
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Oncol. , 05 March 2025
Sec. Hematologic Malignancies
Volume 15 - 2025 | https://doi.org/10.3389/fonc.2025.1531928
Background: Multiple myeloma (MM) is a complex hematological malignancy characterized by the clonal expansion of plasma cells in the bone marrow. Emerging studies have emphasized the importance of lipid metabolism, which is closely associated with the survival, proliferation, and drug resistance of tumor cells. The hypoxic environment in the bone marrow (BM) contributes to metabolic reprogramming in MM cells, including alterations in metabolite levels, changes in metabolic enzyme activity, and metabolic shifts. Cancer cells possess the ability to adapt their metabolism in order to fulfill their continuously increasing energy demands. In this review, we will discuss the alterations in lipid metabolism during the development of MM, and their reciprocal interactions with the tumor microenvironment.
In recent years, the role of lipid metabolism in MM has become a focal point of research. MM cells exhibit imbalances in lipid processing, which is manifested in multiple aspects (1–5). Firstly, there are alterations in lipogenesis. Key enzymes like fatty acid synthase (FASN) are over - expressed (6, 7), leading to increased de novo fatty acid synthesis. This provides the necessary lipids for rapid cell membrane expansion during MM cell proliferation. Secondly, lipid uptake and trafficking are disrupted. MM cells enhance their ability to take up fatty acids from the microenvironment, especially from bone marrow adipocytes (BMAs) (8). BMAs, as energy reservoirs, are exploited by MM cells. They secrete fatty acids that are avidly taken up by MM cells through specific transporters such as fatty acid - binding proteins (FABPs) and fatty acid transporter proteins (FATPs) (9, 10). Thirdly, the tricarboxylic acid (TCA) cycle, which is closely related to lipid metabolism, is also perturbed (11, 12). This leads to an imbalance in energy production and metabolite generation, further fueling the survival and growth of MM cells.Moreover, lipid metabolism in MM also has a profound impact on the tumor microenvironment (TME). Lipid - induced oxidative stress in the TME can damage the DNA, proteins, and lipids within MM cells, but at the same time, MM cells adapt to this stress by upregulating antioxidant defense mechanisms. In addition, lipid - rich tumor - associated macrophages (TAMs) play a crucial role in immune evasion. M2 - polarized TAMs secrete immunosuppressive factors like IL - 10 and TGF - β, express immune checkpoint molecules such as PD - L1, and phagocytose immune cells, all of which contribute to creating an immunosuppressive environment that benefits MM cell survival (13).
The insights into lipid metabolism in MM have opened up new avenues for developing novel therapies. Targeting lipid metabolism has emerged as a promising strategy (5). For example, inhibitors of lipogenesis, such as FASN inhibitors, can disrupt the abnormal lipid synthesis in MM cells. By blocking FASN, the production of palmitate is reduced, which in turn disrupts the lipid raft structure and pro - survival signaling pathways like AKT/mTOR (14–16). This leads to the induction of apoptosis in MM cells. Another approach is to target lipid uptake. Inhibiting the transporters involved in fatty acid uptake can starve MM cells of essential lipid nutrients. For instance, interfering with the function of FABPs or FATPs can prevent MM cells from efficiently taking up fatty acids from BMAs. Furthermore, modulating the TCA cycle can also be a viable strategy. By regulating the key enzymes in the TCA cycle, the energy metabolism of MM cells can be disrupted, making them more vulnerable to treatment. In addition, lipid - based biomarkers and metabolic imaging techniques, combined with artificial intelligence, hold great potential for personalized medicine. They can help in early diagnosis, accurate prognosis, and predicting treatment responses, enabling more tailored treatment strategies for individual MM patients.
Despite the significant progress, there are still aspects of lipid metabolism in MM that remain under - explored (17). The interaction between lipid metabolism and the immune microenvironment is a complex and not fully understood area (1, 18). Although we know that lipid - induced oxidative stress and lipid - rich TAMs affect the immune response, the detailed molecular mechanisms underlying how lipid metabolism precisely regulates immune cell functions in the context of MM are unclear. For example, how the lipid composition of MM cells and the TME affects the activation, differentiation, and effector functions of T cells, B cells, and NK cells requires further investigation.
Moreover, the high heterogeneity of MM poses challenges in understanding lipid metabolism. Different MM subtypes may have distinct lipid metabolic profiles, and how to precisely target these differences for effective treatment is yet to be fully elucidated. Also, the crosstalk between different lipid metabolic pathways and their combined effects on MM cell biology and the TME are still not well - characterized. Addressing these unstudied aspects will be crucial for the development of more effective and targeted therapies for MM.
Multiple myeloma (MM) is a complex hematological malignancy, and imbalances in lipid metabolism has emerged as a fundamental characteristic, underlying various metabolic abnormalities that are central to its pathophysiology. MM cells exhibit a distinct profile of abnormal lipid metabolism, with alterations spanning multiple aspects of lipid homeostasis. This includes a significant upregulation of de novo lipogenesis, enhanced lipid uptake, abnormal formation of lipid droplets, and disrupted lipid trafficking processes (19).
Emerging evidence highlights the critical role of transcriptional and post-transcriptional regulators in mediating lipid metabolic imbalances in multiple myeloma (MM). Sterol regulatory element-binding proteins (SREBPs), particularly SREBP-1c, act as master transcriptional regulators of lipogenic enzymes such as acetyl-CoA carboxylase (ACC) and fatty acid synthase (FASN), which are frequently upregulated in MM cells to sustain aberrant lipid biosynthesis (20). Post-transcriptional modulation by non-coding RNAs further amplifies lipid metabolic reprogramming. For instance, miR-27a-3p directly suppresses the expression of stearoyl-CoA desaturase (SCD), a key enzyme in monounsaturated fatty acid synthesis, thereby altering membrane fluidity and drug sensitivity in MM cells (21). Targeting these regulators—via pharmacological SREBP inhibitors or RNA-based therapies—represents a promising strategy to disrupt lipid dependency in MM, potentially enhancing therapeutic efficacy.
Mitochondrial dynamics and bioenergetic dysfunction are increasingly implicated in MM-associated lipid metabolic alterations. Recent studies reveal imbalances of mitochondrial fission/fusion machinery in MM cells, characterized by reduced expression of fusion regulators and elevated activity of dynamin-related protein 1 (Drp1), a driver of mitochondrial fragmentation (22). Excessive mitochondrial fission impairs fatty acid β-oxidation (FAO) by disrupting cristae structure and electron transport chain efficiency, forcing MM cells to rely on de novo lipogenesis for survival. Intriguingly, pharmacological inhibition of Drp1 not only restores mitochondrial homeostasis but also suppresses lipogenic gene expression, suggesting a mechanistic link between mitochondrial dynamics and lipid anabolism (23). These findings position mitochondrial regulators as dual modulators of bioenergetic and lipid metabolic pathways in MM.
Adipokines play a pivotal role in the intricate relationship between lipid metabolism and the progression of MM, with adiponectin being particularly prominent. Adiponectin is an adipokine deeply involved in fatty acid metabolism. In MM, plasma cells downregulate the expression of adiponectin. Adiponectin possesses anti - tumor properties, and one of its primary functions is to inhibit the production of IL - 6 (24–26). Under normal physiological conditions, an appropriate level of adiponectin can effectively regulate the immune microenvironment and suppress the growth and proliferation of tumor cells.
However, in MM patients, the reduction in adiponectin levels disrupts this balance. The decrease in adiponectin levels leads to the upregulation of IL - 6. IL - 6, in turn, promotes the growth and survival of myeloma cells, thereby driving disease progression. A profound understanding of the signaling pathways of adiponectin and other adipokines offers the potential for therapeutic innovation. By developing drugs that can mimic the functions of adiponectin or therapies that can upregulate adiponectin expression, it may be possible to block the tumor - promoting effects mediated by IL - 6. For example, researchers could design a specific agonist that mimics the binding of adiponectin to its corresponding receptor, thereby activating the downstream anti - tumor signaling pathways. Alternatively, a drug that can regulate gene expression to promote the synthesis of adiponectin and restore its normal level in the body could be developed. This would inhibit the growth of MM cells and open up new avenues for the treatment of MM (27–30).
One of the most tangible manifestations of the dysregulated lipid metabolism in Multiple Myeloma (MM) is the alteration in serum lipid levels. To comprehensively understand these changes, a multitude of well - designed clinical investigations have been meticulously carried out.
During the active phase of MM, a consistent finding is that patients exhibit lower high - density lipoprotein cholesterol (HDL - C) levels. This phenomenon has been firmly supported by a substantial amount of research data. In 14 independent studies (31–44), the HDL - C levels in patients with active MM were measured to range from 26 to 52.4 mg/dl. In contrast, the HDL - C levels in the control group, consisting of healthy individuals, spanned from 38.6 to 58 mg/dl. Simultaneously, triglyceride levels in MM patients are significantly elevated at the time of diagnosis and remain so throughout the active disease stage. This abnormal elevation of triglyceride levels not only reflects the derangement of lipid metabolism in the diseased state but also poses a potential threat to the cardiovascular health of patients. High levels of triglycerides are well - established as a major risk factor for cardiovascular diseases. When comparing different International Staging System (ISS) stages of MM with healthy individuals, significant differences in low - density lipoprotein cholesterol (LDL - C), HDL - C, and total cholesterol levels are clearly observable. For instance, in a large - scale study involving 307 MM patients, the prognostic significance of several plasma markers was comprehensively evaluated. Serum cholesterol, LDL, HDL, apolipoprotein A1 (Apo A1), and apolipoprotein B1 (Apo B1) all demonstrated significant statistical significance in the analysis of variance (ANOVA) (P<0.001, P<0.001, P = 0.002, P<0.001, and P<0.001, respectively). Serum ApoA1, in particular, showed significant differences between stage I and stage II, as well as between stage II and stage III (P = 0.007 and P = 0.002, respectively). Moreover, Apo A1 levels were significantly associated with progression - free survival (PFS) and disease - specific survival (CSS) (Hazard Ratio HR: 0.484 and 0.344, 95% Confidence Interval CI: 0.255 - 0.922 and 0.152 - 0.779, P = 0.028 and P = 0.011) (44). These results suggest that Apo A1 levels can serve not only as an important reference indicator for MM staging but also as a crucial biomarker for assessing the survival prognosis of patients. In another in - depth study focusing on the correlation between lipid expression levels and multiple myeloma, a potential causal relationship was discovered between triglyceride (TG) levels and MM risk (Odds Ratio OR: 0.67, 95% Confidence Interval CI: 0.46 - 0.98, P = 0.038) (45).
In summary, MM patients demonstrate distinct patterns of serum lipid level changes across different disease stages, including the active phase, remission stage, and various ISS stages. HDL - C levels decrease during the active phase, while triglyceride levels increase, and both return to normal during remission. Comparing different ISS - staged patients with healthy individuals reveals significant differences in multiple lipid indicators. Additionally, certain lipid - related biomarkers such as Apo A1 are closely associated with patient prognosis, and there is a potential causal link between TG levels and MM risk. These cumulative research findings not only contribute to a deeper understanding of the pathophysiological mechanisms of MM but also offer valuable lipid - related biomarkers for the diagnosis, staging, and prognosis assessment of MM, providing a robust theoretical basis for the formulation of clinical treatment strategies.
Obesity is an established risk factor for the development and progression of MM. Obesity leads to an increase in the number and volume of bone marrow adipocytes (BMAs) (46–48)., which is directly associated with an elevated risk of MM. In patients with monoclonal gammopathy of undetermined significance (MGUS), a precursor to MM, lipid - reactive clonal immunoglobulins are often present, and obesity in these patients further exacerbates the risk of progression to MM. In animal models, obesity - induced lipid imbalances and diet - induced obesity can promote the development of a myeloma - like syndrome.
In MM, abnormal lipid metabolism confers several advantages to cancer cells. The overproduction of lipids, enhanced lipid uptake, and altered lipid droplet dynamics contribute to the enhanced survival, proliferation, and energy generation of cancer cells. Additionally, dysregulated lipid metabolism disrupts the redox balance within MM cells, leading to increased oxidative stress and further tumor progression. MM cells can also reprogram BMAs, which impacts bone lesions and the tumor microenvironment. Due to promoter methylation, reprogrammed adipocytes have lower levels of peroxisome proliferator - activated receptor γ (PPARγ), and this reduction is associated with an inability to reverse bone lesions after disease remissionγ) (49).
Based on these associations, for high - risk patient populations such as obese individuals or those with MGUS, targeting lipid metabolism pathways could be a viable and effective therapeutic strategy. By developing drugs that target key lipid metabolism enzymes or modulating adipokine signaling pathways, it is possible to intervene in the development and progression of MM. For instance, inhibiting key enzymes involved in lipid synthesis can reduce the lipid resources available to cancer cells, thereby inhibiting their growth. Modulating adipokine signaling pathways, such as those related to adiponectin, is expected to restore the balance of the immune microenvironment and suppress the growth of tumor cells. This not only provides a new direction for the treatment of MM but also offers hope for improving the treatment outcomes of MM patients. Through precise intervention in lipid metabolism, more effective control and treatment of MM may be achieved.
As shown in Figure 1, the reprogramming of bone marrow adipocytes (BMAs) by multiple myeloma (MM) cells to manipulate the tumor microenvironment (TME) is a complex and crucial process. MM cells interact with BMAs, altering their gene expression and functional status (48). Bone marrow adipocytes (BMA) are cells in the bone microenvironment that have been implicated in promoting anti-apoptosis and multiple myeloma disease progression in MM cells by activating NF-κB signaling through IL-6 secretion (50). In one study, BMAs were found to have higher expression of adipocyte differentiation-related genes DLK1, DGAT1, FABP4, and FASN, which may lead to higher secretion of adipose-related factors by BMAs and promote MM cell proliferation and growth (6). An increase in FABP4 gene expression facilitates the binding and transportation of fatty acids within BMAs (6). In tumors with a high frequency of bone metastases, BMAs exert their pro-tumorigenic effects mainly through the regulation of genes related to lipid metabolism, including FABP4, PPARγ and CD36 (6, 51). BMAs have been found to secrete a number of molecules important for supporting MM cells in the bone marrow, and to directly recruit MM cells through monocyte chemotactic protein 1 and stromal cell-derived factor 1α (46). Once these fatty acids are taken up by MM cells, they can generate a large amount of adenosine triphosphate (ATP) through the β - oxidation pathway, providing energy support for the rapid proliferation of MM cells (52–54). Vascular endothelial growth factor (VEGF) and hepatocyte growth factor (HGF) are multifunctional cytokines that stimulate angiogenesis during tumor neovascularization. VEGF is secreted by MM cells, which induce MM cell proliferation and stimulate IL-6 expression by microvascular endothelial cells and bone marrow stromal cells (55, 56).
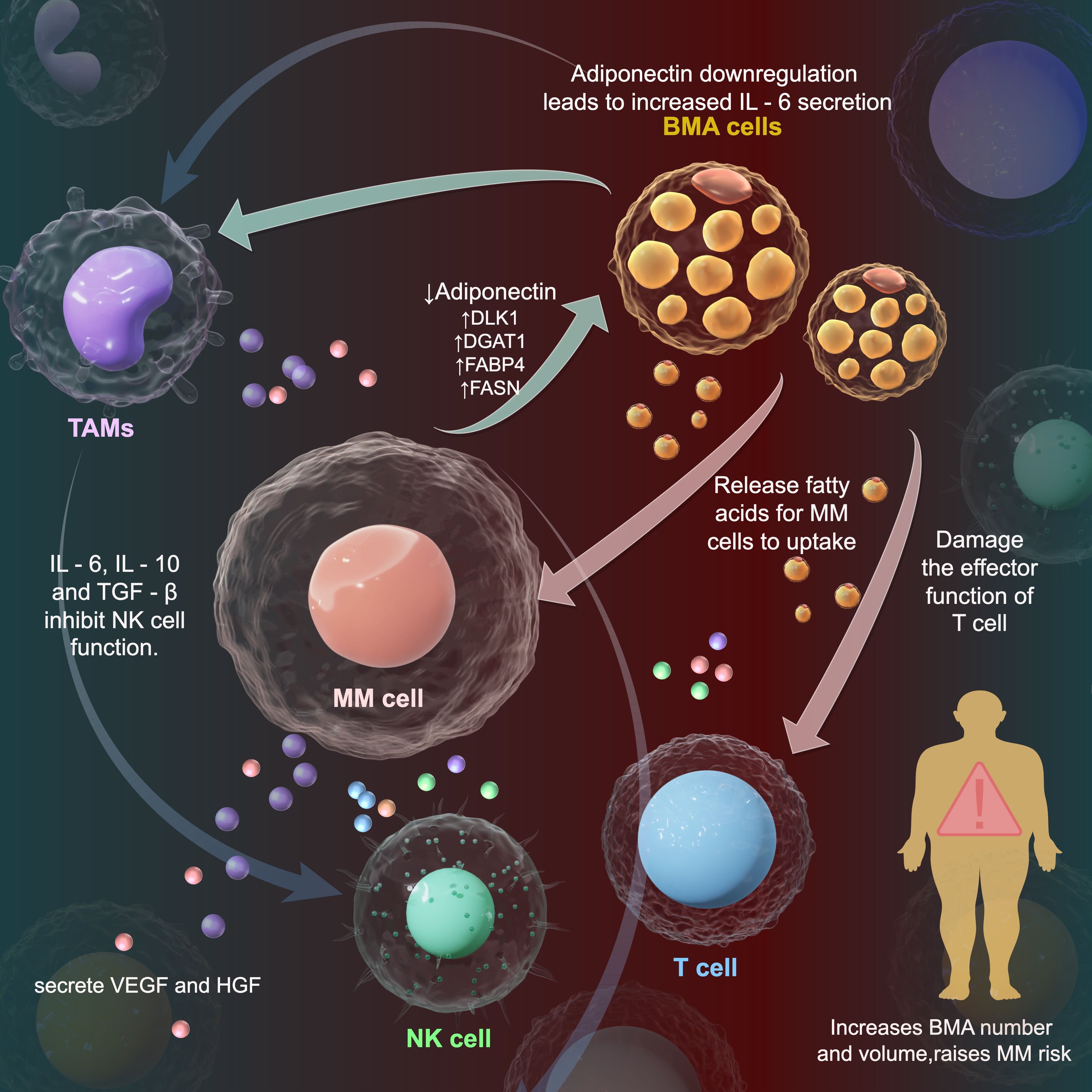
Figure 1. Interaction of myeloma cells, bone marrow adipocytes and tumor microenvironment in multiple myeloma. This diagram shows immune - regulatory and lipid - related mechanisms of multiple myeloma (MM) cells in the tumor microenvironment (TME). MM cells interact with bone marrow adipocytes (BMAs). BMAs release fatty acids for MM cells to make lipid droplets and extracellular vesicles. In MM patients, adiponectin (anti - tumor adipokine) level drops, while IL - 6 level rises, creating a microenvironment for myeloma cell growth. Oxidized fatty acids in vesicles enter tumor - infiltrating T cells, harming their functions.MM cells polarize tumor - associated macrophages (TAMs) into M2 type. M2 - TAMs secrete IL - 10 and TGF - β. IL - 10 suppresses the immune response, TGF - β weakens NK cell cytotoxicity, promoting tumor growth. Decreases, while the level of IL - 6 increases, facilitating the growth of myeloma cells.
In the intricate pathological mechanism of multiple myeloma, lipid - induced oxidative stress plays a pivotal role, especially in promoting tumor cell growth and inhibiting immune cell functions. This process profoundly influences the development of the disease oxidative stress in MM cells (57).
Once the lipid metabolism in the tumor microenvironment (TME) becomes abnormal, a series of cascading reactions are triggered, with the generation of a large amount of reactive oxygen species (ROS) being a crucial starting point. When the ROS level rises sharply, an oxidative stress state ensues, creating a special environment for the growth of myeloma cells. In this environment, multiple signaling pathways within myeloma cells are abnormally activated. For example, the mitogen - activated protein kinase (MAPK) signaling pathway is activated by oxidative stress, which promotes the expression of genes related to myeloma cell proliferation, such as Cyclin D1. The over - expression of this protein accelerates the cell cycle process, enabling myeloma cells to divide and proliferate more rapidly, thus driving tumor growth.
The inhibitory effect of oxidative stress on immune cells is also remarkable. For T cells, lipid - induced oxidative stress disrupts their normal signal transduction process. The T - cell receptor (TCR) signaling pathway is inhibited under oxidative stress, making it difficult for T cells to be effectively activated. As a result, T cells cannot fully exert their cytotoxic effects and fail to recognize and kill myeloma cells in a timely manner. Natural killer (NK) cells are also severely affected. Oxidative stress reduces the expression of activation receptors on the surface of NK cells, such as NKp46, while increasing the expression of inhibitory receptors. This significantly impairs the cytotoxic function of NK cells, rendering them unable to effectively perform immune surveillance and elimination of myeloma cells.
Moreover, oxidative stress also affects the chemotaxis and migration abilities of immune cells. Under normal circumstances, immune cells can accurately migrate to the tumor site to exert immune functions under the guidance of chemokines. However, in the lipid - induced oxidative stress environment, the expression of chemokine receptors on the surface of immune cells is down - regulated, weakening their responsiveness to chemokines and preventing them from migrating normally into the tumor microenvironment. This further weakens the ability of the immune system to attack myeloma cells.
In multiple myeloma, lipid - induced oxidative stress plays a key role in promoting the development of the disease by promoting tumor cell growth and inhibiting immune cell functions. In - depth exploration of this mechanism is of great significance for developing more effective treatment strategies to break the growth advantage of tumor cells and restore the normal functions of immune cells.
In the complex pathophysiological landscape of myeloma development, the tumor microenvironment serves as a critical arena where multiple myeloma (MM) cells orchestrate a series of intricate processes to manipulate the immune system, with lipid - associated mechanisms playing a central role. Tumor-associated macrophages (TAM) are an important component of the bone marrow (BM) in multiple myeloma (MM) patients, accounting for approximately 10% of the BM (58). Apart from the well - studied interactions between MM cells and bone marrow adipocytes (BMAs), M2 - polarized tumor - associated macrophages (TAMs) emerge as key players in this immune - regulatory drama (59, 60). As is well known, the M1 TAM subtype can stimulate the Th - 1 immune response and exert an anti - tumor effect (13), while M2 TAM has low antigen - presenting ability and can promote tumor progression by inducing immunosuppression and angiogenesis (58, 61–65). However, MM cells can induce the polarization of TAM into the tumor - promoting M2 phenotype, causing them to secrete anti - inflammatory molecules, including IL - 10 and tumor growth factor (TGF) - β.
Tumor - infiltrating T cells have their effector functions impaired due to lipid peroxidation and mitochondrial stress triggered by lipid droplets and extracellular vesicles rich in oxidized fatty acids derived from MM cells (66). In this process, MM cells utilize the fatty acids taken up from BMAs to synthesize lipid droplets and extracellular vesicles. After the oxidized fatty acids carried by these substances enter tumor - infiltrating T cells, they disrupt the normal lipid metabolism balance within T cells, trigger mitochondrial stress responses, and ultimately lead to the inability of T cells to exert normal immune - killing functions. In multiple myeloma (MM), transforming growth factor-β (TGF-β) produced by plasma cells (67), regulatory T cells (Tregs) (68), or possibly myeloid - derived suppressor cells (MDSCs) (69) can downregulate natural killer (NK) cell activation receptors and impair NK cell function (70, 71). Elevated levels of interleukin - 6 (IL - 6) and interleukin - 10 (IL - 10) have also been observed in MM (72, 73). These cytokines act as growth factors for plasma cells and promote the development of an NK - resistant tumor phenotype by inhibiting NK cell activity (74, 75). In essence, by regulating the lipid metabolism of TAMs and the subsequent release of immunosuppressive factors, MM cells skillfully manipulate the immune response within the tumor microenvironment. There are complex lipid - metabolism - related connections between MM cells and various cells in the microenvironment (13, 76, 77).
Statins, by inhibiting HMG - CoA reductase in the mevalonate pathway, block the biosynthesis of cholesterol and isoprenoid intermediates (78–85). Preclinical studies, such as those on simvastatin, have demonstrated that it can induce mitochondrial dysfunction and endoplasmic reticulum stress in MM cells, subsequently activating the caspase - 3 - mediated apoptotic pathway. This interference with the MM cell membrane integrity and Ras/Rho protein is oprenylation disrupts normal cell functions, subjecting MM cells to metabolic stress and eventually leading to apoptosis (86). FASN, a key enzyme in fatty acid de novo synthesis highly expressed in MM cells, when inhibited, leads to reduced palmitate synthesis, disrupted lipid raft structure, and impaired pro - survival signaling like the AKT/mTOR pathway. Additionally, it induces ROS accumulation and an imbalance in the Bax/Bcl - 2 ratio, ultimately triggering mitochondria - dependent apoptosis and imposing significant metabolic stress on MM cells (87). The PI3K/Akt/mTOR pathway, which is well - known for its role in lipid metabolism, affects this process in multiple ways. For example, Akt can activate fatty acid synthase (FASN) and increase lipid uptake by cells. Conversely, mTOR complex 1 (mTORC1) can inhibit fatty acid β - oxidation, a catabolic process, by inhibiting AMP - activated protein kinase (AMPK) and its downstream targets, including PGC - 1α and SIRT1. Consequently, therapeutic strategies targeting this pathway have been extensively studied, leading to the development of several drugs. These drugs, commonly referred to as PI3K inhibitors, Akt inhibitors, or mTOR inhibitors depending on their position in the pathway, can inhibit one or more enzymes in the pathway, disrupting signaling and inhibiting the growth and survival of cancer cells. For instance, mTOR inhibitors like rapamycin and its derivatives, known as rapalogs, can block mTOR enzyme activity, thereby reducing protein synthesis and cell growth. These drugs have demonstrated clinical effects in certain cancers, such as kidney and breast cancer, and are currently under investigation for MM and other cancers. For example, silymarin has been found to induce apoptosis in MM cells through the PI3K/Akt/mTOR signaling pathway (88), and AE - 848, a novel small molecule compound, effectively induces apoptosis in human MM cells by regulating NF - κB and PI3K/Akt/mTOR signaling pathways (89).CPT1 inhibitors act by blocking fatty acid oxidation through inhibiting CPT1. This results in ATP depletion and AMPK hyperactivation in MM cells, inducing the transition from autophagy to apoptosis. By interfering with lipid metabolic homeostasis, these drugs cause MM cells to experience an energy crisis, oxidative stress, and membrane signaling disorders, all of which contribute to the induction of cell death (10).
By interfering with lipid metabolic homeostasis, these drugs cause MM cells to experience an energetic crisis, oxidative stress (ROS accumulation), and membrane signaling disorders. Eventually, they induce cell death via endogenous apoptotic pathways or exogenous death receptor pathways. In addition, metabolic stress enhances the sensitivity of MM cells to proteasome inhibitors, indicating the potential for co - administration (90).
Combining drugs targeting lipid metabolism with other medications represents a highly promising treatment approach. For the combination of lipid - targeted drugs and proteasome inhibitors, examples like simvastatin (a statin) combined with bortezomib (a proteasome inhibitor) show that simvastatin inhibits HMG - CoA reductase (91), disrupts the lipid raft structure of MM cell membranes, and interferes with survival signaling pathways, while bortezomib induces endoplasmic reticulum stress and apoptosis, and the combination can enhance the sensitivity of endoplasmic reticulum stress induced by bortezomib and overcome drug resistance; In another study, the combination of lovastatin and MG-132 was found to be synergistically cytotoxic to MM.1S and RPMI8226 cells. The addition of lovastatin also increased the percentage of apoptotic cells compared to MG-132 alone. These results suggest that PI-induced lipid accumulation may confer acquired resistance to MM cells and, therefore, lovastatin may exert a synergistic anti-tumor proliferative effect with PI by reducing intracellular lipid levels (92).
In the treatment of multiple myeloma, significant progress has been made with monoclonal antibodies and immunotherapy. However, instances where lipid-targeted drugs are combined with these therapies remain relatively rare. In recent years, the application of monoclonal antibodies in multiple myeloma has become a research hotspot. Particularly, monoclonal antibodies targeting CD38 and SLAMF7, such as daratumumab (daratumumab) and ibrutinib (elotuzumab), have shown promising efficacy in patients with relapsed/refractory multiple myeloma (RRMM) (93). In addition, bispecific antibodies (BsAbs), as a novel immunotherapy approach, have shown potential in the treatment of multiple myeloma. BsAbs can bind to both the CD3 subunit of T-cell receptor complexes and antigens on tumor cells, thereby activating T cells and killing tumor cells (94). These antibodies have demonstrated high overall response rates (ORRs) in clinical trials, and efforts are actively underway to improve their efficacy and tolerability.
Despite this, research combining lipid-targeted drugs with monoclonal antibodies or immunotherapies is still in its infancy. Existing studies primarily focus on the combination of monoclonal antibodies and immunotherapies, such as CAR-T cell therapy and bispecific antibodies, which show promising prospects in the treatment of multiple myeloma (95). Future research may explore the synergistic effects of lipid-targeted drugs with these immunotherapies to enhance therapeutic outcomes and reduce side effects.
Based on ongoing trials or novel compounds targeting lipid metabolism, as shown in Table 1, there are currently multiple drugs with diverse mechanisms of action and trial statuses being explored for the treatment of multiple myeloma (MM).
Opaganib exerts its action by specifically targeting Sphingosine Kinase (SphK). Through the inhibition of SphK2, it effectively reduces the production of Sphingosine - 1 - Phosphate (S1P). This dual - effect not only induces apoptosis in multiple myeloma (MM) cells but also significantly enhances their sensitivity to proteasome inhibitors. The initial results of its Phase I clinical trial have provided evidence of both safety and potential efficacy (96).TVB - 2640 is centered on Fatty Acid Synthase (FASN). By inhibiting FASN, it blocks the process of fatty acid synthesis, thereby triggering endoplasmic reticulum stress and apoptosis in MM cells. Moreover, it has the capacity to overcome drug resistance (97). Currently, Phase I/II clinical trials are being carried out to comprehensively evaluate its combination with other drugs. Statins act on 3 - Hydroxy - 3 - Methylglutaryl Coenzyme A Reductase, thereby suppressing the mevalonate pathway and cholesterol synthesis. They potentially impede the proliferation of MM cells by regulating the Ras/MAPK pathway. Although retrospective clinical studies have demonstrated that combination therapy can extend the survival of MM patients (98) dedicated Phase III clinical trials are still lacking.Idelalisib targets Phosphoinositide 3 - Kinase (PI3K), inhibiting the PI3K/Akt/mTOR pathway to disrupt the survival and proliferation signals of MM cells (99). However, early - stage clinical trials were terminated due to limited efficacy and immune - related toxicity. Elesclomol impacts iron metabolism and lipid peroxidation. It induces the accumulation of Reactive Oxygen Species (ROS), promotes lipid peroxidation, and ultimately causes ferroptosis in MM cells (100). Pre - clinical studies have indicated that its combination with bortezomib shows effectiveness, yet it has not yet entered the stage of MM clinical trials.
These drugs represent a significant step forward in the development of novel therapies for MM. They hold great transformative potential as they target key aspects of MM cell biology, especially lipid metabolism. However, challenges remain, such as optimizing dosing regimens, minimizing side - effects, and determining the best combination strategies. Overcoming these challenges will be crucial to translate these promising findings into effective treatments for MM patients.
Lipidomics, a powerful systems - biology approach, enables researchers to comprehensively analyze lipid molecule types and their intricate metabolic pathways within cells, tissues, or entire organisms. In the field of multiple myeloma (MM), its significance cannot be overemphasized. MM cells are characterized by abnormal lipid metabolism, which is intricately linked to tumor cell proliferation, survival, and the thorny issue of drug resistance. Through meticulous lipidomic analysis, specific lipid metabolites and pathways closely related to MM, such as sphingolipids, glycerophospholipids, and cholesterol metabolism pathways, can be precisely identified (101). These identifications are not only crucial for understanding the disease mechanism but also serve as potential therapeutic targets, laying the foundation for the development of lipid - targeted therapies.
Artificial Intelligence (AI) technologies are making remarkable progress in the medical field, especially in the crucial area of predicting patient responses to specific treatments. By seamlessly integrating patients’ genomic, lipidomic, and clinical data, AI can construct highly sophisticated predictive models. For example, machine - learning algorithms can sift through vast amounts of lipidomics data to identify biomarkers associated with treatment responses. This newly - acquired ability to predict which patients are more likely to benefit from lipid - targeted therapies has significantly propelled the development of personalized medicine, bringing us closer to the goal of tailoring treatment plans for MM patients.
The combined power of lipidomics and AI provides a potent toolkit for biomarker discovery and patient stratification in MM patients. Lipidomics can accurately pinpoint specific lipid metabolites or pathways that are dysregulated in MM. AI - driven analysis then uses these findings to stratify patients into distinct subgroups. Some lipid metabolites can act as reliable indicators of high sensitivity to specific lipid - targeted therapies, while others may be closely associated with drug resistance (102, 103). This patient stratification is of great significance for optimizing treatment plans. Clinicians can formulate treatment plans based on the characteristics of individual patients, achieving more effective and efficient treatment.
One of the most pressing challenges in lipid - targeted therapies is ensuring high treatment specificity. Minimizing off - target effects on normal tissues is of utmost importance. Since lipid metabolism is a fundamental process in all cells, developing therapies that can specifically target the abnormal lipid metabolism in MM cells without disrupting the normal functions of normal cells is a formidable task. Scientists are actively exploring various strategies, such as designing drugs with high - affinity binding to the specific lipid pathways or metabolites that are dysregulated in MM cells. Nanoparticle - based drug delivery systems are also under intensive investigation to enhance the specific delivery of lipid - targeted drugs to MM cells, thereby reducing the impact on normal tissues. Drug resistance is a major obstacle in the treatment of MM, especially in lipid - targeted therapies. MM cells often develop resistance over time, leading to disappointing treatment outcomes. Combination therapies present a promising solution. By combining lipid - targeted drugs with other therapeutic agents, such as proteasome inhibitors, monoclonal antibodies, or immunotherapies, we can simultaneously target multiple aspects of MM cell survival mechanisms, reducing the likelihood of cells developing drug resistance. In addition, adaptive therapies that target the metabolic plasticity of MM cells are also being studied. These therapies can adapt to the dynamic changes in the lipid metabolism of MM cells, ensuring the continuous effectiveness of treatment.
There is an urgent need for large - scale research to validate the efficacy of lipid - metabolism - based interventions. Well - designed clinical trials involving a large number of patients are crucial for accurately evaluating the true potential of lipid - targeted therapies. These trials should not only focus on treatment outcomes but also closely monitor the safety and long - term effects of these therapies. Large - scale research can provide valuable insights into the optimal dosage, treatment duration, and combination strategies of lipid - targeted therapies. Integrating lipid - targeted therapies into the framework of personalized medicine is of great significance. Using lipidomics and AI for biomarker discovery and patient stratification helps to formulate highly personalized treatment plans. By understanding the unique lipid metabolism characteristics of individual patients, clinicians can make more informed choices about the most suitable lipid - targeted therapies. This personalized approach can lead to better treatment outcomes, reduce side effects, and ultimately improve the quality of life of MM patients.
In conclusion, the current body of research on the role of lipid metabolism in multiple myeloma (MM) has firmly established its fundamental importance in the pathogenesis and progression of the disease. Imbalanced lipid metabolism in MM is a complex phenomenon, characterized by alterations in lipogenesis, disruptions in lipid uptake and trafficking, and perturbations within the tricarboxylic acid (TCA) cycle. These metabolic aberrations drive the reprogramming of energy metabolism, biomass production, and redox homeostasis within MM cells. Concurrently, the aberrant expression and activity of key lipid metabolism enzymes and transporters have been identified, underscoring the pivotal role of this imbalances in MM’s development and making lipid metabolism an enticing therapeutic target.
This imbalances not only enables MM cell survival, proliferation, and drug resistance but also exerts a profound influence on the tumor microenvironment (TME) and immune responses. For instance, lipid - induced oxidative stress in the TME can damage cellular components in MM cells and impact immune cell function, while lipid - rich tumor - associated macrophages (TAMs) promote immune evasion through multiple mechanisms such as secreting immunosuppressive factors and expressing immune checkpoint molecules.
Targeting lipid metabolism has emerged as a highly promising approach for MM treatment. Strategies like inhibitors of lipogenesis, lipid uptake, and the modulation of the TCA cycle, as well as those involving the inhibition of fatty acid synthesis, promotion of fatty acid oxidation, modulation of lipid signaling pathways, modification of the bone marrow (BM) microenvironment, and regulation of lipid - mediated communication within the TME, have shown great potential in preclinical studies and early - phase clinical trials. These approaches hold the potential to enhance patient outcomes by selectively eliminating MM cells, overcoming drug resistance, and minimizing adverse effects. Additionally, lipid - based biomarkers and metabolic imaging techniques, when combined with artificial intelligence, offer new tools for more accurate diagnosis, prognosis, and treatment response prediction, and can assist in patient stratification for personalized treatment. Meanwhile, in multiple myeloma (MM), angiogenesis and immunosuppression are two key features of the tumor microenvironment (TME). Therapeutic strategies targeting vascular endothelial growth factor (VEGF) and hepatocyte growth factor (HGF) have shown potential in disrupting these processes. VEGF is an important angiogenic factor that plays a crucial role in the progression of multiple myeloma. By inhibiting VEGF, the formation of tumor blood vessels can be reduced, thereby limiting tumor growth and spread (104). In addition, the HGF/MET pathway is also considered an important therapeutic target in multiple myeloma. HGF not only promotes the proliferation and survival of tumor cells but also increases angiogenesis and immunosuppression through interactions with the microenvironment. Abnormal activity of this pathway is associated with poor patient outcomes; therefore, targeting the HGF/MET pathway may help improve treatment efficacy (105). By targeting both VEGF and HGF simultaneously, the tumor immune microenvironment (TIME) can be more effectively reshaped to enhance the immune system’s response to tumors. Dual suppression promotes immune activation and complements immunotherapies such as anti-PD-1 or anti-BCMA (55).This strategy not only helps inhibit tumor angiogenesis but may also boost the effectiveness of immunotherapy by reducing the accumulation of suppressor cells (106). Therefore, combining anti-angiogenic and immunomodulatory treatments could provide a more comprehensive treatment approach for multiple myeloma patients. Integrating with lipid metabolism - related targets could offer a highly promising strategy for achieving long - term and effective disease control.
However, translating lipid metabolism research into clinical practice is fraught with challenges. The intricate nature of lipid metabolism pathways, the high degree of heterogeneity in MM, and the complex interplay between lipid metabolism and the TME pose significant obstacles. Ensuring the selectivity and specificity of treatments to target malignant plasma cells while sparing normal cells, managing drug resistance, and optimizing drug delivery systems are crucial steps for the successful implementation of lipid - metabolism - targeted therapies. Looking forward, future research should be dedicated to unraveling the complex mechanisms underlying lipid metabolism in MM and identifying novel therapeutic targets. Combination therapies that integrate lipid - metabolism - targeted agents with existing treatments, immunotherapeutic approaches, and personalized medicine strategies hold great promise for improving patient outcomes and surmounting drug resistance. The integration of lipid metabolism profiling into clinical decision - making processes will be essential for tailoring treatment strategies to individual patients.
In summary, the study of lipid metabolism in MM has provided invaluable insights into the disease’s biology and therapeutic potential. Targeting the dysregulated lipid metabolism pathways in MM cells and their interactions within the TME represents a highly promising avenue for future research and clinical interventions. Sustained efforts in this field will undoubtedly contribute to the development of innovative treatment strategies and, ultimately, improved outcomes for patients with MM.
HQW: Investigation, Methodology, Writing – original draft, Conceptualization. JZ: Investigation, Methodology, Writing – original draft. HR: Conceptualization, Methodology, Resources, Writing – original draft. LC: Methodology, Supervision, Writing – original draft. JR: Conceptualization, Investigation, Methodology, Writing – original draft. CL: Supervision, Writing – review & editing. HKW: Supervision, Writing – review & editing. LZ: Funding acquisition, Project administration, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by National Natural Science Foundation of China (82372313, 82102482, 82072371), Leading Talent Project of Shanghai Huangpu District (2020-1-28), Program of Shanghai Academic/Technology Research Leader (Grant No.23XD1404900), Shanghai Health and Medical Development Foundation under Grant (201972), and Shanghai Science and Technology Committee under Grant (21ZR1478200).
Figure support was provided by Figdraw.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Torcasio R, Gallo Cantafio ME, Ikeda RK, Ganino L, Viglietto G, Amodio N. Lipid metabolic vulnerabilities of multiple myeloma. Clin Exp Med. (2023) 23:3373–90. doi: 10.1007/s10238-023-01174-2
2. Gavriatopoulou M, Paschou SA, Ntanasis-Stathopoulos I, Dimopoulos MA. Metabolic disorders in multiple myeloma. Int J Mol Sci. (2021) 22(21):11430. doi: 10.3390/ijms222111430
3. Panaroni C, Fulzele K, Mori T, Siu KT, Onyewadume C, Maebius A, et al. Multiple myeloma cells induce lipolysis in adipocytes and uptake fatty acids through fatty acid transporter proteins. Blood. (2022) 139(6):876–88. doi: 10.1182/blood.2021013832
4. Oudaert I, van der Vreken A, Maes A, De Bruyne E, De Veirman K, Vanderkerken K, et al. Metabolic cross-talk within the bone marrow milieu: focus on multiple myeloma. Exp Hematol Oncol. (2022) 11(1):49. doi: 10.1186/s40164-022-00303-z
5. Petrusca DN, Lee KP, Galson DL. Role of sphingolipids in multiple myeloma progression, drug resistance, and their potential as therapeutic targets. Front Oncol. (2022) 12:925807. doi: 10.3389/fonc.2022.925807
6. Wei X, Zhang Y, Wang Z, He Y, Ju S, Fu J. Bone marrow adipocytes is a new player in supporting myeloma cells proliferation and survival in myeloma microenvironment. Transl Oncol. (2024) 40:101856. doi: 10.1016/j.tranon.2023.101856
7. Jurczyszyn A, Czepiel J, Gdula-Argasinska J, Pasko P, Czapkiewicz A, Librowski T, et al. Plasma fatty acid profile in multiple myeloma patients. Leuk Res. (2015) 39:400–5. doi: 10.1016/j.leukres.2014.12.010
8. Masarwi M, Deschiffart A, Ham J, Reagan MR. Multiple myeloma and fatty acid metabolism. JBMR Plus. (2019) 3:e10173. doi: 10.1002/jbm4.10173
9. Farrell M, Fairfield H, D'amico A, Murphy CS, Falank C, Marinac CR, et al. Fatty acid binding proteins as a novel therapeutic target in multiple myeloma. Blood. (2021) 138:1569. doi: 10.1182/blood-2021-146902
10. Farrell M, Fairfield H, Karam M, D'amico A, Murphy CS, Falank C, et al. Targeting the fatty acid binding proteins disrupts multiple myeloma cell cycle progression and MYC signaling. Elife. (2023) 12:e81184. doi: 10.7554/eLife.81184.sa2
11. Roman-Trufero M, Auner HW, Edwards CM. Multiple myeloma metabolism - a treasure trove of therapeutic targets? Front Immunol. (2022) 13:897862. doi: 10.3389/fimmu.2022.897862
12. Gonsalves WI, Jang JS, Jessen E, Hitosugi T, Evans LA, Jevremovic D, et al. In vivo assessment of glutamine anaplerosis into the TCA cycle in human pre-malignant and Malignant clonal plasma cells. Cancer Metab. (2020) 8(1):29. doi: 10.1186/s40170-020-00235-4
13. Cencini E, Sicuranza A, Ciofini S, Fabbri A, Bocchia M, Gozzetti A. Tumor-associated macrophages in multiple myeloma: key role in disease biology and potential therapeutic implications. Curr Oncol. (2023) 30:6111–33. doi: 10.3390/curroncol30070455
14. Okawa Y, Hideshima T, Ikeda H, Raje N, Vallet S, Kiziltepe T, et al. Fatty acid synthase is a novel therapeutic target in multiple myeloma. Br J Haematol. (2008) 141(5):659–71. doi: 10.1111/j.1365-2141.2008.07114.x
15. Okawa Y, Hideshima T, Ikeda H, Raje N, Vallet S, Kiziltepe T, et al. Inhibition of fatty acid synthase induces anti-tumor effect in multiple myeloma. J Clin Oncol. (2008) 26:19522. doi: 10.1111/j.1365-2141.2008.07114.x
16. Wang W, Wu D. Fatty acid synthase inhibitor induces apoptosis and inhibits proliferation in multiple myeloma cells. Blood. (2004) 104:4849. doi: 10.1182/blood.V104.11.4849.4849
17. Chen J, Zaal EA, Berkers CR, Ruijtenbeek R, Garssen J, Redegeld FA. Omega-3 fatty acids DHA and EPA reduce bortezomib resistance in multiple myeloma cells by promoting glutathione degradation. Cells. (2021) 10(9):2287. doi: 10.3390/cells10092287
18. Fairfield H, Costa S, Falank C, Farrell M, Murphy CS, D'amico A, et al. Multiple myeloma cells alter adipogenesis, increase senescence-related and inflammatory gene transcript expression, and alter metabolism in preadipocytes. Front Oncol. (2020) 10:584683. doi: 10.3389/fonc.2020.584683
19. Allegra A, Murdaca G, Mirabile G, Gangemi S. Protective effects of high-density lipoprotein on cancer risk: focus on multiple myeloma. Biomedicines. (2024) 12(3):514. doi: 10.3390/biomedicines12030514
20. Morelli E, Fulciniti M, Samur MK, Ribeiro CF, Wert-Lamas L, Henninger JE, et al. A MIR17HG-derived long noncoding RNA provides an essential chromatin scaffold for protein interaction and myeloma growth. Blood. (2023) 141:391–405. doi: 10.1182/blood.2022016892
21. Gallo Cantafio ME, Torcasio R, Viglietto G, Amodio N. Non-coding RNA-dependent regulation of mitochondrial dynamics in cancer pathophysiology. Noncoding RNA. (2023) 9(1):16. doi: 10.3390/ncrna9010016
22. D'aquila P, Ronchetti D, Gallo Cantafio ME, Todoerti K, Taiana E, Fabiani F, et al. Epigenetic regulation of mitochondrial quality control genes in multiple myeloma: A sequenom massARRAY pilot investigation on HMCLs. J Clin Med. (2021) 10(6):1295. doi: 10.3390/jcm10061295
23. Torcasio R, Gallo Cantafio ME, Veneziano C, De Marco C, Ganino L, Valentino I, et al. Targeting of mitochondrial fission through natural flavanones elicits anti-myeloma activity. J Transl Med. (2024) 22(1):208. doi: 10.1186/s12967-024-05013-
24. Ajuwon KM, Spurlock ME. Adiponectin inhibits LPS-induced NF-kappaB activation and IL-6 production and increases PPARgamma2 expression in adipocytes. Am J Physiol Regul Integr Comp Physiol. (2005) 288:R1220–5. doi: 10.1152/ajpregu.00397.2004
25. Klein B, Tarte K, Jourdan M, Mathouk K, Moreaux J, Jourdan E, et al. Survival and proliferation factors of normal and Malignant plasma cells. Int J Hematol. (2003) 78:106–13. doi: 10.1007/BF02983377
26. Wolf AM, Wolf D, Rumpold H, et al. Adiponectin induces the anti-inflammatory cytokines IL-10 and IL-1RA in human leukocytes [J. Biochem Biophys Res Commun. (2004) 323:630–5. doi: 10.1016/j.bbrc.2004.08.145
27. Fedorenko A, Lishko PV, Kirichok Y. Mechanism of fatty-acid-dependent UCP1 uncoupling in brown fat mitochondria. Cell. (2012) 151:400–13. doi: 10.1016/j.cell.2012.09.010
28. Mckillop IH, Girardi CA, Thompson KJ. Role of fatty acid binding proteins (FABPs) in cancer development and progression. Cell Signal. (2019) 62:109336. doi: 10.1016/j.cellsig.2019.06.001
29. Rodriguez-Otero P, Paiva B, San-Miguel JF. Roadmap to cure multiple myeloma. Cancer Treat Rev. (2021) 100:102284. doi: 10.1016/j.ctrv.2021.102284
30. Kyle RA, Rajkumar SV. Multiple myeloma. Blood. (2008) 111:2962–72. doi: 10.1182/blood-2007-10-078022
31. Dehghani M, Hobbi AM, Haghighat S, Fariba K, Ramzi M, Vojdani R, et al. Glucocorticoid induced diabetes and lipid profiles disorders amongst lymphoid Malignancy survivors. Diabetes Metab Syndr. (2020) 14:1645–9. doi: 10.1016/j.dsx.2020.08.027
32. Ellidag HY, Eren E, Aydin O, Yildirim M, Sezer C, Yilmaz N, et al. Multiple myeloma: relationship to antioxidant esterases. Med Princ Pract. (2014) 23:18–23. doi: 10.1159/000355826
33. Ellidag HY, Aydin O, Eren E, Yilmaz N, Ergin M. Decreased HDL-dependent paraoxonase and arylesterase enzyme activity may indicate a worse prognosis in multiple myeloma. Asian Pac J Cancer Prev. (2014) 15:9847–51. doi: 10.7314/APJCP.2014.15.22.9847
34. Faridvand Y, Oskuyi AE, Khadem-Ansari MH. Serum 8-isoprostane levels and paraoxonase 1 activity in patients with stage I multiple myeloma. Redox Rep. (2016) 21:204–8. doi: 10.1179/1351000215Y.0000000034
35. Hachem H, Favre G, Ghalim N. Quantitative abnormalities of lipoprotein particles in multiple myeloma. J Clin Chem Clin Biochem. (1987) 25:675–9. doi: 10.1515/cclm.1987.25.10.675
36. Hachem H, Favre G, Soula G. Evidence for qualitative abnormalities in high-density lipoproteins from myeloma patients: the presence of amyloid A protein could explain HDL modifications. Biochim Biophys Acta. (1988) 963(2):271–7. doi: 10.1016/0005-2760(88)90291-3
37. Hungria VT, Brandizzi LI, Chiattone CS, Bydlowski SP, Maranhao RC. Metabolism of an artificial emulsion resembling chylomicrons in patients with multiple myeloma. Leuk Res. (1999) 23:637–41. doi: 10.1016/S0145-2126(99)00083-1
38. Kabat GC, Kim MY, Chlebowski RT, et al. Serum lipids and risk of obesity-related cancers in postmenopausal women. Cancer Causes Control. (2018) 29:13–24. doi: 10.1007/s10552-017-0991-y
39. Kuliszkiewicz-Janus M, Baczynski S. Chemotherapy-associated changes in 31P MRS spectra of sera from patients with multiple myeloma. NMR BioMed. (1995) 8:127–32. doi: 10.1007/s10552-017-0991-y
40. Kuliszkiewicz-Janus M, Małecki R, Mohamed AS. Lipid changes occuring in the course of hematological cancers. Cell Mol Biol Lett. (2008) 13(3):465–74. doi: 10.2478/s11658-008-0014-9
41. Levy Y, Aviram M, Spira G, Tatarsky I, Brook GJ, Carter A. Plasma cholesterol concentration and extra lipid band in monoclonal gammopathies. Postgrad Med J. (1984) 60:449–53. doi: 10.1136/pgmj.60.705.449
42. Lin ZS, Qin AB, Wang SX, Yu XJ, Dong B, Xiong ZY, et al. A non-invasive differential diagnostic model for light chain cast nephropathy in newly diagnosed multiple myeloma patients with renal involvement: a multicenter study. J Nephrol. (2021) 34:1169–77. doi: 10.1007/s40620-020-00926-7
43. Yavasoglu I, Tombuloglu M, Kadikoylu G, Donmez A, Cagirgan S, Bolaman Z. Cholesterol levels in patients with multiple myeloma. Ann Hematol. (2008) 87:223–8. doi: 10.1007/s00277-007-0375-6
44. Liang L, Li J, Fu H, Liu X, Liu P. Identification of high serum apolipoprotein A1 as a favorable prognostic indicator in patients with multiple myeloma. J Cancer. (2019) 10:4852–9. doi: 10.7150/jca.31357
45. Zhu W, Charwudzi A, Li Q, Zhai Z, Hu L, Pu L, et al. Lipid levels and multiple myeloma risk: insights from Meta-analysis and mendelian randomization. Lipids Health Dis. (2024) 23:299. doi: 10.1186/s12944-024-02289-5
46. Trotter TN, Gibson JT, Sherpa TL, Gowda PS, Peker D, Yang Y. Adipocyte-lineage cells support growth and dissemination of multiple myeloma in bone. Am J Pathol. (2016) 186:3054–63. doi: 10.1016/j.ajpath.2016.07.012
47. Allegra A, Innao V, Gerace D, Allegra AG, Vaddinelli D, Bianco O, et al. The adipose organ and multiple myeloma: Impact of adipokines on tumor growth and potential sites for therapeutic intervention. Eur J Intern Med. (2018) 53:12–20. doi: 10.1016/j.ejim.2018.05.033
48. Liu Z, Xu J, He J, Liu H, Lin P, Wan X, et al. Mature adipocytes in bone marrow protect myeloma cells against chemotherapy through autophagy activation. Oncotarget. (2015) 6:34329–41. doi: 10.18632/oncotarget.v6i33
49. Liu H, He J, Koh SP, Zhong Y, Liu Z, Wang Z, et al. Reprogrammed marrow adipocytes contribute to myeloma-induced bone disease. Sci Transl Med. (2019) 11(494):eaau9087. doi: 10.1126/scitranslmed.aau9087
50. Harmer D, Falank C, Reagan MR. Interleukin-6 interweaves the bone marrow microenvironment, bone loss, and multiple myeloma. Front Endocrinol (Lausanne). (2018) 9:788. doi: 10.3389/fendo.2018.00788
51. Luo G, He Y, Yu X. Bone marrow adipocyte: an intimate partner with tumor cells in bone metastasis. Front Endocrinol (Lausanne). (2018) 9:339. doi: 10.3389/fendo.2018.00339
52. Elsaadi S, Steiro I, Abdollahi P, Vandsemb EN, Yang R, Slordahl TS, et al. Targeting phosphoglycerate dehydrogenase in multiple myeloma. Exp Hematol Oncol. (2021) 10:3. doi: 10.1186/s40164-020-00196-w
53. Bayir H, Anthonymuthu TS, Tyurina YY, Patel SJ, Amoscato AA, Lamade AM, et al. Achieving life through death: redox biology of lipid peroxidation in ferroptosis. Cell Chem Biol. (2020) 27:387–408. doi: 10.1016/j.chembiol.2020.03.014
54. Lakshman A, Painuly U, Rajkumar SV, et al. Natural history of multiple myeloma with de novo del(17p). Blood Cancer J. (2019) 9:32. doi: 10.1038/s41408-019-0191-y
55. Rao L, De Veirman K, Giannico D, Saltarella I, Desantis VA, Frassanito MA, et al. Targeting angiogenesis in multiple myeloma by the VEGF and HGF blocking DARPin((R)) protein MP0250: a preclinical study. Oncotarget. (2018) 9:13366–81. doi: 10.18632/oncotarget.24351
56. Iwasaki T, Sano H. Predicting treatment responses and disease progression in myeloma using serum vascular endothelial growth factor and hepatocyte growth factor levels. Leuk Lymphoma. (2003) 44:1347–51. doi: 10.1080/1042819031000083262
57. Mahmud I, Tian G, Wang J, Hutchinson TE, Kim BJ, Awasthee N, et al. DAXX drives de novo lipogenesis and contributes to tumorigenesis. Nat Commun. (2023) 14:1927. doi: 10.1038/s41467-023-37501-0
58. Sun J, Park C, Guenthner N, Gurley S, Zhang L, Lubben B, et al. Tumor-associated macrophages in multiple myeloma: advances in biology and therapy. J Immunother Cancer. (2022) 10. doi: 10.1136/jitc-2021-003975
59. Shao N, Qiu H, Liu J, Xiao D, Zhao J, Chen C, et al. Targeting lipid metabolism of macrophages: A new strategy for tumor therapy. J Adv Res. (2025) 68:99–114. doi: 10.1016/j.jare.2024.02.009
60. Qiao X, Hu Z, Xiong F, Yang Y, Peng C, Wang D, et al. Lipid metabolism reprogramming in tumor-associated macrophages and implications for therapy. Lipids Health Dis. (2023) 22:45. doi: 10.1186/s12944-023-01807-1
61. Gong D, Shi W, Yi SJ, Chen H, Groffen J, Heisterkamp N. TGFbeta signaling plays a critical role in promoting alternative macrophage activation. BMC Immunol. (2012) 13:31. doi: 10.1186/1471-2172-13-31
62. Tan IL, Arifa RDN, Rallapalli H, Kana V, Lao Z, Sanghrajka RM, et al. CSF1R inhibition depletes tumor-associated macrophages and attenuates tumor progression in a mouse sonic Hedgehog-Medulloblastoma model. Oncogene. (2021) 40:396–407. doi: 10.1038/s41388-020-01536-0
63. Solinas G, Schiarea S, Liguori M, Fabbri M, Pesce S, Zammataro L, et al. Tumor-conditioned macrophages secrete migration-stimulating factor: a new marker for M2-polarization, influencing tumor cell motility. J Immunol. (2010) 185:642–52. doi: 10.4049/jimmunol.1000413
64. Colegio OR, Chu NQ, Szabo AL, Chu T, Rhebergen AM, Jairam V, et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature. (2014) 513:559–63. doi: 10.1038/nature13490
65. Maller O, Drain AP, Barrett AS, Borgquist S, Ruffell B, Zakharevich I, et al. Tumour-associated macrophages drive stromal cell-dependent collagen crosslinking and stiffening to promote breast cancer aggression. Nat Mater. (2021) 20:548–59. doi: 10.1038/s41563-020-00849-5
66. Zhang S, Lv K, Liu Z, Zhao R, Li F. Fatty acid metabolism of immune cells: a new target of tumour immunotherapy. Cell Death Discovery. (2024) 10:39. doi: 10.1038/s41420-024-01807-9
67. Urashima M, Ogata A, Chauhan D, Hatziyanni M, Vidriales MB, Dedera DA, et al. Transforming growth factor-beta1: differential effects on multiple myeloma versus normal B cells. Blood. (1996) 87:1928–38. doi: 10.1182/blood.V87.5.1928.1928
68. Beyer M, Kochanek M, Giese T, Endl E, Weihrauch MR, Knolle PA, et al. In vivo peripheral expansion of naive CD4+CD25high FoxP3+ regulatory T cells in patients with multiple myeloma. Blood. (2006) 107:3940–9. doi: 10.1182/blood-2005-09-3671
69. Van Valckenborgh E, Schouppe E, Movahedi K, De Bruyne E, Menu E, De Baetselier P, et al. Multiple myeloma induces the immunosuppressive capacity of distinct myeloid-derived suppressor cell subpopulations in the bone marrow. Leukemia. (2012) 26:2424–8. doi: 10.1038/leu.2012.113
70. Li H, Han Y, Guo Q, Zhang M, Cao X. Cancer-expanded myeloid-derived suppressor cells induce anergy of NK cells through membrane-bound TGF-beta 1. J Immunol. 182(1):240–9. doi: 10.4049/jimmunol.182.1.240
71. Castriconi R, Cantoni C, Della Chiesa M, Vitale M, Marcenaro E, Conte R, et al. Transforming growth factor beta 1 inhibits expression of NKp30 and NKG2D receptors: consequences for the NK-mediated killing of dendritic cells. Proc Natl Acad Sci U.S.A. (2003) 100:4120–5. doi: 10.1038/leu.2012.113
72. Sharma A, Khan R, Joshi S, Kumar L, Sharma M. Dysregulation in T helper 1/T helper 2 cytokine ratios in patients with multiple myeloma. Leuk Lymphoma. (2010) 51:920–7. doi: 10.3109/10428191003699563
73. Bataille R, Jourdan M, Zhang XG, Klein B. Serum levels of interleukin 6, a potent myeloma cell growth factor, as a reflect of disease severity in plasma cell dyscrasias. J Clin Invest. (1989) 84(6):2008–11. doi: 10.1172/JCI114392
74. Tsuruma T, Yagihashi A, Hirata K, Torigoe T, Araya J, Watanabe N, et al. Interleukin-10 reduces natural killer (NK) sensitivity of tumor cells by downregulating NK target structure expression. Cell Immunol. (1999) 198:103–10. doi: 10.1006/cimm.1999.1586
75. Cifaldi L, Prencipe G, Caiello I, Bracaglia C, Locatelli F, De Benedetti F, et al. Inhibition of natural killer cell cytotoxicity by interleukin-6: implications for the pathogenesis of macrophage activation syndrome. Arthritis Rheumatol. (2015) 67:3037–46. doi: 10.1002/art.v67.11
76. Jaitin DA, Adlung L, Thaiss CA, Weiner A, Li B, Descamps H, et al. Lipid-associated macrophages control metabolic homeostasis in a trem2-dependent manner. Cell. (2019) 178:686–98.e14. doi: 10.1016/j.cell.2019.05.054
77. Manier S, Sacco A, Leleu X, Ghobrial IM, Roccaro AM. Bone marrow microenvironment in multiple myeloma progression. J BioMed Biotechnol. (2012) 2012:157496. doi: 10.1155/2012/157496
78. Van Der Spek E, Bloem AC, Van De Donk NW, Bogers LH, Van Der Griend R, Kramer MH, et al. Dose-finding study of high-dose simvastatin combined with standard chemotherapy in patients with relapsed or refractory myeloma or lymphoma. Haematologica. (2006) 91:542–5. doi: 10.1016/j.cell.2019.05.054
79. Sanfilippo KM, Keller J, Gage BF, Luo S, Wang TF, Moskowitz G, et al. Statins are associated with reduced mortality in multiple myeloma. J Clin Oncol. (2016) 34:4008–14. doi: 10.1200/JCO.2016.68.3482
80. Hus M, Grzasko N, Szostek M, Pluta A, Helbig G, Woszczyk D, et al. Thalidomide, dexamethasone and lovastatin with autologous stem cell transplantation as a salvage immunomodulatory therapy in patients with relapsed and refractory multiple myeloma. Ann Hematol. (2011) 90:1161–6. doi: 10.1007/s00277-011-1276-2
81. Van De Donk NW, Kamphuis MM, Lokhorst HM, Bloem AC. The cholesterol lowering drug lovastatin induces cell death in myeloma plasma cells. Leukemia. (2002) 16:1362–71. doi: 10.1038/sj.leu.2402501
82. Gronich N, Drucker L, Shapiro H, Radnay J, Yarkoni S, Lishner M, et al. Simvastatin induces death of multiple myeloma cell lines. J Investig Med. (2004) 52:335–44. doi: 10.1177/108155890405200534
83. Wong WW, Clendening JW, Martirosyan A, Boutros PC, Bros C, Khosravi F, et al. Determinants of sensitivity to lovastatin-induced apoptosis in multiple myeloma. Mol Cancer Ther. (2007) 6:1886–97. doi: 10.1158/1535-7163.MCT-06-0745
84. Dmoszynska A, Podhorecka M, Rolinski J, et al. Influence of lovastatin on BCL-2 and BAX expression by plasma cells and T lymphocytes in short-term cultures of multiple myeloma bone marrow mononuclear cells. Pol J Pharmacol. (2004) 56:485–9. doi: 10.1136/jim-52-05-34
85. Slawinska-Brych A, Zdzisinska B, Mizerska-Dudka M, Kandefer-Szerszen M. Induction of apoptosis in multiple myeloma cells by a statin-thalidomide combination can be enhanced by p38 MAPK inhibition. Leuk Res. (2013) 37:586–94. doi: 10.1016/j.leukres.2013.01.022
86. Gronich N, Rennert G. Beyond aspirin-cancer prevention with statins, metformin and bisphosphonates. Nat Rev Clin Oncol. (2013) 10:625–42. doi: 10.1038/nrclinonc.2013.169
87. Svensson RU, Parker SJ, Eichner LJ, Kolar MJ, Wallace M, Brun SN, et al. Inhibition of acetyl-CoA carboxylase suppresses fatty acid synthesis and tumor growth of non-small-cell lung cancer in preclinical models. Nat Med. (2016) 22:1108–19. doi: 10.1038/nm.4181
88. Feng N, Luo J, Guo X. Silybin suppresses cell proliferation and induces apoptosis of multiple myeloma cells via the PI3K/Akt/mTOR signaling pathway. Mol Med Rep. (2016) 13:3243–8. doi: 10.3892/mmr.2016.4887
89. Xu Y, Feng X, Zhou Q, Jiang W, Dai Y, Jiang Y, et al. Novel small molecular compound AE-848 potently induces human multiple myeloma cell apoptosis by modulating the NF-kappaB and PI3K/akt/mTOR signaling pathways. Onco Targets Ther. (2020) 13:13063–75. doi: 10.2147/OTT.S270090
90. Soriano GP, Besse L, Li N, Kraus M, Besse A, Meeuwenoord N, et al. Proteasome inhibitor-adapted myeloma cells are largely independent from proteasome activity and show complex proteomic changes, in particular in redox and energy metabolism. Leukemia. (2016) 30:2198–207. doi: 10.1038/leu.2016.102
91. Fuchs D, Berges C, Opelz G, Daniel V, Naujokat C. HMG-CoA reductase inhibitor simvastatin overcomes bortezomib-induced apoptosis resistance by disrupting a geranylgeranyl pyrophosphate-dependent survival pathway. Biochem Biophys Res Commun. (2008) 374:309–14. doi: 10.1016/j.bbrc.2008.07.012
92. Xu G, Huang S, Peng J, Gao X, Li M, Yu S, et al. Targeting lipid metabolism in multiple myeloma cells: Rational development of a synergistic strategy with proteasome inhibitors. Br J Pharmacol. (2021) 178:4741–57. doi: 10.1111/bph.v178.23
93. Moré S, Petrucci MT, Corvatta L, Fazio F, Offidani M, Olivieri A. Monoclonal antibodies: leading actors in the relapsed/refractory multiple myeloma treatment. Pharm (Basel). (2020) 13(12):426. doi: 10.3390/ph13120426
94. Tacchetti P, Barbato S, Mancuso K, Mancuso K, Zamagni E, Cavo M, et al. Bispecific antibodies for the management of relapsed/refractory multiple myeloma. Cancers (Basel). (2024) 16(13):2337. doi: 10.3390/cancers16132337
95. Szlasa W, Dybko J. Current status of bispecific antibodies and CAR-T therapies in multiple myeloma. Int Immunopharmacol. (2024) 134:112043. doi: 10.1016/j.intimp.2024.112043
96. Kang Y, Sundaramoorthy P, Gasparetto C, Feinberg D, Fan S, Long G, et al. Phase I study of opaganib, an oral sphingosine kinase 2-specific inhibitor, in relapsed and/or refractory multiple myeloma. Ann Hematol. (2023) 102:369–83. doi: 10.1007/s00277-022-05056-7
97. Zvida-Bloch T, Muchtar E, Salmon-Divon M, Dispenzieri A, Bender B, Duek A, et al. Targeting the PI3K/AKT pathway and fatty acid synthase in AL amyloidosis: mechanistic insights and therapeutic implications. Blood. (2024) 144:3288. doi: 10.1182/blood-2024-206682
98. Chiu BCH, Chen J-H, Yen Y-C, Calip GS, Chien C-R, Ahsan H, et al. Long term statin use and risk of multiple myeloma among 15.5 million Taiwanese adults: A retrospective cohort study. Blood. (2015) 126:4198. doi: 10.1182/blood.V126.23.4198.4198
99. Kahl BS, Spurgeon SE, Furman RR, Flinn IW, Coutre SE, Brown JR, et al. A phase 1 study of the PI3Kdelta inhibitor idelalisib in patients with relapsed/refractory mantle cell lymphoma (MCL). Blood. (2014) 123:3398–405. doi: 10.1182/blood-2013-11-537555
100. Tsvetkov P, Detappe A, Cai K, Keys HA-O, Brune Z, Ying W, et al. Mitochondrial metabolism promotes adaptation to proteotoxic stress. Nat Chem Biol. (2019) 5(7):681–9. doi: 10.1038/s41589-019-0291-9
101. Mohamed A, Collins J, Jiang H, Molendijk J, Stoll T, Torta F, et al. Concurrent lipidomics and proteomics on Malignant plasma cells from multiple myeloma patients: Probing the lipid metabolome. PLoS One. (2020) 15:e0227455. doi: 10.1371/journal.pone.0227455
102. Wang H, Chen B, Shao R, Liu W, Xiong L, Li L, et al. A new prediction model integrated serum lipid profile for patients with multiple myeloma. J Cancer. (2022) 13:1796–807. doi: 10.7150/jca.69321
103. Maekawa K, Ri M, Nakajima M, Sekine A, Ueda R, Tohkin M, et al. Serum lipidomics for exploring biomarkers of bortezomib therapy in patients with multiple myeloma. Cancer Sci. (2019) 110:3267–74. doi: 10.1111/cas.v110.10
104. Ferrarini M, Ferrero E. Proteasome inhibitors and modulators of angiogenesis in multiple myeloma. Curr Med Chem. (2011) 18:5185–95. doi: 10.2174/092986711798184316
105. Gambella M, Palumbo A, Rocci A. MET/HGF pathway in multiple myeloma: from diagnosis to targeted therapy? Expert Rev Mol Diagn. (2015) 15:881–93. doi: 10.1111/cas.14178
Keywords: multiple myeloma, lipid metabolism, therapeutic targets, tumor, hematology
Citation: Wang H, Zhang J, Ren H, Chen L, Ren J, Liu C, Wu H and Zhou L (2025) Lipid metabolism in multiple myeloma: pathogenesis, therapeutic opportunities, and future directions. Front. Oncol. 15:1531928. doi: 10.3389/fonc.2025.1531928
Received: 21 November 2024; Accepted: 11 February 2025;
Published: 05 March 2025.
Edited by:
Satoshi Yoshihara, Hyogo Medical University, JapanReviewed by:
Nicola Amodio, Magna Græcia University, ItalyCopyright © 2025 Wang, Zhang, Ren, Chen, Ren, Liu, Wu and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lin Zhou, bHlubnpob3UzNkAxMjYuY29t; Hongkun Wu, d2hrMDIxOEAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.