
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol., 11 February 2025
Sec. Neuro-Oncology and Neurosurgical Oncology
Volume 15 - 2025 | https://doi.org/10.3389/fonc.2025.1533114
Background and importance: Leiomyosarcoma is a rare and aggressive malignant tumor with a high potential for relapse and metastasis. Correct and timely diagnosis is critical for effective treatment, yet it is often challenging due to the diverse clinical presentations. This case report highlights the significance of early identification and the consequences of delayed diagnosis in scalp leiomyosarcoma.
Clinical presentation: We present the case of a 39-year-old woman with a scalp neoplasm. Initially, the diagnosis was missed, leading to a delay in surgical intervention. The tumor demonstrated a locally aggressive course, infiltrating the skull and dura mater. Upon admission, the scalp tumor was promptly excised. This case provides valuable insights into the varied symptoms and presentations of scalp leiomyosarcoma, which can aid in the recognition of this condition.
Conclusion: This report underscores the importance of considering leiomyosarcoma in the differential diagnosis of scalp masses, particularly when the etiology is unclear. Early recognition and intervention are essential to prevent locally invasive growth and potential metastasis, emphasizing the need for a high index of suspicion among healthcare professionals.
Leiomyosarcoma (LMS) is a malignant tumor that originates from smooth muscle cells and constitutes a relatively rare subtype of soft tissue sarcomas (STS) (1, 2). According to the currently available epidemiological data, head and neck soft tissue sarcomas (HNSTS) account for only 5%-10% of all STS. Within this category, head and neck leiomyosarcoma constitutes approximately 7% of HNSTS (3). This disease is more prevalent among middle-aged individuals; however, its diverse clinical presentations and low incidence rate often result in clinicians having limited understanding, potentially leading to misdiagnosis or delayed diagnosis. This case report and literature review aims to examine the epidemiological, clinical, anatomical, treatment, and prognostic aspects of LMS.
A 39-year-old woman presented to our hospital for the first time with a scalp mass accompanied by headaches and dizziness. The patient reported that 15 years ago, she first discovered a painless, soft mass approximately 3 centimeters in diameter on the occipital scalp. During this period, the patient underwent a Computerized tomography (CT) scan at a local hospital, which revealed a diagnosis of scalp hemangioma. Consequently, due to the patient’s impoverished family circumstances, surgical treatment was not financially viable at that time, and no additional treatments were pursued. Over the past year, the diameter of the lesion rapidly increased to 12 centimeters and gradually became hard. In addition, the patient has no family history and psychosocial background, as well as any history of associated genetic conditions. Concurrently, she experienced intermittent, stabbing headaches primarily in the occipital region, as well as vertigo characterized by a spinning sensation and unsteadiness while standing. Physical examination revealed localized scalp folds and a hard, painless mass measuring 12 centimeters in diameter on the occipital and parietal regions (Figure 1). CT with 3D reconstruction showed that the tumor was located in the right occipital and parietal areas, associated with severe bone destruction (Figures 2A-C). The lesion extended from the scalp to the dura mater. Although magnetic resonance imaging (MRI) revealed mixed tumor signals and compression of the adjacent brain (Figure 2D), it remained uncertain whether the brain parenchyma was invaded. CT angiography (CTA) with 3D reconstruction demonstrated the vascularity of the lesion, indicating that the tumor was hypervascular (Figures 3A-C). The CTA findings suggested that the lesion might be an hemangioma. To obstruct the tumor’s blood supply, the patient underwent occipital artery embolization (Figures 3D, E). Seventy-two hours post-embolization, the majority of the tumor was excised (Figures 3F, G); although the dura mater was invaded, several portions near the transverse sinus or sigmoid sinus remained intact, and the tumor did not invade the brain parenchyma. In addition to hemangioma, we also considered the following differential diagnoses: neurogenic tumors (such as neurofibroma and schwannoma), trichilemmoma, basal cell carcinoma and squamous cell carcinoma, lipoma and liposarcoma, fibroma and fibrosarcoma, malignant fibrous histiocytoma, rhabdomyosarcoma, and metastatic disease, particularly from melanoma or other common cancers. However, based on the lesion’s location, the patient’s clinical presentation, radiological features (including the tumor’s morphology, margins, density/signal characteristics, and enhancement pattern), as well as the patient’s medical history and risk factors, our preliminary clinical impression suggests that the tumor may be an atypical malignant neoplasm originating from a hemangioma.
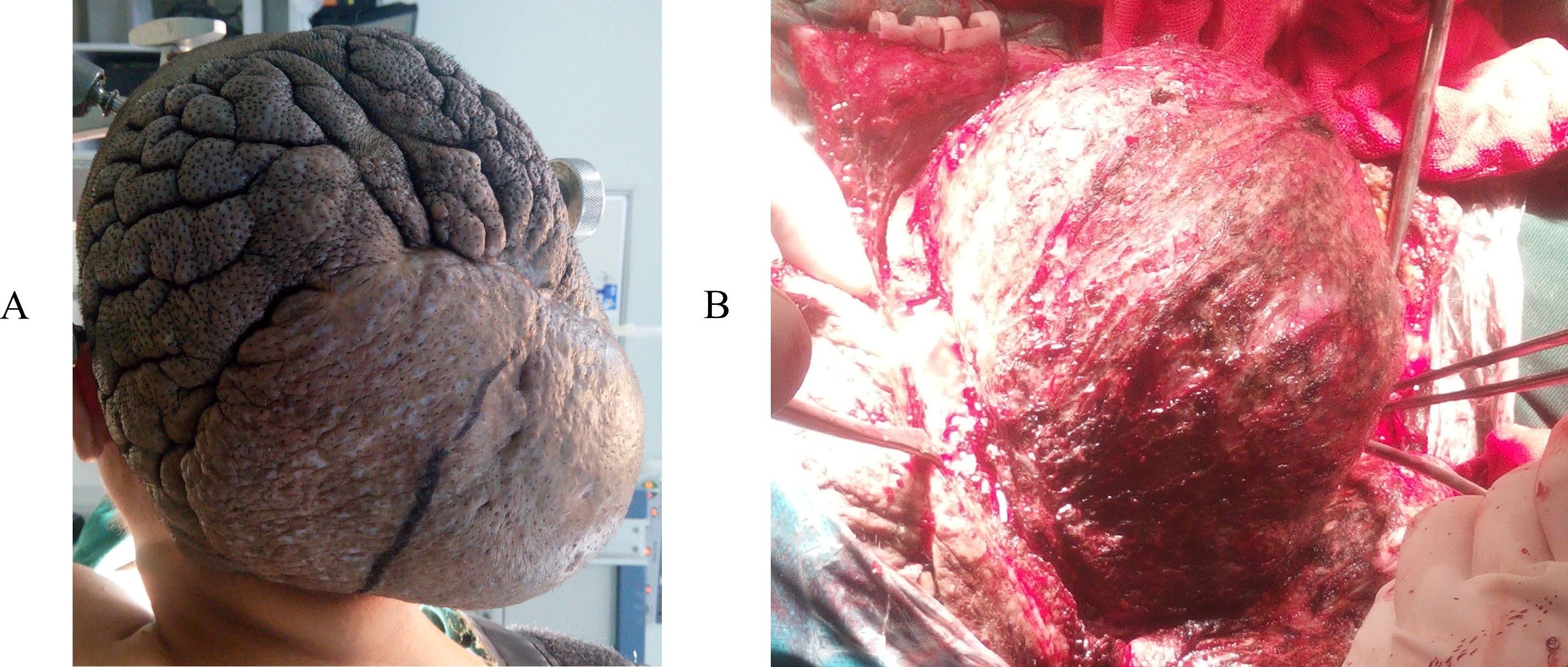
Figure 1. (A) Local cutis verticis gyrata and a 12cm hard, painless mass in the occipital and vertex region. The lesion was burgeoning. The tumor was exposed during the operation (B).
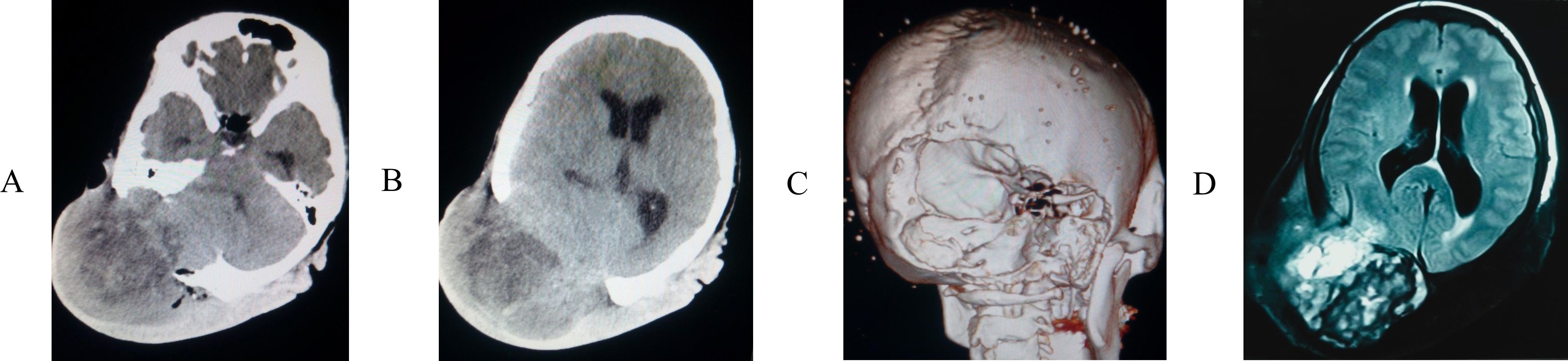
Figure 2. The computed tomography (CT) scan (A, B) showed that the tumor located on the right side of the occipital and vertex region, 3D reconstruction (C) revealed the skull was severely destroyed. (D) Magnetic resonance imaging (MRI) revealed the tumor signal was mixed and that neighboring brain tissue was compressed, poorly circumscribed lesion with infiltrative border.
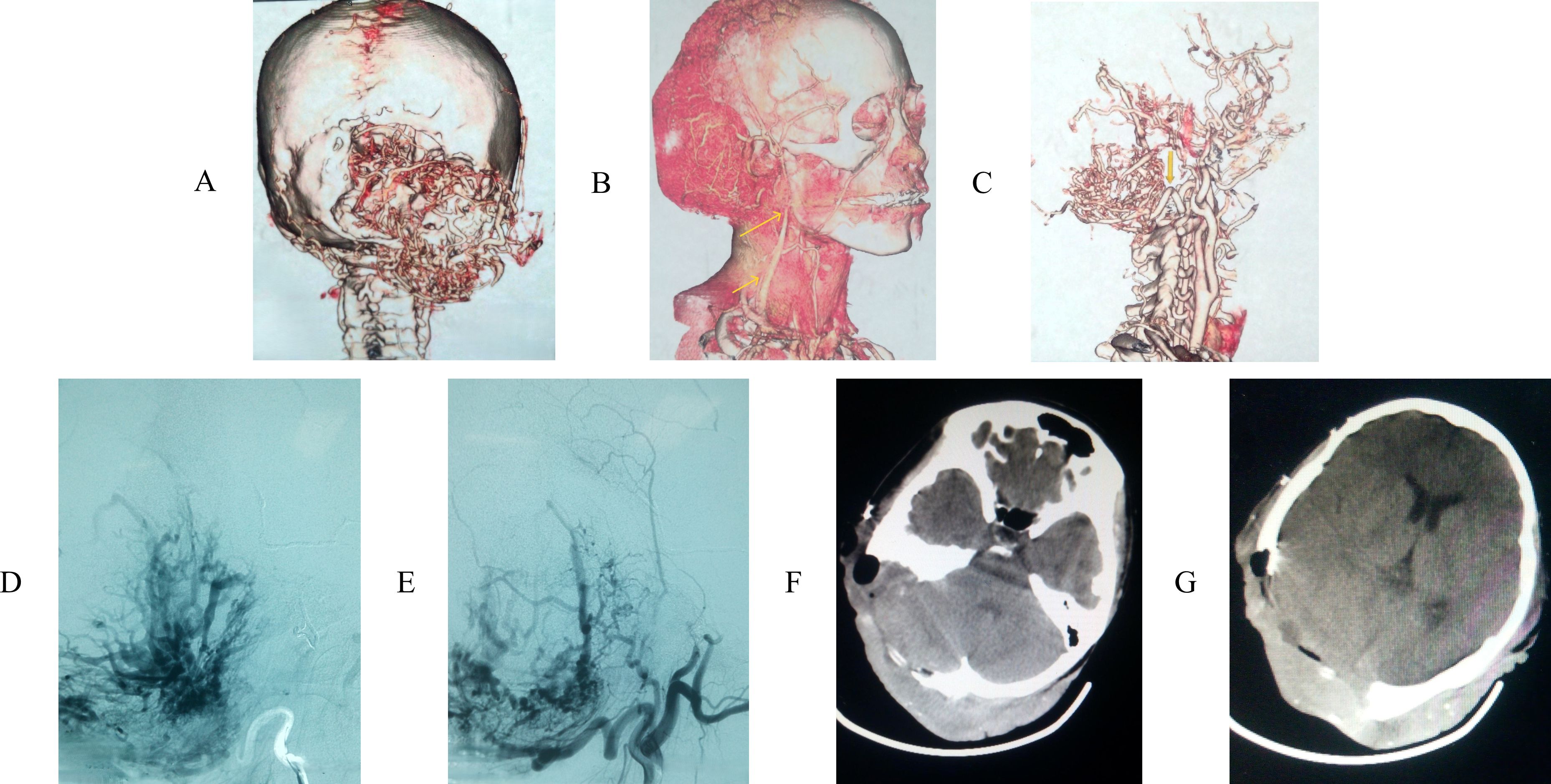
Figure 3. (A) CT angiography (CTA) with 3D reconstruction revealed tumor was hypervascula, leading to an angiomatous lesion with skull invasion. (B) CTA presented the lesion’s vascularization. (C) the lesion’s main vascularization were occipital artery (bottom arrow) and arteriae auricularis posterior (upper arrow). (D, E) the patient accepted embolization therapy of occipital. (F, G) most parts of the tumor were removed.
The histopathological results (Figure 4A) revealed that the tumor consisted of interwoven bundles of spindle cells exhibiting marked cytological atypia and eosinophilic cytoplasm. Key findings included densely stained nuclei, moderate mitotic activity (with a mitotic count of 10 per 10 high-power fields (HPFs)), and less than 50% of the observed area showing tumor necrosis (Figures 4B, C). The expression level of Ki67 was determined to exceed 15%. Immunohistochemical staining revealed diffuse expression of H-calmodulin, smooth muscle actin (SMA), and vimentin in the tumor cells, with a positive result for H-calmodulin (Figures 4D–F). Expression of estrogen receptor (ER), progesterone receptor (PR), epithelial membrane antigen (EMA), CD34, desmin, SMA, Bcl-2, and myoglobin were all negative. The differential diagnoses in histopathology primarily encompassed other spindle cell lesions, including spindle cell carcinoma, desmoplastic melanoma, superficial fibular sarcoma, malignant peripheral nerve sheath tumor, and vascular tumors. For the diagnosis of leiomyosarcoma (LMS), at least two muscle-specific immunohistochemical markers are necessary for confirmation, and in our examination, three markers exhibited diffuse expression. Furthermore, to rule out other spindle cell lesions, immunohistochemical staining was conducted, which included markers such as ER, PR, EMA, CD34, desmin, SMA, Bcl-2, and myoglobin, all of which were negative in leiomyosarcoma (LMS). Consequently, in light of the pathological findings and the patient’s prolonged history of a scalp mass, the tumor was classified as a primary scalp leiomyosarcoma.
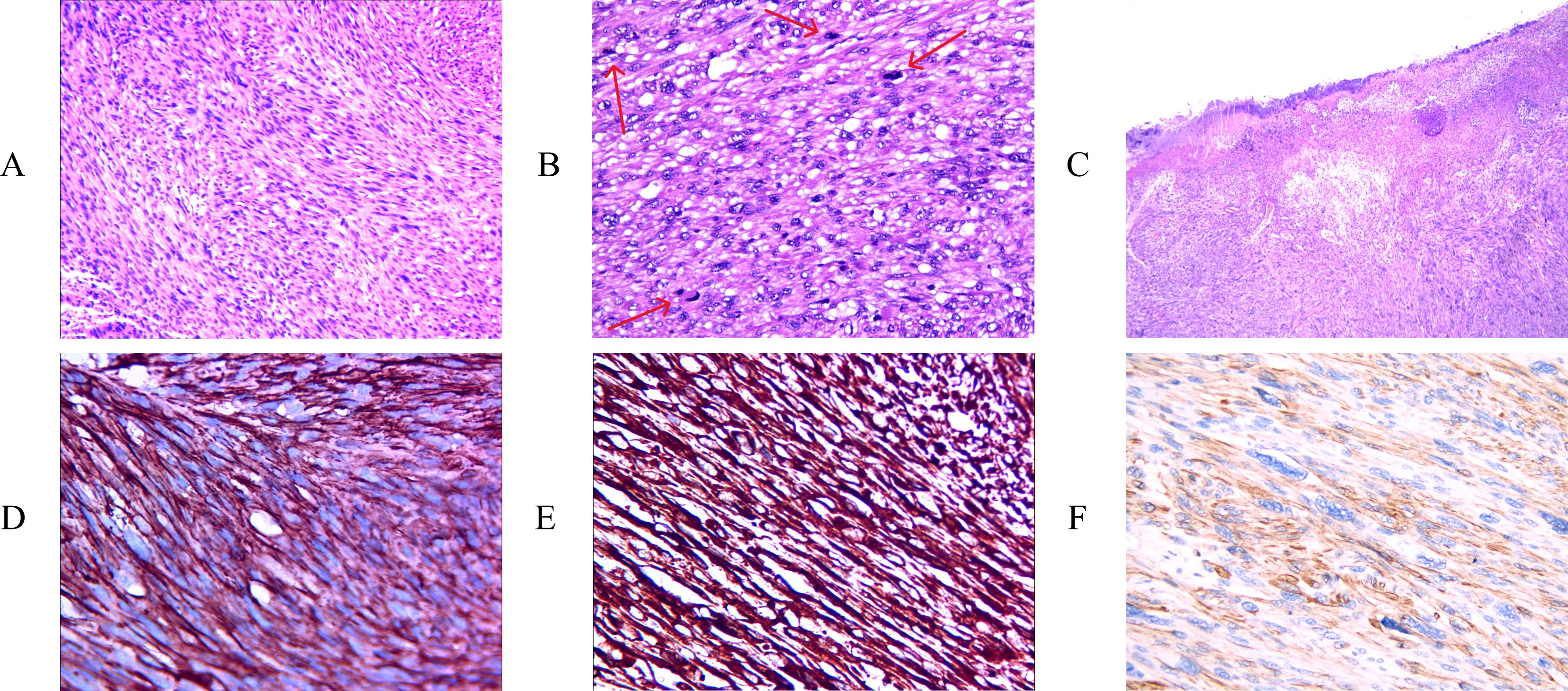
Figure 4. H&E staining showing interlacing fascicles of elongated spindle cells with cytological atypia and abundant eosinophilic cytoplasm [(A) X100]. High-power microscopic examination showing moderate mitosis. The arrows are annotated as mitosis. [(B) X200], marked nuclear atypia and tumoral necrosis [(C) X40]. Immunohistochemical staining of, SMA [(D) X400], and vimentin [(E) X400], H-caldesmon [(F) X400] were positive.
Because it was a malignant sarcoma, the patient received radiation therapy and chemotherapy in the hospital one month after the operation. Due to the lack of a comprehensive systemic examination, the presence of tumor metastasis remains uncertain. However, over the past four months, the patient has not reported any symptoms related to tumor invasion or metastasis. Furthermore, significant abnormalities were not found on CT scans of the head and chest.
Leiomyosarcoma (LMS) is an extremely rare malignant tumor of smooth muscle origin, with an incidence below two per million (4, 5). The most common site of its occurrence is the retroperitoneal region. Superficial LMS is divided into two subtypes: cutaneous and subcutaneous (6, 7). The subcutaneous type, which grows relatively fast and usually does not result in epidermal changes such as ulceration or discoloration (8), arises from the smooth muscle lining of arterioles and veins in the subcutaneous tissue (9). Due to the scalp’s rich vascularity (10), LMS in the scalp is more likely to cause metastasis and local recurrence (3, 11). According to literature reports, the common sites of metastatic spread include the lungs, colon, kidneys, ovaries, uterine cervix, and oral cavity (12–14). This case belongs to the subcutaneous type. Physically, the lesion did not protrude beyond the dermis, and no ulceration was observed, except for local cutis verticis gyrata. Based on imaging and clinical symptoms, we have not yet found direct evidence of tumor metastasis in this patient.
Under normal conditions, LMS may present as a slowly enlarging, firm, nonulcerated, painless mass (15). Its physical appearance can be deceptive and may be mistaken for a benign tumor. Presenting signs and symptoms are nonspecific and usually correspond to the location where the tumor arises. Some scholars argue that pain is the most common symptom of LMS, occurring in 80%–95% of patients (16). Pruritus, burning, and bleeding are also common. In our case, the patient experienced a headache, dizziness, instability while standing, and no tenderness. These positive symptoms may be associated with compression of the brain by the lesion and invasion of the scalp lesion into the dura mater. Larger lesions may exhibit focal areas of hemorrhage and necrosis. According to a large review of LMS of the superficial soft tissues, lesions of soft tissue origin measuring 2.5 cm or larger are more likely to be malignant (17).
The diagnosis of this rare malignant tumor must be based on histologic and ultrastructural examination. LMS is characterized by poorly circumscribed interlacing fascicles of elongated spindle-shaped cells with prominent blunt-ended nuclei and abundant eosinophilic cytoplasm in the subcutaneous tissue (18). Immunohistochemical identification of desmin, vimentin, actin, and myoglobin is helpful in diagnosis. LMS is characterized by the co-expression of vimentin, desmin, and muscle-specific actin. Tumors that exhibit one mitotic figure per five HPFs are considered malignant (19). The diagnostic value of progesterone receptor (PR) expression is still being actively researched. PR expression is useful in distinguishing LMS from smooth muscle tumors of uncertain malignant potential (STUMP), leiomyoma (LM), and atypical leiomyoma (ALM) (20–22). Thus, PR expression may aid in effectively distinguishing both ALMs and STUMP from LMS. Desmin staining results are variable, with an inverse relationship to the tumor’s vascularity. In this case, immunohistochemical staining of H-caldesmon, smooth muscle actin (SMA), and vimentin were positive, while desmin, myoglobin, and PR expression were negative (15). Combining hematoxylin-eosin and immunohistochemical staining, this case is most consistent with LMS.
The prognosis of scalp LMS is generally good, with the five-year survival rate of noncutaneous LMS reported to be 70.5% (23). Local recurrence rates range from 20%–35%, and the prognosis may be associated with tumor site, size, grade, and mitotic figures (24). There is no uniform standard for the treatment of scalp LMS, but maximal tumor resection is the treatment of choice, and surgical treatment can significantly improve the prognosis (25). Metastatic or recurrent LMS is managed with surgery and adjuvant radiochemotherapy, though no standard treatment protocol has been established so far. But the role of adjuvant chemotherapy has not been well understood, and no overall survival advantage has been shown in prospective studies (26). The treatment approach for leiomyosarcoma typically depends on the tumor’s size, location, metastatic status, and the patient’s overall health (25). Surgical resection is the most frequently employed treatment modality. In the case of localized leiomyosarcoma, surgical intervention is the treatment of choice, with the objective of achieving complete excision of the tumor along with a margin of normal tissue to ensure a negative surgical margin. However, for superficial leiomyosarcoma, Mohs Micrographic Surgery (MMS) is more effective, with reports indicating that the recurrence rate following MMS is significantly lower than that following wide local excision (27). Radiotherapy is another modality, categorized into preoperative and postoperative applications. Preoperative radiotherapy is primarily utilized to reduce tumor size, facilitating surgical excision; postoperative radiotherapy aims to eradicate residual tumor cells that may remain after surgery, thereby mitigating the risk of local recurrence (28, 29). LMS, particularly uterine LMS, demonstrates high responsiveness to chemotherapeutic agents. Chemotherapy, typically employing doxorubicin- or gemcitabine-based regimens, is effective in controlling metastatic leiomyosarcoma and extending survival (30). Additionally, novel treatment modalities for LMS are emerging, including targeted DNA repair therapies, immunotherapies, and innovative combinations of chemical agents.
Scalp LMS is an uncommon and aggressive soft tissue neoplasm that presents significant diagnostic and therapeutic challenges owing to its unique anatomical location and high rate of recurrence. Through the analysis of this clinical case of primary scalp LMS complemented by a literature review, the objective is to enhance clinicians’ comprehension of the disease and to prevent diagnostic omissions in clinical practice. Further research is warranted to improve the early diagnosis and treatment strategies for scalp LMS.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
The studies involving humans were approved by Second Hospital of Lanzhou University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
SG: Conceptualization, Formal Analysis, Investigation, Methodology, Resources, Software, Visualization, Writing – original draft. PL: Conceptualization, Data curation, Formal Analysis, Methodology, Software, Validation, Visualization, Writing – original draft. JL: Data curation, Formal Analysis, Funding acquisition, Resources, Writing – review & editing. WY: Formal Analysis, Resources, Software, Supervision, Writing – review & editing. SY: Conceptualization, Data curation, Funding acquisition, Methodology, Resources, Validation, Visualization, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Workman AD, Farquhar DR, Brody RM, Parasher AK, Carey RM, Purkey MT, et al. Leiomyosarcoma of the head and neck: A 17&8208;year single institution experience and review of the National Cancer Data Base. Head Neck. (2018) 40:756–62.
2. Kazlouskaya V, Lai YC. Leiomyosarcoma of the skin: review of the literature with an emphasis on prognosis and management. Int J Dermatol. (2019) 59:165–72. doi: 10.1111/ijd.14705
3. Saluja TS. Leiomyosarcoma_ Prognostic outline of a rare head and neck Malignancy. Oral Oncol. (2019) 95:100–5. doi: 10.1016/j.oraloncology.2019.06.010
4. Zieschang H, Koch R, Wirth MP, Froehner M. Leiomyosarcoma of the urinary bladder in adult patients: A systematic review of the literature and meta-analysis. Urol Int. (2019) 102:96–101. doi: 10.1159/000494357
5. Wang W, Hong J, Meng J, Wu H, Shi M, Yan S, et al. Nomograms predict cancer-specific and overall survival of patients with primary limb leiomyosarcoma. J Orthop Res. (2019) 37:1649–57. doi: 10.1002/jor.24298
6. Wong GN, Webb A, Gyorki D, McCormack C, Tran P, Ngan SY, et al. Cutaneous leiomyosarcoma: dermal and subcutaneous. Australas J Dermatol. (2020) 61:243–9. doi: 10.1111/ajd.13307
7. Helbig D, Dippel E, Erdmann M, Frisman A, Kage P, Leiter U, et al. S1-guideline cutaneous and subcutaneous leiomyosarcoma. JDDG J Dtsch Dermatol Ges. (2023) 21:555–63. doi: 10.1111/ddg.14989
8. Giorgi VD. Cutaneous leiomyosarcoma: A clinical, dermoscopic, pathologic case study. Exp Oncol. (2019) 41:80–81. doi: 10.32471/exp-oncology.2312-8852.vol-41-no-1.12816
9. Pearce H, Yu-Cherng C, Rose BE, Jonczak E, Grossman J, D’Amato GZ, et al. Association of mutational status with metastatic pattern, race, ethnicity, and overall survival (OS) in leiomyosarcoma (LMS). J Clin Oncol. (2024) 42:e23553. doi: 10.1200/JCO.2024.42.16_suppl.e23553
10. Patel S, Bhosle R, Das P, Krishnan P. Management of delayed presentation of scalp loss with double rotation flaps and multiple calvarial drilling. J Neurosci Rural Pract. (2023) 14:762–4. doi: 10.25259/JNRP_140_2023
11. Carr MJ, Sun J, Adams WA, Dugan MM, Naqvi SMH, Kim Y, et al. Grade of primary cutaneous leiomyosarcoma dictates risk for metastatic spread and disease-specific mortality. Cancer Control J Moffitt Cancer Cent. (2023) 30:10732748231206957. doi: 10.1177/10732748231206957
12. Dai Y, Zhang Y, Ke X, Liu Y, Zang C. Cutaneous metastasis from cervical cancer to the scalp and trunk: a case report and review of the literature. J Med Case Rep. (2023) 17:435. doi: 10.1186/s13256-023-04171-x
13. Kannan S, Chong HH, Chew B, Ferguson JD, Galloway E, McCulloch T, et al. Leiomyosarcoma in the extremities and trunk wall: systematic review and meta-analysis of the oncological outcomes. World J Surg Oncol. (2022) 20:124. doi: 10.1186/s12957-022-02584-4
14. Rastogi A, Singh P, Gupta S, Durgapal P, Gupta M. An unusual case of brain metastases from leiomyosarcoma of scalp. Neurol India. (2023) 71:336–7. doi: 10.4103/0028-3886.375427
15. Bala M, Ray A, Saraf A. Leiomyosarcoma of mandible: A diagnostic dilemma; case report and review of literature. Indian J Otolaryngol Head Neck Surg. (2019) 71:848–51. doi: 10.1007/s12070-019-01584-3
16. Øines MN, Smith HG, Preisler L, Penninga L. Leiomyosarcoma of the abdomen and retroperitoneum; a systematic review. Front Surg. (2024) 11:1375483. doi: 10.3389/fsurg.2024.1375483
17. Bregy A, Lim J, Lohman R, Kane J, Prasad D, Qiu J, et al. Primary leiomyosarcoma of the calvarium with intracranial extension: a case report. Indian J Surg Oncol. (2020) 11:165–9. doi: 10.1007/s13193-020-01129-z
18. Mongardini FM, Paolicelli M, Catauro A, Conzo A, Flagiello L, Nesta G, et al. Outcomes and follow-up trends in adrenal leiomyosarcoma: A comprehensive literature review and case report. J Clin Med. (2024) 13:3499. doi: 10.3390/jcm13123499
19. Pinto A. Uterine smooth muscle tumors: an overview. Adv Anat Pathol. (2024) 31:397–410. doi: 10.1097/PAP.0000000000000446
20. Guo E, Li C, Hu Y, Zhao K, Zheng Q, Wang L. Leiomyoma with bizarre nuclei: A current update. Int J Womens Health. (2022) 14:1641–56. doi: 10.2147/IJWH.S388278
21. Huo L, Wang D, Wang W, Cao D, Yang J, Wu M, et al. Oncologic and reproductive outcomes of uterine smooth muscle tumor of uncertain Malignant potential: A single center retrospective study of 67 cases. Front Oncol. (2020) 10:647. doi: 10.3389/fonc.2020.00647
22. Tarique U, Cyr DP, Morosi C, Dickson BC, Greco G, Swallow CJ, et al. On the origin of abdominal venous leiomyosarcomas: the role of the sex-hormone drainage pathways. World J Oncol. (2024) 15:758–68. doi: 10.14740/wjon1884
23. Pan M, Zhou M, Xie L, Bui N, Ganjoo K. Recent advances in sarcoma therapy: new agents, strategies and predictive biomarkers. J Hematol OncolJ Hematol Oncol. (2024) 17:124. doi: 10.1186/s13045-024-01650-6
24. Lee RM. A closer look at the natural history and recurrence patterns of high-grade truncal/extremity leiomyosarcomas: A multi-institutional analysis from the US Sarcoma Collaborative. Surg Oncol. (2020) 34:292–7. doi: 10.1016/j.suronc.2020.06.003
25. Wellings EP, Tibbo ME, Rose PS, Folpe AL, Houdek MT. Treatment outcome of superficial leiomyosarcoma. J Surg Oncol. (2021) 123:127–32. doi: 10.1002/jso.26262
26. Hickman A. Not all leiomyosarcomas are the same: how to best classify LMS. Curr Treat Options Oncol. (2023) 24:327–37. doi: 10.1007/s11864-023-01067-2
27. Murphy-Chutorian B, Routt E, Vinelli G, Ciocon D. A systematic review of the treatment of superficial leiomyosarcoma with mohs micrographic surgery. Dermatol Surg. (2019) 45:1437. doi: 10.1097/DSS.0000000000001992
28. Diggs A, Sia TY, Huang Y, Gockley A, Melamed A, Khoury-Collado F, et al. Utilization and outcomes of adjuvant therapy for stage II and III uterine leiomyosarcoma. Gynecol Oncol. (2022) 166:308–16. doi: 10.1016/j.ygyno.2022.05.018
29. Lebas A, Le Fèvre C, Waissi W, Chambrelant I, Brinkert D, Noël G. Prognostic factors in extremity soft tissue sarcomas treated with radiotherapy: systematic review of the literature. Cancers. (2023) 15:4486. doi: 10.3390/cancers15184486
Keywords: leiomyosarcoma, sarcoma, scalp, surgery, case report
Citation: Gao S, Liu P, Liu J, Yang W and Yang S (2025) Primary leiomyosarcoma of the scalp: a case report and review of the literature. Front. Oncol. 15:1533114. doi: 10.3389/fonc.2025.1533114
Received: 23 November 2024; Accepted: 23 January 2025;
Published: 11 February 2025.
Edited by:
Dimitrije Brasanac, University of Belgrade, SerbiaReviewed by:
Ekta Dhamija, All India Institute of Medical Sciences, IndiaCopyright © 2025 Gao, Liu, Liu, Yang and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jixing Liu, eXlnbWxpdUAxMjYuY29t
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.