
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 25 February 2025
Sec. Gastrointestinal Cancers: Hepato Pancreatic Biliary Cancers
Volume 15 - 2025 | https://doi.org/10.3389/fonc.2025.1554711
This article is part of the Research TopicLiver Cancer Awareness Month 2024: Current Progress and Future Prospects on Advances in Primary Liver Cancer Investigation and TreatmentView all 18 articles
Background: Unresectable hepatocellular carcinoma (HCC) presents significant treatment challenges. While locoregional therapies (LT) and tyrosine kinase inhibitors (TKI) offer some benefits, prognosis remains poor. Immune checkpoint inhibitors (ICI) have shown promise in other oncological settings, suggesting potential benefits in HCC treatment regimens.
Methods: This retrospective study analyzed 232 patients diagnosed with unresectable HCC at West China Hospital from January 2019 to December 2023. Patients were categorized into two treatment groups: LT+TKI and LT+TKI+ICI. All patients underwent standardized locoregional treatments and first-line TKIs, with the latter group also receiving ICIs. The primary endpoints measured were overall survival (OS) and progression-free survival (PFS). Survival analysis utilized Kaplan-Meier estimates and Cox regression models.
Results: The LT+TKI+ICI group demonstrated significantly improved survival outcomes compared to the LT+TKI group. Median OS was 28 ± 3.9 months in the LT+TKI+ICI group versus 21 ± 3.0 months in the LT+TKI group, with corresponding 6-, 12-, and 24-month OS rates of 96.8%, 79.3%, and 59.4% versus 85.8%, 71.5%, and 44.1%, respectively (HR, 0.64; 95% CI, 0.449-0.913; P = 0.014). Median PFS also favored the LT+TKI+ICI group (11 ± 1.1 months vs. 7 ± 0.76 months; HR, 0.60; 95% CI, 0.452-0.805; P<0.001). Multivariable analysis identified LT+TKI, vascular invasion, and metastasis as independent risk factors for poorer survival outcomes.
Conclusions: Adding ICI to LT and TKI significantly extends both OS and PFS in patients with unresectable HCC. These findings suggest that integrating ICI into treatment protocols could be beneficial in managing unresectable HCC, particularly for patients with vascular invasion.
Hepatocellular carcinoma (HCC) is the most common primary liver malignancy and represents a significant global health challenge due to its high morbidity and mortality rates, it is the sixth most common cancer worldwide and becomes the fourth leading cause of cancer-related death (1–5). HCC may be treatable in early stages by resection, liver transplantation, or ablation (6). However, patients are typically identified at intermediate or advanced stages due to a lack of symptoms. Despite advances in diagnostic techniques and therapeutic interventions, the prognosis for patients with unresectable HCC remains poor (7, 8).
For the patient with advanced HCC, multiple guidelines recommend sorafenib as the first-line treatment due to its efficacy and safety (9). The evolution of systemic therapies for HCC has notably included the adoption of other tyrosine kinase inhibitors (TKIs) such as lenvatinib, which have become staples in the treatment of unresectable HCC (10).
Recent developments in cancer immunotherapy have introduced immune checkpoint inhibitors (ICIs) that target inhibitory receptors on T cells, such as PD-1 and CTLA-4, thus enhancing the immune system’s ability to eliminate cancer cells (11, 12). Currently, several immune checkpoint inhibitors have been approved by the FDA for the treatment of hepatocellular carcinoma (HCC) (13). In 2022, nivolumab, the first immune checkpoint inhibitor, was approved as a second-line treatment option for patients with sorafenib-pretreated HCC (14). Additionally, pembrolizumab, another PD-1 inhibitor, was approved as an alternative treatment for patients with sorafenib-resistant HCC (15). Prior to the clinical trial of IMbrave150, sorafenib was the standard first-line systemic therapy for unresectable advanced HCC. However, the results of the IMbrave150 trial demonstrated that the combination of atezolizumab and bevacizumab significantly improved OS and PFS compared to sorafenib in patients with unresectable HCC (16). Based on these findings, the Barcelona Clinic Liver Cancer (BCLC) staging system and treatment guidelines now recommend atezolizumab plus bevacizumab as the first-line systemic therapy for advanced stage (C) HCC (7). The integration of ICIs into the treatment landscape of HCC, particularly in combination with TKIs and locoregional therapies such as transarterial chemoembolization (TACE), has opened new avenues for improving clinical outcomes.
However, research on the combination of these three regimens has remained limited. Yuan Y et al. (17) conducted a retrospective study in HCC patients with PVTT who received either triple therapy (TACE + targeted therapy + immune therapy) or TACE alone, the results demonstrate that triple therapy group showed significantly improved OS, PFS and overall response rate (ORR) compare to the TACE group, and the grade 3/4 adverse events rates were similar between the two groups. Jin ZC et al. (18) also conducted a large, multi-center, retrospective study included a total of 1244 patients with advanced HCC who received either TACE+TKI+ICI treatment or only TKI+ICI treatment. The results showed that the PFS and OS for TACE combined with targeted and immune therapy were significantly better than those for the combination of targeted and immune therapy alone. According to the researches above, we believe that for patients with unresectable HCC, the combination of locoregional therapy (LT) and systemic therapy may offer improved disease control and prolonged overall survival. Given the promising outcomes of ICIs when used in combination with TKIs in the clinical trial mentioned above, We hypothesize that the integration of ICI can enhance the antitumor efficacy of traditional dual therapy of LT and TKI. Consequently, we designed this study to evaluate the comparative efficacy of dual therapy, which consists of LT combined with TKI, versus triple therapy of the addition of ICI to the regimen of LT and TKI.
This retrospective analysis involved 232 patients with a diagnosis of unresectable HCC, treated at West China Hospital between January 2019 and December 2023. The diagnosis was established through pre-treatment enhanced computed tomography (CT) or magnetic resonance imaging (MRI), each independently evaluated by two experienced radiologists. All imaging studies were completed within two weeks prior to initiation of treatment. In instances where the imaging-based diagnosis remained ambiguous, ultrasound-guided liver biopsy was employed to ascertain the diagnosis. Criteria for unresectability were determined by a multidisciplinary team and included (1): infeasibility of R0 resection, (2) a remaining liver volume less than 30% in non-cirrhotic patients or less than 40% in cirrhotic patients, and/or an indocyanine green clearance rate exceeding 15%.
Inclusion criteria for the study were as follows: (1) primary unresectable HCC as evidenced by MRI or CT imaging characteristics, (2) Child–Pugh class A or B liver function, and an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1, (3) treatment that included both locoregional therapy (LT) and tyrosine kinase inhibitors (TKI), with or without immune checkpoint inhibitors. Exclusion criteria were: (1) presence of any malignant tumor other than HCC, (2) incomplete medical records, (3) loss to follow-up within three months post-treatment.
The patients were divided into two groups. The LT+TKI group underwent standard locoregional treatments such as TACE, stereotactic body radiotherapy (SBRT) or radiofrequency ablation (RFA), followed by first-line tyrosine kinase inhibitors including sorafenib or lenvatinib based on individual clinical evaluations. The LT+TKI+ICI group received identical locoregional and TKI therapies, supplemented with an ICI (sintilimab, tislelizumab, and camrelizumab) administered according to established guidelines.
Patients were monitored at intervals ranging from three to six months following the initial treatment. Clinical evaluations, imaging to evaluate disease progression, tests to monitor liver function and pertinent tumor markers such as AFP or PIVKA-II were conducted regularly. The primary endpoint of this study was overall survival (OS), and the secondary endpoint was progression-free survival (PFS). Both endpoints were calculated from the initiation of treatment to the date of death or the date of first documented disease progression, respectively. Treatment-related adverse events were recorded according to the National Cancer Institute Common Terminology Criteria for Adverse Events 4.0 (19). Patients who were lost to follow-up during the study period were considered censored at their last hospital visit.
Categorical variables were presented with frequency and percentage. The Pearson chi-square test and Fisher’s exact test were employed for comparing categorical variables between groups. The survival curves of OS and PFS in the entire cohort and subgroup analysis were analyzed utilizing the Kaplan–Meier method with the Log rank test. All statistical tests were two-sided, and P < 0.05 was considered statistically significant. In Cox regression, variables with P <0.1 were included in the multivariate regression, with a P <0.05 considered statistically significant. All data analyses were conducted using R version 4.4.1, and SPSS Version 29.0.1.
The study enrolled 232 patients who were evaluated by multidisciplinary team (MDT) experts as having unresectable HCC, comprising 107 individuals who underwent locoregional therapy and tyrosine kinase inhibitor treatment (LT+TKI group) and 125 individuals who underwent locoregional therapy and tyrosine kinase inhibitor plus immune checkpoint inhibitor treatment (LT+TKI+ICI group). The median follow-up time in the LT+TKI group was 16 (range: 9–26) months and 17 (range: 11.5–25) months in the LT+TKI+ICI group. There were 71 (66.4%) death events occurred in the LT+TKI group and 55 (44.0%) in the LT+TKI+ICI group. As presented in Table 1, critical baseline data were analyzed, including age, gender, ALT and AST levels, ALBI, Child-Pugh score, vascular invasion, AFP and PIVKA-II levels, the number and size of tumors in the liver, metastasis, CNLC stage, hepatitis and cirrhosis. The baseline characteristics exhibited no significant differences between the two groups, with the exception of gender. Compared to the LT+TKI group, the LT+TKI+ICI group had a lower proportion of female patients enrolled.
As shown in Figure 1A, the median OS was 21 ± 3.0 and 28 ± 3.9 months in the LT+TKI and LT+TKI+ICI groups, respectively. The 6-, 12-, and 24-month OS rates were 85.8%, 71.5%, and 44.1% in the LT+TKI group, and 96.8%, 79.3%, and 59.4% in the LT+TKI+ICI group, respectively. The LT+TKI+ICI group demonstrated a significantly improved OS compared to the LT+TKI group (HR, 0.64; 95% CI, 0.449-0.913; P = 0.014).
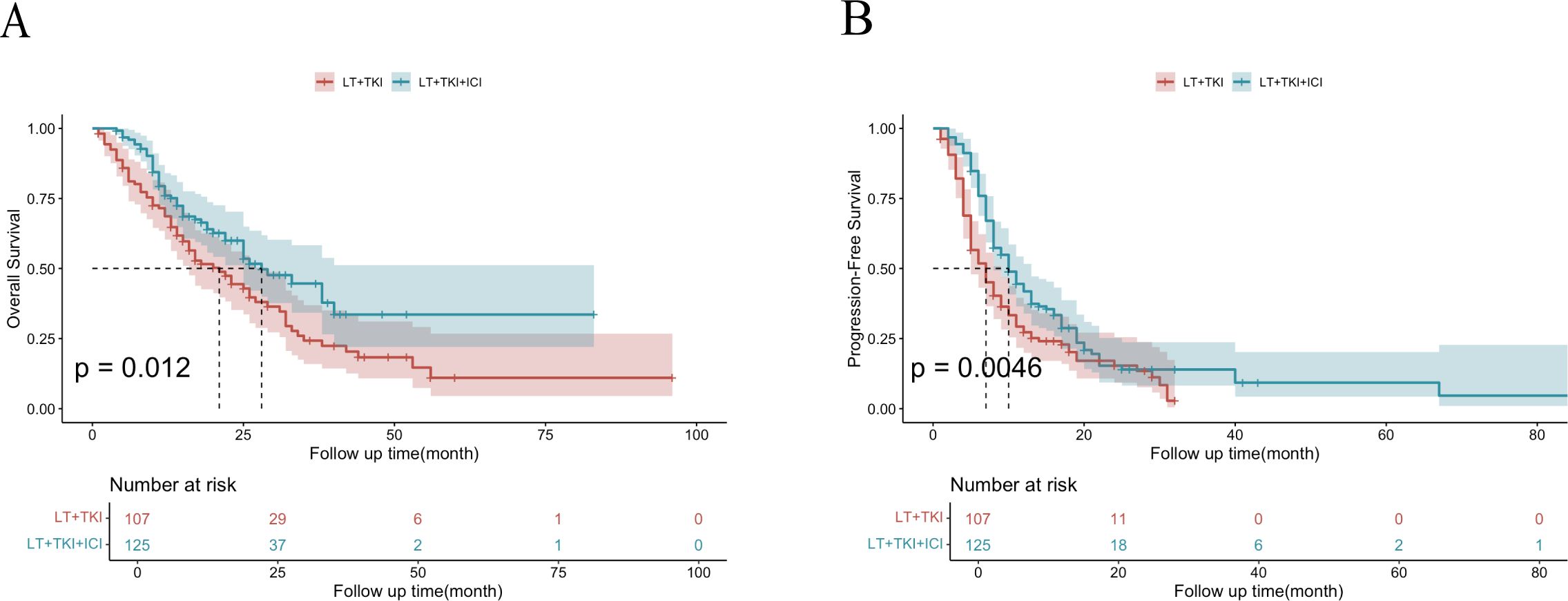
Figure 1. Kaplan-Meier curves of OS (A) and PFS (B) for the entire cohort of patients in the LT+TKI and LT+TKI+ICI groups.
As shown in Figure 1B, the median PFS was 7 ± 0.76 and 11 ± 1.1 months in the LT+TKI and LT+TKI+ICI groups, respectively. The 3-, 6-, and 12-month PFS rates were 90.6%, 64.0%, and 41.5% in the LT+TKI group, and 97.6%, 86.9%, and 60.5% in the LT+TKI+ICI group, respectively. The LT+TKI+ICI group also demonstrated a significantly longer PFS compared to the LT+TKI group (HR, 0.60; 95% CI, 0.452-0.805; P< 0.001).
Univariable and multivariable analysis of OS are conducted. Multivariable analysis indicate that LT+TKI therapy (HR, 1.72; 95% CI, 1.20-2.46; P = 0.003), vascular invasion (HR, 1.97; 95% CI,1.29-3.02; P = 0.002), lymphatic metastasis (HR, 1.59; 95% CI, 1.08-2.34; P= 0.019), metastasis (HR, 1.74; 95% CI, 1.14-2.66; P = 0.01), and PIVKA >1000mAU/mL (HR, 1.50; 95% CI,1.01-2.24; P = 0.045) were independent risk factors associated with poorer OS (Table 2).
Univariable and multivariable analysis of PFS are conducted as well. Multivariable analysis indicate that LT+TKI therapy (HR, 1.65; 95% CI, 1.24-2.21; P< 0.001), metastasis (HR, 1.50; 95% CI, 1.04-2.18; P = 0.032), age < 50 (HR, 1.38; 95% CI,1.01-1.88; P = 0.041) were independent risk factors associated with poorer PFS (Supplementary Table S1).
Forest plot analysis in subgroups was conducted. The results indicate a significantly improved OS in the LT+TKI+ICI group compared to the LT+TKI group for patients with the following characteristics: age >50, male, ALT >40u/ml, ALBI grade 1, Child-Pugh score class A, PIVKA-II ≤ 1000 mAu/ml, number of tumors ≤3, size of tumor ≤10cm, vascular invasion, no intrahepatic metastasis, no lymphatic metastasis, no extrahepatic metastasis, no cirrhosis, and HBV infection (Figure 2A). PFS was significantly prolonged in the LT+TKI+ICI group compared to the LT+TKI group for patients who were male, had ALT >40u/ml, AST >40u/ml, ALBI grade 1, number of tumors ≤3, size of tumor ≤10cm, vascular invasion, no extrahepatic metastasis, and HBV infection (Figure 2B).
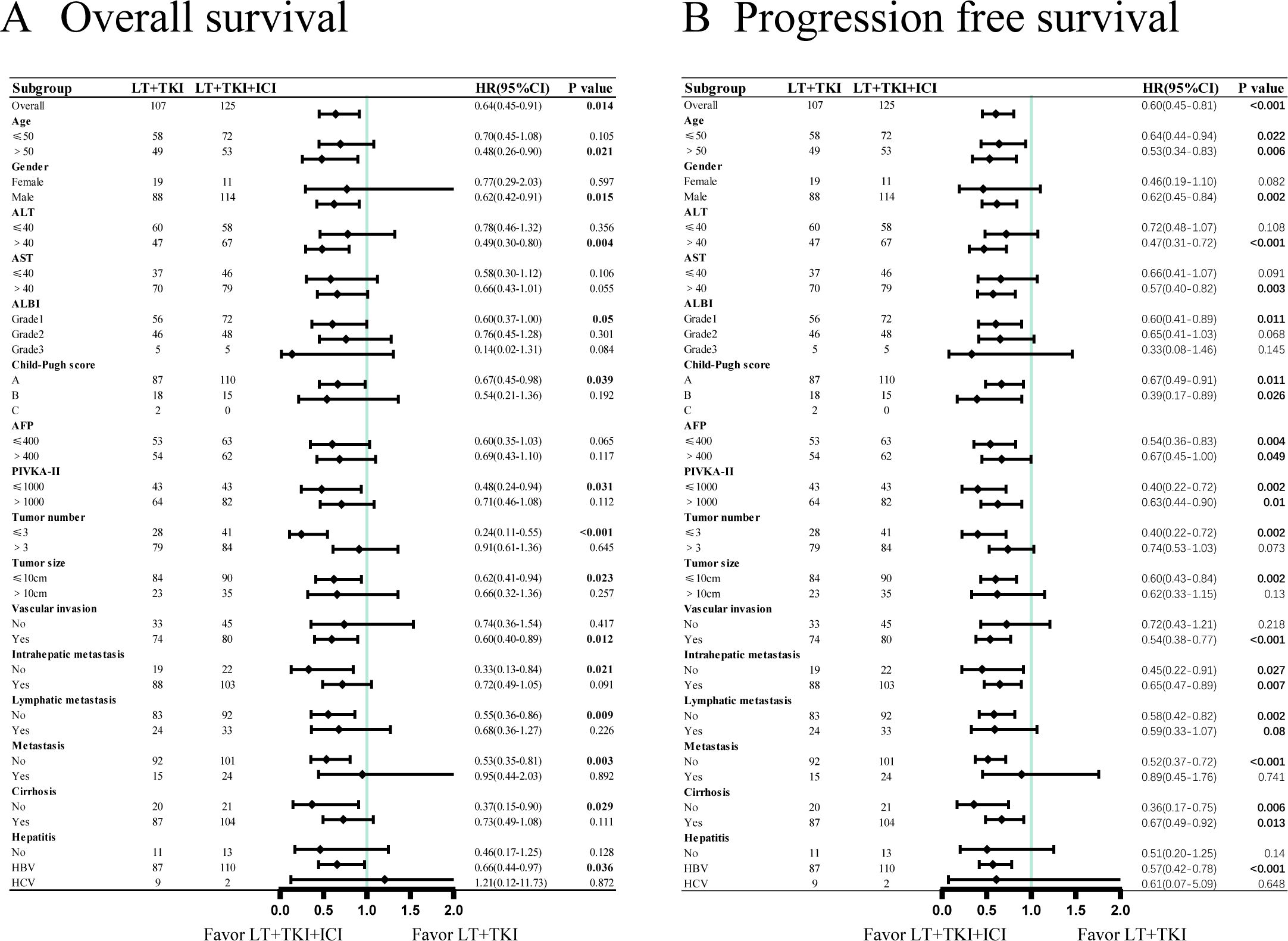
Figure 2. Subgroup analyses of (A) overall survival and (B) progression-free survival in the patient subgroups.
To further elucidate the impact of vascular invasion on prognosis, we conducted a subgroup analysis comprising 78 individuals without vascular invasion and 154 individuals with vascular invasion. The median OS of patients with vascular invasion was 15.0 ± 1.63 and 22.0 ± 2.78 months in the LT+TKI group and LT+TKI+ICI group, respectively, and 34.0 ± 2.86 and 38.0 ± 2.71 months in the two groups, respectively, without vascular invasion. The results indicated that the addition of ICI led to a significantly improved OS in patients with vascular invasion (HR, 0.60; 95% CI, 0.40-0.89; P=0.012) (Figure 3A). However, in the subgroup of patients without vascular invasion, the OS of the LT+TKI group and LT+TKI+ICI group showed no significant difference (HR, 0.74; 95% CI, 0.36-1.54; P=0.417) (Figure 3B). The PFS analysis demonstrated a similar trend, with the median PFS of patients with vascular invasion being 6.0 ± 0.65 and 10.0 ± 1.02 months in the LT+TKI group and LT+TKI+ICI group, respectively, and 11.0 ± 2.06 and 12.0 ± 1.39 months in the two groups, respectively, without vascular invasion. The addition of ICI also prolonged PFS in patients with vascular invasion (HR, 0.54; 95% CI, 0.38-0.77; P<0.001) (Figure 3C). In the subgroup of patients without vascular invasion, the PFS of the LT+TKI group and LT+TKI+ICI group showed no significant difference (HR, 0.72; 95% CI, 0.43-1.21; P=0.218) (Figure 3D).
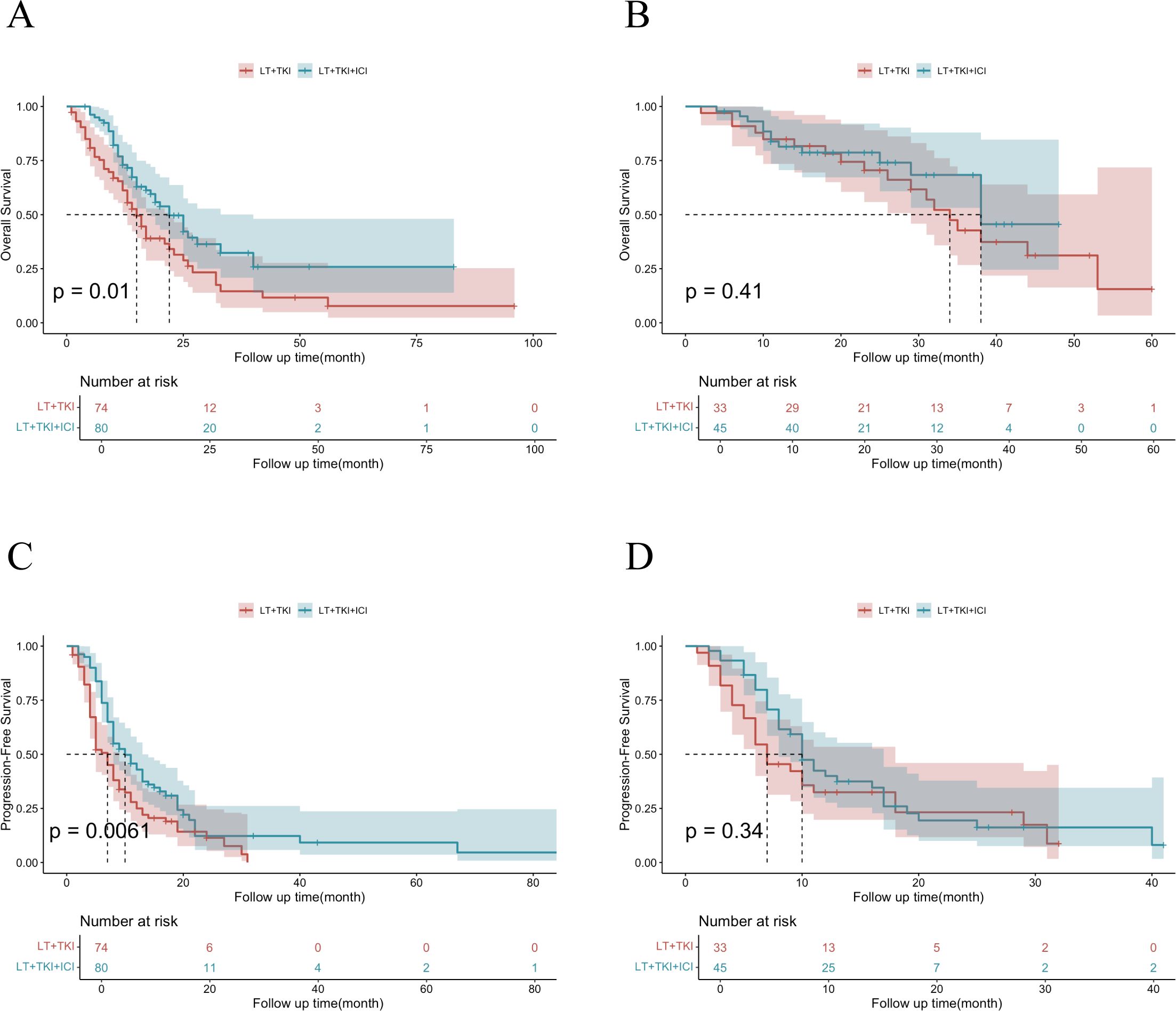
Figure 3. Survival analysis of subgroups stratified by Vascular Invasion.Kaplan-Meier curves of OS in patients with vascular invasion (A) and without vascular invasion (B) in the LT+TKI and LT+TKI+ICI groups. Kaplan-Meier curves of PFS in patients with vascular invasion (C) and without vascular invasion (D) in the LT+TKI and LT+TKI+ICI groups.
We also conducted a subgroup analysis stratified by PIVKA-II level and extrahepatic metastasis. In the subgroup of different PIVKA-II level, the results indicated that the addition of ICI led to a significantly improved OS in patients with low PIVKA-II level (40 vs 26 months; P=0.026) (Supplementary Figure S1A). However, in the subgroup of patients with high PIVKA-II level, the OS of the LT+TKI group and LT+TKI+ICI group showed no significant difference (25 vs 17 months; P=0.10) (Supplementary Figure S1B). Nevertheless, the PFS analysis demonstrated a same trend, the addition of ICI also prolonged PFS in patients with low PIVKA-II level (11 vs 6 months; P=0.0098) (Supplementary Figure S1C). In the subgroup of patients with high PIVKA-II level, the PFS of the LT+TKI group and LT+TKI+ICI group showed no significant difference (10 vs 7 months; P=0.11) (Supplementary Figure S1D). And in the subgroup of extrahepatic metastasis, the results indicated that the addition of ICI led to a significantly improved OS in patients without extrahepatic metastasis (40 vs 23 months; P=0.0022) (Supplementary Figure S2B). However, in the subgroup of patients who have extrahepatic metastasis, the OS of the LT+TKI group and LT+TKI+ICI group showed no significant difference (12 vs 12 months; P=0.89) (Supplementary Figure S2A). However, the PFS analysis demonstrated a contrast trend, the addition of ICI led to a significantly improved PFS in patients with extrahepatic metastasis (8.5 vs 4 months; P<0.001) (Supplementary Figure S2C). However, in the subgroup of patients without extrahepatic metastasis, the PFS of the LT+TKI group and LT+TKI+ICI group showed no significant difference (11 vs 8 months; P=0.051) (Supplementary Figure S2D).
The adverse events in the two groups are listed in Table 3. Overall, no death events occurred because of Treatment-related adverse events (TRAEs) during the follow-up period and there was no significant difference between the LT+TKI and LT+TKI+ICI groups in the types of severe TRAEs. The incidence of severe treatment-related adverse events (TRAEs) remained low in both groups, and patients who reported severe TRAEs temporarily discontinued TKI or ICI administration until the adverse effects were alleviated or resolved.
The present study rigorously evaluated the efficacy of adding an ICI to the established regimen of locoregional therapy and TKI in patients with unresectable HCC. Our findings demonstrate a significant improvement in both OS and PFS in the group receiving the combined therapy of LT+TKI+ICI, compared to those who received only LT+TKI therapy. Specifically, the median OS increased from 21 months in the LT+TKI group to 28 months in the LT+TKI+ICI group, accompanied by a superior PFS of 11 months compared to 7 months in the LT+TKI group. Further subgroup analyses revealed that patients with vascular invasion in the unresectable HCC cohort might derive greater benefits from the addition of ICI. These results indicate the potential of ICIs to enhance the therapeutic landscape for HCC, suggesting that combining immunotherapy with locoregional therapy and TKI may provide a more effective strategy for the treatment of unresectable HCC.
For the treatment of unresectable HCC, the only proven therapy was sorafenib, a tyrosine kinase inhibitor. Recently, the synergistic effect of an immune checkpoint inhibitor, atezolizumab plus bevacizumab outperformed sorafenib alone in terms of survival, making it the recommended first-line therapy. Other multikinase inhibitors, lenvatinib and regorafenib, were also recommended as first and second-line drugs, respectively (6, 20–22). Ann-Lii Cheng et al. (16) reported that a combination of atezolizumab and bevacizumab significantly improved both OS(19.2 months [95% CI 17.0–23.7] vs 13.4 months [95% CI 11.4–16.9]) and PFS (6.9 months [95% CI 5.7-8.6] vs 4.3 months [95% CI 4.0-5.6]) compared to the monotherapy with sorafenib. These findings highlight the potential of immunotherapy as a pivotal component of future therapeutic strategies for advanced HCC. Shen et al. (23) reported enhanced disease control and survival rates when combining TACE with sintilimab and lenvatinib, supporting the notion that multi-modal therapy can be beneficial. Similarly, Wang et al. (24) observed improved outcomes in infiltrative HCC with the addition of ICIs to hepatic arterial infusion chemotherapy and lenvatinib.
These studies above indicate the multiple therapy may enhanced the efficacy of individual treatments. HCC and other solid tumor cells can avoid the immune system attack by inducing the expression of immune checkpoints in T cells. ICIs are monoclonal antibodies that could block the interaction of immune checkpoint proteins with their ligands, thereby enhance the anti-tumor immune response by preventing the inactivation of T cells and restoring immune recognition and immune attack (25, 26). Historically, it was presumed that immune interventions were incompatible with conventional chemotherapies. However, emerging evidence indicates that standard chemotherapy agents might actually induce immunogenicity in the tumor microenvironment and the immune system itself. For instance, chemotherapeutic agents such as oxaliplatin, frequently utilized in TACE or hepatic artery infusion chemotherapy (HAIC), have been shown to upregulate HLA class I expression in tumor cells, associated with the proliferation and activation of cytotoxic T cells (27). Furthermore, TACE could induce tissue hypoxia that results in the upregulation of vascular endothelial growth factor (VEGF), which may lead to tumor revascularization and local recurrence (28), TKIs target the VEGF pathway, downregulate the VEGF expression, potentially inducing vascular normalization. Consequently, the combination of TACE with anti-angiogenic agents may potentially delay tumor progression or recurrence (17). In addition, the vascular normalization can enhance blood perfusion and reduce vascular leakage, leading to improved CD8+ T-cell infiltration in tumor tissues, as demonstrated in various animal models (29–32), which may enhance the efficacy of immune therapy theoretically. Additionally, ICIs can enhance anti-tumor activity by activating CD8+ T-cell function, thereby improving the tumor-killing efficacy of LT and TKIs. Based on these insights, the combination of a triple therapy regimen combining ICIs, LT, and TKIs may be crucial for maximizing therapeutic outcomes in advanced unresectable HCC.
Numerous studies have focused on the integration of ICIs with traditional therapies for HCC to investigate potential improvements in survival outcomes for patients ineligible for curative resection. Kelley RK et al. (33) enrolled the 837 patients who were randomly assigned to combination treatment of cabozantinib plus atezolizumab (n=432), sorafenib (n=217), or single-agent cabozantinib (n=188), the median progression-free survival was 6.8 months (99% CI 5.6-8.3) in the combination treatment group versus 4.2 months (2.8-7.0) in the sorafenib group (HR 0.63, 99% CI 0.44-0.91, P=0.0012). Median overall survival was 15.4 months (96% CI 13.7-17.7) in the combination treatment group versus 15.5 months (12.1-not estimable) in the sorafenib group (HR 0.90, 95% CI 0.69-1.18; P=0.44). Locoregional therapy such as TACE or ablation were considered suitable for patients with intermediate stage HCC. However, it has also been utilized extensively beyond this stage and has become a commonly employed non-surgical treatment option for various stages of HCC due to its effectiveness and widespread availability (23). The previous study shows TACE plus Lenvatinib and sintilimab leads to a satisfied median overall survival of 23.6 months (95%CI 22.2-25.0 months) (34). Jin ZC et al. (18) analysis 1244 patients received ICI-VEGF with or without TACE. the result showed TACE-ICI-VEGF group exhibited a significantly improved median OS (22.6 months [95% CI: 21.2-23.9] vs 15.9 months [14.9-17.8]; P < 0.0001). In our study, median OS was 28 ± 3.9 months in the LT+TKI+ICI group versus 21 ± 3.0 months in the LT+TKI group (HR, 0.64; 95% CI, 0.449-0.913; P = 0.014). Median PFS also favored the LT+TKI+ICI group (11 ± 1.1 months vs 7 ± 0.76 months; HR, 0.60; 95% CI, 0.452-0.805; P<0.001). Based on the findings, the combination of LT, TKI and ICI shows benefits in the treatment of unresectable HCC.
In our study cohort, patients with vascular invasion appeared to derive greater benefit from treatment compared to those without such invasion. Shen L et al. (35)reported TACE plus SBRT could provide improved OS and PFS in the patients with macrovascular invasion. SBRT is an advanced radiation modality that can concentrate a high radiation dose precisely on the tumor in a few fractions, provide a high local control rate (>80%) for tumor thrombosis, while TACE provides good control of tumors outside the radiation field as a complement (36). Additionally, the incorporation of a TKI and an ICI may further enhance control over the systemic tumor burden and improve the efficacy of locoregional therapies (8).
While our study provides valuable insights into the benefits of combining ICIs with locoregional therapies and TKIs in treating unresectable HCC, it has several limitations. As a retrospective analysis, our study is subject to inherent biases associated with such study designs, including selection and information bias, because the treatment was determined by doctors’ clinical judgement, patients’ tolerance and family economic affordability. We tried our best to minimize this bias by enrolling patients with specific inclusion and exclusion criteria, and all enrolled patients have been evaluated by a fixed-member MDT team to ensure treatment consistency. However, prospective randomized controlled trials are still needed to validate our findings. Our data derived from a single institution may not be generalizable to a broader population due to specific demographic and treatment practice variations. The relatively short follow-up period may not fully capture long-term survival outcomes and late emerging effects of the therapy combinations.
Integrating immune checkpoint inhibitors (ICIs) with locoregional therapies (LT) and tyrosine kinase inhibitors (TKIs) signifies a major advancement in treating unresectable hepatocellular carcinoma (HCC). This study highlights a triple therapy regimen’s potential to improve survival outcomes for advanced unresectable HCC patients, emphasizing ICIs’ role in enhancing established treatments. Our findings may set the stage for future investigations into the integration of immunotherapy with traditional HCC therapies, potentially leading to more refined and effective treatment protocols for this challenging condition.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by The Ethic Committee of West China Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
JB: Data curation, Formal analysis, Investigation, Visualization, Writing – original draft. ZL: Formal analysis, Investigation, Software, Writing – review & editing. DH: Data curation, Investigation, Writing – review & editing. LL: Data curation, Investigation, Writing – review & editing. JH: Methodology, Project administration, Resources, Writing – review & editing. XW: Methodology, Project administration, Resources, Writing – review & editing. QL: Methodology, Project administration, Resources, Writing – review & editing. JZ: Conceptualization, Resources, Supervision, Validation, Writing – review & editing. YZ: Conceptualization, Funding acquisition, Resources, Supervision, Validation, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1554711/full#supplementary-material
1. Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. (2021) 7:6. doi: 10.1038/s41572-020-00240-3
2. Villanueva A. Hepatocellular carcinoma. N Engl J Med. (2019) 380:1450–62. doi: 10.1056/NEJMra1713263
3. Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR. A global view of hepatocellular carcinoma: Trends, risk, prevention and management. Nat Rev Gastroenterol Hepatology. (2019) 16:589–604. doi: 10.1038/s41575-019-0186-y
4. Vogel A, Meyer T, Sapisochin G, Salem R, Saborowski A. Hepatocellular carcinoma. Lancet. (2022) 400:1345–62. doi: 10.1016/S0140-6736(22)01200-4
5. Rossari F, Tada T, Suda G, Shimose S, Kudo M, Yoo C, et al. Disease etiology impact on outcomes of hepatocellular carcinoma patients treated with atezolizumab plus bevacizumab: A real-world, multicenter study. Liver Cancer. (2024) 13(15):522–36. doi: 10.1159/000537915
6. Leowattana W, Leowattana T, Leowattana P. Systemic treatment for unresectable hepatocellular carcinoma. World J Gastroenterol. (2023) 29:1551–68. doi: 10.3748/wjg.v29.i10.1551
7. Reig M, Forner A, Rimola J, Ferrer-Fábrega J, Burrel M, Garcia-Criado Á, et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J hepatology. (2021) 76:681. doi: 10.1016/j.jhep.2021.11.018
8. Chiang CL, Chan KSK, Chiu KWH, Lee FAS, Chen W, Wong NSM, et al. Complete response to locoregional therapy plus immunotherapy for hepatocellular carcinoma. JAMA Oncol. (2024) 10(11):1548–53. doi: 10.1001/jamaoncol.2024.4085
9. Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. (2008) 359:378–90. doi: 10.1056/NEJMoa0708857
10. Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet (London England). (2018) 391:1163–73. doi: 10.1016/S0140-6736(18)30207-1
11. Cariani E, Missale G. Immune landscape of hepatocellular carcinoma microenvironment: Implications for prognosis and therapeutic applications. Liver International: Off J Int Assoc Study Liver. (2019) 39:1608–21. doi: 10.1111/liv.14192
12. Sangro B, Sarobe P, Hervás-Stubbs S, Melero I. Advances in immunotherapy for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatology. (2021) 18:525–43. doi: 10.1038/s41575-021-00438-0
13. Psilopatis I, Damaskos C, Garmpi A, Sarantis P, Koustas E, Antoniou EA, et al. FDA-approved monoclonal antibodies for unresectable hepatocellular carcinoma: what do we know so far? Int J Mol Sci. (2023) 24(3):2685. doi: 10.3390/ijms24032685
14. Yau T, Park JW, Finn RS, Cheng AL, Mathurin P, Edeline J, et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. (2022) 23:77–90. doi: 10.1016/S1470-2045(21)00604-5
15. Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. (2018) 19:940–52. doi: 10.1016/S1470-2045(18)30351-6
16. Cheng AL, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Updated efficacy and safety data from IMbrave150: Atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol. (2022) 76:862–73. doi: 10.1016/j.jhep.2021.11.030
17. Yuan Y, He W, Yang Z, Qiu J, Huang Z, Shi Y, et al. TACE-HAIC combined with targeted therapy and immunotherapy versus TACE alone for hepatocellular carcinoma with portal vein tumour thrombus: a propensity score matching study. Int J Surg (Lond Engl). (2023) 109:1222–30. doi: 10.1097/JS9.0000000000000256
18. Jin ZC, Chen JJ, Zhu XL, Duan XH, Xin YJ, Zhong BY, et al. Immune checkpoint inhibitors and anti-vascular endothelial growth factor antibody/tyrosine kinase inhibitors with or without transarterial chemoembolization as first-line treatment for advanced hepatocellular carcinoma (CHANCE2201): A target trial emulation study. EClinicalMedicine. (2024) 72:102622. doi: 10.1016/j.eclinm.2024.102622
19. Dueck AC, Mendoza TR, Mitchell SA, Reeve BB, Castro KM, Rogak LJ, et al. Validity and reliability of the US national cancer institute’s patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE). JAMA Oncol. (2015) 1:1051–9. doi: 10.1001/jamaoncol.2015.2639
20. Brown ZJ, Tsilimigras DI, Ruff SM, Mohseni A, Kamel IR, Cloyd JM, et al. Management of hepatocellular carcinoma: A review. JAMA surgery. (2023) 158:410–20. doi: 10.1001/jamasurg.2022.7989
21. Chidambaranathan-Reghupaty S, Fisher PB, Sarkar D. Hepatocellular carcinoma (HCC): Epidemiology, etiology and molecular classification. Adv Cancer Res. (2021) 149:1–61. doi: 10.1016/bs.acr.2020.10.001
22. Miura R, Ono A, Yano S, Amioka K, Naruto K, Yamaoka K, et al. Real-world efficacy and safety of durvalumab–tremelimumab as second-line systemic therapy after atezolizumab–bevacizumab in unresectable hepatocellular carcinoma. Med (baltimore). (2024) 103:e39289. doi: 10.1097/MD.0000000000039289
23. Shen C, Jiang W, Chen R, Li L, Wu Y, Tan L, et al. Transarterial chemoembolization combined with sintilimab and lenvatinib for the treatment of unresectable hepatocellular carcinoma: A retrospective study. J Cancer Res Clin Oncol. (2024) 150:427. doi: 10.1007/s00432-024-05949-2
24. Wang W, Li R, Li H, Wang M, Wang J, Wang X, et al. Addition of immune checkpoint inhibitor showed better efficacy for infiltrative hepatocellular carcinoma receiving hepatic arterial infusion chemotherapy and lenvatinib: A multicenter retrospective study. ImmunoTargets Ther. (2024) 13:399–412. doi: 10.2147/ITT.S470797
25. Liu Z, Liu X, Liang J, Liu Y, Hou X, Zhang M, et al. Immunotherapy for hepatocellular carcinoma: Current status and future prospects. Front Immunol. (2021) 12:765101. doi: 10.3389/fimmu.2021.765101
26. Zhang Y, Zhang Z. The history and advances in cancer immunotherapy: Understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell Mol Immunol. (2020) 17:807–21. doi: 10.1038/s41423-020-0488-6
27. Liu WM, Fowler DW, Smith P, Dalgleish AG. Pre-treatment with chemotherapy can enhance the antigenicity and immunogenicity of tumours by promoting adaptive immune responses. Br J Cancer. (2010) 102:115–23. doi: 10.1038/sj.bjc.6605465
28. Chang Y, Jeong SW, Young Jang J, Jae Kim Y. Recent updates of transarterial chemoembolilzation in hepatocellular carcinoma. Int J Mol Sci. (2020) 21:8165. doi: 10.3390/ijms21218165
29. Kuo HY, Khan KA, Kerbel RS. Antiangiogenic–immune-checkpoint inhibitor combinations: Lessons from phase III clinical trials. Nat Rev Clin Oncol. (2024) 21:468–82. doi: 10.1038/s41571-024-00886-y
30. Manning EA, Ullman JGM, Leatherman JM, Asquith JM, Hansen TR, Armstrong TD, et al. A vascular endothelial growth factor receptor-2 inhibitor enhances antitumor immunity through an immune-based mechanism. Clin Cancer Research: Off J Am Assoc Cancer Res. (2007) 13:3951–9. doi: 10.1158/1078-0432.CCR-07-0374
31. Kikuchi H, Matsui A, Morita S, Amoozgar Z, Inoue K, Ruan Z, et al. Increased CD8+ T-cell infiltration and efficacy for multikinase inhibitors after PD-1 blockade in hepatocellular carcinoma. J Natl Cancer Inst. (2022) 114:1301–5. doi: 10.1093/jnci/djac051
32. Starzer AM, Wolff L, Popov P, Kiesewetter B, Preusser M, Berghoff AS. The more the merrier? Evidence and efficacy of immune checkpoint- and tyrosine kinase inhibitor combinations in advanced solid cancers. Cancer Treat Rev. (2024) 125:102718. doi: 10.1016/j.ctrv.2024.102718
33. Kelley RK, Rimassa L, Cheng AL, Kaseb A, Qin S, Zhu AX, et al. Cabozantinib plus atezolizumab versus sorafenib for advanced hepatocellular carcinoma (COSMIC-312): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. (2022) 23:995–1008. doi: 10.1016/S1470-2045(22)00326-6
34. Cao F, Yang Y, Si T, Luo J, Zeng H, Zhang Z, et al. The efficacy of TACE combined with lenvatinib plus sintilimab in unresectable hepatocellular carcinoma: A multicenter retrospective study. Front Oncol. (2021) 11:783480. doi: 10.3389/fonc.2021.783480
35. Shen L, Xi M, Zhao L, Zhang X, Wang X, Huang Z, et al. Combination therapy after TACE for hepatocellular carcinoma with macroscopic vascular invasion: Stereotactic body radiotherapy versus sorafenib. Cancers. (2018) 10:516. doi: 10.3390/cancers10120516
Keywords: unresectable hepatocellular carcinoma, locoregional therapy, tyrosine kinase inhibitor, immune checkpoint inhibitor, system therapy
Citation: Bu J, Li Z, Hu D, Lan L, Huang J, Wang X, Li Q, Zhou J and Zeng Y (2025) Enhanced efficacy of immune checkpoint inhibitors combined locoregional therapy and tyrosine kinase inhibitors in the treatment of unresectable hepatocellular carcinoma: A single - center retrospective study. Front. Oncol. 15:1554711. doi: 10.3389/fonc.2025.1554711
Received: 02 January 2025; Accepted: 04 February 2025;
Published: 25 February 2025.
Edited by:
Rodrigo Xavier Das Neves, National Cancer Institute (NIH), United StatesReviewed by:
Chenguang Su, Affiliated Hospital of Chengde Medical University, ChinaCopyright © 2025 Bu, Li, Hu, Lan, Huang, Wang, Li, Zhou and Zeng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yong Zeng, emVuZ3lvbmdAbWVkbWFpbC5jb20uY24=; Jin Zhou, emhvdWppbjA5NkAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.