
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol. , 13 February 2025
Sec. Genitourinary Oncology
Volume 15 - 2025 | https://doi.org/10.3389/fonc.2025.1508600
 Simran Makker1
Simran Makker1 Rayan Rammal2
Rayan Rammal2 Ping Gu3
Ping Gu3 Guido Dalbagni4
Guido Dalbagni4 Hikmat Al-Ahmadie2
Hikmat Al-Ahmadie2 Narasimhan P. Agaram2
Narasimhan P. Agaram2 Gopa Iyer3
Gopa Iyer3 Ritesh R. Kotecha3*
Ritesh R. Kotecha3*While gastrointestinal stromal tumors (GISTs) often arise within the GI tract, it is well known that GISTs may also rarely emanate outside of the digestive system. Prior case reports have documented various primary sites in non-GI organs [extra-intestinal GIST (EGIST)], yet only one report has described a localized GIST of renal origin. Here, we describe a patient who presented with bilateral renal masses who was found to have a large unresectable renal GIST tumor treated with imatinib. We discuss treatment experience and response with systemic therapy and describe molecular data to contextualize this ultra-rare presentation within the landscape of EGIST tumors.
Gastrointestinal stromal tumors (GISTs) represent the most common soft-tissue mesenchymal tumor within the GI tract. While these tumors usually arise within the stomach and small intestine, a subset of GISTs (<5%) originate in other organ sites (1), as detailed in several case reports (2–4). As most GIST tumors have identifiable driver alterations that characterize the natural history of these molecular subtypes and overall guide treatment strategy, studying EGISTs sheds light on common biology associated with tumorigenesis. To our knowledge, only one prior case report describes a patient diagnosed with a localized renal GIST who subsequently underwent nephrectomy and adjuvant imatinib therapy (5). Here, we present a patient diagnosed with bilateral renal masses found to have a large, unresectable, KIT exon 11-mutant, renal GIST tumor treated with imatinib. We discuss the clinical presentation, with emphasis on bilateral renal masses, treatment experience, and molecular findings to contextualize this rare tumor presentation in the landscape of EGIST tumors.
A 78-year-old previously healthy man presented for medical care due to worsening abdominal pain and distension. He previously had been undergoing routine health examinations and prostate serum antigen monitoring for benign prostate hypertrophy and had no previously documented family history of cancer. At initial evaluation, he underwent an abdominal ultrasound that showed a large abdominal mass. A subsequent contrast-enhanced computed tomography (CT) abdomen/pelvis demonstrated a large right partially necrotic mass (16.8 × 12.7 cm) with associated compression of the proximal ureter and a largely necrotic 4.5 × 4.1 cm mid-left renal mass. He therefore sought care at our institution, where an F-fluorodeoxyglucose (FDG) positron emission tomography (PET)/CT showed a peripherally hypermetabolic right renal mass measuring 17.6 × 12.9 cm with a maximum standardized uptake value (SUV) of 11.5 and a 5.2 × 4.8 cm mildly peripherally avid left renal mass (SUV 2.9) (Figure 1A). Laboratory data at presentation indicated normal renal function with a serum creatinine of 1.1 mg/dL and mild anemia (hemoglobin 11.0 g/dL) and a normal lactate dehydrogenase (145 U/L).
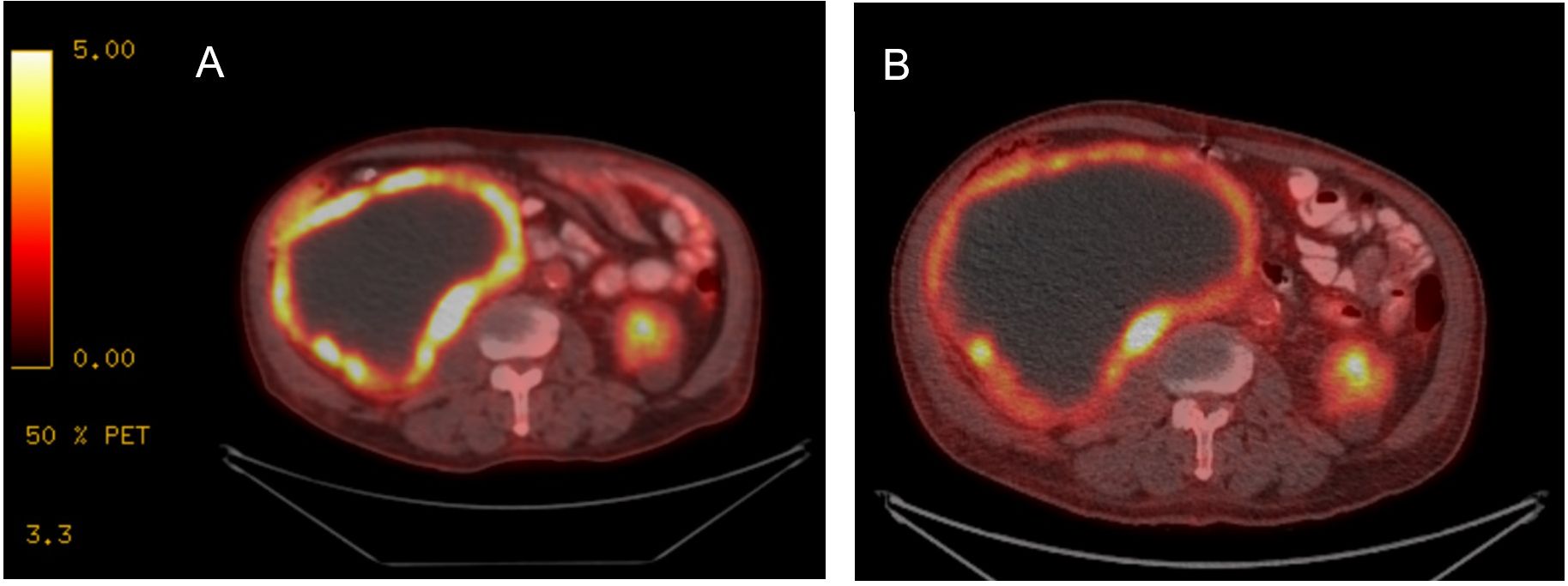
Figure 1. FDG PET/CT with treatment response to imatinib. (A) At diagnosis, FDG PET/CT imaging demonstrated a large right renal tumor with peripheral enhancement and necrosis. (B) After treatment initiation, while tumor size is comparably similar, peripheral avidity has decreased, consistent with overall treatment response.
The patient underwent a CT-guided right renal core needle biopsy, and pathology demonstrated a GIST, spindle cell type, with spindle cells arranged in fascicles in a myxoid background. Tumor necrosis was present. By immunohistochemistry (IHC), the tumor showed strong, diffuse positivity for DOG1 (ANO1) and CD117, and negativity for SMA, desmin, HMB45, Mel-A, CDK4, MDM2, S100, CKAE1/AE3, CK7, racemase, and PAX8 (Figure 2). Expression of H3K27me by IHC was retained. The mitotic activity was 12 per 10 high-power fields, and the Ki-67 index was 15%. Next-generation targeted panel sequencing by MSK-IMPACT of the tumor specimen detected a KIT exon 11 mutation (D579del) and alterations in NF1, TP53, RB1, and PTCH1, with loss of PTEN and RAC2 by copy number (Figure 3). Copy number analysis by FACETs suggested widespread loss of heterozygosity.

Figure 2. (A) Microscopic examination reveals a neoplastic proliferation of spindle cells arranged in fascicles and in a myxoid background, with mitotic figures (arrow, hematoxylin and eosin stain, 200× magnification). The tumor cells are negative for (B) PAX8 while diffusely and strongly positive for (C) CD117 and (D) DOG1 (200×).
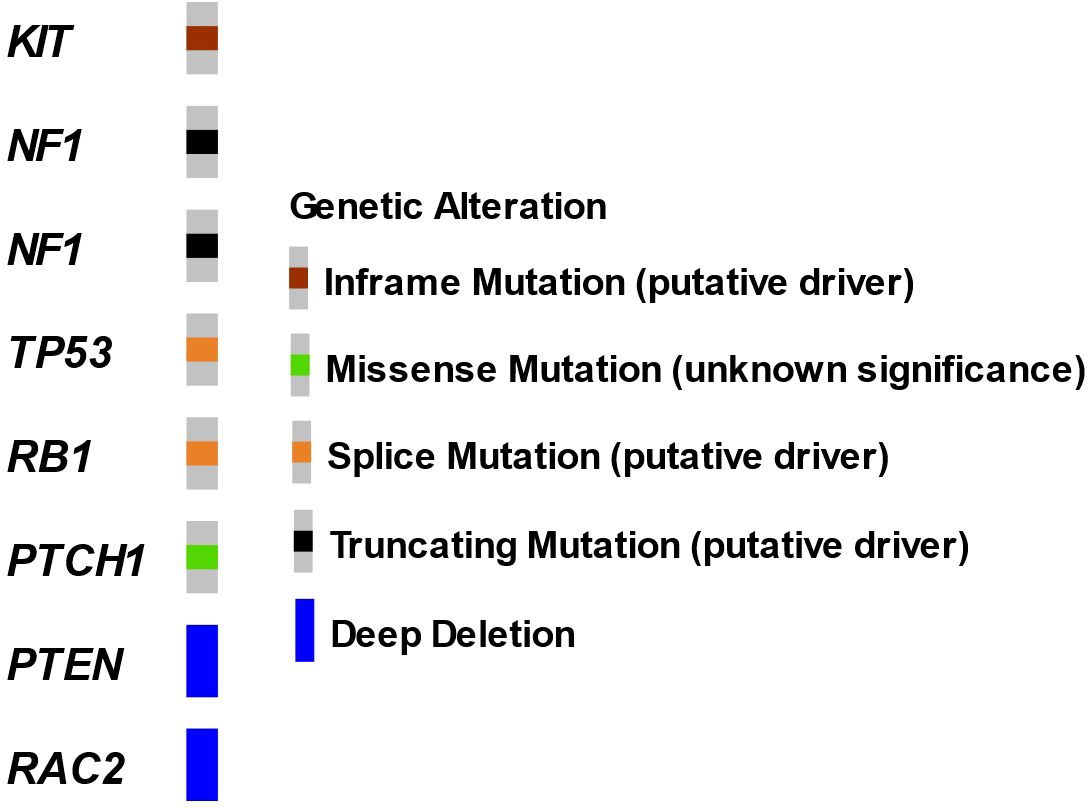
Figure 3. Oncoprint of renal GIST tumor by MSK-IMPACT. Next-generation sequencing performed on a core renal tumor biopsy specimen.
The patient underwent evaluation by urology and medical oncology, and based on the presence of bilateral renal masses and the large unresectable nature of the proven GIST tumor, the left renal tumor was not biopsied as the results would not impact initial disease management. He was initiated on systemic therapy with imatinib 400 mg orally daily with subsequent dose reduction to 400 mg every other day for tolerability. A short-interval FDG PET/CT obtained 4 weeks after treatment initiation revealed that the peripherally hypermetabolic right GIST tumor was slightly larger (measured 19.2 × 13.8 cm) but had decreased hypermetabolic activity within the posterior medial wall of the mass (SUV 6.7), consistent with treatment effect (Figure 1B). The other peripherally avid left renal mass was similar in tumor measurement and avidity. He has continued imatinib with 6 months of follow-up given ongoing clinical benefit and follows up in the outpatient oncology clinic (Figure 4).
We report a case of bilateral renal masses initially suspicious for renal cell carcinoma (RCC) and ultimately found to be an unresectable renal GIST tumor. Although the presence of bilateral renal masses poses both diagnostic and therapeutic challenges, we present this case to highlight a rare presentation of an EGIST tumor. While the retroperitoneum remains a common site for EGIST tumors, which can lead to indirect invasion of the kidney, radiographic and pathologic findings in this case pointed towards a renal origin. In a previously reported renal GIST case (5), the patient had presented with a large solitary renal tumor accompanied by hydronephrosis. He underwent nephrectomy and was treated with adjuvant imatinib. In contrast, in our case, the patient was started on upfront systemic therapy with imatinib, and short-interval post-treatment PET/CT showed clinical response. Molecular analysis identified an oncogenic KIT alteration, which may portend sensitivity to tyrosine kinase inhibitor (TKI) therapy. To our knowledge, this is the first case report highlighting imatinib therapy with data showing tumor response for renal EGIST, and the first report showing molecular features associated with a renal EGIST tumor site.
GISTs are mesenchymal tumors that arise from interstitial cells of Cajal (ICC) that exhibit functional heterogeneity across different organ sites (6). ICC-like cells have been observed in other tissues including the cardiovascular system (7), lungs (8), skin (9), mammary glands (10), liver and biliary system (11), male and female reproductive systems (12), placenta (7), prostate (13), urinary bladder, renal pelvis, and uretero-pelvic junction (7, 13, 14). ICC-like cells can modulate immune response, regulate blood flow, and play a role in angiogenesis through the production of vascular endothelial growth factor (VEGF), extracellular matrix organization, and cell migration (10, 15), and harbor electrical capacity that can influence contractile activity (16). In the urinary system, these cells may conduct and amplify the pacemaker signals generated by atypical smooth muscle cells and modulate signal transduction (17) and may vary in density and distribution by location in the urinary system and in the setting of pathology (18).
Patients with bilateral renal masses pose both diagnostic and therapeutic challenges. In the diagnostic differential, these tumors may represent either a second primary or a metastasis from the contralateral kidney. In this case, biopsy of the small left renal tumor was deferred as immediate clinical management would not be impacted and followed clinically to ascertain etiology based upon concordant or discordant response to systemic therapy. Bilateral renal tumors are seen in up to 5% of patients with RCC, and in these situations, tumor resection is often limited by the need to preserve renal function postoperatively. In the context of bilateral tumors, hereditary syndromes, including von Hippel Lindau, are usually invoked, prompting germline testing (19). In this individual, germline testing did not detect a known pathogenic alteration with increased hereditary risk. Interestingly, Carney-Stratakis syndrome, characterized by germline mutations in SDHB, SDHC, or SDHD, is a hereditary syndrome associated with GIST and paraganglioma, and may also be associated with SDH-mutant RCC. Therefore, when evaluating patients with the potential for both entities, it is important to consider if there remains a disease overlap with rare presentations.
The presence of KIT or PDGFRA gain-of-function alterations are highly specific for GIST tumors, found in up to 85% of these tumors, and these alterations also guide treatment decision-making (20–22), with KIT mutations comprising 75%–80% of cases (23–25). In the remaining 15% of GISTs, alterations in a variety of genes have been reported, including NF1, SDH, BRAF, KRAS, PIK3CA, and ETV6::NTRK3 gene fusion (26–31). Notably, this patient was found to have a co-mutation in NF1 and deletion of PTEN. GISTs harboring KIT mutations, particularly those with exon 11 mutations, are associated with improved response to imatinib therapy. While prior reports have detailed that E-GIST tumors harbor similar molecular profiles to GIST tumors, TP53 alterations, as seen in our patient, appear less commonly in conventional GIST and are usually found in more aggressive, advanced stage tumors. Outside of genomic features, elevated Ki67 (as seen in our case) also suggests higher risk disease and appears to be more typical in EGIST tumor sites.
FDG PET/CT is an essential tool for staging and early treatment response evaluation in GIST tumors. GISTs typically exhibit strong FDG uptake; moreover, the sensitivity and positive predictive value of PET/CT are 86% and 98%, respectively, with false-negative results mainly attributed to small lesions that are below PET scan resolution (32). PET/CT is also a useful imaging modality to assess early response to imatinib treatment as tumor size alone is unreliable for assessing response to TKI therapy treatment. GIST tumors may not change significantly in size and may even grow while responding to imatinib administration (33), and cystic and density changes can precede tumor shrinkage. One study demonstrated that the overall GIST disease burden evaluated according to changes in size, density, and number of tumor nodules and intralesional vasculature correlated best with the reduction of maximum SUV on FDG PET/CT scans (34). This was apparent initially during this case with reduction in avidity occurring soon after treatment initiation.
In sum, we present a case of bilateral FDG avid renal masses with subsequent identification of a renal GIST tumor. Treatment with imatinib yielded early and durable systemic therapeutic benefit, and molecular analysis revealed a KIT exon 11 mutation that portends favorable sensitivity to imatinib systemic therapy. This case highlights that renal GIST tumors have similar molecular profiles to conventional GIST tumors and systemic therapy approaches including precision medical therapies can similarly impact treatment response in this rare subtype.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
SM: Writing – original draft, Writing – review & editing, Data curation. RR: Data curation, Writing – review & editing. PG: Writing – review & editing. GD: Writing – review & editing. HA-A: Supervision, Writing – review & editing. NA: Supervision, Writing – review & editing. GI: Supervision, Writing – review & editing. RK: Conceptualization, Supervision, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work, in part, was supported by the NIH/NCI Cancer Center Support Grant (P30 CA008748). RK is supported, in part, by a Department of Defense Early Career Investigator grant (KCRP-AKCI, W81XWH-21-1-0942).
RK reports consulting or advisory role for Eisai and Guidepoint and institutional research funding from Pfizer, Takeda, Novartis, Allogene Therapeutics, Exelixis, Xencor, and ArsenalBio. GI reports consulting or advisory role for Mirati Therapeutics, Janssen, Bayer, Basilea, Flare Therapeutics, and LOXO and Lilly; speaker’s fees from Gilead Sciences and the Lynx Group, and institutional research funding from Mirati Therapeutics, Inc., Novartis, Debiopharm Group, Bayer, Janssen, Flare Therapeutics, Seagen, Aadi Bioscience, and LOXO at Lilly.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Søreide K, Sandvik OM, Søreide JA, Giljaca V, Jureckova A, Bulusu VR. Global epidemiology of gastrointestinal stromal tumours (GIST): A systematic review of population-based cohort studies. Cancer Epidemiol. (2016) 40:39–46. doi: 10.1016/j.canep.2015.10.031
2. Yi JH, Park BB, Kang JH, Hwang IG, Shin DB, Sym SJ, et al. Retrospective analysis of extra-gastrointestinal stromal tumors. World J Gastroenterol. (2015) 21:1845–50. doi: 10.3748/wjg.v21.i6.1845
3. El Charif MH, Amro S, Boulos F, Khalife M, Shamseddine A, Assi H, et al. Extra-gastrointestinal stromal tumors (EGISTs): A case report for a mischief entity. Med (Baltimore). (2023) 102:e33394. doi: 10.1097/MD.0000000000033394
4. Hatipoğlu E. Extragastrointestinal stromal tumor (EGIST): A 16-year experience of 13 cases diagnosed at a single center. Med Sci Monit. (2018) 24:3301–6. doi: 10.12659/MSM.907654
5. Karlidag I, Ozbay E, Colakerol A, Dizibuyuk OF, Yenigurbuz S, Ozyuvali E. A rare case of renal gastrointestinal stromal tumor. Urol Case Rep. (2019) 24:100881. doi: 10.1016/j.eucr.2019.100881
6. Sweet T, Abraham CM, Rich A. Origin and development of interstitial cells of cajal. Int J Dev Biol. (2024) 68:93–102. doi: 10.1387/ijdb.240057ar
7. Wang Q, Huang ZP, Zhu Y, Fu F, Tian L. Contribution of interstitial cells of cajal to gastrointestinal stromal tumor risk. Med Sci Monit. (2021) 27:e929575. doi: 10.12659/MSM.929575
8. Liu J, Cao Y, Song Y, Huang Q, Wang F, Yang W, et al. Telocytes in liver. Curr Stem Cell Res Ther. (2016) 11:415–9. doi: 10.2174/1574888x10666150630112035
9. Rusu MC, Mirancea N, Mănoiu VS, Vâlcu M, Nicolescu MI, Păduraru D. Skin telocytes. Ann Anat. (2012) 194:359–67. doi: 10.1016/j.aanat.2011.11.007
10. Klein M, Csöbönyeiová M, Danišovič Ľ, Lapides L, Varga I. Telocytes in the female reproductive system: up-to-date knowledge, challenges and possible clinical applications. Life (Basel). (2022) 12:267. doi: 10.3390/life12020267
11. Chen L, Yu B. Telocytes and interstitial cells of Cajal in the biliary system. J Cell Mol Med. (2018) 22:3323–9. doi: 10.1111/jcmm.13643
12. Wang JP, Ding GF, Wang QZ. Interstitial cells of Cajal mediate excitatory sympathetic neurotransmission in Guinea pig prostate. Cell Tissue Res. (2013) 352:479–86. doi: 10.1007/s00441-013-1572-3
13. Balikci O, Turunç T, Bal N, Çelik H, Özkardeş H. Comparison of Cajal-like cells in pelvis and proximal ureter of kidney with and without hydronephrosis. Int Braz J Urol. (2015) 41:1178–84. doi: 10.1590/S1677-5538.IBJU.2014.0427
14. Wishahi M, Mehena AA, Elganzoury H, Badawy MH, Hafiz E, El-Leithy T. Telocyte and Cajal cell distribution in renal pelvis, ureteropelvic junction (UPJ), and proximal ureter in normal upper urinary tract and UPJ obstruction: reappraisal of the aetiology of UPJ obstruction. Folia Morphol (Warsz). (2021) 80:850–6. doi: 10.5603/FM.a2020.0119
15. López-Pingarrón L, Almeida H, Pereboom-Maicas D, García JJ. Pathophysiological implications of interstitial cajal-like cells (ICC-like) in uterus: A comparative study with gastrointestinal ICCs. Curr Issues Mol Biol. (2023) 45:7557–71. doi: 10.3390/cimb45090476
16. Aleksandrovych V, Walocha JA, Gil K. Telocytes in female reproductive system (human and animal). J Cell Mol Med. (2016) 20:994–1000. doi: 10.1111/jcmm.12843
17. McHale NG, Hollywood MA, Sergeant GP, Shafei M, Thornbury KT, Ward SM. Organization and function of ICC in the urinary tract. J Physiol. (2006) 576:689–94. doi: 10.1113/jphysiol.2006.116657
18. Samaranayake UMJE, Mathangasinghe Y, Liyanage UA, de Silva MVC, Samarasinghe MC, Abeygunasekera S, et al. Variations in the density and distribution of cajal like cells associated with the pathogenesis of ureteropelvic junction obstruction: A systematic review and meta-analysis. Front Surg. (2021) 8:721143. doi: 10.3389/fsurg.2021.721143
19. von Mehren M, Kane JM, Riedel RF, Sicklick JK, Pollack SM, Agulnik M, et al. NCCN guidelines® Insights: gastrointestinal stromal tumors, version 2. 2022. J Natl Compr Canc Netw. (2022) 20:1204–14. doi: 10.6004/jnccn.2022.0058
20. Fülöp E, Marcu S, Milutin D, Borda A. Gastrointestinal stromal tumors: review on morphology, diagnosis and management. Rom J Morphol Embryol. (2009) 50:319–26.
21. Heinrich MC, Corless CL, Duensing A, McGreevey L, Chen CJ, Joseph N, et al. PDGFRA activating mutations in gastrointestinal stromal tumors. Science. (2003) 299:708–10. doi: 10.1126/science.1079666
22. Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. (1998) 279:577–80. doi: 10.1126/science.279.5350.577
23. Huss S, Künstlinger H, Wardelmann E, Kleine MA, Binot E, Merkelbach-Bruse S, et al. A subset of gastrointestinal stromal tumors previously regarded as wild-type tumors carries somatic activating mutations in KIT exon 8 (p. D419del). Mod Pathol. (2013) 26:1004–12. doi: 10.1038/modpathol.2013.47
24. Pasini B, McWhinney SR, Bei T, Matyakhina L, Stergiopoulos S, Muchow M, et al. Clinical and molecular genetics of patients with the Carney-Stratakis syndrome and germline mutations of the genes coding for the succinate dehydrogenase subunits SDHB, SDHC, and SDHD. Eur J Hum Genet. (2008) 16:79–88. doi: 10.1038/sj.ejhg.5201904
25. Sbaraglia M, Businello G, Bellan E, Fassan M, Dei Tos AP. Mesenchymal tumours of the gastrointestinal tract. Pathologica. (2021) 113:230–51. doi: 10.32074/1591-951X-309
26. Agaram NP, Wong GC, Guo T, Maki RG, Singer S, Dematteo RP, et al. Novel V600E BRAF mutations in imatinib-naive and imatinib-resistant gastrointestinal stromal tumors. Genes Chromosomes Cancer. (2008) 47:853–9. doi: 10.1002/gcc.20589
27. Brenca M, Rossi S, Polano M, Gasparotto D, Zanatta L, Racanelli D, et al. Transcriptome sequencing identifies ETV6-NTRK3 as a gene fusion involved in GIST. J Pathol. (2016) 238:543–9. doi: 10.1002/path.4677
28. Hostein I, Faur N, Primois C, Boury F, Denard J, Emile JF, et al. BRAF mutation status in gastrointestinal stromal tumors. Am J Clin Pathol. (2010) 133:141–8. doi: 10.1309/AJCPPCKGA2QGBJ1R
29. Lasota J, Felisiak-Golabek A, Wasag B, Kowalik A, Zięba S, Chłopek M, et al. Frequency and clinicopathologic profile of PIK3CA mutant GISTs: molecular genetic study of 529 cases. Mod Pathol. (2016) 29:275–82. doi: 10.1038/modpathol.2015.160
30. Miettinen M, Fetsch JF, Sobin LH, Lasota J. Gastrointestinal stromal tumors in patients with neurofibromatosis 1: a clinicopathologic and molecular genetic study of 45 cases. Am J Surg Pathol. (2006) 30:90–6. doi: 10.1097/01.pas.0000176433.81079.bd
31. Miranda C, Nucifora M, Molinari F, Conca E, Anania MC, Bordoni A, et al. KRAS and BRAF mutations predict primary resistance to imatinib in gastrointestinal stromal tumors. Clin Cancer Res. (2012) 18:1769–76. doi: 10.1158/1078-0432.CCR-11-2230
32. Gayed I, Vu T, Iyer R, Johnson M, Macapinlac H, Swanston N, et al. The role of 18F-FDG PET in staging and early prediction of response to therapy of recurrent gastrointestinal stromal tumors. J Nucl Med. (2004) 45:17–21.
33. Chen MY, Bechtold RE, Savage PD. Cystic changes in hepatic metastases from gastrointestinal stromal tumors (GISTs) treated with Gleevec (imatinib mesylate). AJR Am J Roentgenol. (2002) 179:1059–62. doi: 10.2214/ajr.179.4.1791059
34. Choi H, Charnsangavej C, Macapinlac HA, Burgess MA, Patel SR, Chen LL, et al. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J Clin Oncol. (2007) 25:1753–9. doi: 10.1200/JCO.2006.07.3049
Keywords: gastrointestinal stromal tumor, GIST, renal mass, imatinib, KIT
Citation: Makker S, Rammal R, Gu P, Dalbagni G, Al-Ahmadie H, Agaram NP, Iyer G and Kotecha RR (2025) Case report: Clinical and molecular features of renal gastrointestinal tumor. Front. Oncol. 15:1508600. doi: 10.3389/fonc.2025.1508600
Received: 09 October 2024; Accepted: 17 January 2025;
Published: 13 February 2025.
Edited by:
Sanja Stifter-Vretenar, Skejby Sygehus, DenmarkReviewed by:
Fabio Grizzi, Humanitas Research Hospital, ItalyCopyright © 2025 Makker, Rammal, Gu, Dalbagni, Al-Ahmadie, Agaram, Iyer and Kotecha. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ritesh R. Kotecha, a290ZWNoYXJAbXNrY2Mub3Jn
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.