- 1Laboratory of Molecular Translational Medicine, Center for Translational Medicine, Key Laboratory of Birth Defects and Related Diseases of Women and Children (Sichuan University), Ministry of Education, West China Second University Hospital, Sichuan University, Chengdu, Sichuan, China
- 2Department of Pathology, West China Second University Hospital, Sichuan University, Chengdu, Sichuan, China
Background: Numerous researches have investigated the correlation between single nucleotide polymorphisms (SNPs) in the transcription factor forkhead box protein 3 (Foxp3) gene and the development of various cancers. However, the relationship of Foxp3 polymorphism and bladder cancer (BC) remain unclear.
Method: This hospital-based case-control study enrolled a total of 316 patients diagnosed with BC and 643 healthy controls. Two Foxp3 SNPs (rs3761548 C/A, rs5902434 del/ATT) were selected, and genotyping of the samples was performed using the polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) technique. SPSS and online SNPstats software were used to determine the disparities between groups.
Results: For the rs3761548 C/A polymorphism, patients with the CA/AA genotype showed a notable decrease in the case group (22.1% versus 34.8%, P = 0.003, OR = 0.61, 95%CI = 0.44-0.85), and the heterozygous CA genotype presented a distinctly lower risk for BC (P = 0.0003, OR = 0.43, 95%CI = 0.26-0.70). Notably, individuals who were homozygous for the AA genotype demonstrated a markedly lower overall survival (OS) rate compared to those with the CC/CA genotypes (P = 0.03, OR = 5.89, 95%CI = 1.23-28.15), after adjusting for factors such as age, gender, smoking status, tumor grade, metastasis, and clinical stage. For the rs5902434 del/ATT polymorphism, a decreased risk was observed across the codominant and over-dominant models with statistical significance (codominant model: P = 0.01, OR = 0.61, 95%CI = 0.42-0.89; over-dominant model: P = 0.004, OR = 0.60, 95%CI = 0.42-0.85), and no significant association was observed between the rs5902434 polymorphism and patient’s OS rate.
Conclusions: Our findings indicate that Foxp3 polymorphisms may be associated with BC susceptibility, and that rs3761548 could potentially serve as an independent risk factor for the OS rate.
1 Introduction
Bladder cancer (BC) ranks the tenth most commonly diagnosed cancer worldwide as reported by the International Agency for Research on Cancer (IARC), with 573,278 new cases as well as 212,536 deaths in 2020 (1). Notably, China has the highest incidence and mortality rates for BC in Asia among 2020, with 85694 new cases and 39393 deaths (2). Its epidemiological distributions varies among sex, area and age in China. The incidence and mortality rates of BC are disproportionately higher in men, approximately four times that of women, which in line with the global pattern (1). The urban patients are 1.4 times as high as that in rural areas, and the increased incidence through the country is partly owing to the aging after 45 years old and the abusing of cigarette in recent years (3). Consequently, evaluating the accurate and cost-effective screening biomarkers and controlling the tobacco use have become breakthroughs in solving the problem. Non-muscle-invasive bladder cancer (NMIBC) constitutes approximately 75% of all BCs and typically has a more favorable prognosis. In contrast, muscle-invasive bladder cancer (MIBC) presents a more lethal phenotype, with a 5-year survival rate of approximately 50%, even with aggressive treatment strategies (4, 5). Encouragingly, advancements in treatment modalities, including endoscopic resection, adjuvant chemotherapy instillation, and intravesical immunotherapy, have led to a significant reduction in BC mortality rates (6).
Tobacco smoking is recognized as one of the most significant carcinogens associated with BC, although Schistosoma haematobium infection and other risk factors may also play a substantial role in certain populations (7, 8). Recent genome-wide association studies have explored the interactions between smoking and single nucleotide polymorphisms (SNPs) in BC patients, yet no conclusive link has been established (9). Genetic factors are increasingly acknowledged as critical elements in the pathogenesis of BC. Numerous family-based studies have indicated that individuals with a family history of BC are approximately twice as likely to develop the disease, underscoring the familial aggregation of BC and its significant genetic component (10–13). Furthermore, the unchecked proliferation of tumor cells is closely associated with the tumor’s ability to evade immune surveillance, a process that involves the participation of regulatory immune cell populations (14, 15).
Regulatory T (Treg) cells are pivotal for the mechanisms of tumor immune based on their immunosuppressive functions. They may contribute to the failure of tumor immunotherapy (16, 17). Specifically, CD4+ Treg cells express the transcription factor gene known as forkhead box P3 (Foxp3), located on the X chromosome at Xp11.23, and regulates T cell activation and function via downregulating the cytokine production (18, 19). The polymorphic variants within the Foxp3 gene induce autoimmune diseases, potentially through decreasing the number of functional CD4+CD25+ Tregs (20). These genetic variations may also influence tumor progression by regulating the tumor microenvironment. Numerous researches have investigated the correlation between SNPs in the Foxp3 gene and the development of various cancers (21).
Tregs are known to regulate the proliferation and activation of immune cells in the tumor microenvironment, which can affect the patient survival rates. The decrease in Treg cell function leads to a disruption in the immune homeostasis (20). However, increasing evidence suggests that Tregs have paradoxical prognostic effects on BC, which has partly been attributed to the misidentification of specific biomarkers, and the inflammatory profile of the tumor (22). Immunohistochemical studies have demonstrated an increase in the number of ghrelin-induced Foxp3+ Treg cells within BC tissues, which suppress the activity of immune system against BC (23). In contrast, a robust correlation between reduced frequency of Treg cells and an unfavorable prognosis in post-surgical BC patients has been identified by Jóźwicki et al. (24). These opposite findings indicate that the specific role of Foxp3+ Treg cells in the development of BC remains to be fully elucidated.
We consulted the NCBI database and found that the rs3761548 polymorphism in the Foxp3 gene has been linked to malignancies such as colorectal cancer, gastric adenocarcinoma, and endometrial cancer (21). And the rs5902434 polymorphism has been proven to be associated with the onset of various diseases such as unexplained recurrent spontaneous abortion, allogenic hematopoietic stem cell transplantation, chronic obstructive pulmonary disease (25–27). Besides, both of the two loci are located in the promoter region of the Foxp3 gene, which might play a potential role in the regulation of Foxp3 expression. Consequently, the present study aimed to explore whether the two specific polymorphic variants (rs3761548 and rs5902434) contributed to the progression of BC within the Chinese Han population.
2 Materials and methods
2.1 Subjects and characteristics
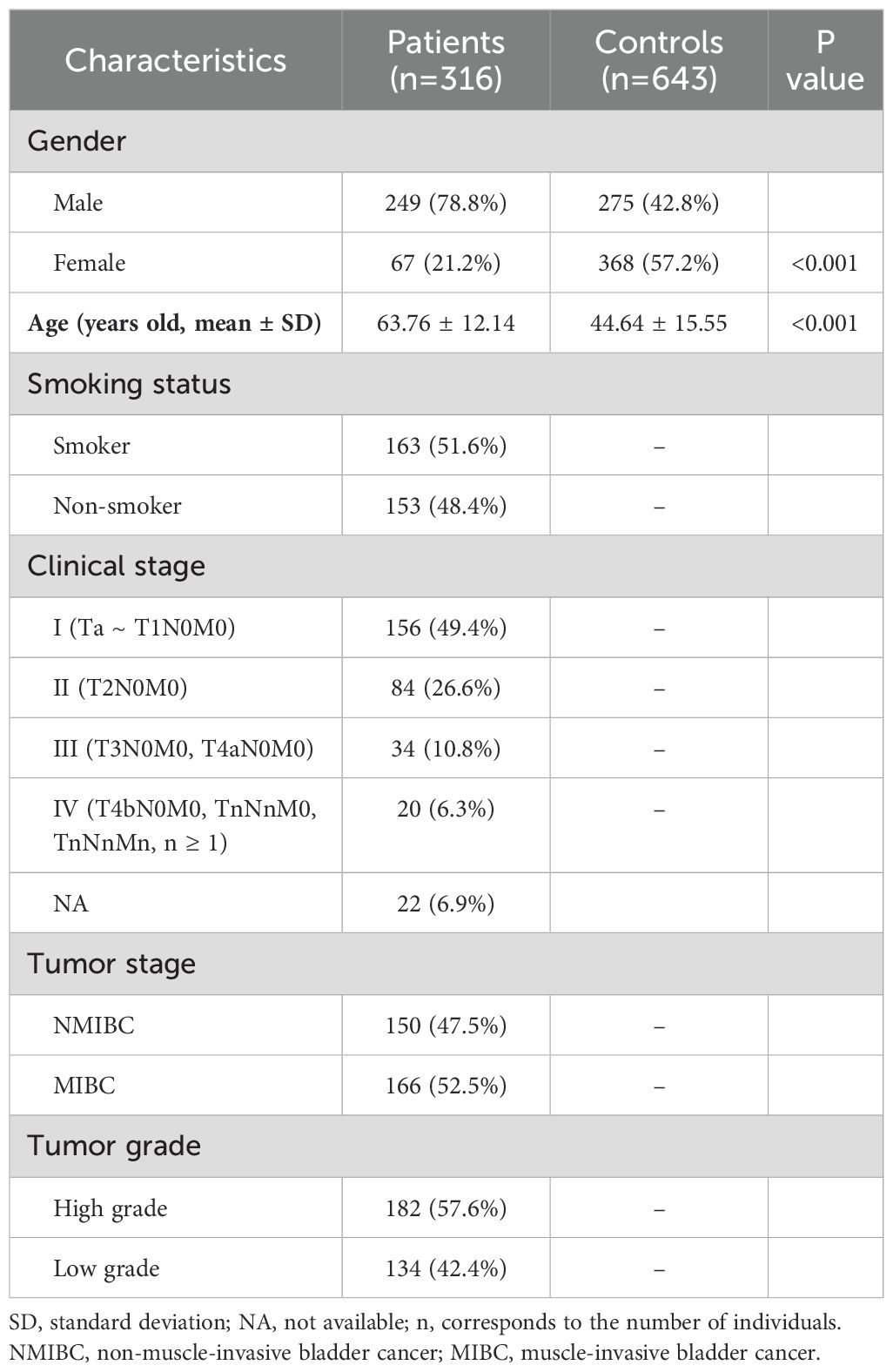
This case-control study enrolled a total of 316 patients diagnosed with BC, with a mean age of 63.76 ± 12.14 years, as well as 643 healthy controls with a mean age of 44.64 ± 15.55 years, from the West China Hospital of Sichuan University between 2001 and 2012. The study protocol was approved by the hospital’s ethics committee, and all participants provided informed consent. Patients with a prior history of cancer, autoimmune or infectious diseases, or those who had undergone radiotherapy or chemotherapy were excluded, as these conditions could potentially confound the study results. Healthy controls were recruited from the hospital’s department of physical examination, and selected based on the absence of genetic relationships between participants. Individuals with a personal or family history of BC or other severe illnesses were also excluded from the control group. A follow-up protocol was established, consisting of telephone calls every six months for a duration of five years. The histopathological analysis was performed to confirm the presence of tumor tissues in resected specimens obtained from the patients. Clinical characteristic data were extracted from medical records and are detailed in Table 1.
2.2 Gene selection and genotyping
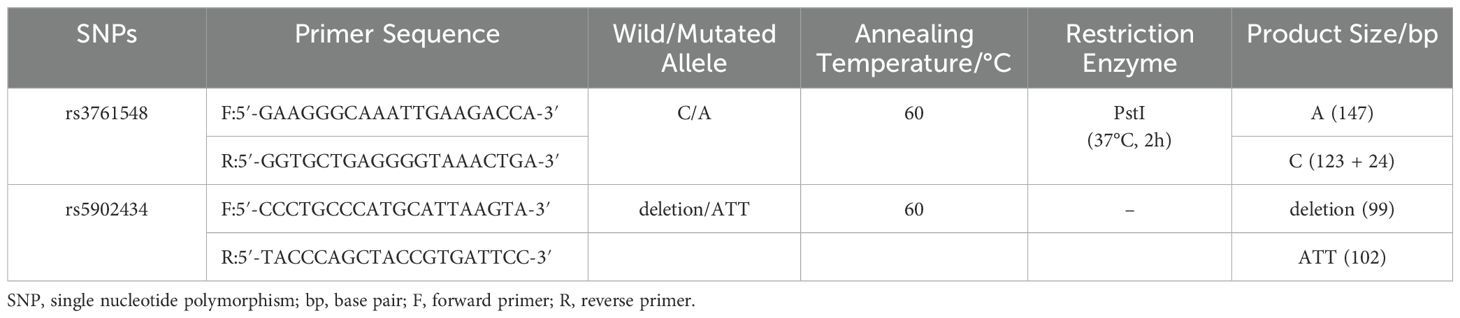
Two SNPs (rs3761548 C/A, rs5902434 del/ATT) were identified from CHB population sample data of the HapMap Project through the SNPinfo software (28). Primers for polymerase chain reaction (PCR) were designed using Primer 3 web version 4.1.0. (http://primer3.ut.ee/) (29).
Genomic DNA was extracted from a 200 μL sample of EDTA-anticoagulated peripheral blood taken from each participant, using a DNA isolation kit (BioTeke, China). The genotyping of the samples were performed using the polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) technique. Amplification of genomic DNA fragments was conducted in a total volume of 10 μL, including 5 μL of 2x Power Taq PCR Master Mix (BioTeke, China), 4 picomoles of each primer, and 100 ng of genomic DNA. The specific primer sequences and the PCR conditions are detailed in Table 2. Subsequently, the PCR products for rs3761548 were digested using the restriction enzyme, as indicated in Table 2. In contrast, the rs5902434 fragments were directly separated by electrophoresis on a 15% polyacrylamide gel, and then the gels for both polymorphisms were stained using a 1.5 g/L silver nitrate solution (Figure 1). Finally, the genotypes were verified by DNA sequencing analysis. Approximately 10% of the samples were randomly chosen for repeat assays, which confirmed the initial results with 100% concordance.
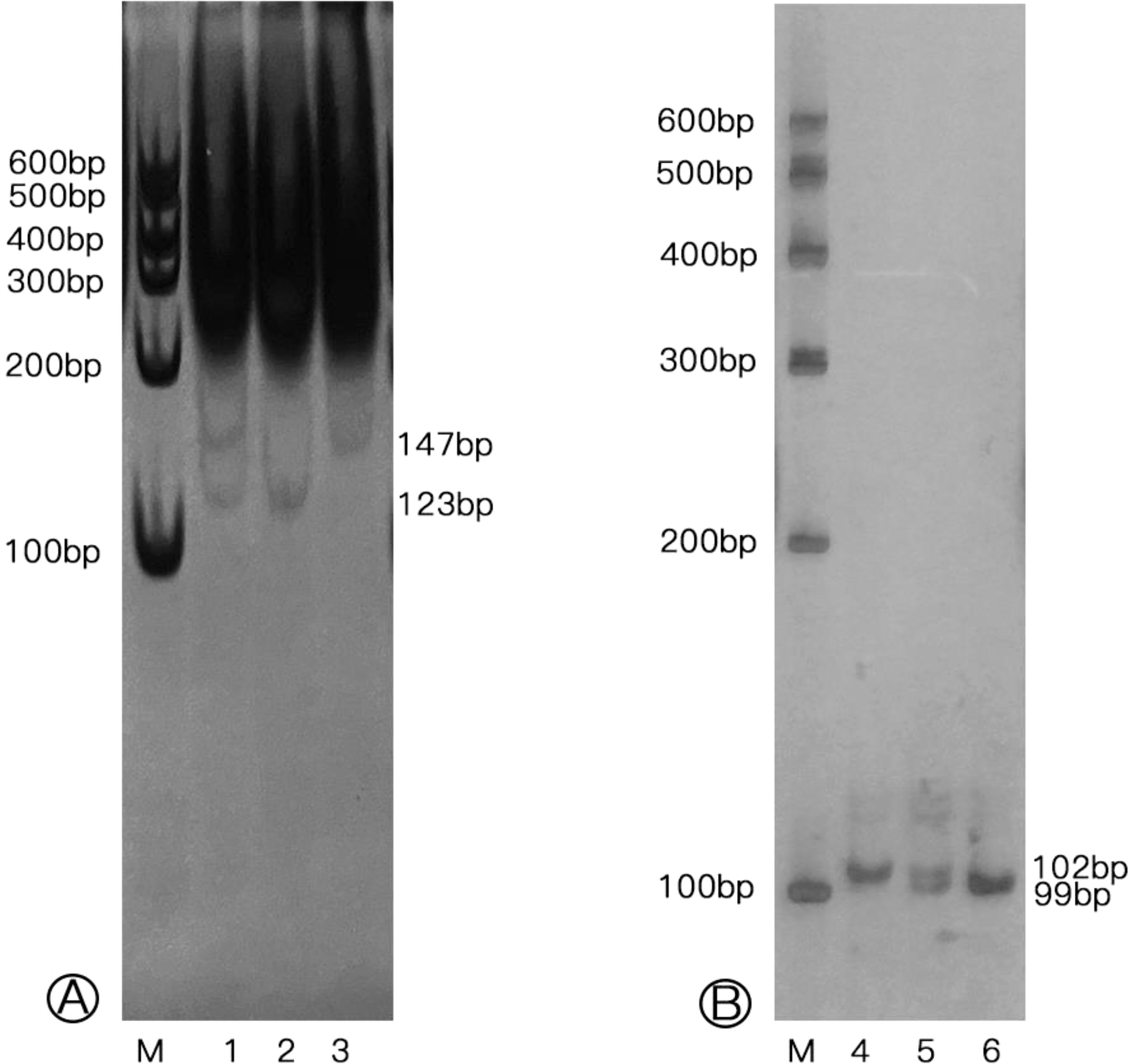
Figure 1. Genotypes of Foxp3 rs3761548 and rs5902434 polymorphisms in individuals. (A) Genotypes of rs3761548 polymorphism (M: Marker; Lane 1: CA genotype; Lane 2: CC genotype; Lane 3: AA genotype); (B) Genotypes of rs5902434 polymorphism (M: Marker; Lane 4: ATT/ATT genotype; Lane 5: -/ATT genotype; Lane 6: -/- genotype).
2.3 Statistical analysis
Data analysis was conducted using SPSS software, version 20.0 (SPSS Inc., Chicago, IL, USA). The online SNPstats software (www.snpstats.net/start.htm) was used to determine the disparities in allele and genotypic distributions between the case and control groups. It was achieved by directly counting genotype frequencies across various genetic models, including codominant, dominant, recessive, and over-dominant models (30). The Hardy-Weinberg equilibrium was assessed using a chi-squared test. Odds ratios (OR) and 95% confidence intervals (CI) were calculated to detect the correlation between specific genotypes and clinical characteristics. Univariate survival analysis was performed using Kaplan-Meier plots and the log-rank test, while multivariate survival analysis was conducted using the Cox proportional hazards regression model. P < 0.05 was set as the statistical significance threshold.
3 Results
3.1 Foxp3 genetic distribution and BC susceptibility
The allele and genotype distributions for the two SNPs adhered to the assumption of Hardy-Weinberg equilibrium (P > 0.05) and are presented in Table 3 after adjusted by gender. In the case of rs3761548 C/A polymorphism, the frequency of A allele within BC patients was decreased compared to that in controls with statistical significance (18.5% versus 24.4%, P = 0.01, OR = 0.72, 95%CI = 0.56-0.93), and it was in line with the distribution of CA/AA genotypes in the dominant model (22.1% versus 34.8%, P = 0.003, OR = 0.61, 95%CI = 0.44-0.85), which indicated a significantly lower BC risk of CA/AA genotypes carriers. The heterozygous CA genotype of rs3761548 presented a distinctly decreased risk for BC in the codominant model (P = 0.001, OR = 0.42, 95%CI = 0.25-0.68) and the over-dominant model (P = 0.0003, OR = 0.43, 95%CI = 0.26-0.70) when in contrast with the homozygous CC/AA genotypes.
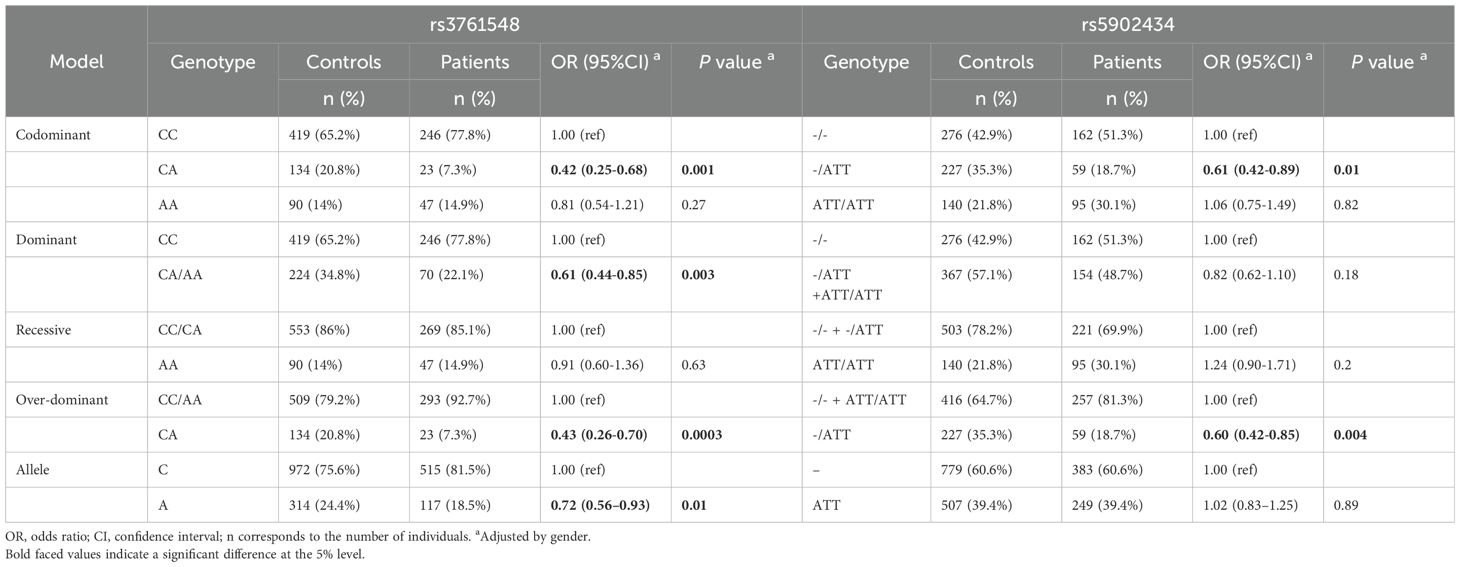
Table 3. The association between bladder cancer risk and the distribution of SNPs in FOXP3 among patients and controls.
For the rs5902434 del/ATT polymorphism, a decreased susceptibility to BC was observed across the codominant and over-dominant models with statistical significance (codominant model: P = 0.01, OR = 0.61, 95%CI = 0.42-0.89; over-dominant model: P = 0.004, OR = 0.60, 95%CI = 0.42-0.85).
3.2 Foxp3 SNPs and subgroup analyses
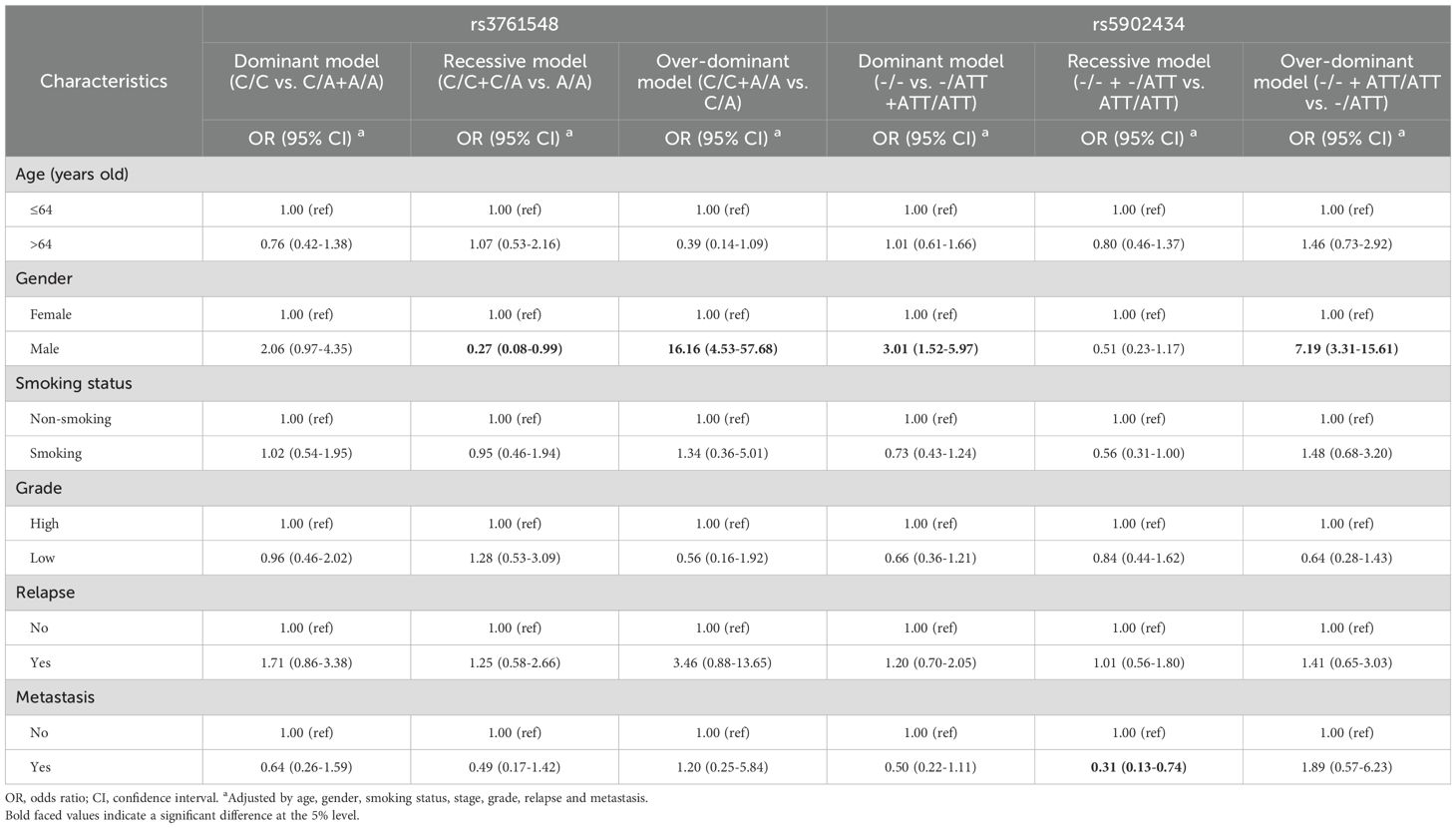
Stratified analyses were conducted to examine the distribution of Foxp3 genotypes among BC patients relative to various demographic and clinical characteristic groups, including age (≤64 years and >64 years), gender (female and male), smoking status, tumor grade (high and low), as well as the presence of relapse and metastasis. The results are presented in Table 4. No significant differences were observed across most subgroups for the two SNPs, with the exception of gender and metastasis status. Regarding the rs3761548 polymorphism, male patients with the CA genotype demonstrated a higher risk of BC in contrast with females, with an adjusted OR of 16.16 (95%CI = 4.53-57.68) after adjusting for variables such as age, smoking status, tumor stage, grade, relapse, and metastasis. A similar pattern was observed for the del/ATT heterozygotes in the rs5902434 polymorphism, where the OR was 7.19 (95%CI = 3.31-15.61). Notably, considering the location of Foxp3 gene (located on X chromosome) and the shortage of female patient number, the results of gender analysis should be approached dialectically, because this might present a gender preference and lead a result with bias. Individuals homozygous for the ATT allele in rs5902434 (ATT/ATT) displayed a reduced risk of metastasis when compared to those with del/del and del/ATT genotypes, with an OR of 0.31 (95%CI = 0.13-0.74).
3.3 Foxp3 genotypes and survival analyses
In the current study, a total of 316 BC patients were enrolled in a follow-up plan that included telephone calls every 6 months for a period of 5 years. By the end of the follow-up period, 51 patients (16.1%, NMIBC: 13; MIBC: 38) were dead. To assess overall survival (OS), Kaplan-Meier analysis and Cox regression analysis was conducted, revealing no significant correlation between the OS rate of BC patients and the two SNPs (P > 0.05).
It is well recognized that the prognosis of NMIBC and MIBC is significantly different following treatment. To delve deeper into the outcomes of BC patients, this study conducted a Cox multivariate survival analysis to investigate the outcomes of NMIBC and MIBC patients. The findings are presented in Table 5. According to the Cox multivariate survival analysis, individuals who were homozygous for the AA genotype at rs3761548 demonstrated a markedly lower OS rate compared to those with the CC/CA genotypes (P = 0.03, HR = 5.89, 95%CI = 1.23-28.15), after adjusting for factors such as age, gender, smoking status, tumor grade, metastasis, and clinical stage. No significant association was observed with the rs5902434 polymorphism.
4 Discussion
Foxp3 is considered a hallmark molecule of Tregs, functioning as a transcriptional regulator that controls the activity of Treg by directly regulating the expression of multiple genes (19). Mutations in the Foxp3 gene could potentially lead to a decrease in Treg cell function as well as the secretion of inhibitory cytokines, leading to a disruption in the immune homeostasis and the development of serious autoimmune diseases (20). In recent years, there has been a growing focus on the regulation and influence of Foxp3 on disease pathophysiology. Genetic polymorphisms with the Foxp3 gene may functionally or quantitatively alter the protein, thereby influencing the risk of developing certain disease.
Previous studies have explored the interaction between cancer risk and the polymorphism of Foxp3 gene. In 2013, He et al. demonstrated that the A allele of Foxp3 rs3761548 increased the risk of non-small cell lung cancer (31). Subsequently, in a study by Chen et al., the Foxp3 gene polymorphism at rs3761548 was found to be a contributing factor to the high susceptibility to colorectal cancer within the Chinese population (32). In 2019, Nazanin et al. also found that the AA genotype and A allele of Foxp3 rs3761548 were linked to a higher risk of prostate cancer incidence (33).
In contrast, a study on endometrial cancer has reported a positive effect of Foxp3 polymorphisms, where the CA heterozygotes of rs3761548 were found to have a protective role, and the ATT/ATT genotype of rs5902434 was associated with a reduced risk of endometrial cancer (34). The findings above suggest that the influence of Foxp3 polymorphisms on cancer susceptibility may vary significantly between different types of cancer. To the best of our knowledge, the current study is the first investigation into the association between Foxp3 gene polymorphisms and both the susceptibility to and prognosis of BC. Our results revealed that heterozygotes for both rs3761548 and rs5902434 polymorphisms are linked to a reduced risk of BC susceptibility, which was in line with the aforementioned study even the gender preference existed (males occupying 80% of the incidence). Additionally, we observed that AA homozygotes for the rs3761548 polymorphism had a notably lower OS rate among BC patients which was coincidence with the results of non-small cell lung cancer, colorectal cancer, and prostate cancer. The paradoxical prognostic effects of Foxp3 gene polymorphisms on tumors were in line with the role of Tregs on BC.
Similar self-contradictory phenomenon has been observed in research concerning the relationship between Foxp3 expression and BC prognosis. Horn et al. pointed out that an increased ratio of Foxp3/CD3 was associated with slightly shorter OS in BC patients (35). In contrast, Winerdal et al. found that while Foxp3 expression in BC cells correlated with decreased long-term survival, a higher infiltration of Foxp3 + tumor-infiltrating lymphocytes (TILs) was correlated with better survival outcomes, highlighting the complex role of TILs in cancer progression (36). Furthermore, in 2018, the same researchers suggested that Treg-mediated suppression of matrix metalloproteinase 2 within the MIBC tumor microenvironment may be a potential mechanism underlying the positive prognostic impact of tumor-infiltrating Tregs (22).
Within the tumor microenvironment, Tregs are known to enhance the proliferation and activation of immune cells, which can lead to improved patient survival rates. It suggests that cellular interactions within the tumor microenvironment may exert a greater impact on patient survival than the intrinsic subtypes of the tumor itself (37). In the present study, we demonstrated that mutations in the Foxp3 gene were associated with a poorer prognosis in NMIBC, which might be attributable to the potential dysfunction of Tregs resulting from the disrupted expression of the mutated Foxp3 gene.
In conclusion, this study presents an initial exploration into the relationship between Foxp3 polymorphisms and BC. Our findings indicate that Foxp3 polymorphisms may be associated with the risk of BC susceptibility, and that rs3761548 could potentially serve as an independent risk factor for the OS rate. However, this study has several limitations. At first, the sample size, especially the female patients, was insufficient because of the higher BC incident rate of male than that of female and the genetic patten of X-linked gene, which might affect the reliability and veracity of the results, although we have conducted Cochran’s and Mantel-Haenszel statistics during analyses to adjust the gender parameter. Secondly, the lack of information on the smoking status of controls might introduce bias in this study since smoking is a known risk factor for BC. And more, the definitive impact of these two SNPs on the protein level of Foxp3 remains unclear. Therefore, further research is necessary to comprehensively elucidate this relationship.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Medical Research Ethics Committee of West China Second University Hospital, Sichuan University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
QL: Investigation, Methodology, Software, Validation, Writing – original draft, Writing – review & editing. YZ: Methodology, Validation, Writing – review & editing. MS: Methodology, Validation, Writing – review & editing. YS: Methodology, Writing – review & editing. YW: Formal Analysis, Software, Writing – review & editing. BZ: Conceptualization, Resources, Supervision, Writing – review & editing. LZ: Conceptualization, Data curation, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China (No. 32171264, 81974365 and 81974226) and the Sichuan Science and Technology Program (No. 2023ZYD0124).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Ferlay J EM, Lam F, et al. Global Cancer Observatory: Cancer Today (2020). International Agency for Research on Cancer. Available online at: http://gco.iarc.fr/today (Accessed November 25, 2020).
3. Li HZ, Zheng RS, Du LB, Zhang SW, Zhu C, Wei WW, et al. Bladder cancer incidence, mortality and temporal trends in China. Zhonghua Zhong Liu Za Zhi. (2021) 43:293–8 doi: 10.3760/cma.j.cn112152-20200421-00362
4. EM M. Urothelial tumors of the bladder. In: Wein A, editor. Campbell-Walsh Urology, 9th, vol. 3 . Elsevier/Saunders, Philadelphia (2007). p. 2406–45.
5. Nielsen ME, Smith AB, Meyer AM, Kuo TM, Tyree S, Kim WY, et al. Trends in stage-specific incidence rates for urothelial carcinoma of the bladder in the United States: 1988 to 2006. Cancer. (2014) 120:86–95. doi: 10.1002/cncr.v120.1
6. Babjuk M, Burger M, Zigeuner R, Shariat SF, van Rhijn BW, Compérat E, et al. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: update 2013. Eur Urol. (2013) 64:639–53. doi: 10.1016/j.eururo.2013.06.003
7. Antoni S, Ferlay J, Soerjomataram I, Znaor A, Jemal A, Bray F. Bladder cancer incidence and mortality: A global overview and recent trends. Eur Urol. (2017) 71:96–108. doi: 10.1016/j.eururo.2016.06.010
8. Cumberbatch MG, Rota M, Catto JW, La Vecchia C. The role of tobacco smoke in bladder and kidney carcinogenesis: A comparison of exposures and meta-analysis of incidence and mortality risks. Eur Urol. (2016) 70:458–66. doi: 10.1016/j.eururo.2015.06.042
9. Rothman N, Garcia-Closas M, Chatterjee N, Malats N, Wu X, Figueroa JD, et al. Baris D et al: A multi-stage genome-wide association study of bladder cancer identifies multiple susceptibility loci. Nat Genet. (2010) 42:978–84. doi: 10.1038/ng.687
10. Koutros S, Decker KL, Baris D, Pardo LA, Johnson A, Hosain GMM, et al. Bladder cancer risk associated with family history of cancer. Int J Cancer. (2021) 148:2915–23. doi: 10.1002/ijc.v148.12
11. Kunze E, Chang-Claude J, Frentzel-Beyme R. Life style and occupational risk factors for bladder cancer in Germany. A case-control study. Cancer. (1992) 69:1776–90. doi: 10.1002/1097-0142(19920401)69:7<1776::AID-CNCR2820690721>3.0.CO;2-P
12. Martin C, Leiser CL, O’Neil B, Gupta S, Lowrance WT, Kohlmann W, et al. Familial cancer clustering in urothelial cancer: A population-based case-control study. J Natl Cancer Institute. (2018) 110:527–33. doi: 10.1093/jnci/djx237
13. Randi G, Pelucchi C, Negri E, Talamini R, Galeone C, Franceschi S, et al. Family history of urogenital cancers in patients with bladder, renal cell and prostate cancers. Int J Cancer. (2007) 121:2748–52. doi: 10.1002/ijc.v121:12
14. Drake CG, Jaffee E, Pardoll DM. Mechanisms of immune evasion by tumors. Adv Immunol. (2006) 90:51–81. doi: 10.1016/S0065-2776(06)90002-9
15. Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol. (2007) 25:267–96. doi: 10.1146/annurev.immunol.25.022106.141609
16. Khattri R, Cox T, Yasayko S-A, Ramsdell F. : An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol. (2003) 4:337–42. doi: 10.1038/ni909
17. Nishikawa H, Sakaguchi S. Regulatory T cells in tumor immunity. Int J Cancer. (2010) 127:759–67. doi: 10.1002/ijc.v127:4
18. Wang L, Liu R, Li W, Chen C, Katoh H, Chen GY, et al. Somatic single hits inactivate the X-linked tumor suppressor FOXP3 in the prostate. Cancer Cell. (2009) 16:336–46. doi: 10.1016/j.ccr.2009.08.016
19. Hori S, Nomura T Fau - Sakaguchi S, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. (2003) 299:1057–61. doi: 10.1126/science.1079490
20. Wildin RS, Smyk-Pearson S, Filipovich AH. Clinical and molecular features of the immunodysregulation, polyendocrinopathy, enteropathy, X linked (IPEX) syndrome. J Med Genet. (2002) 39:537–45. doi: 10.1136/jmg.39.8.537
21. Zhang X, Li J, Qin X, Li S, Liang D. The effect of FOXP3 genetic polymorphisms on correlations with hepatitis B virus-hepatocellular carcinoma: A case-control study. Heliyon. (2023) 10:e23660. doi: 10.1016/j.heliyon.2023.e23660
22. Winerdal ME, Krantz D, Hartana CA, Zirakzadeh AA, Linton L, Bergman EA, et al. Hansson J et al: Urinary Bladder Cancer Tregs Suppress MMP2 and Potentially Regulate Invasiveness. Cancer Immunol Res. (2018) 6:528–38. doi: 10.1158/2326-6066.CIR-17-0466
23. Karimi M, Heshmati MA-O, Fattahi S, Bagheri NA-O, Alibeigi FA-O, Taheri F, et al. The relation between the ghrelin receptor and FOXP3 in bladder cancer. Biotech Histochem. (2021) 96:287–95. doi: 10.1080/10520295.2020.1799074
24. Jóźwicki W, Brożyna AA, Siekiera J, Slominski AT. Frequency of CD4+CD25+Foxp3+ cells in peripheral blood in relation to urinary bladder cancer Malignancy indicators before and after surgical removal. Oncotarget. (2016) 7:11450–62. doi: 10.18632/oncotarget.v7i10
25. Chu S, Zhong X, Zhang J, Lai X, Xie J, Li Y. Four SNPs and systemic level of FOXP3 in smokers and patients with chronic obstructive pulmonary disease. COPD. (2016) 13:760–6. doi: 10.1080/15412555.2016.1192112
26. Nam M, Shin S, Park KU, Kim I, Yoon SS, Kwon TA-OX, et al. Association of FOXP3 single nucleotide polymorphisms with clinical outcomes after allogenic hematopoietic stem cell transplantation. Ann Lab Med. (2018) 38:591–8. doi: 10.3343/alm.2018.38.6.591
27. Wu Z, You Z, Zhang C, Li Z, Su X, Zhang X, et al. Association between functional polymorphisms of Foxp3 gene and the occurrence of unexplained recurrent spontaneous abortion in a Chinese Han population. Clin Dev Immunol. (2012) 2012:896458. doi: 10.1155/2012/896458
28. Xu Z, Taylor JA. SNPinfo: integrating GWAS and candidate gene information into functional SNP selection for genetic association studies. Nucleic Acids Res. (2009) 37:W600–605. doi: 10.1093/nar/gkp290
29. Untergasser A, Cutcutache I, Koressaar T, Ye J, Faircloth BC, Remm M, et al. Primer3–new capabilities and interfaces. Nucleic Acids Res. (2012) 40:e115. doi: 10.1093/nar/gks596
30. Solé X, Guinó E, Valls J, Iniesta R, Moreno V. SNPStats: a web tool for the analysis of association studies. Bioinformatics. (2006) 22:1928–9. doi: 10.1093/bioinformatics/btl268
31. He YQ, Bo Q, Yong W, Qiu ZX, Li YL, Li WM. FoxP3 genetic variants and risk of non-small cell lung cancer in the Chinese Han population. Gene. (2013) 531:422–5. doi: 10.1016/j.gene.2013.08.066
32. Chen L, Yu Q, Liu B, Zhu L, Zhu L. Association of FoxP3 rs3761548 polymorphism with susceptibility to colorectal cancer in the Chinese population. Med Oncol. (2014) 31:374. doi: 10.1007/s12032-014-0374-0
33. Chatrabnous N, Ghaderi A, Ariafar A, Razeghinia MS, Nemati M, Jafarzadeh A. Serum concentration of interleukin-35 and its association with tumor stages and FOXP3 gene polymorphism in patients with prostate cancer. Cytokine. (2019) 133:221–7. doi: 10.1016/j.cyto.2018.07.006
34. You D, Wang Y, Zhang Y, Li Q, Yu X, Yuan M, et al. Association of Foxp3 promoter polymorphisms with susceptibility to endometrial cancer in the Chinese Han women. Medicine. (2018) 97:e0582. doi: 10.1097/MD.0000000000010582
35. Horn T, Laus J, Seitz AK, Maurer T, Schmid SC, Wolf P, et al. The prognostic effect of tumor-infiltrating lymphocytic subpopulations in bladder cancer. World J Urol. (2016) 34:181–7. doi: 10.1007/s00345-015-1615-3
36. Winerdal ME, Marits P Fau - Winerdal M, Winerdal M Fau - Hasan M, Hasan M Fau - Rosenblatt R, Rosenblatt R Fau - Tolf A, Selling K, et al. FOXP3 and survival in urinary bladder cancer. BJU Int. (2011) 108:1672–8. doi: 10.1111/j.1464-410X.2010.10020.x
Keywords: Foxp3, polymorphism, prognosis, non-muscle-invasive bladder cancer, tumor microenvironment
Citation: Li Q, Zhang Y, Su M, Song Y, Wang Y, Zhou B and Zhang L (2025) Nucleotide variation in Foxp3 gene and prognosis of bladder cancer: a case-control study. Front. Oncol. 15:1506900. doi: 10.3389/fonc.2025.1506900
Received: 06 October 2024; Accepted: 08 January 2025;
Published: 28 January 2025.
Edited by:
Syed Sameer Aga, King Saud bin Abdulaziz University for Health Sciences, Saudi ArabiaReviewed by:
Wensheng Zhang, Xavier University of Louisiana, United StatesAmir Salek Farrokhi, Pasteur Institute of Iran (PII), Iran
Copyright © 2025 Li, Zhang, Su, Song, Wang, Zhou and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lin Zhang, emhhbmdsaW5Ac2N1LmVkdS5jbg==
 Qin Li
Qin Li Yan Zhang
Yan Zhang Min Su
Min Su Yaping Song1
Yaping Song1