- Department of Urology, the First Affiliated Hospital of Chongqing Medical University, Chongqing, China
Background: There are discrepancies between the results of different studies regarding the prognostic role of circulating Chromogranin A (CgA) in prostate cancer. Therefore, we conducted a meta-analysis of the available findings to explore the value of circulating Chromogranin A in the prognosis of prostate cancer.
Methods: We systematically searched the PubMed, Embase, Web of Science, Cochrane Library, and Clinical Trials databases for studies on the relationship between CgA and survival outcomes in prostate cancer from inception until December 2024, and we focused on articles detecting circulating CgA, with the primary endpoints of the studies being overall survival (OS), and progression-free survival (PFS).
Results: Of the 2049 articles retrieved, 10 articles met our inclusion criteria, involving a total of 1445 patients. Elevated circulating CgA was associated with poorer OS (HR=1.82, 95% CI: 1.38–2.41; p<0.001) and PFS (HR=2.04, 95% CI: 1.42–2.94; p<0.001). However, no correlation was found between post-treatment circulating CgA changes and OS (HR=0.95, 95% CI: 0.66–1.37; p=0.767).
Conclusion: Circulating CgA is a predictive marker of poor survival outcomes in prostate cancer However, the sample size of the current study is small and larger studies are needed to further validate this in the future.
Introduction
In the United States, prostate cancer is the most diagnosed cancer (excluding non-melanoma skin cancers) and is the second leading cause of cancer deaths among men in the U.S. The cancer incidence of prostate cancer is increasing by 3% per year from 2014 through 2019. It is estimated that more than 280,000 men were diagnosed with prostate cancer in 2023 and more than 34,000 died from prostate cancer (1, 2).
Adenocarcinoma accounts for 90–95% of the pathological staging of prostate cancer and is characterized by androgen receptor (AR) and prostate-specific antigen (PSA) expression (3, 4). In the development of prostate cancer, cell survival is dependent on androgens and androgen receptors, therefore, androgen deprivation therapy (ADT) combined with anti-androgen therapy is widely used in prostate cancer treatment (5). Prostate cancer patients initially respond to hormone therapy, but the duration of the response lasts anywhere from a few months to a few years, eventually leading to castration-resistant prostate cancer (CRPC) (6).
The predominant subtype in CRPC is AR-positive adenocarcinoma (CRPC-adeno), but approximately 10–17% of patients treated with ADT or anti-androgen therapy exhibit a neuroendocrine differentiation (NE) phenotype, and these tumors are classified as neuroendocrine prostate cancer (NEPC) (4, 7). New-onset neuroendocrine prostate cancer (NEPC) is rare, accounting for under 2% of diagnosed prostate cancers. Neuroendocrine prostate cancer is characterized by the expression of neuroendocrine markers, such as Chromogranin A (CgA), synaptophysin (SYP), and neural cell adhesion molecules (CD56). It also exhibits deficiency in androgen receptor (AR) and prostate-specific antigen (PSA), and NEPC tends to be more aggressive, has a poorer prognosis, and is associated with hormone therapy resistance (4, 7).
There are different views on the origin of prostate cancer NE cells, with one view being that they are derived from pluripotent stem cells. Benign prostate tissue includes epithelial cells, including secretory epithelial cells, basal cells, and neuroendocrine cells, the first two of which are the major components of the prostate epithelium, with neuroendocrine cells accounting for approximately 1% of the entire epithelial cell population. Although these three types of cells markedly differ in marker expression and hormone regulation, they share a common origin as pluripotent stem cells, and it is a current view that prostate cancer NE cells are derived from pluripotent stem cells (4, 8). Another view is that prostate cancer NE cells arise from prostate cancer lineage plasticity. Prostate adenocarcinoma cells lose their adenocarcinoma characteristics and develop a neuroendocrine phenotype during androgen deprivation therapy (ADT), suggesting that it is driven by lineage plasticity (4, 9, 10). Although the source of NE cells in prostate cancer is difficult to determine and more studies are needed to further clarify it in the future, neuroendocrine differentiation (NED) correlates with tumor progression, and the measurement of serum NE markers can objectively respond to the neuroendocrine differentiation of tumor cell populations, among which CgA is a widely used serum marker.
Chromogranin A (CgA) is an acidic, hydrophilic secretory protein with a molecular weight of 48 kD (11) that belongs to the granin family (12). It was first found in the secretory granules of adrenal chromaffin granulocytes, which are widely distributed in the neuroendocrine system, including normal tissues and tumor tissues, and involved in energy metabolism, immunoregulation, tissue repair, and other processes. It is suggested that circulating CgA is associated with positive immunohistochemical CgA expression (13) and may serve as a supplement to PSA and provide important information on prostate cancer disease prognosis (14, 15). In contrast, other studies conclude that there is no correlation between circulating CgA and prostate cancer prognosis (16, 17).
Controversy exists regarding the value of circulating CgA in prostate cancer prognosis. Therefore, this study aimed to meta-analyze published results to investigate whether circulating CgA can provide useful prognostic information in prostate cancer.
Methods
Search strategy
This systematic review and meta-analysis is reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement (18). And we are registered in the International prospective register of systematic reviews(ID: CRD42023492830). A systematic search of the PubMed, Embase, Web of Science, Cochrane Library, and Clinical Trials databases for articles on the prognostic role of Chromogranin A (CgA) in prostate cancer was performed from inception through December 2024. We searched the following combination of Medical Subject Headings (Mesh) and related keywords: “Prostatic Neoplasms [Mesh] or Prostate Neoplasms or Prostate Cancer or Prostatic Cancer” andC “hromogranin A or CgA or CHGA”.
Inclusion and exclusion criteria
We developed inclusion criteria based on the PICOS principles (P: population, I: intervention, C: comparison, O: outcome, S: study design): (1) Population: Patients diagnosed with prostate cancer by histopathologic examination; (2) Intervention: To detect CgA during the course of a disease, we focused on studies that have detected serum or plasma CgA; (3) Comparison: Comparing patients with elevated CgA to patients without elevated CgA to study the impact on survival outcomes; (4) Outcome: The study endpoints were overall survival (OS), progression-free survival (PFS), and the results were presented as hazard ratio (HR) and corresponding 95% confidence interval (CI); (5) Study design: We have no restrictions on the article study design. The exclusion criteria were as follows: no outcome metrics, no study of the correlation between CgA and survival outcomes in prostate cancer, as well as reviews, conference abstracts, commentaries, letters, and animal testing were excluded.
Quality assessment and data extraction
Titles and abstracts were reviewed by two independent researchers, articles that met the inclusion criteria were retrieved in full text, and quality assessment and data extraction were completed. The decision was made after discussion with a third researcher if there was a disagreement between them.
Two independent researchers extracted the following data from the article based on a pre-designed table: Authors, publication date, country, study design, number of patients, age, CgA values, and disease stage,outcome indicators.The quality of included studies were assessed using the Newcastle-Ottawa Scale (NOS) (a score of 7–9 is considered a high-quality study, a score of 4–6 is a medium-quality study, and a score of less than 4 is considered a low-quality study). Differences between the two researchers were resolved by consensus.
Statistical analysis
We performed a meta-analysis using Stata (version 15.0) to pool the results of the included studies to calculate the overall hazard ratio (HR) and their 95% confidence interval (CI). Assessing heterogeneity across studies using the I2 test (cochrane classification: 0% to 40%: might not be important, 30% to 60%: may represent moderate heterogeneity, 50% to 90%: may represent substantial heterogeneity, 75% to 100%: considerable heterogeneity). A fixed-effects model was used if I2 <50%, while a random-effects model was used if I2 >50%. Meta-regression and subgroup analysis were performed to find sources of heterogeneity when it was apparent. We evaluated publication bias using the Egger’s test, which suggests the presence of publication bias if the P value is <0.05. Sensitivity analysis were performed using the trim-and-fill method to assess the robustness of the results. Of the 10 papers included, Patients in the Szarvas et al. study (19) were divided into three cohorts based on treatment modalities, comprising radical prostatectomy cohort, docetaxel cohort, and abiraterone/enzalutamide cohorts. The radical prostatectomy cohort was excluded from our study due to different study outcomes. The other two cohorts existed independently of each other as separate observation cohorts. Therefore, the docetaxel cohort and abiraterone/enzalutamide cohorts were considered as two separate outcomes to be combined in the summary analysis of results.
Result
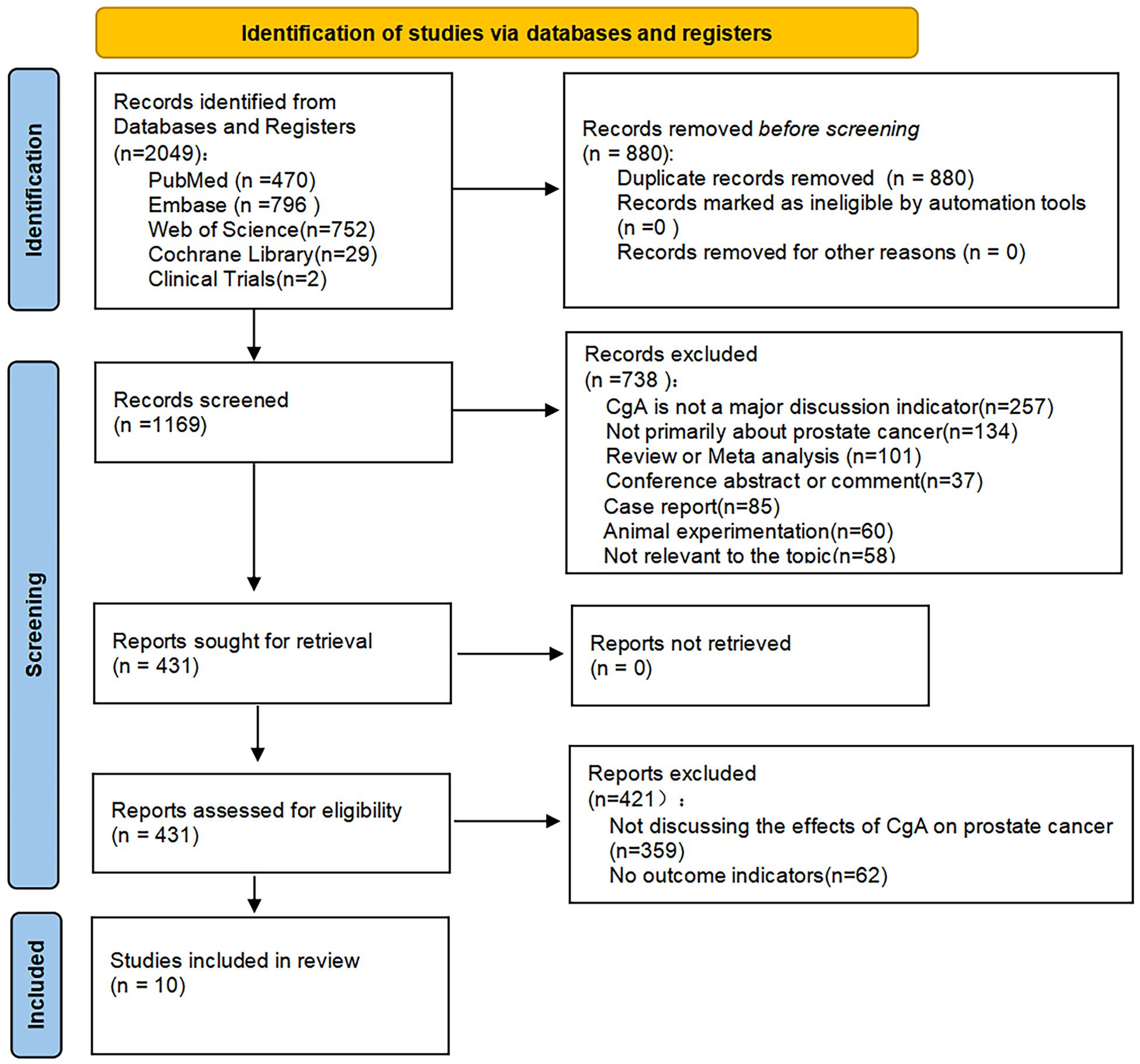
By searching several databases, a total of 2049 studies were retrieved, 880 duplicates were excluded, and a total of 738 studies were excluded by reading the titles and abstracts for the following reasons: Not primarily about CgA or prostate cancer, not consistent with research topic, reviews, meeting abstracts, comments, letters, and animal testing, 421 of the remaining studies were excluded because they did not discuss the relationship between CgA and prostate cancer prognosis or had no relevant outcome indicators. A total of 10 articles were eventually included in our study (17, 19–27) (Figure 1).
Study characteristics and quality assessment
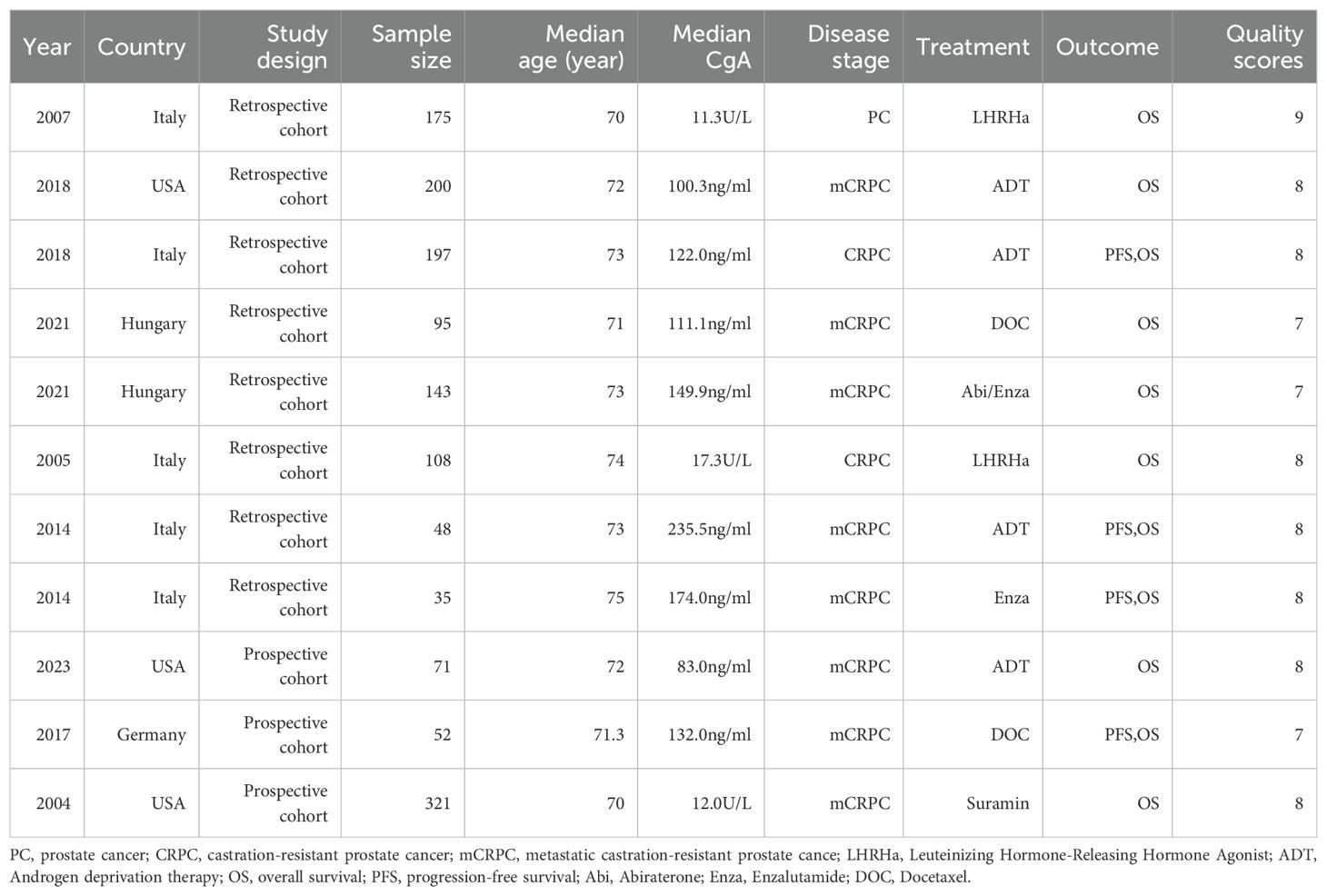
A total of 10 studies involving 1,445 patients from 4 countries were included (17, 19–27). Articles were published between 2004 and 2023, with five studies from Italy, three from the United States, one from Hungary, and one from Germany. A total of 7 retrospective studies and 3 prospective studies were used with the median age of patients between 70–75 years old. Ten study outcomes were overall survival (OS) and four were progression-free survival (PFS), with two studies looking at the impact of post-treatment CgA changes on survival outcomes. We evaluated the quality of the included studies using the Newcastle-Ottawa Scale (NOS), and all studies scored between 7 and 9 as high-quality studies, Table 1 shows the characteristics of each included study (Table 1).
Synthesis of results
Eight of these papers discuss the relationship between circulating CgA and OS (17, 19–24, 26). A combination of the data suggests that elevated circulating CgA negatively correlates with OS (HR=1.82, 95% CI: 1.38–2.41; p<0.001). There was heterogeneity between studies (I2 = 72.5%, p <0.001); therefore, random effects models were used to combine the analysis (Figure 2A). We performed meta-regression and subgroup analysis to find the source of heterogeneity. In our included literature, two studies by Conteduca et al. (23, 24) compared patients with circulating CgA elevations of more than three times the upper normal limit or elevations of less than three times the upper limit of normal levels to explore the impact on survival outcomes. This grouping may have yielded more significant results compared with groupings in other studies, which may have contributed to the source of heterogeneity. Therefore, we grouped these two studies into one category and performed a multifactorial meta-regression of the sample size, different CgA grouping methods, country, and treatment modality of each study. The results suggested that the grouping method of CgA may be a source of heterogeneity (P=0.043) (Figure 3). We subsequently performed subgroup analysis according to the different groupings of CgA with several studies other than Conteduca et al. as subgroup 1 (17, 19–22, 25–27) and two studies by Conteduca et al. as subgroup 2 (23, 24). The results showed no significant heterogeneity between subgroup 1 studies (I2 = 45.0%, p=0.091) and no heterogeneity between subgroup 2 studies (I2 = 0.0%, p=0.637). Results in both subgroups suggest that elevated CgA is negatively associated with OS (subgroup 1, HR=1.47, 95% CI: 1.20–1.79; p<0.001; subgroup 2, HR=3.85, 95% CI: 2.4–6.19; p<0.001) (Figure 2B). Therefore, different ways of grouping CgA were considered possible sources of heterogeneity.
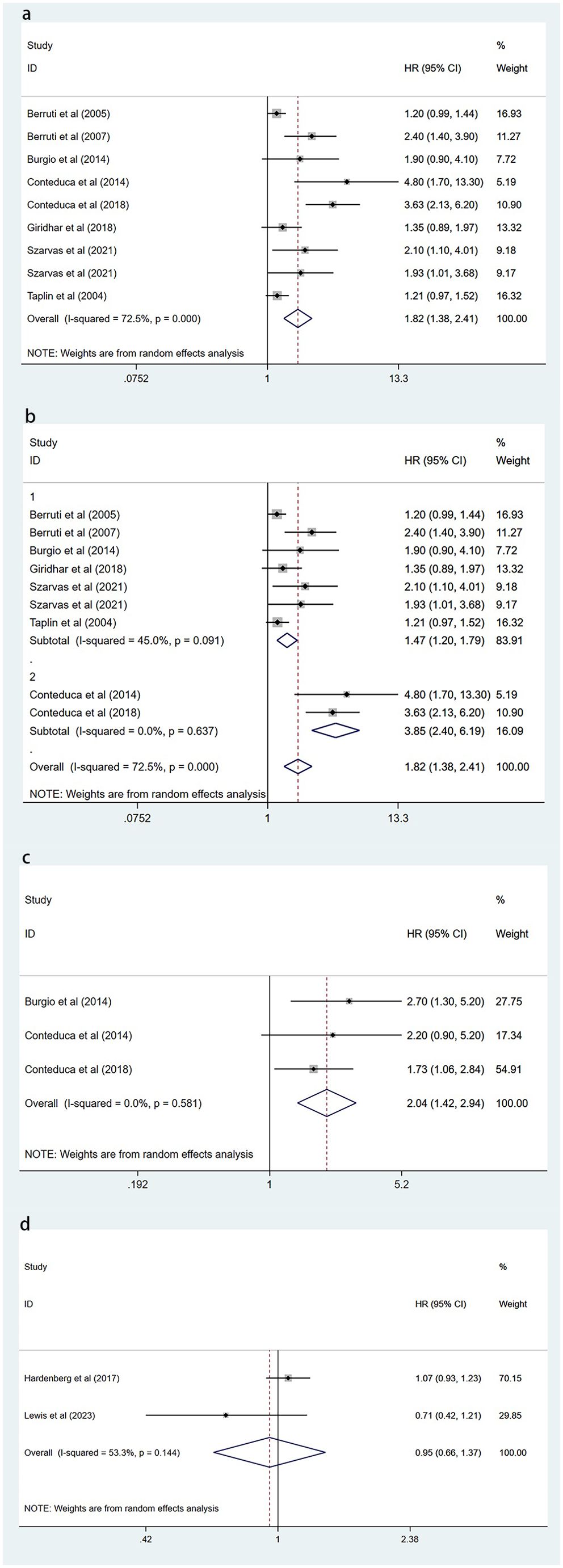
Figure 2. Forest plot of the effect of circulating CgA on survival outcomes. (A) Forest plot comparing elevated circulating CgA with OS. (B) Forest plot comparing elevated circulating CgA with OS in different subgroups. (C) Forest plot comparing elevated circulating CgA with PFS. (D) Forest plot comparing circulating CgA changes with OS. CgA, chromogranin A; OS, overall survival; PFS, progression-free survival; HR, hazard ratio; CI, confidence interval.
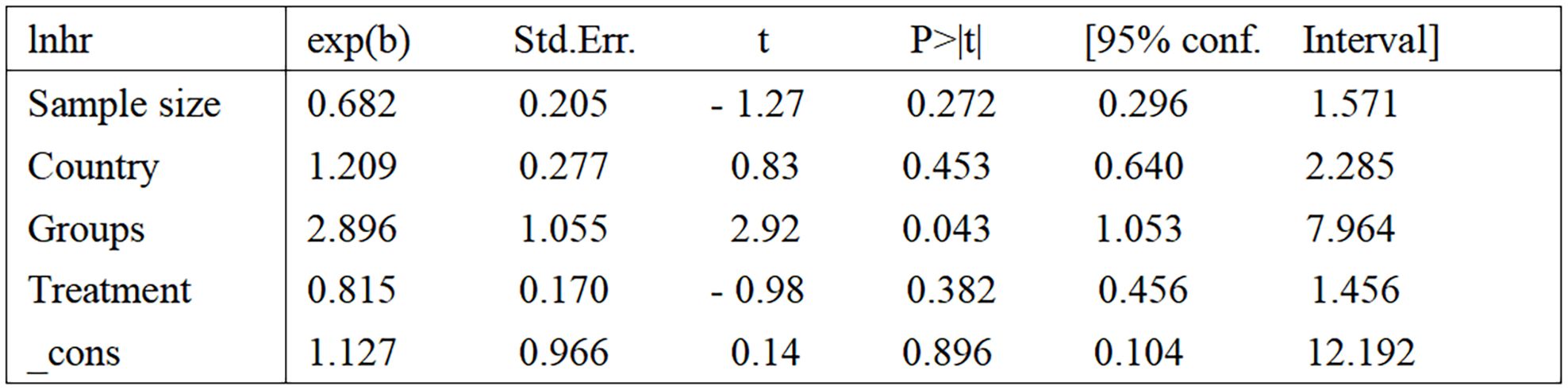
Figure 3. Results of meta-regression. samplesize (1=Sample size less than 100, 2=Sample size greater than 100); country (1=Italy, 2=USA, 3=Hungary); groups [1=several studies other than Conteduca et al. (17, 19–22, 25–27), 2=two studies by Conteduca et al. (23, 24)]; treatment (1=LHRHa, 2=ADT, 3=Other treatments). LHRHa, Leuteinizing Hormone-Releasing Hormone Agonist; ADT, Androgen deprivation therapy.
There are three publications examining the effect of elevated circulating CgA on PFS (22–24). One publication examines the impact of post-treatment CgA changes on PFS (27). Data from three articles examining the relationship between elevated circulating CgA and survival outcomes were pooled and showed that elevated circulating CgA was negatively associated with PFS (HR=2.04, 95% CI: 1.42–2.94; p<0.001) (Figure 2C).
Two articles examined the association between post-treatment changes in circulating CgA and OS (25, 27). The pooled results showed no correlation between post-treatment CgA elevation and OS (HR=0.95, 95% CI: 0.66-1.37; P=0.767) (Figure 2D). This suggested that elevated CgA after treatment does not lead to poorer OS.
The latter two outcomes were limited in subgroup analysis, sensitivity analysis, and risk of bias evaluation because of the small number of included studies.
Publication bias
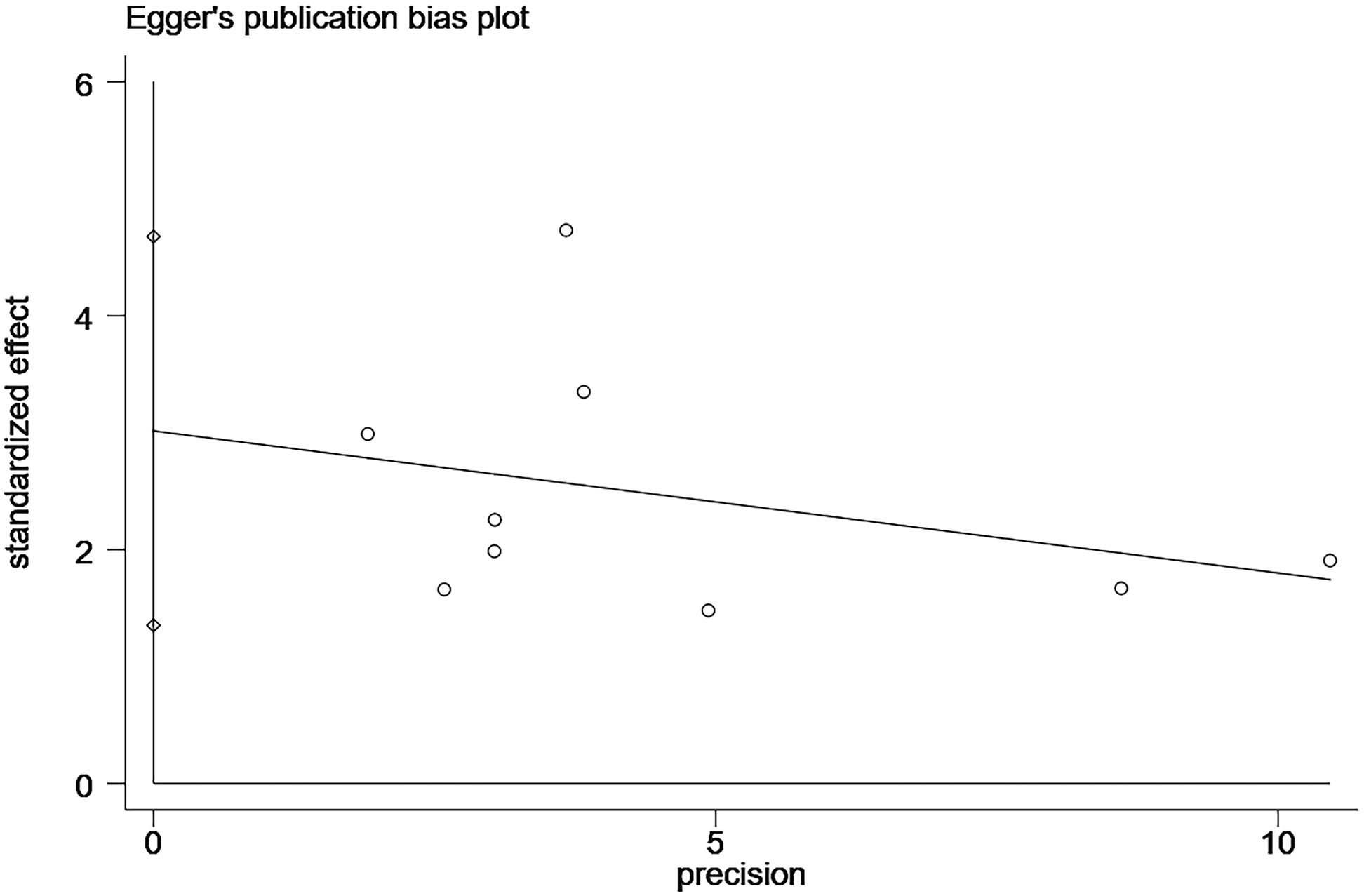
In the study related to the effect of elevated CgA on OS, we found publication bias using the Egger’s test (P=0.004; Figure 4; Supplementary Figure S1). A common source of publication bias is that our study sample was limited to published studies, but published studies often provide positive findings.
Sensitivity analysis
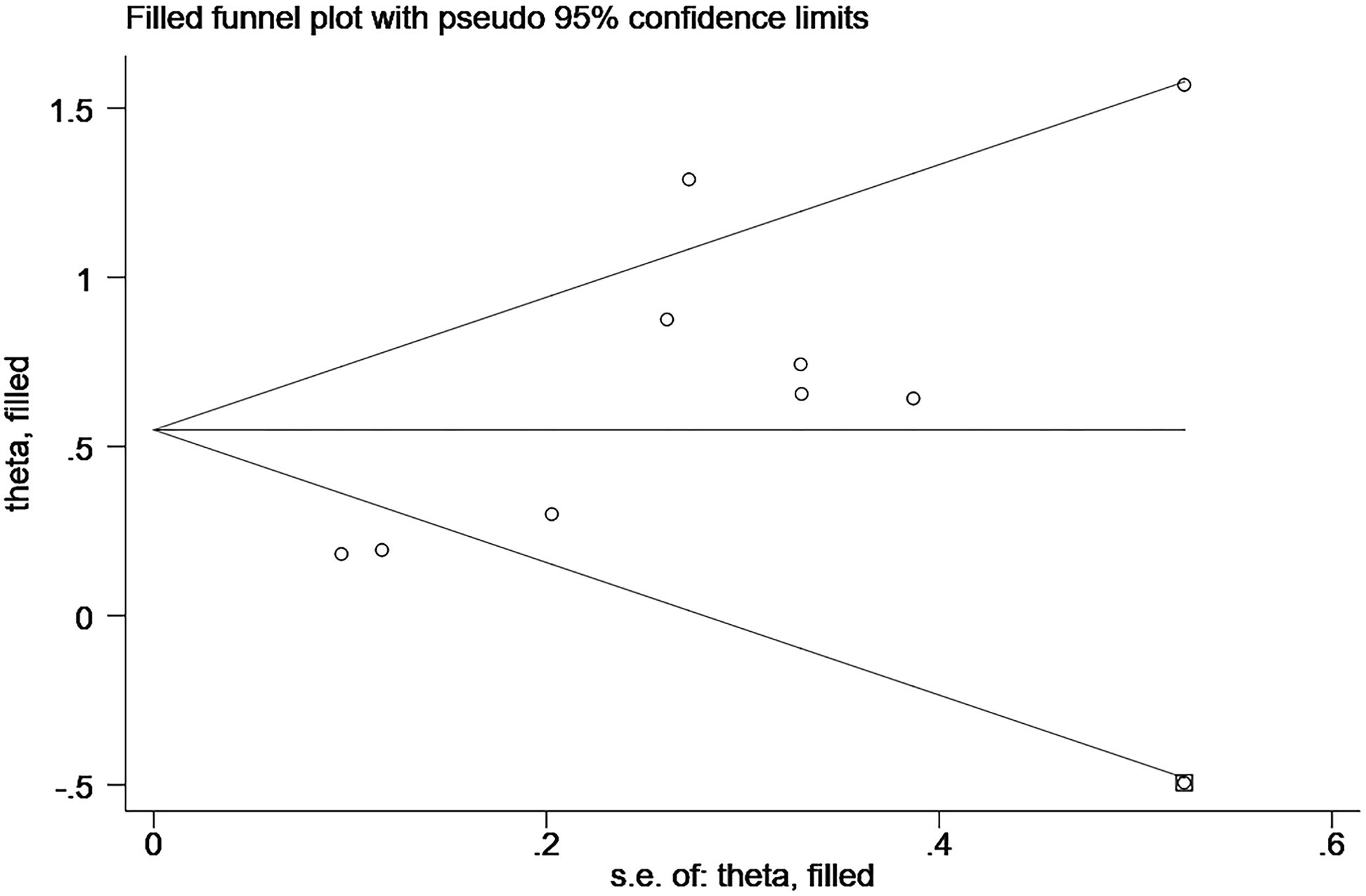
We performed sensitivity analysis using the trim-and-fill method, and the results after the trim-and-fill method were consistent with the results of our original study, all suggest a negative correlation between elevated CgA and OS (before trim-and-fill method HR=1.82, 95% CI: 1.38–2.41, p<0.001; after trim-and-fill method HR=1.73, 95% CI: 1.32–2.28, p<0.001; Figure 5; Supplementary Figure S2). It is suggested that our study did not affect the assessment of the results, although it was subject to publication bias.
Discussion
Our study aimed to investigate the prognostic role of circulating CgA in prostate cancer, with a meta-analysis of 10 included studies (17, 19–27). Elevated circulating CgA is associated with poorer OS and PFS, but post-treatment CgA changes did not correlate with OS.
Four of the included literature suggest a negative correlation between elevated circulating CgA and OS (19, 20, 23, 24), three suggest a critical significance of elevated circulating CgA with shorter OS (21, 22, 26). And one article suggests no correlation between elevated CgA and shorter OS, but CgA showed prognostic significance in patients with a Gleason score ≥8 (HR=2.19, 95% CI: 1.16–3.85; p=0.0169) (17). Two study results suggested that elevated CgA was associated with poorer PFS (22, 24). One literature suggests that elevated CgA is critically associated with worse PFS (23). Combined with the results of our pooled analyses, we suggest that elevated circulating CgA is suggestive of poorer OS and PFS in prostate cancer, and the results of our meta-analysis and sensitivity analysis reflect rationality and reliability.
Of the total literature, two papers focused on the impact of post-treatment changes in circulating CgA on survival outcomes (25, 27). These two studies focused on the impact of post-treatment CgA elevation from baseline values on outcomes using patients’ CgA values at the time of the first specimen collection as the baseline value, and both studies suggested that post-treatment CgA elevation from baseline values did not correlate with OS. However, Hardenberg et al. (27) found that elevated CgA from baseline values after treatment was associated with worse PFS (HR=1.136, 95% CI: 0.999–1.291; p=0.052). Furthermore, patients with CgA elevations greater than 100% ULN had a trend toward lower OS and PFS in cycles 1 to 3, but it was not an independent predictor of OS in multivariate analyses. We believe that there are several factors that may account for the lack of correlation found between changes in circulating CgA after treatment and survival outcomes. The first is the heterogeneity of tumors, including differences in biologic characteristics and the degree of neuroendocrine differentiation. Prostate cancer is highly heterogeneous, and tumor cells from different patients have great differences in gene expression and biological behavior. Even after the same treatment, the regulatory mechanisms of CgA synthesis and release in tumor cells from different patients are different. Some tumor cells may proliferate and metastasis independently of CgA related biological processes. Therefore, the change of CgA level cannot reflect the progress of tumor, and thus cannot be correlated with survival outcome. In addition, the degree of neuroendocrine differentiation of prostate cancer varies, and only part of the tumor cells with neuroendocrine differentiation characteristics will secrete a large amount of CgA. If the proportion of neuroendocrine cells in the tumor is low, then the change of CgA level has limited effect on the overall tumor progression, and it is difficult to significantly correlate with the survival outcome. The second is the limitations of CgA detection, including the differences in detection methods and the lack of standardized detection methods. Different methods have differences in sensitivity and specificity, resulting in the accuracy and comparability of test results. Even if the samples from the same patient are tested in different laboratories, different CgA levels may be obtained, which interferes with the analysis of its correlation with survival outcomes. With regard to the impact of elevated circulating CgA on survival outcomes after treatment, the number of studies is small, and more studies are needed to further clarify this in the future.
Androgen deprivation therapy(ADT) is now widely used in prostate cancer treatment, and it was suggested that ADT induces neuroendocrine differentiation(NED) in prostate cancer (28). There are many different theories about why NED occurs. Han et al. (10) showed that expression of the transcription factor FOXA2 (which drives the transition of prostate cancer glands to the lineage plasticity) was significantly induced under ADT. Zhang et al. (29) suggest that ADT leads to the activation of CREB (cAMP response element-binding protein), which affects the neuroendocrine differentiation of prostate cancer through the CREB-EZH2-TSP1 pathway (EZH2: enhancer of zeste homolog 2, TSP1: thrombospondin-1, THBS1).
Liu et al (30) suggests that ADT induces leukemia inhibitory factor (LIF) expression, and LIF promotes neuroendocrine differentiation through activation of prostatic tumor promoter (ZBTB46). Enriquez et al (31) suggests that ADT leads to downregulation of the stromal cell protein SPARC in stromal cells, and that downregulation of SPARC causes stromal cells to release IL6, which is a NED inducer that drives neuroendocrine differentiation in prostate cancer. There are many more studies on the mechanisms by which NED occurs in prostate cancer, and it is not yet clear which mechanism predominates.
The presence of NED is often indicative of a poor prognosis, and the detection of circulating CgA reflects the neuroendocrine differentiation of tumor cells and has the advantages of simplicity and reproducibility, which can be considered as a biomarker of prognostic value and provides useful information for treating the disease. The biological role of CgA in prostate cancer progression includes the following aspects. First, CgA can promote the proliferation of prostate cancer cells by activating intracellular signaling pathways. Studies have found that CgA can bind to specific receptors on the surface of prostate cancer cells and activate the downstream PI3K-Akt signaling pathway. This signaling pathway plays a key role in cell growth, proliferation, and survival. When activated, Akt protein can phosphorylate a series of substrates and promote cell cycle progression, allowing cells to enter S phase from G1 phase, thereby accelerating cell proliferation (32). Second, CgA can enhance the invasion and metastasis of prostate cancer cells, which is reflected in the regulation of the expression of migration and invasion related molecules in prostate cancer cells. On the one hand, it can induce an increase in the expression of matrix metalloproteinases (MMPs). MMPs can degrade extracellular matrix components and create conditions for tumor cell migration and invasion (33). On the other hand, CgA may affect the expression of intercellular adhesion molecules. It can down-regulate the expression of E-cadherin, which is an important intercellular adhesion molecule. The reduction of E-cadherin expression will weaken the adhesion force between cells, and make tumor cells more likely to leave the primary tumor and migrate and metastasize (34). Third, it can regulate the tumor microenvironment. CgA has a regulatory effect on immune cells in the tumor microenvironment. It can inhibit the anti-tumor activity of immune cells, thereby helping tumor cells escape immune surveillance. For example, CgA can inhibit the proliferation and cytotoxicity of T lymphocytes and reduce their killing effect on tumor cells. At the same time, CgA can also promote the polarization of tumor-associated macrophages to M2 type. M2 type macrophages have immunosuppressive function and can secrete a variety of cytokines to promote tumor growth, angiogenesis and metastasis (35).
However, circulating CgA is affected by a number of diseases and medications, such as hypertension, heart failure, renal failure,and inflammatory bowel disease, and the use of proton pump inhibitors can cause an elevation of circulating CgA (36), which would be a confounding factor in the clinical work.
CgA is abnormally expressed in a variety of tumors, such as pheochromocytoma, small-cell lung cancer, medullary thyroid carcinoma, pancreatic islet cell tumors, and prostate cancers. Nowadays, many studies suggest that the increased release of CgA from neuroendocrine tumor cells is involved in the regulation of tumor growth and progression and that circulating CgA is a useful serum marker for diagnosing various types of neuroendocrine tumors (12, 37, 38). In Baudin et al’s (39) study, circulating CgA and NSE were measured in patients with neuroendocrine tumors (NET) and non-NET patients, and an analysis comparing them concluded that CgA appeared to be more reflective of tumor progression than NSE, suggesting that CgA should be used as a marker for screening patients with NET. In Campana et al’s (11) study, plasma CgA values were compared between NET and non-NET patients, and it was found that plasma CgA levels were higher in NET patients, and CgA levels were higher in patients with diffuse disease than in patients with localized disease.
There are also studies on the diagnostic value of circulating CgA in the diagnosis of prostate cancer, but there are differences between the results, with some studies showing that circulating CgA levels are higher in prostate cancer patients than in non-prostate cancer patients (40–42). However, other studies suggest that circulating CgA levels are not significantly different between the two and that detection of circulating CgA does not provide useful value in diagnosing prostate cancer (43–45). If the detection methods of CgA are standardized and the tumor heterogeneity is classified, CgA is likely to play a very important role as a key factor in the prognosis of prostate cancer. We think we can establish a prognosis evaluation system for prostate cancer combined with CgA, as follows: For low-risk prostate cancer, if the patient has normal CgA level, low PSA level (such as PSA < 10 ng/mL), Gleason score ≤6, and clinical stage T1-T2a, it can be judged as low-risk prostate cancer. Such patients have a good prognosis and a high 5-year survival rate. Active surveillance strategy should be considered, and CgA, PSA, prostate ultrasound and digital rectal examination should be regularly reviewed. If the CgA level was slightly elevated, and the PSA level was between 10-20 ng/mL, Gleason score was 7, and clinical stage was T2b-T2c, it was classified as intermediate-risk prostate cancer. The prognosis of these patients is moderate, and the treatment methods such as radical prostatectomy and radiotherapy can be selected according to the specific condition of the patient. The level of CgA should be closely monitored after treatment. If CgA continues to increase, the prognosis may be worse. If the level of CgA was significantly increased, accompanied by PSA > 20 ng/mL, Gleason score ≥8, clinical stage of T3-T4 or regional lymph node metastasis, it was considered as high-risk prostate cancer and highly suspected as neuroendocrine prostate cancer. These patients have a poor prognosis and usually require comprehensive treatment, such as surgery combined with radiotherapy, chemotherapy, and endocrine therapy. CgA was monitored dynamically during the treatment. If CgA did not decrease significantly or continued to increase, the treatment effect was not good, and the treatment strategy should be changed in time. More studies are needed to clarify the diagnostic role of circulating CgA in prostate cancer.
We evaluated the prognostic role of circulating CgA in prostate cancer through a systematic and comprehensive search of databases and performed subgroup and sensitivity analysis to demonstrate the reliability and stability of the results. Our study has some limitations. First, the sample size was not large enough, and second, CgA was measured differently between studies, which may lead to the presence of bias. Only two of the included studies focused on the impact of post-treatment changes in circulating CgA on outcomes. Therefore, more and larger studies may be needed in the future to confirm the prognostic role of circulating CgA in prostate cancer.
Conclusion
Overall, elevated circulating CgA was associated with poorer OS and PFS in prostate cancer, according to our pooled results. This suggested that circulating CgA can provide prognostic value in prostate cancer. However, no correlation was found between post-treatment changes in circulating CgA and survival outcomes and more detailed and comprehensive studies with large sample sizes grouped according to clinical differences between patients are needed to further validate the prognostic role of circulating CgA in the future.
Author contributions
XT: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Supervision, Validation, Visualization, Writing – original draft. ZL: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Supervision, Validation, Visualization, Writing – original draft. LS: Data curation, Investigation, Methodology, Software, Supervision, Validation, Visualization, Writing – original draft. HZ: Formal analysis, Project administration, Supervision, Validation, Visualization, Writing – original draft. SS: Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Software, Supervision, Validation, Visualization, Writing – review & editing. DW: Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by grants from the doctoral program of the first affiliated hospital of Chongqing Medical University (CYYY-BSYJSCXXM-202332).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1521558/full#supplementary-material
Supplementary Figure 1 | Plot of the Egger’s test for publication bias.
Supplementary Figure 2 | Sensitivity analysis by trim-and-fill method.
References
1. Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. (2023) 73:17–48. doi: 10.3322/caac.21763
2. Pinsky PF, Parnes H. Screening for prostate cancer. N Engl J Med. (2023) 388:1405–14. doi: 10.1056/NEJMcp2209151
3. Cheng Q, Butler W, Zhou Y, Zhang H, Tang L, Perkinson K, et al. Pre-existing castration-resistant prostate cancer-like cells in primary prostate cancer promote resistance to hormonal therapy. Eur Urol. (2022) 81:446–55. doi: 10.1016/j.eururo.2021.12.039
4. Wang Y, Wang Y, Ci X, Choi SYC, Crea F, Lin D, et al. Molecular events in neuroendocrine prostate cancer development. Nat Rev Urol. (2021) 18:581–96. doi: 10.1038/s41585-021-00490-0
5. Debes JD, Tindall DJ. Mechanisms of androgen-refractory prostate cancer. N Engl J Med. (2004) 351:1488–90. doi: 10.1056/NEJMp048178
6. Watson PA, Arora VK, Sawyers CL. Emerging mechanisms of resistance to androgen receptor inhibitors in prostate cancer. Nat Rev Cancer. (2015) 15:701–11. doi: 10.1038/nrc4016
7. Liu S, Alabi BR, Yin Q, Stoyanova T. Molecular mechanisms underlying the development of neuroendocrine prostate cancer. Semin Cancer Biol. (2022) 86:57–68. doi: 10.1016/j.semcancer.2022.05.007
8. Bonkhoff H. Neuroendocrine differentiation in human prostate cancer. Morphogenesis, proliferation and androgen receptor status. Ann Oncol. (2001) 12 Suppl 2:S141–4. doi: 10.1093/annonc/12.suppl_2.s141
9. Chakraborty G, Gupta K, Kyprianou N. Epigenetic mechanisms underlying subtype heterogeneity and tumor recurrence in prostate cancer. Nat Commun. (2023) 14:567. doi: 10.1038/s41467-023-36253-1
10. Han M, Li F, Zhang Y, Dai P, He J, Li Y, et al. Foxa2 drives lineage plasticity and kit pathway activation in neuroendocrine prostate cancer. Cancer Cell. (2022) 40:1306–1323.e8. doi: 10.1016/j.ccell.2022.10.011
11. Campana D, Nori F, Piscitelli L, Morselli-Labate AM, Pezzilli R, Corinaldesi R, et al. Chromogranin a: is it a useful marker of neuroendocrine tumors? J Clin Oncol. (2007) 25:1967–73. doi: 10.1200/JCO.2006.10.1535
12. Taupenot L, Harper KL, O’Connor DT. The chromogranin-secretogranin family. N Engl J Med. (2003) 348:1134–49. doi: 10.1056/NEJMra021405
13. Bollito E, Berruti A, Bellina M, Mosca A, Leonardo E, Tarabuzzi R, et al. Relationship between neuroendocrine features and prognostic parameters in human prostate adenocarcinoma. Ann Oncol. (2001) 12 Suppl 2:S159–64. doi: 10.1093/annonc/12.suppl_2.s159
14. Vashchenko N, Abrahamsson PA. Neuroendocrine differentiation in prostate cancer: implications for new treatment modalities. Eur Urol. (2005) 47:147–55. doi: 10.1016/j.eururo.2004.09.007
15. Berruti A, Dogliotti L, Mosca A, Gorzegno G, Bollito E, Mari M, et al. Potential clinical value of circulating chromogranin a in patients with prostate carcinoma. Ann Oncol. (2001) 12:S153–7. doi: 10.1093/annonc/12.suppl_2.s153
16. Masieri L, Lanciotti M, Gontero P, Marchioro G, Mantella A, Zaramella S, et al. The prognostic role of preoperative chromogranin a expression in prostate cancer after radical prostatectomy. Arch Ital Urol Androl. (2012) 84:17–21.
17. Giridhar KV, Sanhueza C, Hillman DW, Alkhateeb H, Carlson R, Tan W, et al. Serum chromogranin-a-based prognosis in metastatic castration-resistant prostate cancer. Prostate Cancer Prostatic Dis. (2018) 21:431–7. doi: 10.1038/s41391-018-0046-9
18. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The prisma 2020 statement: an updated guideline for reporting systematic reviews. Bmj. (2021) 372:n71. doi: 10.1136/bmj.n71
19. Szarvas T, Csizmarik A, Fazekas T, Hüttl A, Nyirády P, Hadaschik B, et al. Comprehensive analysis of serum chromogranin a and neuron-specific enolase levels in localized and castration-resistant prostate cancer. Bju Int. (2021) 127:44–55. doi: 10.1111/bju.15086
20. Berruti A, Mosca A, Porpiglia F, Bollito E, Tucci M, Vana F, et al. Chromogranin a expression in patients with hormone naïve prostate cancer predicts the development of hormone refractory disease. J Urol. (2007) 178:838–43; quiz 1129. doi: 10.1016/j.juro.2007.05.018
21. Berruti A, Mosca A, Tucci M, Terrone C, Torta M, Tarabuzzi R, et al. Independent prognostic role of circulating chromogranin a in prostate cancer patients with hormone-refractory disease. Endocr Relat Cancer. (2005) 12:109–17. doi: 10.1677/erc.1.00876
22. Burgio SL, Conteduca V, Menna C, Carretta E, Rossi L, Bianchi E, et al. Chromogranin a predicts outcome in prostate cancer patients treated with abiraterone. Endocr Relat Cancer. (2014) 21:487–93. doi: 10.1530/ERC-14-0071
23. Conteduca V, Burgio SL, Menna C, Carretta E, Rossi L, Bianchi E, et al. Chromogranin a is a potential prognostic marker in prostate cancer patients treated with enzalutamide. Prostate. (2014) 74:1691–6. doi: 10.1002/pros.22890
24. Conteduca V, Scarpi E, Salvi S, Casadio V, Lolli C, Gurioli G, et al. Plasma androgen receptor and serum chromogranin a in advanced prostate cancer. Sci Rep. (2018) 8:15442. doi: 10.1038/s41598-018-33774-4
25. Lewis AR, Costello BA, Quevedo F, Pagliaro LC, Sanhueza C, Weinshilboum RM, et al. Dynamic assessment of serum chromogranin a and treatment response with abiraterone acetate in metastatic castration-resistant prostate cancer. Prostate. (2023) 83:649–55. doi: 10.1002/pros.24498
26. Taplin ME, George DJ, Halabi S, Sellers WR, Sanford B, Hennessy KT, et al. Prognostic significance of plasma chromogranin a levels in hormone-refractory prostate cancer patients treated an cancer and leukemia group b (calgb) 9480. J Clin Oncol. (2004) 22S:396S–S. doi: 10.1200/jco.2004.22.90140.4557
27. Von Hardenberg J, Schwartz M, Werner T, Werner T, Fuxius S, Müller M, et al. Prospective evaluation of neuromediator dynamics in castration-resistant prostate cancer patients during docetaxel. Anticancer Res. (2017) 37:5117–24. doi: 10.21873/anticanres.11931
28. Hirano D, Okada Y, Minei S, Takimoto Y, Nemoto N. Neuroendocrine differentiation in hormone refractory prostate cancer following androgen deprivation therapy. Eur Urol. (2004) 45:586–92; discussion 592. doi: 10.1016/j.eururo.2003.11.032
29. Zhang Y, Zheng D, Zhou T, Song H, Hulsurkar M, Su N, et al. Androgen deprivation promotes neuroendocrine differentiation and angiogenesis through creb-ezh2-tsp1 pathway in prostate cancers. Nat Commun. (2018) 9:4080. doi: 10.1038/s41467-018-06177-2
30. Liu YN, Niu S, Chen WY, Zhang Q, Tao Y, Chen WH, et al. Leukemia inhibitory factor promotes castration-resistant prostate cancer and neuroendocrine differentiation by activated zbtb46. Clin Cancer Res. (2019) 25:4128–40. doi: 10.1158/1078-0432.CCR-18-3239
31. Enriquez C, Cancila V, Ferri R, Sulsenti R, Fischetti I, Milani M, et al. Castration-induced downregulation of sparc in stromal cells drives neuroendocrine differentiation of prostate cancer. Cancer Res. (2021) 81:4257–74. doi: 10.1158/0008-5472.CAN-21-0163
32. Gong J, Lee J, Akio H, Schlegel ,PN, Shen R. Attenuation of apoptosis by chromogranin A-induced Akt and survivin pathways in prostate cancer cells. Endocrinology. (2007) 148:4489–99. doi: 10.1210/en.2006-1748
33. Niedworok C, Tschirdewahn S, Reis H, Lehmann N, Szücs M, Nyirády P, et al. Serum chromogranin A as a complementary marker for the prediction of prostate cancer-specific survival. Pathol. (2017) 23(3):643–50. doi: 10.1007/s12253-016-0171-5
34. McKeithen D, Graham T, Chung LW, Odero-Marah V. Snail transcription factor regulates neuroendocrine differentiation in LNCaP prostate cancer cells. Prostate. (2010) 70:982–92. doi: 10.1002/pros.21132
35. Huang L, Xie Y, Jiang S, Dai T, Xu Z, Shan H. Insights into immune microenvironment and therapeutic targeting in androgen-associated prostate cancer subtypes. Sci Rep. (2024) 14:18036. doi: 10.1038/s41598-024-68863-0
36. Mahata SK, Corti A. Chromogranin a and its fragments in cardiovascular, immunometabolic, and cancer regulation. Ann N Y Acad Sci. (2019) 1455:34–58. doi: 10.1111/nyas.14249
37. Colombo B, Curnis F, Foglieni C, Monno A, Arrigoni G, Corti A. Chromogranin a expression in neoplastic cells affects tumor growth and morphogenesis in mouse models. Cancer Res. (2002) 62:941–6.
38. Helle KB, Corti A, Metz-Boutigue MH, Tota B. The endocrine role for chromogranin a: a prohormone for peptides with regulatory properties. Cell Mol Life Sci. (2007) 64:2863–86. doi: 10.1007/s00018-007-7254-0
39. Baudin E, Gigliotti A, Ducreux M, Ropers J, Comoy E, Sabourin JC, et al. Neuron-specific enolase and chromogranin a as markers of neuroendocrine tumours. Br J Cancer. (1998) 78:1102–7. doi: 10.1038/bjc.1998.635
40. Berruti A, Dogliotti L, Mosca A, Bellina M, Mari M, Torta M, et al. Circulating neuroendocrine markers in patients with prostate carcinoma. Cancer. (2000) 88:2590–7. doi: 10.1002/1097-0142(20000601)88:11<2590::aid-cncr23>3.0.co;2-d
41. Reis LO, Vieira LF, Zani EL, Denardi F, de Oliveira LC, Ferreira U. Assessment of serum chromogranin-a as prognostic factor in high-risk prostate cancer. J Investig Med. (2010) 58:957–60. doi: 10.231/JIM.0b013e3181f5d610
42. Hirano D, Minei S, Sugimoto S, Yamaguchi K, Yoshikawa T, Hachiya T, et al. Implications of circulating chromogranin a in prostate cancer. Scand J Urol Nephrol. (2007) 41:297–301. doi: 10.1080/00365590701303934
43. Mearini L, Zucchi A, Scarponi E, Nunzi E, Aglietti MC, Bini V, et al. Correlation between age and chromogranin a determination in prostate diseases. Cancer biomark. (2011) 10:117–23. doi: 10.3233/CBM-2012-0237
44. Marszalek M, Wachter J, Ponholzer A, Leitha T, Rauchenwald M, Madersbacher S. Insulin-like growth factor 1, chromogranin a and prostate specific antigen serum levels in prostate cancer patients and controls. Eur Urol. (2005) 48:34–9. doi: 10.1016/j.eururo.2005.03.020
Keywords: prostate cancer, circulating Chromogranin A, neuroendocrine, meta-analysis, prognostic
Citation: Tang X, Liu Z, Song L, Zhu H, Su S and Wang D (2025) Prognostic value of circulating Chromogranin A in prostate cancer: a systematic review and meta-analysis. Front. Oncol. 15:1521558. doi: 10.3389/fonc.2025.1521558
Received: 02 November 2024; Accepted: 20 January 2025;
Published: 05 February 2025.
Edited by:
Parames C. Sil, Bose Institute, IndiaReviewed by:
Rajesh Gunage, Boston Children’s Hospital and Harvard Medical School, United StatesPriyanka Sharma, University of Texas MD Anderson Cancer Center, United States
Senthilkumar Ravichandran, University of Alabama at Birmingham, United States
Copyright © 2025 Tang, Liu, Song, Zhu, Su and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Delin Wang, ZGx3YW5nd3NAc2luYS5jb20=; Shuai Su, c3VzaHVhaTkzMDgwOUAxNjMuY29t
†These authors have contributed equally to this work
 Xiaoying Tang†
Xiaoying Tang† Zhenyu Liu
Zhenyu Liu Huixuan Zhu
Huixuan Zhu Delin Wang
Delin Wang