- 1Department of Neurosurgery, The First Affiliated Hospital of China Medical University, Shenyang, Liaoning, China
- 2Department of Organ and Tissue Anatomy, Hamamatsu University School of Medicine, Hamamatsu, Shizuoka, Japan
- 3Department of Optical Neuroanatomy, Hamamatsu University School of Medicine, Hamamatsu, Shizuoka, Japan
- 4Imaging technician in Charge, Shenyang Renren Kangye Hospital, Shenyang, Liaoning, China
Background: Cavernous malformations are common vascular abnormalities of the central nervous system, but cavernous malformations of the cerebral aqueduct are rare. The choice of treatment is influenced by various factors.
Case Description: We report two cases of midbrain cavernous malformations. Both cases involved midbrain lesions obstructing the cerebral aqueduct, leading to obstructive hydrocephalus. The primary symptoms and complaints of the patients were related to hydrocephalus. Prior to surgery, patients underwent comprehensive imaging evaluations and received endoscopic third ventriculostomy rather than tumor resection. Both patients had favorable recoveries. We also reviewed the literature and discussed the choice of treatment strategies.
Conclusion: Cavernous malformations are slow-progressing central nervous system lesions with a relatively benign natural course. When selecting a treatment strategy, clinicians should carefully consider the underlying cause of the patient’s primary symptoms and the specific objectives of the surgery. Avoiding overly aggressive resection that fails to address the main symptoms and potentially causes irreversible damage is crucial.
1 Introduction
Cerebral cavernous malformations (CCMs) are common vascular malformations of the central nervous system. CCMs occur in both sporadic and familial forms, frequently affecting young adults, typically between the ages of 20 and 50 (1–3). The sporadic form is generally associated with a single isolated lesion, while the familial form is linked to multiple lesions and mutations in three specific genes: CCM1, CCM2, and CCM3. Familial cerebral cavernous malformations (FCCM) are inherited in an autosomal dominant manner due to heterozygous mutations in one of these three genes, with approximately 40-60% of FCCM cases being attributed to this mode of inheritance (4–6). The natural course of cavernous malformations is relatively benign, with about 21% of patients remaining asymptomatic. However, depending on the location and size of the lesions, patients may present with clinical symptoms such as seizures, headaches, neurological deficits, and intracerebral hemorrhage (7–9). Most cavernous malformations are supratentorial, with the incidence of midbrain CCMs being around 9%-35% (10, 11). Here, we report two rare cases of midbrain aqueductal CCMs and discuss them in the context of a literature review.
2 Materials and methods
2.1 General data
Retrieve and collect the complete treatment and follow-up data of patients with deep cerebral cavernous hemangiomas, who presented primarily with symptoms related to hydrocephalus, treated in our hospital from January 2024 to August 2024.
2.2 Literature review
To gather relevant literature, we conducted a search in PubMed for English-language articles published between 2002 and 2024 using the Boolean search terms: “(cavernous angioma OR cavernous malformation OR cavernous hemangioma OR cavernoma OR cerebral cavernous malformations) AND (brain OR cerebral OR intracranial OR brainstem).” A total of 5,135 related papers were retrieved. We specifically selected adult CCM cases located in the midbrain and adjacent structures (midbrain, thalamus, and third ventricle) that provided detailed descriptions of patient conditions, lesion locations, treatment plans, and outcomes. Considering that resective treatment is undoubtedly the first-choice therapy in certain cases,we excluded cases involving acute hemorrhage, mass effect leading to specific neurological deficits, or other conditions severely impacting quality of life. Finally, we selected 14 relevant articles, including a total of 15 patients for the literature review.
3 Case report
3.1 Case 1
The patient is a 21-year-old male with a long history of headaches accompanied by vision decline and a past history of seizures. Over the past two months, he experienced significant worsening of his vision. Fundoscopic examination revealed normal intraocular pressure in both eyes, papilledema, and optic atrophy. No other significant abnormalities were detected on physical examination. MRI scans (Figures 1A–F) showed supratentorial ventricular enlargement, periventricular interstitial edema, and a flattening of the sulci and cisterns. Multiple intracranial lesions appeared isointense on T1-weighted imaging and exhibited mixed hyperintense and hypointense signals on T2-weighted imaging, without enhancement. These lesions displayed patchy short T1 signals internally and surrounding ring-like hypointense signals. The entrance of the midbrain aqueduct was obstructed, with downward displacement of the third ventricle floor, empty sella syndrome, and shallowing of the pontine and basal cisterns. Using cine phase-contrast MRI, cerebrospinal fluid (CSF) flow velocities at the membrane structure above and below the midbrain aqueduct were assessed (Supplementary Video S1), indicating complete obstruction of the aqueduct.
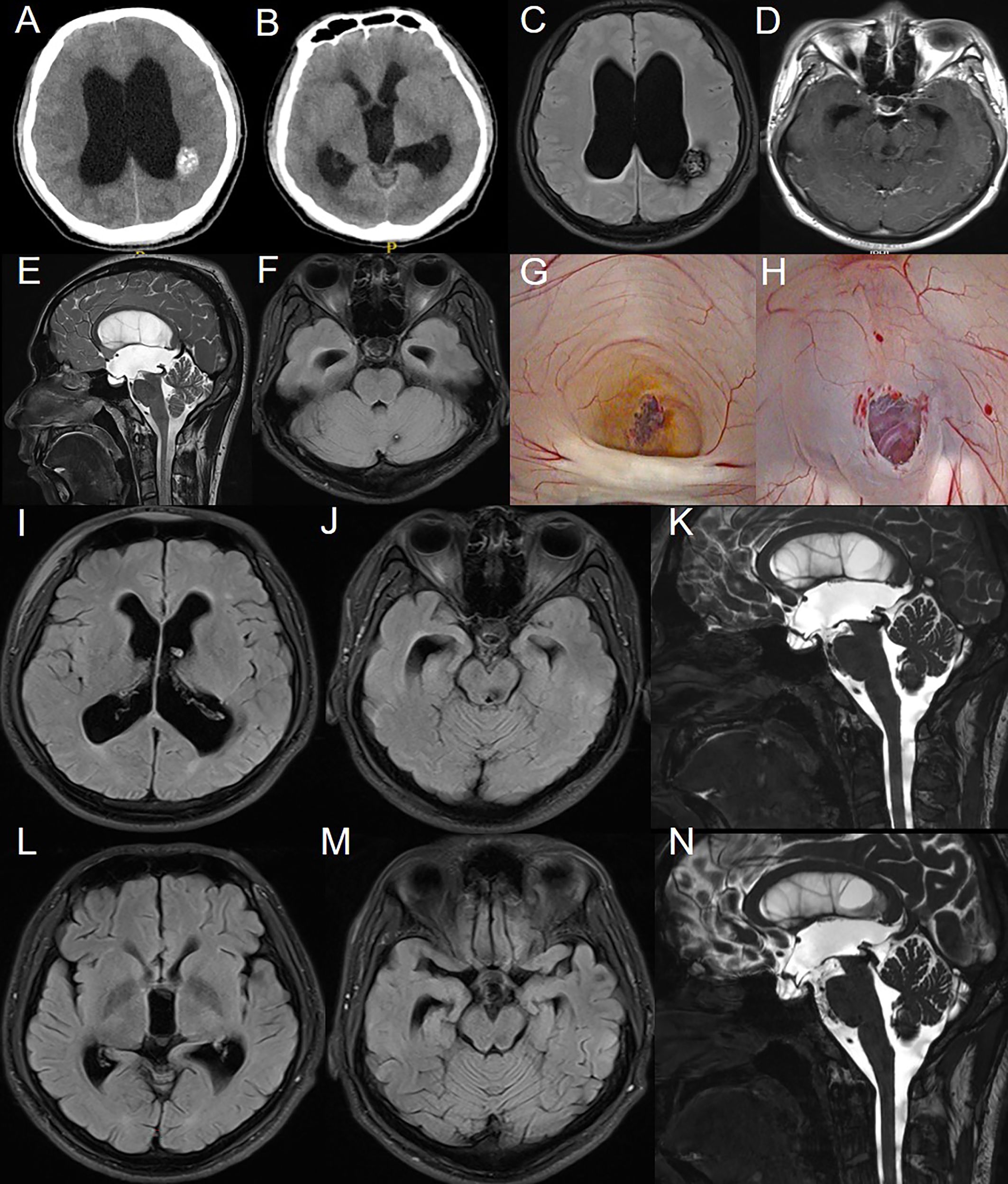
Figure 1. Preoperative imaging examination, intraoperative findings, and postoperative follow-up of the patient in Case 1. (A-F): CT and MRI reveal multiple intracranial masses and clear signs of obstructive hydrocephalus. (G): CCM obstructing the cerebral aqueduct with surrounding hemosiderin deposition. (H): After balloon catheter puncture and fistula creation, the fistula was patent, and the basilar artery showed good pulsation. (I-K): MRI results on the third day postoperatively. (L-N): MRI results at the third-month postoperative follow-up.
An endoscopic third ventriculostomy (ETV) was performed using a flexible neuroendoscope to establish a CSF shunt, opening the prepontine cistern. Intraoperative ventricular exploration was consistent with chronic hydrocephalus (Figures 1G, H), showing a fenestrated septum pellucidum, enlarged foramen of Monro, downward displacement of the third ventricle, and anterior displacement of the lamina terminalis. The cavernous malformation obstructing the entrance to the midbrain aqueduct was clearly identified, with hemosiderin deposits indicative of prior hemorrhage. A third ventriculostomy was performed under the microscope, with fenestration of the Liliequist’s membrane in the basal cisterns, and a robust pulsation of the basilar artery was observed through the stoma.
Follow-up MRI on postoperative day 3 (Figures 1I–K) showed improvement compared to preoperative imaging, with decreased ventricular enlargement and deepening of the cerebral sulci and cisterns. The floor of the third ventricle was elevated, and the empty sella syndrome was alleviated, with the stoma remaining patent. This was further confirmed by cine phase-contrast MRI (Supplementary Video S2).
At the 3-month postoperative follow-up MRI (Figures 1L–N), the stoma remained patent, with further reduction in ventricular enlargement and resolution of periventricular interstitial edema. Cine phase-contrast MRI demonstrated clear CSF flow from the third ventricle floor to the prepontine cistern (Supplementary Video S3). The patient reported significant relief from headaches, with no seizure episodes observed since surgery, although there was no notable improvement in vision.
At 11.5 months post-surgery, a telephone follow-up was conducted. The patient’s headache symptoms had resolved, and there have been no seizures to date. Regarding vision impairment, the patient regularly attends a rehabilitation center for treatment and reports slight improvement in vision compared to before.
3.2 Case 2
The patient is a 74-year-old female who had recovered from a midbrain hemorrhage treated conservatively nine years ago, with no residual neurological deficits. Eight months ago, she began experiencing multiple episodes of transient loss of consciousness followed by falls, with no recollection of the events afterward. Approximately one and a half months ago, she developed gait instability and gradually worsening cognitive function. About a month ago, she started experiencing urinary incontinence, which progressed to fecal incontinence ten days before admission. On physical examination, the patient was found to have anisocoria with the left pupil measuring 3.5 mm in diameter and non-reactive to light, while the right pupil was 3.0 mm in diameter and reactive to light. She exhibited left ptosis, with the left eye in an abducted position and restricted adduction. Muscle strength was graded 4/5 in both upper limbs and 3/5 in both lower limbs. CT scans (Figure 2A) showed bilateral ventricular enlargement with a mixed-density mass in the brainstem obstructing the midbrain aqueduct. MRI (Figures 2B–E) revealed shallow supratentorial sulci and cisterns, ventricular enlargement, downward displacement of the third ventricle floor, and a mixed signal mass in the brainstem, with localized narrowing and obstruction of the midbrain aqueduct, consistent with obstructive hydrocephalus.
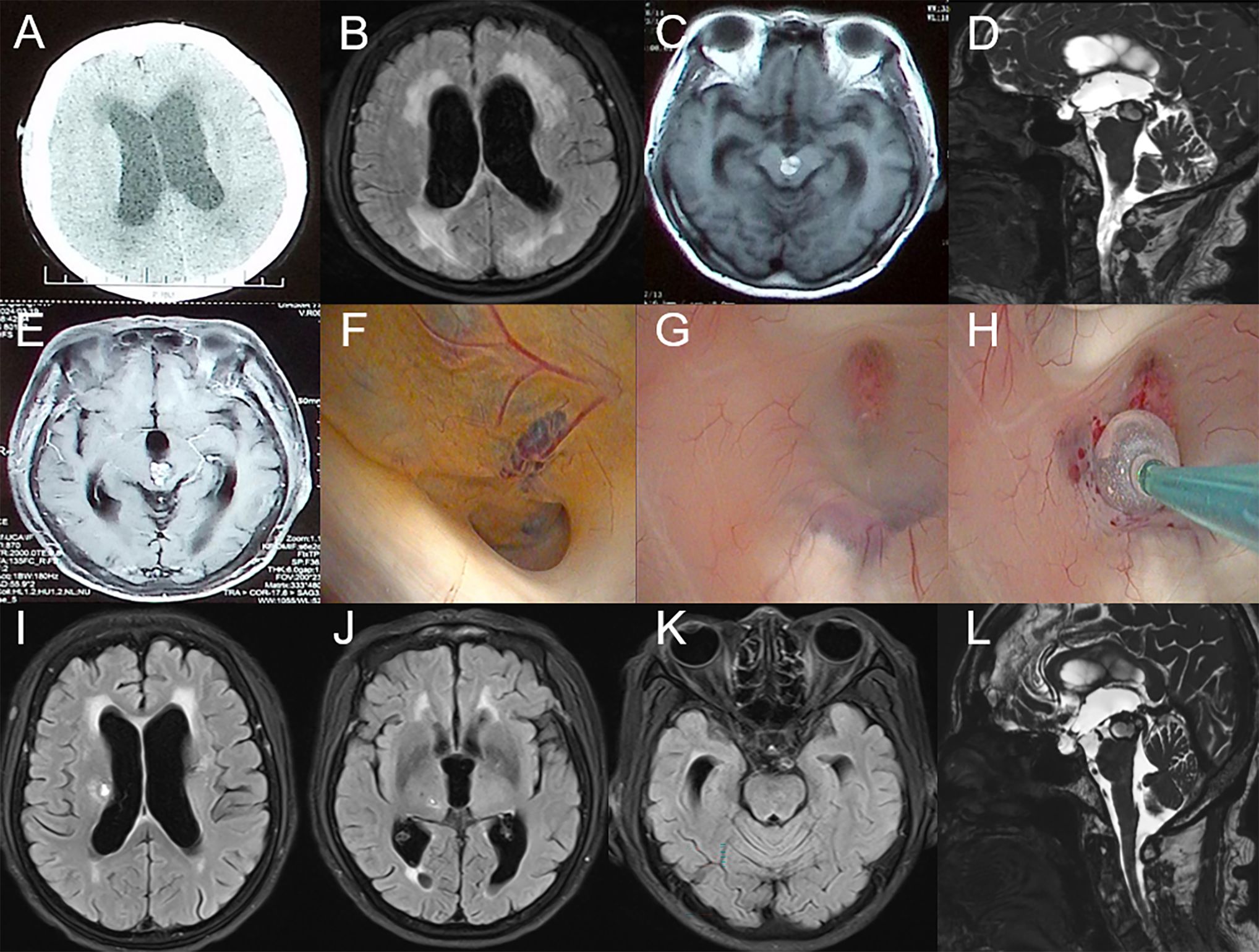
Figure 2. Preoperative imaging examination, intraoperative findings, and postoperative follow-up of the patient in Case 2. (A-E): CT and MRI reveal a mass in the midbrain and clear signs of obstructive hydrocephalus. (F): CCM obstructing the cerebral aqueduct with surrounding hemosiderin deposition. (G-H): Select the appropriate site and create the fistula. (I-L): MRI results on the seventh day postoperatively.
An endoscopic third ventriculostomy (ETV) was performed, creating a stoma at the floor of the third ventricle to communicate with the prepontine cistern. Intraoperative findings (Figures 2F–H) were consistent with hydrocephalus, showing ventricular enlargement and downward displacement of the third ventricle. The midbrain aqueduct was obstructed by a cavernous malformation, with hemosiderin deposits indicative of prior hemorrhage. A third ventriculostomy was created, and the Liliequist’s membrane in the basal cisterns was fenestrated, with robust basilar artery pulsations observed through the stoma.
Follow-up MRI on postoperative day 7 (Figures 2I–L) showed improved ventricular enlargement, with deepened sulci and cisterns and reduced interstitial edema compared to preoperative imaging. The floor of the third ventricle was elevated, and the stoma remained patent. Cine phase-contrast MRI (Supplementary Video S4) confirmed these findings. The patient’s hydrocephalus-related symptoms were significantly alleviated compared to preoperative status, with reported relief of pyramidal tract-related symptoms. She was subsequently transferred to a rehabilitation facility for further treatment.
At 5 months post-surgery, the patient’s symptoms had significantly improved. Compared to one-week post-surgery, urinary and fecal incontinence had completely resolved, cognitive function was normal, and the patient was able to walk independently. Imaging results were consistent with expectations (Supplementary Figure S1).
4 Literature review
A total of 14 articles, encompassing 15 cases, met these criteria (12–25), with details summarized in Table 1. In these cases, nearly all patients presented with hydrocephalus caused by CCM mass effect, with no specific neurological deficits directly attributable to the tumor itself. Among the 15 reported cases, 7 patients received treatment solely targeting hydrocephalus symptoms, including endoscopic third ventriculostomy (ETV) and ventriculoperitoneal (VP) shunting. Of these, 6 cases achieved favorable outcomes with significant symptom improvement, while 1 case had a poor prognosis due to postoperative hemorrhage within hours after surgery. In Case 5, although surgical resection was performed, only the thalamic lesion among multiple CCMs was removed for diagnostic purposes. The midbrain lesion causing hydrocephalus was left untreated and managed instead with ETV to address the hydrocephalus. Excluding Case 5, 6 cases involved CCM resection. Among them, Cases 9 and 15 underwent simultaneous CCM resection and ETV, both achieving good outcomes. However, 4 patients showed no significant postoperative improvement and even had poor prognoses. Notably, Case 4 required a second surgery for VP shunting due to postoperative hydrocephalus. Additionally, one patient with multiple CCMs presenting with vertigo achieved satisfactory outcomes and long-term follow-up through conservative management alone.
In conclusion, for CCMs located in the midbrain and adjacent structures, neurosurgeons should exercise caution when choosing treatment options. The literature review suggests that the benefits of resective treatment for tumors in such conditions are relatively limited. In contrast, treatments targeting the primary issue, hydrocephalus, through approaches like ETV, often result in better prognoses.
5 Discussion
Based on imaging examinations and intraoperative endoscopic exploration, both cases were ultimately diagnosed as cavernous malformations (CCMs). Case 1 involved multiple lesions, while Case 2 featured a solitary lesion in an elderly patient. In both cases, the midbrain lesions obstructed the cerebral aqueduct, leading to obstructive hydrocephalus. The progression of hydrocephalus was gradual, with significant dilation of the third ventricle. In Case 1, the patient’s improvement in vision progressed slowly post-surgery. We believe this is due to the gradual progression of the patient’s hydrocephalus, which differs from the effects caused by acute intracranial pressure elevation in the short term. Therefore, the treatment of obstructive hydrocephalus alone did not show immediate effectiveness. However, the imaging results and the complete resolution of the patient’s headache symptoms also confirm the efficacy of our treatment. In Case 2, despite the obvious mass effect causing corticospinal tract symptoms, such as ptosis and restricted eye movement, the primary cause of the patient’s symptoms was obstructive hydrocephalus, which significantly impacted the patient’s quality of life. Recent studies suggest that conservative treatment also has a positive impact on CCM (26). In a recent cohort study involving 265 CCM patients treated conservatively with a follow-up period of at least 6 months, the results showed that most conservatively treated CCM patients did not experience symptomatic hemorrhage during the follow-up, and few required intervention, with death due to CCM being rare (27). Additionally, for symptomatic or recurrently hemorrhaging brainstem CCMs, surgical treatment is an important option (28). In conclusion, for our patient, choosing endoscopic third ventriculostomy (ETV) to treat obstructive hydrocephalus effectively alleviated the patient’s symptoms while avoiding the potential harm caused by surgery itself. This approach is both reasonable and meaningful.
The diagnosis of familial multiple cavernous malformations (FCCM) requires meeting one or more of the following criteria: (1) presence of multiple CCMs (≥5); (2) at least two family members diagnosed with CCM; (3) mutation in one of the three genes associated with FCCM (29). Typically, CCMs appear as “popcorn-like” lesions with mixed high and low signals on T1- and T2-weighted MRI sequences (30). The MRI appearance of CCMs is influenced by the time interval since hemorrhage, leading Zabramski et al. to classify CCMs into four types (2). Although CCMs are generally considered to have a relatively benign natural course, some patients may experience focal neurological deficits, which can sometimes be irreversible (1, 31, 32).
In recent years, cohort studies on surgical treatment of CCMs have increased, with treatment options including traditional craniotomy, neuroendoscopic surgery, gamma knife, and laser interstitial thermal therapy, all showing favorable outcomes (33–36). For incidentally discovered, asymptomatic CCMs, conservative management—primarily periodic monitoring considering the patient’s age and lesion location—is generally preferred over immediate surgical resection (37–40). The timing of surgical intervention for symptomatic CCMs, especially those causing neurological deficits, remains controversial (11, 41, 42). This is particularly true for midbrain CCMs, where some reports suggest that aggressive surgical treatment does not significantly increase the risk of adverse outcomes (43–45). For intraventricular CCMs, although studies have indicated a high hemorrhage propensity (46), the resection of CCMs in the fourth ventricle or nearby areas carries significant risks (17). This is particularly unacceptable for patients whose primary symptoms are hydrocephalus or those who are asymptomatic. Therefore, when making treatment decisions, factors such as the size, location, mass effect, and surgical risks associated with the CCM must be carefully considered. For symptomatic CCMs located in superficial brain areas, or for patients with recurrent symptomatic hemorrhages even when the lesion is deep-seated, surgical resection is a reasonable choice (47, 48).
Certainly, this study also has objective limitations. Most notably, the sample size is small, with only two patients. This is a retrospective study rather than a standard cohort study or a controlled trial. Factors such as the surgical skills and decision-making ability of the lead surgeon may also influence the generalizability of the final results. Furthermore, the lack of preoperative quality-of-life assessments for the patients further limits the conclusions of this study. Future research will focus on larger-scale prospective studies, with comprehensive quality-of-life indicators and longitudinal follow-up, to better understand the impact of surgery on patient outcomes and improve clinical strategies in future studies. Finally, we also hope for multicenter studies, especially those including diverse nationalities and ethnicities, as this will provide more generalizable and meaningful results for this type of research.
6 Conclusion
As a slow-progressing central nervous system lesion with a relatively benign natural course, the treatment strategy for CCMs is influenced by various factors. While the hemorrhage risk of CCMs cannot be overlooked, overly aggressive surgical approaches may cause greater harm to patients. Our cases and the literature review offer new perspectives and insights into surgical decision-making, contributing to better treatment outcomes and prognosis. By demonstrating the effectiveness of addressing hydrocephalus as the primary cause of symptoms rather than pursuing aggressive surgical resection of cavernous malformations, this study supports a more conservative treatment approach in similar cases. This approach not only reduces the risk of potential surgical complications but also emphasizes the importance of individualized patient care. Clinicians are encouraged to carefully evaluate the primary cause of symptoms and consider less invasive options when appropriate. This is particularly important when dealing with younger patients or elderly patients, where optimizing treatment outcomes while preserving quality of life is especially crucial. The patients we reported on benefitted from the surgeries and have been followed up long-term, further supporting the value of this conservative approach.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
Author contributions
HY: Conceptualization, Data curation, Methodology, Writing – original draft. LD: Conceptualization, Data curation, Methodology, Writing – original draft. JG: Data curation, Writing – original draft. YBW: Data curation, Writing – original draft. YW: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1513254/full#supplementary-material
Supplementary Figure 1 | Follow-up imaging results of Case 2 at 5 months post-surgery.
Supplementary Video 1 | Preoperative cerebrospinal fluid (CSF) cine phase-contrast MRI of Case 1.
Supplementary Video 2 | Postoperative day 3 cerebrospinal fluid (CSF) cine phase-contrast MRI of Case 1.
Supplementary Video 3 | Postoperative 3-month cerebrospinal fluid (CSF) cine phase-contrast MRI of Case 1.
Supplementary Video 4 | Postoperative day 7 cerebrospinal fluid (CSF) cine phase-contrast MRI of Case 2.
References
1. Robinson JR, Awad IA, Little JR. Natural history of the cavernous angioma. J Neurosurg. (1991) 75:709–14. doi: 10.3171/jns.1991.75.5.0709
2. Zabramski JM, Wascher TM, Spetzler RF, Johnson B, Golfinos J, Drayer BP, et al. The natural history of familial cavernous malformations: results of an ongoing study. J Neurosurg. (1994) 80:422–32. doi: 10.3171/jns.1994.80.3.0422
3. Cohen DS, Zubay GP, Goodman RR. Seizure outcome after lesionectomy for cavernous malformations. J Neurosurg. (1995) 83:237–42. doi: 10.3171/jns.1995.83.2.0237
4. Hayman LA, Evans RA, Ferrell RE, Fahr LM, Ostrow P, Riccardi VM. Familial cavernous angiomas: natural history and genetic study over a 5-year period. Am J Med Genet. (1982) 11:147–60. doi: 10.1002/ajmg.1320110205
5. Dubovsky J, Zabramski JM, Kurth J, Spetzler RF, Rich SS, Orr HT, et al. A gene responsible for cavernous malformations of the brain maps to chromosome 7q. Hum Mol Genet. (1995) 4:453–8. doi: 10.1093/hmg/4.3.453
6. Craig HD, Günel M, Cepeda O, Johnson EW, Ptacek L, Steinberg GK, et al. Multilocus linkage identifies two new loci for a mendelian form of stroke, cerebral cavernous malformation, at 7p15-13 and 3q25.2-27. Hum Mol Genet. (1998) 7:1851–8. doi: 10.1093/hmg/7.12.1851
7. Kivelev J, Niemelä M, Hernesniemi J. Characteristics of cavernomas of the brain and spine. J Clin Neurosci. (2012) 19:643–8. doi: 10.1016/j.jocn.2011.08.024
8. Prasad R, Saha S, Mishra OP, Srivastava A. Multiple cavernous malformations with hemorrhage of brain. Indian J Pediatr. (2014) 81:1246–7. doi: 10.1007/s12098-014-1354-2
9. Efe IE, Aydin OU, Alabulut A, Celik O, Aydin K. Multiple large-size cystic cerebral cavernomas. World Neurosurg. (2020) 139:410–4. doi: 10.1016/j.wneu.2020.04.189
10. Fritschi JA, Reulen HJ, Spetzler RF, Zabramski JM. Cavernous malformations of the brain stem. A review of 139 cases. Acta Neurochir (Wien). (1994) 130:35–46. doi: 10.1007/BF01405501
11. Kondziolka D, Lunsford LD, Kestle JR. The natural history of cerebral cavernous malformations. J Neurosurg. (1995) 83:820–4. doi: 10.3171/jns.1995.83.5.0820
12. Cristini A, Fischer C, Sindou M. Tectal plate cavernoma-a special entity of brainstem cavernomas: case report. Surg Neurol. (2004) 61:474–8. doi: 10.1016/S0090-3019(03)00487-7
13. Bulluss KJ, Wood M, Smith P, Trost N, Murphy MA. Cavernous haemangioma presenting with obstructive hydrocephalus. J Clin Neurosci. (2005) 12:660–3. doi: 10.1016/j.jocn.2004.11.005
14. Darwish B, Boet R, Finnis N, Smith N. Third ventricular cavernous haemangioma. J Clin Neurosci. (2005) 12:601–3. doi: 10.1016/j.jocn.2004.08.025
15. Giannetti AV. Purely neuroendoscopic resection of an intraventricular cavernous angioma: case report. J Neurol Surg A Cent Eur Neurosurg. (2013) 74:47–50. doi: 10.1055/s-0032-1325632
16. Belousova OB, Okishev DN, Ignatova TM, Balashova MS, Boulygina ES. Hereditary multiple cerebral cavernous malformations associated with Wilson disease and multiple lipomatosis. World Neurosurg. (2017) 105:1034.e1–1034.e6. doi: 10.1016/j.wneu.2017.06.002
17. Feletti A, Dimitriadis S, Pavesi G. Cavernous angioma of the cerebral aqueduct. World Neurosurg. (2017) 98:876.e15–876.e22. doi: 10.1016/j.wneu.2016.11.096
18. Kulason KO, Schneider JR, Rahme R, Ratzon F, Anderson TA, Shatzkes DR, et al. Suprasellar and third ventricular cavernous malformation: Lessons learned in differential diagnosis and surgical planning. Surg Neurol Int. (2017) 8:251. doi: 10.4103/sni.sni_229_17
19. Li J, He J, Liu L, Zhou L. Anterior endoscopic transcortical approach to a pineal region cavernous hemangioma. Neurosurg Focus Video. (2021) 5:V15. doi: 10.3171/2021.4.FOCVID215
20. Loh DD-L, Chen MW, Lim JX, Keong NCH, Kirollos RW. Endoscopic excision of an aqueduct of Sylvius cavernoma causing obstructive hydrocephalus: technical note. Br J Neurosurg. (2022) 38(6):1475–1478. doi: 10.1080/02688697.2021.2024501
21. Kashaf N, Afeena AFA, Lim CC, Jaafar RJ, Hailani I. Multiple cerebral cavernous malformation: an unusual cause of vertigo. Indian J Otolaryngol Head Neck Surg. (2024) 76:4997–5000. doi: 10.1007/s12070-024-04893-4
22. Lee H-J, Lee S-U, Park E, Kim J-S. Cavernous malformation in the fourth ventricle: trivial findings but grave prognosis. Acta Neurol Belg. (2024) 124:1085–7. doi: 10.1007/s13760-023-02464-y
23. Liu S, Sun C, Chen P, Yang H, Xie T, Huang J, et al. Endoscopic occipital transtentorial approach for dorsal midbrain cavernous malformation: technical notes with illustrative case. World Neurosurg. (2024) 194:123460. doi: 10.1016/j.wneu.2024.11.043
24. Salem R, Almutairi OT, Albrahim M, Alomar N. Cerebral cavernous malformation bleeding following cerebrospinal fluid diversion surgery: A case report and literature review. Cureus. (2024) 16:e58689. doi: 10.7759/cureus.58689
25. Yang W, He C. Intraventricular peritoneal shunt for obstructive hydrocephalus caused by cavernous hemangioma of the aqueduct of sylvius. Balkan Med J. (2025) 42:75–6. doi: 10.4274/balkanmedj.galenos.2024.2024-5-86
26. da Fontoura Galvão G, Verly G, Valença P, Domingues FS, da Silva MR, Marcondes J. Early and long-term outcome of surgical versus conservative management for intracranial cerebral cavernous malformation: Meta-analysis of reconstructed time-to-event data. Clin Neurol Neurosurg. (2024) 246:108567. doi: 10.1016/j.clineuro.2024.108567
27. Sandmann ACA, Kempeneers MA, van den Berg R, Verbaan D, Vandertop WP, Coutinho JM. Clinical course of patients with conservatively managed cerebral cavernous malformations. Eur Stroke J. (2024) 9:667–75. doi: 10.1177/23969873241246868
28. Lu J, Li Z, Deng H, Shi G, Wang W, You C, et al. Treatment modalities and outcomes in brainstem cavernous malformations: A large multicenter observational cohort study. Stroke. (2024) 55:1151–60. doi: 10.1161/STROKEAHA.123.046203
29. Mespreuve M, Vanhoenacker F, Lemmerling M. Familial multiple cavernous malformation syndrome: MR features in this uncommon but silent threat. J Belg Soc Radiol. (2016) 100:51. doi: 10.5334/jbr-btr.938
30. Hegde AN, Mohan S, Lim CCT. CNS cavernous haemangioma: “popcorn” in the brain and spinal cord. Clin Radiol. (2012) 67:380–8. doi: 10.1016/j.crad.2011.10.013
31. Simard JM, Garcia-Bengochea F, Ballinger WE, Mickle JP, Quisling RG. Cavernous angioma: a review of 126 collected and 12 new clinical cases. Neurosurgery. (1986) 18:162–72. doi: 10.1227/00006123-198602000-00008
32. Steiger HJ, Markwalder TM, Reulen HJ. Clinicopathological relations of cerebral cavernous angiomas: observations in eleven cases. Neurosurgery. (1987) 21:879–84. doi: 10.1227/00006123-198712000-00016
33. Gamboa NT, Karsy M, Iyer RR, Bollo RJ, Schmidt RH. Stereotactic laser interstitial thermal therapy for brainstem cavernous malformations: two preliminary cases. Acta Neurochir (Wien). (2020) 162:1771–5. doi: 10.1007/s00701-020-04316-7
34. Nagata Y, Takeuchi K, Yamamoto T, Mizuno A, Wakabayashi T. Fully endoscopic transcylinder trans-magendie foraminal approach for fourth ventricular cavernoma: A technical case report. World Neurosurg. (2020) 142:104–7. doi: 10.1016/j.wneu.2020.06.171
35. Hu Y-J, Zhang L-F, Ding C, Tian Y, Chen J. Gamma knife radiosurgery for cavernous malformations of basal ganglia and thalamus: A retrospective study of 53 patients. Stereotact Funct Neurosurg. (2021) 99:273–80. doi: 10.1159/000510108
36. Catapano JS, Benner D, Rhodenhiser EG, Rumalla K, Graffeo CS, Srinivasan VM, et al. Safety of brainstem safe entry zones: comparison of microsurgical outcomes associated with superficial, exophytic, and deep brainstem cavernous malformations. J Neurosurg. (2023) 139:113–23. doi: 10.3171/2022.9.JNS222012
37. Del Curling O, Kelly DL, Elster AD, Craven TE. An analysis of the natural history of cavernous angiomas. J Neurosurg. (1991) 75:702–8. doi: 10.3171/jns.1991.75.5.0702
38. Moriarity JL, Wetzel M, Clatterbuck RE, Javedan S, Sheppard JM, Hoenig-Rigamonti K, et al. The natural history of cavernous malformations: a prospective study of 68 patients. Neurosurgery. (1999) 44:1166–71.
39. Raychaudhuri R, Batjer HH, Awad IA. Intracranial cavernous angioma: a practical review of clinical and biological aspects. Surg Neurol. (2005) 63:319–28. doi: 10.1016/j.surneu.2004.05.032
40. Al-Shahi Salman R, Hall JM, Horne MA, Moultrie F, Josephson CB, Bhattacharya JJ, et al. Untreated clinical course of cerebral cavernous malformations: a prospective, population-based cohort study. Lancet Neurol. (2012) 11:217–24. doi: 10.1016/S1474-4422(12)70004-2
41. Sandalcioglu IE, Wiedemayer H, Secer S, Asgari S, Stolke D. Surgical removal of brain stem cavernous malformations: surgical indications, technical considerations, and results. J Neurol Neurosurg Psychiatry. (2002) 72:351–5. doi: 10.1136/jnnp.72.3.351
42. Moultrie F, Horne MA, Josephson CB, Hall JM, Counsell CE, Bhattacharya JJ, et al. Outcome after surgical or conservative management of cerebral cavernous malformations. Neurology. (2014) 83:582–9. doi: 10.1212/WNL.0000000000000684
43. Dammers R, Delwel EJ, Krisht AF. Cavernous hemangioma of the mesencephalon: tonsillouveal transaqueductal approach. Neurosurgery. (2009) 64:296–9. doi: 10.1227/01.NEU.0000341530.36757.20
44. Tsuji Y, Kar S, Bertalanffy H. Microsurgical management of midbrain cavernous malformations: predictors of outcome and lesion classification in 72 patients. Oper Neurosurg (Hagerstown). (2019) 17:562–72. doi: 10.1093/ons/opz026
45. Huang C, Bertalanffy H, Kar S, Tsuji Y. Microsurgical management of midbrain cavernous malformations: does lesion depth influence the outcome? Acta Neurochir (Wien). (2021) 163:2739–54. doi: 10.1007/s00701-021-04915-y
46. Kivelev J, Niemelä M, Kivisaari R, Hernesniemi J. Intraventricular cerebral cavernomas: a series of 12 patients and review of the literature. J Neurosurg. (2010) 112:140–9. doi: 10.3171/2009.3.JNS081693
47. Zhang S, Li H, Liu W, Hui X, You C. Surgical treatment of hemorrhagic brainstem cavernous malformations. Neurol India. (2016) 64:1210–9. doi: 10.4103/0028-3886.193825
Keywords: cavernous malformations, midbrain, hydrocephalus, endoscopic third ventriculostomy, case report
Citation: Han Y, Liang D, Guo J, Wang Y and Wang Y (2025) Exploration of treatment strategies for cerebral cavernous malformations: two case reports on non-resection treatment and literature review. Front. Oncol. 15:1513254. doi: 10.3389/fonc.2025.1513254
Received: 18 October 2024; Accepted: 10 January 2025;
Published: 28 January 2025.
Edited by:
Shinji Kawabata, Osaka Medical and Pharmaceutical University, JapanReviewed by:
Chandrashekhar Deopujari, Bombay Hospital Institute of Medical Sciences, IndiaSaja Albanaa, University of Baghdad, Iraq
Copyright © 2025 Han, Liang, Guo, Wang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yong Wang, d2FuZ3lvbmdkbEAxMjYuY29t
†These authors have contributed equally to this work
 Yibo Han
Yibo Han Dong Liang
Dong Liang Jing Guo4
Jing Guo4 Yibao Wang
Yibao Wang Yong Wang
Yong Wang