- Prof Brien Holden Eye Research Centre, LV Prasad Eye Institute, LV Prasad Marg, Hyderabad, India
Ocular tuberculosis (TB) is frequently considered as intraocular inflammation in the setting of latent TB, owing mainly to the absence of microbiological evidence of Mycobacterium tuberculosis in ocular fluid samples. Even though such lack of microbiological evidence, and of systemic signs of active TB disease, are suggestive of latent TB infection, molecular and rare histopathologic evidence of mycobacteria in the eye, and favourable response of ocular inflammation to anti-TB therapy point to the presence of active infection in ocular TB. Here, we discuss how intraocular inflammation in ocular TB is not merely an immunologic response to bacilli, but an active tuberculosis infection. We will discuss the reason for the frequent absence of microbiological evidence of TB in the eye in ocular TB and the diagnostic hierarchy to arrive at the diagnosis of this infectious uveitis entity.
Introduction
Let us think of two of the greatest scientific theories in human history – the theory of evolution and the Big Bang theory. While there are several pointers to their existence, there is no direct proof for either of them. Yet, scientists across the world continue to believe in them and are engaged in cutting edge research based on these theories. It would be reasonable to state, that in science, the absence of evidence does not always imply the evidence of absence. Although it may appear counterintuitive, the above aphorism can be applied to the practice of evidence-based medicine (EBM) too. This assumption is based on the fact that EBM is not merely “cookbook” medicine based on a compendium of irrefutable evidence (1). Instead, EBM requires the integration of clinical expertise with the “best available external evidence”. The external evidence can be drawn from the basic sciences, and from studies on diagnostic accuracy and therapeutic efficacy. In this way, the absence of evidence such as randomized clinical trial does not necessarily amount to the evidence of absence in the practice of EBM.
One condition where the above reasoning can find application in ophthalmology is ocular tuberculosis (TB). It’s causative organism, Mycobacterium tuberculosis (Mtb) was first identified in the eye in 1883, only a year after Robert Koch first discovered the bacillus (2). Yet ophthalmologists continue to associate the clinical manifestations of ocular TB with latent Mtb infection in the patient (3–5). It is assumed that the absence of microbiological evidence of Mtb infection in the ocular fluids of these patients, is sufficient evidence for the absence of mycobacterial infection in these eyes. Put differently, the absence of evidence becomes the evidence of absence. Since these patients have immunological and/or radiological evidence of systemic TB infection, but no active pulmonary TB, it is also assumed that the manifestations of ocular TB are associated with latent TB infection. This has led to a unique situation; wherein ocular TB is possibly the only human infectious disease that is associated with a latent infection.
In this perspective, we will first understand the divergence between the concepts of ocular TB and latent TB, and how intraocular inflammation could be induced by Mtb despite its absence in ocular fluids. We will then analyze how ocular TB [and other extrapulmonary TB (EPTB)] are not always associated with active pulmonary TB (PTB). Finally, we will focus on the tests for TB immunoreactivity – tuberculin skin test (TST) and interferon gamma release assays (IGRA) – and discuss ways to interpret them meaningfully for the diagnosis of ocular TB. Some of the terms that have been used frequently in this review have been explained in Box 1.
What is Ocular TB?
The past two decades have seen a significant evolution in our understanding of ocular TB. During this period, ocular TB has been defined by clinical criteria that include characteristic ocular signs, ancillary evidence of systemic TB infection (immunological or radiological tests) and the exclusion of non-TB entities, and not necessarily histopathological or microbiological evidence of TB (6, 7). In broad terms, this definition has been endorsed by the multinational Collaborative Ocular Tuberculosis Study (COTS) (8), as well as the Standardization of Uveitis Nomenclature (SUN) Working Group (9). The range of ocular signs identified as ocular TB is wider and more inclusive in diagnostic criteria (such as the COTS), while it is more restricted in the classification criteria recently published by the SUN Working Group. In addition, the COTS group has termed the disease as “ocular TB” to connect the anatomical structure involved (eye) with the pathogen (Mtb) that requires specific anti-microbial therapy.
Despite the availability of these nomenclature and diagnostic criteria, alternative definitions of ocular TB continue to appear in ophthalmic literature from time to time. The terms “TB-uveitis” or “TB-associated uveitis” have been used selectively for patients with uveitis and active systemic TB, or when the intraocular inflammation resolves with anti-TB therapy (ATT) alone (12, 13). ‘Active’ ocular TB has been used in the context of patients microbiological or polymerase chain reaction (PCR) evidence of Mtb in ocular fluids (13). The remaining patients, who comprise the majority, and neither have active systemic TB nor any evidence of Mtb in ocular fluids, have been labelled as uveitis associated with latent TB infection (3–5, 12, 13).
Latent TB Infection and its Incompatibility With Ocular TB
The World Health Organization (WHO) defines latent TB as a state of persistent immune response to stimulation by mycobacterial antigens without evidence of clinically active manifest TB (10). It makes no reference to the biological state of the organism within the host. If we consider microbiologically proven TB alone as “active manifest TB”, then most ocular TB patients who present only with immunological evidence of TB, and not microbiological evidence, will be labelled as latent TB. However, this assumption misses on several important facts that are not congruent with latent infection. Firstly, ocular TB frequently resolves (or does not recur) following treatment with ATT indicating the presence of actively proliferating mycobacteria (3–5). Secondly, granulomatous inflammation and molecular/microbiologic evidence of Mtb that have been reported in various clinical subtypes of ocular TB (14), are representative of the immune reaction against Mtb bacilli and cannot be explained by latent TB infection.
The final argument against latent TB infection in ocular TB is related to the current understanding of the bacterial state in latent infection. The standard narrative for TB has been that a third of the world’s population has latent infection with Mtb, and 5-10% of those infected develop disease due to ‘reactivation’ of the latent infection (10). The latent infection is defined by TB immunoreactivity – TST or IGRA (in the absence of clinical disease) – and it is therefore convenient to label anyone with positive TST/IGRA results and no ‘obvious’ TB disease as latent TB. This narrative has now been challenged through literature review of epidemiological data, from the pre- and post-antibiotic eras (11, 15). It has been shown that most TB disease occurs within 18-24 months of infection, and there is no special bacterial state (such as dormancy) during the asymptomatic phase of TB. In fact, in both latent (asymptomatic) and active TB, the organisms appear to be a mix of active and non-replicating bacteria, and the metabolic state during latency is like the replicative state (16). In summary, the presence of ATT-responsive intraocular inflammation, and the timelines of progression from Mtb infection to disease, effectively rule out any role of latent infection in the pathogenesis of ocular TB.
It needs to be emphasized here that the presence of TB immunoreactivity due to memory T-cells does not necessarily indicate presence of viable bacteria in the body. Memory T-cells are maintained by a slow, but steady process of self-renewal that is not antigen-dependent (17). While these cells may themselves be relatively short lived (30-160 days), the immunologic memory tends to last longer (half-life of 8-15 years) (18). The implication for ocular TB is that the mere presence of positive TST/IGRA tests does not guarantee the presence of viable Mtb in the patient. Additionally, the elimination of bacteria by the host immune response or by ATT will not influence the TST/IGRA outcomes in the patient.
Possible Mechanisms of Ocular TB in the ‘Absence’ of Microbiological Evidence of Mtb Infection
There are several explanations to the absence of Mtb in the ocular fluids, and of active systemic TB, in most cases diagnosed as ocular TB. The best explanation for both the above observations can be obtained from the available histopathological studies of ocular TB specimens. Here are key points that emerged from a clinicopathological study of enucleated eyes from 42 patients that were histopathologically proven to be ocular TB (19).
1. Acid fast organisms were found in 37 of 42 specimens in ocular or ocular adnexal structures. The ocular specimens were typically paucibacterial with only 1-2 organisms noted in the entire specimen, mostly in the vicinity of giant cells or an area of necrosis.
2. Nine of 42 also had Mtb in other organs, four of which were only in extrapulmonary organs.
3. Seven (40%) of the 17 available tuberculin skin test (TST) reports were negative, while eight (57%) of 14 chest radiographs were normal.
These data clearly explain why microbiological or even molecular evidence of Mtb is rarely found in aqueous or vitreous samples of patients with ocular TB. If the organism is so sparse in the ocular tissues, it would be even more unlikely in the adjacent ocular fluids. Additionally, the blood retinal barriers (even if partially disrupted due to the inflammation) would restrict the passage of organisms from the underlying tissues into the ocular fluids.
An obvious question that emerges from this data is what drives the widespread inflammatory response in the eye, if such few Mtb are present in ocular tissues. Current evidence points at two possible mechanisms that are not directly related to actively replicating Mtb. The first one is autoimmunity against retinal antigens. Flow cytometric studies of vitreous T-cells of patients with ocular TB, have revealed a highly pro-inflammatory cytokine response against retinal autoantigens, apart from that against Mtb antigens (20). How Mtb infection triggers the autoimmune response in the eye is not clear, but it is likely that the autoimmunity augments the inflammatory response in the eye. The role of autoimmunity has also been highlighted in TB affecting other organs, including pulmonary TB (21). For example, in the lungs, the extensive pathology far outweighs the number of bacteria found in the tissues.
The second possible mechanism is the inflammation induced by bacterial products. There are several indications to the existence of this mechanism. In experiments performed nearly a century ago, injection of heat-killed Mtb into internal carotid arteries of rabbits could induce inflammation in nearly all ocular tissues (22). Furthermore, heat-killed Mtb has been used as component of Complete Freund’s Adjuvant, along with retinal autoantigens for inducing experimental autoimmune uveitis (23). More recently, in vitro and animal studies have demonstrated innate immune response in the retinal pigment epithelium to mycobacterial antigen and double-stranded RNA (24). Together, it appears that replicating Mtb, even if present in numbers too few to be detected in the ocular fluids, can be supported by a retinal autoimmune response, and by bacterial products, in inducing intraocular inflammation.
Absence of Systemic TB in Patients With Ocular TB
Since microbiological evidence of ocular TB infection is rarely found, an evidence of systemic TB infection, is critical to the diagnosis of ocular TB. This could either be an active TB disease (PTB or EPTB), or as in most cases, immunoreactivity to TB antigens. As noted above, even in histopathologically-proven ocular TB, active systemic TB may not be present in all cases (19). This phenomenon is not unique to ocular TB alone. Even among other forms of EPTB, PTB has not been found in more than half the cases (25). Hence the question arises, how does ocular TB, or any other EPTB, occur in the absence of pulmonary disease. The answer probably lies in the extrapulmonary niches of Mtb infection that exist in nearly every organ of the body (26). These include both professional phagocytic cells and other intracellular niches, present in different organs. Among these, the bone marrow stem cells (mesenchymal and hemopoietic) are of particular interest, since they not only harbor the infection, but also disseminate it to other parts of the body. In the eye, the retinal pigment epithelial (RPE) cells may have a significant role, since they can phagocytose Mtb, to a similar extent as macrophages (27). Mtb-laden RPE cells survive longer in the presence of Mtb as compared to traditional phagocytes, thus becoming ideal reservoirs of the infection in the eye (27). To summarize, Mtb can exist and disseminate within the host, in the absence of pulmonary disease. Absence of PTB in a suspected case of ocular TB should not deter the diagnosis, rather it should stimulate a search for EPTB elsewhere in the body. The relative importance of each of the types of evidence of Mtb infection that are used in diagnosis of ocular TB is given in Box 2.
How do we Interpret the Available Evidence for the Diagnosis of Ocular TB?
The supportive evidences for the diagnosis of ocular TB (Box 2) follow a hierarchy, whereby the strongest possible evidence is also the rarest in clinical practice. In this hierarchy, microbiological evidence of Mtb in ocular tissue or fluid samples remains the strongest, yet the rarest association of mycobacterial infection with intraocular inflammation. Similarly, the amplification of mycobacterial DNA from ocular samples, or the presence of active PTB or EPTB in patients with relevant ocular signs, are diagnostic of ocular TB, unless there is clear evidence for an alternative diagnosis. However, in clinical practice, the immune response to Mtb antigens as tested by TST (in vivo) or IGRA (in vitro) remains the most commonly used evidence, for the diagnosis of ocular TB. These tests measure the memory T-cell response to Mtb, that follows the development of adaptive immunity against the organism. However, neither test is able to distinguish between infection and active disease, or between present and past infection. As noted above, the presence of TB immunoreactivity does not necessarily confirm continued TB infection in the body (15). Hence, the utility of these tests in the diagnosis of ocular TB is closely linked to the context in which they are performed. This includes the presence of appropriate clinical signs and exclusion of non-TB entities. In mathematical terms, the value of the context in which a diagnostic test is applied is measured by the Bayes’ theorem (31). With this theorem, the pre-test probability of the disease (prevalence in general population), and the sensitivity and specificity of that test, are used to calculate the post-test probability of having a disease after the test is performed.
What might be the impact of the Bayes’ theorem on the interpretation of TB immunoreactivity for the diagnosis of ocular TB? Firstly, it means that these tests cannot be used in isolation for the screening of ocular TB, especially in low endemic populations. For example, a study from the United States showed that the post-test probability of ocular TB is only 1% in a patient with uveitis and a positive TST (32). However, the post-test probability increased to 30.3%, when TST was used for screening foreign-born patients for ocular TB in the United States (33). In high endemic populations too, the clinical presentation will influence the pre-test probability and thereby the post-test probability of ocular TB. For example, in a patient with non-granulomatous anterior uveitis with inflammatory joint pain, or with bilateral exudative retinal detachment suggestive of Vogt-Koyanagi-Harada disease, a positive TST will be of little value even in a TB-endemic country. Conversely, a higher pre-test probability of TB disease will lower the cut-off value for a positive test. Thus the Centre for Disease Control recommends a TST cut-off value of 15 mm of induration for non-endemic countries and 10 mm for TB-endemic countries (34). The cut off is even lower at 5 mm if there is a history of recent TB contact, previous TB disease or immunosuppression (e.g. HIV, organ transplantation). It will be interesting to investigate if this analogy can be applied to highly predictive signs of ocular TB such as serpiginous-like choroiditis, to lower the cut-off value in a given population. Table 1 highlights some of the common myths and facts associated with TB immunoreactivity tests.
A more difficult situation arises when the TB immunoreactivity tests are negative despite the tell-tale signs of TB in the eye. This can be a challenge since these tests are often the only link connecting the ocular disease to TB infection. It has been demonstrated that even in culture confirmed PTB, 10-40% of HIV-negative individuals could have negative TST/IGRA test (42). A meta-analysis of published literature revealed that IGRAs have a sensitivity of ~70-90% for the diagnosis of active TB disease, and it may be still lower in high endemic settings, and with advanced age (43). Furthermore, in CNS TB, up to two thirds of possible TB cases could be IGRA negative (29). Hence, it is possible that a significant number of ocular TB patients will also test negative for TB immunoreactivity. Figure 1 describes one such case that was misdiagnosed on the basis of a negative TST result at initial presentation. In such situations, one option could be testing both TST and IGRA in every ocular TB suspect, as recommended by the COTS guidelines (44, 45). Those who test negative for both TST and IGRA despite strong clinical suspicion, should be followed up closely for persistent or recurrent inflammation, and retested at appropriate timepoints.
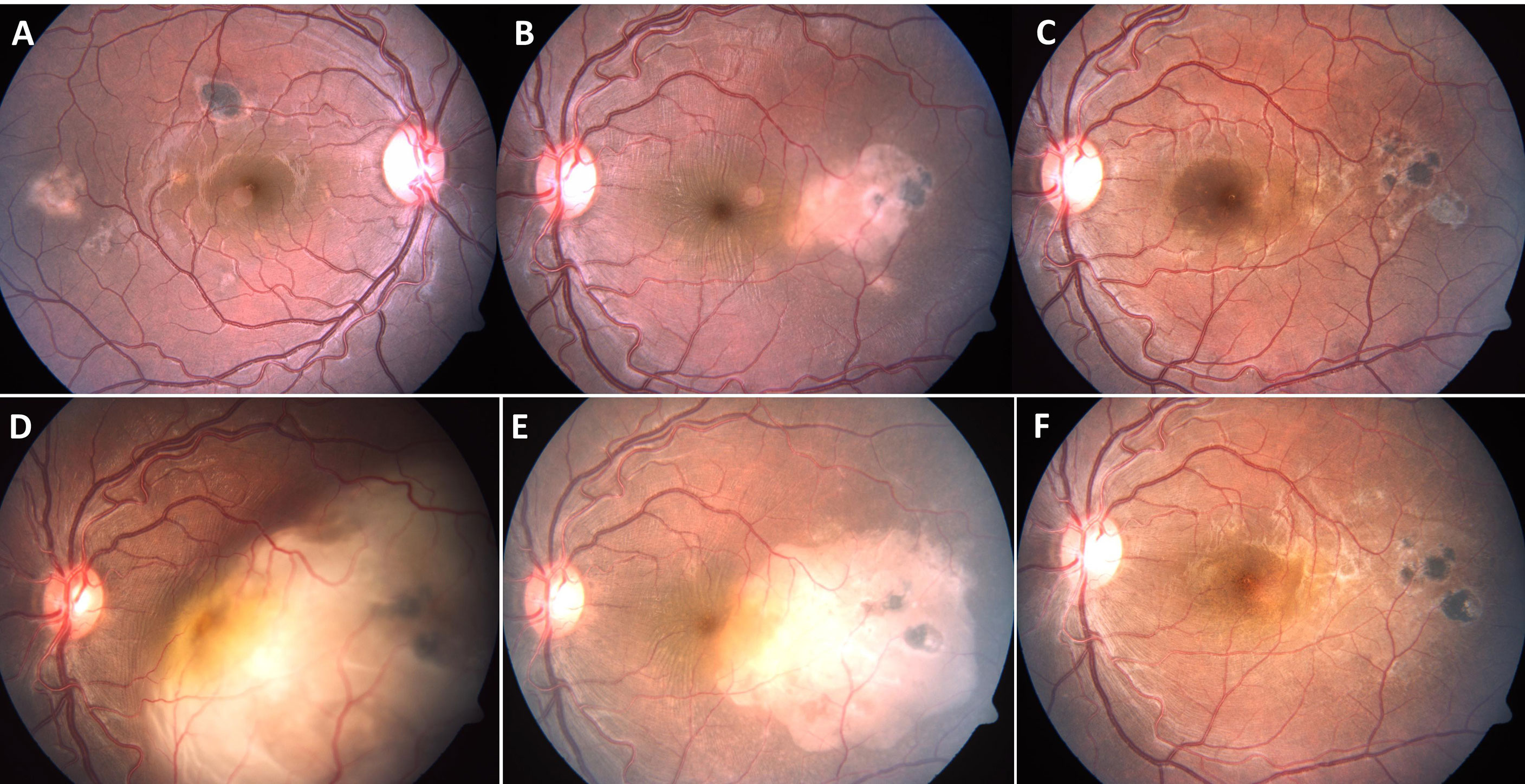
Figure 1 A 28-year-old immunocompetent male presented with yellowish white subretinal lesions temporal to macula in both eyes (A, B). The patient had negative tuberculin skin test, chest X-ray and tests for syphilis. He was treated with a course of oral corticosteroids, tapered over four months. The lesions resolved completely in both eyes (C), left eye). The patient presented eight months after completion of treatment with rapid loss of vision in left eye. The left eye fundus showed large choroidal granuloma, eight disc diameters in size, with overlying serous detachment (D). The right eye did not have any active lesions. The patient, a migrant labour, reported tuberculosis (TB) contact two years ago. He was not further investigated (though warranted) and started on four drug anti-TB therapy (without any corticosteroids). There was marked decrease in size of the lesion within just two weeks of therapy (E), and near total resolution of the lesion, after five weeks (F).
Way Forward
Ocular TB is likely to remain a clinical diagnosis, at least in the near future, despite ongoing research on the molecular diagnosis of this condition. This is not unusual for diseases that lack a gold standard (46). For example, case definitions based on composite clinical criteria are routinely used in other paucibacillary forms of TB such as childhood and central nervous system TB (47, 48). The primary goal of these criteria is to ensure early recognition of the disease and timely initiation of ATT. The recent COTS consensus guidelines for the initiation of ATT in various forms of ocular TB, are a step in that direction (44, 45). Additional studies are required to validate the role of ATT prospectively and to determine the appropriate duration of ATT. There is also a need to differentiate between different clinical phenotypes of ocular TB, based on morphological appearance (e.g., choroidal granuloma, serpiginous-like choroiditis and retinal vasculitis) or between acute, chronic and recurrent disease. It is possible that each of these could have different pathomechanisms and therefore, different diagnostic and therapeutic requirements.
In conclusion, ocular TB and latent TB infection are mutually exclusive entities. The former is an ocular inflammatory disease requiring anti-microbial therapy, and the latter, cannot have any clinical symptoms – in the lungs or elsewhere in the body. Yet the marker of latent TB infection – TB immunoreactivity, for reasons described above, is critical to the diagnosis of ocular TB. The tests for TB immunoreactivity – TST and IGRA, should however be interpreted in the context of the clinical scenario.
Author Contributions
SB was responsible for the conception, design, and writing of the manuscript.
Funding
DBT-Wellcome Trust India Alliance Intermediate Fellowship in Clinical and Public Health Research (#IA/CPHI/18/1/503975); Hyderabad Eye Research Foundation.
Conflict of Interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Handling editor NR declared a past co-authorship with the author SB.
Box 1. Glossary of terms (non-alphabetical).
TB immunoreactivity: Indirect evidence of present or past infection with Mycobacterium tuberculosis (Mtb) as inferred by a detectable adaptive immune response to Mtb antigens (on tuberculin skin test or interferon gamma release assay) (6).
Latent TB: State of persistent immune response to stimulation by mycobacterial antigens without evidence of clinically active manifest TB – in the lungs, or any other extrapulmonary organ (including the eye) (10).
Active TB: Evidence of progressive disease of the lung and/or other organs generally accompanied by a positive culture for Mtb and/or roentgenographic findings and/or histopathology consistent with TB (11).
Systemic infection: Ongoing Mtb infection in lungs and/or extrapulmonary organs (excluding the eye for the purpose of this discussion) that may or may not be associated with clinically active manifest disease.
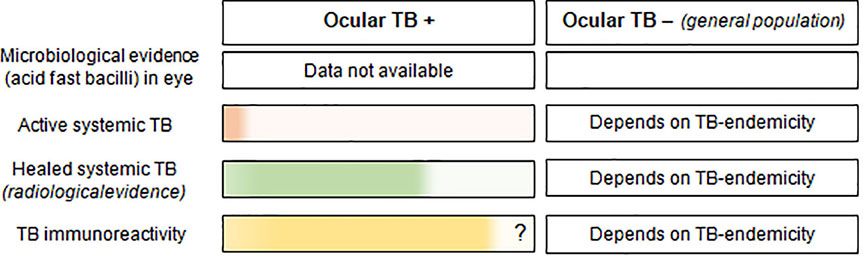
Box 2. Proportion of different types of evidence of Mycobacterium tuberculosis infection (in color), available in in patients with and without ocular TB disease.
Explanation
1. Microbiological evidence in the eye: There is no real epidemiological data available on the proportion of patients with microbiological evidence of acid-fast bacilli in ocular tissues, since this investigation is rarely done in patients with ocular TB.
2. Active systemic TB (including both pulmonary and extra-pulmonary TB): This data is drawn from the Collaborative Ocular TB Study (COTS) in which symptoms suggestive of TB (chronic cough, hemoptysis, weight loss and night sweats) were found in only 8% of patients (28). An additional 23.3% had a past history of pulmonary or extrapulmonary TB.
3. Healed systemic TB: Again, drawing from the COTS data, healed or inactive pulmonary TB was found on chest computed tomography scans in 68.6% patients (28). The number of ocular TB patients with healed/inactive extra-pulmonary TB remains unknown.
4. TB immunoreactivity: While a positive TB immunoreactivity test is currently considered sine qua non for the diagnosis of ocular TB, a significant number of true ocular TB patients (data unavailable) may test negative (Figure 1). 28.8% of patients with extrapulmonary TB tested negative for interferon gamma release assay (IGRA) (29), and 40% of histopathologically proven ocular TB patients had negative tuberculin test (19). Conversely, many patients with non-TB uveitis can have positive TB immunoreactivity, the number depending on TB endemicity in the population. When tested in all uveitis patients, the IGRA positivity ranged from 14.4% in the United States (13), to 36% in Thailand (30), in both cases being higher than the IGRA positivity in the respective general populations. Thus, a significant number of patients without ocular TB, can test positive for TB-immunoreactivity.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
Sanchita Mitra (for comments on the manuscript).
References
1. Sackett DL, Rosenberg WM, Gray JA, Haynes RB, Richardson WS. Evidence Based Medicine: What It Is and What it Isn’t. BMJ (1996) 312(7023):71–2. doi: 10.1136/bmj.312.7023.71
2. von Michel J. Über Iris Und Iritis. Albrecht Von Graefes Arch Klin Exp Ophthalmol (1881) 27:171–282. doi: 10.1007/BF01693194
3. Bansal R, Gupta A, Gupta V, Dogra MR, Bambery P, Arora SK. Role of Anti-Tubercular Therapy in Uveitis With Latent/Manifest Tuberculosis. Am J Ophthalmol (2008) 146(5):772–9. doi: 10.1016/j.ajo.2008.06.011
4. Ang M, Hedayatfar A, Wong W, Chee SP. Duration of Anti-Tubercular Therapy in Uveitis Associated With Latent Tuberculosis: A Case-Control Study. Br J Ophthalmol (2012) 96(3):332–6. doi: 10.1136/bjophthalmol-2011-300209
5. Tomkins-Netzer O, Leong BCS, Zhang X, Lightman S, McCluskey PJ. Sydney-London Latent Ocular TB Study GroupEffect of Antituberculous Therapy on Uveitis Associated With Latent Tuberculosis. Am J Ophthalmol (2018) 190:164–70. doi: 10.1016/j.ajo.2018.03.032
6. Gupta V, Gupta A, Rao NA. Intraocular Tuberculosis–an Update. Surv Ophthalmol (2007) 52(6):561–87. doi: 10.1016/j.survophthal.2007.08.015
7. Gupta A, Sharma A, Bansal R, Sharma K. Classification of Intraocular Tuberculosis. Ocul Immunol Inflamm (2015) 23(1):7–13. doi: 10.3109/09273948.2014.967358
8. Agrawal R, Agarwal A, Jabs DA, Kee A, Testi I, Mahajan S, et al. Standardization of Nomenclature for Ocular Tuberculosis - Results of Collaborative Ocular Tuberculosis Study (COTS) Workshop. Ocul Immunol Inflamm (2019) 10:1–11. doi: 10.1080/09273948.2019.1653933
9. Standardization of Uveitis Nomenclature (SUN) Working Group. Classification Criteria for Tubercular Uveitis. Am J Ophthalmol (2021) 228:142–51. doi: 10.1016/j.ajo.2021.03.040
10. World Health Organization. Latent Tuberculosis Infection: Updated and Consolidated Guidelines for Programmatic Management. World Health Organization (2018) https://apps.who.int/iris/handle/10665/260233.
11. Behr MA, Edelstein PH, Ramakrishnan L. Revisiting the Timetable of Tuberculosis. BMJ (2018) 362:k2738. doi: 10.1136/bmj.k2738
12. Groen-Hakan F, van Laar JAM, Bakker M, van Hagen PM, Hardjosantoso H, Rothova A. Prevalence of Positive QuantiFERON-TB Gold In-Tube Test in Uveitis and its Clinical Implications in a Country Nonendemic for Tuberculosis. Am J Ophthalmol (2020) 211:151–8. doi: 10.1016/j.ajo.2019.11.009
13. Yakin M, Kesav N, Cheng SK, Caplash S, Gangaputra S, Sen HN. The Association Between QuantiFERON-TB Gold Test and Clinical Manifestations of Uveitis in the United States. Am J Ophthalmol (2021) 230:181–7. doi: 10.1016/j.ajo.2021.04.024
14. Basu S, Elkington P, Rao NA. Pathogenesis of Ocular Tuberculosis: New Observations and Future Directions. Tuberculosis (Edinb) (2020) 124:101961. doi: 10.1016/j.tube.2020.101961
15. Behr MA, Edelstein PH, Ramakrishnan L. Is Mycobacterium Tuberculosis Infection Life Long? BMJ (2019) 367:l5770. doi: 10.1136/bmj.l5770
16. Dutta NK, Karakousis PC. Latent Tuberculosis Infection: Myths, Models, and Molecular Mechanisms. Microbiol Mol Biol Rev (2014) 78(3):343–71. doi: 10.1128/MMBR.00010-14
17. Urdahl KB, Shafiani S, Ernst JD. Initiation and Regulation of T-Cell Responses in Tuberculosis. Mucosal Immunol (2011) 4(3):288–93. doi: 10.1038/mi.2011.10
18. Macallan DC, Borghans JA, Asquith B. Human T Cell Memory: A Dynamic View. Vaccines (2017) 5(1):5. doi: 10.3390/vaccines5010005
19. Wroblewski KJ, Hidayat AA, Neafie RC, Rao NA, Zapor M. Ocular Tuberculosis: A Clinicopathologic and Molecular Study. Ophthalmol (2011) 118(4):772–7. doi: 10.1016/j.ophtha.2010.08.011
20. Tagirasa R, Parmar S, Barik MR, Devadas S, Basu S. Autoreactive T Cells in Immunopathogenesis of TB-Associated Uveitis. Invest Ophthalmol Vis Sci (2017) 58(13):5682–91. doi: 10.1167/iovs.17-22462
21. Elkington P, Tebruegge M, Mansour S. Tuberculosis: An Infection-Initiated Autoimmune Disease? Trends Immunol (2016) 37(12):815–8. doi: 10.1016/j.it.2016.09.007
22. Finnoff WC. Changes in Eyes of Rabbits Following Injection of Dead Tubercle Bacilli Into Common Carotid Artery. Am J Ophthalmol (1924) 7:365–72. doi: 10.1016/S0002-9394(24)90818-X
23. Caspi RR, Silver PB, Luger D, Tang J, Cortes LM, Pennesi G, et al. Mouse Models of Experimental Autoimmune Uveitis. Ophthalmic Res (2008) 40(3-4):169–74. doi: 10.1159/000119871
24. Basu S, Fowler BJ, Kerur N, Arnvig KB, Rao NA. NLRP3 Inflammasome Activation by Mycobacterial ESAT-6 and dsRNA in Intraocular Tuberculosis. Microb Pathog (2018) 114:219–24. doi: 10.1016/j.micpath.2017.11.044
25. Qian X, Nguyen DT, Lyu J, Albers AE, Bi X, Graviss EA. Risk Factors for Extrapulmonary Dissemination of Tuberculosis and Associated Mortality During Treatment for Extrapulmonary Tuberculosis. Emerg Microbes Infect (2018) 7(1):102. doi: 10.1038/s41426-018-0106-1
26. Mayito J, Andia I, Belay M, Jolliffe DA, Kateete DP, Reece ST, et al. Anatomic and Cellular Niches for Mycobacterium Tuberculosis in Latent Tuberculosis Infection. J Infect Dis (2019) 219(5):685–94. doi: 10.1093/infdis/jiy579
27. Nazari H, Karakousis PC, Rao NA. Replication of Mycobacterium Tuberculosis in Retinal Pigment Epithelium. JAMA Ophthalmol (2014) 132(6):724–9. doi: 10.1001/jamaophthalmol.2014.270
28. Agrawal R, Gunasekeran DV, Raje D, Agarwal A, Nguyen QD, Kon OM, et al. Global Variations and Challenges With Tubercular Uveitis in the Collaborative Ocular Tuberculosis Study. Invest Ophthalmol Vis Sci (2018) 59(10):4162–71. doi: 10.1167/iovs.18-24102
29. Kim YJ, Kang JY, Kim SI, Chang MS, Kim YR, Park YJ. Predictors for False-Negative QuantiFERON-TB Gold Assay Results in Patients With Extrapulmonary Tuberculosis. BMC Infect Dis (2018) 18(1):457. doi: 10.1186/s12879-018-3344-x
30. Pathanapitoon K, Kunavisarut P, Sirirungsi W, Rothova A. Looking for Ocular Tuberculosis: Prevalence and Clinical Manifestations of Patients With Uveitis and Positive QuantiFERON®-TB Gold Test. Ocul Immunol Inflamm (2018) 26(6):819–26. doi: 10.1080/09273948.2016.1245760
31. Goodman SN. Toward Evidence-Based Medical Statistics2: The Bayes Factor. Ann Intern Med (1999) 130(12):1005–13. doi: 10.7326/0003-4819-130-12-199906150-00019
32. Rosenbaum JT, Wernick R. The Utility of Routine Screening of Patients With Uveitis for Systemic Lupus Erythematosus or TuberculosisA Bayesian Analysis. Arch Ophthalmol (1990) 108(9):1291–3. doi: 10.1001/archopht.1990.01070110107034
33. Hong BK, Khanamiri HN, Bababeygy SR, Rao NA. The Utility of Routine Tuberculosis Screening in County Hospital Patients With Uveitis. Br J Ophthalmol (2014) 98(8):1091–5. doi: 10.1136/bjophthalmol-2013-303937
34. . Available at: https://www.cdc.gov/tb/publications/factsheets/testing/skintesting.pdf (Accessed September 19, 2021).
35. Farhat M, Greenaway C, Pai M, Menzies D. False-Positive Tuberculin Skin Tests: What is the Absolute Effect of BCG and non-Tuberculous Mycobacteria? Int J Tuberc Lung Dis (2006) 10(11):1192–204.
36. Al Zahrani K, Al Jahdali H, Menzies D. Does Size Matter? Utility of Size of Tuberculin Reactions for the Diagnosis of Mycobacterial Disease. Am J Respir Crit Care Med (2000) 162Pt 1):1419–22. doi: 10.1164/ajrccm.162.4.9912048
37. Menzies D. Interpretation of Repeated Tuberculin TestsBoosting, Conversion, and Reversion. Am J Respir Crit Care Med (1999) 159(1):15–21. doi: 10.1164/ajrccm.159.1.9801120
38. Sharma SK, Vashishtha R, Chauhan LS, Sreenivas V, Seth D. Comparison of TST and IGRA in Diagnosis of Latent Tuberculosis Infection in a High TB-Burden Setting. PloS One (2017) 12(1):e0169539. doi: 10.1371/journal.pone.0169539
39. Ang M, Kiew SY, Wong WL, Chee SP. Discordance of Two Interferon-γ Release Assays and Tuberculin Skin Test in Patients With Uveitis. Br J Ophthalmol (2014) 98(12):1649–53. doi: 10.1136/bjophthalmol-2014-305229
40. Chee CB, KhinMar KW, Gan SH, Barkham TM, Koh CK, Shen L, et al. Tuberculosis Treatment Effect on T-Cell Interferon-Gamma Responses to Mycobacterium Tuberculosis-Specific Antigens. Eur Respir J (2010) 36(2):355–61. doi: 10.1183/09031936.00151309
41. Denkinger CM, Pai M, Patel M, Menzies D. Gamma Interferon Release Assay for Monitoring of Treatment Response for Active Tuberculosis: An Explosion in the Spaghetti Factory. J Clin Microbiol (2013) 51(2):607–10. doi: 10.1128/JCM.02278-12
42. Dewan PK, Grinsdale J, Kawamura LM. Low Sensitivity of a Whole-Blood Interferon-Gamma Release Assay for Detection of Active Tuberculosis. Clin Infect Dis (2007) 44(1):69–73. doi: 10.1086/509928
43. Menzies D, Pai M, Comstock G. Meta-Analysis: New Tests for the Diagnosis of Latent Tuberculosis Infection: Areas of Uncertainty and Recommendations for Research. Ann Intern Med (2007) 146(5):340–54. doi: 10.7326/0003-4819-146-5-200703060-00006
44. Agrawal R, Testi I, Mahajan S, Yuen YS, Agarwal A, Kon OM, et al. Collaborative Ocular Tuberculosis Study Consensus Guidelines on the Management of Tubercular Uveitis-Report 1: Guidelines for Initiating Antitubercular Therapy in Tubercular Choroiditis. Ophthalmol (2021) 128(2):266–76. doi: 10.1016/j.ophtha.2020.01.008
45. Agrawal R, Testi I, Bodaghi B, Barisani-Asenbauer T, McCluskey P, Agarwal A, et al. Collaborative Ocular Tuberculosis Study Consensus Guidelines on the Management of Tubercular Uveitis-Report 2: Guidelines for Initiating Antitubercular Therapy in Anterior Uveitis, Intermediate Uveitis, Panuveitis, and Retinal Vasculitis. Ophthalmol (2021) 128(2):277–87. doi: 10.1016/j.ophtha.2020.06.052
46. Coggon D, Martyn C, Palmer KT, Evanoff B. Assessing Case Definitions in the Absence of a Diagnostic Gold Standard. Int J Epidemiol (2005) 34:949–52. doi: 10.1093/ije/dyi012
47. Graham SM, Ahmed T, Amanullah F, Browning R, Cardenas V, Casenghi M, et al. Evaluation of Tuberculosis Diagnostics in Children, 1: Proposed Clinical Case Definitions for Classification of Intrathoracic Tuberculosis Disease. Consensus an Expert Panel J Infect Dis (2012) 205:S199–208. doi: 10.1093/infdis/jis008
Keywords: ocular TB, latent infection, pulmonary TB, TB immunoreactivity, tuberculin skin test, interferon gamma release assay
Citation: Basu S (2022) Absence of Evidence as The Evidence Of Absence: The Curious Case of Latent Infection Causing Ocular Tuberculosis. Front. Ophthalmol. 2:874400. doi: 10.3389/fopht.2022.874400
Received: 12 February 2022; Accepted: 31 March 2022;
Published: 02 May 2022.
Edited by:
Narsing Rao, University of Southern California, United StatesReviewed by:
Hossein Nazari, University of Minnesota Twin Cities, United StatesJyotirmay Biswas, Sankara Nethralaya, India
Copyright © 2022 Basu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Soumyava Basu, YmFzdUBsdnBlaS5vcmc=; ZXlldGFsa0BnbWFpbC5jb20=
 Soumyava Basu
Soumyava Basu