
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 14 February 2025
Sec. Gastrointestinal Cancers: Colorectal Cancer
Volume 15 - 2025 | https://doi.org/10.3389/fonc.2025.1524172
This article is part of the Research Topic Cancer screening and “virtuous” health behaviors: the contribution of behavioral economics View all 4 articles
Objectives: Despite the implementation of colorectal cancer (CRC) screening programs in many regions worldwide over the past few decades, the cost-effectiveness of these programs has been questioned owing to their acceptance rates. In this study, we evaluated the cost-effectiveness of screening strategies, quantified the impact of colonoscopy acceptance rates, and analyzed the underlying factors driving individual preferences.
Methods: The cost-effectiveness of three strategies—no screening, sequential two-step screening (fecal immunochemical test and risk assessment, followed by colonoscopy), and colonoscopy screening—was evaluated from a societal perspective. This assessment was conducted using a decision-tree Markov model with the incremental cost-effectiveness ratio as the primary evaluation criterion.
Results: Sequential screening was more cost-effective than colonoscopy screening (19,335 vs. 27,379 United States dollars per quality-adjusted life year). Ideal sequential screening could prevent 32.2%(691/2147) CRC deaths, whereas colonoscopy screening at the same colonoscopy acceptance rate (20.3%) could prevent 17.6%(377/2147) CRC deaths. When the acceptance rate of direct colonoscopy surpasses the threshold of 37.2%, the resulting health benefits likely outweigh those achieved using a the sequential two-step screening approach.
Conclusions: Sequential screening is recommended for individuals in areas with constrained screening resources or during the early stages of regional screening program implementation. However, once screening habits are established, transitioning to direct colonoscopy screening becomes more favorable. Notably, reducing colonoscopy costs is the principal factor for enhancing an individual’s willingness to undergo the procedure.
Globally, colorectal cancer (CRC) is the third most prevalent cancer and the second leading cause of cancer deaths (1). CRC also imposes a significant economic burden, ranking third among all cancers (2). Some patients with CRC present with typical symptoms, such as constipation, bloody stool, anorexia, nausea, and vomiting, whereas approximately 50% of these patients are asymptomatic (3). Thus, it is difficult for many patients with CRC to recognize the onset of the disease, with most presenting with advanced-stage disease and some even having metastasis (4). Patients with advanced CRC have a poor quality of life, unfavorable prognosis, and substantial treatment costs, such as chemotherapy (5–7). Therefore, reducing the incidence of CRC and increasing the rate of early diagnosis is vital.
Since 1975, large CRC screening programs have been launched in several countries, including the United States, some in Europe, Australia, and South Korea (8–11). Screening methods include guaiac fecal occult blood tests, fecal immunochemical tests (FITs), total colonoscopy, and flexible sigmoidoscopy (9). Nationwide screening programs have reduced the CRC incidence and CRC-specific mortality rates (12–14), whereas both rates have increased in regions lacking national screening programs, particularly in Asia (15, 16). More recently, however, colonoscopy screening programs have been reported to be ineffective in reducing CRC incidence and mortality rates (17).
Many countries have long-standing CRC screening programs launched as part of their national public health policies (8–11), whereas in others, including China, these programs, including colonoscopy, are currently being implemented. Therefore, examining the effectiveness of these screening programs is critical. Actual screening and medical insurance at a from an eastern Chinese city were examined to determine the need for screening, select the most appropriate screening strategy, and suggest methods to enhance screening implementation.
A social perspective was selected as the research perspective, a model-based design was used, and residents of Huzhou City aged 50–75 years were identified as the target population. Based on the CRC screening project for key populations, 804,180 residents of Huzhou City were estimated to be aged 50–75 years. During the modeling phase, the population was set at 804,180 individuals, and the time horizon was set at 10 years. The incremental cost-effectiveness ratios (ICERs) were calculated to determine the value of each screening method, and the optimal screening strategy was determined by comparing the ICERs. The variability and uncertainty were assessed using one-way sensitivity, probabilistic sensitivity, and scenario analyses.
Ethical considerations were in accordance with the Declaration of Helsinki. The study protocol was reviewed and approved by the Institutional Review Board approval # 2022-12-1019, and was also registered internationally (IRB00002408 & FWA00002399).
This study was conducted in Huzhou City, Zhejiang Province, China. The total population of Huzhou is 2.68 million, with the Han ethnic group accounting for 96.57% of the population. The overall sex ratio (calculated as the number of males per 100 females) is 109.04 and 18.70% of the population is aged 60 years or above. The per capita gross domestic product (GDP) of Huzhou is approximately 17,324 United States dollars (USD). The number of healthcare workers in Huzhou is approximately 11.74 per 1,000 people, and the number of hospital beds is approximately 6.37 per 1,000 people. According to data from 14 cancer registries in Zhejiang Province, the incidence rate of CRC is approximately 41.75 per 100,000 people and the mortality rate due to CRC is approximately 17.02 per 100,000 people.
Our CRC screening strategy consisted of three approaches: no screening, sequential screening, and direct colonoscopy (1). No screening: No proactive measures are taken, and patients are diagnosed when they seek medical attention following symptom manifestation. (2) Sequential screening: This two-step process uses a two-sample FIT and risk assessment questionnaire. A positive result in the FIT (20μg of human hemoglobin per gram of stool, with samples taken 1 week apart) or a high-risk designation from the questionnaire leads to a recommendation for colonoscopy. (3) Direct colonoscopy: Individuals who undergo a colonoscopy without prior screening.
The risk assessment questionnaire included seven indicators: history of colorectal polyposis, familial adenomatous polyposis, age, sex, family history of CRC, smoking, and body mass index (Table 1). These were drawn from the Chinese CRC screening guidelines and validated through a meta-analysis. We further validated these indicators based on the detection results in our study.
The CRC screening program began in 2019 and initially targeted a small subset of the population. In 2020, screening was officially launched with the aim of selecting one-fifth of the local target population (aged 45–74 years) annually. Within a 5-year interval, individuals within the target population will not be duplicated; however, new participants who meet the age criterion may still be included. Beginning in 2020, data from all individuals became accessible for analysis, and this study used data from 2020 to 2022. Owing to the large scale of the data, the electronic entry and verification processes exceeded 1year, making the 2022 data the most recently available. Participants were recruited from community hospitals and rural health service centers by doctors who typically invited residents to participate in the screening through posted flyers and phone notifications. This study employed a model-based approach with parameters derived from the following primary sources. The Huzhou Key Population Colorectal Cancer Screening Project was critical to our dataset, as it incorporates a two-step sequential screening strategy (Additional File 1 in Supplementary Material). Through this project, we obtained acceptance rates for preliminary screening and subsequent colonoscopy-based evaluations, diagnostic results for the participants, and the costs associated with the initial screening. Data from the Health Insurance Bureau provided insights into treatment expenses related to early-stage CRC, advanced CRC, and metastatic advanced CRC, along with colonoscopy fees. Data from the Seventh Population Census were used to establish life tables (Additional File 2 in Supplementary Material). Additionally, information from the Huzhou Municipal Bureau of Statistics contributed to our understanding of Huzhou’s GDP and other foundational demographic and infrastructural data. For parameters not readily available from the aforementioned data sources, we relied on data from relevant literature (Table 2).
In the model, the health status of individuals was classified into three categories: non-CRC, CRC, and death. Among the patients who underwent sequential screening, 20.3% were classified as high-risk and 79.0% as low-risk based on primary screening. Of the 15,019 high-risk individuals who underwent colonoscopy, 411 (2.7%) were diagnosed with CRC. The probability of developing CRC in high-risk individuals who refused colonoscopy was assumed to be the same as that in individuals who underwent colonoscopy. The sensitivity of sequential primary screening was set at 83% based on a meta-analysis of 40 publications on individuals screened for CRC according to the Chinese screening guidelines, which reported a sensitivity of 83% for the FIT (18).Consequently, the probability of CRC in the low-risk population was expected to be 0.6%, resulting in an estimated 1.0% (8,042/804,180) in the same age range (50–75 years) in the Huzhou natural population. Early CRC was defined as cancer limited to the mucosa or invading the submucosa only (19, 20). Advanced CRC was defined as cancer that had penetrated the submucosa with metastasis or local progression. Of the 411 patients in the screening population diagnosed with CRC, 269 (65.5%) had early-stage (stage I) tumors, and 142 (34.5%)had advanced-stage (stages II–IV) tumors, with none having metastases (stage IV). In comparison, of the 10,150 self-referred patients with CRC, 433 (4.3%) had early-stage CRC and 9,717 (95.7%) had advanced-stage tumors. Although the databases did not report metastases, the rate was estimated to be 20% based on the results of other studies. The cancer stage was used in the model to determine weight costs and healthcare outcomes.
The incidence of CRC in the general population has been reported to be 0.002 and decreases following colonoscopy (21, 22). The standardized incidence rates of CRC were 19.77 per 100,000 person-years 0–5 years after colonoscopy and 35.21 per 100,000 person-years 5–10 years after colonoscopy (22).
The duration for the decision-tree Markov model was set at 10 years. However, patients with CRC were not followed up; rather, data from a study that included a similarly aged population from similar regions with a 10-year follow-up period were used (23). In the present study, survival at 10 years was considered a cure. The 10-year survival rates of patients with CRC stages I–IV were 83.8%, 70.1%, 57.3%, and 8.7%, respectively. The stage-weighted cure rates were 76.9% in patients with CRC detected through screening and 53.6% in self-referred patients with CRC. The 1-, 3-, 5-, and 10-year stage-weighted CRC death rates were 2.4%, 7.8%, 13.1%, and 23.1%, respectively, among the screened patients, and 9.2%, 24.9%, 34.8%, and 46.4%, respectively, among those who were self-referred.
Costs included direct and indirect costs. Screening costs included 5 USD for the risk questionnaire and FIT and 195 USD for the colonoscopy. The total costs hospitalization costs for patients with early and advanced-stage CRC, with and without metastases, were calculated based on the medical insurance records of Huzhou City in 2020. The mean ± standard deviation (SD) direct costs to patients with early-stage CRC were 3249 ± 3,932 USD. In contrast, the mean ± SD direct costs to patients with advanced-stage CRC comprised 1,751 ± 1,831USD for hospitalization and 1,024 ± 742 USD for adjuvant treatment, the latter of which was administered six times to patients without metastasis and 12 times to patients with metastasis. Direct nonmedical and indirect costs were based on productivity costs. These costs were calculated based on the length of hospital stay, costs to the patient and one caregiver, and the per capita income in Huzhou. The average length of hospitalization was 11.7 ± 10.1 days for patients with early CRC In contrast, for patients with advanced CRC, the average hospitalization and adjuvant treatment lengths were 11.8 ± 12.0 days and 3.6 ± 3.7 days, respectively. In 2020, employees in Huzhou City earned an average of 37 USD per day. In accordance with the China Guidelines for Pharmacoeconomic Evaluations, the cost discount was set at 5% and the sensitivity analysis ranged from 0%–8% (24). The same discount rate was applied to the health outcome index. To simplify the model, all CRC stages were included and weighted in different populations. After weighting, the mean cost for individuals diagnosed with CRC through screening was set at 6,286 USD, whereas the mean cost for individuals diagnosed through self-referred visits was set at 11,672 USD.
Health outcomes were determined by assessing the quality of life of patients with different stages of CRC in the same regions. These outcomes were based on the EuroQol five-dimension five-level index scores, in which the health outcomes of patients with CRC stages I–IV were set at 0.893, 0.821, 0.698, and 0.637, respectively, weighted according to the proportion of patients with these CRC stages in different populations (25). The stage-weighted CRC health effectiveness was set at 0.85 for patients diagnosed by screening and 0.74 for self-referred patients. The incremental cost, quality-adjusted life years (QALYs), and ICER were calculated.
A decision-tree Markov model was established to estimate the impact of different colorectal screening strategies on disease burden, QALYs, and costs for individuals aged 50–75 years in Huzhou City (Figure 1). A decision tree was developed at the beginning of the model, with individuals free to choose whether to undergo screening. The participants were allowed to enter in any state and could transition to death from any health state. Sequential primary screening could provide positive or negative results; based on this probability, individuals with positive primary screening results were assigned to undergo or refuse colonoscopy. The model compared the ICER to the target population’s decision to initiate screening or not, as well as to various screening strategies. Individuals in the cohort were divided into three categories: healthy, CRC, and deceased. The probability of a person with a given set of primary screening results being in a category was determined based on the results of the screening project. Statistical analyses were performed using TreeAge Pro Healthcare 2020 software (TreeAge Pro 2020, R1. TreeAge Software, Williamstown, MA, USA) and R Statistical Software (version 4.2.1; R Foundation for Statistical Computing, Vienna, Austria). A checklist for this guidance is provided in Additional File 4 in Supplementary Material.
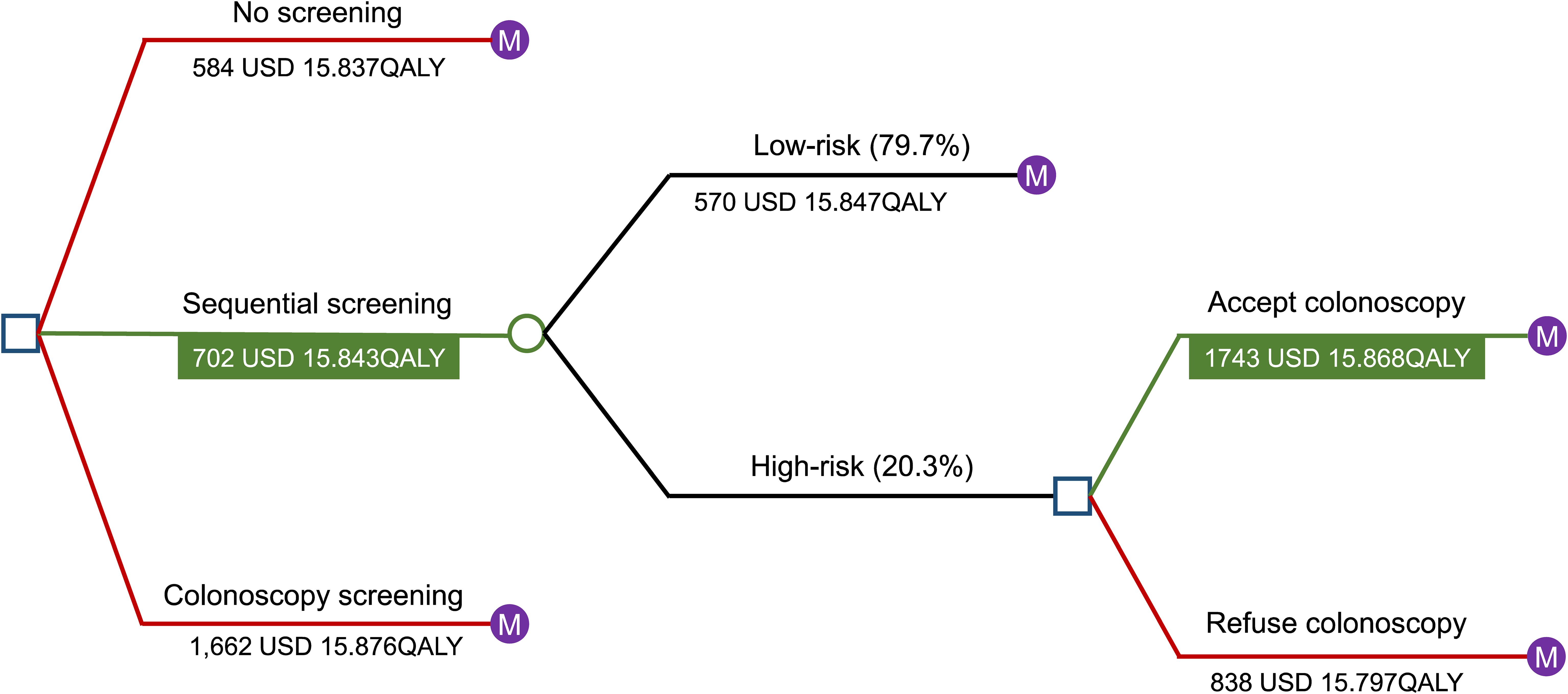
Figure 1. Results of the Markov decision tree analysis. A decision-tree Markov model was established to estimate the impact of different colorectal screening strategies on disease burden, QALYs, and costs for individuals aged 50–75 years in Huzhou City. Individuals in the cohort were divided into three categories: healthy, CRC, and deceased. A decision tree was created at the beginning of the model, with participants free to choose whether to undergo screening. Participants were allowed to enter in any state and could transition to death from any health state. Sequential primary screening could provide positive or negative results, and based on probability, individuals with positive primary screening results were assigned to undergo or refuse colonoscopy. The model compared the ICER to the target population’s decision to initiate screening or not, as well as to various screening strategies. Abbreviations: USD, United States dollar; QALYs, quality-adjusted life years; CRC, colorectal cancer; ICER, incremental cost-effectiveness ratio.
This study included 175,550 individuals who underwent screening; of these, 35,555 (20.3%) were evaluated as high-risk. Of these high-risk individuals, 15,019 (42.2%) underwent colonoscopy, of whom, 411 (2.7%) were diagnosed with CRC. Colonoscopy acceptance rates were associated with the questionnaire scores and the number of FIT-positive results. Among individuals with a high risk, those with 0, 1, and 2 FIT positive results had colonoscopy acceptance rates of 26.3% (3,007/11,449), 41.3% (1,181/2,859), and 65.7% (435/662), respectively. Among individuals with questionnaire scores of 0-4 points, those with one and two FIT-positive results had colonoscopy acceptance rates of 46.8% (7,332/15,665) and 62.3% (3,064/4,920), respectively. The colonoscopy acceptance rate was primarily affected by the number of FIT positive results. Patients who were divided into questionnaire-positive(5 points), FIT-positive, and doubly-positive on primary screening had early CRC rates of 83.3% (30/36), 64.1% (207/323), and 61.5% (32/52), respectively, with the proportion of early-stage tumors being higher in the questionnaire-positive group than in the FIT-positive (P questionnaire vs. P FIT =0.033) and doubly positive (P questionnaire vs. P questionnaire + FIT=0.049) groups.
Screening benefits were evaluated in different situations. The ICER showed that sequential screening was more cost-effective (Table 3, Figure 1). According to situation 1 (actual results of the CRC screening project in key populations), only 8.6% of the entire population underwent colonoscopy, resulting in the avoidance of 292 (13.6%) deaths from CRC, whereas colonoscopy screening would have prevented 160 (7.5%) CRC deaths. According to situation 2 (ideal sequential screening), 691 (32.2%) deaths from CRC would be avoided, whereas the same colonoscopy acceptance rate in patients screened by colonoscopy would prevent 377 (17.6%) deaths from CRC. To reduce the number of CRC deaths to the same level as that in situation 2, the colonoscopy acceptance rate in individuals screened by colonoscopy would have to increase to 37.2%.
The one-way sensitivity analysis of the four decisions, using the ICER as the criterion enabled the selection of the five factors with the greatest influence on each outcome (Figure 2).The resulting tornado diagram shows the cost of colonoscopy had a major impact on individuals who underwent screening. The sensitivity of sequential primary screening had the greatest impact on the choice between sequential and colonoscopy screenings. In patients with positive initial screening results, the cost of colonoscopy affects the decision to continue with the procedure. The incremental cost-effectiveness scatter plot demonstrates that sequential and direct colonoscopy screening is superior to no screening at the current threshold. Notably, individuals who test positive during the initial sequential screening should be referred for colonoscopy. The probabilistic sensitivity analysis results are reported in Additional File 3 in Supplementary Material.
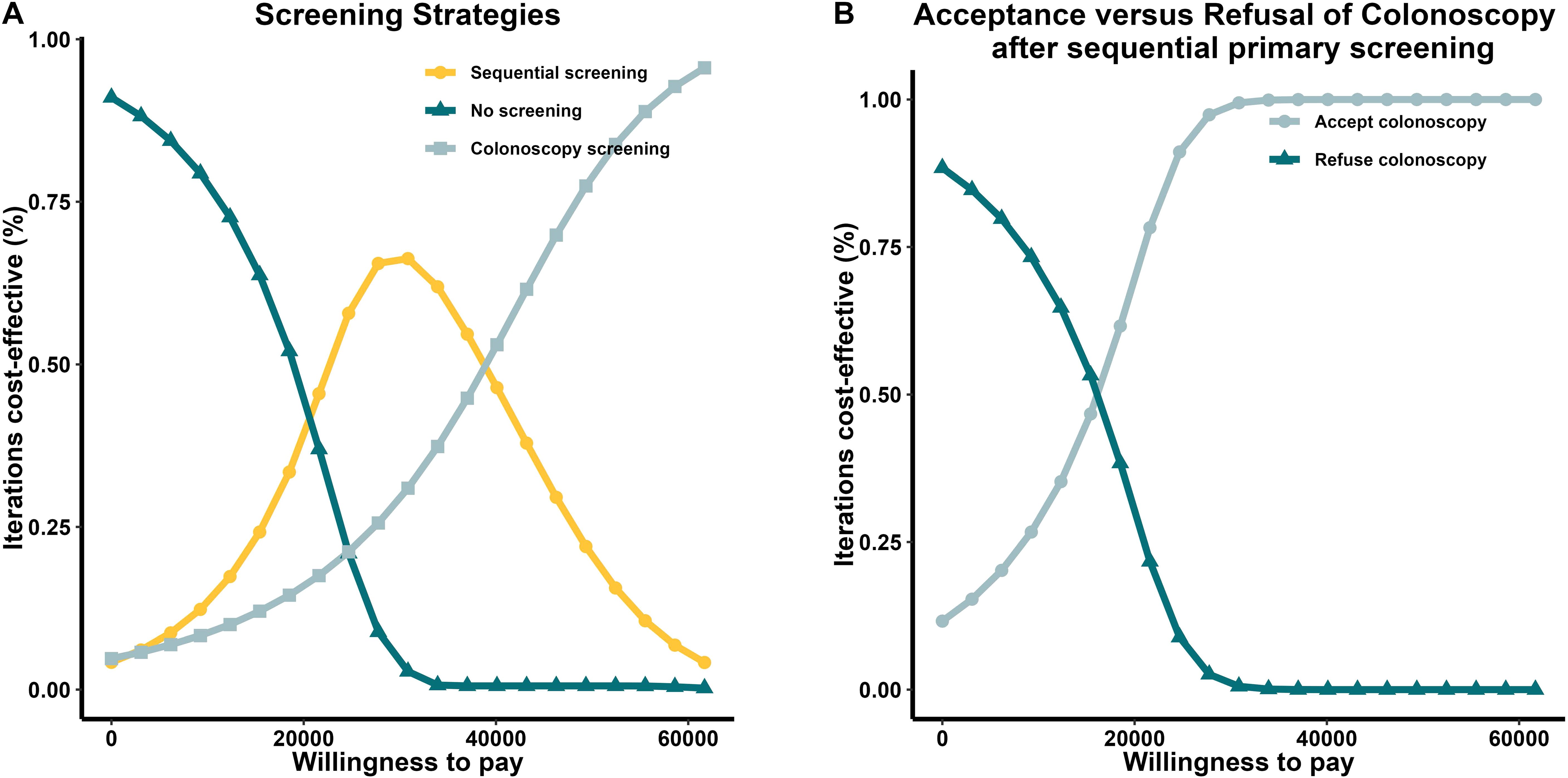
Figure 2. Tornado diagram. A one-way sensitivity analysis of the four decisions included in this study, using the ICER as the criterion, enabled the selection of the five factors with the greatest influence on each outcome. The resulting tornado diagram showed that the cost of colonoscopy had a major impact on individuals who underwent screening. The sensitivity of sequential primary screening had the greatest impact on the choice between sequential and colonoscopy screenings. Abbreviations: EV, economic value; ICER, incremental cost-effectiveness ratio.
The cost-effectiveness acceptance curves and the per capita GDP of 17,324USD showed that sequential primary screening was preferred when the willingness-to-pay (WTP) was within approximately one to two times the per capita GDP range. In contrast, colonoscopy screening was more likely to be chosen when the WTP was greater than two times the per capita GDP. Comparisons of acceptance versus refusal after sequential primary screening showed that, when the WTP was close to the per capita GDP, individuals were more likely to proceed to colonoscopy, the second step of sequential screening (Figure 3).
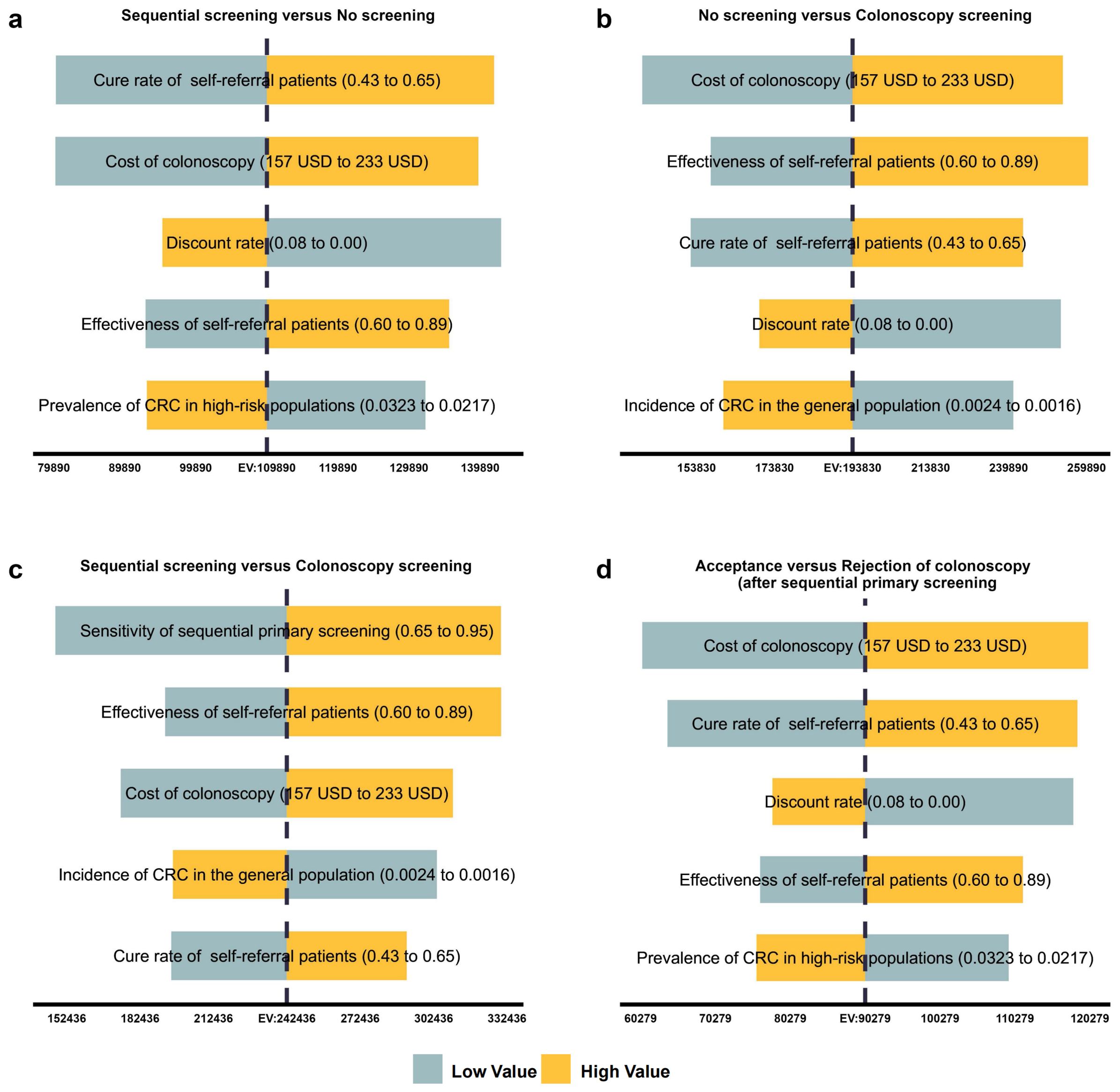
Figure 3. Cost-effectiveness acceptability curve. Results of the CE acceptance curves and the per capita (GDP) of 17,324 USD showed that when the WTP was within approximately one to two times the per capita GDP range, sequential primary screening was preferred. In contrast, when the WTP was greater than two times the per capita GDP, colonoscopy screening was more likely to be chosen. Comparisons of acceptance versus refusal after sequential primary screening showed that when the WTP was close to the per capita GDP, individuals were more likely to proceed to colonoscopy, the second step of sequential screening. CE, cost-effectiveness; GDP, gross domestic product; USD, United States dollar; WTP, willingness-to-pay.
By integrating a substantial volume of screening and medical insurance data, we employed a decision-tree Markov model to comprehensively assess the cost-effectiveness of three CRC screening strategies: no screening, sequential two-step screening, and colonoscopy screening. The findings revealed that colonoscopy and initial sequential screening were cost-effective. According to the ICER criterion, sequential screening is the preferred option, and it is essential to underscore the existence of an upper limit to the health benefits achievable through a two-step screening approach. Therefore, this approach is particularly suitable for the initial phases of screening implementation. With increased screening duration and higher acceptance rates among the population, transitioning to direct colonoscopy screening is recommended. One of the primary considerations for enhancing the individual acceptance rates of colonoscopy is the reduction in colonoscopy costs.
The main health benefit of screening is the early diagnosis of CRC. The data from this study show that the proportion of early-stage patients identified through screening was higher than that of self-referred patients. Early diagnosis resulted in a cure rate over nine times higher than in patients with metastases but also to provide a superior quality of life (23, 25, 26). In addition, early diagnosis resulted in a shorter treatment period, with accumulated hospital stays of approximately 11.7 days in early-stage patients, 33.4 days in advanced-stage patients without metastases, and approximately 55 days in advanced-stage patients with metastases. The costs of early diagnosis are also lower, with medical insurance data showing that the costs in early-stage patients were approximately 41.2% of those in advanced-stage patients without metastases and 23.2% of those in advanced-stage patients with metastases.
Nationwide colonoscopy screening programs for CRC have been introduced in developed regions such as Europe and the United States (8–11). Screening reduces the incidence and mortality of CRC (16). More recently, however, the effectiveness of colonoscopy has been questioned, with screening being found to be ineffective in reducing the incidence and mortality of CRC (17). This study suggests that the acceptance rate of colonoscopy may be the main factor affecting the benefits of screening. In this study, only 42% of the participants in the sequential screening group underwent colonoscopy. If individuals in the screening group who refused colonoscopy were excluded, the incidence and death rates of CRC would have been reduced by 31% and 50%, respectively (17). Similar outcomes were obtained in the present study, indicating that the acceptance rate of colonoscopy substantially affects screening effectiveness. Based on a questionnaire assessment, only 26.3% of individuals in the FIT-negative but high-risk group underwent colonoscopy, which may be even lower in the general population. Similar studies in China may find that the colonoscopy acceptance rate is below 26.3%, indicating that colonoscopy screening alone may not be suitable for developing countries preparing to implement new screening strategies.
Sequential screening was defined as a low-cost, non-invasive primary screening, consisting of risk questionnaires and FITs, followed by colonoscopy in high-risk individuals. This strategy allows high-risk individuals to undergo colonoscopy at the same cost, making it more cost-effective from social and healthcare system perspectives. Owing to the increasing incidence of CRC in developing nations (27), this cost-effective screening strategy should be widely promoted.
The present study also found that the results of sequential primary screening can affect individuals’ willingness to undergo a second-step colonoscopy. Therefore, in nations that have implemented colonoscopy screening programs, primary screening of those unwilling to undergo colonoscopy may directly increase the acceptance rate. This strategy can improve the colonoscopy acceptance rate in countries where this rate has stabilized and is no longer increasing. The sensitivity analysis results also suggest the future use of more sensitive primary screening methods (18). Although the high-risk questionnaire did not increase patient acceptance of colonoscopy, it effectively identified more patients with early-stage CRC. Improving and promoting the questionnaire may increase screening acceptance rates.
The present study has several limitations. Because we chose some parameters from the literature and despite our efforts to select high-quality research and consider the study areas and target populations, bias was inevitable. The screening data were obtained from records collected in Huzhou between 2020 and 2022. When selecting screening participants, we focused exclusively on residents of Huzhou aged 45–74 years to enhance the generalizability of the findings. However, selection bias was unavoidable, precluding the elimination of bias among the research participants. Individuals with confidence in their health or those declining participation in our screening because of other factors, such as regular health checkups at their workplace, might have still opted to forego the screening, even after being recruited. Second, although we analyzed the direct non-medical and indirect labor costs to patients and caregivers resulting from patient hospitalization, we omitted other costs, including food and accommodation, transportation, nutrition, and informal care. Thus, the costs of CRC and the cost-effectiveness of screening might have been underestimated. Third, the potential risks of colonoscopy are among the reasons individuals resist this procedure. In this study, only two of the 15,019 patients who underwent colonoscopy experienced moderate bleeding, and none experienced serious complications. However, the model did not account for these events, which might have resulted in an overestimation of the health advantages in the screening group. Finally, because the CRC incidence rate in the low-risk groups could not be obtained during the modeling stage, it was replaced by the incidence rate in the general population. This could have overestimated the incidence of CRC diagnoses and deaths in the sequential screening group while underestimating the health benefits.
In developing nations, CRC screening is still in its infancy. Future studies should include additional data to enhance the described model and provide more precise results. Individual-based microsimulation models are also under development. Future studies should include additional characteristics resulting in more accurate analyses of the cost-effectiveness of screening and the development of more effective screening strategies.
We evaluated the cost-effectiveness of three CRC screening strategies: no screening, sequential two-step screening, and colonoscopy. Sequential screening is more cost-effective than colonoscopy screening, especially in regions with limited resources or low colonoscopy acceptance rates. However, colonoscopy screening is more advantageous than sequential screening in preventing CRC deaths when costs are not a barrier. Therefore, we recommend sequential screening as a feasible and effective approach for individuals residing in regions with limited resources or in the initial phases of implementing regional screening programs. In areas where regional colonoscopy screening has been implemented, utilizing the FIT for preliminary assessment can significantly improve the acceptance rates of colonoscopy in individuals who decline direct colonoscopy, thus effectively increasing the colonoscopy acceptance rate.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by the Institutional Review Board (IRB) with the approval number IRB # 2022-12-1019, and the study was also registered internationally with the IRB registration number IRB00002408 & FWA00002399. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. The manuscript presents research on animals that do not require ethical approval for their study.
YF: Conceptualization, Formal Analysis, Funding acquisition, Methodology, Supervision, Writing – original draft, Writing – review & editing. HL: Conceptualization, Formal Analysis, Funding acquisition, Methodology, Supervision, Writing – original draft, Writing – review & editing. AX: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Validation, Writing – original draft, Writing – review & editing. ZY: Conceptualization, Formal Analysis, Funding acquisition, Methodology, Supervision, Writing – original draft, Writing – review & editing. PZ: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Validation, Writing – original draft, Writing – review & editing. WW: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Validation, Writing – original draft, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was funded by Huzhou Science and Technology Research Plan Project (2024GYB54), Key Laboratory of Emergency Detection for Public Health of Huzhou. The funders had no role in the study design, data collection and analysis, decision to publish, or manuscript preparation.
The authors would like to thank all participants for their valuable contributions to this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1524172/full#supplementary-material
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Luengo-Fernandez R, Leal J, Gray A, Sullivan R. Economic burden of cancer across the European Union: a population-based cost analysis. Lancet Oncol. (2013) 14:1165–74. doi: 10.1016/S1470-2045(13)70442-X
3. Juul JS, Hornung N, Andersen B, Laurberg S, Olesen F, Vedsted P. The value of using the faecal immunochemical test in general practice on patients presenting with non-alarm symptoms of colorectal cancer. Br J Cancer. (2018) 119:471–9. doi: 10.1038/s41416-018-0178-7
4. Lu W, Pan X, Dai S, Fu D, Hwang M, Zhu Y, et al. Identifying stage II colorectal cancer recurrence associated genes by microarray meta-analysis and building predictive models with machine learning algorithms. J Oncol. (2021) 2021:6657397. doi: 10.1155/2021/6657397
5. Drageset S, Lindstrom TC, Underlid K. I just have to move on": Women's coping experiences and reflections following their first year after primary breast cancer surgery. Eur J Oncol Nurs. (2016) 21:205–11. doi: 10.1016/j.ejon.2015.10.005
6. Cunningham D, Atkin W, Lenz HJ, Lynch HT, Minsky B, Nordlinger B, et al. Colorectal cancer. Lancet. (2010) 375:1030–47. doi: 10.1016/S0140-6736(10)60353-4
7. Yajima S, Shimizu H, Sakamaki H, Ikeda S, Ikegami N, Murayama JI. Real-world cost analysis of chemotherapy for colorectal cancer in Japan: detailed costs of various regimens during the entire course of chemotherapy. BMC Health Serv Res. (2016) 16:2. doi: 10.1186/s12913-015-1253-x
8. Mandel JS, Bond JH, Church TR, Snover DC, Bradley GM, Schuman LM, et al. Reducing mortality from colorectal cancer by screening for fecal occult blood. Minnesota Colon Cancer Control Study. N Engl J Med. (1993) 328:1365–71. doi: 10.1056/NEJM199305133281901
9. Basu P, Ponti A, Anttila A, Ronco G, Senore C, Vale DB, et al. Status of implementation and organization of cancer screening in The European Union Member States-Summary results from the second European screening report. Int J Cancer. (2018) 142:44–56. doi: 10.1002/ijc.31043
10. Kim SY, Kim HS, Kim YT, Lee JK, Park HJ, Kim HM, et al. Colonoscopy versus fecal immunochemical test for reducing colorectal cancer risk: A population-based case-control study. Clin Transl Gastroenterol. (2021) 12:e00350. doi: 10.14309/ctg.0000000000000350
11. Lew JB, St John DJB, Xu XM, Greuter MJE, Caruana M, Cenin DR, et al. Long-term evaluation of benefits, harms, and cost-effectiveness of the National Bowel Cancer Screening Program in Australia: a modelling study. Lancet Public Health. (2017) 2:e331–40. doi: 10.1016/S2468-2667(17)30105-6
12. Hillyer GC, Neugut AI. Where does it FIT? The roles of fecal testing and colonoscopy in colorectal cancer screening. Cancer. (2015) 121:3186–9. doi: 10.1002/cncr.29459
13. Senore C, Haug U. Faecal immunochemical tests have the potential for correctly ruling out colorectal cancer in symptomatic patients. BMJ Evid Based Med. (2018) 23:113–4. doi: 10.1136/bmjebm-2018-110901
14. Sharaf RN, Ladabaum U. Comparative effectiveness and cost-effectiveness of screening colonoscopy vs. sigmoidoscopy and alternative strategies. Am J Gastroenterol. (2013) 108:120–32. doi: 10.1038/ajg.2012.380
15. Zhiqin W, Palaniappan S, Raja Ali RA. Inflammatory bowel disease-related colorectal cancer in the asia-pacific region: past, present, and future. Intest Res. (2014) 12:194–204. doi: 10.5217/ir.2014.12.3.194
16. Bray F, Ferlay J, Laversanne M, Brewster DH, Gombe Mbalawa C, Kohler B, et al. Cancer Incidence in Five Continents: Inclusion criteria, highlights from Volume X and the global status of cancer registration. Int J Cancer. (2015) 137:2060–71. doi: 10.1002/ijc.29670
17. Bretthauer M, Loberg M, Wieszczy P, Kalager M, Emilsson L, Garborg K, et al. Effect of colonoscopy screening on risks of colorectal cancer and related death. N Engl J Med. (2022) 387:1547–56. doi: 10.1056/NEJMoa2208375
18. National Cancer Center, China, Expert Group of the Development of China Guideline for the Screening, Early Detection and Early Treatment of Colorectal Cancer. China guideline for the screening, early detection and early treatment of colorectal cancer (2020, Beijing). Zhonghua Zhong Liu Za Zhi. (2021) 43:16–38. doi: 10.3760/cma.j.cn112152-20210105-00010
19. Kashida H, Kudo SE. Early colorectal cancer: concept, diagnosis, and management. Int J Clin Oncol. (2006) 11:1–8. doi: 10.1007/s10147-005-0550-5
20. Hong SW, Byeon JS. Endoscopic diagnosis and treatment of early colorectal cancer. Intest Res. (2022) 20:281–90. doi: 10.5217/ir.2021.00169
21. Brenner H, Chen C. The colorectal cancer epidemic: challenges and opportunities for primary, secondary and tertiary prevention. Br J Cancer. (2018) 119:785–92. doi: 10.1038/s41416-018-0264-x
22. Pilonis ND, Bugajski M, Wieszczy P, Franczyk R, Didkowska J, Wojciechowska U, et al. Long-term colorectal cancer incidence and mortality after a single negative screening colonoscopy. Ann Intern Med. (2020) 173:81–91. doi: 10.7326/M19-2477
23. Cheng E, Blackburn HN, Ng K, Spiegelman D, Irwin ML, Ma X, et al. Analysis of survival among adults with early-onset colorectal cancer in the national cancer database. JAMA Netw Open. (2021) 4:e2112539. doi: 10.1001/jamanetworkopen.2021.12539
24. Chinese Pharmaceutical Association. China guidelines for pharmacoeconomic evaluations (2021). Available online at: https://www.cpa.org.cn/cpadmn/attached/file/20201203/1606977380634185.pdf (Accessed April 22, 2021).
25. Huang W, Yang J, Liu Y, Liu C, Zhang X, Fu W, et al. Assessing health-related quality of life of patients with colorectal cancer using EQ-5D-5L: a cross-sectional study in Heilongjiang of China. BMJ Open. (2018) 8:e022711. doi: 10.1136/bmjopen-2018-022711
26. Huang W, Yu H, Liu C, Liu G, Wu Q, Zhou J, et al. Assessing health-related quality of life of chinese adults in heilongjiang using EQ-5D-3L. Int J Environ Res Public Health. (2017) 14:224. doi: 10.3390/ijerph14030224
Keywords: cost-effectiveness, analysis, colorectal cancer, screening, survey
Citation: Fu Y, Li H, Xu A, Yang Z, Zhang P and Wang W (2025) Cost-effectiveness analysis of sequential two-step screening versus direct colonoscopy screening for colorectal cancer: a large-scale survey in Eastern China. Front. Oncol. 15:1524172. doi: 10.3389/fonc.2025.1524172
Received: 07 November 2024; Accepted: 27 January 2025;
Published: 14 February 2025.
Edited by:
Zhaohui Jin, Mayo Clinic, United StatesReviewed by:
Adina Turcu-Stiolica, University of Medicine and Pharmacy of Craiova, RomaniaCopyright © 2025 Fu, Li, Xu, Yang, Zhang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peng Zhang, aHpqa3pwQDE2My5jb20=; Weibing Wang, d3diQGZ1ZGFuLmVkdS5jbg==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.