
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 14 March 2025
Sec. Surgical Oncology
Volume 15 - 2025 | https://doi.org/10.3389/fonc.2025.1487477
Objective: This study identified the trends and clinical significance of anemia and ferritin status 1 year postoperatively in patients with long-term survival and analyzed clinicopathological factors and preoperative nutritional/inflammatory conditions associated with anemia of chronic disease (ACD) development.
Methods: Between March 2009 and December 2018, 2,976 patients who underwent curative gastrectomy for gastric cancer without recurrence or mortality within postoperative 1 year were included. The patients were categorized into four groups; non-iron deficiency without anemia, iron deficiency without anemia, iron deficiency anemia (IDA), and ACD based on postoperative 1 year ferritin and hemoglobin.
Results: The overall incidence of anemia was 36.5% (n=1,086). The prevalence of IDA and ACD was 12.7% (n=377) and 23.8 (n=709), respectively, at postoperative 1 year. Patients with ACD were significantly older, had higher ECOG, increased early complications, and were at a more advanced stage than the other groups. The overall survival (OS) of ACD was significantly lower than that of the other groups (p < 0.001), especially for stages I and III. The presence of ACD was a significant risk factor for overall (hazard ratio [HR] = 1.832, p < 0.001), disease-free (HR= 1.714, p = 0.003), and cancer-specific (HR= 1.690, P = 0.015) survival. Older age, advanced disease stage, low preoperative prognostic nutritional index, preoperative anemia, and early postoperative complications were significant risk factors for ACD.
Conclusions: Relationship between ferritin and Hb at postoperative 1 year is a significant prognostic factor for survival in patients with gastric cancer. Particularly, ACD may be a specific predictor of gastric cancer. Therefore, clinicians need to pay attention to ACD status and prevent the risk factors for its development during long-term postoperative follow-up.
According to the 2021 Cancer Statistics of Korea, gastric cancer is one of the fourth common cancers and the fourth leading cause of cancer-related deaths in Korea (1) whereas it is the fifth most common cancer and the fifth leading cause of cancer-related deaths worldwide based on GLOBOCAN 2022 (2).
Traditionally, curative gastrectomy has remained the cornerstone of treatment for resectable gastric cancer (3). However, anemia has emerged as a prevalent post-gastrectomy complication, often attributed to iron, vitamin B12, or folate deficiencies, either in isolation or synergistically (4, 5). These deficiencies typically arise from factors such as malabsorption, diminished dietary intake, or chronic gastric mucosal bleeding (4, 5).
Several studies have indicated a potential relationship between post-gastrectomy anemia, nutritional deficiencies, and unfavorable prognostic outcomes (6, 7). However, most prior investigations have predominantly relied on hemoglobin (Hb) levels as the sole indicator to assess the prevalence of anemia following gastrectomy (7, 8). Furthermore, studies simultaneously examining the occurrence of iron-deficiency anemia (IDA) and anemia of chronic disease (ACD) in post-gastrectomy state are scarce, and the correlation between these types of anemia and gastric cancer prognosis has been scarcely investigated.
Ferritin, a pivotal iron-binding protein, reflects the iron reserves within the body (9). Recent findings have underscored its pivotal involvement in critical processes including cancer cell proliferation, angiogenesis, immunosuppression, carcinogenesis, and treatment resistance (10). Elevated serum ferritin levels have consistently been correlated with poorer survival outcomes across various malignancies, such as lung, colorectal, breast, pancreatic, and hepatocellular carcinomas (11–14). However, the prognostic significance of serum ferritin levels in gastric cancer remains controversial. Literature regarding the prognostic significance of serum ferritin levels, specifically in patients with gastric cancer undergoing gastrectomy, is scarce (15, 16).
Therefore, this study aimed to classify post-gastrectomy patients (long-term survivors of gastric cancer) based on the occurrence patterns of anemia and ferritin levels at postoperative 1 year, and to assess their clinical impact on gastric cancer prognosis. Additionally, the clinicopathological factors, preoperative nutritional status, and inflammatory conditions associated with ACD were analyzed.
Medical records of patients with gastric cancer who underwent curative gastrectomy at Seoul St. Mary’s Hospital and Yeouido St. Mary’s Hospital between March 2009 and December 2018 were reviewed. Patients with remnant gastric cancer who underwent total gastrectomy, hematologic disease, cancer recurrence, or death within postoperative 1 year; underwent Whipple procedure; and those who had no postoperative 1 year laboratory data were excluded. After exclusion, 2,976 patients were enrolled in this study. This study was approved by the Institutional Review Board (XC22RIDI0045) and was performed in line with the principles of the Declaration of Helsinki.
Anemia was defined as Hb < 12 g/dL in women and < 13 g/dL in men according to the World Health Organization (WHO) criteria. Iron deficiency was defined as a serum ferritin level < 15 ng/mL, regardless of anemia. ACD was defined as anemia with serum ferritin level ≥ 15 ng/mL. The patients were categorized into four groups; non-iron deficiency without anemia, iron deficiency without anemia (ID), IDA, and ACD based on postoperative 1 year ferritin and Hb level. The clinicopathological features and long-term prognoses of these groups were comparatively analyzed.
The platelet-to-lymphocyte ratio (PLR) was defined as platelet count divided by lymphocyte count. The neutrophil-to-lymphocyte ratio (NLR) was defined as neutrophil count divided by lymphocyte count. The prognostic nutritional index (PNI) was calculated using the following formula: 10 × serum albumin level (g/dL) + 0.005 × peripheral blood lymphocyte count. Patients were classified into high and low groups according to the median PNI value.
Body mass index (BMI) was calculated using the following formula: weight (kg)/height2 (m2). Patients were classified according to BMI, based on the WHO definition of obesity for Asians, as follows: underweight (BMI < 18.5 kg/m2), normal/overweight (18.5 ≤ BMI < 25.0 kg/m2), or obese (BMI ≥ 25.0 kg/m2).
Continuous and categorical variables were compared using the independent t-test and chi-square test, respectively. Kaplan–Meier curves were used to estimate overall survival (OS), disease-free survival (DFS), and cancer-specific survival (CSS). The survival rates of the groups were compared using log-rank test. A Cox regression model was used to identify variables influencing OS, DFS, and CSS. Logistic regression analysis was performed to assess the factors independently associated with the presence of ACD. In all analyses, P < 0.05 was considered to indicate statistical significance. All statistical analyses were performed using the SPSS software (version 18.0; SPSS Inc., Chicago, IL, USA).
The clinicopathological characteristics and prevalence of anemia are summarized in Table 1. Of the 2,976 patients, 1,912 (642.%) were male. Majority were presented with ECOG 0 or 1 (n = 2870; 96.4%). Overall, 2,358 (79.2%) and 2,474 (83.1%) patients underwent partial gastrectomy and duodenal non-passing anastomosis, respectively. A number of postoperative complications within 30-day after operation was 644 (21.6%). The prevalence of stage I, II, and III among all patients was 75.0%, 14.1%, and 10.9%, respectively. The frequency of preoperative and adjuvant chemotherapy were 0.8% (n = 25) and 19.2% (n = 572), respectively.
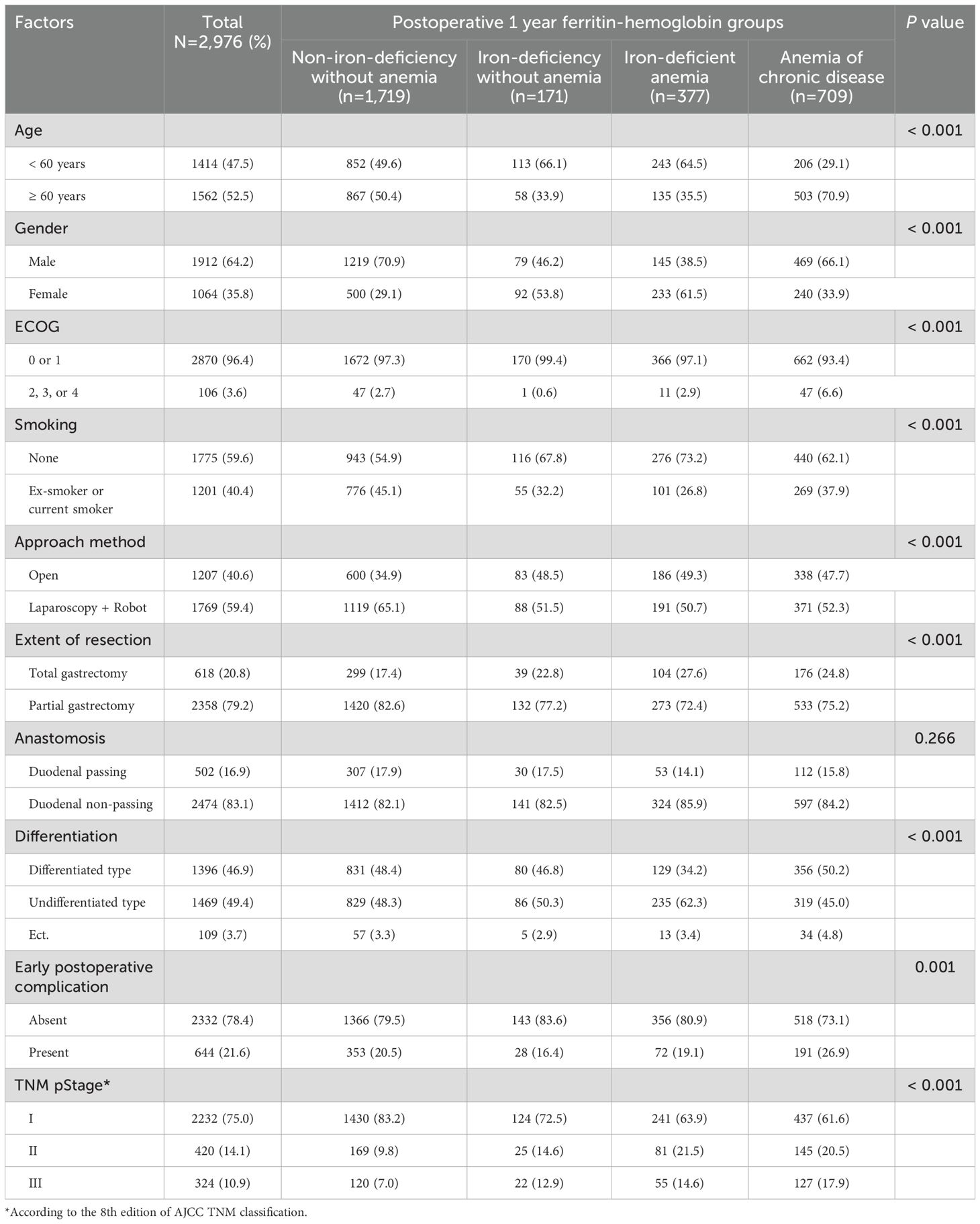
Table 1. Association of the patients’ clinicopathological characteristics with the postoperative 1 year ferritin-hemoglobin groups.
The overall incidence of anemia was 36.5% (n = 1,086). The prevalence of ID and ACD was 18.4% (n = 548) and 23.8% (n = 709), respectively, at postoperative 1 year after gastrectomy. Among patients in the ID group, 68.8% (n = 377) had anemia. Overall, the prevalence of IDA was 12.7% (n = 377) at postoperative 1 year.
The relationships between the clinicopathological characteristics and anemia groups are summarized in Table 1. The prevalence rate of older age and higher ECOG grade were significantly higher in the ACD group (p < 0.001 and p < 0.001, respectively). Higher TNM stage was significantly associated with the ACD group (p < 0.001). The early postoperative complication rate was significantly higher in the ACD group than in the other groups (p = 0.001). IDA occurred significantly more frequently in female, non-smokers, patients with total gastrectomy and undifferentiated type (p < 0.001, p < 0.001, p < 0.001, and p < 0.001, respectively). The prevalence of partial gastrectomy was significantly higher in patients with ID without anemia (p < 0.001). The patients who underwent duodenal non-passing anastomosis tended to have IDA, but it was not significant (p = 0.266).
The OS rate in the ACD group was significantly lower than that in the other groups (p < 0.001), especially in stage I and III disease (p < 0.001 and p = 0.009, respectively) (Figure 1). The CSS and DFS rates in the ACD group were significantly lower than those in the other groups (p < 0.001 and p < 0.001, respectively), especially for stage III disease (p = 0.034 and p = 0.027, respectively) (Figures 2, 3).
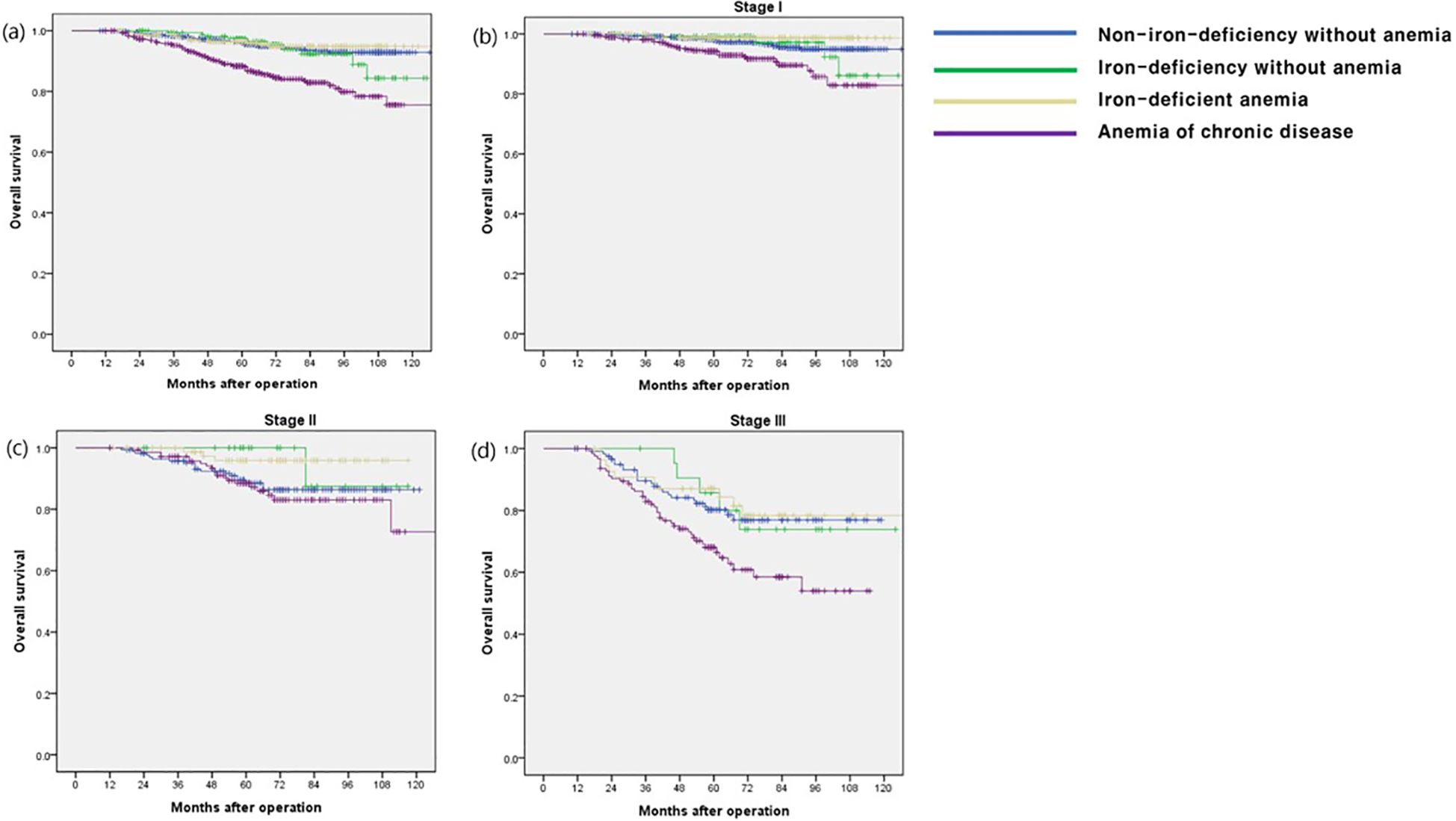
Figure 1. Overall survival according to postoperative 1 year ferritin-hemoglobin groups. (a) Total patients (p < 0.001), Non-iron-deficiency without anemia vs. Iron-deficiency without anemia (p = 0.704), Non-iron-deficiency without anemia vs. Iron-deficient anemia (p = 0.615), Non-iron-deficiency without anemia vs. Anemia of chronic disease (p < 0.001); (b) stage I (p < 0.001), Non-iron-deficiency without anemia vs. Iron-deficiency without anemia (p = 0.894), Non-iron-deficiency without anemia vs. Iron-deficient anemia (p = 0.170), Non-iron-deficiency without anemia vs. Anemia of chronic disease (p < 0.001); (c) stage II (p = 0.074) Non-iron-deficiency without anemia vs. Iron-deficiency without anemia (p = 0.268), Non-iron-deficiency without anemia vs. Iron-deficient anemia (p = 0.044), Non-iron-deficiency without anemia vs. Anemia of chronic disease (p = 0.560); (d) stage III (p = 0.009) Non-iron-deficiency without anemia vs. Iron-deficiency without anemia (p = 0.914), Non-iron-deficiency without anemia vs. Iron-deficient anemia (p = 0.663), Non-iron-deficiency without anemia vs. Anemia of chronic disease (p = 0.008).
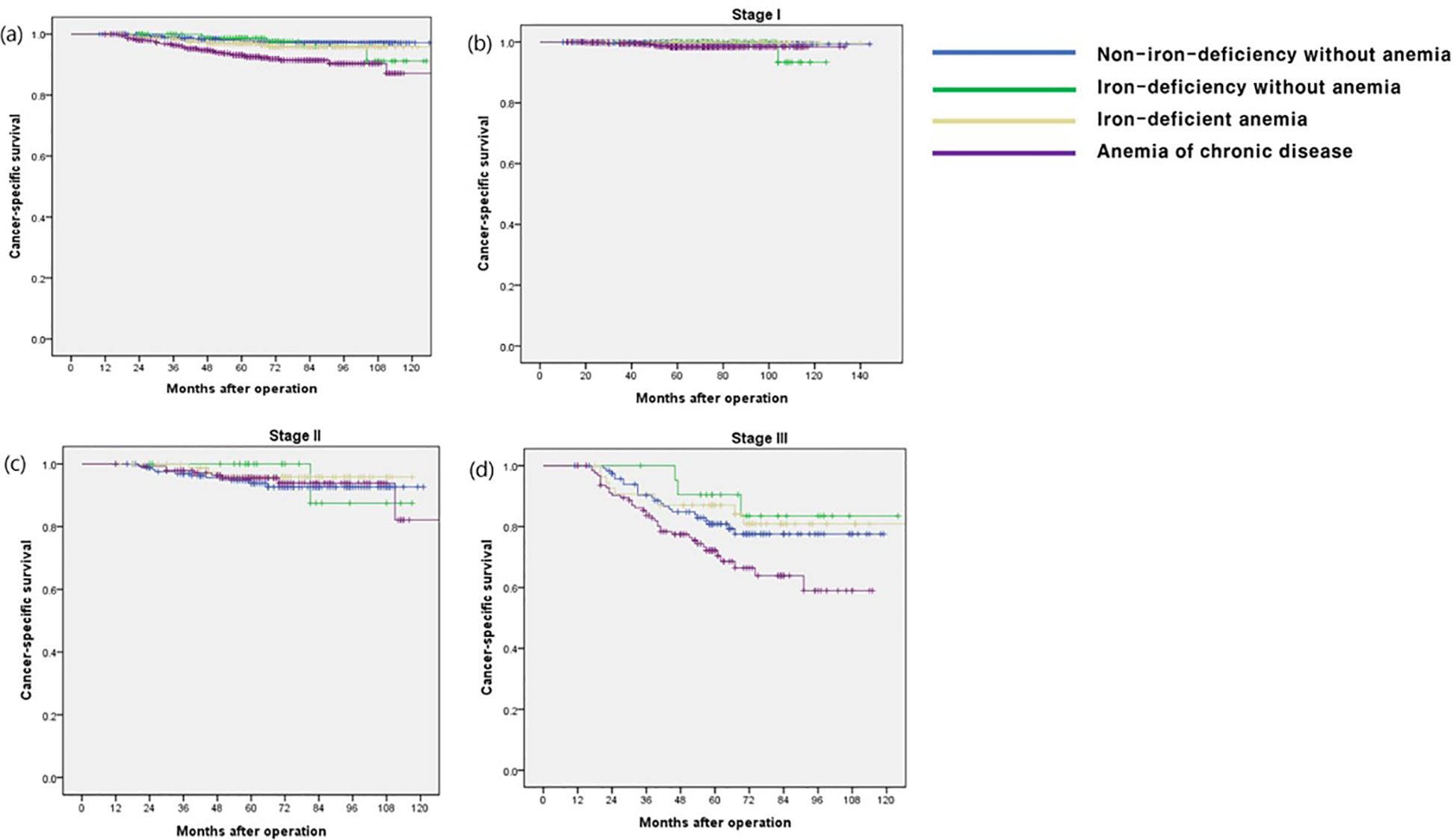
Figure 2. Cancer-specific survival according to postoperative 1 year ferritin-hemoglobin groups. (a) Total patients (p < 0.001) Non-iron-deficiency without anemia vs. Iron-deficiency without anemia (p = 0.697), Non-iron-deficiency without anemia vs. Iron-deficient anemia (p = 0.211), Non-iron-deficiency without anemia vs. Anemia of chronic disease (p < 0.001); (b) stage I (p = 0.124) Non-iron-deficiency without anemia vs. Iron-deficiency without anemia (p = 0.636), Non-iron-deficiency without anemia vs. Iron-deficient anemia (p = 0.979), Non-iron-deficiency without anemia vs. Anemia of chronic disease (p = 0.023); (c) stage II (p = 0.859), Non-iron-deficiency without anemia vs. Iron-deficiency without anemia (p =0.682), Non-iron-deficiency without anemia vs. Iron-deficient anemia (p = 0.411), Non-iron-deficiency without anemia vs. Anemia of chronic disease (p = 0.795); (d) stage III (p = 0.027) Non-iron-deficiency without anemia vs. Iron-deficiency without anemia (p = 0.392), Non-iron-deficiency without anemia vs. Iron-deficient anemia (p = 0.568), Non-iron-deficiency without anemia vs. Anemia of chronic disease (p = 0.035).
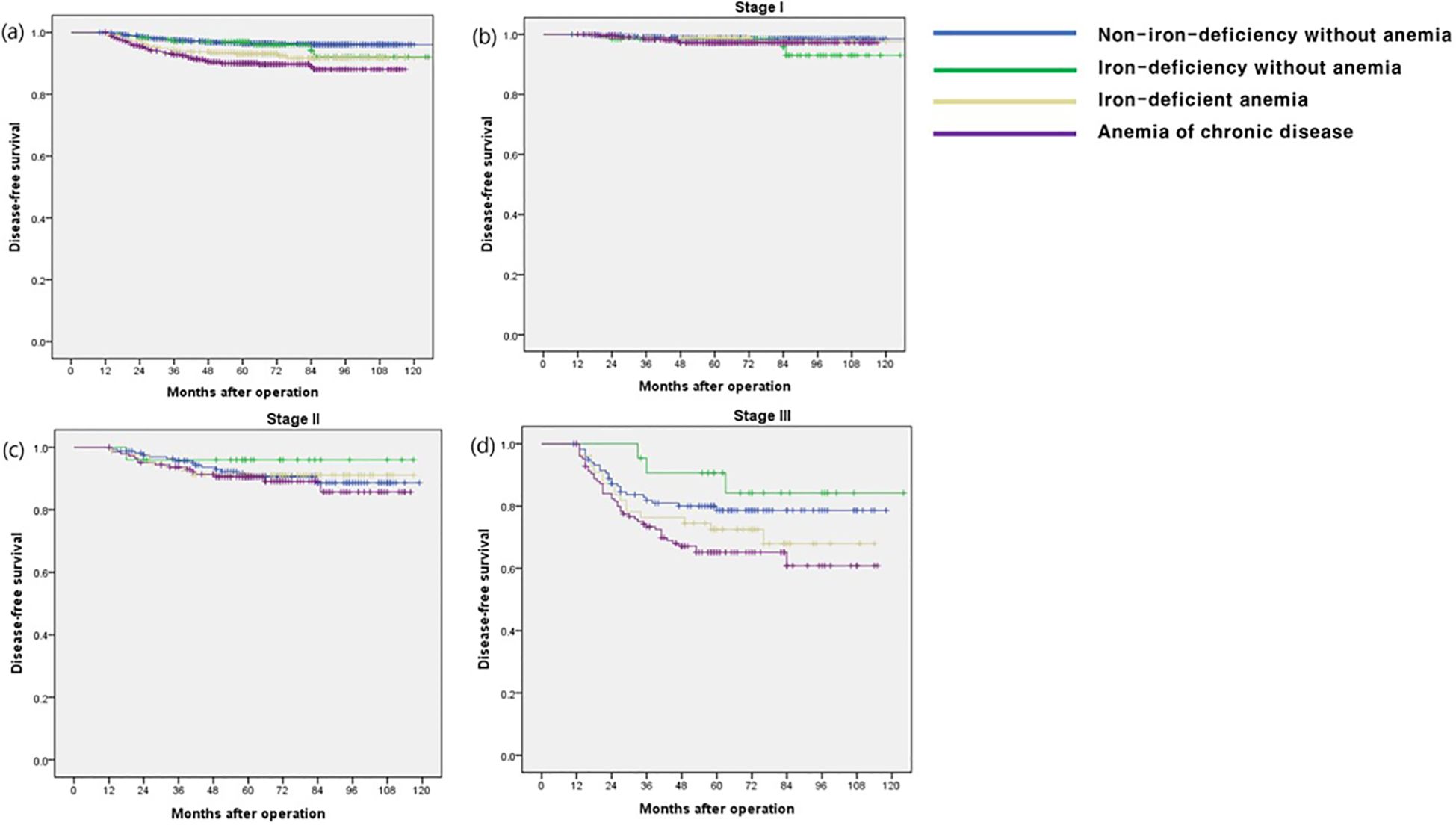
Figure 3. Disease-free survival according to postoperative 1 year ferritin-hemoglobin groups. (a) Total patients (p < 0.001) Non-iron-deficiency without anemia vs. Iron-deficiency without anemia (p =0.446), Non-iron-deficiency without anemia vs. Iron-deficient anemia (p = 0.001), Non-iron-deficiency without anemia vs. Anemia of chronic disease (p < 0.001); (b) stage I (p = 0.189) Non-iron-deficiency without anemia vs. Iron-deficiency without anemia (p = 0.118), Non-iron-deficiency without anemia vs. Iron-deficient anemia (p = 0.698), Non-iron-deficiency without anemia vs. Anemia of chronic disease (p = 0.070); (c) stage II (p = 0.772), Non-iron-deficiency without anemia vs. Iron-deficiency without anemia (p = 0.423), Non-iron-deficiency without anemia vs. Iron-deficient anemia (p = 0.908), Non-iron-deficiency without anemia vs. Anemia of chronic disease (p = 0.620); (d) stage III (p = 0.034) Non-iron-deficiency without anemia vs. Iron-deficiency without anemia (p = 0.379), Non-iron-deficiency without anemia vs. Iron-deficient anemia (p = 0.273), Non-iron-deficiency without anemia vs. Anemia of chronic disease (p =0.016).
In the multivariate Cox regression analysis, older age, higher ECOG grade, higher TNM stage, and ACD were independent risk factors for OS. In terms of CSS and DFS, higher TNM stage was an independent risk factor. In addition, the presence of ACD was a significant independent risk factor for OS [hazard ratio (HR) = 1.832; p < 0.001], CSS (HR, 1.690; p = 0.015), and DFS (HR, 1.714; p = 0.003) (Tables 2, Supplementary Tables S1, S2).
In multivariate logistic regression analysis, older age and higher TNM stage were independent risk factors for ACD (p < 0.001 and p < 0.001, respectively). The patients with lower PNI, presence of preoperative anemia, and early postoperative complications were significant risk factors for ACD (p = 0.026, p < 0.001, and p = 0.008, respectively) (Table 3).
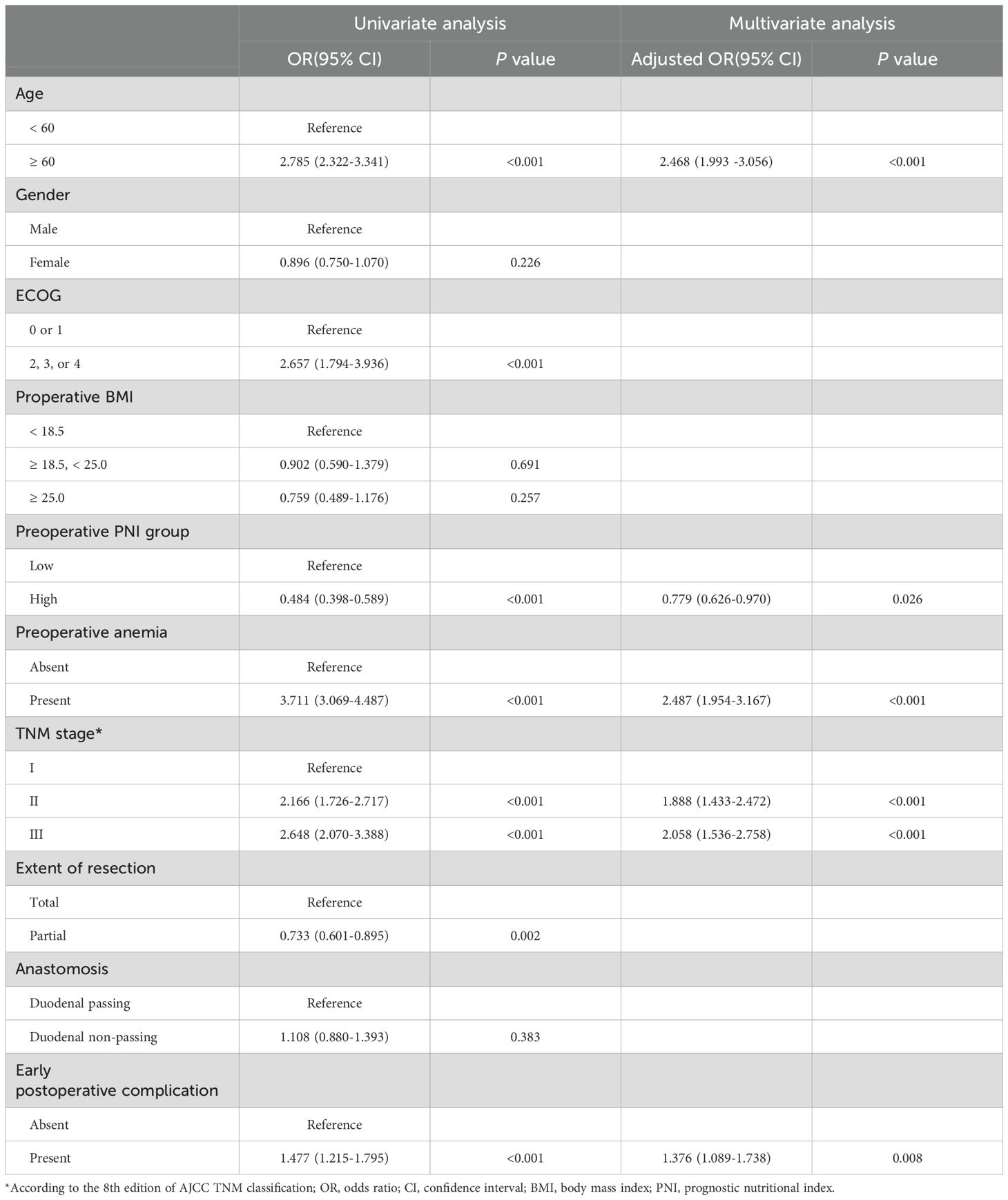
Table 3. Univariate and multivariate analyses of factors for the prediction of anemia of chronic disease.
The correlations between ACD and preoperative nutritional and inflammatory conditions are summarized in Table 4. In patients with ACD at postoperative 1 year, the total protein, albumin, triglyceride, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, Hb, iron, and calcium levels were significantly lower than the preoperative laboratory results (p < 0.001, p < 0.001, p = 0.001, p < 0.001, p = 0.006, p < 0.001, p = 0.026, and p < 0.001, respectively). Systemic inflammatory markers such as NLR and PLR were not significantly related to ACD occurrence (Table 4).
This study highlights a notable incidence of post-gastrectomy anemia, with a higher prevalence of ACD than IDA. Patients diagnosed with ACD tended to be older, had higher ECOG scores, experienced more early postoperative complications, and frequently had a more advanced stages of gastric cancer than the other groups. Importantly, the presence of ACD emerged as a significant risk factor for poor long-term OS, particularly evident in stages I and III.
Various manifestations of post-gastrectomy anemia in gastric cancer include association with morbidity and mortality, decreased quality of life, and poor functional status among patients with cancer (5, 17). Anemia impairs quality of life by causing a variety of symptoms ranging from mild headache and fatigue to impaired work capacity to cognitive impairment (5, 18).
In this study, the overall incidence of anemia was 36.6%, with IDA and ACD prevalences of 12.7% and 23.9%, respectively, at postoperative 1 year. Approximately eight studies had defined post-gastrectomy anemia and reported its incidence (5, 7, 8, 19–23). Similar to the current study, only two out of approximately eight studies specifically subclassified post-gastrectomy anemia and reported its detailed incidence (5, 20). According to the study published in 2012, the risk of anemia increases with time and affected 27% of patients at 1 year and 37% at 2 years after gastrectomy among patients with early gastric cancer (20). In this study, IDA predominated at approximately 12%, with ACD being < 5%. The discrepancy in the occurrence rates of ACD in our study is likely attributable to differences in the established ferritin cutoff values. Their study differed from ours in that it focused exclusively on anemia in early gastric cancer. In our study, at postoperative 1 year, 30.5% of stage I patients experienced anemia, showing similar results upon detailed analysis (data not-shown). In a study conducted in 2016, the occurrence of anemia was reported from 1 to 5 years post-gastrectomy, distinguishing only between IDA and vitamin B12 deficient anemia. The incidence of anemia was reported to be 18.7% at postoperative 1 year, with < 10% of cases with IDA, excluding preoperative anemia patients from the analysis (5).
Furthermore, other studies have reported heterogeneous incidence rates of anemia (7, 22). For instance, one study in 2019 reported a 27.4% incidence of anemia at 1 year post-gastrectomy (22), whereas another study in 2018 focusing solely on patients with stage I gastric cancer for up to 5 years post-surgery reported a 7.1% incidence of anemia at 1 year post-surgery (7). Additionally, other studies have reported post-gastrectomy incidence rates according to sex, extent of gastric resection, and reconstruction method (8, 19, 21). Exclusion criteria varied across studies, contributing to the observed heterogeneous results.
Interestingly, most of these studies have shown considerable interest in the incidence of anemia based on the reconstruction methods (7, 21, 24–26). Among them, Imamura et al. found that the reconstruction method independently influences post-gastrectomy anemia, with type Billroth-I anastomosis associated with less decline in Hb levels (24). While our study also examined anastomosis types, we did not observe any significant differences.
Several studies have analyzed anemia as a prognostic factor in gastric cancer (7, 27–31). However, most studies tend to focus on preoperative or pre-treatment anemia, with limited postoperative data available (27–31). To the best of our knowledge, only one previous study has investigated the correlation between post-gastrectomy anemia and long-term survival in patients with gastric cancer. In that study, patients with anemia had a significantly poorer long-term prognosis. However, the study did not provide detailed data for each anemia type (7).
In our study, ferritin level was an important parameter for the classification of anemia subtypes. Ferritin is the principal iron-binding protein, a transporter, and a recycler of iron (9, 10). Ferritin serves as a reservoir for iron, thus a decrease in its levels reflects iron deficiency, while elevated ferritin levels typically signify the presence of ACD (9, 10, 32). Ferritin not only aids in iron transport and recycling but also plays a role in cellular defense against oxidative stress and inflammation (10, 33). Increased serum ferritin levels are associated with various conditions including liver diseases, infections, inflammatory conditions, and malignancy (9, 10, 33). In particular, ferritin can be produced by the tumor microenvironment in solid tumors (10). Therefore, ferritin is considered an oncofetal protein and has been implicated as a tumor marker in lung, breast, and renal cancers (34–36). In recent years, there has been an increased interest in ferritin as a marker of iron overload, reflecting oxidative stress, the pro-inflammatory role of iron in carcinogenesis, and pathological nutritional status (9, 10, 32, 33, 37). Furthermore, serum ferritin levels have been suggested to have diagnostic value and are also useful for evaluating treatment efficacy and prognosis in various cancer (11–14, 34–36, 38). However, the prognostic role of serum ferritin in gastric cancer remains controversial. Approximately only two studies have demonstrated the prognostic role of ferritin in gastric cancer (15, 16). One study compared pre- and post-treatment ferritin levels in patients with gastric cancer, observing an increase in ferritin levels in non-responsive cases and a significant decrease in responsive cases (16). Another study found a positive correlation between serum ferritin levels and sarcopenia, and a high serum ferritin level was an independent poor prognostic factor (15). Recent studies have suggested that combining multiple tumor markers may improve the prognostic accuracy for gastric cancer patients. For instance, a study demonstrated that a combination of three important tumor markers—CEA, CA72-4, and CA19-9—provided significantly better prognostic value than individual markers alone (39). In addition, emerging research also emphasizes the role of BMI in the prognosis and treatment response of cancer patients (40). This suggests that a multifactorial approach, incorporating ferritin along with other biomarkers, may provide more robust insights into the long-term prognosis of gastric cancer patients in the future.
Several studies have contributed to our understanding of the association between elevated ferritin levels and poor prognosis in gastric cancer (15, 41). A 2020 study concluded that iron overload may be related to muscle loss in patients with gastric cancer cachexia and that elevated ferritin levels are associated with muscle wasting (41). In a 2023 study, preoperative serum ferritin levels were associated with sarcopenia and the prognosis of gastric cancer (15).
Other studies on gastric cancer have explored the different aspects of ferritin levels (42–44). In 2023, a systematic review and meta-analysis investigated the diagnostic significance of ferritin levels in gastric cancer (42). A 2020 study examined postoperative ferritin levels as a nutritional factor (43), whereas a 2022 study identified postoperative ferritin deficiency as a common complication in patients with gastric cancer, analyzing the predictive factors for ferritin deficiency (44).
Our study has several strengths. First, it was based on reliable large-scale data collected from multiple centers over an extended period. Second, parameters such as Hb and ferritin, which were presented in our study, were routinely assessed during follow-up, requiring no additional effort to evaluate. These markers are easy to measure, minimally invasive, and cost effective. Third, our study makes a significant contribution by classifying post-gastrectomy anemia types and elucidating their association with long-term prognosis in gastric cancer patients. We found that the classification of anemia is crucial for identifying factors that influence long-term outcomes. Specifically, our detailed categorization based on hemoglobin and ferritin levels provides valuable insights into the prognostic implications of anemia, highlighting its potential utility as a marker for monitoring gastric cancer progression after gastrectomy. This nuanced approach to anemia classification represents a key innovation of our study, offering a novel perspective that may improve clinical management and patient outcomes. Fourth, our study provides valuable clinical insights into early interventions for high-risk patients, particularly emphasizing the importance of nutritional support, such as iron supplementation or dietary adjustments, to prevent further anemia progression and improve long-term outcomes. Additionally, we highlight the need for close monitoring, including radiologic and tumor marker assessments, to detect potential recurrence in high-risk patients. These findings contribute to a more comprehensive approach to managing post-gastrectomy anemia and improving the overall prognosis of gastric cancer patients. Therefore, Hb and ferritin levels may serve as valuable alternative markers for monitoring disease progression during follow-up. Lastly, our study uniquely concentrated on ACD in post-gastrectomy patients and investigated its impact on long-term survival, analyzing the risk factors for ACD occurrence for the first time to our knowledge. This enables clinicians to identify and prevent efforts aimed at understanding and preventing the risk factors for the development of ACD during long-term postoperative follow-up.
Despite its strengths, our study has several limitations. First, owing to its retrospective nature, inherent biases may have influenced the findings. Second, the lack of standardized protocols and the variability in anemia management, combined with the retrospective design of this study, limited the ability to control for treatment variables related to postoperative anemia, such as iron supplementation and blood transfusion, which may have influenced the postoperative 1-year anemia status of patients. Due to the retrospective study, we encountered challenges in systematically tracking the exact timing and details of iron supplementation or blood transfusions after surgery. While some patients may have received treatment for anemia, based on clinical discretion, we were unable to collect comprehensive data on these interventions for all patients. Third, the absence of time-dependent serial tests for post-gastrectomy anemia represents another limitation. Finally, this study relied on limited laboratory results for anemia. In particular, vitamin B12 results was excluded from the results of this study. There have been investigations into the role of total iron-binding capacity and hematocrit levels in gastric cancer prognosis. Therefore, comprehensive, well-designed prospective studies are warranted to address these limitations and enhance our understanding of post-gastrectomy anemia in patients with gastric cancer in the future.
The relationship between ferritin and Hb at postoperative 1 year are significant prognostic factor for overall survival in patients with gastric cancer. In particular, the presence of ACD can be a more specific predictor of gastric cancer. Therefore, clinicians should pay attention to and correct ACD in outpatient clinics during postoperative follow-up.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by The Catholic University of Korea, Catholic Medical Center. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because at present, obtaining consent from research participants is practically infeasible. There are no indications that participants would refuse consent, and waiving the consent process will have minimal impact on them. The participants have already undergone surgery, treatment, and tests at the hospital, so the research does not add additional risk or negatively affect their dignity, rights, or welfare. The study will use clinical information obtained from medical records and past tests, avoiding the collection of unnecessary personal data. Any inadvertently collected personal information will be handled with strict security and confidentiality. Personal data will not be disclosed outside the research team, and databases will be maintained and disposed of according to established regulations in a coded format to ensure data integrity. The research team will adhere to the Helsinki Declaration and make every effort to uphold the ethical standards of medical research.
EK: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. KS: Conceptualization, Data curation, Investigation, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing. DK: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Research Foundation of Korea (NRF-2022R1C1C1011459) Grant funded by Korean Government.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1487477/full#supplementary-material
1. Park EH, Jung KW, Park NJ, Kang MJ, Yun EH, Kim HJ, et al. Cancer statistics in korea: incidence, mortality, survival, and prevalence in 2021. Cancer Res Treat. (2024) 56:357–71. doi: 10.4143/crt.2024.253
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
2. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2024) 74(3):229–63. doi: 10.3322/caac.21834
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
3. Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2021. Gastric Cancer. (2023) 26:1–25. doi: 10.1007/s10120-022-01331-8
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
4. Carrillo Lozano E, Osés Zárate V, Campos Del Portillo R. Nutritional management of gastric cancer. Endocrinol Diabetes Nutr (Engl Ed). (2021) 68:428–38. doi: 10.1016/j.endien.2020.09.005
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
5. Jun JH, Yoo JE, Lee JA, Kim YS, Sunwoo S, Kim BS, et al. Anemia after gastrectomy in long-term survivors of gastric cancer: A retrospective cohort study. Int J Surg. (2016) 28:162–8. doi: 10.1016/j.ijsu.2016.02.084
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
6. Xishan Z, Ye Z, Feiyan M, Liang X, Shikai W. The role of prognostic nutritional index for clinical outcomes of gastric cancer after total gastrectomy. Sci Rep. (2020) 10:17373. doi: 10.1038/s41598-020-74525-8
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
7. Kim JH, Bae YJ, Jun KH, Chin HM. The prevalence and clinical significance of postgastrectomy anemia in patients with early-stage gastric cancer: A retrospective cohort study. Int J Surg. (2018) 52:61–6. doi: 10.1016/j.ijsu.2018.02.037
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
8. Kim KH, Park DJ, Park YS, Ahn SH, Park DJ, Kim HH. Actual 5-year nutritional outcomes of patients with gastric cancer. J Gastric Cancer. (2017) 17:99–109. doi: 10.5230/jgc.2017.17.e12
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
9. Garcia-Casal MN, Pasricha SR, Martinez RX, Lopez-Perez L, Peña-Rosas JP. Serum or plasma ferritin concentration as an index of iron deficiency and overload. Cochrane Database Syst Rev. (2021) 5:Cd011817. doi: 10.1002/14651858.CD011817.pub2
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
10. Liang W, Ferrara N. Iron metabolism in the tumor microenvironment: contributions of innate immune cells. Front Immunol. (2020) 11:626812. doi: 10.3389/fimmu.2020.626812
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
11. Lee S, Eo W, Jeon H, Park S, Chae J. Prognostic significance of host-related biomarkers for survival in patients with advanced non-small cell lung cancer. J Cancer. (2017) 8:2974–83. doi: 10.7150/jca.20866
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
12. Demir H, Beypinar I, Urvay S, Davarcı SE, Baykara M. Prognostic role of pre-operative serum ferritin level in stage 2 colon cancer. Eur Rev Med Pharmacol Sci. (2021) 25:6473–9. doi: 10.26355/eurrev_202111_27091
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
13. Lin S, Fang Y, Lin Y, Mo Z, Hong X, Jian Z, et al. Meta-analysis of the prognostic value of pretreatment serum ferritin in hepatobiliary and pancreas (HBP) cancers. BMJ Open. (2021) 11:e040801. doi: 10.1136/bmjopen-2020-040801
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
14. Wu SJ, Zhang ZZ, Cheng NS, Xiong XZ, Yang L. Preoperative serum ferritin is an independent prognostic factor for liver cancer after hepatectomy. Surg Oncol. (2019) 29:159–67. doi: 10.1016/j.suronc.2019.05.013
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
15. Zhou D, Zeng C, Zhang L, Gao X, Li G, Wang X. Serum ferritin is associated with sarcopenia and predicts long-term survival for gastric cancer undergoing radical gastrectomy. Eur J Gastroenterol Hepatol. (2023) 35:1341–8. doi: 10.1097/MEG.0000000000002659
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
16. Huang X, Wang Q, Chen Q, Ye J, Chen G, Wu Q. Analysis of serum ferritin level and its clinical value in advanced gastric cancer. J Biosci Med (Irvine). (2022) 10:1–6. doi: 10.4236/jbm.2022.101001
17. Yu W, Chung HY. Quality of life and nutritional outcomes of billroth I and billroth II reconstruction. J Korean Gastric Cancer Assoc. (2002) 2:91–5. doi: 10.5230/jkgca.2002.2.2.91
18. Brittenham GM, Moir-Meyer G, Abuga KM, Datta-Mitra A, Cerami C, Green R, et al. Biology of anemia: A public health perspective. J Nutr. (2023) 153 Suppl 1:S7–s28. doi: 10.1016/j.tjnut.2023.07.018
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
19. Lim J, Yoo MW, Kang SY, Park HS. Long-term changes in the metabolic and nutritional parameters after gastrectomy in early gastric cancer patients with overweight. Asian J Surg. (2019) 42:386–93. doi: 10.1016/j.asjsur.2018.06.011
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
20. Lim CH, Kim SW, Kim WC, Kim JS, Cho YK, Park JM, et al. Anemia after gastrectomy for early gastric cancer: long-term follow-up observational study. World J Gastroenterol. (2012) 18:6114–9. doi: 10.3748/wjg.v18.i42.6114
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
21. Cho M, Son T, Kim HI, Noh SH, Choi S, Seo WJ, et al. Similar hematologic and nutritional outcomes after proximal gastrectomy with double-tract reconstruction in comparison to total gastrectomy for early upper gastric cancer. Surg Endosc. (2019) 33:1757–68. doi: 10.1007/s00464-018-6448-x
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
22. Yang IJ, Kim D-W, Jee YS. Risk factor of anemia after a gastrectomy in patients with gastric cancer. Surg Metab Nutr. (2019) 10:15–9. doi: 10.18858/smn.2019.10.1.15
23. Jung MJ, Kim H-I, Hw C, Hy Y, Kim CB. Pre- and post-gastrectomy anemia in gastric cancer patients. Korean J Clin Oncol. (2011) 7:88–95. doi: 10.14216/kjco.11024
24. Imamura T, Komatsu S, Ichikawa D, Kosuga T, Okamoto K, Konishi H, et al. Reconstruction method as an independent risk factor for the postoperative decrease in hemoglobin in stage I gastric cancer. J Gastroenterol Hepatol. (2016) 31:959–64. doi: 10.1111/jgh.2016.31.issue-5
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
25. Kim JH, Bae YJ, Jun KH, Chin HM. Long-term trends in hematological and nutritional status after gastrectomy for gastric cancer. J Gastrointest Surg. (2017) 21:1212–9. doi: 10.1007/s11605-017-3445-7
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
26. Jun B, Nian L, Shan H, Hong-Jun Y, Heng-Yi D, Wu W, et al. Effects of different gastrointestinal reconstruction techniques on nutrition, anemia, and quality of life in laparoscopic distal gastrectomy for gastric cancer. Acta Cir Bras. (2022) 37:e370408. doi: 10.1590/acb370408
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
27. Huang XZ, Yang YC, Chen Y, Wu CC, Lin RF, Wang ZN, et al. Preoperative anemia or low hemoglobin predicts poor prognosis in gastric cancer patients: A meta-analysis. Dis Markers. (2019) 2019:7606128. doi: 10.1155/2019/7606128
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
28. Jiang J, Ouyang J, Liu S, Chen J, Zhang H, Wang C, et al. The prognostic impact of pretreatment anemia in patients with gastric cancer and nonhypoalbuminemia undergoing curative resection: a retrospective study. Ann Transl Med. (2021) 9:1046. doi: 10.21037/atm-21-1649
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
29. Chang CC, Sun JT, Chen JY, Chen YT, Li PY, Lee TC, et al. Impact of peri-operative anemia and blood transfusions in patients with gastric cancer receiving gastrectomy. Asian Pac J Cancer Prev. (2016) 17:1427–31. doi: 10.7314/APJCP.2016.17.3.1427
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
30. Kunishige T, Migita K, Matsumoto S, Wakatsuki K, Nakade H, Aoki S, et al. The prognostic significance of preoperative anemia in gastric cancer patients. In Vivo. (2022) 36:2314–22. doi: 10.21873/invivo.12962
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
31. Liu X, Qiu H, Huang Y, Xu D, Li W, Li Y, et al. Impact of preoperative anemia on outcomes in patients undergoing curative resection for gastric cancer: a single-institution retrospective analysis of 2163 Chinese patients. Cancer Med. (2018) 7:360–9. doi: 10.1002/cam4.2018.7.issue-2
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
32. Madu AJ, Ughasoro MD. Anaemia of chronic disease: an in-depth review. Med Princ Pract. (2017) 26:1–9. doi: 10.1159/000452104
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
33. Moreira AC, Mesquita G, Gomes MS. Ferritin: an inflammatory player keeping iron at the core of pathogen-host interactions. Microorganisms. (2020) 8(4):589. doi: 10.3390/microorganisms8040589
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
34. Kirkali Z, Esen AA, Kirkali G, Güner G. Ferritin: a tumor marker expressed by renal cell carcinoma. Eur Urol. (1995) 28:131–4. doi: 10.1159/000475037
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
35. Zhou J, Diao X, Wang S, Yao Y. Diagnosis value of combined detection of serum SF, CEA and CRP in non-small cell lung cancer. Cancer Manag Res. (2020) 12:8813–9. doi: 10.2147/CMAR.S268565
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
36. Fadavi P, Nafisi N, Hariri R, Novin K, Sanei M, Razzaghi Z, et al. Serum ferritin, vitamin D and pathological factors in breast cancer patients. Med J Islam Repub Iran. (2021) 35:162. doi: 10.47176/mjiri.35.162
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
37. Velasco-Rodriguez R, Perez-Hernandez MG, Mora-Brambila AB, Bazan-Arellano DA, Vasquez C. Serum ferritin and nutritional status in older adults at eldercare facilities. J Nutr Health Aging. (2012) 16:525–8. doi: 10.1007/s12603-012-0013-4
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
38. Ramírez-Carmona W, Díaz-Fabregat B, Yuri Yoshigae A, Musa de Aquino A, Scarano WR, de Souza Castilho AC, et al. Are serum ferritin levels a reliable cancer biomarker? A systematic review and meta-analysis. Nutr Cancer. (2022) 74:1917–26. doi: 10.1080/01635581.2021.1982996
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
39. Zhang R, Chen X, Chen G, Zhao Z, Wei Y, Zhang F, et al. Combined use of tumor markers in gastric cancer: A novel method with promising prognostic accuracy and practicality. Ann Surg Oncol. (2023) 30:8561–71. doi: 10.1245/s10434-023-14194-9
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
40. Nie RC, Chen GM, Wang Y, Yuan SQ, Zhou J, Duan JL, et al. Association between body mass index and survival outcomes in patients treated with immune checkpoint inhibitors: meta-analyses of individual patient data. J Immunother. (2021) 44:371–5. doi: 10.1097/CJI.0000000000000389
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
41. Zhou D, Zhang Y, Mamtawla G, Wan S, Gao X, Zhang L, et al. Iron overload is related to muscle wasting in patients with cachexia of gastric cancer: using quantitative proteome analysis. Med Oncol. (2020) 37:113. doi: 10.1007/s12032-020-01439-w
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
42. Deng D, Zhang Y, Zhang R, Yi J, Dong J, Sha L, et al. Circulating proteins and metabolite biomarkers in gastric cancer: A systematic review and meta-analysis. Arch Med Res. (2023) 54:124–34. doi: 10.1016/j.arcmed.2022.12.012
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
43. Veeralakshmanan P, Tham JC, Wright A, Bolter M, Wadhawan H, Humphreys LM, et al. Nutritional deficiency post esophageal and gastric cancer surgery: A quality improvement study. Ann Med Surg (Lond). (2020) 56:19–22. doi: 10.1016/j.amsu.2020.05.032
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
Keywords: anemia, ferritin, hemoglobin, gastrectomy, gastric cancer
Citation: Kim EY, Song KY and Kim DJ (2025) Post-gastrectomy anemia and ferritin dynamics: key determinants of prognosis and clinical management in patients with gastric cancer. Front. Oncol. 15:1487477. doi: 10.3389/fonc.2025.1487477
Received: 28 August 2024; Accepted: 24 February 2025;
Published: 14 March 2025.
Edited by:
Sharon R. Pine, University of Colorado Anschutz Medical Campus, United StatesReviewed by:
Run-Cong Nie, Sun Yat-sen University Cancer Center (SYSUCC), ChinaCopyright © 2025 Kim, Song and Kim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dong Jin Kim, ZGpkamNhcEBjYXRob2xpYy5hYy5rcg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.