
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Oncol., 11 February 2025
Sec. Cancer Molecular Targets and Therapeutics
Volume 15 - 2025 | https://doi.org/10.3389/fonc.2025.1488289
Vanilloid1 (TRPV1), a subfamily of transient receptor channels, is one of the non-selective calcium channels, which is a bridge between cellular response and extracellular environmental networks, and is involved in a variety of pathophysiological processes. It is also involved in the process of cancer occurrence and progression, and researchers are revealing its role in cancer. In this paper, we review the expression and significance of TRPV1 receptor in various cancer cell types, the role of TRPV1 in the apoptosis-proliferation balance, cancer cell invasion and metastasis, and tumor micro-environment, with emphasis on the mechanisms by which TRPV1 receptor mediates inflammatory response, immune system, and thus regulates cancer. We discussed the latest directions and current challenges of TRPV1 receptor-targeting therapy for cancer, and summarized the odorous traditional herbs that modulate TRPV1 receptors, with a view to developing anti-tumor drugs targeting TRPV1 receptors in the future.
According to the latest Global Cancer Statistics Report (1), there were nearly 20 million new cancer cases (including non-melanoma skin cancer) and 9.7 million deaths from cancer (including non-melanoma skin cancer) in 2022, cancer has become a public health problem that seriously jeopardizes the health of human beings. Most patients diagnosed with cancer are in the middle and late stage. However, among the treatment methods, surgical complications, radiotherapy and chemotherapy toxic side effects are large, which seriously affects the quality of life of patients. At present, the treatment of cancer is transitioning from traditional surgery, chemotherapy and radiotherapy to new immunotherapy, targeted therapy and ion channel therapy. Finding new targets or low-toxicity and high-efficiency drugs for cancer treatment has always been a hot field of medical research. TRPV1 has emerged as a potential target for cancer treatment due to its role in regulating tumor growth, cell apoptosis, and the tumor micro-environment.
Capsaicin receptor - transient receptor potential vanillate receptor (TRPV1) is a non-selective cation channel that can be activated by temperature, physical and chemical stimuli (2). Its activation regulates a variety of biological responses, such as apoptosis and proliferation (3), metabolism and glucose homeostasis control (4), as well as nociception and body temperature. Recent studies have shown that TRPV1 receptor plays an important role in tumor biology through a variety of pathways. However, their specific mechanisms and roles in different types of cancer are still not fully understood, and TRPV1 modulators still have a long way to go before becoming anti-tumor drugs due to their side effects. We believe that TRPV1 is a heat-activated channel that can be activated by some odorous traditional herbs. TRPV1 receptor is a popular target for cancer therapy, and these odorous traditional herbs may also be potential drugs for cancer prevention and treatment.
This review aims to summarize the mechanisms by which TRPV1 receptors influence cancer progression, including their role in tumor cell proliferation, apoptosis, invasion, and tumor micro-environment. In addition, the anti-tumor potential of odorous traditional herbs that regulate TRPV1 is also discussed, hoping to develop anti-tumor drugs targeting TRPV1 receptors in the future.
Transient receptor potential vanilloid receptor was the first member of the transient receptor potential cation channel family to be discovered, it was cloned from the rat dorsal root ganglion by Caterina et al. in 1997 (5), and later formally named TRPV1 (6). TRPV1 is a multi-modal injury receptor that can be activated or variably modulated by temperature, physical, and chemical stimuli (7). In addition to capsaicin, its thermal sensitivity is activated by other endogenous substances (ATP, bradykinin, nerve growth factor, inflammatory mediators such as arachidonic acid amide and prostaglandins) and exogenous physical or chemical stimuli (camphor, resinaceous toxins, vanilloid toxins 1-3, ginger, and ethanol) (8). Upon activation of the post-cation channel TRPV1, extracellular Ca2+ flows into cells and releases intracellular cisterna Ca2+, resulting in increased intracellular Ca2+ concentration and mediating various basic cellular activities, such as muscle cell contraction, neuronal activity and transmitter release, cell proliferation and apoptosis (9).
TRPV1 transmembrane protein is a homologous tetramer composed of four identical subunits assembled around a central water pore, each consisting of six transmembrane domains (S1-S6), a long N-terminal region, and a short C-terminal region (10, 11). There is a short hydrophobic hole between S5 and S6 that mediates the passage of ions; The N-terminal region contains several phosphorylation sites and six ankyloprotein repeats that bind calmodulin and ATP; The C-terminal region contains the TRP region, multiple calmodulin binding regions, and endogenous substance binding sites, which can bind protein kinase C (PKC), Ca2+/calmodulin-dependent protein kinase II (CaMKII), and phosphatidylinositol-4,5 diphosphate (PIP2) (12). Compound binding to these sites modulates TRPV1 sensitivity and function, we need to elucidate their ligand structures and corresponding TRPV1 sites, mechanisms of action in the cancer process, and final effects. (The structure of TRPV1 is shown in Figure 1).
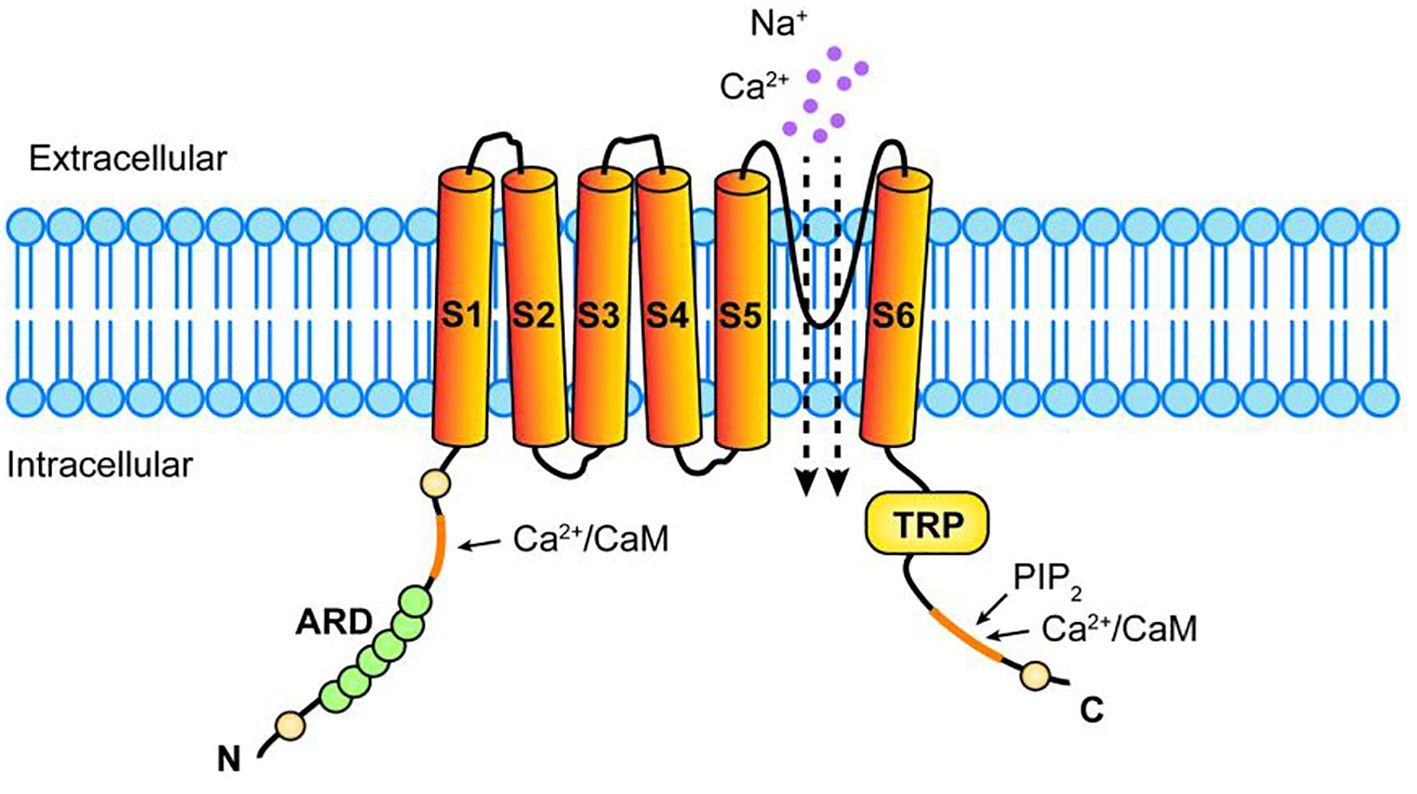
Figure 1. Structure of TRPV1 receptor. TRPV1 consists of four identical subunits assembled around a central hydrophobic pore, each consisting of six transmembrane domains (S1-S6), a long N-terminal region, and a relatively short C-terminal region. A hydrophobic pore mediates ionic passage between S5 and S6, N-terminal region has several phosphorylation sites and 6 ankyloprotein repeats (ARD) that bind calmodulin (CaM),The C-terminal region has TRP region, multiple CaM binding regions and endogenous substance binding sites, which can bind phosphatidylinositol-4,5 diphosphate (PIP2), Ca2+ and so on.
TRPV1 mRNA and protein are expressed in many cancer cell lines, and the expression levels in different cancer tissues are significantly different from those in their normal tissues, suggesting that it is involved in key processes of cancer progression. Weber LV et al. found that the expression of TRPV1 in breast cancer tissues and cells was significantly higher than that of healthy breast tissues, and that the average expression level was the highest in the triple-negative breast cancer subtypes (13). TRPV1 expression in cervical cancer tissues was higher than that in precancerous epithelial tissues and normal epithelial tissues, and its up-regulated expression was significantly correlated with more advanced tumor stage and poorer tumor grading. Over-expression of TRPV1 significantly increased the proliferation and clonogenic ability of cervical cancer cells (14).
However, the expression level of TRPV1 in some cancer tissues was lower than that in their normal tissues. It was found that the expression of TRPV1 in melanoma tissues and cell lines was significantly lower than that in nevus tissues and normal melanocytes (15). The expression of TRPV1 was lower in cervical squamous cell carcinoma (CSCC) and cervical adenocarcinoma than in normal group, and lower in CSCC than in cervical adenocarcinoma group (16).
Apparently the expression level of TRPV1 in each cancer cell line is highly heterogeneous, and the expression of TRPV1 is associated with different tumor micro-environments and different cancer-associated pathways. The expression level of TRPV1 also changed in the same cancer tissue, which was related to the grade of cancer tissue, pathological stage and tumor stage. Therefore, the expression level of TRPV1 can be used for the diagnosis and prognosis of cancer. The changes in TRPV1 expression, the mechanism of change and its role depend on different cancer cell types, which still need further study. (Compared with normal tissues, the changes and significance of TRPV1 expression level in cancer tissues are shown in Table 1).
Intracellular Ca2+ is involved in the process of apoptosis and cell proliferation, and is a second messenger that influences the proliferation-apoptosis balance (33). Therefore, Ca2+ influx into the cytoplasm after activation of TRPV1 will change the balance between apoptosis and proliferation signaling pathways, which may promote the development of cancer and may also have anti-tumor effects.
After binding of exogenous agonists to TRPV1, the flow of Ca2+ from the cytoplasm into cells is a common step between apoptosis and proliferation pathways. TRPV1 activation passes through the mitochondrial pathway, the endoplasmic reticulum pathway, and the exogenous death receptor pathway, ultimately leading to activation of the protease caspase 3, which mediates apoptosis through nuclear activity (34). The mechanism by which TRPV1 promotes cell proliferation is still under investigation, and two mechanisms have been derived: (1) TRPV1 activation causes ATP to be released into the extracellular space, where ATP binds to the G-protein-coupled receptor P2Y2, initiating a kinase signaling cascade that activates serine/threonine kinase (Akt); (2) TRPV1 trans-activates the EGFR, which results in the activation of a series of protein signals that activate extracellular signal-regulated kinase1/2 (ERK1/2). Ultimately Akt and ERK 1/2 MAPK promote cell proliferation through nuclear activity (35).
The activation of TRPV1 affects apoptosis or proliferation through different and competitive pathways, which may explain why TRPV1 is differently expressed in different types of cancer and the reason and effect of the expression change. The higher expression of TRPV1 in more malignant cancer cell lines may be due to the fact that it mainly promotes cell proliferation; On the contrary, the lower expression of TRPV1 in the more malignant cancer cell lines may be due to the fact that it mainly plays a role in promoting apoptosis. Therefore, it is promising to regulate TRPV1 to restore the balance of the cell proliferation-apoptosis signaling pathway to achieve anti-cancer effects. For example, TRPV1 was found to be over-expressed in intestinal epithelial HCT116 cells, and TRPV1 inhibited EGFR-induced proliferation of epithelial cells through activation of Ca2+/calpain and protein tyrosine phosphatase 1B (PTP1B) (25). Over expression of TRPV1 or the action of its agonist capsaicin can inhibit melanoma growth by activating p53 and inducing apoptosis (15).
TRPV1 also regulates the apoptosis-proliferation balance through mechanisms other than Ca2+ signaling, which may be related to the interaction of cellular receptors and cytokines in the tumor micro-environment (35), it may also be related to the type of agonist, the type of cancer, and the inflammatory response. Therefore, TRPV1 may play a multi-directional role in cancer tissue.
Cancer invasive metastasis is an important cause of mortality in malignant tumors, matrix metalloproteinases (MMPs) promote cancer cell migration by degrading extracellular matrix (ECM), basement membrane (BM) and remodeling intercellular adhesion. It can promote epithelial-mesenchymal transition (EMT), apoptosis of anticancer cells and promote angiogenesis in tumor micro-environment. Currently, It has been found that TRPV1 receptor can inhibit the invasion and metastasis of cancer cells by regulating MMPs (36). XuS et al. (26) found that capsaicin activation of TRPV1 down-regulates the expression of key EMT transcription factors Snail1 and Twist1, and also down-regulates the expression of MMPs-2 and MMPs-9, and ultimately significantly inhibits the migration, invasion and adhesion of bCPAP cells in thyroid papillary carcinoma. Robert Ramer et al. (37) found that cannabidiol up-regulated intercellular adhesion molecule-1 through cannabinoid receptor, TRPV1 receptor, and p42/44 mitogen-activated protein kinase in lung cancer cell lines A549, H358, and H460, inhibiting lung cancer cell invasion. Nuray Erin et al. (38) found that the TRPV1 agonist olvanil activated TRPV1-containing sensory nerve fibers, enhanced T-cell responses, and significantly inhibited breast cancer metastasis. This anti-breast cancer metastatic effect may be mediated through the neuroimmune pathway. In fact, MMPs are also involved in the immune response, and perhaps it plays a role in TRPV1’s anti-breast cancer metastasis through the neuroimmune pathway, which needs to be further investigated.
In addition, We need to pay attention to the fact that TRPV1 regulators can inhibit cancer cell migration and invasion through non-TRPV1 pathways. For example, in bladder cancer cells (39), Capsaicin reduces the deacetylase of SIRT1, enhances the acetylation of cortactin and β-catenin, thereby reducing the activation of MMP-2 and MMP-9, and ultimately leads to the migration disturbance of bladder cancer cells.
Tumor is not only a simple collection of cancer cells, but also constitutes the tumor micro-environment (TME) with its internal and external environment. TME is mainly composed of tumor Extracellular matrix (ECM), Tumor vascular system, Cancer-associated fibroblasts (CAF), immune system and inflammation, which jointly affect the occurrence and development of tumors (40). There is an association between TRPV1 and the tumor micro-environment (The assciation is shown in Figure 2).
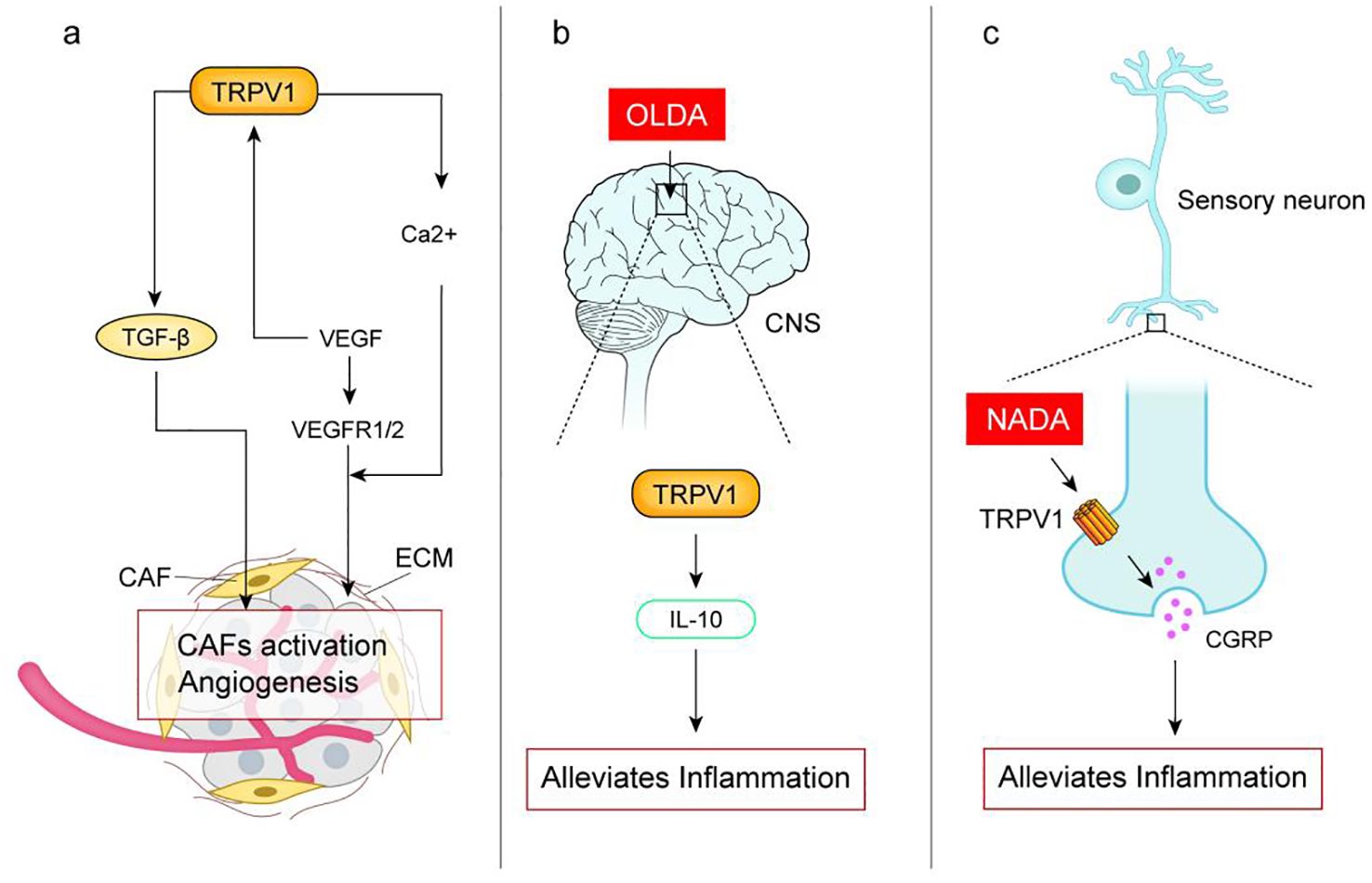
Figure 2. (A) Activation of TRPV1 upregulates the expression of transforming growth factor TGF-β, which recruits and activates cancer-associated fibroblasts (CAF), which regulate the formation of ECM ands promote tumor proliferation and metastasis; Vascular endothelial growth factor (VEGF) secreted by human uveal melanoma cells activates TRPV1 leading to intracellular Ca2+ inward flow, which is essential for angiogenesis. VEGF binds to VEGFR1/2 can induce proliferation and migration of vascular endothelial cells and promote angiogenesis. (B) TRPV1 endogenous agonist N-oleoyldopamine (OLDA) reduces inflammatory response by activating the central nervous system TRPV1 induces IL-10; (C) TRPV1 endogenous agonist N-dopamine (NADA) activates peripheral sensory neurons TRPV1 inhibits the release of calcitonin gene-related peptide (CGRP) thereby reducing acute inflammation. Studies have shown the pro-inflammatory effects of TRPV1, which may be related to the fact that activation of TRPV1 in different tissues produces different effects, or may be related to the type of agonist.
TRPV1 may regulate TGF-β/CAF and influence cancer development. CAF are a major component of the ECM, which regulates ECM formation and promotes tumor proliferation and metastasis (41). TGF-β, a transforming growth factor secreted by tumors, recruits and activates CAFs. Bodo et al. (42) demonstrated that capsaicin activation of TRPV1 up-regulated the expression of TGF-β2 mRNA and protein in human hair follicles, but the mechanism remains unclear. In TRPV1 knockout animal models, TGF-β1 expression was attenuated (43). These suggest that TRPV1 may regulate transforming growth factor TGF-β, modulating CAF, and thus be involved in cancer development.
TRPV1 is involved in tumor angiogenesis by regulating the expression and function of VEGF and its receptors. Tumor vascular system is a key process in tumor growth and metastasis, tumor cells or ECM can release key proteins vascular endothelial growth factor (VEGF) to bind to VEGFR1/2, induce vascular endothelial cell proliferation and migration, and promote angiogenesis (44). VEGF secreted by human uveal melanoma cells was found to activate TRPV1 function, leading to intracellular Ca2+ endocytosis to endothelial cells, and intracellular Ca2+ endocytosis is required for angiogenesis (45). Studies have shown that in TRPV1-/- mice, the NF-kB and STAT3 signaling pathways are overactivated, leading to a group of inflammatory factors (including IL-1 and IL-6) as well as invasive factors (such as MMP9 upregulation) that contribute to the development of cancer (46). NF-kB and STAT3 are known to be regulators of VEGF in several tumors (47) and may be involved in the regulation of VEGF expression by TRPV1. However, TRPV1 regulates the expression and function of VEGF and its receptors and is involved in tumor angiogenesis process, which is still uncertain and needs further study.
TRPV1 is expressed not only in cancer cells, but also in sensory neurons, the central nervous system, and a variety of immune cells (lymphocytes, dendritic cells, macrophages, and neutrophils). It is involved in the cancer process by regulating inflammatory responses, neuroimmune pathways, and immune cell function.
According to the current study, TRPV1 has a contradictory role in the inflammatory process. TRPV1 was found to be pro-inflammatory: TRPV1 is over-expressed in inflamed tissues (48), the use of TRPV1 antagonists or gene ablation attenuates the inflammatory response (49); TRPV1-expressing peripheral sensory neurons produce and release pro-inflammatory neuropeptides from their peripheral terminals, such as substance P (SP) and calcitonin gene-related peptide (CGRP), which subsequently cause neurogenic inflammation promoting cancer progression (50).
However, TRPV1 also has anti-inflammatory effects. Its activation may secrete neuropeptides with anti-inflammatory properties such as growth inhibitors (51). TRPV1 endogenous agonists such as N-oleoyldopamine (OLDA) induce IL-10 by activating TRPV1 in the central nervous system to reduce inflammatory responses and improve endotoxemia and sepsis outcomes (52). N-dopamine (NADA) reduces acute inflammation by activating TRPV1 (53). TRPV1 modulation of inflammation occurs at multiple levels and is associated with many regulatory proteins and inflammatory mediators, which leads to different final outcomes. Ca2+ plays a key role in the activation, differentiation, proliferation, cytokine secretion and effector functions of immune cells. TRPV1 acts as a calcium channel to regulate the function of immune cells (54). TRPV1 has been associated with macrophage-induced defense mechanisms. Activation of TRPV1 by Evodiamine or Capsaicin in mouse macrophages attenuates the inflammatory response induced by TNF-α, while CPZ, a TRPV1 antagonist, eliminated this anti-inflammatory effect (55).
Dendritic cells (DCs) are cells of the innate immune system responsible for antigen presentation, stimulation of T cells, and shaping the adaptive immune system. Activation of TRPV1 by Capsaicin stimulated the maturation and migration of DCs to lymph nodes in mice. In addition, TRPV1 was found to induce the release of calcitonin gene-related peptide (CGRP), a neuropeptide that regulates DC activation and T helper cell type 1 polarization (56). In the context of inflammatory and autoimmune diseases, TRPV1 has a broader role in T cells, where it is involved in TCR signaling, T cell proliferation and differentiation, and cytokine production (57).
TRPV1 activation may be important for maintaining the dynamic balance of immunity, however the exact molecular pathways by which TRPV1 regulates inflammation and immunity need to be further investigated. In conclusion, from the point of view of targeting TME and utilizing the immune system against tumors, TRPV1 receptor is a good choice.
TRPV1 receptor is a popular target for the development of anti-cancer drugs. Pharmacological modulators of specific TRPV1 channels may promote or induce cancer cell death, and may also be effective in reducing cancer cell proliferation, invasion, and metastasis. In addition, it can achieve anti-cancer effects by regulating the tumor micro-environment. TRPV1 modulators can enhance the effect of chemoradiotherapy, and the synergistic use of TRPV1 modulator can reduce the required dose of chemoradiotherapy to control cancer and reduce the toxic side effects of chemoradiotherapy. TRPV1 agonist combined with chemoradiotherapy is a feasible strategy for cancer treatment (58). However, the systemic use of TRPV1 modulators causes side effects such as hyperthermia, burns and respiratory injury (59), which severely limits its application. Only low-concentration cream or transdermal patch types of topical analgesic preparations are currently in clinical use (60). The key point is how to ensure the anti-cancer effect of TRPV1 modulators while overcoming their side effects. Some studies have combined TRPV1 modulators with the latest drug delivery systems in the hope that precise localized drug delivery can avoid the activation of systemic TRPV1 receptors (61, 62); Others have been devoted to the development of new structural variants of TRPV1 modulators (63). However, all the current studies are still in the experimental stage, and there is still a long way to go before practical clinical application.
We may be able to turn our attention to the potential of traditional herbs, which traditional Chinese medicine categorizes as odorous traditional herbs that elicit odorous flavors and heat sensations, and use them to treat tumors (64, 65). TRPV1 receptors are activated by a number of irritating substances and temperatures above 43°C. Studies have shown that many odorous traditional herbs activate the TRPV1 receptor. The efficacy of odorous traditional herbs is highly similar to the physiological functions mediated by TRPV1 receptors, such as dispelling cold and relieving pain (66). Capsaicin, an extract of odorous traditional herbs, activates the TRPV1 receptor against tumors (67). Gingerol, an extract of ginger (68), has anti-tumor effects, studies have shown that gingerol can activate TRPV1 (69), but no one has studied whether it is anti-tumor through the TRPV1 receptor pathway. Curcumin, an extract of turmeric, also has anti-tumor effects (70), studies have shown that curcumin can antagonize TRPV1 receptors (71). The TRPV1 receptor is a popular target for treating cancer, We hypothesize that these odorous herbs can modulate the TRPV1 receptor to exert anti-tumor effects, which needs further research and may be a more promising direction for finding anti-cancer drugs that target TRPV1 receptors. We summarize the currently known herbs that regulate TRPV1 receptors, as shown in Table 2:
TRPV1 is a multifunctional cell receptor that is widely distributed in human tissues and can sense various stimuli and be converted into calcium-based cascades, a variety of physiological and pathological processes based on it are being discovered. TRPV1 receptors are involved in the development of cancer in various ways, and the specific mechanisms have not been fully elucidated. The occurrence of tumor is a multi-factor, multi-step, complex and long biological process. In the process of development, the uneven distribution and effects of environmental factors and the randomness of gene mutations lead to high heterogeneity of tumors. TRPV1 receptor is also affected by these factors in the occurrence and development of various types of cancer, activating different factors and molecular pathways to produce different effects. Therefore, activation of TRPV1 has different effects on different types of cancers, which needs to be further investigated.
The TRPV1 receptor is a biomarker for predicting and diagnosing cancer, Its expression levels in cancer tissues are highly heterogeneous. It is necessary to study its progression in specific cancer types, determine the range of its expression levels and the relationship between its changes and prognosis. A recent study (24) revealed for the first time the expression, clinical value and potential mechanism of TRPV1 in pancarcinoma, which is also a direction.
In terms of the regulation of the apoptosis-proliferative balance of cancer cells and the tumor micro-environment, we need to consider that TRPV1 receptor is involved in the cancer process in multiple ways, the results of the studies may be different, which is the reason why many studies have conflicting results. When designing relevant experiments, we should carefully consider the type of cancer cell, the type of experiment (in vivo or in vitro), the dose, concentration and duration of action of TRPV1 modulator, etc. We also need to circumvent the effects of the non-TRPV1 pathway of TRPV1 modulators on cancer and the effects of other TRPV1 cancer pathways so that the experimental results can be accurate. At present, the research on the role of TRPV1 in cancer is still in the initial stage. How to control multiple confounding factors in the experiment to make the results accurate is the current problem. In addition, it needs to overcome its serious side effects in humans.
There is no doubt that manipulating the TRPV1 receptor could treat cancer, and the possibilities are endless. We think it is very promising to find suitable medicines from traditional herbs, because traditional herbs have been practiced for thousands of years and have a safe dose range, they can activate TRPV1 receptors while avoiding side effects. We summarized the currently known odorous herbs that can regulate TRPV1 receptors, hoping to develop anti-cancer drugs targeting TRPV1 receptors in the future.
MZ: Conceptualization, Data curation, Formal analysis, Project administration, Resources, Writing – original draft. ZW: Data curation, Validation, Writing – original draft. SL: Funding acquisition, Project administration, Software, Writing – review & editing. YL: Data curation, Writing – original draft. YG: Project administration, Visualization, Writing – original draft. ML: Conceptualization, Methodology, Validation, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Fund Project: National Natural Science Foundation of China (No. : 82274423).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
2. Roper SD. TRPs in taste and chemesthesis. Handb Exp Pharmacol. (2014) 223:827–71. doi: 10.1007/978-3-319-05161-1_5
3. Omari SA, Adams MJ, Geraghty DP. TRPV1 channels in immune cells and hematological Malignancies. Adv Pharmacol. (2017) 79:173–98. doi: 10.1016/bs.apha.2017.01.002
4. Suri A, Szallasi A. The emerging role of TRPV1 in diabetes and obesity. Trends Pharmacol Sci. (2008) 29:29–36. doi: 10.1016/j.tips.2007.10.016
5. Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. (1997) 389:816–24. doi: 10.1038/39807
6. Montell C, Birnbaumer L, Flockerzi V, Bindels RJ, Bruford EA, Caterina MJ, et al. A unified nomenclature for the superfamily of TRP cation channels. Mol Cell. (2002) 9:229–31. doi: 10.1016/s1097-2765(02)00448-3
7. Holzer P. The pharmacological challenge to tame the transient receptor potential vanilloid-1 (TRPV1) nocisensor. Br J Pharmacol. (2008) 155:1145–62. doi: 10.1038/bjp.2008.351
8. Sharma SK, Vij AS, Sharma M. Mechanisms and clinical uses of capsaicin. Eur J Pharmacol. (2013) 720:55–62. doi: 10.1016/j.ejphar.2013.10.053
9. Oh SJ, Lim JY, Son MK, Ahn JH, Song KH, Lee HJ, et al. TRPV1 inhibition overcomes cisplatin resistance by blocking autophagy-mediated hyperactivation of EGFR signaling pathway. Nat Commun. (2023) 14:2691. doi: 10.1038/s41467-023-38318-7
10. Tominaga M, Tominaga T. Structure and function of TRPV1. Pflugers Arch. (2005) 451:143–50. doi: 10.1007/s00424-005-1457-8
11. Lishko PV, Procko E, Jin X, Phelps CB, Gaudet R. The ankyrin repeats of TRPV1 bind multiple ligands and modulate channel sensitivity. Neuron. (2007) 54:905–18. doi: 10.1016/j.neuron.2007.05.027
12. Garcia-Sanz N, Fernandez-Carvajal A, Morenilla-Palao C, Planells-Cases R, Fajardo-Sanchez E, Fernandez-Ballester G, et al. Identification of a tetramerization domain in the C terminus of the vanilloid receptor. J Neurosci. (2004) 24:5307–14. doi: 10.1523/JNEUROSCI.0202-04.2004
13. Weber LV, Al-Refae K, Wolk G, Bonatz G, Altmuller J, Becker C, et al. Expression and functionality of TRPV1 in breast cancer cells. Breast Cancer (Dove Med Press). (2016) 8:243–52. doi: 10.2147/BCTT.S121610
14. Han GH, Chay DB, Nam S, Cho H, Chung JY, Kim JH. The combination of transient receptor potential vanilloid type 1 (TRPV1) and phosphatase and tension homolog (PTEN) is an effective prognostic biomarker in cervical cancer. Int J Gynecol Pathol. (2021) 40:214–23. doi: 10.1097/PGP.0000000000000677
15. Wang Z, Dong J, Tian W, Qiao S, Wang H. Role of TRPV1 ion channel in cervical squamous cell carcinoma genesis. Front Mol Biosci. (2022) 9:980262. doi: 10.3389/fmolb.2022.980262
16. Yang Y, Guo W, Ma J, Xu P, Zhang W, Guo S, et al. Downregulated TRPV1 expression contributes to melanoma growth via the calcineurin-ATF3-p53 pathway. J Invest Dermatol. (2018) 138:2205–15. doi: 10.1016/j.jid.2018.03.1510
17. Kalogris C, Caprodossi S, Amantini C, Lambertucci F, Nabissi M, Morelli MB, et al. Expression of transient receptor potential vanilloid-1 (TRPV1) in urothelial cancers of human bladder: relation to clinicopathological and molecular parameters. Histopathology. (2010) 57:744–52. doi: 10.1111/j.1365-2559.2010.03683.x
18. Miao X, Liu G, Xu X, Xie C, Sun F, Yang Y, et al. High expression of vanilloid receptor-1 is associated with better prognosis of patients with hepatocellular carcinoma. Cancer Genet Cytogenet. (2008) 186:25–32. doi: 10.1016/j.cancergencyto.2008.05.011
19. Sanchez MG, Sanchez AM, Collado B, Malagarie-Cazenave S, Olea N, Carmena MJ, et al. Expression of the transient receptor potential vanilloid 1 (TRPV1) in LNCaP and PC-3 prostate cancer cells and in human prostate tissue. Eur J Pharmacol. (2005) 515:20–7. doi: 10.1016/j.ejphar.2005.04.010
20. Gao N, Yang F, Chen S, Wan H, Zhao X, Dong H. The role of TRPV1 ion channels in the suppression of gastric cancer development. J Exp Clin Cancer Res. (2020) 39:206. doi: 10.1186/s13046-020-01707-7
21. Li L, Chen C, Xiang Q, Fan S, Xiao T, Chen Y, et al. Transient receptor potential cation channel subfamily V member 1 expression promotes chemoresistance in non-small-cell lung cancer. Front Oncol. (2022) 12:773654. doi: 10.3389/fonc.2022.773654
22. Beider K, Rosenberg E, Dimenshtein-Voevoda V, Sirovsky Y, Vladimirsky J, Magen H, et al. Blocking of Transient Receptor Potential Vanilloid 1 (TRPV1) promotes terminal mitophagy in multiple myeloma, disturbing calcium homeostasis and targeting ubiquitin pathway and bortezomib-induced unfolded protein response. J Hematol Oncol. (2020) 13:158. doi: 10.1186/s13045-020-00993-0
23. Omari SA, Geraghty DP, Khalafallah AA, Venkat P, Shegog YM, Ragg SJ, et al. Optimized flow cytometric detection of transient receptor potential vanilloid-1 (TRPV1) in human hematological Malignancies. Med Oncol. (2022) 39:81. doi: 10.1007/s12032-022-01678-z
24. Lah TT, Majc B, Novak M, Susnik A, Breznik B, Porcnik A, et al. The cytotoxic effects of cannabidiol and cannabigerol on glioblastoma stem cells may mostly involve GPR55 and TRPV1 signaling. Cancers (Basel). (2022) 14:5918. doi: 10.3390/cancers14235918
25. de Jong PR, Takahashi N, Harris AR, Lee J, Bertin S, Jeffries J, et al. Ion channel TRPV1-dependent activation of PTP1B suppresses EGFR-associated intestinal tumorigenesis. J Clin Invest. (2014) 124:3793–806. doi: 10.1172/JCI72340
26. Xu S, Zhang L, Cheng X, Yu H, Bao J, Lu R. Capsaicin inhibits the metastasis of human papillary thyroid carcinoma BCPAP cells through the modulation of the TRPV1 channel. Food Funct. (2018) 9:344–54. doi: 10.1039/c7fo01295k
27. Zuo HL, Ma YM, Li YF, Li XD, Wang HS, Jiang W. Clinical significance of capsaicin receptor TRPV1 expression in endometrial cancer tissues. Chin J Cancer Prev Treat. (2017) 24:745–9. doi: 10.16073/j.cnki.cjcpt.2017.11.005
28. Huang J, Liu J, Qiu L. Transient receptor potential vanilloid 1 promotes EGFR ubiquitination and modulates EGFR/MAPK signaling in pancreatic cancer cells. Cell Biochem Funct. (2020) 38:401–8. doi: 10.1002/cbf.3483
29. Huang R, Wang F, Yang Y, Ma W, Lin Z, Cheng N, et al. Recurrent activations of transient receptor potential vanilloid-1 and vanilloid-4 promote cellular proliferation and migration in esophageal squamous cell carcinoma cells. FEBS Open Bio. (2019) 9:206–25. doi: 10.1002/2211-5463.12570
30. Kiss F, Kormos V, Szoke E, Kecskes A, Toth N, Steib A, et al. Functional transient receptor potential ankyrin 1 and vanilloid 1 ion channels are overexpressed in human oral squamous cell carcinoma. Int J Mol Sci. (2022) 23:1921. doi: 10.3390/ijms23031921
31. Zheng L, Dou X, Song H, Gao R, Tang X. TRPV1 acts as a Tumor Suppressor and is associated with Immune Cell Infiltration in Clear Cell Renal Cell Carcinoma: evidence from integrated analysis. J Cancer. (2020) 11:5678–88. doi: 10.7150/jca.45918
32. Hudhud L, Rozmer K, Kecskes A, Pohoczky K, Bencze N, Buzas K, et al. Transient receptor potential Ankyrin 1 ion channel is expressed in osteosarcoma and its activation reduces viability. Int J Mol Sci. (2024) 25:3760. doi: 10.3390/ijms25073760
33. Pardo LA, Stuhmer W. The roles of K+ channels in cancer. Nat Rev Cancer. (2014) 14:39–48. doi: 10.1038/nrc3635
34. Zhai K, Liskova A, Kubatka P, Busselberg D. Calcium entry through TRPV1: A potential target for the regulation of proliferation and apoptosis in cancerous and healthy cells. Int J Mol Sci. (2020) 21:4117. doi: 10.3390/ijms21114177
35. Wu YY, Liu XY, Zhuo DX, Huang HB, Zhang FB, Liao SF. Decreased expression of TRPV1 in renal cell carcinoma: association with tumor Fuhrman grades and histopathological subtypes. Cancer Manag Res. (2018) 10:1647–55. doi: 10.2147/CMAR.S166390
36. Ip SW, Lan SH, Lu HF, Huang AC, Yang JS, Lin JP, et al. Capsaicin mediates apoptosis in human nasopharyngeal carcinoma NPC-TW 039 cells through mitochondrial depolarization and endoplasmic reticulum stress. Hum Exp Toxicol. (2012) 31:539–49. doi: 10.1177/0960327111417269
37. Ramer R, Bublitz K, Freimuth N, Merkord J, Rohde H, Haustein M, et al. Cannabidiol inhibits lung cancer cell invasion and metastasis via intercellular adhesion molecule-1. FASEB J. (2012) 26:1535–48. doi: 10.1096/fj.11-198184
38. Erin N, Akman M, Aliyev E, Tanriover G, Korcum AF. Olvanil activates sensory nerve fibers, increases T cell response and decreases metastasis of breast carcinoma. Life Sci. (2022) 291:120305. doi: 10.1016/j.lfs.2022.120305
39. Islam A, Yang YT, Wu WH, Chueh PJ, Lin MH. Capsaicin attenuates cell migration via SIRT1 targeting and inhibition to enhance cortactin and beta-catenin acetylation in bladder cancer cells. Am J Cancer Res. (2019) 9:1172–82.
40. Kaymak I, Williams KS, Cantor JR, Jones RG. Immunometabolic interplay in the tumor microenvironment. Cancer Cell. (2021) 39:28–37. doi: 10.1016/j.ccell.2020.09.004
41. Kalluri R. The biology and function of fibroblasts in cancer. Nat Rev Cancer. (2016) 16:582–98. doi: 10.1038/nrc.2016.73
42. Bodo E, Biro T, Telek A, Czifra G, Griger Z, Toth BI, et al. A hot new twist to hair biology: involvement of vanilloid receptor-1 (VR1/TRPV1) signaling in human hair growth control. Am J Pathol. (2005) 166:985–98. doi: 10.1016/S0002-9440(10)62320-6
43. Nidegawa-Saitoh Y, Sumioka T, Okada Y, Reinach PS, Flanders KC, Liu CY, et al. Impaired healing of cornea incision injury in a TRPV1-deficient mouse. Cell Tissue Res. (2018) 374:329–38. doi: 10.1007/s00441-018-2878-y
44. Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. (2003) 9:669–76. doi: 10.1038/nm0603-669
45. Murata M. Inflammation and cancer. Environ Health Prev Med. (2018) 23:50. doi: 10.1186/s12199-018-0740-1
46. Vinuesa AG, Sancho R, Garcia-Limones C, Behrens A, ten Dijke P, Calzado MA, et al. Vanilloid receptor-1 regulates neurogenic inflammation in colon and protects mice from colon cancer. Cancer Res. (2012) 72:1705–16. doi: 10.1158/0008-5472.CAN-11-3693
47. Gao P, Niu N, Wei T, Tozawa H, Chen X, Zhang C, et al. The roles of signal transducer and activator of transcription factor 3 in tumor angiogenesis. Oncotarget. (2017) 8:69139–61. doi: 10.18632/oncotarget.19932
48. Bujak JK, Kosmala D, Szopa IM, Majchrzak K, Bednarczyk P. Inflammation, cancer and immunity-implication of TRPV1 channel. Front Oncol. (2019) 9:1087. doi: 10.3389/fonc.2019.01087
49. Liu T, Wang G, Tao H, Yang Z, Wang Y, Meng Z, et al. Capsaicin mediates caspases activation and induces apoptosis through P38 and JNK MAPK pathways in human renal carcinoma. BMC Cancer. (2016) 16:790. doi: 10.1186/s12885-016-2831-y
50. Erin N, Szallasi A. Carcinogenesis and metastasis: focus on TRPV1-positive neurons and immune cells. Biomolecules. (2023) 13:983. doi: 10.3390/biom13060983
51. Walcher L, Budde C, Bohm A, Reinach PS, Dhandapani P, Ljubojevic N, et al. TRPM8 activation via 3-iodothyronamine blunts VEGF-induced transactivation of TRPV1 in human uveal melanoma cells. Front Pharmacol. (2018) 9:1234. doi: 10.3389/fphar.2018.01234
52. Joffre J, Wong E, Lawton S, Lloyd E, Nguyen N, Xu F, et al. N-Oleoyl dopamine induces IL-10 via central nervous system TRPV1 and improves endotoxemia and sepsis outcomes. J Neuroinflammation. (2022) 19:118. doi: 10.1186/s12974-022-02485-z
53. Lawton SK, Xu F, Tran A, Wong E, Prakash A, Schumacher M, et al. N-arachidonoyl dopamine modulates acute systemic inflammation via nonhematopoietic TRPV1. J Immunol. (2017) 199:1465–75. doi: 10.4049/jimmunol.1602151
54. Feske S, Wulff H, Skolnik EY. Ion channels in innate and adaptive immunity. Annu Rev Immunol. (2015) 33:291–353. doi: 10.1146/annurev-immunol-032414-112212
55. Zhao JF, Ching LC, Kou YR, Lin SJ, Wei J, Shyue SK, et al. Activation of TRPV1 prevents OxLDL-induced lipid accumulation and TNF-alpha-induced inflammation in macrophages: role of liver X receptor alpha. Mediators Inflamm. (2013) 2013:925171. doi: 10.1155/2013/925171
56. Assas MB, Wakid MH, Zakai HA, Miyan JA, Pennock JL. Transient receptor potential vanilloid 1 expression and function in splenic dendritic cells: a potential role in immune homeostasis. Immunology. (2016) 147:292–304. doi: 10.1111/imm.12562
57. Majhi RK, Sahoo SS, Yadav M, Pratheek BM, Chattopadhyay S, Goswami C. Functional expression of TRPV channels in T cells and their implications in immune regulation. FEBS J. (2015) 282:2661–81. doi: 10.1111/febs.13306
58. Akhilesh, Uniyal A, Gadepalli A, Tiwari V, Allani M, Chouhan D, et al. Unlocking the potential of TRPV1 based siRNA therapeutics for the treatment of chemotherapy-induced neuropathic pain. Life Sci. (2022) 288:120187. doi: 10.1016/j.lfs.2021.120187
59. Dumitrache MD, Jieanu AS, Scheau C, Badarau IA, Popescu GDA, Caruntu A, et al. Comparative effects of capsaicin in chronic obstructive pulmonary disease and asthma (Review). Exp Ther Med. (2021) 22:917. doi: 10.3892/etm.2021.10349
60. Iftinca M, Defaye M, Altier C. TRPV1-targeted drugs in development for human pain conditions. Drugs. (2021) 81:7–27. doi: 10.1007/s40265-020-01429-2
61. Li T, Jiang S, Zhang Y, Luo J, Li M, Ke H, et al. Nanoparticle-mediated TRPV1 channel blockade amplifies cancer thermo-immunotherapy via heat shock factor 1 modulation. Nat Commun. (2023) 14:2498. doi: 10.1038/s41467-023-38128-x
62. Zhen X, Xie C, Jiang Y, Ai X, Xing B, Pu K. Semiconducting photothermal nanoagonist for remote-controlled specific cancer therapy. Nano Lett. (2018) 18:1498–505. doi: 10.1021/acs.nanolett.7b05292
63. Maximiano TKE, Carneiro JA, Fattori V, Verri WA. TRPV1: Receptor structure, activation, modulation and role in neuro-immune interactions and pain. Cell Calcium. (2024) 119:102870. doi: 10.1016/j.ceca.2024.102870
64. Hu GS, Xia MJ, Li ML, You FM, Yan R, Pan L. odorous medicine for the treatment of tumors. Hubei J Traditional Chin Med. (2019) 41:47–9.
65. Ma F. Study on the anti-tumor effect of Xinwen Jiebiao and Wenzhong Dispersed Cold Traditional Chinese Medicine. Tianjin: Tianjin Medical University (2011).
66. Wang X, Zhang YL, Wang Y, Ren ZZ, Bao HJ, Qiao YJ. Study on the relationship between TRPV1 ion channels and odorous medicinal properties of traditional Chinese medicine. China J Chin Materia Medica. (2014) 39:2422–7. doi: 10.4268/cjcmm20141314
67. Muratovska N, Silva P, Pozdniakova T, Pereira H, Grey C, Johansson B, et al. Towards engineered yeast as production platform for capsaicinoids. Biotechnol Adv. (2022) 59:107989. doi: 10.1016/j.bioteChadv.2022.107989
68. Mahomoodally MF, Aumeeruddy MZ, Rengasamy KRR, Roshan S, Hammad S, Pandohee J, et al. Ginger and its active compounds in cancer therapy: From folk uses to nano-therapeutic applications. Semin Cancer Biol. (2021) 69:140–9. doi: 10.1016/j.semcancer.2019.08.009
69. Yin Y, Dong Y, Vu S, Yang F, Yarov-Yarovoy V, Tian Y, et al. Structural mechanisms underlying activation of TRPV1 channels by odorous compounds in gingers. Br J Pharmacol. (2019) 176:3364–77. doi: 10.1111/bph.14766
70. Wang W, Li M, Wang L, Chen L, Goh BC. Curcumin in cancer therapy: Exploring molecular mechanisms and overcoming clinical challenges. Cancer Lett. (2023) 570:216332. doi: 10.1016/j.canlet.2023.216332
71. Yeon KY, Kim SA, Kim YH, Lee MK, Ahn DK, Kim HJ, et al. Curcumin produces an antihyperalgesic effect via antagonism of TRPV1. J Dent Res. (2010) 89:170–4. doi: 10.1177/0022034509356169
72. Zou R, Zhou Y, Lu Y, Zhao Y, Zhang N, Liu J, et al. Preparation, pungency and bioactivity transduction of piperine from black pepper (Piper nigrum L.): A comprehensive review. Food Chem. (2024) 456:139980. doi: 10.1016/j.foodchem.2024.139980
73. Greenshields AL, Doucette CD, Sutton KM, Madera L, Annan H, Yaffe PB, et al. Piperine inhibits the growth and motility of triple-negative breast cancer cells. Cancer Lett. (2015) 357:129–40. doi: 10.1016/j.canlet.2014.11.017
74. Tian KM, Li JJ, Xu SW. Rutaecarpine: A promising cardiovascular protective alkaloid from Evodia rutaecarpa (Wu Zhu Yu). Pharmacol Res. (2019) 141:541–50. doi: 10.1016/j.phrs.2018.12.019
75. Jiang ZB, Huang JM, Xie YJ, Zhang YZ, Chang C, Lai HL, et al. Evodiamine suppresses non-small cell lung cancer by elevating CD8(+) T cells and downregulating the MUC1-C/PD-L1 axis. J Exp Clin Cancer Res. (2020) 39:249. doi: 10.1186/s13046-020-01741-5
76. Macpherson LJ, Geierstanger BH, Viswanath V, Bandell M, Eid SR, Hwang S, et al. The pungency of garlic: activation of TRPA1 and TRPV1 in response to allicin. Curr Biol. (2005) 15:929–34. doi: 10.1016/j.cub.2005.04.018
77. Catanzaro E, Canistro D, Pellicioni V, Vivarelli F, Fimognari C. Anticancer potential of allicin: A review. Pharmacol Res. (2022) 177:106118. doi: 10.1016/j.phrs.2022.106118
78. Harb AA, Bustanji YK, Almasri IM, Abdalla SS. Eugenol reduces LDL cholesterol and hepatic steatosis in hypercholesterolemic rats by modulating TRPV1 receptor. Sci Rep. (2019) 9:14003. doi: 10.1038/s41598-019-50352-4
79. Teixeira C, Pereira RB, Pinto NFS, Coelho CMM, Fernandes MJG, Fortes AG, et al. Eugenol beta-amino/beta-alkoxy alcohols with selective anticancer activity. Int J Mol Sci. (2022) 23:3759. doi: 10.3390/ijms23073759
80. Chen X, Sun W, Gianaris NG, Riley AM, Cummins TR, Fehrenbacher JC, et al. Furanocoumarins are a novel class of modulators for the transient receptor potential vanilloid type 1 (TRPV1) channel. J Biol Chem. (2014) 289:9600–10. doi: 10.1074/jbc.M113.536862
81. Xu WW, Huang ZH, Liao L, Zhang QH, Li JQ, Zheng CC, et al. Direct targeting of CREB1 with imperatorin inhibits TGFbeta2-ERK signaling to suppress esophageal cancer metastasis. Adv Sci (Weinh). (2020) 7:2000925. doi: 10.1002/advs.202000925
82. Guo R, Liu ZH, Wang WL, Gao W, Tao XG, An ZJ, et al. Effect of artemisia leaf alcohol extract on the heat-sensitive channel TRPV1. J Basic Chin Med. (2019) 25:314–6 + 98. doi: 10.19945/j.cnki.issn.1006-3250.2019.03.041
83. Yu JJ. Study on the analgesic mechanism of asarum regulation of TRPV1 ion channels. Nanjing: Nanjing University of Traditional Chinese Medicine (2019).
84. Zhao M, Wen Y, Yang Y, Pan H, Xie S, Shen C, et al. (-)-Asarinin alleviates gastric precancerous lesions by promoting mitochondrial ROS accumulation and inhibiting the STAT3 signaling pathway. Phytomedicine. (2024) 126:155348. doi: 10.1016/j.phymed.2024.155348
85. Li Y, Zeng J, Tian YH, Hou Y, Da H, Fang J, et al. Isolation, identification, and activity evaluation of diterpenoid alkaloids from Aconitum sinomontanum. Phytochemistry. (2021) 190:112880. doi: 10.1016/j.phytochem.2021.112880
Keywords: TRPV1, cancer, tumor micro-environment, odorous traditional herbal medicines, apoptosis
Citation: Zhang M, Wang Z, Liu S, Li Y, Gong Y and Liu M (2025) New options for targeting TRPV1 receptors for cancer treatment: odorous Chinese herbal medicine. Front. Oncol. 15:1488289. doi: 10.3389/fonc.2025.1488289
Received: 29 August 2024; Accepted: 23 January 2025;
Published: 11 February 2025.
Edited by:
Andy T. Y. Lau, Shantou University, ChinaReviewed by:
Yan-Ming Xu, Shantou University, ChinaCopyright © 2025 Zhang, Wang, Liu, Li, Gong and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Min Liu, bGl1bWluNjk1OEAxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.