
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Oncol. , 05 March 2025
Sec. Gastrointestinal Cancers: Gastric and Esophageal Cancers
Volume 15 - 2025 | https://doi.org/10.3389/fonc.2025.1515502
Background: Postoperative pulmonary complications in gastric cancer surgery significantly impact patient recovery and prognosis. These complications, including infections, can increase hospital stays and costs, and even lead to death. Numerous risk factors are involved, such as age, smoking history, and lung function. Although preventive measures exist, a unified and effective strategy is lacking. Therefore, researching and implementing effective prevention measures is crucial for improving patients’ postoperative quality of life and survival rates.
Aim: To collate and summarize the best available evidence for the prevention of pulmonary complications in patients undergoing gastric cancer surgery, thereby providing a reference for the clinical development of relevant intervention strategies.
Methods: A literature search was conducted in databases including BMJ Best Practice, UpToDate, JBI, Cochrane Library, PubMed, Embase, the Ontario Nurses Registration Network, the U.S. National Clinical Practice Guidelines, and MedLine, for documents related to the prevention of pulmonary complications in gastric cancer surgery patients. The search period extended from the inception of these databases to July 25, 2024. The quality of the literature was evaluated according to the standards of the Joanna Briggs Institute (JBI) Evidence-Based Health Care Center, and evidence was extracted from the included documents.
Results: A total of 27 documents were ultimately included. The extracted content encompassed three areas: preoperative assessment, risk prevention and intervention measures, totaling 31 best evidences across five categories. The findings of our study underscore the significance of comprehensive preoperative assessments, such as the ARISCAT index for pulmonary risk evaluation, and stress the importance of preoperative interventions like inspiratory muscle training, smoking cessation, and oral care in mitigating postoperative pulmonary complications (PPCs) following gastric cancer surgery. We also advocate for the adoption of protective lung ventilation strategies during surgery and continuous pulse oximetry monitoring postoperatively, along with targeted treatments for specific complications.
Conclusion: The best evidence extracted for the prevention of complications in gastric cancer surgery patients serves as a basis for evidence-based practice for the prevention of pulmonary complications in this patient group. Further research topics on pulmonary complications of gastric cancer, we recommend further optimization of preoperative assessment tools, investigation into the efficacy of smoking cessation programs, comparative studies on intraoperative ventilation strategies, development of postoperative rehabilitation programs, and research into culturally and resource-sensitive interventions to broaden the global applicability of these practices.
Gastric cancer (GC) is one of the most common malignant tumors globally, ranking fifth among all cancers and third as a cause of cancer-related death (1). In 2018, it accounted for 5.7% of new cancer cases and 8.2% of cancer-related deaths (2). Comprehensive treatment centered around surgical resection continues to play a pivotal role in the management of gastric cancer (3). However, the incidence of postoperative complications after gastric cancer surgery ranges from 12.9% to 40.1% and is considered a significant factor affecting patient prognosis (3–5). Postoperative pulmonary complications (PPCs) are a common postoperative complication and the most frequent medium to long-term complication following major surgery, with an incidence rate of 1 to 23% (6, 7). Postoperative Pulmonary Complications (PPCs) refer to a variety of respiratory complications that occur after surgery. These complications primarily include, but are not limited to, pulmonary infections, respiratory failure, pleural effusions, atelectasis (lung collapse), pneumothorax, bronchospasm, aspiration pneumonia, pulmonary edema, and acute respiratory distress syndrome (ARDS). PPCs not only significantly impact patient recovery and mortality but also increase the demand for mechanical ventilation, intensive care, and prolonged hospital stays, thereby adding to the burden on healthcare services (8, 9). Therefore, the management of pulmonary complications has become one of the focal points in the perioperative management of gastric cancer and is paramount in perioperative nursing care.
Studies have indicated that postoperative pulmonary infections in gastric cancer patients can lead to recurrence and poor prognosis (10). Numerous risk factors contribute to the occurrence of pulmonary complications after gastric cancer surgery, with advanced age being a significant predictor of postoperative pneumonia and a leading cause of mortality, these risk factors also include smoking history, lung function, cardiovascular disease, diabetes, nutritional status, obesity, immunosuppression, and genetic factors, which can result in prolonged mechanical ventilation, extended hospital stays, increased mortality risk, poor prognosis, higher medical costs, and reduced quality of life. (11). Additionally, the surgical field for gastric cancer, particularly total gastrectomy, is close to the diaphragm, which can easily stimulate the diaphragm and lead to a reflexive decrease in its function, thereby affecting the recovery of postoperative pulmonary ventilation (12). Postoperative pain in gastric cancer patients can also result in poor spontaneous sputum expectoration, hindering the recovery of pulmonary function. Most gastric cancer patients suffer from malnutrition, and conditions such as anemia and hypoproteinemia can lead to pulmonary interstitial edema, affecting gas exchange and causing hypoxemia (13). The presence of a nasogastric tube postoperatively increases the incidence of pulmonary infection, as it can irritate the throat during swallowing, causing mucosal edema and airway compression, which impairs ventilation. Moreover, sputum can easily adhere to the tube’s walls during coughing, leading to bacterial colonization and an increased risk of pulmonary infection. The tube can also stimulate the throat and induce vomiting, raising the risk of aspiration (14, 15). Furthermore, factors such as prolonged surgery time, intraoperative bleeding, laparoscopic surgery, concurrent abdominal complications, and general anesthesia intubation all contribute to an increased incidence of pulmonary complications after gastric cancer surgery (13, 16).
With the advent of enhanced-recovery protocols, pulmonary rehabilitation during the perioperative period has gradually gained the attention of clinical researchers, reducing the occurrence of pulmonary complications after gastric cancer surgery to some extent. However, the current clinical practice lacks a systematic and standardized management process for perioperative pulmonary rehabilitation, and there is no universally recognized and effective intervention measures (13). Therefore, inconsistent guidance on the timing and duration of interventions, our study systematically synthesizes the best available evidence on postoperative complications in gastric cancer patients to provide patient-centered recommendations, address the optimal timing and duration of key interventions, and develop a comprehensive management strategy for postoperative pulmonary complications prevention in gastric cancer surgery patients, aiming to improve patient outcomes and reduce the healthcare burden.
This summary of best evidence was registered on Fudan University Evidence Based Nursing Center. The registered number is ES20245327.
We used the PIPOST method as a guide to identify research questions (17), the evidence-based question was constructed as follows: Target Population (P): Patients in the perioperative period of gastric cancer surgery; Intervention (I): Preventive measures related to the occurrence of pulmonary infections; Practitioners (P): Clinical medical staff providing guidance for gastric cancer patients; Outcome (O): The incidence of postoperative pulmonary infections; Setting (S): Inpatient wards and specialty clinics; Type of Evidence (T): Best practice manuals, guidelines, expert consensuses, evidence summaries, recommended practices, and systematic reviews published in Chinese and English.
Chinese and English search terms were determined in accordance with the “6S” evidence pyramid model, searching from the most reliable sources downwards (18). Databases and websites including BMJ Best Practice, UpToDate, JBI, Cochrane Library, PubMed, Embase, Ontario Nurses Registration Network, U.S. National Clinical Practice Guidelines Database, and MedLine were searched.
Using the PIPOST approach, we developed a comprehensive search strategy for our research. The search utilized both subject words and free words. Subject words were” Stomach Neoplasms, Gastrointestinal Neoplasms, Digestive System Neoplasms, Pneumonia, Bacterial Healthcare-Associated Pneumonia, Guideline”. Free words were (“ Stomach Neoplasm*, Stomach tumor*, Stomach cancer*, Stomach carcinoma*, gastric cancer*, gastric Neoplasm*, gastric tumor*, gastric carcinoma*”) and (“pulmonary complications, pulmonary comorbidities, pulmonary infection, respiratory infection, lower respiratory infection, pneumonia, new pneumonia, new pulmonary infection, hospital-acquired pneumonia, aspiration pneumonia” and “guideline, Practice Guideline, Best Practice*, Recommendation*, Consensus*, Experts Opinion*, Systematic Reviews, Evidence Summaries, Best Practice Manuals”). This structured approach ensures a thorough exploration of relevant literature and guidelines related to stomach neoplasms and pneumonia.
A representative search strategy in Databases is presented in Table 1. The search period was from the inception of these databases to July 25, 2024.
To ensure our study includes the most relevant and high-quality evidence, we have established detailed inclusion criteria that focus on patients of all ages undergoing gastric cancer surgery, prioritize literature on preventive measures for postoperative pulmonary complications, consider only Chinese and English documents, emphasize high-quality study designs, limit our search to publications from the inception of databases until July 25, 2024, exclude abstracts without full texts, and prioritize literature with clear clinical applicability to provide scientific guidance for preventing pulmonary complications after gastric cancer surgery.
The quality assessment of guidelines was conducted using the updated Clinical Guidelines Research and Evaluation System II (AGREE II) from 2012 (19); expert consensus was evaluated using the quality assessment tool for expert opinions and professional consensus articles from the Australian JBI Evidence-Based Practice Center (2016); systematic reviews were assessed using the systematic review tool from the Australian JBI Evidence-Based Practice Center (2016); the quality assessment of clinical decisions, best practices, and evidence summaries traced back to the original literature they were based on, with appropriate evaluation standards selected based on the type of literature.
Two researchers trained in systematic review methodology independently searched, screened, and extracted data, cross-checking after the initial search. The preliminary search results were imported into EndNote X9 for deduplication, followed by full-text screening based on the titles and abstracts, and finally, the full texts were rescreened to include the final set of articles. Disagreements were resolved through consensus by a third researcher. A total of 27 articles were included in the study (see Figure 1).
Two researchers independently read each included article, extracted evidence content, and organized and summarized the evidence by theme. The study prioritized evidence-based evidence, high-quality evidence, and the most recently published authoritative literature. The evidence was graded using the Australian JBI Evidence-Based Nursing Center’s pre-grading system and evidence recommendation level system (2014 edition), in conjunction with an expert argumentation meeting that considered the “FAME” framework (feasibility, appropriateness, meaningfulness, and effectiveness) and the JBI evidence recommendation level principle. In this framework, Level A represents a strong recommendation, while Level B indicates a weak recommendation. The expert argumentation meeting involved the participation of 10 experts, including 5 gastrointestinal surgeons, 3 oncology nurses, and 2 anesthesiologists. All 10 experts possess senior professional titles and hold a master’s degree or higher. Evidence was categorized into levels 1 to 5, with level 1 being the highest and level 5 the lowest, based on the type of research design and the consensus reached by the expert panel. Three members of the research team, trained in systematic review methodology and evidence-based nursing, have extensive experience in evidence extraction and grading, and were part of the expert argumentation process.
In this study, the literature screening process began with an initial database search, identifying 3270 records, and an additional 20 records were identified through other sources, totaling 3291 records. After deduplication, 3052 records remained. Following the acquisition of full texts, 346 were excluded due to inappropriate literature types (154), population mismatch (118), or non-compliant methods (74). Subsequently, 377 records were further screened. During the full-text assessment phase, an additional 4 were excluded due to the inability to extract valid information (2) or population mismatch (2). Ultimately, 27 studies were included in the final analysis. This flowchart clearly illustrates how the research team rigorously sifted through a large number of initial literature to select high-quality documents that meet the research criteria, ensuring the reliability and validity of the study’s results. The literature screening flowchart is shown in Figure 1. The general characteristics of the included literature are shown in Table 2.
The quality of the guidelines was assessed using the 2012 updated Clinical Guidelines Research and Evaluation System II (AGREE II). A total of three guidelines were included in this study. The Intraclass Correlation Coefficient (ICC) values for the assessment results from four evaluators were all greater than 0.75, indicating a high level of consistency among the evaluators. The recommendation levels were as follows: A grade (directly recommended) for areas scoring 60% or above; B grade (requires modification and improvement) for areas with scores below 60% but with at least three areas scoring 30% or above; and C grade (not recommended for the time being) for areas with at least three scores below 30%. Overall, the quality of the three guidelines was high, with detailed standardized scores and recommendation levels presented in Table 3.
The quality of expert consensus documents was evaluated using the quality assessment tool for expert opinions and professional consensus articles from the Australian JBI Evidence-Based Practice Center (2016). A total of five expert consensus documents were included in this study, with specific evaluation results shown in Table 4.
The quality of systematic reviews was assessed using the systematic review tool from the Australian JBI Evidence-Based Practice Center (2016). A total of ten systematic reviews were included in this study, with specific evaluation results shown in Table 5.
Clinical decisions and evidence summaries were included based on the extraction of original literature from high-quality systematic reviews or randomized controlled trials. A total of four clinical decisions and five evidence summaries were included in this study.
All literature was independently assessed by two researchers trained in standardized evidence-based methodology. In cases of disagreement, a decision was made in consultation with a third teacher experienced in evidence-based methodology and clinical practice.
Evidence was extracted, evaluated, and organized to generate 31 pieces of evidence for the prevention of postoperative pulmonary complications following gastric cancer surgery. This comprehensive set includes preoperative assessment, risk prevention measures and intervention strategies. These areas are categorized into five distinct groups.
Two researchers, trained in systematic evidence review methods, utilized the 2014 version of the Joanna Briggs Institute (JBI) evidence pre-grading system to classify the included evidence. The evidence was graded on a scale of 1 to 5, with 1 representing the highest level of evidence and 5 the lowest. For detailed grading,refer to Table 6.
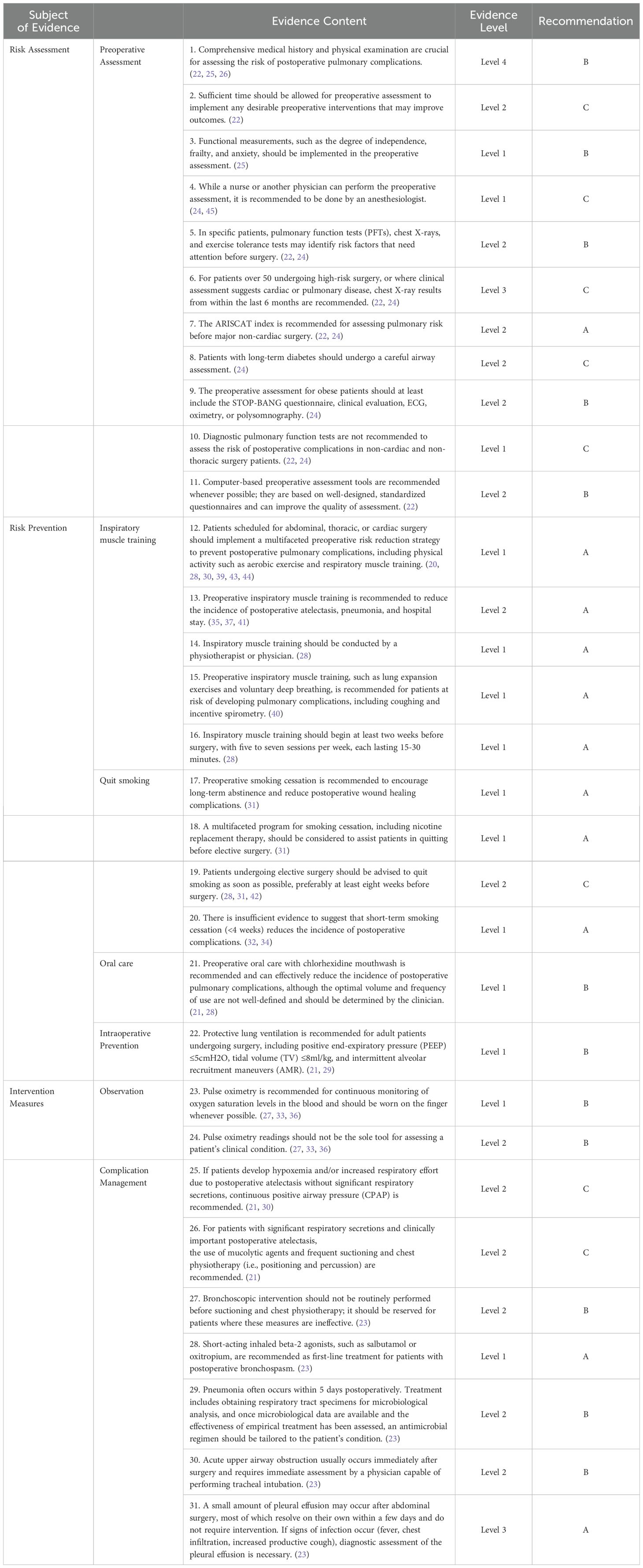
Table 6. Best evidence summary for preventing postoperative pulmonary complications after gastric cancer surgery.
The National Surgical Quality Improvement Program (NSQIP) reported a 6% incidence of postoperative pulmonary complications (PPCs) in 165,196 patients who underwent major abdominal surgery, with these patients facing higher mortality, ICU admission rates, and longer hospital stays. An additional NSQIP study revealed a 10.25% mortality rate within 30 days post-surgery among outpatients with PPCs. Postoperative lung volume reduction, particularly after thoracic and upper abdominal surgeries, increases PPC risk by causing restrictive lung volume decrease, reduced tidal volume, loss of sighing respiration, and elevated respiratory rates. Gastric cancer surgery, especially total gastrectomy, is prone to irritate the diaphragm, affecting pulmonary ventilation recovery.
In China, there’s a recognized need for improved emphasis on preventing PPCs in gastric cancer surgery, with current practices lacking standardized management for risk assessment and intervention. This study compiles evidence to aid medical staff in managing PPCs post-gastric cancer surgery, aiming to early identify high-risk patients and implement preventive measures to enhance medical care quality. It includes relevant strategies like preoperative aerobic training and respiratory exercises, essential for respiratory muscle preparation in gastric cancer patients, to be tailored to clinical practice.
Postoperative pulmonary complications (PPCs) are a significant concern following gastric cancer surgery, necessitating a standard risk assessment as part of preoperative evaluations. The ARISCAT score, developed by Canet J et al., is a recommended tool for this purpose, incorporating seven risk factors: age, recent respiratory infection, preoperative SpO2, anemia, surgical site, duration, and emergency status. It stratifies patients into low (≤26 points), medium (27-44 points), and high (≥45 points) risk categories. Wang Xiaomei’s external validation confirmed its efficacy in major abdominal surgeries.
To enhance the accuracy of risk assessment and personalized preoperative preparation, it is advisable for anesthesiologists, given their expertise in anesthesia management, to conduct these evaluations. The ARISCAT score’s inclusion of both preoperative and intraoperative variables facilitates timely postoperative assessments and interventions. Interdisciplinary collaboration, involving medical staff and anesthesiologists, is essential for a comprehensive approach to pulmonary complication prevention, ensuring that patients receive tailored recommendations that address their overall health needs.
Evidence 12 to 20 highlights preventive strategies for gastric cancer surgery patients, with a focus on inspiratory muscle training (Evidence 12-16). This training, guided by professionals like physiotherapists, enhances respiratory muscle strength, exercise capacity, and alleviates dyspnea, starting at least two weeks pre-surgery with 5-7 sessions weekly. The optimal timing for intervention is tailored to individual patient circumstances. Preoperative smoking cessation (Evidence 17-18) is crucial, with long-term cessation recommended to reduce postoperative complications, though the effectiveness of short-term cessation is debated. Patients are advised to quit at least eight weeks before elective surgery to mitigate pulmonary risks. Preoperative oral care, particularly with chlorhexidine mouthwash, is emphasized to reduce oral pathogens and the risk of aspiration pneumonia. The frequency and volume of mouthwash use should be determined by oral hygiene status and clinical judgment. Intraoperatively, protective lung ventilation is advised for adult patients, including PEEP ≤5cmH2O, TV ≤8ml/kg, and AMR, to prevent alveolar overexpansion and atelectasis. The development and adjustment of personalized ventilation strategies are essential for patient safety and recovery.
In summary, a comprehensive preoperative regimen that includes inspiratory muscle training, smoking cessation, oral hygiene, and protective lung ventilation is vital for mitigating pulmonary complications in gastric cancer surgery, emphasizing the need for personalized care to optimize patient outcomes.
Evidence 21 to 31 emphasizes critical intervention measures for preventing postoperative pulmonary complications (PPCs) after gastric cancer surgery. Key strategies include continuous monitoring with pulse oximeters to detect oxygen saturation levels below 95%, prompting immediate medical intervention and comprehensive patient assessment. Early detection and management of PPCs are essential for patient recovery.
For managing specific complications, physical therapy, such as sputum suction and chest percussion, is recommended for patients with respiratory tract secretions or atelectasis. Postoperative bronchospasm is treated with short-acting inhaled beta-2 agonists like salbutamol or oxitropium. Pneumonia treatment should be guided by microbiological analysis to ensure rational and targeted antibiotic use.
In cases of acute upper airway obstruction post-surgery, prompt assessment by a doctor skilled in tracheal intubation is crucial. While minor postoperative pleural effusions may resolve without intervention, signs of infection necessitate diagnostic assessment. These measures underscore the importance of proactive and tailored approaches to managing PPCs for optimal patient outcomes.
We acknowledge the importance of comparing and contrasting different evidence sources, particularly when there are conflicting viewpoints. Such an analysis is crucial for understanding how these differences may impact clinical practice, especially in the context of gastric cancer surgery. For example, while some guidelines advocate for aggressive preoperative pulmonary rehabilitation, others prioritize optimizing nutritional status before surgery. Similarly, there are differing opinions on the optimal duration of smoking cessation before surgery, with some sources suggesting a longer duration for better outcomes, while others emphasize the immediate need for surgery due to the aggressive nature of gastric cancer. The differences in these recommendations can lead to variations in clinical practice. For instance, the approach to preoperative preparation may differ significantly between institutions, potentially affecting patient outcomes. To reconcile these differences, we have taken the following steps. Firstly, we have contextualized each recommendation within the specific patient population and surgical context it was derived from, highlighting the need for tailored approaches based on individual patient needs. Secondly, we have synthesized the evidence to identify common themes and best practices that can be universally applied, while also acknowledging the areas where further research is needed to resolve discrepancies. Last but not least, we emphasize the importance of clinical judgment in interpreting these guidelines, advocating for a flexible approach that considers the unique circumstances of each patient.
Gastric cancer patients may experience postoperative recovery and complications differently due to factors such as malnutrition and the impact of surgery on gastrointestinal function. Therefore, the applicability of the evidence must consider these unique aspects of gastric cancer treatment and recovery. Given the specific challenges faced by gastric cancer patients, it is crucial to tailor intervention measures to this patient population. This includes considerations for preoperative optimization of nutritional status, postoperative pain management protocols that facilitate pulmonary toilet, and targeted pulmonary rehabilitation programs. The evidence must be critically assessed and adapted to the specific needs of gastric cancer surgery patients. This involves considering local epidemiological data, resource constraints, and cultural sensitivities to ensure that the interventions are both relevant and effective.
The evidence we synthesized spans various surgical procedures, including abdominal, thoracic, and cardiac surgeries. While this broad perspective is valuable, it also means that the evidence may not fully capture the unique aspects of gastric cancer surgery, such as the specific impacts on pulmonary function due to the proximity of the surgery to the diaphragm. The evidence is derived from multiple regions and cultures, which may influence the practices and outcomes. These differences can affect the generalizability of the findings to gastric cancer surgery patients in different settings. The implementation of certain interventions may be limited by the availability of resources, which can vary significantly between healthcare systems. This variability could affect the feasibility of applying the evidence in all clinical environments.
This study summarizes the best evidence for the prevention of postoperative pulmonary complications in patients after gastric cancer surgery. The evidence comes from both domestic and international systematic evaluations, guidelines, and authoritative expert consensuses, and is of a high level with strong credibility. It can provide a basis for decision-making and clinical nursing practice by medical staff in relevant departments. However, the evidence included in this study comes from various countries, and there are differences in culture, environment, and resources in hospitals from different countries. It is recommended that relevant departments, in conjunction with the specific circumstances of their unit and patients, fully consider the applicability and feasibility of the evidence to develop personalized management plans for the prevention of postoperative pulmonary complications in patients after gastric cancer surgery, ensuring patient safety.
ML: Methodology, Writing – original draft, Writing – review & editing, Conceptualization. GF: Methodology, Writing – review & editing. WM: Conceptualization, Methodology, Software, Writing – review & editing. YY: Data curation, Formal analysis, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by Hunan Provincial Health and Family Planning Commission (grant number 20200013) and Hunan Provincial Social Science Achievements Evaluation Committee project (grant number XSP22YBC626).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that Generative AI was used in the creation of this manuscript. The article is to be revised for grammar and translated into English using AI only.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Kinoshita T, Honda M, Matsuki A, Enomoto N, Aizawa M, Nunobe S, et al. Billroth-i vs roux-en-y after distal gastrectomy: a comparison of long-term nutritional status and survival rates from a large-scale multicenter cohort study. Ann Gastroenterol Surg. (2020) 4:142–50. doi: 10.1002/ags3.12309
3. Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Piñeros M, et al. Estimating the global cancer incidence and mortality in 2018: globocan sources and methods. Int J Cancer. (2019) 144:1941–53. doi: 10.1002/ijc.31937
4. Tokunaga M, Kurokawa Y, Machida R, Sato Y, Takiguchi S, Doki Y, et al. Impact of postoperative complications on survival outcomes in patients with gastric cancer: exploratory analysis of a randomized controlled jcog1001 trial. Gastric Cancer. (2021) 24:214–23. doi: 10.1007/s10120-020-01102-3
5. Yu F, Huang C, Cheng G, Xia X, Zhao G, Cao H. Prognostic significance of postoperative complication after curative resection for patients with gastric cancer. J Cancer Res Ther. (2020) 16:1611–6. doi: 10.4103/jcrt.JCRT_856_19
6. Meng Y, Zhao P, Yong R. Modified frailty index independently predicts postoperative pulmonary infection in elderly patients undergoing radical gastrectomy for gastric cancer. Cancer Manag Res. (2021) 13:9117–26. doi: 10.2147/CMAR.S336023
7. Gertsen EC, Goense L, Brenkman H, van Hillegersberg R, Ruurda JP. Identification of the clinically most relevant postoperative complications after gastrectomy: a population-based cohort study. Gastric Cancer. (2020) 23:339–48. doi: 10.1007/s10120-019-00997-x
8. Abbott T, Fowler AJ, Pelosi P, Gama DAM, Møller AM, Canet J, et al. A systematic review and consensus definitions for standardised end-points in perioperative medicine: pulmonary complications. Br J Anaesth. (2018) 120:1066–79. doi: 10.1016/j.bja.2018.02.007
9. Fernandez-Bustamante A, Frendl G, Sprung J, Kor DJ, Subramaniam B, Martinez RR, et al. Postoperative pulmonary complications, early mortality, and hospital stay following noncardiothoracic surgery: a multicenter study by the perioperative research network investigators. JAMA Surg. (2017) 152:157–66. doi: 10.1001/jamasurg.2016.4065
10. Kiuchi J, Komatsu S, Ichikawa D, Kosuga T, Okamoto K, Konishi H, et al. Putative risk factors for postoperative pneumonia which affects poor prognosis in patients with gastric cancer. Int J Clin Oncol. (2016) 21:920–6. doi: 10.1007/s10147-016-0987-8
11. Yang CK, Teng A, Lee DY, Rose K. Pulmonary complications after major abdominal surgery: national surgical quality improvement program analysis. J Surg Res. (2015) 198:441–9. doi: 10.1016/j.jss.2015.03.028
12. Huang YT, Lin YJ, Hung CH, Cheng HC, Yang HL, Kuo YL, et al. The fully engaged inspiratory muscle training reduces postoperative pulmonary complications rate and increased respiratory muscle function in patients with upper abdominal surgery: a randomized controlled trial. Ann Med. (2022) 54:2222–32. doi: 10.1080/07853890.2022.2106511
13. Bai D, Xiang W, Chen XZ, Hu JK. Risk factors of postoperative pulmonary infection of gastric cancer and perioperative intervention measures. Zhonghua Wei Chang Wai Ke Za Zhi. (2021) 24:185–90. doi: 10.3760/cma.j.issn.441530-20200611-00353
14. Lin Y, Sun Z, Wang H, Liu M. The effects of gastrointestinal function on the incidence of ventilator-associated pneumonia in critically ill patients. Open Med (Wars). (2018) 13:556–61. doi: 10.1515/med-2018-0082
15. Huang KS, Pan BL, Lai WA, Bin PJ, Yang YH, Chou CP. Could prokinetic agents protect long-term nasogastric tube-dependent patients from being hospitalized for pneumonia? A nationwide population-based case-crossover study. PloS One. (2021) 16:e0249645. doi: 10.1371/journal.pone.0249645
16. Gebeyehu G, Eshetu A, Aweke S. Incidence and associated factors of postoperative pulmonary complications after abdominal surgery in the public hospital, addis ababa, Ethiopia. Anesthesiol Res Pract. (2022) 2022:8223903. doi: 10.1155/2022/8223903
17. Zhu Z, Hu Y, Xing W, Zhou Y, Gu Y. The composition of different types of evidence based problems. J Nurses Training. (2017) 32:1991–4. doi: 10.16821/j.cnki.hsjx.2017.21.025
18. Dicenso A, Bayley L, Haynes RB. Accessing pre-appraised evidence: fine-tuning the 5S model into a 6S model. Evid Base Nurs. (2007) 12:99–101. doi: 10.1136/ebn.12.4.99-b
19. Jacklin C, Tan M, Sravanam S, Harrison CJ. Appraisal of International Guidelines for Cutaneous Melanoma Management using the AGREE II assessment tool. JPRAS Open. (2022) 31:31114–122. doi: 10.1016/j.jpra.2021.11.002
20. Joyce MF. Overview of prehabilitation for surgical patients. Available online at: https://www-uptodate-com.webvpn.sjlib.cn/contents/overview-of-prehabilitation-for-surgical-patients?search=Overview%20of%20prehabilitation%20for%20surgical%20patients&source=Out%20of%20date%20-%20zh-Hans&selectedTitle=1%7E150 (Accessed September 20, 2024).
21. Smetana GM. Strategies to reduce postoperative pulmonary complications in adults(2024). Available online at: https://www-uptodate-com.webvpn.sjlib.cn/contents/search?search=Strategies%20to%20reduce%20postoperative%20pulmonary%20complications%20in%20adults&sp=0&searchType=PLAIN_TEXT&source=USER_INPUT&searchControl=TOP_PULLDOWN&autoComplete=false (Accessed August 25, 2024).
22. Smetana GM. Evaluation of perioperative pulmonary risk(2024). Available online at: https://www-uptodate-com.webvpn.sjlib.cn/contents/zh-Hans/evaluation-of-perioperative-pulmonary-risk?search=Evaluation%20of%20perioperative%20pulmonary%20risk&source=search_result&selectedTitle=1%7E150&usage_type=default&display_rank=1 (Accessed September 20, 2024).
23. Conde MV. Postoperative airway and pulmonary complications in adults management following initial stabilization(2024). Available online at: https://www-uptodate-com.webvpn.sjlib.cn/contents/zh-Hans/postoperative-airway-and-pulmonary-complications-in-adults-management-following-initial-stabilization?search=Postoperative%20airway%20and%20pulmonary%20complications%20in%20adults%20management%20following%20initial%20stabilization&source=search_result&selectedTitle=1%7E150&usage_type=default&display_rank=1 (Accessed August 25, 2024).
24. De Hert S, Staender S, Fritsch G, Hinkelbein J, Afshari A, Bettelli G, et al. Pre-operative evaluation of adults undergoing elective noncardiac surgery: updated guideline from the european society of anaesthesiology. Eur J Anaesthesiol. (2018) 35:407–65. doi: 10.1097/EJA.0000000000000817
25. Chinese Society of Surgery, Chinese Society of Anesthesiology. Clinical practice guidelines for ERAS in China, (2021) (IV). Chin J Anesthesiology. (2021) 41:1053–60. doi: 10.12290/xhyxzz.20210004
26. Joshi GP, Abdelmalak BB, Weigel WA, Harbell MW, Kuo CI, Soriano SG, et al. American society of anesthesiologists practice guidelines for preoperative fasting: carbohydrate-containing clear liquids with or without protein, chewing gum, and pediatric fasting duration-a modular update of the 2017 american society of anesthesiologists practice guidelines for preoperative fasting. Anesthesiology. (2023) 138:132–51. doi: 10.1097/ALN.0000000000004381
27. Zaccagnini M. Postoperative respiratory depression (prevention): pulse oximetry and capnography(2022). Available online at: http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=jbi&NEWS=N&AN=JBI172 (Accessed October 14, 2024).
28. Overall, B. D. B. Postoperative pulmonary complications: preoperative strategies(2024). Available online at: http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=jbi&NEWS=N&AN=JBI15884 (Accessed October 14, 2024).
29. Overall, B. D. B, Mevin SB. Postoperative pulmonary complications: intraoperative prevention strategies(2024). Available online at: http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=jbi&NEWS=N&AN=JBI15885 (Accessed October 14, 2024).
30. Overall, B. D. B. Postoperative pulmonary complications: postoperative strategies(2024). Available online at: http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=jbi&NEWS=N&AN=JBI15886 (Accessed October 14, 2024).
31. Pamaiahgari PB. Preoperative smoking cessation interventions(2022). Available online at: http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=jbi&NEWS=N&AN=JBI8153 (Accessed October 14, 2024).
32. Myers K, Hajek P, Hinds C, Mcrobbie H. Stopping smoking shortly before surgery and postoperative complications: a systematic review and meta-analysis. Arch Intern Med. (2011) 171:983–9. doi: 10.1001/archinternmed.2011.97
33. Pedersen T, Nicholson A, Hovhannisyan K, Møller AM, Smith AF, Lewis SR. Pulse oximetry for perioperative monitoring. Cochrane Database Syst Rev. (2014) 2014:CD002013. doi: 10.1002/14651858.CD002013
34. Thomsen T, Villebro N, Møller AM. Interventions for preoperative smoking cessation. Cochrane Database Syst Rev. (2014), 3:CD002294. doi: 10.1002/14651858.CD002294
35. Katsura M, Kuriyama A, Takeshima T, Fukuhara S, Furukawa TA. Preoperative inspiratory muscle training for postoperative pulmonary complications in adults undergoing cardiac and major abdominal surgery. Cochrane Database Syst Rev. (2015) 2015:CD010356. doi: 10.1002/14651858.CD010356
36. Lam T, Nagappa M, Wong J, Singh M, Wong D, Chung F. Continuous pulse oximetry and capnography monitoring for postoperative respiratory depression and adverse events: a systematic review and meta-analysis. Anesth Analg. (2017) 125:2019–29. doi: 10.1213/ANE.0000000000002557
37. Wang LH, Zhu RF, Gao C, Wang SL, Shen LZ. Application of enhanced recovery after gastric cancer surgery: an updated meta-analysis. World J Gastroenterol. (2018) 24:1562–78. doi: 10.3748/wjg.v24.i14.1562
38. Hughes MJ, Hackney RJ, Lamb PJ, Wigmore SJ, Christopher DD, Skipworth R. Prehabilitation before major abdominal surgery: a systematic review and meta-analysis. World J Surg. (2019) 43:1661–8. doi: 10.1007/s00268-019-04950-y
39. Lau C, Chamberlain RS. Prehabilitation programs improve exercise capacity before and after surgery in gastrointestinal cancer surgery patients: a meta-analysis. J Gastrointest Surg. (2020) 24:2829–37. doi: 10.1007/s11605-019-04436-1
40. Odor PM, Bampoe S, Gilhooly D, Creagh-Brown B, Moonesinghe SR. Perioperative interventions for prevention of postoperative pulmonary complications: systematic review and meta-analysis. BMJ. (2020) 368:m540. doi: 10.1136/bmj.m540
41. Assouline B, Cools E, Schorer R, Kayser B, Elia N, Licker M. Preoperative exercise training to prevent postoperative pulmonary complications in adults undergoing major surgery. A systematic review and meta-analysis with trial sequential analysis. Ann Am Thorac Soc. (2021) 18:678–88. doi: 10.1513/AnnalsATS.202002-183OC
42. Mortensen K, Nilsson M, Slim K, Schäfer M, Mariette C, Braga M, et al. Consensus guidelines for enhanced recovery after gastrectomy: enhanced recovery after surgery (eras®) society recommendations. Br J Surg. (2014) 101:1209–29. doi: 10.1002/bjs.9582
43. Rochester CL, Vogiatzis I, Holland AE, Lareau SC, Marciniuk DD, Puhan MA, et al. An official american thoracic society/european respiratory society policy statement: enhancing implementation, use, and delivery of pulmonary rehabilitation. Am J Respir Crit Care Med. (2015) 192:1373–86. doi: 10.1164/rccm.201510-1966ST
44. Carli F, Silver JK, Feldman LS, Mckee A, Gilman S, Gillis C, et al. Surgical prehabilitation in patients with cancer: state-of-the-science and recommendations for future research from a panel of subject matter experts. Phys Med Rehabil Clin N Am. (2017) 28:49–64. doi: 10.1016/j.pmr.2016.09.002
45. Gelb AW, Morriss WW, Johnson W, Merry AF. World health organization-world federation of societies of anaesthesiologists (who-wfsa) international standards for a safe practice of anesthesia. Can J Anaesth. (2018) 65:698–708. doi: 10.1213/ANE.0000000000002927
Keywords: gastric cancer, pulmonary complications, prevention, best evidence, intervention strategies
Citation: Li M, Fu G, Mo W and Yan Y (2025) Summary of best evidence for prevention of postoperative pulmonary complications after surgery for patients undergoing gastric cancer operations. Front. Oncol. 15:1515502. doi: 10.3389/fonc.2025.1515502
Received: 23 October 2024; Accepted: 17 February 2025;
Published: 05 March 2025.
Edited by:
Sharon R. Pine, University of Colorado Anschutz Medical Campus, United StatesReviewed by:
David Morris, University of New South Wales, AustraliaCopyright © 2025 Li, Fu, Mo and Yan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuanyuan Yan, MTQ0Mjg2Njg0M0BxcS5jb20=
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.