
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ophthalmol., 27 June 2022
Sec. Inflammatory Eye Diseases
Volume 2 - 2022 | https://doi.org/10.3389/fopht.2022.916718
This article is part of the Research TopicRising Stars in Inflammatory Eye Disease: 2022View all 4 articles
 Laurie W. van der Merwe1*†
Laurie W. van der Merwe1*† Candice Snyders2†
Candice Snyders2† Martin Kidd3
Martin Kidd3 Novel N. Chegou2†
Novel N. Chegou2† Gerhard Walzl2†
Gerhard Walzl2† Derrick P. Smit1†
Derrick P. Smit1†Aim: To investigate the role of the chemokines CXCL13, CXCL10 and CXCL8 in the diagnosis of ocular- and neurosyphilis by examining the serum, aqueous humour (AH) and cerebrospinal fluid (CSF) of patients with ocular syphilis.
Methods: An observational descriptive study was performed prospectively at Tygerberg Academic Hospital in Cape Town, South Africa from 1 February 2018 till 31 January 2021 which enrolled 23 participants. 14 Patients were male and 9 female, 15 patients were HIV positive, and all patients were newly diagnosed with ocular syphilis. Upon diagnosis of ocular syphilis, the HIV status of each patient was determined, and 3 samples (AH, serum and CSF) were collected to measure the levels of CXCL13, CXCL10 and CXCL8 in each. All patients were treated with 14 days of intravenous Penicillin G and topical corticosteroid drops for uveitis.
Results: The mean concentrations of all 3 biomarkers were higher in the AH and CSF than in the serum. The mean concentrations of the 3 measured biomarkers were markedly different when comparing both AH and CSF levels to serum levels. The level of CXCL13 measured in the AH correlated well with the concentrations found in the CSF of patients with neurosyphilis. In patients with neurosyphilis, mean AH levels of CXCL13 and CXCL10 were markedly higher than in serum while mean CSF levels of CXCL10 were also markedly higher than in serum. Also, the AH/serum ratio of CXCL13 and CXCL10, as well as the CSF/serum ratio of CXCL10, was much higher in patients with neurosyphilis than without. In patients with HIV infection, mean AH CXCL13 levels were much higher than in patients without HIV infection.
Conclusion: The levels of CXCL13, CXCL10 and CXCL8 in the AH of patients with neurosyphilis are similar to previously reported levels in the CSF of patients with neurosyphilis and can potentially be an adjunct in the diagnosis of ocular syphilis. Patients with ocular syphilis who tested negative for neurosyphilis with conventional CSF testing showed features of neurosyphilis when analysing the CSF chemokines.
The incidence of syphilis, caused by the spirochaete Treponema pallidum, decreased towards the end of the 20th century when penicillin became available and public health measures improved (1). However, since the start of the 21st century, an increasing number of cases of syphilis and ocular syphilis were recorded (1–8). The Centers for Disease Control and Prevention (CDC) reported a 74% increase in syphilis cases in the United States between 2015 and 2019 (2). The risk factors most frequently documented are male sex, homosexuality and intravenous drug abuse, but the infection rates are also increasing in females and heterosexual individuals (1–8). In 3 studies from South Africa, syphilis was identified as the cause of uveitis in 7% to 16.2% of cases (9–11).Ocular syphilis is diagnosed when ocular inflammation occurs in conjunction with a serologically confirmed systemic syphilis infection, and other causes for inflammation have been excluded. The relationship between ocular syphilis and neurosyphilis has been much debated, but it is assumed that, due to the embryological origin of the retina from the central nervous system, ocular syphilis is a clinical feature of neurosyphilis. Although this is widely accepted for the involvement of the optic nerve and retina, it is disputed whether anterior uveitis represents an entity apart from neurosyphilis (12, 13).
The CDC recommends that a baseline examination of the cerebrospinal fluid (CSF) should be performed for all patients with ocular syphilis, and treatment should commence as for symptomatic neurosyphilis, with 18–24 million units intravenous aqueous crystalline penicillin G per day for 10–14 days, even with a normal CSF examination (14) Alternatives to intravenous aqueous crystalline penicillin G would be intramuscular procaine penicillin G 2.4 million units daily plus Probenecid 500 mg orally four times per day, both for 10 – 14 days. If a patient is allergic to penicillin Ceftriaxone 1 -2 g daily as intravenous or intramuscular injection is advised (14). Ocular syphilis can, nevertheless, manifest as various ocular syndromes, and no differentiation is made regarding the type of ocular involvement.
However, the International Ocular Syphilis Study Group surveyed a group of ophthalmologists, which comprised 103 ophthalmologists from 35 countries, and found that only 40.8% of the participants performed routine CSF examination, 49.5% only tested the CSF if certain clinical findings were present (optic nerve involvement, HIV or other) and 9.7% never examined the CSF (7). Only 10.7% performed PCR tests for T pallidum on ocular fluid (7). All participants diagnosed patients with ocular syphilis by serological testing and clinical presentation, and 95% of the participants treated patients with this diagnosis with antibiotics prescribed for 10 days or more, but only 61.8% of participants treated ocular syphilis with intravenous aqueous penicillin G and 24.5% treated with intramuscular benzathine penicillin G (duration not specified) (7).
PCR and immunoblot analysis of ocular fluid have been used to aid in the diagnosis of ocular syphilis, either as a part of the diagnostic workup or in cases where serological results were inconclusive (14–16). In non-ocular syphilis the sensitivity of PCR to detect T. pallidum is reported as 72.8% and specificity of 95.5% (17). In a study by Smit et al. none of the 13 participants diagnosed with ocular syphilis tested PCR positive for ocular syphilis on AH sampling, whereas immunoblot assays confirmed syphilis in 3 of the cases (13). Other case series reported variable sensitivity of PCR on ocular fluid for detecting T. pallidum (15, 16). In 1 series, PCR testing of vitreous humor produced positive results even after repeated PCR testing of AH was negative (15). In the other, PCR testing of AH was positive in 3 of 5 cases (16). It appears that the sensitivity of PCR on ocular fluid in ocular syphilis is thus less than that of non-ocular syphilis and it is still uncertain whether vitreous sampling may provide a higher yield than AH (15). The CSF findings of patients with ocular and/or neurosyphilis can be confusing, as 30–70% of patients with neurosyphilis can have a false negative Venereal Disease Research Laboratory (VDRL) test, especially in advanced syphilis (2, 18, 19). Patients co-infected with HIV also pose a unique diagnostic challenge as HIV can cause a CSF pleocytosis (18). VDRL testing of the CSF is seen as the gold standard for diagnosing neurosyphilis, but the high false negative rate has led to the development of additional diagnostic criteria as proposed on the UpToDate (UTD) database, which we used as guidelines for diagnosing neurosyphilis in this study (20).
The chemokine CXCL13 is part of the CXC chemokine family, consisting of 1 amino-acid residue enclosed by the 2 N-terminal cysteines, and is also known as B-lymphocyte chemokine 1 (BLC-1) or B-cell attracting chemokine-1 (BCA-1). CXCL13 is secreted by secondary lymphoid tissue, dendritic cells and lymph nodes and is involved in the regulation and migration of B-cells, correlating well with the concentrations of intrathecal B- and T-cells (21–23),and has been investigated as a biomarker for confirmed and asymptomatic neurosyphilis (18, 21–25).
CXCL13 has been found to be upregulated in the CSF of patients with spirochaetal neuroinflammation related to neuroborreliosis and neurosyphilis. This has also been observed in patients with other neuroinflammatory disorders such as multiple sclerosis, cryptococcus infections, CNS lymphoma and asymptomatic HIV infection (18, 23, 25–27). The CXCL13 levels in the CSF of patients with neurosyphilis exceeded 250 pg/ml and was second only to the concentrations found in patients with neuroborreliosis (26).
The aim of our study was to determine if CXCL13, CXCL10 and CXCL8 can be used to facilitate the diagnosis of ocular syphilis by examining the concentrations of these biomarkers in the serum, aqueous humour (AH) and CSF of patients with ocular syphilis and correlating these results with the clinical presentation, HIV status and routine serological and CSF tests, and to assess the association between ocular syphilis and neurosyphilis. To the best of our knowledge, the AH and CSF levels of these chemokines have not previously been measured in patients with ocular syphilis.
An observational descriptive study was performed prospectively at Tygerberg Academic Hospital, Cape Town, South Africa, from 1 February 2018 till 31 January 2021. All patients presenting to the eye clinic with ocular syphilis were recruited if they were older than 18 years, had a positive serum T. pallidum antibodies (TPA) result, had an RPR titre of ≥8 and had confirmed ocular inflammation. Patients were excluded from this study if they declined to participate or had another identified cause for the uveitis. Written informed consent was obtained from all participants.
Permission for the study was granted by the Health Research Ethics Committee of the University of Stellenbosch (N13/10/146). Upon diagnosis of ocular syphilis, AH, serum and CSF samples were collected before the initiation of treatment. The HIV status of each patient was ascertained, if they were already on antiretroviral treatment they were also included in the study. All relevant clinical and demographic data were documented, and the patients were treated according to the CDC guidelines. All samples were barcoded and registered on an electronic database before being placed in an automated cryostorage system (Hamilton BIOS, Hamilton Storage GmbH, Bonaduz, Switzerland). Prior to analysis, the samples were removed from cryostorage, thawed, and analysed in a controlled environment. Biomarker concentrations were evaluated using the Luminex®XMAP technology platform. The Luminex® platform is a bead-based system and permits simultaneous analysis of several biomarkers in one specimen, based on the principles of flow cytometry. It delivers fast, cost-effective results with up to 500 analytes measurable in one microplate well.
The following biomarkers were measured using the R&D Human Magnetic Luminex Screening Assay kits (R&D Systems Inc, BioTechne, Minneapolis, USA): C-Reactive protein (CRP), CCL4 (MIP-1 beta), CCL24 (Eotaxin-2/MPIF-2), CXCL7 (NAP-2), CXCL10 (IP-10/CRG-2), IFN-gamma, IL-2, IL-6, IL-10, IL-12 p70, CXCL13 and CXCL8. Apo AI and Apo CIII concentrations were assessed using the APOMAG-62K Milliplex Map Human Apolipoprotein Magnetic Bead Panel (Merck Millipore [EMD Millipore] Billerica, MA, USA). For this study the levels of CXCL13, CXCL10 and CXCL8 were measured in the AH, CSF and serum.
Biomarker levels were compared between sources using mixed model ANOVA. In these analyses the patients were included as a random effect, and sample type, groupings like neurosyphilis (yes/no) were included as fixed effects. Normal probability plots were inspected for deviations from normality, and in suspect cases transformations were done but the results were similar to the untransformed analyses. Thus, only untransformed results were reported. For post hoc analyses Fisher least significant difference (LSD) were used.
A total of 23 patients with an age range of 22–56 years were included in our study. The most common clinical feature was anterior uveitis (11 patients, 47%), followed by panuveitis, optic neuropathy and intermediate uveitis. CSF features consistent with neurosyphilis were present in 56.5% (13 patients) of the participants, and 65.2% (15 patients) were also infected with HIV. (Table 1).
The mean concentrations of the 3 biomarkers were measured, and the levels in the eye, central nervous system and blood were compared.
For all 3 biomarkers the mean concentrations were higher in the AH and CSF than the serum (Figures 1–3). Even though the mean concentrations of all 3 chemokines were consistently higher in the AH than the CSF, the differences in mean concentrations between the AH and CSF were insignificant (CXCL13 p=0.39, CXCL10 p=0.06, CXCL8 p=0.13). In contrast, there were marked differences between the mean concentrations of all 3 biomarkers in the AH and serum (CXCL13 p=0.01, CXCL10 p<0.01, CXCL8 p<0.01). When comparing the mean values of all 3 biomarkers in the CSF and serum, there was a noticeable trend for the mean concentrations to be higher in the CSF than the serum (CXCL13 p=0.09, CXCL10 p=0.05, CXCL8 p=0.06).
When considering all patients with ocular syphilis, the overall AH/serum ratio was 3.33 for CXCL13, 84.07 for CXCL10 and 16.89 for CXCL8.
Participants were diagnosed with neurosyphilis using CSF test results according to the UpToDate guidelines for patients with and without HIV (20). Different algorithms are used to diagnose neurosyphilis depending on the HIV status of patients:
● A positive CSF VDRL result confirms the diagnosis in both groups.
● In HIV-positive patients:
o with a non-reactive CSF VDRL, a CSF lymphocyte count of >20 cells/μl suggests a diagnosis of probable neurosyphilis.
o If the CSF lymphocyte count is between 6–20 cells/μL, but the CD4 count is <200/μL, the patient is receiving antiretroviral (ARV) therapy and the HIV viral load is <50 copies/mL, the patient would be diagnosed with probable neurosyphilis.
o If any of the preceding criteria are negative, treatment for probable neurosyphilis should be initiated if the CSF fluorescent treponemal antibody-absorption test result is positive (20).
● In patients who tested negative for HIV with a negative VDRL result, a lymphocyte count of >5 cells/μL or CSF protein level >45 mg/dL suggests a diagnosis of probable neurosyphilis (20).
For participants with and without neurosyphilis, there were no significant differences in the mean concentrations of CXCL13 in the AH (p=0.8), serum (p=0.88) and CSF (p=0.52) (Figure 4). However, the mean CXCL13 concentration in the AH was higher than in the serum of participants with neurosyphilis (p=0.02), while there was only a trend for the mean CXCL13 concentration to be higher in the CSF than the serum in these participants (p=0.07) (Table 2).
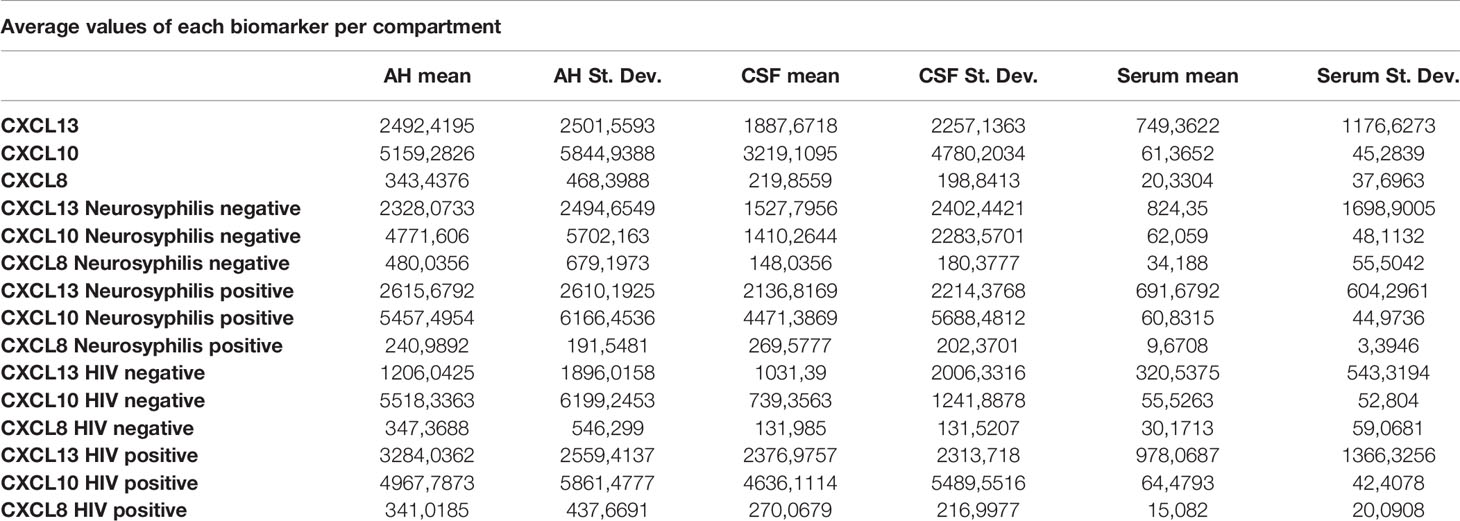
Table 2 Actual values of each individual chemokine for each compartment also showing values corrected for neurosyphilis and HIV status.
There were also no differences in the mean concentrations of CXCL10 in the AH (p=0.71), serum (p=1) and CSF (p=0.11) of participants both with and without neurosyphilis. However, in patients with neurosyphilis, the mean CXCL10 concentrations were higher in the AH (p<0.01) and in the CSF (p=0.01) than the serum (Figure 5 and Table 2).
The mean concentrations of CXCL8 did not differ between the CSF (p=0.33) and serum (p=0.84) of patients with and without neurosyphilis, but the CXCL8 levels trended higher in the AH of participants without neurosyphilis (p=0.06). The mean AH concentrations of CXCL8 in participants without neurosyphilis were also higher than serum concentrations in participants both with and without neurosyphilis (Figure 6 and Table 2).
In patients with ocular syphilis and neurosyphilis the AH/serum ratio for CXCL13, CXCL10 and CXCL8 was 3.78, 89.72 and 24.92 respectively while in patients with ocular syphilis without neurosyphilis the AH/serum ratio for CXCL13, CXCL10 and CXCL8 was 2.82, 76.89 and 14.04 respectively.
There was no significant difference in the mean CXCL13 concentrations in the CSF (p=0.13) and serum (p=0.45) between patients with and without HIV, but the CXCL13 levels were significantly higher in the AH of participants infected with HIV (p=0.02) (Figure 7). The mean concentrations of CXCL13 in the AH of participants infected with HIV were also higher than the serum concentrations in participants both with and without neurosyphilis (p<0.01) and the CSF concentrations of participants not infected with HIV (p=0.01) (Table 2).
The mean concentrations of CXCL10 in the AH (p=0.77) and serum (p=1.00) did not differ between participants with and without HIV, although there was a trend for CXCL10 levels to be higher in the CSF of participants infected with HIV (p=0.05) (Figure 8). The mean CSF concentration of CXCL10 was higher than the serum concentration of participants both with (p<0.01) and without (p=0.02) HIV, whereas the mean AH concentrations of CXCL10 in both groups were higher than the serum concentration of participants with (p=0.01) and without (p<0.01) HIV (Table 2).
The mean concentrations of CXCL8 did not differ between the AH (p=0.96), CSF (p=0.29) or serum (p=0.91) for participants both with and without HIV (Figure 9). The mean serum concentrations of CXCL8 in patients with HIV were markedly lower than the AH concentrations of participants with (p<0.01) and without (p=0.01) HIV, and the mean serum concentrations of CXCL8 in HIV negative patients were also lower than the AH concentrations of participants with (p=0.02) and without (p=0.04) HIV (Table 2).
Assessment of the mean chemokine levels of patients that presented with anterior uveitis, panuveitis, optic neuropathy and intermediate uveitis (Table 3) showed all groups except the patients with optic neuropathy to have significantly elevated levels of chemokines in the AH and CSF, especially when compared to the serum. All 4 groups had mean CSF CXCL13 levels above 250 pg/mL.
On analysis of the sample values of each patient (Table 4), 21 CSF samples (95%) had a CSF CXCL13 level of more than 10 pg/mL, and 12 samples (54%) had a CSF CXCL13 level of more than 250 pg/mL. For 11 of the 21 participants (50%) the CXCL13 CSF to serum ratio was more than 1.
Analysis of the CXCL13 AH to serum ratio showed 14 patients to have a ratio of more than 1 (71.4%). Not one of the three patients with optic neuropathy had a ratio of 1 or more. An increased ratio was evident in 89% of patients with anterior uveitis, 62% with panuveitis and in the single patient with intermediate uveitis.
Of the 21 available samples, 17 (81%) had AH CXCL13 levels of more than 250 pg/mL, with the lowest levels in the patients with optic neuritis. Except for 2 of the patients with optic neuropathy, all patients had an AH CXCL13 level of more than 10 pg/mL.
Marra et al. found that, compared to patients with uncomplicated syphilis, patients with neurosyphilis who also tested positive for HIV had increased CSF and serum concentrations of CXCL13 despite CSF pleocytosis (18). They found that using a CSF CXCL13 cut-off value of 10 pg/ml had a significantly higher sensitivity (sensitivity 90%; specificity 37%) for diagnosing symptomatic neurosyphilis than using VDRL tests. Using a CXCL13 cut-off value of 250 pg/ml (sensitivity 41%; and specificity 79%) or a CSF to serum ratio of >1 (sensitivity 58%; specificity 84%) was comparable to CSF VDRL testing (18). The CXCL13 concentrations in the CSF decline after treatment for neurosyphilis is initiated and can be used as an indicator for the response to treatment (18, 19, 21, 26).
Mothapo et al. measured the levels of CXCL13 in the CSF of patients with neurosyphilis and uncomplicated syphilis who tested positive or negative for HIV. They used a positive CSF rapid plasma reagin (RPR) test, and not the clinical symptoms, to diagnose neurosyphilis and found a CXCL13 cut-off value of 76.3 pg/ml to be 50% sensitive and 90% specific for diagnosing neurosyphilis (22). The use of CXCL13 as an adjunctive test for patients who tested HIV-negative has been shown to be valuable (22), with significantly elevated levels of CXCL13 in the CSF compared with the serum, indicating intrathecal CXCL13 production in patients with neurosyphilis (21, 25). Zeng et al. could not identify a cut-off value for serum CXCL13 concentrations to differentiate between neurosyphilis and other infectious (viral and cryptococcal) and non-infections central nervous system (CNS) pathology, but the CSF CXCL13 levels were markedly increased in the neurosyphilis group compared with the other groups (25).
Wang et al. identified 3 potential biomarkers for the diagnosis of neurosyphilis. These were CXCL13, CXCL10, also known as interferon-gamma-induced protein 10 (IP10) and CXCL8, also known as interleukin 8 (IL8). The chemokines CXCL8 and CXCL10 also belong to the CXC chemokine subfamily (19). CXCL10 is induced by IFN-γ and promotes adhesion of T-cells, attracts mononuclear cells and enhances innate immunity. CXCL8 enhances the inflammatory cascade involved in the breakdown of the blood brain barrier. Both CXCL10 and CXCL8 are inflammatory chemokines (19). Wang et al. investigated 36 chemokines in the CSF of patients with neurosyphilis who tested negative for HIV and compared it with that of patients with uncomplicated systemic syphilis. They found that CXCL13, CXCL10 and CXCL8 levels were significantly increased in the neurosyphilis group. The optimal cut-off values were 256.4 pg/ml, 163.1 pg/ml and 48.1 pg/ml for CXCL13, CXCL10 and CXCL8, respectively. Above these values CSF CXCL13 was 85.4% sensitive and 89.1% specific for diagnosing neurosyphilis, and CXCL10 and CXCL8 were both 79% sensitive, with a specificity of 90.1% and 91.1%, respectively (19). The sensitivity and specificity of the CSF to serum ratio of CXCL13, CXCL10 and CXCL8 correlated with these values (19). In addition, the decrease in the CSF levels of CXCL10 and CXCL8 was consistent with the decrease of CXCL13 levels after treatment for neurosyphilis was initiated (19).
In our study, the biomarkers CXCL13, CXCL10 and CXCL8 show a trend to be consistently elevated in the AH and CSF of patients with ocular syphilis, with no significant differences between these two compartments. This appears to confirm the relationship between ocular syphilis and neurosyphilis, showing that the intraocular fluid mirrors the intrathecal immunological biomarker milieu in ocular syphilis. The wide variability in the range of the levels and the small study group can exaggerate the mean values, and further studies are needed to confirm these findings.
There were marked differences in the mean concentrations of the 3 biomarkers in the AH and serum (CXCL13 p=0.01, CXCL10 p<0.01 and CXCL8 p<0.01), with higher levels in the AH than the serum. This may indicate that the eye does not merely reflect the serum chemokines that move across the blood-ocular barrier but rather that it is a site of inflammation that produces chemokines.
When comparing patients with and without neurosyphilis, the mean biomarker values, especially CXCL13, did not show significant differences.
The following statistically significant findings became apparent when we compared the group diagnosed as having neurosyphilis with the group diagnosed as not having neurosyphilis: The mean CXCL13 concentration was higher in the AH than the serum of participants with neurosyphilis (p=0.02). The mean CXCL10 concentrations were higher in the AH (p<0.01) and CSF (p=0.01) than in the serum of patients with neurosyphilis. The mean AH concentrations of CXCL8 in participants who were not diagnosed with neurosyphilis were higher than the serum concentrations in participants both with and without neurosyphilis.
In patients with ocular syphilis and neurosyphilis the AH/serum ratios for CXCL13, CXCL10 and CXCL8 were consistently higher than those in patients with ocular syphilis but no neurosyphilis. Further research is required to determine whether or not these ratios could potentially become useful in future in deciding whether someone with ocular syphilis may or may not have concurrent neurosyphilis. Using CXCL13 as an example, the overall AH/serum ratio is 3.33 whereas that for patients without concurrent neurosyphilis is only 2.82 and for patients with concurrent neurosyphilis it is 3.78. Similarly, the overall CSF/serum ratio for CXCL10 is 52.45 where for patients without neurosyphilis it is 22.72 and for those with neurosyphilis it is 73.51. Higher values of the ratio therefore appear to favour a diagnosis of neurosyphilis and lower values the opposite.
Patients with HIV showed elevated CXCL13, CXCL10 and CXCL8 levels when compared to patients without HIV. The mean CXCL10 levels of patients with HIV and without HIV were higher in the AH (p<0.01 and p=0.02) and CSF (p=0.01 and p<0.01) than in the serum. In patients with and without HIV the mean AH level were markedly elevated compared to the serum of HIV positive (p<0.01 and p=0.01) and HIV negative (p=0.02 and p=0.04) patients.
The same trend occurred when dividing the HIV-positive and HIV-negative groups into patients with and without neurosyphilis. The HIV-positive group had higher levels of CXCL13, CXCL10 and CXCL8, but this was not statistically influenced by the neurosyphilis status in these patients with ocular syphilis. The significance of this finding is limited by the small number of patients included in this study.
Using a CSF CXCL13 value of more than 250 pg/mL independently to make the diagnosis of neurosyphilis, would have resulted in 54% being classified as having neurosyphilis, which correlates with the 56.5% of the study sample who have been diagnosed with neurosyphilis. If a CSF CXCL13 level of more than 250 pg/mL was added as an additional criterion to the current UTD guidelines for diagnosing neurosyphilis, another four patients would have been classified as having neurosyphilis. The addition of CXCL13 measurements in the routine testing of our patients would, therefore, have made a difference in the classification of our patients to have neurosyphilis or not. Eighty-one per cent of the patients had AH CXCL13 levels of more than 250 pg/mL
The group of patients with optic neuropathy had the lowest level of AH CXCL13, which confirms the clinical diagnosis of an optic neuritis without intraocular inflammation (uveitis). The CSF results of one patient was inconsistent, with a negative FTA Abs for neurosyphilis, but with the other CSF tests indicating neurosyphilis (CSF CXCL13 level of 5600 pg/mL, 44 lymphocytes, 0.95 g/L protein, CXCL13 CSF to serum ratio of 614, serum RPR of 32). This raises the question whether this patient had a false negative CSF FTA Abs.
With the advances made in recent years regarding the diagnostic capabilities in ascertaining the causes of uveitis, we are moving away from idiopathic uveitis, with more patients receiving targeted treatment for an identified cause. We now propose that the diagnostic certainty for ocular syphilis can also be improved by analysing the AH as an adjunct to the serological testing. This can lead to better outcomes with targeted treatment, especially for patients with HIV, who can have several other causes for infectious uveitis and who also have a higher risk for systemic syphilis.
Although further controlled trials for groups with and without inflammation need to be performed to validate the finding of the biomarker levels in the AH, the above results have a twofold significance. Firstly, it shows that the chemokines measured in the AH of patients with ocular syphilis correlate to the values previously found in the CSF of patients with neurosyphilis, and that patients with ocular syphilis have elevated CSF levels of CXCL13, CXCL10 and CXCL8, as expressed in the mean values (Figures 1–3). The finding that more than 95% of our patients had a CSF CXCL13 concentration of more than 10 pg/mL also supports the guidelines of the CDC that all patients with ocular syphilis should be treated for neurosyphilis. Secondly, adjunctive analysis of the CXCL13, CXCL10 and CXCL8 levels in the AH of patients with uveitis can be of value in the diagnosis and treatment of uveitis, either as part of a routine workup and assessment of the response to treatment or to confirm or refute a diagnosis of ocular syphilis.
To the best of our knowledge, this is the first study that examined the chemokines CXCL13, CXCL10 and CXCL8 in the AH, CSF, and serum of patients with ocular syphilis. We suggest that the AH can be sampled in addition to testing the serum to confirm the diagnosis of ocular syphilis. This is a novel diagnostic approach for ocular syphilis, and further studies need to be performed to confirm the results and to establish the biomarker levels in normal control groups and patients with other uveitis aetiologies.
Our findings also show that the analysis of the chemokines CXCL13, CXCL10 and CXCL8 in the CSF can indicate the occurrence of neuroinflammation in patients who do not meet the criteria for being diagnosed with neurosyphilis. The addition of CXCL13 analysis in the routine testing of our patients would have resulted in 4 more patients being diagnosed with neurosyphilis. This supports the current guidelines by the CDC that all patients with ocular syphilis need to be treated with the neurosyphilis regime of 10–14 days of intravenous penicillin G.
CXCL13, CXCL10 and CXCL8 showed AH levels equivalent to previously reported levels in the CSF of patients with neurosyphilis and can potentially be an adjunct in the diagnosis and management of ocular syphilis. Patients with ocular syphilis diagnosed as not having neurosyphilis with conventional CSF testing showed features of neurosyphilis with analysis of the CSF chemokines. This supports the CDC guidelines that all patients with ocular syphilis should be treated with the neurosyphilis regime.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by the Health Research Ethics Committee of the University of Stellenbosch (N13/10/146). The patients/participants provided their written informed consent to participate in this study.
LM was responsible for patient consent, collection of samples and clinical information, literature review and preparation of the article. DS was responsible for conceptualisation of the research and editing of the final article. GW, CS and NC were responsible for analysing the samples and editing the final manuscript. MK was responsible for statistical analysis and interpretation as well as editing the final manuscript. All authors contributed to the article and approved the submitted version.
This research was funded exclusively by internal research funds of the Division of Ophthalmology, Faculty of Medicine and Health Sciences, Stellenbosch University, Cape Town, South Africa.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Ghanem K, Ram S, Rice P. The Modern Epidemic of Syphilis. NEJM (2020) 382(9):845–54. doi: 10.1056/NEJMra1901593
2. Bowen V, Davis D, Fleming M, Grey J, Grier L, Harvey A, et al.Sexually Transmitted Disease Surveillance, 2019. In: Centers for Disease Control and Prevention. Available at: https://www.cdc.gov/std/statistics/2019/default.htm.
3. Zhang T, Zhu Y, Xu G. Clinical Features and Treatments of Syphilitic Uveitis: A Systematic Review and Meta-Analysis. J Ophthalmol (2017) 2017:1–15. doi: 10.1155/2017/6594849
4. Doris J, Saha K, Jones N, Sukthanka A. Ocular Syphilis: The New Epidemic. Eye (2005) 20(6):703–5. doi: 10.1038/sj.eye.6701954
5. Hughes E, Guzowski M, Simunovic M, Hunyor A, McCluskey P. Syphilitic Retinitis and Uveitis in HIV-Positive Adults. Clin Exp Ophthalmol (2010) 38(9):851–6. doi: 10.1111/j.1442-9071.2010.02383.x
6. Eslami M, Noureddin G, Pakzad-Vaezi K, Warner S, Grennan T. Resurgence of Ocular Syphilis in British Columbia Between 2013–2016: A Retrospective Chart Review. Can J Ophthalmol (2020) 55(2):179–84. doi: 10.1016/j.jcjo.2019.11.002
7. Oliver G, Stathis R, Furtado J, Arantes T, McCluskey P, Matthews J, et al. Current Ophthalmology Practice Patterns for Syphilitic Uveitis. Br J Ophthalmol (2019) 103(11):1645–9. doi: 10.1136/bjophthalmol-2018-313207
8. Schulz D, Orr S, Johnstone, Devlin M, Sheidow T, Bursztyn L, et al. The Many Faces of Ocular Syphilis: Case-Based Update on Recognition, Diagnosis, and Treatment. Can J Ophthalmol (2021) 56(5):283–93. doi: 10.1016/j.jcjo.2021.01.006
9. Rautenbach W, Steffen J, Smit D, Lecuona K, Esterhuizen T. Patterns of Uveitis at Two University-Based Referral Centres in Cape Town, South Africa. Ocul Immunol Inflamm (2017) 27(6):868–74. doi: 10.1080/09273948.2017.1391954
10. Smit D, Esterhuizen T, Meyer D, de Boer J, de Groot-Mijnes J. The Etiology of Intraocular Inflammation in HIV Positive and HIV Negative Adults at a Tertiary Hospital in Cape Town, South Africa. Ocul Immunol Inflamm (2018) 27(2):203–10. doi: 10.1080/09273948.2018.1476555
11. Schaftenaar E, Meenken C, Baarsma G, Khosa N, Luijendijk A, McIntyre J, et al. Uveitis Is Predominantly of Infectious Origin in a High HIV and TB Prevalence Setting in Rural South Africa. Br J Ophthalmol (2016) 100(10):1312–6. doi: 10.1136/bjophthalmol-2016-308645
12. Aldave A, King J, Cunningham E. Ocular Syphilis. Curr Opin Ophthalmol (2001) 12(6):433–41. doi: 10.1097/00055735-200112000-00008
13. Smit D, De Graaf M, Meyer D, de Groot-Mijnes J. Immunoblot and Polymerase Chain Reaction to Diagnose Ocular Syphilis and Neurosyphilis in HIV-Positive and HIV-Negative Patients. Ocul Immunol Inflamm (2020) 28(7):1049–55. doi: 10.1080/09273948.2019.1698753
14. Workowski K, Bachmann L, Chan P, Johnston C, Muzny C, Park I, et al. STI Treatment Guidelines. In: Centers for Disease Control and Prevention. Available at: https://www.cdc.gov/mmwr/volumes/70/rr/rr7004a1.htm.
15. Troutbeck R, Chhabra R, Jones N. Polymerase Chain Reaction Testing of Vitreous in Atypical Ocular Syphilis. Ocul Immunol Inflamm (2013) 21(3):227–30. doi: 10.3109/09273948.2013.770887
16. Cornut P, Sobas C, Perard L, De Bats F, Salord H, Manificat H, et al. Detection of Treponema Pallidum in Aqueous Humor by Real-Time Polymerase Chain Reaction. Ocul Immunol Inflamm (2011) 19(2):127–8. doi: 10.3109/09273948.2010.531175
17. Heymans R, van der Helm J, De Vries H, Fennema H, Coutinho R, Bruisten S, et al. Clinical Value of Treponema Pallidum Real-Time PCR for Diagnosis of Syphilis. J Clin Microbiol (2010) 48(2). doi: 10.1128/JCM.00720-09
18. Marra C, Tantalo L, Sahi S, Maxwell C, Lukehart S. CXCL13 as a Cerebrospinal Fluid Marker for Neurosyphilis in HIV-Infected Patients With Syphilis. Sex Transm Dis (2010) 37(5):283–7. doi: 10.1097/OLQ.0b013e3181d877a1
19. Wang C, Wu K, Yu Q, Zhang S, Gao Z, Liu Y, et al. CXCL13, CXCL10 and CXCL8 as Potential Biomarkers for the Diagnosis of Neurosyphilis Patients. Sci Rep (2016). doi: 10.1038/srep33569
20. Marra C, Gonzalez-Scarano F. Neurosyphilis: Diagnostic Algorithims. Available at: https://www.uptodate.com.
21. Yan Y, Wang J, Qu B, Zhang Y, Wei Y, Liu H, et al. CXCL13 and TH1/Th2 Cytokines in the Serum and Cerebrospinal Fluid of Neurosyphilis Patients. Medicine (2017) 96(47):e8850. doi: 10.1097/MD.0000000000008850
22. Mothapo K, Verbeek M, van der Velden L, Ang C, Koopmans P, van der Ven A, et al. Has CXCL13 an Added Value in Diagnosis of Neurosyphilis? J Clin Microbiol (2015) 53(5):1693–6. doi: 10.1128/JCM.02917-14
23. Kowarik M, Cepok S, Sellner J, Grummel V, Weber M, Korn T, et al. CXCL13 Is the Major Determinant for B Cell Recruitment to the CSF During Neuroinflammation. J Neuroinflam (2012) 9(1). doi: 10.1186/1742-2094-9-93
24. Hu R, Lu C, Lu S, Hu Y, Ma H, Lai W, et al. Value of CXCL13 in Diagnosing Asymptomatic Neurosyphilis in HIV-Infected Patients. Int J STD AIDS (2015) 27(2):141–6. doi: 10.1177/0956462415577229
25. Zeng Y, Lin Y, Zhang N, Zou C, Zhang H, Peng F, et al. CXCL13 Chemokine as a Promising Biomarker to Diagnose Neurosyphilis in HIV-Negative Patients. SpringerPlus (2016) 5(1). doi: 10.1186/s40064-016-2462-4
26. Dersch R, Hottenrott T, Senel M, Lehmensiek V, Tumani H, Rauer S, et al. The Chemokine CXCL13 is Elevated in the Cerebrospinal Fluid of Patients With Neurosyphilis. Fluids Barriers CNS (2015) 12(1). doi: 10.1186/s12987-015-0008-8
Keywords: CXCL13, CXCL10, CXCL8, neurosyphilis, ocular syphilis, uveitis
Citation: van der Merwe LW, Snyders C, Kidd M, Chegou NN, Walzl G and Smit DP (2022) CXCL13, CXCL10 and CXCL8 as Indicators of Ocular and Neurological Involvement in Patients With Ocular Syphilis: An Observational Descriptive Study. Front. Ophthalmol. 2:916718. doi: 10.3389/fopht.2022.916718
Received: 09 April 2022; Accepted: 24 May 2022;
Published: 27 June 2022.
Edited by:
Daniel Vitor Vasconcelos-Santos, Universidade Federal de Minas Gerais, BrazilReviewed by:
Padmaja Sudhakar, University of Kentucky, United StatesCopyright © 2022 van der Merwe, Snyders, Kidd, Chegou, Walzl and Smit. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Laurie W. van der Merwe, bGF1cmlld2lpZC52ZG1lcndlQGdtYWlsLmNvbQ==
†ORCID: Laurie van der Merwe, orcid.org/0000-0002-4437-5248
Derrick Smit, orcid.org/0000-0003-3206-8184
Candice Snyders, orcid.org/0000-0002-8571-6029
Gerhard Walzl, orcid.org/0000-0003-2487-125X
Novel Chegou, orcid.org/0000-0002-8690-1699
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.