
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 05 March 2025
Sec. Surgical Oncology
Volume 15 - 2025 | https://doi.org/10.3389/fonc.2025.1533378
This article is part of the Research Topic Advances in Esophageal Cancer: Treatment Updates and Future Challenges View all 5 articles
 Ke-xun Li1,2†
Ke-xun Li1,2† Si-miao Lu1,3†
Si-miao Lu1,3† Chang-ding Li1,4†
Chang-ding Li1,4† Cheng-hao Wang1
Cheng-hao Wang1 Jia-hua Lv5,6
Jia-hua Lv5,6 Qi-feng Wang5,6
Qi-feng Wang5,6 Yun-chao Huang2
Yun-chao Huang2 Yong-tao Han1
Yong-tao Han1 Xue-feng Leng1*
Xue-feng Leng1* Lin Peng1*
Lin Peng1*Background: Esophagectomy is the primary treatment for localized esophageal squamous cell carcinoma (ESCC). Intraoperative thoracic duct ligation (TDL) has been suggested as an adjunct to reduce the risk of postoperative chylothorax in patients with ESCC, but its effect on long-term oncologic outcomes remains uncertain.
Methods: Data from the Sichuan Cancer Hospital and Institute Esophageal Cancer Case Management Database were analyzed for patients treated between 2010 and 2017. Participants were classified into TDL and non-TDL groups. Univariate Cox regression analyses and propensity score matching (PSM) were used to identify independent risk factors for overall survival (OS).
Results: A total of 2,510 patients were included, with 2,095 in the TDL group and 415 in the non-TDL group. The median follow-up was 63.97 months. No significant differences in OS were observed between the TDL and non-TDL groups (HR: 1.13; 95% CI: 0.96–1.31; P = 0.13). After PSM, the analysis continued to show no significant differences between the groups (P = 0.72).
Conclusion: Intraoperative TDL during esophagectomy did not significantly impact long-term OS in patients with ESCC.
Esophageal cancer is a highly aggressive malignancy with poor overall survival (OS), posing a significant global health challenge. Esophageal squamous cell carcinoma (ESCC) is the predominant histological subtype, particularly in East Asian countries (1, 2). Despite advancements in multimodal treatment strategies centered around esophagectomy (3–6), ESCC remains associated with high rates of lymph node metastasis (LNM) and poor long-term OS (7, 8).
Esophagectomy is the primary curative approach for localized ESCC (9, 10), but long-term outcomes vary based on several factors (11–13), necessitating individualized treatment plans (14–16). However, this procedure carries a notable risk of postoperative complications, such as chylothorax - the leakage of lymphatic fluid into the pleural space- which can lead to severe metabolic and nutritional imbalances, prolonged hospitalization and increased mortality (17, 18).
To mitigate the risk of chylothorax, intraoperative thoracic duct ligation (TDL) has been proposed as an adjunct to esophagectomy. TDL may lower the incidence of chylothorax-related complications (19–21), however, its effect on long-term survival in patients with ESCC remains uncertain, and high-quality studies on this topic are limited in China.
This retrospective cohort study aimed to assess the long-term OS of ESCC patients undergoing esophagectomy with or without intraoperative TDL.
Data for this retrospective analysis were collected from the Sichuan Cancer Hospital and Institute Esophageal Cancer Case Management Database (SCCH-ECCM) covering cases from January 2010 to December 2017. The study included patients diagnosed with ESCC who underwent esophagectomy at the institution. Demographic and pathological data were reviewed, and disease staging was conducted according to the AJCC 8th edition TNM system. Patients were followed post-treatment, with assessments every 3 months for the first 2 years and every 6 months for years 3 to 5. OS was calculated from the date of diagnosis to either the last follow-up or death. A total of 2,510 patients with ESCC who underwent esophagectomy between 2010 and 2017 met the inclusion criteria. Criteria excluded patients with non-squamous cell carcinoma, tumors outside the thoracic region, incomplete resection, distant metastasis, early-stage disease, or missing data (Figure 1). OS was assessed from diagnosis to the most recent follow-up in March 2021. Smoking history: Patients who reported smoking at least once per day for a duration exceeding one year. Drinking history: Patients who reported consuming alcohol at least once per day for a duration exceeding one year. We hope this clarification addresses your concerns.
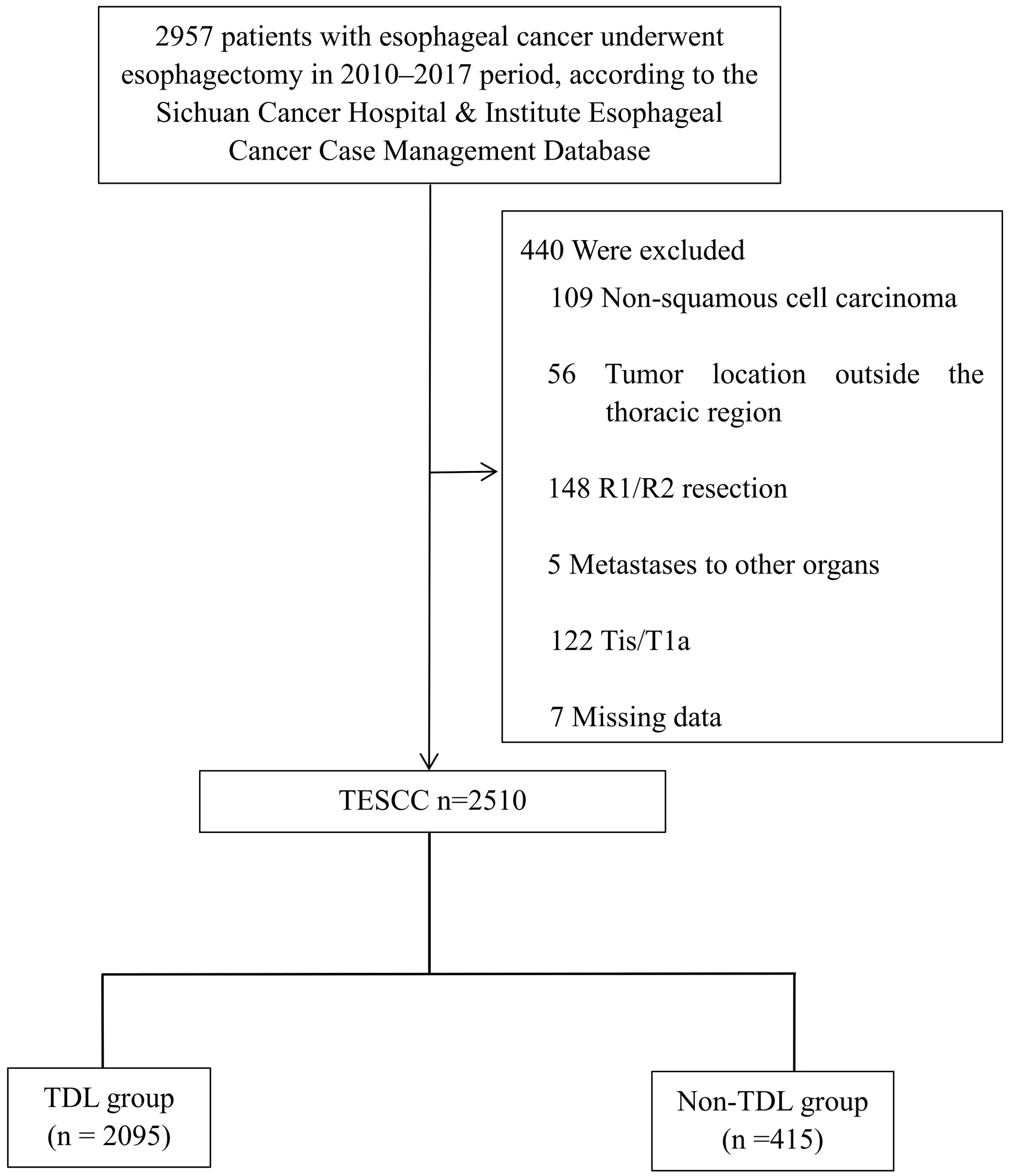
Figure 1. CONSORT diagram of patient selection. TESCC, thoracic esophageal squamous cell carcinoma; TDL, thoracic duct ligation.
Patients were divided into two groups based on intraoperative TDL status. The TDL group included patients who had esophagectomy with concurrent intraoperative TDL, including cases where incidental thoracic duct (TD) injury during surgery required ligation. The Non-TDL group comprised patients who underwent esophagectomy without TDL, where no incidental TD injury occurred, and the duct was preserved intentionally. Clinical outcomes and survival data were compared between groups according to TNM stages as per the AJCC 8th edition.
This study was conducted in accordance with the Declaration of Helsinki (2013 revision) and approved by the SCCHEC-02-2024-191 Ethics Committee at Sichuan Cancer Hospital.
Categorical variables were presented as percentages and compared between the TDL and Non-TDL groups using chi-square or Fisher’s exact tests, as appropriate. The primary outcome of interest was OS), estimated using Kaplan-Meier survival curves and assessed with the log-rank test. To minimize confounding and selection bias, propensity score matching (PSM) was applied. Propensity scores were calculated with a logistic regression model using treatment (TDL vs. Non-TDL) as the dependent variable and relevant covariates, including age, sex and other significant baseline characteristics, as independent variables. A 1:1 nearest-neighbor matching algorithm without replacement was employed, with a calliper width of 0.2 times the standard deviation of the logit of the propensity score. Covariate balance between matched groups was evaluated using standardized mean differences, with values below 0.1 indicating satisfactory balance. Univariable and multivariable Cox proportional hazards regression models were used to identify independent risk factors associated with OS, with hazard ratios (HRs) and 95% confidence intervals (CIs) reported. Sensitivity analyses were conducted using stabilized inverse probability of treatment weighting (IPTW), overlap weighting (OW) and standardized mortality ratio weighting (SMRW) methods to confirm robustness. Adjusted HRs and 95% CIs were calculated with these alternative weighting approaches. All statistical analyses were conducted using SPSS version 23.0 (Chicago, IL, USA) and RStudio with R version 4.3.0. A two-sided P value < 0.05 was considered statistically significant.
Data from 2,510 patients with ESCC were analyzed retrospectively. As shown in Figure 1, 82.1% (2,060/2,510) were male and 17.9% (450/2,510) were female, with approximately 57.5% (1,443/2,510) of cases above stage III (Table 1). Among these patients, 2,095 were in the TDL group and 415 were in the Non-TDL group.
Further analyses include recurrence in the upper mediastinum, recurrence in the residual esophagus, anastomotic recurrence, distant organ and bone metastasis, lymph node (LN) recurrence in the mediastinal and supraclavicular zones, as well as recurrence and metastasis in the abdominal organs, peritoneal space, peritoneum, and abdominal LNs. Additionally, mortality rates at 30 days, 90 days, and six months were incorporated into Table 2. The outcomes indicated that patients in the TDL group exhibited significantly higher rates of recurrence in the upper mediastinum (P=0.013) and LN recurrence in the mediastinal and supraclavicular zones (P=0.041) compared to those in the Non-TDL group. However, for the remaining outcome measures, no statistically significant differences were observed between the two groups. After PSM to mitigate potential confounding factors, we found that there were no statistically significant differences in any of the recurrence and metastasis indicators between the TDL and Non-TDL groups. This suggests that while the TDL group exhibited higher rates of certain types of recurrence initially, these differences were not sustained when accounting for baseline characteristics through PSM.
The median follow-up period was 63.97 months. The TDL group had a median OS of 43.80 months (95% CI: 38.83–48.77), while the Non-TDL group had a median OS of 50.03 months. The 1-, 3-, and 5-year OS rates were 86%, 56% and 45% for the TDL group, and 87%, 60% and 47% for the Non-TDL group (HR: 1.13; 95% CI: 0.96–1.31; P = 0.13; Figure 2). Post-PSM, no significant differences were observed between groups (HR: 0.96; 95% CI: 0.96–1.04; P = 0.72; Figure 3A). Similar results were obtained with IPTW, OW and SMRW methods (HR: 0.93; 95% CI: 0.93–1.08; P = 0.55; Figure 3B; HR: 0.88; 95% CI: 0.99–1.01; P = 0.93; Figure 3C; and HR: 1.14; 95% CI: 0.88–1.14; P = 0.19; Figure 3D). Figure 3E illustrated that for 1:1 PSM, IPTW, and OW methods, the standardized mean difference for all variables is less than 0.02.
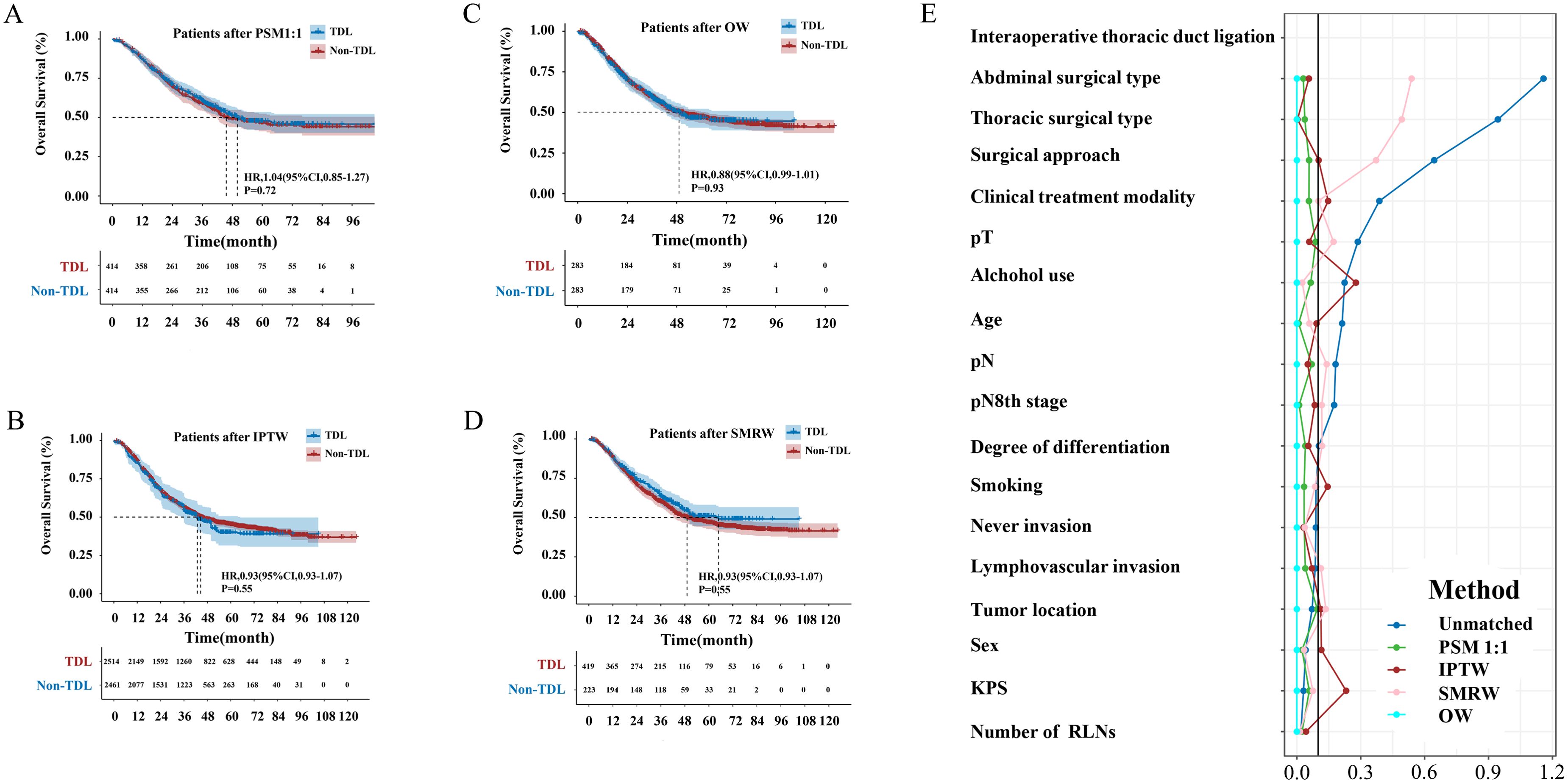
Figure 3. (A) Overall survival curves of TDL and Non-TDL groups after PSM; (B) Overall survival curves of TDL and Non-TDL groups after IPTW; (C) Overall survival curves of TDL and Non-TDL groups after OW; (D) Overall survival curves of TDL and Non-TDL groups after SMRW; (E) Standardized in the subjects stratified by characteristic.
Univariate analysis identified several factors significantly impacting OS after esophagectomy: alcohol use (P < 0.001), smoking status (P < 0.001), age (P = 0.004), KPS scores (P < 0.001), sex (P < 0.001), thoracic and abdominal surgical type (P < 0.001 and P = 0.024, respectively), surgical approach (P = 0.004), tumor grade (P < 0.001), lymphovascular and nerve invasion (P < 0.001 each), pathological T and N categories (P < 0.001) and TNM stage (P < 0.001; Figure 4). Multivariate analysis indicated that alcohol use (P = 0.003), age (P < 0.001), KPS scores (P = 0.022), tumor grade (P = 0.003), lymphovascular invasion (P = 0.047) and pathological T and N categories (P < 0.001 each) were independently associated with OS post-esophagectomy (Figure 4).

Figure 4. Univariate and multivariate Cox regression analyses of factors affecting patient survival.
This retrospective cohort study investigated the impact of TDL on the long-term OS of patients with ESCC who underwent esophagectomy. While previous research has indicated the potential benefits of TDL in minimizing chylothorax incidence, our findings did not reveal a significant difference in OS between patients who underwent TDL and those who did not. This suggests that the role of TDL in enhancing long-term survival outcomes in ESCC treatment may be limited. Although TDL theoretically reduces chylothorax-related complications, the lack of significant disparity in OS between the TDL and Non-TDL groups indicates that TDL may not substantially affect long-term prognosis (19–21). This conclusion held even after rigorous statistical adjustments using PSM and various weighting methods, indicating that the decision to perform TDL may not influence survival outcomes in patients with ESCC. The study further identified several independent factors affecting OS post-esophagectomy, including alcohol use, smoking, age, KPS scores, sex, surgical approach, tumor grade, lymphovascular invasion, nerve invasion and TNM stage.
The absence of a significant difference in OS between the two groups underscores the complexity of factors impacting survival outcomes in ESCC. While surgical innovations aim to reduce perioperative risks and enhance short-term outcomes, long-term survival is more intricately linked to factors such as tumor stage, patient health, genetic predispositions and the effectiveness of adjunctive treatments like chemotherapy, radiotherapy and immunotherapy (22–25).
Before PSM, patients in the TDL group indeed exhibited higher rates of upper mediastinal recurrence (P=0.013) and mediastinal/supraclavicular LN recurrence (P=0.041). We hypothesize that this discrepancy may be attributed to baseline imbalances in TNM staging between the two cohorts before matching. Specifically, the TDL group showed a tendency toward more advanced disease stages at initial diagnosis, which could predispose this population to increased locoregional recurrence risk. Importantly, these differences were mitigated after PSM adjustment, suggesting that tumor stage rather than TDL status itself may be the primary driver of recurrence patterns.
The current treatment paradigm for ESCC primarily focuses on a multimodal approach centered around surgery, with additional neoadjuvant therapies like radiotherapy, chemotherapy and immunotherapy. This is followed by adjuvant chemotherapy or immunotherapy for maintenance (26–29). Lymphadenectomy and TDL are crucial components of the surgical procedure. Lymphadenectomy is essential for accurate staging and potentially curative treatment, as LN involvement is a key prognostic factor in ESCC. Comprehensive LN removal allows for precise staging and may eradicate micrometastatic disease, thereby enhancing survival (30–32). While TDL is effective in reducing chylothorax incidence, its impact on long-term survival remains inconclusive (19–21). Additional large-scale, multicenter studies are warranted to establish the role of TDL in ESCC treatment, especially concerning long-term survival, to better inform surgical decisions and improve patient outcomes.
A study in Japan investigated the impact of the TD and surrounding LN resection on short-term and long-term outcomes, as well as postoperative nutritional status, in patients with esophageal cancer undergoing esophagectomy. Among the 145 patients (27.0%) who underwent TD and surrounding LN resection (33), those in the resection group had more advanced clinical stages and more frequently received preoperative treatment. Additionally, these patients experienced longer surgical time and greater intraoperative blood loss. Complications classified as Clavien–Dindo Grade II, along with pulmonary complications, were more prevalent in the resection group. Multivariate analysis identified TD resection as an independent risk factor for pulmonary complications and indicated a possible association with Clavien–Dindo Grade II complications. Despite a higher count of thoracic anatomical LNs resected in the resection group, OS rates remained similar across all stages regardless of TD resection. Furthermore, one year postoperatively, the nutritional status between the groups was comparable. The findings suggest that extensive LN resection combined with TD resection may not improve prognostic outcomes and could increase postoperative complications, potentially affecting survival negatively (33). Extended LN dissection alongside TD resection may correlate with higher rates of chylothorax and other complications (33–35). It was noted that chylothorax, in particular, can delay chemotherapy initiation, potentially diminishing the survival benefits of aggressive resection.
In 2023, a national study in Japan further analyzed TD resection outcomes using data from the Comprehensive Registry of Esophageal Cancer in Japan, including 12,237 patients who underwent esophagectomy between 2007 and 2012. Results showed no significant OS improvement in patients who had TD resection compared to those who did not. Interestingly, while the TD resection group had a higher retrieval of mediastinal nodes and lower rates of LN recurrence, this did not translate to better survival outcomes. On the contrary, these patients had an increased incidence of distant metastasis. This finding challenges the assumed preventive benefit of TD resection in esophageal cancer, suggesting that this approach may elevate the risk of distant metastasis despite its theoretical advantages in LN management. The increased retrieval of mediastinal nodes in the TD resection group, although indicating a more thorough surgical clearance, did not correlate with enhanced survival. This observation underscores the complex relationship between the extent of surgical intervention and prognosis in esophageal cancer. Hou and colleagues’ study provided valuable insights by categorizing patients into those who underwent prophylactic TDL and those who did not, allowing for a clear comparison of outcomes. Contrary to the expected benefits, there was no significant difference in the incidence of postoperative chylothorax between the two groups. This challenges the commonly held rationale for TDL, which is often performed to prevent this specific complication. Similarly, Aiolfi et al.’s meta-analysis reinforces these findings, highlighting the ongoing debate surrounding the efficacy of TDL in esophageal cancer surgeries. By analyzing data from a substantial patient cohort, the meta-analysis showed that TDL does not significantly reduce the risk of postoperative chylothorax, thus questioning the procedure’s protective role. Moreover, both studies consistently indicate a concerning impact of TDL on long-term survival, with patients undergoing the procedure experiencing reduced overall survival compared to those who did not. These findings collectively call into question the routine implementation of prophylactic TDL in esophagectomy. Rather than uniformly benefiting patients, TDL may inadvertently compromise long-term survival outcomes (34–38).
This study has several limitations. As a retrospective analysis, it is subject to inherent biases, including selection bias and uncontrolled confounding factors. Although PSM was employed to reduce these biases, residual confounders may still be present. Furthermore, the single-institution setting limits the generalizability of the findings to other populations or healthcare systems. Multicenter studies with larger sample sizes are essential to confirm these results. Additionally, this study focused solely on the impact of TDL on long-term OS, without assessing other critical outcomes such as disease-free survival or quality of life. These outcomes are important for a holistic evaluation of TDL’s effectiveness. The significance of preoperative therapy, especially in patients with T2/T3N0-1 esophageal squamous cell carcinoma, has been acknowledged in the CROSS study. Even the results of CROSS and NEOCRTEC5010 studies were used as guidelines to recommend preoperative neoadjuvant therapy for patients with locally advanced esophageal cancer (4, 35), China’s own data of esophageal squamous cell carcinoma (NEOCRTEC5010 study) was published in 2018 and the first guideline of Chinese Society Of Clinical Oncology (CSCO) on diagnosis and treatment of esophageal cancer published in 2019. In the data we included from 2010 to 2017, real-world clinical practice primarily involved surgery alone for resectable tumors. However, over the past 5 years, the proportion of neoadjuvant therapy has gradually increased in our center and China. In future studies, we will consider including more patients who have undergone neoadjuvant therapy and investigate the potential impact of such treatments on patient OS outcomes. Future research should include a broader range of outcomes to provide a more comprehensive assessment of TDL’s impact.
This retrospective cohort study found no statistically significant difference in long-term OS between patients with ESCC who underwent esophagectomy with intraoperative TDL and those who did not. While locoregional control remains important, the increased risk of complications—especially those affecting timely systemic therapy—may offset theoretical oncologic advantages. This supports the recommendation for individualized surgical strategies prioritizing both short-term recovery and long-term survival.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
This study was conducted in accordance with the Declaration of Helsinki (2013 revision) and approved by the SCCHEC-02-2024-191 Ethics Committee at Sichuan Cancer Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because This is a retrospective study.
K-XL: Conceptualization, Supervision, Validation, Writing – review & editing, Writing – original draft. S-ML: Conceptualization, Validation, Writing – review & editing, Formal Analysis. C-DL: Conceptualization, Writing – review & editing, Data curation. C-HW: Conceptualization, Writing – review & editing. J-HL: Conceptualization, Writing – review & editing. Q-FW: Conceptualization, Writing – review & editing. Y-CH: Conceptualization, Writing – review & editing. Y-TH: Conceptualization, Writing – review & editing, Funding acquisition. X-FL: Conceptualization, Funding acquisition, Writing – review & editing, Supervision, Validation. LP: Conceptualization, Supervision, Validation, Writing – review & editing, Resources.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the grants from the National Natural Science Foundation of China (82472663); the International Cooperation Projects of Science and Technology Department of Sichuan Province grant number 2024YFHZ0322; the Chengdu Science and Technology Bureau Key Research Project (2024-YF05-00797-SN); the Sichuan Key Research and Development Project from Science and Technology Department of Sichuan Province grant number 2023YFS0044, 2023YFQ0056 and 2022YFQ0008; and Sichuan Province Clinical Key Specialty Construction Project ([2022]70).
We appreciated SCCH-ECCM Database for providing the original study data. We thank Bullet Edits Limited for the linguistic editing and proofreading of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
ESCC, esophageal squamous cell carcinoma; RT, radiotherapy; CRT, chemoradiotherapy; OS, overall survival; TDL, thoracic duct ligation; LN, lymph node; LNM, lymph node metastasis; HRs, hazard ratios; CIs, confidence intervals; TD, thoracic duct; PSM, propensity score matching; KPS, Karnofsky Performance Status; IPTW, inverse probability of treatment weighting; SMRW, standardized mortality ratio weighting; OW, overlap weighting.
1. Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. (2023) 73:17–48. doi: 10.3322/caac.21763
2. Han B, Zheng R, Zeng H, Wang S, Sun K, Chen R, et al. Cancer incidence and mortality in China, 2022. J Natl Cancer Center. (2024) 4(1):47–53. doi: 10.1016/j.jncc.2024.01.006
3. Yan X, Duan H, Ni Y, Zhou Y, Wang X, Qi H, et al. Tislelizumab combined with chemotherapy as neoadjuvant therapy for surgically resectable esophageal cancer: A prospective, single-arm, phase II study (TD-NICE). Int J Surg. (2022) 103:106680. doi: 10.1016/j.ijsu.2022.106680
4. Shapiro J, van Lanschot J, Hulshof M, van Hagen P, van Berge HM, Wijnhoven B, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol. (2015) 16:1090–98. doi: 10.1016/S1470-2045(15)00040-6
5. Kelly RJ, Ajani JA, Kuzdzal J, Zander T, Van Cutsem E, Piessen G, et al. Adjuvant nivolumab in resected esophageal or gastroesophageal junction cancer. N Engl J Med. (2021) 384:1191–203. doi: 10.1056/NEJMoa2032125
6. Zhou N, Mitchell KG, Corsini EM, Truong V, Antonoff MB, Mehran RJ, et al. Analysis of trimodal and bimodal therapy in a selective-surgery paradigm for locally advanced oesophageal squamous cell carcinoma. Br J Surg. (2021) 108:1207–15. doi: 10.1093/bjs/znab162
7. Li K, Nie X, Li C, He W, Wang C, Du K, et al. Mapping of lymph node metastasis and efficacy index in thoracic esophageal squamous cell carcinoma: A large-scale retrospective analysis. Ann Surg Oncol. (2023) 30:5856–65. doi: 10.1245/s10434-023-13655-5
8. Tachimori Y, Ozawa S, Numasaki H, Matsubara H, Shinoda M, Toh Y, et al. Efficacy of lymph node dissection by node zones according to tumor location for esophageal squamous cell carcinoma. Esophagus. (2016) 13:1–07. doi: 10.1007/s10388-015-0515-3
9. Ajani JA, D'Amico TA, Bentrem DJ, Chao J, Corvera C, Das P, et al. Esophageal and esophagogastric junction cancers, version 2.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. (2019) 17:855–83. doi: 10.6004/jnccn.2019.0033
10. Kitagawa Y, Ishihara R, Ishikawa H, Ito Y, Oyama T, Oyama T, et al. Esophageal cancer practice guidelines 2022 edited by the Japan esophageal society: part 1. Esophagus. (2023) 20:343–72. doi: 10.1007/s10388-023-00993-2
11. Sakowitz S, Bakhtiyar SS, Mallick S, Yanagawa J, Benharash P. Travel to high-volume centers and survival after esophagectomy for cancer. JAMA Surg. (2024) 160(1):19–27. doi: 10.1001/jamasurg.2024.5009
12. Jeon WJ, Park D, Al-Manaseer F, Chen YJ, Kim JY, Liu B, et al. Survival and treatment patterns in stage II to III esophageal cancer. JAMA Netw Open. (2024) 7:e2440568. doi: 10.1001/jamanetworkopen.2024.40568
13. Li C, Zhao S, Zheng Y, Han Y, Chen X, Cheng Z, et al. Preoperative pembrolizumab combined with chemoradiotherapy for oesophageal squamous cell carcinoma (PALACE-1). Eur J Cancer. (2021) 144:232–41. doi: 10.1016/j.ejca.2020.11.039
14. Solomon D, Deeb AL, Tarabine K, Xie Y, Mazzola E, Zhao L, et al. Predicting outcomes in esophageal adenocarcinoma following neoadjuvant chemoradiation: Interactions between tumor response and survival. J Thorac Cardiovasc Surg. (2024) 168:278–89. doi: 10.1016/j.jtcvs.2023.11.015
15. Li K, Leng X, Peng L. Resected lymph nodes and survival of patients with esophageal squamous cell carcinoma in pT2 and pT3. Int J Surg. (2024) 110:1879–81. doi: 10.1097/JS9.0000000000001023
16. Li K, Li C, Nie X, He W, Du K, Liu K, et al. Surgical vs nonsurgical treatment for esophageal squamous cell carcinoma in patients older than 70 years: a propensity score matching analysis. J Gastrointest Surg. (2024) 28:611–20. doi: 10.1016/j.gassur.2024.01.010
17. Zarei M, Montazer M, Shakeri BOS, Jahanshahlou F, Hosseini MS. Postesophagectomy chylothorax: a review of the risk factors, diagnosis, and management. Ann Med Surg (Lond). (2023) 85:2781–86. doi: 10.1097/MS9.0000000000000809
18. Fujisawa K, Ohkura Y, Ueno M, Yago A, Shimoyama H, Udagawa H. Nutritional outcomes of thoracic duct resection for radical esophagectomy by assessing body composition changes in one year: A single-center retrospective study. Ann Surg Oncol. (2021) 28:8414–25. doi: 10.1245/s10434-021-10222-8
19. Orringer MB, Bluett M, Deeb GM. Aggressive treatment of chylothorax complicating transhiatal esophagectomy without thoracotomy. Surgery. (1988) 104:720–26.
20. Wurnig PN, Hollaus PH, Ohtsuka T, Flege JB, Wolf RK. Thoracoscopic direct clipping of the thoracic duct for chylopericardium and chylothorax. Ann Thorac Surg. (2000) 70:1662–65. doi: 10.1016/s0003-4975(00)01921-4
21. Merigliano S, Molena D, Ruol A, Zaninotto G, Cagol M, Scappin S, et al. Chylothorax complicating esophagectomy for cancer: a plea for early thoracic duct ligation. J Thorac Cardiovasc Surg. (2000) 119:453–57. doi: 10.1016/s0022-5223(00)70123-1
22. Li K, Du K, Li C, He W, Lu S, Liu K, et al. Impact of metastatic lymph nodes on survival of patients with pN1-category esophageal squamous cell carcinoma: A long-term survival analysis. Ann Surg Oncol. (2024) 31(6):3794–802. doi: 10.1245/s10434-024-15019-z
23. Okawa Y, Sasagawa S, Kato H, Johnson TA, Nagaoka K, Kobayashi Y, et al. Immuno-genomic analysis reveals eosinophilic feature and favorable prognosis of female non-smoking esophageal squamous cell carcinomas. Cancer Lett. (2024) 581:216499. doi: 10.1016/j.canlet.2023.216499
24. Goto H, Oshikiri T, Kato T, Sawada R, Harada H, Urakawa N, et al. The influence of preoperative smoking status on postoperative complications and long-term outcome following thoracoscopic esophagectomy in prone position for esophageal carcinoma. Ann Surg Oncol. (2023) 30:2202–11. doi: 10.1245/s10434-022-12898-y
25. Kashiwagi M, Ojima T, Hayata K, Kitadani J, Takeuchi A, Kuroi A, et al. Risk factors for chronic atrial fibrillation development after esophagectomy for esophageal cancer. J Gastrointest Surg. (2022) 26:2451–59. doi: 10.1007/s11605-022-05493-9
26. Yang X, Yin H, Zhang S, Jiang T, Gu J, Jiao H, et al. Perioperative outcomes and survival after neoadjuvant immunochemotherapy for locally advanced esophageal squamous cell carcinoma. J Thorac Cardiovasc Surg. (2025) 169(1):289–300. doi: 10.1016/j.jtcvs.2024.06.020
27. Ng CB, Chiu CH, Yeh CJ, Chang YC, Hou MM, Tseng CK, et al. Temporal trends in survival outcomes for patients with esophageal cancer following neoadjuvant chemoradiotherapy: A 14-year analysis. Ann Surg Oncol. (2024) 31:6652–61. doi: 10.1245/s10434-024-15644-8
28. Yang Y, Liu J, Liu Z, Zhu L, Chen H, Yu B, et al. Two-year outcomes of clinical N2-3 esophageal squamous cell carcinoma after neoadjuvant chemotherapy and immunotherapy from the phase 2 NICE study. J Thorac Cardiovasc Surg. (2024) 167:838–47. doi: 10.1016/j.jtcvs.2023.08.056
29. Yang H, Liu H, Chen Y, Zhu C, Fang W, Yu Z, et al. Neoadjuvant chemoradiotherapy followed by surgery versus surgery alone for locally advanced squamous cell carcinoma of the esophagus (NEOCRTEC5010): A phase III multicenter, randomized, open-label clinical trial. J Clin Oncol. (2018) 36:2796–803. doi: 10.1200/JCO.2018.79.1483
30. Florea IB, Shersher DD. Do nodal disease patterns and approach to lymphadenectomy affect survival in patients with esophageal squamous cell carcinoma? Reinvigorating an age-old debate. Ann Surg Oncol. (2024) 31:3584–86. doi: 10.1245/s10434-024-15176-1
31. Li B, Zhang Y, Miao L, Ma L, Luo X, Zhang Y, et al. Esophagectomy with three-field versus two-field lymphadenectomy for middle and lower thoracic esophageal cancer: long-term outcomes of a randomized clinical trial. J Thorac Oncol. (2021) 16:310–17. doi: 10.1016/j.jtho.2020.10.157
32. Zheng YZ, Li XQ, Wang JY, Yang H, Wen J, Zhai WY, et al. Impact of examined lymph node count for esophageal squamous cell carcinoma in patients who underwent right transthoracic esophagectomy. Ann Surg Oncol. (2021) 28:3025–33. doi: 10.1245/s10434-020-09217-8
33. Yoshida N, Nagai Y, Baba Y, Miyamoto Y, Iwagami S, Iwatsuki M, et al. Effect of resection of the thoracic duct and surrounding lymph nodes on short- and long-term and nutritional outcomes after esophagectomy for esophageal cancer. Ann Surg Oncol. (2019) 26:1893–900. doi: 10.1245/s10434-019-07304-z
34. Oshikiri T, Numasaki H, Oguma J, Toh Y, Watanabe M, Muto M, et al. Prognosis of patients with esophageal carcinoma after routine thoracic duct resection: A propensity-matched analysis of 12,237 patients based on the comprehensive registry of esophageal cancer in Japan. Ann Surg. (2023) 277:e1018–25. doi: 10.1097/SLA.0000000000005340
35. Oshikiri T, Numasaki H, Oguma J, Toh Y, Watanabe M, Muto M, et al. Is thoracic duct resection necessary for esophageal squamous cell carcinoma patients treated with neoadjuvant chemoradiotherapy? A propensity-matched analysis based on the comprehensive registry of esophageal cancer in Japan. Ann Surg Oncol. (2023) 30:2691–98. doi: 10.1245/s10434-022-12891-5
36. Aiolfi A, Bona D, Cali M, Manara M, Rausa E, Bonitta G, et al. Does thoracic duct ligation at the time of esophagectomy impact long-term survival? An individual patient data meta-analysis. J Clin Med. (2024) 13. doi: 10.3390/jcm13102849
37. Dos SC, Dos SL, Tavares G, Tristao LS, Orlandini MF, Serafim M, et al. Prophylactic thoracic duct obliteration and resection during esophagectomy: What is the impact on perioperative risks and long-term survival? A systematic review and meta-analysis. J Surg Oncol. (2022) 126:90–8. doi: 10.1002/jso.26827
Keywords: esophageal squamous cell carcinoma, intraoperative thoracic duct ligation, esophagectomy, overall survival, propensity score matching
Citation: Li K-x, Lu S-m, Li C-d, Wang C-h, Lv J-h, Wang Q-f, Huang Y-c, Han Y-t, Leng X-f and Peng L (2025) Long-term survival outcomes of esophageal squamous cell carcinoma with intraoperative thoracic duct ligation: a large-scale propensity score matching analysis. Front. Oncol. 15:1533378. doi: 10.3389/fonc.2025.1533378
Received: 23 November 2024; Accepted: 14 February 2025;
Published: 05 March 2025.
Edited by:
Airazat M. Kazaryan, Østfold Hospital, NorwayReviewed by:
Alessio Vagliasindi, Oncological Center of Basilicata (IRCCS), ItalyCopyright © 2025 Li, Lu, Li, Wang, Lv, Wang, Huang, Han, Leng and Peng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xue-feng Leng, doc.leng@uestc.edu.cn; Lin Peng, doclinpeng@163.com
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.