (−0.6 to −2.2) compared with no treatment group (21). The effect of TENS on sleep has not yet been examined for this clinical trial. Given that the lower limit of the 95% CI included small effects (−0.4 to −0.6) of TENS on resting pain, a reliable and responsive measure of restorative sleep may be needed to detect improvement.
The first aim of this study was to examine the reliability and validity of the PROMIS sleep measures in women with fibromyalgia. We hypothesized that the PROMIS short forms would demonstrate superior psychometric properties (reliability, validity) compared with the PSQI and actigraphy in women with fibromyalgia. The second aim was to determine the responsiveness of the PROMIS sleep short forms to daily TENS intervention in women with fibromyalgia compared with the PSQI and actigraphy over a 4-week period. In parallel with previously published findings on the effect of TENS on pain (21) we hypothesized that Active-TENS would have a greater effect on restorative sleep than the use of Placebo-TENS or No-TENS, and that this effect would be best detected by the PROMIS sleep measures.
Materials and Methods
Study Design
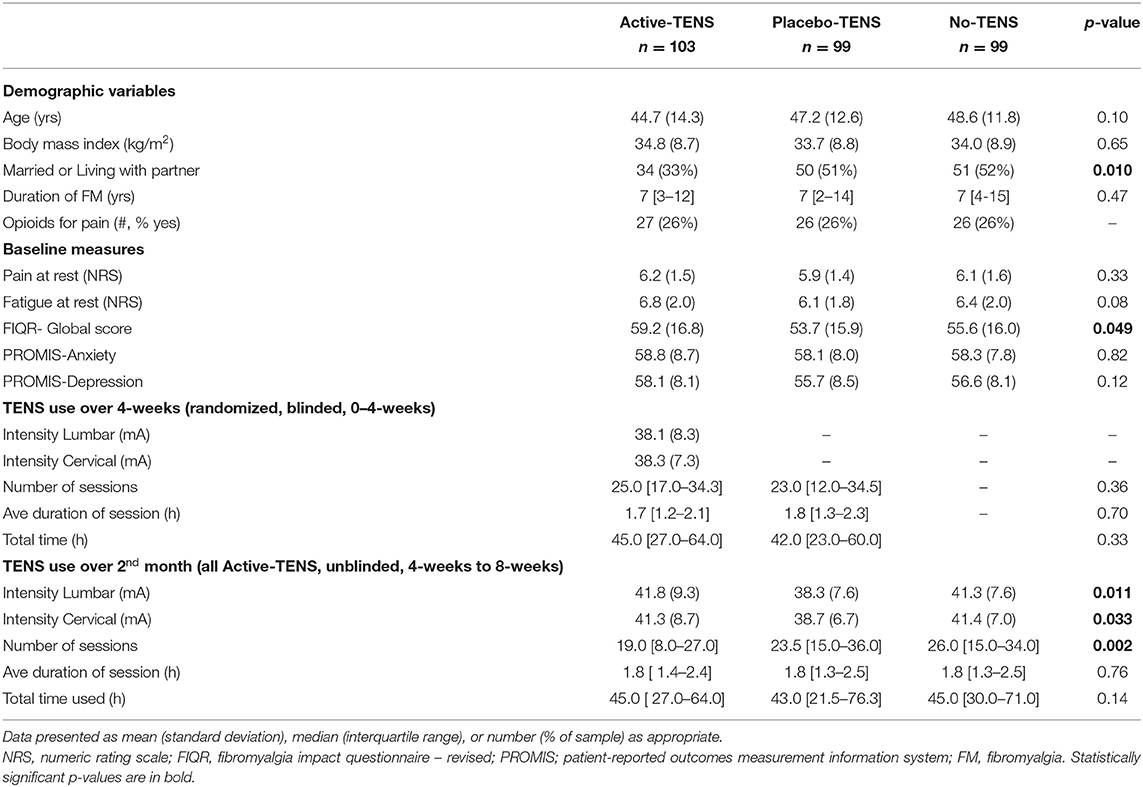
This is a secondary analysis of data collected during a phase II randomized, double-blinded, placebo-controlled dual-site clinical trial. The Fibromyalgia Activity Study with TENS (FAST) was conducted at two university medical centers (NCT01888640) (21). The participants completed four visits over a 9-week period (Figure 1) (22). The first visit was for in-person screening and consent. The participants had to be diagnosed with fibromyalgia by a physician and meet the 1990 American College of Rheumatology (ACR) criteria for fibromyalgia. Additional inclusion criteria were women, aged 18–70 years, who reported stable medication use for the last 4 weeks and projected stable treatment for the next 2 months. Exclusion criteria were: TENS use in the last 5 years; pain rating of <4/10 on the verbal numerical pain rating scale; serious or unstable medical or psychiatric condition that would preclude participation in the study (surgery in the past or upcoming 3 months, undergoing diagnostic testing for an undiagnosed condition, instability of a condition that is not part of the eligibility criteria); pacemaker; neuropathic or autoimmune disorder; spinal fusion or metal implants in the spine; allergy to adhesive or nickel; pregnancy; epilepsy; and inability to walk 6 min without assistance. At 0 weeks, the participants completed a second screening for eligibility and began the randomized treatment for 4 weeks. The primary endpoint of 4 weeks was chosen; because clinically, physical therapists re-evaluate their patients every 30 days for insurance to document progress and determine if a change in the plan of care is needed. At 4 weeks, all the participants were unblinded and were provided with an Active-TENS unit. At eight-weeks, the final outcome assessment was carried out.
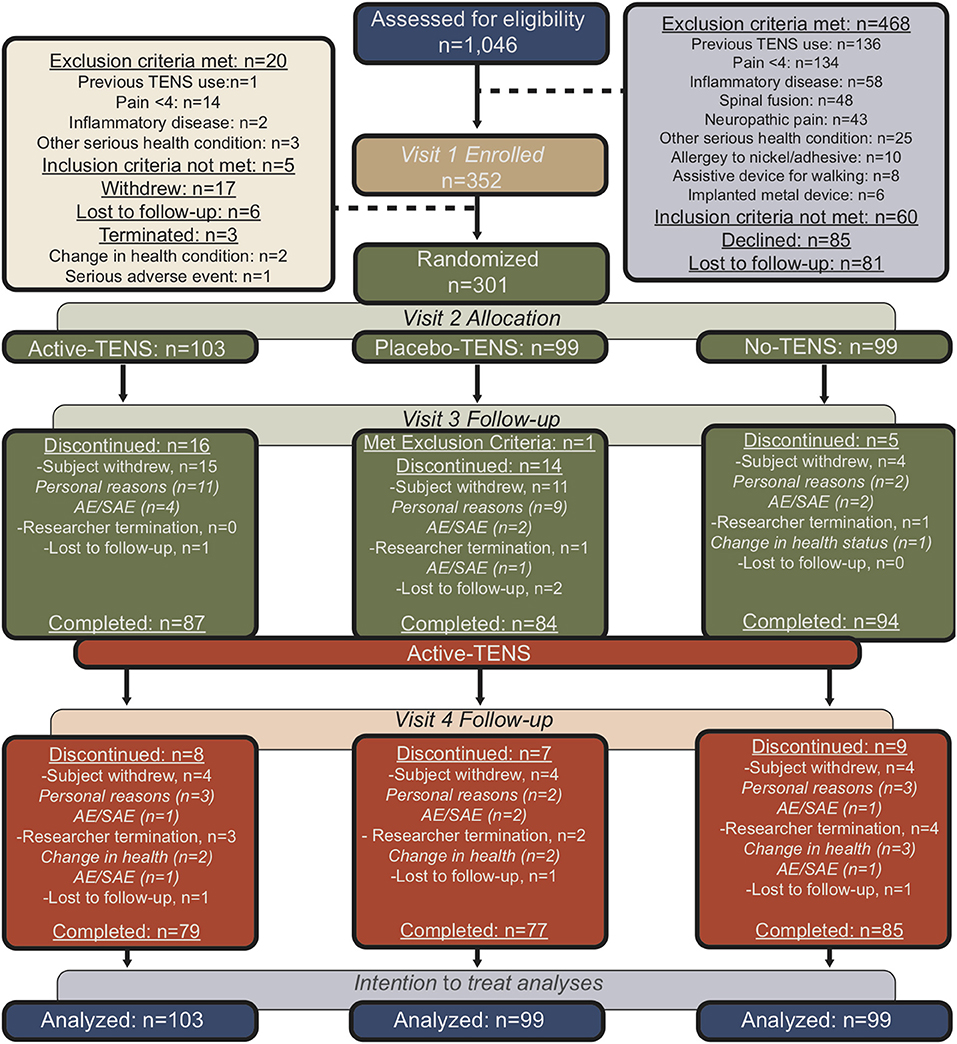
Figure 1. Flow diagram for participants in the trial using the Consolidated Standards of Reporting Trials guidelines.
Participants
The participants were recruited from university clinics and surrounding communities using mass email, posting flyers, and referring clinicians (21). They were provided free parking, mileage reimbursement, and payment for time (21). All the participants continued current treatments prescribed by their healthcare provider and were told not to change medications during the study. All of them provided written informed consent to participate in this study, which was approved by the institutional review boards of both universities.
Randomization
Between September 2013 and February 2018, 1,046 women were assessed for eligibility, 352 participants were enrolled, and 301 were randomly assigned to one of three groups: Active-TENS (n = 103), Placebo-TENS (n = 99), or No-TENS (n = 99; Figure 1). Randomization was stratified by geographical location and opioid status (opioid vs. non-opioid). The participants were classified as opioid users if they had taken an opioid at least 5/7 days per week for the last 30 days. Randomization was then performed separately within each stratum using permuted block randomization with blocks of six and nine.
Blinding
To blind the outcome assessors, a mock TENS unit was used in the No-Treatment group during evaluation visits. The participants had a study bag to hide their study materials, and they were asked to not discuss their treatment with the outcome assessor. To blind the participants, only TENS-naïve individuals were eligible to participate and a Placebo-TENS unit was provided. In addition, a nearly identical script was used for patient education, except for a line that differed between the Active-TENS and Placebo-TENS groups. The Active-TENS group was asked to indicate when they could first feel the TENS and then when the intensity was a strong, comfortable, non-painful sensation. In contrast, the Placebo-TENS group was only asked when they could first feel the TENS, and this was the maximum TENS intensity that they were instructed to use when setting up the unit throughout the 4 weeks. Both groups were informed that as the treatment continues they may experience no change or an increase or decrease in the way the TENS feels. This accounted for the lack of sensation experienced in the Placebo-TENS group throughout the remainder of the session. At 4 weeks, the outcome assessors and participants were asked to guess the treatment group allocation.
Transcutaneous Electrical Nerve Stimulation Intervention
An EMPI-Select TENS (DJO Global, Vista, CA, United States) unit was used to deliver treatment through butterfly electrodes placed on the upper and lower back. To be appropriate for non-opioid and opioid users, the TENS parameters alternated between low and high frequencies (2 to 125 Hz) to activate not only μ-opioid receptors targeted by pharmaceuticals but also δ-opioid receptors (23–27). The intensity was set to strong and comfortable (28). The Placebo-TENS unit delivered current for 45 s ramping down to 0 Hz in the last 15 s and remained at 0 Hz for the remainder of the session (29). The participants were instructed to use TENS at least 2 h per day during activity at home for 4 weeks. They were provided education that TENS was most effective when combined with exercise. Therefore, they were encouraged to use TENS to enhance their ability to complete moderate to vigorous activities. The participants were discouraged from using TENS as a passive treatment during rest and during sleep for safety. The TENS unit collected information on a range of usage, such as number of sessions, average duration of session, and intensity.
Outcome Measures
Demographics and Potential Covariates
Participant characteristics listed in Table 1 were collected at 0 weeks. Disease impact was measured with the Fibromyalgia Impact Questionnaire-Revised (FIQR) (30).
Patient-Reported Outcomes
Restorative sleep was examined using three questionnaires: the PROMIS Sleep Disturbance (short form 8b, range 28.9–76.5) (10), PROMIS Sleep-Related Impairment (short form 8a, range 30–80) (10), and the PSQI (range 0–21) (7, 9). Fatigue was examined using the PROMIS Fatigue (short form 7a, range 29.4–83.2) (14). For these PROMIS short forms, a t-score of 60 represents 1 SD worse than the general population mean t-score of 50. While the minimally important difference (MID) has not been established for all PROMIS measures, the MID for fatigue is 3 to 5 points for cancer patients (31).
Actigraph-Measured Outcomes
Accelerometry (ActiSleep+, ActiGraph, Pensacola, FL, United States) was used to measure sleep. The participants wore the accelerometer on their nondominant wrist for 7 days prior to each time point with 0 weeks indicating sleep during the week prior to baseline measures and 4 weeks indicating sleep during the fourth week of study participation. To interpret actigraph sleep data, the participants kept a log of when they went to bed and got up each day. Validation of reported times was performed using the actigraph data. For “In Bed,” if a spike in activity (count ≥ 1,000 for a period of 5 min or longer) occurred within the stated bedtime, then the bedtime was adjusted to the beginning of the next consecutive 15 min of inactivity (32). For “Out of Bed,” if a spike in activity (count ≥ 1000 for a period of 5 min or longer) was within the stated bedtime, then the “Out of Bed” time was adjusted to indicate it was prior to the spike in activity. Three actigraph-measured sleep variables were chosen for analysis total sleep time, sleep efficiency, and wake after sleep onset (33). The actigraph-measured sleep variables have demonstrated concurrent validity with the gold standard of polysomnography (32, 33). The Cole–Kripke algorithm was used to distinguish “Sleep Onset” from wakefulness after the “In bed” time (32). Total Sleep Time (TST) was defined as the number of minutes between “Sleep Onset” and time “Out of Bed.” Sleep efficiency was defined as TST as a percentage of the time in bed (time difference between “In Bed” and “Out of Bed”). Wake After Sleep Onset (WASO) was defined as the number of minutes awake between “Sleep Onset” and “Out of Bed.”
Statistical Analysis
Baseline characteristics were compared between groups using one-way ANOVA for continuous variables and Pearson's Chi-squared test for categorical variables. To examine test–retest reliability, we examined the intra-class correlation (ICC) over the 4-week period when no treatment was administered within the No-TENS group. Interpretation of the ICC estimates was based on >0.75 indicating good to excellent reliability and 0.5 to 0.75 indicating moderate reliability (34). Since reliability is a prerequisite for validity, measures with poor test–retest reliability (ICC < 0.50) in the TENS group were not used in subsequent analyses (34). For internal consistency, we examined the inter-item correlations and Cronbach's alpha for the total questionnaire. We also reported the potential improvement in the inter-item correlation if a specific item was deleted to evaluate the impact of each item on the Cronbach's alpha for the total questionnaire. To examine convergent validity, we examined the Pearson correlations between patient-reported outcomes.
A Linear mixed model for repeated measures (0, 4, and 8 weeks) was used to examine (1) the effect of time within each group (0 to 4 weeks and 0 to 8 weeks); (2) the effect of treatment*time interaction from 0 to 4 weeks (SAS version 9.4, SAS/STAT 14.3). To correct for multiple comparisons, Bonferroni adjustment was applied for time (adjusted for six tests) and treatment*time (adjusted for three tests). In addition to the p-values, estimates of mean change or mean difference between groups with adjusted 95% CIs were computed. Differences between groups at baseline were entered as covariates of the model. When there was a significant interaction between group and treatment, further analyses examined if this effect was moderated by opioid use. In fitting the linear mixed model, the Akaike Information Criteria (AIC) and Schwarz's Bayesian Information Criteria (BIC) were used to select the covariance structure that best fit these longitudinal measures within the subject. The covariance types that were considered included compound symmetry (CS), heterogeneous CS, first order autoregressive (AR1), and unstructured. From these model parameter estimates and the fitted covariance structure, tests of mean contrast were performed to assess the effect of Active-TENS, compared with Placebo-TENS and No-TENS on sleep outcomes. Statistical significance was defined as p < 0.05, and corresponding 95% CIs are provided for all data.
The linear mixed model performed an intent-to-treat analysis, which included all available data from all the randomized study subjects. Four weeks was the primary endpoint with completion rates ranging from 84 to 95% between groups and 8 weeks completion rates ranged from 90 to 92% (Figure 1). Comparison of the 25 participants that dropped out at 8 weeks with those that had completed follow-up through 8 weeks showed no difference in baseline FIQR, PSQI, and PROMIS short forms, or actigraph-measured sleep. The lack of difference in the sleep measures between those who dropped out and those who did not supports the use of the missing at random statistical assumption, which allows for estimation of the sleep measures based on other variables in the dataset (35). The use of likelihood-based methods (i.e., linear mixed model analysis) for analysis of unbalanced data (with missing values) under the missing at random assumption provides valid estimates for inference. As a post-hoc analysis, we examined the Pearson correlations between changes in sleep with resting pain and duration of TENS use.
Results
Test–Retest Reliability
The PROMIS sleep measures had moderate test–retest reliability in the No-TENS group over a 4-week period with ICCs ranging from.62 to.71 (Table 2). The PSQI global score also had moderate test–retest reliability of.59 (Table 2). The actigraph measures of sleep efficiency, total sleep time, and wake after sleep onset had moderate reliability with ICCs ranging from 0.59 to 0.68.
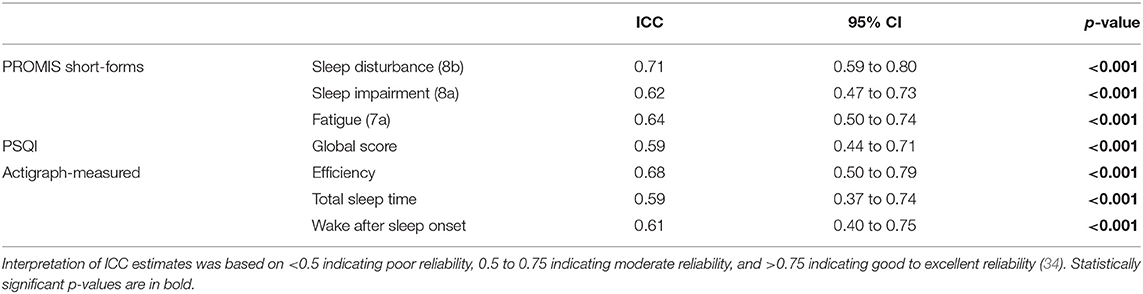
Table 2. Test–retest reliability of sleep measures for the No-TENS group between 0-week and 4-weeks.
Internal Consistency
The PROMIS short forms of Sleep Disturbance and Sleep-Related Impairment had high internal consistency with Cronbach's alpha of 0.92 and 0.89, respectively (Tables 3A, B). The PSQI had low internal consistency with a Cronbach's alpha of.54 (Table 3C). The Cronbach's alpha would increase to 0.66 if component 6 (use of sleeping medication) was deleted, but even with this deletion, the internal consistency would still not reach an acceptable level (0.7).
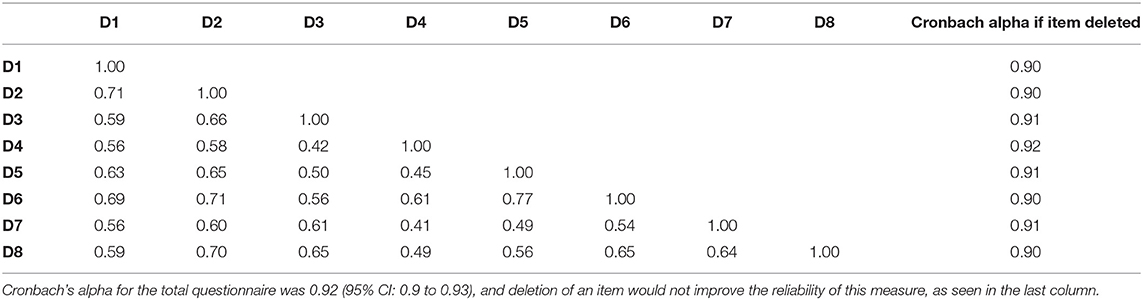
Table 3A. Inter-item correlations for PROMIS Sleep Disturbance with the questions numbered D1 through D8.
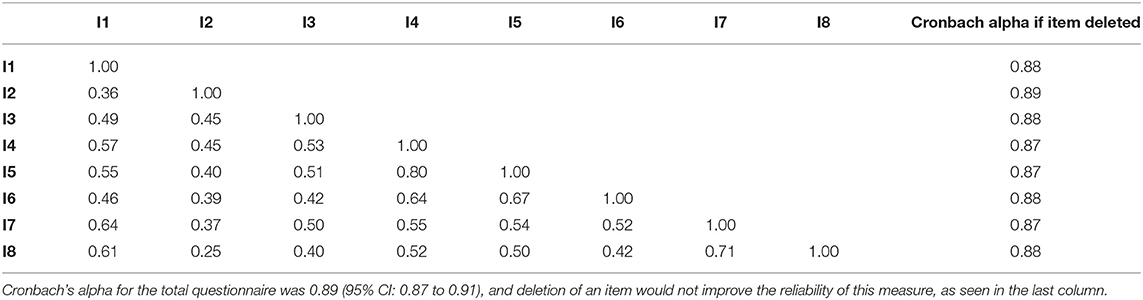
Table 3B. Inter-item correlations for PROMIS Sleep-Related Impairment with the questions numbered I1 through I8.
Convergent Validity
PROMIS Sleep Disturbance was most strongly correlated with the PSQI (r = 0.71), moderately correlated with both PROMIS Sleep-Related Impairment (r = 0.5) and PROMIS Fatigue (r = 0.33), and minimally correlated with actigraph-measured sleep (r < 0.2, Table 4). PROMIS Sleep-Related Impairment was most strongly correlated with PROMIS Fatigue (r = 0.63), moderately correlated with both PROMIS Sleep Disturbance (r = 0.5) and PSQI (r = 0.47), and minimally correlated with actigraph-measured sleep (r < 0.1). Actigraph-measured sleep efficiency and wake after sleep onset were strongly correlated (r = −0.94), but all other correlations between actigraph measures were weak (r = 0.07 to 0.2).
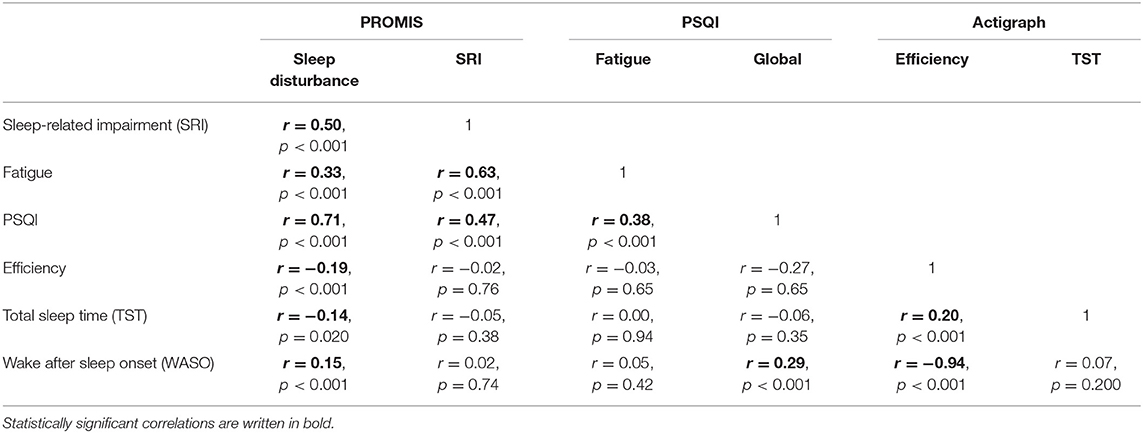
Table 4. Convergent validity between PROMIS short forms, Pittsburg Sleep Quality Index, and Actigraph-measured sleep (efficiency, total sleep time, and wake after sleep onset).
Responsiveness of Sleep Measures During Randomized, Blinded TENS Treatment
Potential covariates were similar between groups, except that the Active-TENS group had a lower rate of being married/living with a partner (p = 0.01) and a higher FIQR score (p = 0.049) compared with the other two groups (Table 1). There were no differences between groups in self-reported measures (PROMIS and PSQI) at baseline, except that the Active-TENS group had a higher level of PROMIS sleep-related impairment than the Placebo-TENS group (p = 0.04, Supplementary Table 1). From 0 to 4 weeks, the PROMIS short-forms were responsive to improvement in restorative sleep and specific to the Active-TENS group but not to Placebo-TENS or No-TENS (Figure 2). The Active-TENS group reported reduced Sleep Disturbance [mean change (95% CI), −1.9 (−3.6 to −0.3) and Sleep-Related Impairment: −3 (−4.6 to −1.4)] at 4 weeks compared with baseline (Supplementary Table 1). In contrast, the PROMIS sleep measures did not significantly improve for the Placebo-TENS Sleep Disturbance: −1.3 (−3 to 0.3); Sleep-Related Impairment: −1.2 (−2.8 to 0.4) and No-TENS Sleep Disturbance: −0.1 (−1.6 to 1.5); Sleep-Related Impairment: −0.2 (−1.7 to 1.4) groups.
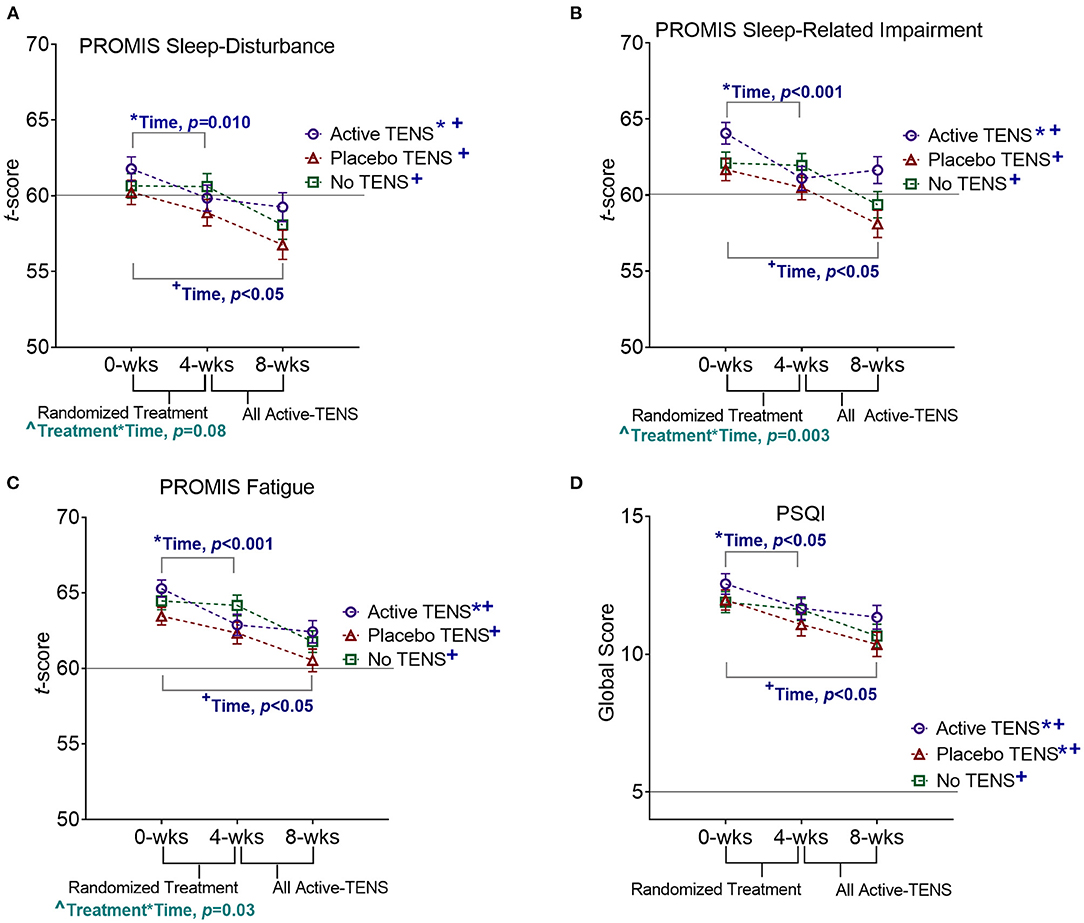
Figure 2. Effects of TENS on (A) PROMIS Sleep Disturbance, (B) Pittsburg Sleep Quality Index (PSQI), (C) PROMIS Sleep-Related Impairment, and (D) PROMIS Fatigue. Statistically significant effects of time (*0 to 4 weeks, and +0 to 8 weeks) and interaction effects of treatment*time(∧) are indicated. For all outcome measures, scores higher than the horizontal line are indicative of nonrestorative sleep.
To further examine the significant interaction between treatment group and time for PROMIS Sleep-Related Impairment and Fatigue short forms, we examined the correlations of these variables with resting pain and duration of TENS use. A greater decrease in resting pain was correlated with a greater reduction in Sleep-Related Impairment [r = 0.21 (95% CI: 0.09 to 0.32)] and Fatigue [r = 0.27 (95% CI: 0.16 to 0.38)]. The significant interactions between treatment group and time became non-significant when resting pain was included in the model (PROMIS Sleep-Related Impairment: p = 0.136, PROMIS Fatigue: p = 0.505, Supplementary Table 1). More minutes per day of TENS use was associated with greater improvement in restorative sleep (PROMIS Sleep-Related Impairment: r = −0.21 (−0.09 to −0.33); PROMIS Fatigue: r = −0.17 (−0.05 to −0.28) when accounting for resting pain at baseline.
The PSQI was responsive but not specific with improvement detected in both the Active-TENS (p = 0.03) and Placebo-TENS (p = 0.04) groups but not in the No-TENS group (p > 0.99, Figure 2, Supplementary Table 2). Actigraphy measures were not sensitive to changes in restorative sleep (p > 0.05 for all measures). There were no significant effects of visit or group*visit for the PSQI or actigraphy measures (p > 0.05). The addition of marital status and baseline FIQR as co-variates in the analysis did not change the significance of these findings (Supplementary Table 3).
Responsiveness of Sleep Measures During Non-randomized, Unblinded Active-Transcutaneous Electrical Nerve Stimulation Treatment
From 4 to 8 weeks, the Placebo-TENS and No-TENS groups had a significant improvement in the PROMIS short forms and the PSQI during the Active-TENS treatment (p < 0.05 for all effects of time at 8 weeks), but there were no changes in actigraph measures (Figure 2, Supplementary Tables 1, 2). The Active-TENS group also had a significant improvement in all self-report measures (time, p < 0.05), indicating sustained improvement with additional 1-month use.
Integrity of Blinding
The outcome assessor was adequately blinded and correctly guessed treatment allocation for 45% of the Active-TENS group, 13% of the Placebo-TENS group, and 20% of the No-TENS group (21). Given a 50% chance of receiving an Active-TENS or Placebo-TENS unit for home use, the Placebo-TENS group was adequately blinded with 49% of the participants correctly guessing their treatment group, but 70% of the Active-TENS group was able to correctly guess group allocation (21). For the sleep measures that differed between the Active-TENS and Placebo-TENS groups, these differences became non-significant among those who incorrectly guessed group allocation [PROMIS Sleep-Related Impairment: 1.37 (−1.8 to 4.59), p = 0.97; PROMIS Fatigue: 1.2 (−1.9 to 4.4), p = 0.377)]. In contrast for participants in the Active-TENS and Placebo-TENS groups who correctly guessed their allocation, the difference remained significant [PROMIS Sleep-Related Impairment: −3.7 (95% CI: −6.21 to −1.3, p < 0.001), PROMIS Fatigue: −2.8 (−5.3 to −0.4), p < 0.001].
Data Availability Statement
The data that support the findings of this study are available from the corresponding author (RLC) upon reasonable request.
Discussion
The PROMIS short forms were reliable, valid, and responsive to change during randomized, blinded treatment in women with fibromyalgia. TENS improved self-reported sleep with the PROMIS short forms but not with the PSQI or actigraph measures after 4 weeks of daily use in women with fibromyalgia. The interpretation of the findings, in which improvements in sleep were detected with some measures but not with others, may be related to the relative psychometric properties of the outcome measures.
Patient-Reported Outcomes Measurement Information System Reliability
The test–retest reliability of all self-reported and actigraph-measured sleep measures was moderate (ICC 0.59 to 0.71), which likely reflects natural fluctuations in sleep over a 4-week period. The test–retest reliability results are within the range of another study that used polysomnography and that reported moderate test–retest reliability at 1 month (sleep efficiency, r = 0.52) in individuals with suspected sleep apnea (36). A previous study that validated the PSQI had found good reliability (r = 0.85) (7), but it was noted that reliability was lower for participants with depression (PSQI sub-components r = 0.19 to 0.83). Similarly, the test–retest reliability of the PROMIS sleep short forms in individuals with rheumatoid arthritis was reported as excellent (r = 0.73 to 0.83) (37). The lower reliability in this study may be due to a longer follow-up (2 days vs. 1 month), higher level of depression (49.1 ± 8.8 vs. 56.8 + 8.2), and the limitation of a smaller sample size (N = 188 vs. N = 99 for the test–retest reliability in the No-TENS group) (21, 37). Since perceptions of nonrestorative sleep, fatigue, and psychological factors are intertwined within an individual and change over time, it may be informative to evaluate changes in sleep within the context of changes in other psychological factors, such as depression.
Consistent with the literature (10, 12, 13, 37), the internal consistency was high for all PROMIS sleep measures (Cronbach's alpha = 0.89 to.91). In contrast, we found the internal consistency of the PSQI to be below the acceptable level of.7 (Cronbach's alpha = 0.54). Published internal consistency measures for the PSQI have varied in the literature from 0.64 to 0.83, and no published studies have reached the level of high internal consistency (Cronbach's alpha ≥0.9) observed with the PROMIS sleep short forms (38). The lower internal consistency of the PSQI is likely due to its design. The PSQI has 18 items that assess seven components of sleep over a 4-week recall period, whereas both the PROMIS Sleep Disturbance and Sleep-Related Impairment have eight items that are focused on a single component of sleep over a 1-week recall period. The higher reliability, supported by differences in internal consistency, of the PROMIS short forms in the sample, potentially allows for greater precision and ability to detect change over time compared with the PSQI.
Patient-Reported Outcomes Measurement Information System Concurrent Validity
PROMIS Sleep Disturbance was most strongly correlated with the PSQI (r = 0.71), which is consistent with the literature validating these measures in other populations (r = 0.83 to 0.85) (6, 10). Given that half of the items on the PSQI (9/18) assess sleep disturbance, it is not surprising that variance in the global PSQI score appears to be primarily driven by sleep disturbance items. PROMIS Sleep-Related Impairment had the strongest correlation with PROMIS Fatigue (r = 0.63), indicating some overlap between these measures in women with fibromyalgia. Correlations between the PROMIS and actigraph-measured sleep were < 0.2, which is similar to previous studies of individuals with fibromyalgia (4, 5). Okifuji et al. (4) found a nearly equal distribution of participants who overestimated their sleep as those who underestimated sleep with self-report (sleep duration, number of awakenings, and feeling refreshed upon awakening) compared with actigraph-based measures. The lack of correspondence between self-report and device-measured sleep in women with fibromyalgia likely indicates that a problem in an aspect of sleep/wakefulness is not reflective of a problem in other aspects.
Responsiveness of Patient-Reported Outcomes Measurement Information System Short Forms to Transcutaneous Electrical Nerve Stimulation
There have been no previously published studies of the effects of TENS on restorative sleep. We have previously reported that daily use of TENS with activity reduces pain in women with fibromyalgia at 4 weeks and that this effect was maintained at 8 weeks (21, 39). The findings of this study on sleep follow a similar pattern of improvement as pain. Given the correlations between pain and sleep, the reduced effect of TENS on sleep when pain was included in the model may be due to the mediation effect. This could be further explored in future studies examining the mediation effect of pain on changes in sleep. Comparing the different self-reported sleep measures in this study, the PSQI detected an improvement at 4 weeks in both the Active-TENS and Placebo-TENS groups, indicating a non-specific effect on sleep. In contrast, the PROMIS measures detected improvement at 4 weeks only in the Active-TENS group, indicating improvement in a specific component of restorative sleep. These findings on responsiveness combined with the higher reliability of the PROMIS measures support the validity of these measures in women with fibromyalgia.
Clinical Relevance
Accurately identifying the factors contributing to nonrestorative sleep can direct treatment decisions for fibromyalgia symptoms, which include both pharmacological (e.g., antidepressants) and non-pharmacological (e.g., cognitive behavioral therapy) options (40, 41). A meta-analysis revealed that a benefit of non-pharmacological treatments is their multi-modal effects that can reduce not only pain but also fatigue and nonrestorative sleep associated with fibromyalgia (41). The effect of TENS on sleep disturbance is moderate (Active-TENS vs. Placebo-TENS, Cohen's d = 0.7) and is within the range of other effective pharmacological and non-pharmacological treatments as reported in the meta-analysis by Perrot et al. (Sleep disturbance, Cohen's d = amitriptyline: 0.7, sodium oxybate: 0.5, cognitive behavioral therapy: 0.4, magnetic cerebral stimulation: 0.5) (41). Although the clinical significance of improvements in restorative sleep during the blinded and unblinded phases of this study is undetermined, the findings support that TENS is a low-cost adjunct to care for women with fibromyalgia who report nonrestorative sleep.
Limitations
A limitation in the generalizability of these findings is that the eligibility criteria only included women with fibromyalgia who met the 1990 ACR criteria. As a secondary measure, study personnel also assessed the 2010 ACR criteria for fibromyalgia. While most participants met both diagnostic criteria, 3/301 participants did not meet the 2010 criteria. The effect of TENS on sleep in a more heterogeneous sample of men and women with fibromyalgia who meet the 2016 ACR criteria for fibromyalgia is unknown.
Another limitation of the study is the inability to achieve sufficient blinding in the Active-TENS group when TENS is used at an adequate intensity (strong and comfortable) to produce analgesia (29, 42). This potential source of bias is challenging to avoid despite the methods utilized in this study to maximize blinding. Correctly guessing treatment allocation may affect TENS use and contribute to the correlation between greater TENS use and a greater improvement in sleep.
The lack of a detected effect on actigraph-measured sleep could be due to a lack of efficacy or a lack of validity. While actigraph-measured sleep has demonstrated concurrent validity with polysomnography (32), it remains unknown how actigraph-measured sleep compares with polysomnography-measured sleep in women with fibromyalgia. Since this study did not assess polysomnography, we can only comment on poor concurrent validity with self-report measures in women with fibromyalgia.
Conclusions
The findings suggest that the PROMIS sleep short forms are reliable and sensitive to changes in sleep in women with fibromyalgia. This is may be due to better precision and/or accurate measurement of particular aspects of restorative sleep than the PSQI. A month of daily TENS use improved self-reported sleep in women with fibromyalgia by a statistically significant amount, although the clinical relevance of this improvement is unclear. TENS may influence self-reported sleep by reducing pain, which allows women with fibromyalgia to improve daytime function and sleep quality at night. TENS is a multi-modal, non-pharmacological treatment option for women with fibromyalgia who report nonrestorative sleep.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics Statement
The studies involving human participants were reviewed and approved by the University of Iowa Institutional Review Board and Vanderbilt University Institutional Review Board. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
BR, DD, CV, MZ, LC, and KS contributed to the conception and design of the study. RC, DD, CV, KG, and JW contributed to data acquisition. RC, BR, MZ, LC, and KS contributed to data analysis and interpretation. All the authors were involved in drafting the article or revising it critically for important intellectual content, and all approved the final version to be published.
Funding
This study was funded by NIH UM1 AR063381, NIH UM1 AR063381-S1, T32 NS045549-12, and K99AR071517, as well as supported by the National Center for Advancing Translational Sciences, Award Number U54TR001356 to University of Iowa and the National Center for Advancing Translational Sciences, Award Number UL1TR000445, to Vanderbilt University Medical Center. Active and Placebo TENS units and electrodes were provided by DJO, Inc.
Conflict of Interest
KS serves as a consultant for Pfizer Consumer Health and Novartis Consumer Healthcare/GSK Consumer Healthcare, had an active grant from the American Pain Society/Pfizer, and receives royalties from IASP Press.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpain.2021.682072/full#supplementary-material
References
1. Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Katz RS, Mease P, et al. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res. (2010) 62:600–10. doi: 10.1002/acr.20140
2. Wu YL, Chang LY, Lee HC, Fang SC, Tsai PS. Sleep disturbances in fibromyalgia: a meta-analysis of case-control studies. J Psychosom Res. (2017) 96:89–97. doi: 10.1016/j.jpsychores.2017.03.011
3. Diaz-Piedra C, Catena A, Sanchez AI, Miro E, Martinez MP, Buela-Casal G. Sleep disturbances in fibromyalgia syndrome: the role of clinical and polysomnographic variables explaining poor sleep quality in patients. Sleep Med. (2015) 16:917–25. doi: 10.1016/j.sleep.2015.03.011
4. Okifuji A, Hare BD. Nightly analyses of subjective and objective (actigraphy) measures of sleep in fibromyalgia syndrome: what accounts for the discrepancy? Clin J Pain. (2011) 27:289–96. doi: 10.1097/AJP.0b013e31820485db
5. Stuifbergen AK, Phillips L, Carter P, Morrison J, Todd A. Subjective and objective sleep difficulties in women with fibromyalgia syndrome. J Am Acad Nurse Pract. (2010) 22:548–56. doi: 10.1111/j.1745-7599.2010.00547.x
6. Buysse DJ, Yu L, Moul DE, Germain A, Stover A, Dodds NE, et al. Development and validation of patient-reported outcome measures for sleep disturbance and sleep-related impairments. Sleep. (2010) 33:781–92. doi: 10.1093/sleep/33.6.781
7. Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. (1989) 28:193–213. doi: 10.1016/0165-1781(89)90047-4
8. Cole JC, Motivala SJ, Buysse DJ, Oxman MN, Levin MJ, Irwin MR. Validation of a 3-factor scoring model for the Pittsburgh sleep quality index in older adults. Sleep. (2006) 29:112–6. doi: 10.1093/sleep/29.1.112
9. Bigatti SM, Hernandez AM, Cronan TA, Rand KL. Sleep disturbances in fibromyalgia syndrome: relationship to pain and depression. Arthritis Rheum. (2008) 59:961–7. doi: 10.1002/art.23828
10. Yu L, Buysse DJ, Germain A, Moul DE, Stover A, Dodds NE, et al. Development of short forms from the PROMIS sleep disturbance and sleep-related impairment item banks. Behav Sleep Med. (2011) 10:6–24. doi: 10.1080/15402002.2012.636266
11. English M, Stoykova B, Slota C, Doward L, Siddiqui E, Crawford R, et al. Qualitative study: burden of menopause-associated vasomotor symptoms (VMS) and validation of PROMIS sleep disturbance and sleep-related impairment measures for assessment of VMS impact on sleep. J Patient Rep Outcom. (2021) 5:37. doi: 10.1186/s41687-021-00289-y
12. Full KM, Malhotra A, Crist K, Moran K, Kerr J. Assessing psychometric properties of the PROMIS sleep disturbance scale in older adults in independent-living and continuing care retirement communities. Sleep Health. (2019) 5:18–22. doi: 10.1016/j.sleh.2018.09.003
13. Lei DK, Yousaf M, Janmohamed SR, Vakharia PP, Chopra R, Sacotte R, et al. Validation of patient-reported outcomes information system sleep disturbance and sleep-related impairment in adults with atopic dermatitis. Bri J Dermatol. (2020) 183:875–82. doi: 10.1111/bjd.18920
14. Merriwether EN, Rakel BA, Zimmerman MB, Dailey DL, Vance CGT, Darghosian L, et al. Reliability and construct validity of the patient-reported outcomes measurement information system (PROMIS) instruments in women with fibromyalgia. Pain Med. (2017) 18:1485–95. doi: 10.1093/pm/pnw187
15. Keskindag B, Karaaziz M. The association between pain and sleep in fibromyalgia. Saudi Med J. (2017) 38:465–75. doi: 10.15537/smj.2017.5.17864
16. Ramlee F, Afolalu EF, Tang NKY. Do people with chronic pain judge their sleep differently? A qualitative study. Behav Sleep Med. (2018) 16:259–71. doi: 10.1080/15402002.2016.1188393
17. Affleck G, Tennen H, Urrows S, Higgins P, Abeles M, Hall C, et al. Fibromyalgia and women's pursuit of personal goals: a daily process analysis. Health Psychol. (1998) 17:40–47. doi: 10.1037/0278-6133.17.1.40
18. Anderson RJ, McCrae CS, Staud R, Berry RB, Robinson ME. Predictors of clinical pain in fibromyalgia: examining the role of sleep. J Pain. (2012) 13:350–8. doi: 10.1016/j.jpain.2011.12.009
19. Kothari DJ, Davis MC, Yeung EW, Tennen HA. Positive affect and pain: mediators of the within-day relation linking sleep quality to activity interference in fibromyalgia. Pain. (2015) 156:540–6. doi: 10.1097/01.j.pain.0000460324.18138.0a
20. Nicassio PM, Moxham EG, Schuman CE, Gevirtz RN. The contribution of pain, reported sleep quality, and depressive symptoms to fatigue in fibromyalgia. Pain. (2002) 100:271–9. doi: 10.1016/S0304-3959(02)00300-7
21. Dailey DL, Vance CG, Rakel BA, Zimmerman MB, Embree J, Merriwether EN, et al. Transcutaneous electrical nerve stimulation reduces movement-evoked pain and fatigue: a randomized, controlled trial. Arthritis Rheumatol. (2020) 72:824–36. doi: 10.1002/art.41170
22. Noehren B, Dailey DL, Rakel BA, Vance CG, Zimmerman MB, Crofford LJ, et al. Effect of transcutaneous electrical nerve stimulation on pain, function, and quality of life in fibromyalgia: a double-blind randomized clinical trial. Phys Ther. (2015) 95:129–40. doi: 10.2522/ptj.20140218
23. Kalra A, Urban MO, Sluka KA. Blockade of opioid receptors in rostral ventral medulla prevents antihyperalgesia produced by transcutaneous electrical nerve stimulation (TENS). J Pharmacol Exp Ther. (2001) 298:257–63.
24. Leonard G, Cloutier C, Marchand S. Reduced analgesic effect of acupuncture-like TENS but not conventional TENS in opioid-treated patients. J Pain. (2011) 12:213–21. doi: 10.1016/j.jpain.2010.07.003
25. Leonard G, Goffaux P, Marchand S. Deciphering the role of endogenous opioids in high-frequency TENS using low and high doses of naloxone. Pain. (2010) 151:215–19. doi: 10.1016/j.pain.2010.07.012
26. Sluka KA, Bailey K, Bogush J, Olson R, Ricketts A. Treatment with either high or low frequency TENS reduces the secondary hyperalgesia observed after injection of kaolin and carrageenan into the knee joint. Pain. (1998) 77:97–102. doi: 10.1016/S0304-3959(98)00090-6
27. Sluka KA, Deacon M, Stibal A, Strissel S, Terpstra A. Spinal blockade of opioid receptors prevents the analgesia produced by TENS in arthritic rats. J Pharmacol Exp Ther. (1999) 289:840–46.
28. Vance CG, Chimenti RL, Dailey DL, Hadlandsmyth K, Zimmerman MB, Geasland KM, et al. Development of a method to maximize the transcutaneous electrical nerve stimulation intensity in women with fibromyalgia. J Pain Res. (2018) 11:2269–78. doi: 10.2147/JPR.S168297
29. Rakel B, Cooper N, Adams HJ, Messer BR, Frey Law LA, Dannen DR, et al. A new transient sham TENS device allows for investigator blinding while delivering a true placebo treatment. J Pain. (2010) 11:230–8. doi: 10.1016/j.jpain.2009.07.007
30. Bennett RM, Friend R, Jones KD, Ward R, Han BK, Ross RL. The revised fibromyalgia impact questionnaire (FIQR): validation and psychometric properties. Arthritis Res Ther. (2009) 11:R120. doi: 10.1186/ar2783
31. Yost KJ, Eton DT, Garcia SF, Cella D. Minimally important differences were estimated for six patient-reported outcomes measurement information system-cancer scales in advanced-stage cancer patients. J Clin Epidemiol. (2011) 64:507–16. doi: 10.1016/j.jclinepi.2010.11.018
32. Cole RJ, Kripke DF, Gruen W, Mullaney DJ, Gillin JC. Automatic sleep/wake identification from wrist activity. Sleep. (1992) 15:461–9. doi: 10.1093/sleep/15.5.461
33. Slater JA, Botsis T, Walsh J, King S, Straker LM, Eastwood PR. Assessing sleep using hip and wrist actigraphy. Sleep Biol Rhythms. (2015) 13:172–80. doi: 10.1111/sbr.12103
34. Koo TK, Li MY. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med. (2016) 15:155–63. doi: 10.1016/j.jcm.2016.02.012
35. Molenberghs G, Kenward M. Missing Data in Clinical Studies. Chichester: John Wiley & Sons (2007).
36. Levendowski DJ, Zack N, Rao S, Wong K, Gendreau M, Kranzler J, et al. Assessment of the test-retest reliability of laboratory polysomnography. Sleep Breath. (2009) 13:163–7. doi: 10.1007/s11325-008-0214-6
37. Bartlett SJ, Orbai AM, Duncan T, DeLeon E, Ruffing V, Clegg-Smith K, et al. Reliability and validity of selected PROMIS measures in people with rheumatoid arthritis. PLoS ONE. (2015) 10:e0138543. doi: 10.1371/journal.pone.0138543
38. Mollayeva T, Thurairajah P, Burton K, Mollayeva S, Shapiro CM, Colantonio A. The Pittsburgh sleep quality index as a screening tool for sleep dysfunction in clinical and non-clinical samples: a systematic review and meta-analysis. Sleep Med Rev. (2016) 25:52–73. doi: 10.1016/j.smrv.2015.01.009
39. Dailey DL, Rakel BA, Vance CG, Liebano RE, Amrit AS, Bush HM, et al. Transcutaneous electrical nerve stimulation reduces pain, fatigue and hyperalgesia while restoring central inhibition in primary fibromyalgia. Pain. (2013) 154:2554–62. doi: 10.1016/j.pain.2013.07.043
40. Martinez MP, Miro E, Sanchez AI, Diaz-Piedra C, Caliz R, Vlaeyen JW, et al. Cognitive-behavioral therapy for insomnia and sleep hygiene in fibromyalgia: a randomized controlled trial. J Behav Med. (2014) 37:683–97. doi: 10.1007/s10865-013-9520-y
41. Perrot S, Russell IJ. More ubiquitous effects from non-pharmacologic than from pharmacologic treatments for fibromyalgia syndrome: a meta-analysis examining six core symptoms. Eur J Pain. (2014) 18:1067–80. doi: 10.1002/ejp.564
Keywords: sleep wake disorders, patient reported outcome measures, reproducibility of results, psychometrics, transcutaneous electric nerve stimulation
Citation: Chimenti RL, Rakel BA, Dailey DL, Vance CGT, Zimmerman MB, Geasland KM, Williams JM, Crofford LJ and Sluka KA (2021) Test–Retest Reliability and Responsiveness of PROMIS Sleep Short Forms Within an RCT in Women With Fibromyalgia. Front. Pain Res. 2:682072. doi: 10.3389/fpain.2021.682072
Received: 17 March 2021; Accepted: 12 May 2021;
Published: 08 June 2021.
Edited by:
Marco L. Loggia, Massachusetts General Hospital and Harvard Medical School, United StatesReviewed by:
Zeynab Alshelh, Massachusetts General Hospital and Harvard Medical School, United StatesDominik Mischkowski, Ohio University, United States
Susanne Becker, University of Zurich, Switzerland
Copyright © 2021 Chimenti, Rakel, Dailey, Vance, Zimmerman, Geasland, Williams, Crofford and Sluka. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ruth L. Chimenti, cnV0aC1jaGltZW50aUB1aW93YS5lZHU=
 Ruth L. Chimenti
Ruth L. Chimenti Barbara A. Rakel
Barbara A. Rakel Dana L. Dailey
Dana L. Dailey Carol G. T. Vance1
Carol G. T. Vance1 Katharine M. Geasland
Katharine M. Geasland Jon M. Williams
Jon M. Williams Leslie J. Crofford
Leslie J. Crofford Kathleen A. Sluka
Kathleen A. Sluka