
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Ophthalmol. , 24 April 2024
Sec. Inflammatory Eye Diseases
Volume 4 - 2024 | https://doi.org/10.3389/fopht.2024.1372429
Introduction: Zoledronate is a commonly prescribed medication to maintain bone health; however, a rare side effect includes ocular inflammation. We report a case of simultaneous anterior uveitis and orbital inflammation associated with zoledronate infusion in a patient with metastatic breast cancer. We also performed a literature search to provide an up-to-date summary of cases with zoledronate-associated ocular inflammation.
Methods: This is a case report with literature review. Literature search (timeline 2010 to 2023) was performed using PubMed with the search team: (zoledronate) AND (uveitis OR scleritis OR orbital inflammation OR ocular inflammation).
Results: A 48-year-old female presented with left eye pain, swelling, and decreased vision 2 days after receiving zoledronic acid infusion. An ophthalmic exam showed non-granulomatous anterior uveitis. CT orbits and ocular ultrasound showed signs of posterior scleritis and orbital inflammation. Ocular inflammation caused by an infection or metastatic cancer was ruled out. The patient was treated with both topical and systemic corticosteroids. Complete resolution of the inflammation occurred after 2.5 weeks.
Conclusion: Orbital inflammation and uveitis are an uncommon side effect of zoledronate but needs to be promptly recognized and treated to prevent sight-threatening complications.
Bisphosphonates are frequently prescribed for patients with osteoporosis, glucocorticoid-induced osteoporosis, multiple myeloma, Paget’s disease, and primary and metastatic bone tumors (1). Zoledronic acid infusion is among the most appealing forms used due to its lower frequency of administration and high potency, which increases compliance among patients unable to tolerate the side effects of oral bisphosphonate. Zoledronic acid infusion is generally well tolerated; however, systemic side effects include flu-like symptoms (fever, headaches, myalgias, and arthralgias), gastritis, hypocalcemia, and osteonecrosis of the jaw (1). Ocular inflammation, which includes both orbital inflammation and uveitis, is an uncommon but a serious side effect of zoledronate, leading to significant ocular pain and decreased vision (2). Common signs of orbital inflammation include conjunctival hyperemia, chemosis, proptosis, and lid edema (2). Unfortunately, these findings are non-specific, making the diagnosis of bisphosphonate-induced inflammation challenging, and physicians need to consider alternative pathologies (e.g., orbital cellulitis or metastatic disease) which can be vision- or life-threatening.
Here we report a severe case of zoledronate-associated orbital inflammation occurring simultaneously with anterior uveitis.
A 48-year-old female presented to Ottawa Hospital Eye Institute (Canada) with a 6-day history of left eye pain, swelling of the upper eyelid, and decreased vision (see Table 1 for the case report timeline). Past medical history included total thyroidectomy (May 2007), radioiodine ablation for papillary thyroid cancer (August 2007), and a recent diagnosis of lobular carcinoma of the breast with metastasis to bone (December 2022). She is on chemotherapy: goserelin (Zoladex, 10.8 mg subcutaneous injection every 3 months; last received December 2, 2022) and palbociclib (100-mg pill daily; started December 2, 2022). She has a history of laser vision corrective surgery (July 2017) but no other ocular disease.
Her ocular symptoms started approximately 48 h after receiving her first intravenous infusion of 4 mg zoledronate (Zometa; medication revived on December 12, 2022) (Figure 1A). She was afebrile and did not report any other zoledronate-related systemic side effects. Before presenting to the eye institute, she was initially treated with topical erythromycin ointment by her family physician for presumed infectious conjunctivitis. She later presented to the emergency department with worsening symptoms where she was given a dose of IV piperacillin/tazobactam for presumed orbital cellulitis before referral to ophthalmology.
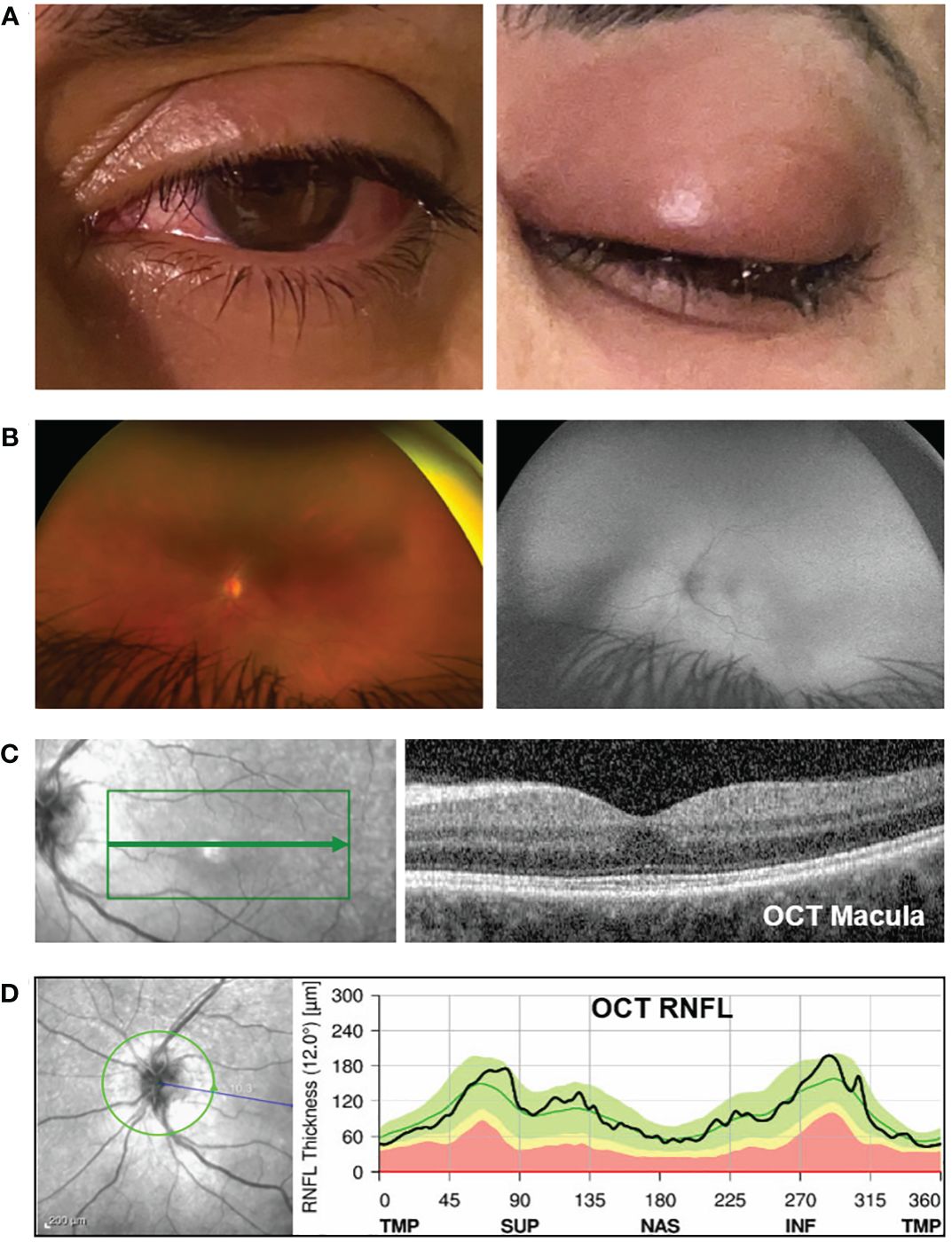
Figure 1 Clinical exam and optical coherence tomography (OCT) analysis. (A) Photograph of the left eye showing peri-orbital swelling, conjunctival injection, and chemosis (day 2 after receiving zoledronate). (B) On the left panel is a color fundus photo (Optos camera) of the left eye showing normal-appearing optic disc, vessels, and retina. The right panel shows the corresponding normal fundus autofluorescence photo. (C) The left image panel shows the en-face fundus photo of the retina (the green box highlights the area within the macula where the OCT scan was captured). The right panel shows the OCT image corresponding to the area indicated by the green arrow on the en-face image. The OCT macula shows normal retina structures, normal foveal contour, and the absence of cystic edema, intraretinal fluid, and subretinal fluid. The average central subfield thickness was measured as 297 µm, which is within the normal range (250–300 µm) (3, 4). (D) The OCT retinal nerve fiber layer (RNFL) analysis shows no optic nerve head swelling. The left panel is the en-face fundus photo showing the optic disc (the green circle indicates where the OCT analysis was captured). The right image panel shows the RNFL thickness along the different regions around the optic disc. TMP, temporal; SUP, superior; NAS, nasal; INF, inferior. The black line is within the green region, indicating that the patient’s RNFL thickness is within the normal range. The abnormal yellow and red regions represent thin RNFL. Anything above the green region would indicate a thickened RNFL, i.e., optic disc swelling.
Her visual acuity in the right eye was 20/20 and in the left eye was 20/80. Intraocular pressure was 15 mmHg in both eyes. Photophobia limited the accurate assessment of relative afferent pupillary defect, but she had decreased color vision in the left eye. Extraocular movements were restricted in all gaze. There was periorbital swelling, ocular pain, diffuse conjunctival injection, and moderate chemosis. The cornea was clear with only mild punctate epithelial erosions and had fine keratic precipitates. The anterior chamber had 4+ cells, 2+ flare, and fibrin deposit on the anterior lens capsule and posterior iris synechiae. The fundus view was hazy, but the posterior segment appeared normal (Figure 1B). Optical coherence tomography (OCT) showed no macular edema or ischemia (Figure 1C); the average central subfield thickness was measured as 297 µm, which is within the normal range (250–300 µm) (3, 4). OCT also did not show optic disc swelling (Figure 1D). There was no evidence of vitritis on B-scan ultrasound, but there was diffuse thickening of the ocular coats (measuring 2 mm at the posterior sclera which is twofold thicker than normal (5); Figure 2). There was also an echoluscent shadow posterior to the sclera (T-sign) suggestive of scleritis and orbital inflammation on B-scan. The CT result demonstrated left periorbital soft tissue swelling, lacrimal gland enlargement, fluid collection along the inferior lateral aspect of the globe with diffuse thickening and enhancement of the sclera, and mild thickening of the left lateral rectus muscle belly (Figure 3). The rest of the orbit, extraocular muscles, and optic nerve sheath complexes were unremarkable. The oncology team, together with ophthalmology and radiology, ruled out orbital metastasis after a careful assessment of the CT orbits; the B-scan ultrasound result also did not show any signs of orbital metastasis. Lastly, no metastatic choroidal lesions were seen on complete ophthalmic exam. She had a negative tuberculin skin test and a negative syphilis screen. There was no leukocytosis. The C-reactive protein (21 mg/dL) and erythrocyte sedimentation rate (27 mm/hr) were elevated. Overall, her clinical presentation was in keeping with a non-specific orbital inflammation (anterior and posterior scleritis, myositis, and dacryoadenitis) with anterior non-granulomatous uveitis.
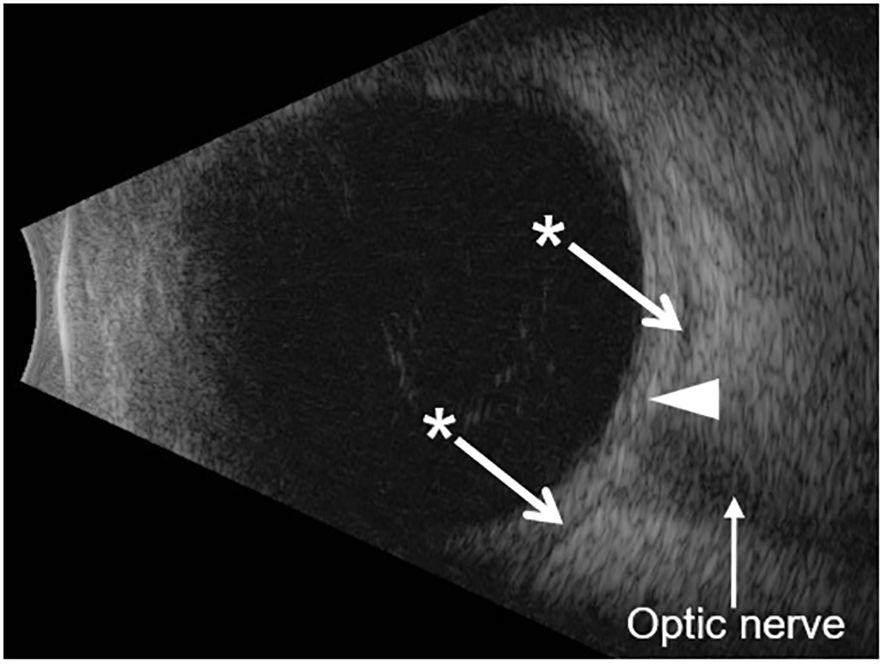
Figure 2 B-scan ocular ultrasound. The B-scan shows diffuse thickening of the ocular coats (white arrowhead). The measurement of the ocular coats taken at the macula (posterior sclera) was 2.0 mm, which is twofold thicker than the normal value (1.0 mm in an average eye axial length of 23–25 mm; the patient’s axial length was 24.5 mm) (5). The asterisk shows hypoechoic regions on either side of the optic nerve which represents sub-Tenon’s fluid. This is seen in cases of posterior scleritis (also called the T-sign).
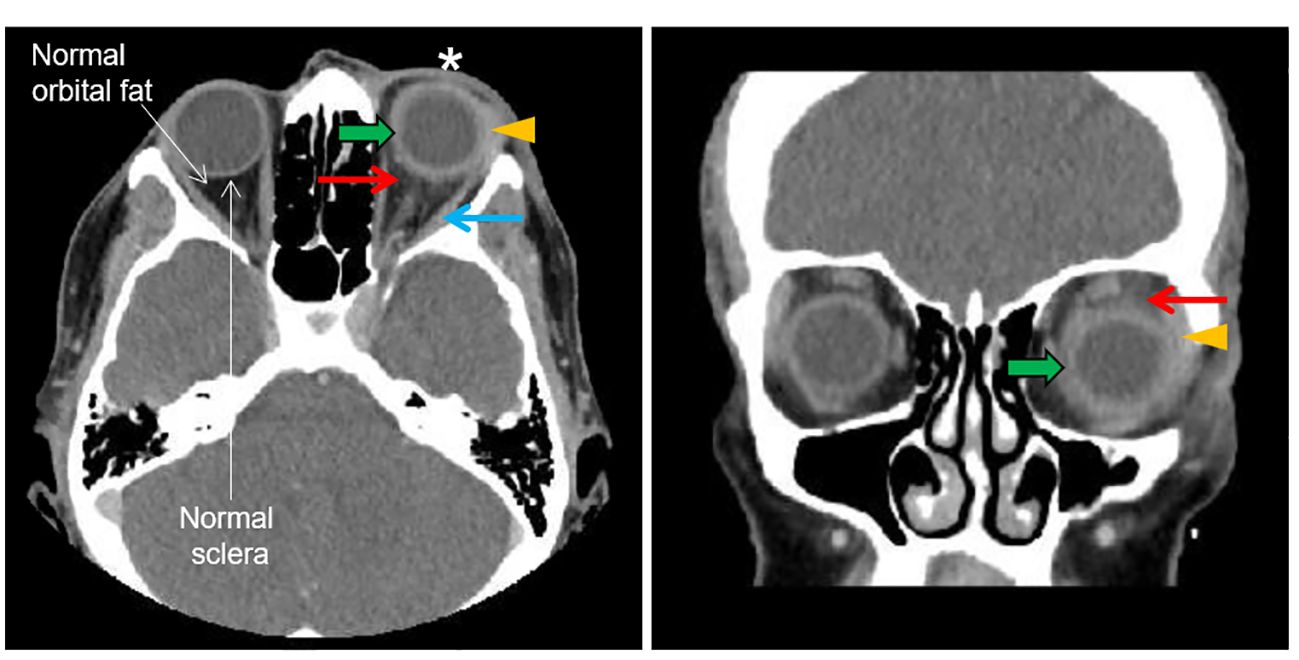
Figure 3 CT orbit. The left and right panels show the transverse and coronal plan, respectively. The asterisk shows left periorbital soft tissue swelling. The orange arrowhead shows lacrimal gland enlargement. The green arrow shows a blurry margin, thickening and enhancement of the sclera. The red arrow shows an increased density of orbital adipose tissue (fat stranding) adjacent to the sclera which is absent in the other eye. The blue arrow shows a mild thickening of the lateral rectus muscle belly.
She was started on topical 1% prednisolone acetate hourly, dexamethasone 0.1% ointment nightly, and 1% cyclopentolate twice daily. The patient was already started on dexamethasone (8 mg PO daily; started ~3 days after receiving zoledronate) as part of her treatment for cancer (Table 1). The course of systemic dexamethasone was extended to 30 days. Upon follow-up at day 3, there was almost complete resolution of periorbital swelling, chemosis, and anterior uveitis (trace cells) and improved visual acuity (20/50). The fundus exam was normal, with no evidence of intermediate uveitis or vasculitis. The remainder of the ocular exam demonstrated mild pigmented deposits on the anterior capsule, few posterior synechiae, and 1+ nuclear sclerosis cataract. The patient continued to improve while tapering off topical steroids with no recurrence of symptoms. Inflammation had completely resolved in 2.5 weeks. Visual acuity returned to normal (20/20) after ~3 weeks. In collaboration with oncology, zoledronate was stopped and she was switched to denosumab.
Over the last decade, multiple case reports and series have shown an association between bisphosphonate and ocular inflammation (6). This association is strengthened by the following: (1) a temporal relationship between starting medication and developing inflammation, (2) symptoms reoccur after rechallenging the patient with the same or alternative bisphosphonate, and (3) cases do not resolve without discontinuing the medication (7). Cases have been reported with the use of pamidronate disodium (8–10), zoledronate (2, 11–13), alendronate sodium (2, 10, 14), risedronate sodium (2, 7, 15), and etidronate sodium (7, 16). While the mechanism of bisphosphonate-associated ocular inflammation is unclear, it is thought to involve the activation of gamma-delta T cells which release inflammatory cytokines (17, 18). These cytokines can produce an immunologic reaction, resulting in uveitis or orbital inflammation. Here we presented a case of severe ocular inflammation occurring a few days after starting zoledronate infusion. Our literature search using PubMed yielded 35 publications (total: 44 cases) on zoledronate-associated ocular inflammation over two decades (2010 to 2023). Supplementary Table 1 shows a summary of the published cases (2, 11–13, 19–49). The patients’ average age is 64.5 years (range, 45–87 years), with 79% female patients and 21% male patients. Furthermore, 45.5% of cases developed only orbital inflammation, 38.6% developed anterior uveitis, and 15.9% had both.
Ocular inflammation most commonly involves the sclera (scleritis) but can also include extraocular muscles (myositis), lacrimal gland (dacryoadenitis), orbital soft tissue (e.g., orbital fat), optic neuritis, and uveal tissue (uveitis) (2, 7, 44, 50, 51). The most common signs and symptoms include pain, blurry vision, lid edema, conjunctival injection, and chemosis (2, 7). These ocular signs/symptoms are non-specific and can often be mistaken for conjunctivitis (52). More severe cases of orbital inflammation can present with proptosis, limited extraocular movements, uveitis, and poor vision (11). Fortunately, ocular inflammation is a rare side effect of bisphosphonate with a reported rate of 0.1%–1.1% of users (10, 50, 53). However, it is important to recognize inflammation early as prompt diagnosis and treatment are vital to prevent vision loss (2, 11). A careful assessment is also required to rule out infectious (e.g., orbital cellulitis, tuberculosis, syphilis, endophthalmitis, herpes simplex virus, varicella-zoster virus, Bartonella henselae, or toxoplasmosis) or non-infectious inflammation (e.g., granulomatosis with polyangiitis, thyroid orbitopathy, sarcoidosis, Behcet’s disease, idiopathic orbital inflammation, orbital metastasis, or primary ocular malignancy), which can be vision- or life-threatening (13). The clinical features and diagnosis of various uveitis conditions are covered in detail in the following references (54–56). Ancillary tests like CT and MRI orbit or B-scan ultrasound are useful in ruling out other orbital pathologies and help in establishing diagnosis (2, 13). However, if the diagnosis is not clear at initial presentation, it is appropriate to start broad-spectrum intravenous antibiotics (e.g., piperacillin–tazobactam or ceftriaxone) for treating orbital cellulitis especially in an immunocompromised patient who is prone to infections (57). Moreover, prior to starting high-dose systemic corticosteroids, it is prudent to rule out underlying infections like tuberculosis or syphilis. In our case, both infectious and non-infectious etiologies were investigated.
Majority of the cases are unilateral (77%; range in other reports: 70%–89%) (2, 58). Inflammation occurs more frequently with a quicker onset (few hours to 14 days) when delivered intravenously compared to the oral route (range: 15–45 days) (2, 7, 10, 50, 58). Two large cohort studies found that the risk for developing ocular inflammation was greater among patients with underlying inflammatory conditions such as seronegative rheumatoid arthritis, Sjogren’s syndrome, ankylosing spondylitis, or pulmonary disease like sarcoidosis (7, 10). Akin to our literature search, up to 60%–70% of reported cases occur in women (2, 58). This is likely the result of prescribing bisphosphonates more commonly to women for treating postmenopausal osteoporosis (53); whether there is a true sex predilection remains unknown and require further studies.
In our case, the patient developed severe diffuse orbital inflammation and uveitis within 48 h of receiving zoledronate infusion. Although less common, acute anterior uveitis can occur simultaneously with orbital inflammation in up to 16%–30% of cases (2, 58). Studies commonly report the presenting visual acuity as normal or mild (<20/50), but more severe vision loss can occur as in our case (20/80) (11, 13). This is akin to the case reported by Umunakwe et al. (11) in which the patient presented with severe anterior uveitis and diffuse scleritis with a visual acuity of 20/200. Fortunately, the orbital inflammation resolves after discontinuing bisphosphonate and initiating systemic (intravenous or oral) corticosteroid therapy (2, 11, 13). Uveitis is treated with topical corticosteroids and cycloplegic agents for pain relief and preventing posterior synechiae (50). Treatment should be initiated promptly to avoid a secondary complication of ocular inflammation such as macular edema, glaucoma, cataracts, and scleral perforation (53). As shown in Supplementary Table 1, nearly all cases report the complete resolution of inflammation (range: 2 days to 3 months) after starting the treatment with systemic steroids (e.g., intravenous methylprednisolone for orbital inflammation), topical steroids (e.g., prednisolone acetate for uveitis), or both (2, 11, 13, 26).
Orbital inflammation can be treated with various corticosteroids including prednisone, oral (or intravenous) dexamethasone, or methylprednisolone. Overall, the choice of corticosteroid and dosing is not standardized. As shown in Supplementary Table 1, various treatment regimens have been used successfully for treating orbital inflammation, such as (1) prednisone only (starting dose of 1 mg/kg, but a range of 30–60 mg daily has also been used) (19, 28, 30, 35, 40), (2) pulse with intravenous methylprednisolone (dosed at 1 mg/kg/day or a single dose of 500 mg or 1 g daily for 1–3 days) followed by prednisone taper (11, 13, 30, 36, 37), and (3) pulse with intravenous dexamethasone (10 mg) followed by prednisone taper (26). In one case report, the patient was started on oral dexamethasone (4 mg every other day) as part of their cancer treatment and did not require a higher dose of steroid (30). In our case, the patient was already started on dexamethasone (8 mg PO daily) as part of her cancer treatment; this is a typical dose used to manage pain flare after radiation treatment of bone metastasis (59). This dose is equivalent to receiving 1 mg/kg prednisone (~55 mg for our patient) which is an adequate dose for treating orbital inflammation as previously reported (see Supplementary Table 1). As such, a pulse treatment with a higher dose of steroid (e.g., 1 g methylprednisolone) was not initiated, which also helped minimize the harmful side effect of steroid such as hypertension, diabetes, weight gain, stomach ulcers, mood disorder (e.g., psychosis), increased susceptibility to infection, and osteoporosis (60). Our patient showed a rapid improvement with the treatment and therefore did not require a higher dose of steroids.
Unfortunately, data regarding rechallenging patients or switching bisphosphonates is contradictory (7, 8, 61, 62). In general, rechallenging should be considered in a case-by-case basis, and the risk and benefits need to be clearly explained to the patient with close monitoring if reinitiated. Given the severity of inflammation in our case, bisphosphonate therapy was switched to denosumab.
In conclusion, severe ocular inflammation is an uncommon side effect of bisphosphonates but needs to be promptly recognized and treated to prevent sight-threatening complications. In addition, the prescribing physician (or the consultant ophthalmologist) should assess for other dangerous orbital pathologies which present in a similar fashion.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
PK: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. IG-C: Data curation, Writing – original draft, Writing – review & editing. JD: Data curation, Writing – review & editing. AT: Supervision, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
We acknowledge and thank Carla Blackburn B.Sc., COMT, ROUB, CDOS, for performing and reporting the B-scan ultrasound.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fopht.2024.1372429/full#supplementary-material
1. Ganesan K, Goyal A, Roane D. Bisphosphonate. Treasure Island (FL: StatPearls Publishing (2023). Available at: https://www.ncbi.nlm.nih.gov/pubmed/29262103.
2. Keren S, Leibovitch I, Ben Cnaan R, Neudorfer M, Fogel O, Greenman Y, et al. Aminobisphosphonate-associated orbital and ocular inflammatory disease. Acta Ophthalmol. (2019) 97:e792–e9. doi: 10.1111/aos.14063
3. Grover S, Murthy RK, Brar VS, Chalam KV. Normative data for macular thickness by high-definition spectral-domain optical coherence tomography (spectralis). Am J Ophthalmol. (2009) 148:266–71. doi: 10.1016/j.ajo.2009.03.006
4. Adhi M, Aziz S, Muhammad K, Adhi MI. Macular thickness by age and gender in healthy eyes using spectral domain optical coherence tomography. PloS One. (2012) 7:e37638. doi: 10.1371/journal.pone.0037638
5. Vurgese S, Panda-Jonas S, Jonas JB. Scleral thickness in human eyes. PloS One. (2012) 7:e29692. doi: 10.1371/journal.pone.0029692
6. Clark EM, Durup D. Inflammatory eye reactions with bisphosphonates and other osteoporosis medications: what are the risks? Ther Adv Musculoskelet Dis. (2015) 7:11–6. doi: 10.1177/1759720X14566424
7. Pazianas M, Clark EM, Eiken PA, Brixen K, Abrahamsen B. Inflammatory eye reactions in patients treated with bisphosphonates and other osteoporosis medications: cohort analysis using a national prescription database. J Bone Miner Res. (2013) 28:455–63. doi: 10.1002/jbmr.1783
8. Fraunfelder FW, Fraunfelder FT, Jensvold B. Scleritis and other ocular side effects associated with pamidronate disodium. Am J Ophthalmol. (2003) 135:219–22. doi: 10.1016/S0002-9394(02)01840-8
9. Subramanian PS, Kerrison JB, Calvert PC, Miller NR. Orbital inflammatory disease after pamidronate treatment for metastatic prostate cancer. Arch Ophthalmol. (2003) 121:1335–6. doi: 10.1001/archopht.121.9.1335
10. French DD, Margo CE. Postmarketing surveillance rates of uveitis and scleritis with bisphosphonates among a national veteran cohort. Retina. (2008) 28:889–93. doi: 10.1097/IAE.0b013e31816576ef
11. Umunakwe OC, Herren D, Kim SJ, Kohanim S. Diffuse ocular and orbital inflammation after zoledronate infusion-case report and review of the literature. Digit J Ophthalmol. (2017) 23:18–21. doi: 10.5693/djo.02.2017.08.002
12. Peterson JD, Bedrossian EH Jr. Bisphosphonate-associated orbital inflammation–a case report and review. Orbit. (2012) 31:119–23. doi: 10.3109/01676830.2011.648818
13. Rahimy E, Law SK. Orbital inflammation after zoledronate infusion: an emerging complication. Can J Ophthalmol. (2013) 48:e11–2. doi: 10.1016/j.jcjo.2012.09.011
14. Mbekeani JN, Slamovits TL, Schwartz BH, Sauer HL. Ocular inflammation associated with alendronate therapy. Arch Ophthalmol. (1999) 117:837–8.
15. Hemmati I, Wade J, Kelsall J. Risedronate-associated scleritis: a case report and review of the literature. Clin Rheumatol. (2012) 31:1403–5. doi: 10.1007/s10067-012-2035-z
16. Fraunfelder FW, Fraunfelder FT. Bisphosphonates and ocular inflammation. N Engl J Med. (2003) 348:1187–8. doi: 10.1056/NEJM200303203481225
17. Thompson K, Roelofs AJ, Jauhiainen M, Monkkonen H, Monkkonen J, Rogers MJ. Activation of gammadelta T cells by bisphosphonates. Adv Exp Med Biol. (2010) 658:11–20. doi: 10.1007/978-1-4419-1050-9_2
18. Nussbaumer O, Gruenbacher G, Gander H, Komuczki J, Rahm A, Thurnher M. Essential requirements of zoledronate-induced cytokine and gammadelta T cell proliferative responses. J Immunol. (2013) 191:1346–55. doi: 10.4049/jimmunol.1300603
19. Missotten G, Verheezen Y. Orbital inflammation after use of zoledronic acid for metastasized prostate carcinoma. Bull Soc Belge Ophtalmol. (2010) 315:23–4.
20. Yeo J, Jafer AK. Zolendronate associated inflammatory orbital disease. N Z Med J. (2010) 123:50–2.
21. Kaur H, Uy C, Kelly J, Moses AM. Orbital inflammatory disease in a patient treated with zoledronate. Endocr Pract. (2011) 17:e101–3. doi: 10.4158/EP11081.CR
22. Ortiz-Perez S, Fernandez E, Molina JJ, Sanchez-Dalmau B, Navarro M, Corretger X, et al. Two cases of drug-induced orbital inflammatory disease. Orbit. (2011) 30:37–9. doi: 10.3109/01676830.2010.535641
23. Belliveau MJ, Almeida DR, Urton TE. Acute anterior uveitis following zoledronic acid infusion for osteoporosis. Can J Ophthalmol. (2012) 47:e22–3. doi: 10.1016/j.jcjo.2012.03.020
24. Boni C, Kordic H, Chaloupka K. Bisphosphonate-associated orbital inflammatory disease and uveitis anterior–a case report and review. Klin Monbl Augenheilkd. (2013) 230:367–9. doi: 10.1055/s-0032-1328337
25. Freitas-Neto CA, de Oliveira Fagundes WB, Ribeiro M Jr., Pacheco KD, Freitas LG, Avila MP. Unilateral uveitis with vitreous haze following zoledronic Acid therapy for osteoporosis. Semin Ophthalmol. (2015) 30:232–4. doi: 10.3109/08820538.2013.839806
26. Vora MM, Rodgers IR, Uretsky S. Nitrogen bisphosphonate-induced orbital inflammatory disease: gamma delta T cells–a report and review of 2 cases. Ophthalmic Plast Reconstr Surg. (2014) 30:e84–5. doi: 10.1097/IOP.0b013e31829f3b46
27. Cavallasca JA, Reyt C. [Acute anterior uveitis associated with the use zoledronic acid]. Medicina (B Aires). (2014) 74:428–9.
28. Muruganandam M, Sandhu H. Orbital inflammation secondary to zoledronic acid, a rare presentation. J Clin Rheumatol. (2016) 22:384. doi: 10.1097/RHU.0000000000000433
29. Tian Y, Wang R, Liu L, Ma C, Lu Q, Yin F. Acute bilateral uveitis and right macular edema induced by a single infusion of zoledronic acid for the treatment of postmenopausal osteoporosis as a substitution for oral alendronate: a case report. BMC Musculoskelet Disord. (2016) 17:72. doi: 10.1186/s12891-016-0926-x
30. Lefebvre DR, Mandeville JT, Yonekawa Y, Arroyo JG, Torun N, Freitag SK. A case series and review of bisphosphonate-associated orbital inflammation. Ocul Immunol Inflamm. (2016) 24:134–9. doi: 10.3109/09273948.2014.942747
31. Haider AS, Burbidge AM, Dunlop A. Bilateral ocular pain and hyperemia in a patient with Paget disease of the bone. JAMA Ophthalmol. (2017) 135:665–6. doi: 10.1001/jamaophthalmol.2016.4921
32. Jun JH. Acute bilateral anterior uveitis after a single intravenous infusion of zoledronic acid in metastatic breast cancer. Korean J Ophthalmol. (2017) 31:368–9. doi: 10.3341/kjo.2017.0018
33. Kennedy T, Sellar PW, Vaideanu-Collins D, Ng J. Two case reports of zoledronic acid-induced uveitis. Age Ageing. (2018) 47:754–5. doi: 10.1093/ageing/afy070
34. Chehade LK, Curragh D, Selva D. Bisphosphonate-induced orbital inflammation: more common than once thought? Osteoporos Int. (2019) 30:1117–20. doi: 10.1007/s00198-019-04850-w
35. Herrera I, Kam Y, Whittaker TJ, Champion M, Ajlan RS. Bisphosphonate-induced orbital inflammation in a patient on chronic immunosuppressive therapy. BMC Ophthalmol. (2019) 19:51. doi: 10.1186/s12886-019-1063-8
36. Tan M, Kalin-Hajdu E, Narayan R, Wong SW, Martin TG. Zoledronic acid-induced orbital inflammation in a patient with multiple myeloma. J Oncol Pharm Pract. (2019) 25:1253–7. doi: 10.1177/1078155218785967
37. Han LS, Weatherhead RG. Zoledronic acid associated orbital inflammation. Clin Exp Ophthalmol. (2020) 48:249–50. doi: 10.1111/ceo.13659
38. Gupta S, Onkar A, Vashisht T. Zoledronic acid induced unilateral anterior uveitis. Indian J Ophthalmol. (2020) 68:2002–3. doi: 10.4103/ijo.IJO_1654_19
39. Anandasayanan K, Malaravan M, Suganthan N. Acute unilateral anterior uveitis following zoledronic acid infusion: A case report. SAGE Open Med Case Rep. (2020) 8:2050313X20944305. doi: 10.1177/2050313X20944305
40. Khalid MF, Micieli J. Zoledronic acid-induced orbital inflammation. BMJ Case Rep. (2021) 14:e245359. doi: 10.1136/bcr-2021-245359
41. Karmiris E, Vasilopoulou MG, Chalkiadaki E. Acute bilateral anterior uveitis following cyclophosphamide/bortezomid/dexamethasone (CyBorD) protocol in a newly diagnosed multiple myeloma patient with concomitant use of zoledronic acid. Ocul Immunol Inflamm. (2021) 29:1328–31. doi: 10.1080/09273948.2020.1745245
42. Faryal R, Hayat A. Keeping an eye on bisphosphonate therapy in myeloma: A case report of ocular inflammation postzoledronic acid infusion. Case Rep Hematol. (2021) 2021:6647277. doi: 10.1155/2021/6647277
43. Jin X, Shou Z, Shao Y, Bian P. Zoledronate-induced acute anterior uveitis: a three-case report and brief review of literature. Arch Osteoporos. (2021) 16:104. doi: 10.1007/s11657-021-00964-z
44. Wolpert LE, Watts AR. Zoledronate-induced anterior uveitis, scleritis and optic neuritis: a case report. N Z Med J. (2021) 134:91–4.
45. Larid G, Inglard C, Mahe J, Roy-Peaud F, Debiais F. Zoledronic acid-induced posterior scleritis associated with inflammatory orbitopathy. Joint Bone Spine. (2022) 89:105445. doi: 10.1016/j.jbspin.2022.105445
46. Jakobsen TS, Funding M. [Bisphosphonate-induced ocular and orbital inflammation]. Ugeskr Laeger. (2022) 184:V03220169.
47. Kaiser KP, Dingerkus VLS, Becker MD, Turgut F. [Acute unilateral fibrinous anterior uveitis after zoledronic acid infusion]. Ophthalmologie. (2023) 121(1):64-67. doi: 10.1007/s00347-023-01870-0
48. Chacko G, Kota S, Kumar S, Ohri N, Omene C, Ganesan S, et al. Uveitis, a rare but important complication of adjuvant zoledronic acid for early-stage breast cancer. Anticancer Drugs. (2023) 34:592–4. doi: 10.1097/CAD.0000000000001503
49. Ankireddypalli AR, Sibley S. Acute iridocyclitis associated with intravenous zoledronic acid: a case report. Cureus. (2023) 15:e43162. doi: 10.7759/cureus.43162
50. Patel DV, Bolland M, Nisa Z, Al-Abuwsi F, Singh M, Horne A, et al. Incidence of ocular side effects with intravenous zoledronate: secondary analysis of a randomized controlled trial. Osteoporos Int. (2015) 26:499–503. doi: 10.1007/s00198-014-2872-5
51. Stack R, Tarr K. Drug-induced optic neuritis and uveitis secondary to bisphosphonates. N Z Med J. (2006) 119:U1888.
52. Statham MO, Sharma A, Pane AR. Misdiagnosis of acute eye diseases by primary health care providers: incidence and implications. Med J Aust. (2008) 189:402–4. doi: 10.5694/j.1326-5377.2008.tb02091.x
53. Etminan M, Forooghian F, Maberley D. Inflammatory ocular adverse events with the use of oral bisphosphonates: a retrospective cohort study. CMAJ. (2012) 184:E431–4. doi: 10.1503/cmaj.111752
54. Seve P, Cacoub P, Bodaghi B, Trad S, Sellam J, Bellocq D, et al. Uveitis: Diagnostic work-up. A literature review and recommendations from an expert committee. Autoimmun Rev. (2017) 16:1254–64. doi: 10.1016/j.autrev.2017.10.010
55. Grillo A, Levinson RD, Gordon LK. Practical diagnostic approach to uveitis. Expert Rev Ophthalmol. (2011) 6:449–59. doi: 10.1586/eop.11.47
56. Rathinam SR, Babu M. Algorithmic approach in the diagnosis of uveitis. Indian J Ophthalmol. (2013) 61:255–62. doi: 10.4103/0301-4738.114092
57. Danishyar A, Sergent SR. Orbital Cellulitis. Treasure Island (FL: StatPearls (2024). Disclosure: Shane Sergent declares no relevant financial relationships with ineligible companies.
58. Gonzalez Barlatay J, Pagano Boza C, Hernandez Gauna GV, Premoli JE. Orbital inflammation caused by aminobisphosphonates. J Ophthalmic Vis Res. (2022) 17:118–22. doi: 10.18502/jovr.v17i1.10176
59. Fabregat C, Almendros S, Navarro-Martin A, Gonzalez J. Pain flare-effect prophylaxis with corticosteroids on bone radiotherapy treatment: A systematic review. Pain Pract. (2020) 20:101–9. doi: 10.1111/papr.12823
60. Yasir M, Goyal A, Sonthalia S. Corticosteroid Adverse Effects. Treasure Island (FL: StatPearls (2024). Disclosure: Amandeep Goyal declares no relevant financial relationships with ineligible companies. Disclosure: Sidharth Sonthalia declares no relevant financial relationships with ineligible companies.
61. Patel DV, Horne A, Mihov B, Stewart A, Reid IR, McGhee CN. The effects of re-challenge in patients with a history of acute anterior uveitis following intravenous zoledronate. Calcif Tissue Int. (2015) 97:58–61. doi: 10.1007/s00223-015-0015-4
Keywords: bisphosphonates, drug-induced ocular inflammation, orbital inflammation, scleritis, uveitis, zoledronate
Citation: Kanda P, Guerrero-Córdova I, Dhillon J and Tsang A (2024) Case report: A severe case of zoledronate-associated diffuse orbital inflammation and uveitis in a patient with metastatic breast cancer. Front. Ophthalmol. 4:1372429. doi: 10.3389/fopht.2024.1372429
Received: 18 January 2024; Accepted: 25 March 2024;
Published: 24 April 2024.
Edited by:
Tarsis G. Ferreira, University of Houston, United StatesReviewed by:
Ali Osman Saatci, Dokuz Eylül University, TürkiyeCopyright © 2024 Kanda, Guerrero-Córdova, Dhillon and Tsang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pushpinder Kanda, cGthbmQwNDJAdW90dGF3YS5jYQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.