
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Ophthalmol. , 06 March 2023
Sec. Pediatric Ophthalmology and Strabismus
Volume 2 - 2022 | https://doi.org/10.3389/fopht.2022.1080869
This article is part of the Research Topic Insights in Pediatric Ophthalmology and Strabismus: 2022 View all 8 articles
Background: Idiopathic congenital nystagmus (ICN) is an inherited disorder characterized by uncontrollable binocular conjugating oscillation. X-linked idiopathic congenital nystagmus is one of the most prevalent types of ICN. Elucidation of the genetic mechanisms involved in ICN will enhance our understanding of its molecular etiology.
Case presentation: We report a girl with uncontrollable binocular oscillation and anomalous head posture, then presented a novel heterozygous missense variant (c.686G>T) within the mutation-rich region of the FERM domain containing 7 (FRMD7) gene in her family member. The girl received occlusion therapy and surgical operation which balanced her binocular vision and corrected the anomalous head posture.
Conclusions: This is the first report on a mutation (c.686G>T) caused the substitution of Arg (R) with Leu (L) at position 229 (p.R229L) of the FRMD7 protein in a patient with ICN.
Idiopathic congenital nystagmus (ICN) is a disorder characterized by uncontrollable binocular conjugating oscillation that occurs in the horizontal or vertical plane of either a jerking or pendular waveform. These pathophysiological events progress commonly at birth or shortly afterward (1). Genetically, ICN is a heterogeneous disease that could be divided into autosomal dominant (2), recessive (3, 4), and X-linked dominant (3), with X-linked type being the most prevalent (5). This disorder may be caused by deregulation of signaling pathways or mutation of a single gene (6). Two major genes, FERM domain containing 7 (FRMD7) and G-protein coupled receptor 143 (GPR143), have been implicated in the pathogenesis of ICN (7, 8). Studies have demonstrated that FRMD7 is expressed in the retina, cerebellum, lateral ventricles, and neurite during development (6). Several distinct X-linked loci have been reported to be associated with congenital nystagmus: Xp11.4-p11.3 (4), Xq26-Xq27 (3), Xp22.3-p22.2 (9), and Xq24-q26.3 (10). Approximately 20–97% of patients with X-linked ICN harbor FRMD7 mutations at Xq26.2 (11).
ICN is highly associated with the occurrence of low visual acuity, which is attributed to formation of an unstable image away from the fovea (1). Hypothetically, ICN could be a developmental response to incomplete high-contrast foveal vision (12). Most patients show a fixed angle where they achieve the best visual acuity and turn their heads in the opposite direction of the null zone. Currently, the pathogenesis of ICN has not been fully established. Although there is no etiological treatment for ICN, surgery is often performed to improve patient symptoms. We investigated a five-generation Chinese family with a history of ICN. Surgery was performed on a child diagnosed with ICN to improve anomalous head posture. A summary of recent studies exploring the association of FRMD7 gene mutation with ICN, as well as the role of FRMD7 plays in the regulation of eye movement is presented in this report.
This case report was approved by the ethics committee of the West China Hospital of Sichuan University and written informed consents were obtained from the patients. A four-year-old Chinese girl who presented with uncontrollable binocular oscillation and anomalous head posture was referred to the West China Hospital of Sichuan University. She had no significant past ophthalmic or medical history. On admission, her corrected visual acuity (BCVA) was 20/66 with +1.00Dc×90° in the right eye and 20/40 with +0.50 Dc×90° in the left eye. Her nystagmus was pendular and horizontal. Her face turned right to get optimal VA. No remarkable abnormalities were observed in the anterior segments of both eyes. Findings of the fundus exam and optical coherence tomography (OCT) revealed that thickness measurements of the retinal layer and macular fovea were normal (Figure 1). Moreover, titmus test showed no near stereopsis. No other general physical and neurological symptoms were observed.

Figure 1 Fundus photography and OCT of the four-year-old Chinese girl. (A) Fundus photography of left eye. (B) Fundus photography of right eye. (C) OCT of the macular fovea and thickness of retinal layer on left eye. (D) OCT of the macular fovea and thickness of retinal layer on right eye.
Evidence from previous studies demonstrate that refractive correction could improve visual acuity and reduce nystagmus in ICN (13).We gave the four-year-old Chinese girl a prescription for glasses and followed up. At one-year follow-up, her BCVA was 20/50 in the right eye and 20/30 in the left eye. Moreover, occlusion was observed in the left eye. At two-year follow-up, monocular BCVA was 20/30 in each eye. Binocular BCVA was 20/20 when she looked left, 20/30 when she looked forward, and 20/66 when she looked right.
Subsequently, surgery was performed on both eyes targeting the internal and external rectus muscles at the age of six. Specifically, the lateral rectus was recessed 10.0 mm from the insertion in the left eye, and the medial rectus was recessed 10.5 mm from the limbus in the right eye. One month after surgery, her compensatory head posture was improved, and the null zone was in the straight-ahead gaze; her binocular BVCA improved to 20/25 when she looked forward; the near and far binocular vision were restored. The titmus test revealed the occurrence of near gross stereopsis. Binocular vision examined using the synoptophore (Inami, Japan) showed a simultaneous perception at 0°, fusional amplitude ranging from -6° to +10°, and far stereopsis in the range of -5° to +10°. Her head posture was corrected to normal. At the age of nine years, her binocular BCVA was 20/25 when she looked forward and left. Surgery could shift the null zone of nystagmus into primary position (14), and recession or tenotomy of the four horizontal muscles could significantly improve visual function and eye movements in ICN patients (15). This study provide evidence that extraocular surgery intervention on two horizontal muscles could independently improve neurological and visual quality to achieve eye repositioning. The intervention on two horizontal muscles which also have been peformed by Dr.Muralidhar in patients with infantile nystagmus syndrome and face turn (16).
The girl in the present case came from a five-generation Chinese family, in which the mother and maternal grandmother had nystagmus, but the other family members did not develop nystagmus. Binocular BCVAs of the patient’s mother and maternal grandmother were 20/50, and both exhibited anomalous head postures. However, they did not undergo surgical treatment. None of the family members showed remarkable structural abnormalities in the eyes. To further investigate the underlying genetic cause of nystagmus, we scheduled a genomic DNA test for member of the family. Consequently, peripheral venous blood samples were collected from each family member after obtaining written informed consent. Genomic DNA was extracted from the blood samples, whole exome was isolated using the Agilent SureSelect Human All Exon Kit and then sequencing on the Agilent Hiseq sequencer. A novel missense heterozygous mutation, c.686G>T, was identified at codon 686 in exon 8 of the FRMD7 gene (Figure 2). The c.686G>T mutation caused a substitution of Arg (R) with Leu (L) at position 229 (p.R229L) of the FRMD7 protein. A gene test revealed that among the 20 family members, four females were affected and two members were carriers (Figure 3). The mutation was detected in the patient’s mother, maternal grandmother, and two sisters of the maternal grandmother (Figure 2).
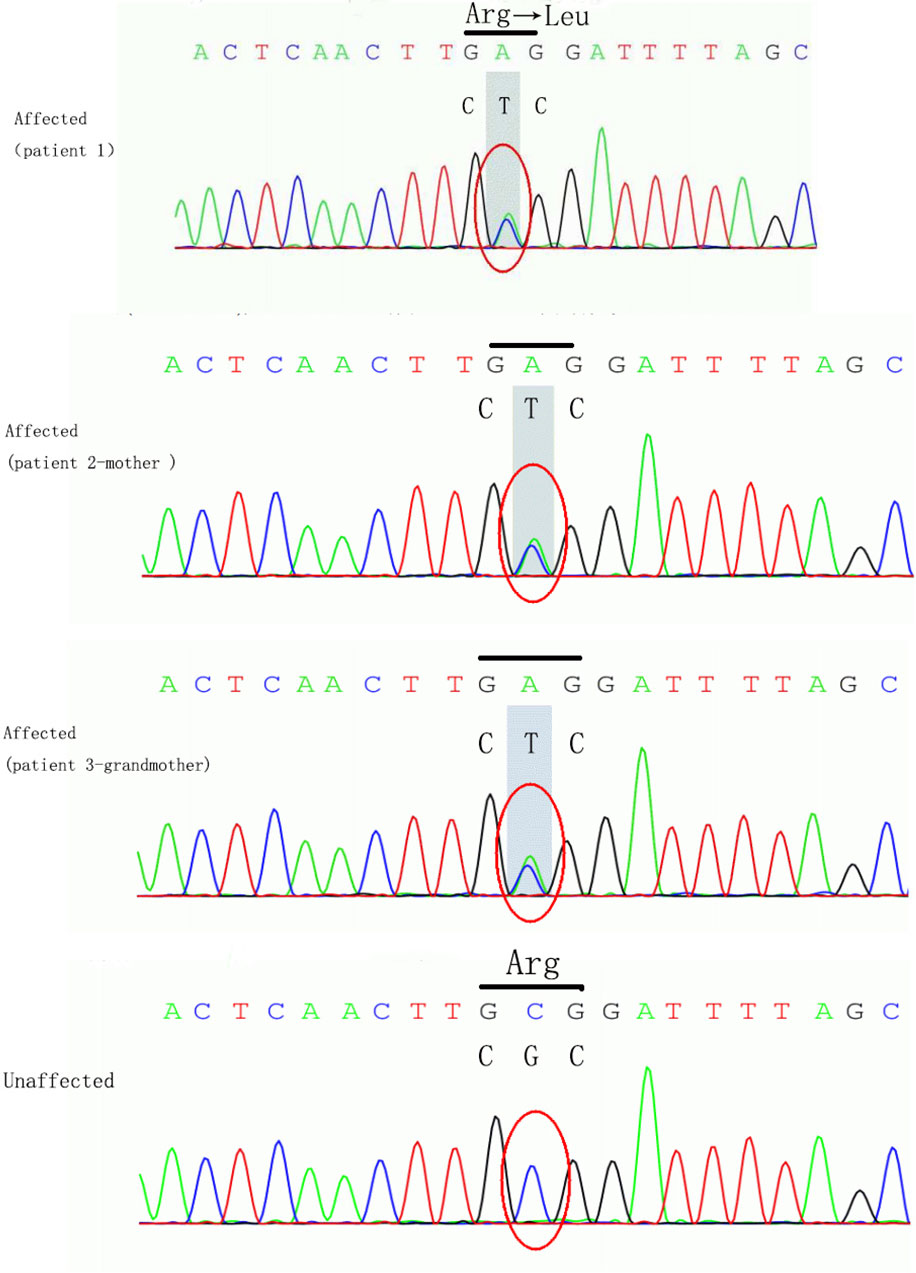
Figure 2 The loci of mutation in FRMD7. The mutation c.686G>T caused a substitution of Arg (R) to Leu (L) at position 229 (p.R229L) of the FRMD7 protein in the girl, her mother and grand-mother.
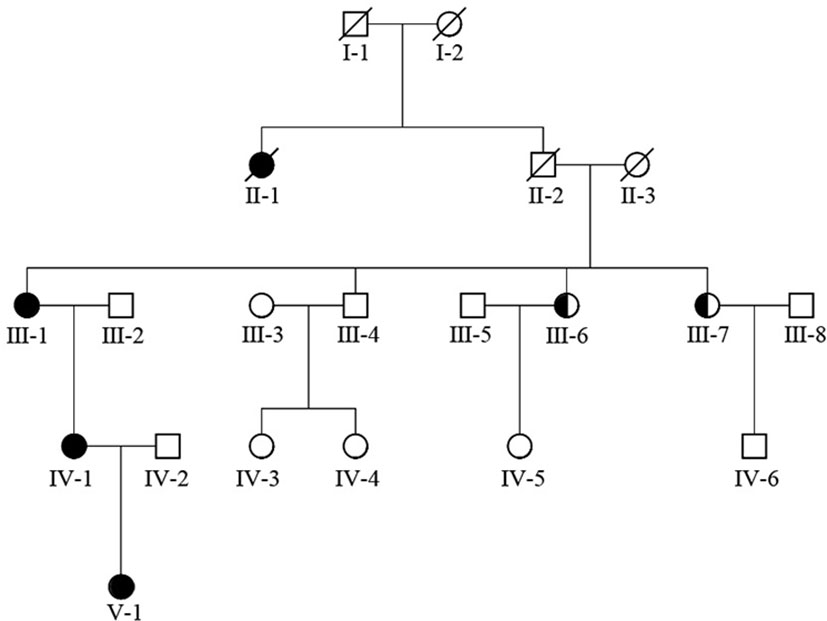
Figure 3 FRMD7 mutations in this family. The family (20 members) included four affected females and two carriers.
Congenital nystagmus is a clinically and genetically heterogeneous disorder that affects vision (1). This disorder is characterized by idiopathic and nystagmus-related disorders. It has been postulated that ICN might be caused by a developmental defect in the brain’s ocular motor regions that control fixation (1).
Here, we identified a novel heterozygous missense mutation of the FRMD7 gene in a Chinese family with ICN. A four-year-old Chinese girl who had ICN underwent surgical treatment to reposition of the minimal intensity zone to the primary position of the eye. The surgery balanced binocular vision and corrected the anomalous head posture at 3-year follow-up. The identified mutation caused substitution of CGC (that codes for Arg) with CTC (that codes for Leu) in eight exons of position of 229 in the FRMD7 gene, c.686G>T. Two other reports of missense mutation of the position 229 have been described in FRMD7 (17, 18). One of them is a missense c.686C>G mutation in exon 8 of the FRMD7 gene, which results in the substitution of Gly(G) for Arg(R) at amino acid position 229 (p.R229G) (17). The other is a missense c.685C>T mutation, which causes the substitution of Arg (R) with Cys (C) at position 229 (p.R229C) in exon 8 of the FRMD7 gene (18). The missense mutations in position 229 of FRMD7 are mostly deleterious and attributed to the pathogenicity (18).The FRMD7 gene, located in chromosome Xq26.2, comprises 12 exons and encodes a polypeptide containing 714 residues (19). The FRMD7 protein, containing FERM-N, FERM-M, FERM-C, and FA structural domains, is a member of the 4.1 superfamily (19, 20). The identified novel mutation was located in the FERM-C domain, which has been reported to have the highest number of mutations compared with all other domains (20). This mutation was also detected in her mother, maternal grandmother, and two sisters of her maternal grandmother.
In our report family, nystagmus was inherited as an apparent X-linked dominant disorder with incomplete penetrance. Among the tested family members, there were four affected females and two obligate female carriers. Studies have been reported X-linked congenital idiopathic nystagmus pedigrees could be represented a penetrance in the range of 30–100% in female members (21, 22). The possible mechanisms for this variable penetrance include skewed X inactivation, genetic modifiers, regulation of other genes, and other factors that influence ocular motor development (22–26).
FRMD7 is mainly detected in neuronal tissue within the afferent arms of the vestibulo-ocular reflex consisting of the optic vesicle, cranial nerve VIII, vestibular ganglia, developing neural retina, and ventricular zone of the optic stalk. It is also selectively expressed in starburst amacrine cells (7, 27, 28). It is involved in the regulation of eye movement, neuronal morphogenesis, synapse function, and neurite growth (29). The FERM domain and the FERM-adjacent domain control plasma membrane and actin cytoskeleton organization suggesting that they are essential to the development of neural system and the brain region that control eye movement (30).
Several FRMD7 isoforms have been shown to play important roles during neuronal differentiation and development. The original form of FRMD7 (FRMD7-FL) and its splice variants FRMD7-S and FRMD7_SV2 are involved in the neuronal development process (29) (31). A search performed on the BLAST tool showed that the FRMD7 protein shared a close homology with FARP1 and FARP2, which modulate the length and the degree of branching of neurites in rat embryonic cortical neurons and reorganize the cytoskeleton (32, 33).
Evidence shows that FRMD7 mutations are associated with the development of nystagmus (34). Besides, FRMD7 mutations could alter retinal direction selectivity, diminish responsiveness at higher stimulus speeds, and eliminate overrepresentation of posterior-motion-preferring cortical cells (35), thereby cause retinal neuron migratory defects such as foveal hypoplasia (36). In addition, they could affect starburst amacrine cells and impair the development of visual motion responses in superior colliculus neurons downstream of the retina (37). This disrupts axogenesis, dendritogenesis, and neuronal guidance in brain areas involved in the control of eye movement (30). FRMD7 promoted neurite elongation by modulating actin cytoskeleton whereas FRMD7 gene knockdown led to a significant reduction in overall neurite length (34, 38), further contributing to the development of neuronal circuit asymmetry and neurological disorders (28, 39). Mutations associated with ICN include missense mutations, null mutations, deletions and insertions, and frameshift mutations. A series of FRMD7 gene mutations linked to ICN are summarized in Table 1.
In conclusion, our study adds to the spectrum of FRMD7 mutations associated with X-linked ICN. We show that FRMD7 mutations underlie the development of X-linked ICN. Surgery could be an effective treatment approach to correct persistent anomalous head posture in ICN patients. However, the molecular mechanisms by which genetic variations cause ICN are unknown, and therefore, further investigations are required to reveal detailed mechanisms that drive the pathogenesis of this hereditary ocular disease.
This study has several potential limitations. First, only one Chinese family was investigated in the study. In addition, we did not examine protein and cell levels to clarify the pathological mechanism by which FRMD7 mutation led to ICN. Furthermore, the follow-up duration was short. Therefore, further studies with longer follow-up duration are needed to validate the efficacy of surgical therapy in ICN patients.
The original contributions presented in the study are included in the article/supplementary materials. Further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by West China Hospital of Sichuan University. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin. Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
XJ and FL carried out the experiments, prepared the figures, and drafted the manuscript. MW performed the gene test. ML and LL participated in its design and edited the manuscript. All authors contributed to the article and approved the submitted version.
This study was supported by the National Natural Science Foundation of China (NSFC, Grant No.281500728). The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication.
We thank the patient in this case study and her family for participating in the study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
ICN, Idiopathic congenital nystagmus; FERM, 4.1 (four point one) and ERM (ezrin, radixin, moesin) proteins; FARP2, FERM, RhoGEF and pleckstrin domain protein 2; FRMD7, FERM domain containing 7; GPR143, G-protein coupled receptor 143; BCVA, Best corrected visual acuity; OCT, Optical coherence tomography; FA, FERM-adjacent (FA) region.
1. Papageorgiou E, McLean RJ, Gottlob I. Nystagmus in childhood. Pediatr Neonatol (2014) 55(5):341–51. doi: 10.1016/j.pedneo.2014.02.007
2. Kerrison JB, Arnould VJ, Barmada MM, Koenekoop RK, Schmeckpeper BJ, Maumenee IH. A gene for autosomal dominant congenital nystagmus localizes to 6p12. Genomics (1996) 33(3):523–6. doi: 10.1006/geno.1996.0229
3. Kerrison JB, Vagefi MR, Barmada MM, Maumenee IH. Congenital motor nystagmus linked to Xq26-q27. Am J Hum Genet (1999) 64(2):600–7. doi: 10.1086/302244
4. Cabot A, Rozet J-M, Gerber S, Perrault I, Ducroq D, Smahi A. A gene for X-linked idiopathic congenital nystagmus (NYS1) maps to chromosome Xp11. 4-p11. 3. Am J Hum Genet (1999) 64(4):1141–6. doi: 10.1086/302324
5. Oetting WS, Armstrong CM, Holleschau AM, DeWan AT, Summers CG. Evidence for genetic heterogeneity in families with congenital motor nystagmus (CN). Ophthalmic Genet (2000) 21(4):227–33. doi: 10.1076/1381-6810(200012)2141-HFT227
6. Kumar A, Gottlob I, Mclean RJ, Thomas S, Thomas MG, Proudlock FA. Clinical and oculomotor characteristics of albinism compared to FRMD7 associated infantile nystagmus. Invest Ophthalmol Visual Sci (2011) 52(5):2306–13. doi: 10.1167/iovs.10-5685
7. Tarpey P, Thomas S, Sarvananthan N, Mallya U, Lisgo S, Talbot CJ, et al. Mutations in FRMD7, a newly identified member of the FERM family, cause X-linked idiopathic congenital nystagmus. Nat Genet (2006) 38(11):1242–4. doi: 10.1038/ng1893
8. Hu J, Liang D, Xue J, Liu J, Wu L. A novel GPR143 splicing mutation in a Chinese family with X-linked congenital nystagmus. Mol Vision (2011) 17:715.
9. Liu JY, Ren X, Yang X, Guo T, Yao Q, Li L, et al. Identification of a novel GPR143 mutation in a large Chinese family with congenital nystagmus as the most prominent and consistent manifestation. J Hum Genet (2007) 52(6):565–70. doi: 10.1007/s10038-007-0152-3
10. Self JE, Ennis S, Collins A, Shawkat F, Harris CM, Mackey DA, et al. Fine mapping of the X-linked recessive congenital idiopathic nystagmus locus at Xq24-q26. 3. Mol Vis (2006) 12(10):1211–6.
11. Penix K, Swanson MW, DeCarlo DK. Nystagmus in pediatric patients: interventions and patient-focused perspectives. Clin Ophthalmol (Auckland NZ) (2015) 9:1527. doi: 10.2147/OPTH.S62786
12. Harris C, Berry D. A developmental model of infantile nystagmus. in seminars in ophthalmology. Taylor & Francis (2006) 21:63–9.
13. Hertle RW. Examination and refractive management of patients with nystagmus. Survey Ophthalmol (2000) 45(3):215–22. doi: 10.1016/S0039-6257(00)00153-3
14. McLean RJ, Gottlob I. The pharmacological treatment of nystagmus: A review. Expert Opin pharmacother (2009) 10(11):1805–16. doi: 10.1517/14656560902978446
15. Hertle RW, Yang D. Clinical and electrophysiological effects of extraocular muscle surgery on patients with infantile nystagmus syndrome (INS). in seminars in ophthalmology. Taylor & Francis (2006) 21:103–10.
16. Muralidhar R, Ramamurthy D. A preliminary study on the outcome of plication augmentation of the augmented anderson procedure for patients with infantile nystagmus syndrome and a face turn. J Curr Ophthalmol (2021) 33(3):330. doi: 10.4103/2452-2325.329065
17. Kaplan Y, Vargel I, Kansu T, Akin B, Rohmann E, Kamaci S, et al. Skewed X inactivation in an X linked nystagmus family resulted from a novel, p. R229G, missense mutation in the FRMD7 gene. Br J Ophthalmol (2008) 92(1):135–41. doi: 10.1136/bjo.2007.128157
18. Bai D, Shi W, Qi Z, Li W, Wei A, Cui Y, et al. Clinical feature and waveform in infantile nystagmus syndrome in children with FRMD7 gene mutations. Sci China Life Sci (2017) 60(7):707–13. doi: 10.1007/s11427-017-9089-5
19. Watkins RJ, Thomas MG, Talbot CJ, Gottlob I, Shackleton S. The role of FRMD7 in idiopathic infantile nystagmus. J Ophthalmol (2012) 2012:1–7. doi: 10.1155/2012/460956
20. Baines AJ. A FERM-adjacent (FA) region defines a subset of the 4.1 superfamily and is a potential regulator of FERM domain function. BMC Genomics (2006) 7(1):1–7. doi: 10.1186/1471-2164-7-85
21. Self JE, Shawkat F, Malpas CT, Thomas NS, Harris CM, Hodgkins PR, et al. Allelic variation of the FRMD7 gene in congenital idiopathic nystagmus. Arch Ophthalmol (2007) 125(9):1255–63. doi: 10.1001/archopht.125.9.1255
22. Thomas S, Proudlock FA, Sarvananthan N, Roberts EO, Awan M, McLean R, et al. Phenotypical characteristics of idiopathic infantile nystagmus with and without mutations in FRMD7. Brain (2008) 131(5):1259–67. doi: 10.1093/brain/awn046
23. Hagen SH, Henseling F, Hennesen J, Savel H, Delahaye S, Richert L, et al. Heterogeneous escape from X chromosome inactivation results in sex differences in type I IFN responses at the single human pDC level. Cell Rep (2020) 33(10):108485. doi: 10.1016/j.celrep.2020.108485
24. Migeon BR. Why females are mosaics, X-chromosome inactivation, and sex differences in disease. Gender Med (2007) 4(2):97–105. doi: 10.1016/S1550-8579(07)80024-6
25. McMullan TF, Collins AR, Tyers AG, Robinson DO. A novel X-linked dominant condition: X-linked congenital isolated ptosis. Am J Hum Genet (2000) 66(4):1455–60. doi: 10.1086/302860
26. Guo X, Li S, Jia X, Xiao X, Wang P, Zhang Q. Linkage analysis of two families with X-linked recessive congenital motor nystagmus. J Hum Genet (2006) 51(1):76–80. doi: 10.1007/s10038-005-0316-y
27. Thomas MG, Crosier M, Lindsay S, Kumar A, Thomas S, Araki M, et al. The clinical and molecular genetic features of idiopathic infantile periodic alternating nystagmus. Brain (2011) 134(3):892–902. doi: 10.1093/brain/awq373
28. Yonehara K, Fiscella M, Drinnenberg A, Esposti F, Trenholm S, Krol J, et al. Congenital nystagmus gene FRMD7 is necessary for establishing a neuronal circuit asymmetry for direction selectivity. Neuron (2016) 89(1):177–93. doi: 10.1016/j.neuron.2015.11.032
29. Li Y, Pu J, Liu Z, Xu S, Jin F, Zhu L, et al. Identification of a novel FRMD7 splice variant and functional analysis of two FRMD7 transcripts during human NT2 cell differentiation. Mol Vision (2011) 17:2986.
30. Salman A, Hutton SB, Newall T, Scott JA, Griffiths HL, Lee H, et al. Characterization of the Frmd7 knock-out mice generated by the EUCOMM/COMP repository as a model for idiopathic infantile nystagmus (IIN). Genes (2020) 11(10):1157. doi: 10.3390/genes11101157
31. Li Y, Pu J, Zhang B. Expression of a novel splice variant of FRMD7 in developing human fetal brains that is upregulated upon the differentiation of NT2 cells. Exp Ther Med (2014) 8(4):1131–6. doi: 10.3892/etm.2014.1916
32. Kubo T, Yamashita T, Yamaguchi A, Sumimoto H, Hosokawa K, Tohyama M. A novel FERM domain including guanine nucleotide exchange factor is involved in rac signaling and regulates neurite remodeling. J Neurosci (2002) 22(19):8504–13. doi: 10.1523/JNEUROSCI.22-19-08504.2002
33. Toyofuku T, Yoshida J, Sugimoto T, Zhang H, Kumanogoh A, Hori M, et al. FARP2 triggers signals for Sema3A-mediated axonal repulsion. Nat Neurosci (2005) 8(12):1712–9. doi: 10.1038/nn1596
34. Thomas MG, Crosier M, Lindsay S, Kumar A, Araki M, Leroy BP, et al. Abnormal retinal development associated with FRMD7 mutations. Hum Mol Genet (2014) 23(15):4086–93. doi: 10.1093/hmg/ddu122
35. Hillier D, Fiscella M, Drinnenberg A, Trenholm S, Rompani SB, Raics Z, et al. Causal evidence for retina-dependent and-independent visual motion computations in mouse cortex. Nat Neurosci (2017) 20(7):960–8. doi: 10.1038/nn.4566
36. Kuht HJ, Maconachie GD, Han J, Kessel L, Van Genderen MM, McLean RJ, et al. Genotypic and Phenotypic Spectrum of Foveal Hypoplasia: A Multicenter Study. Ophthalmology (2022) 129(6):708–18.
37. Ge X, Zhang K, Gribizis A, Hamodi AS, Sabino AM, Crair MC. Retinal waves prime visual motion detection by simulating future optic flow. Science (2021) 373(6553):eabd0830. doi: 10.1126/science.abd0830
38. Pu J, Lu X, Zhao G, Yan Y, Tian J, Zhang B. FERM domain containing protein 7 (FRMD7) upregulates the expression of neuronal cytoskeletal proteins and promotes neurite outgrowth in neuro-2a cells. Mol Vision (2012) 18:1428.
39. Roska B. The first steps in vision: Cell types, circuits, and repair. EMBO Mol Med (2019) 11(3):e10218. doi: 10.15252/emmm.201810218
40. Wang F, Guan H, Liu W, Zhao G, Liu S. Next-generation sequencing identifies a novel frameshift variant in FRMD7 in a Chinese family with idiopathic infantile nystagmus. J Clin Lab Anal (2020) 34(1):e23012. doi: 10.1002/jcla.23012
41. Yan N, Xiao L, Hou C, Guo B, Fan W, Deng Y, et al. X-Linked inheritances recessive of congenital nystagmus and autosomal dominant inheritances of congenital cataracts coexist in a Chinese family: A case report and literature review. BMC Med Genet (2019) 20(1):1–6. doi: 10.1186/s12881-019-0780-4
42. Wang Z, Wang M, Wang C, Lu B. Identification and functional characterization of a novel missense mutation in FRMD7 responsible for idiopathic congenital nystagmus. Acta Biochim Biophys Sin (2019) 51(2):178–84. doi: 10.1093/abbs/gmy161
43. Xiu Y, Yao Y, Yang T, Pan M, Yang H, Fang W, et al. Identification of a novel idiopathic congenital nystagmus−causing missense mutation, p. G296C, in the FRMD7 gene. Mol Med Rep (2018) 18(3):2816–22. doi: 10.3892/mmr.2018.9260
44. Jia X, Zhu X, Li Q, Jia X, Li S, Guo X. Novel mutations of FRMD7 in Chinese patients with congenital motor nystagmus. Mol Med Rep (2017) 16(2):1753–8. doi: 10.3892/mmr.2017.6824
45. Zhao H, Huang X-F, Zheng Z-L, Deng W-L, Lei X-L, Xing D-J, et al. Molecular genetic analysis of patients with sporadic and X-linked infantile nystagmus. BMJ Open (2016) 6(4):e010649. doi: 10.1136/bmjopen-2015-010649
46. AlMoallem B, Bauwens M, Walraedt S, Delbeke P, Zaeytijd De J, Kestelyn P, et al. Novel FRMD7 mutations and genomic rearrangement expand the molecular pathogenesis of X-linked idiopathic infantile nystagmus. Invest Ophthalmol Visual Sci (2015) 56(3):1701–10. doi: 10.1167/iovs.14-15938
47. Gupta S, Pathak E, Chaudhry VN, Chaudhry P, Mishra R, Chandra A, et al. A novel mutation in FRMD7 causes X-linked idiopathic congenital nystagmus in a north Indian family. Neurosci Lett (2015) 597:170–5. doi: 10.1016/j.neulet.2015.04.037
48. Kohmoto T, Okamoto N, Satomura S, Naruto T, Komori T, Hashimoto T, et al. A FRMD7 variant in a japanese family causes congenital nystagmus. Hum Genome Variation (2015) 2(1):1–4. doi: 10.1038/hgv.2015.2
49. Choi J-H, Shin J-H, Seo J-H, Jung J-H, Choi KD. A start codon mutation of the FRMD7 gene in two Korean families with idiopathic infantile nystagmus. Sci Rep (2015) 5(1):1–6. doi: 10.1038/srep13003
50. Zhang X, Ge X, Yu Y, Zhang Y, Wu Y, Luan Y, et al. Identification of three novel mutations in the FRMD7 gene for X-linked idiopathic congenital nystagmus. Sci Rep (2014) 4(1):1–5. doi: 10.1038/srep03745
51. Zhu Y, Zhuang J, Ge X, Zhang X, Wang Z, Sun J, et al. Identifcation of a novel mutation p. I240T in the FRMD7 gene in a family with congenital nystagmus. Sci Rep (2013) 3(1):1–5. doi: 10.1038/srep03084
52. Liu Z, Mao S, Pu J, Ding Y, Zhang B, Ding M. A novel missense mutation in the FERM domain containing 7 (FRMD7) gene causing X-linked idiopathic congenital nystagmus in a Chinese family. Mol Vision (2013) 19:1834.
53. Song F-w, Chen B-B, Sun Z-H, Wu L-P, Zhao S-J, Miao Q, et al. Novel mutation c. 980_983delATTA compound with c. 986C> a mutation of the FRMD7 gene in a Chinese family with X-linked idiopathic congenital nystagmus. J Zhejiang Univ Sci B (2013) 14(6):479–86. doi: 10.1631/jzus.B1200259
54. Radhakrishna U, Ratnamala U, Deutsch S, Bartoloni L, Kuracha MR, Singh R, et al. Novel homozygous, heterozygous and hemizygous FRMD7 gene mutations segregated in the same consanguineous family with congenital X-linked nystagmus. Eur J Hum Genet (2012) 20(10):1032–6. doi: 10.1038/ejhg.2012.60
55. Li N, Wang X, Wang Y, Wang L, Ying M, Han R, et al. Investigation of the gene mutations in two Chinese families with X-linked infantile nystagmus. Mol Vision (2011) 17:461.
56. Du W, Bu J, Dong J, Jia Y, Li J, Liang C, et al. A novel frame-shift mutation in FRMD7 causes X-linked idiopathic congenital nystagmus in a Chinese family. Mol Vision (2011) 17:2765.
57. He X, Gu F, Wang Y, Yan J, Zhang M, Huang S, et al. A novel mutation in FRMD7 causing X-linked idiopathic congenital nystagmus in a large family. Mol Vision (2008) 14:56.
58. Li N, Wang L, Cui L, Zhang L, Dai S, Li H, et al. Five novel mutations of the FRMD7 gene in Chinese families with X-linked infantile nystagmus. Mol Vision (2008) 14:733.
59. He X, Gu F, Wang Z, Wang C, Tong Y, Wang Y, et al. A novel frameshift mutation in FRMD7 causing X-linked idiopathic congenital nystagmus. Genet testing (2008) 12(4):607–13. doi: 10.1089/gte.2008.0070
60. Zhang Q, Xiao X, Li S, Guo X. FRMD7 mutations in Chinese families with X-linked congenital motor nystagmus. Mol Vis (2007) 13:1375–8.
61. Schorderet DF, Tiab L, Gaillard MC, Lorenz B, Klainguti G, Kerrison JB, et al. Novel mutations in FRMD7 in X-linked congenital nystagmus. Hum Mutat (2007) 28(5):525–5. doi: 10.1002/humu.9492
62. Shiels A, Bennett TM, Prince JB, Tychsen L. X-Linked idiopathic infantile nystagmus associated with a missense mutation in FRMD7. (2007).
Keywords: idiopathic congenital nystagmus, FRMD7, X-linked, gene mutation, eye movement
Citation: Liu F, Wang M, Liao M, Liu L and Jiang X (2023) X-linked FRMD7 gene mutation in idiopathic congenital nystagmus and its role in eye movement: A case report and literature review. Front. Ophthalmol. 2:1080869. doi: 10.3389/fopht.2022.1080869
Received: 26 October 2022; Accepted: 28 December 2022;
Published: 06 March 2023.
Edited by:
Rohit Saxena, All India Institute of Medical Sciences, IndiaReviewed by:
Chitra Kannabiran, L V Prasad Eye Institute, IndiaCopyright © 2023 Liu, Wang, Liao, Liu and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoshuang Jiang, amlhbmd4aWFvc2h1YW5nQHdjaHNjdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.