
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol., 07 April 2025
Sec. Hematologic Malignancies
Volume 15 - 2025 | https://doi.org/10.3389/fonc.2025.1484820
Background: Rosai Dorfman disease (RDD), also known as sinus histiocytosis with extensive lymph node involvement, is a rare histiocytosis with unknown etiology. Liver RDD is relatively rare and is often reported in individual cases. Exploring the imaging and pathological features of extrahepatic Rosai Dorfman disease (RDD) and conducting a comprehensive analysis of reported cases in domestic and foreign literature to enhance understanding of this rare disease.
Methods: We collected data from a patient diagnosed with liver RDD in our hospital. In addition, we searched for liver RDD cases through PubMed and conducted a literature review.
Results: Of the patient data used in this study, 1 patient’s data was obtained from our hospital records and 16 were retrieved from literature. There were 6 males and 11 females aged between 2-72 years, with an average age of 33.55 ± 19.38 years. Four patients presented with isolated liver nodules without the involvement of lymph nodes or extranodal organs, two patients presented with a case that involved extranodal organs but not lymph nodes, two patients presented with a case involving lymph nodes but not extranodal organs, eight patients presented with a case involving both lymph nodes and extranodal organs and lastly one patient presented with a case that did not involve either lymph nodes or extranodal organs. Four of the patients underwent enhanced scanning. The results revealed that 2 of them showed low enhancement, and the other 2 showed no significant enhancement. Only 1 case underwent PET-CT, and the results indicated a high uptake. Immunohistochemistry was performed on all 17 cases of liver RDD, and the results were positive for CD1a (-), CD68 (+), and S-100 (+).
Conclusions: Liver RDD can manifest at any age. It presents with nonspecific imaging findings and a challenging preoperative diagnosis. Patients with extranodal hepatic RDD present with typical RDD characteristic immunohistochemistry features such as, high uptake on PET-CT, and no to mild enhancement on contrast-enhanced scans. For an early diagnosis, it is beneficial to fully comprehend these traits.
Rosai Dorfman disease (RDD), sometimes referred to as sinus histiocytosis with large lymphadenopathy (SHML), is a rare non-Langerhans cell histiocytosis that affects the extranodal areas and lymph nodes. Now often known as Rosai Dorfman disease or syndrome (RDD), it was first characterized by Azoury and Rreed. Later, Rosai and Ddorfman classified it as a benign course (1). Typical histological features of RDD include S-100 protein immunostaining positivity and CD1a negativity with tissue cell proliferation and epidermal hyperplasia. The most common site of RDD involvement is the lymph nodes, although extranodal involvement is occasionally noted. A case of extranodal intrahepatic RDD is one in which the liver is affected but no lymph nodes are involved. It is still quite uncommon, with most examples being documented as isolated incidents (2, 3). The most frequently observed extranodal locations are the skin, upper respiratory tract, and soft tissue. Additionally, reports of testes, thyroid gland, mammary glands, kidneys, and central nervous system involvement have been made (4, 5).
Therefore, this report aims to advance knowledge of RDD by comprehensively examining the imaging and clinical features of 17 patients who had been diagnosed with extranodal intrahepatic RDD.
Relevant data of one patient with primary liver RDD confirmed by postoperative pathology in August 2023 were analyzed retrospectively and selected. Further 16 patients’ data diagnosed with liver RDD were collected after conducting a thorough literature review. The study was approved by the Ethics Committee of Shaanxi Province’s Baoji Central Hospital and written informed consent was waived due to the nature of the study.
The patient from our hospital had undergone an upper abdominal ultrasound examination before being transferred to the outpatient department. After hospitalization, plain and three-phase enhanced scans were performed on 256 row single source dual energy CT (GE, Revolution CT Xstream Edition). The parameters of the conventional sequence included instantaneous switching of tube voltage between 80 kVp and 140 kVp, tube current of 200-400 mA, layer thickness and spacing of 5 mm, reconstruction layer thickness of 1.25 mm, detector width of 80mm, tube rotation time of 0.5s, pitch of 0.992:1, and FOV of 35 cm x 35 cm. Iodohexanol (300 mgI/mL, dose of 1.2 mL/kg body mass) was used as the contrast agent for enhanced scanning and was injected at a rate of 3.0-3.5 mL/s through the anterior elbow vein. Following the injection of the contrast agent for 30 s, 60 s, and 120 s, arterial, venous, and delayed phase images were obtained and analyzed by two senior radiologists.
The literature search using keywords “liver” and “Rosai Dorfman”, retrieved a total of 38 articles. After screening, a total of 16 cases of liver RDD were included. The confirmed liver RDD diagnosis data retrieved from our hospital was added, resulting in a total of 17 patients. The inclusion criteria for patients in this study included: (1) Patients diagnosed with liver RDD through pathological examination; (2) Case reports and literature related to liver RDD disease. The exclusion criteria included: (1) Case reports that did not include the full text of the literature, (2) duplicate reports, including the same case reported by the same author in different articles.
Of the 17 patients with liver RDD, 6 were male and 11 were female, ranging in age from 2 to 72 years, with an average age of (33.55 ± 19.38) years; the patient’s prognosis was usually good, with only 2 deaths from the disease (Table 1).
Results from the imaging analysis showed that 4 patients presented with a case of isolated liver nodules without the involvement of lymph nodes or extranodal organs, 2 patients presented with a case involving extranodal organs but not lymph nodes, 2 patients presented a case involving lymph nodes but not extranodal organs, 8 patients presented a case that involved lymph nodes and extranodal organs, and finally, 1 patient presented with a case without involvement of either lymph nodes and extranodal organs. Among them, 4 patients underwent enhanced scanning, of which 2 cases showed low enhancement and 2 cases showed no significant enhancement. Moreover, 2 patients underwent PET-CT and showed a high uptake. as illustrated in Table 2.
A significant quantity of lymphoid tissue proliferation was detected in all 17 individuals, as well as the development of lymphoid follicles. A substantial amount of plasma cells and abundant light-stained cytoplasm were found between the follicles, with huge oval-shaped cells clustered together. Lymphocytes were detected in the cytoplasm in affected cells, and neutrophils, lymphocytes, and plasma cells with intact phagocytic morphology were locally observed. The results from immunohistochemistry showed patients presented with classic immunophenotypical results which were the expression of CD68 (+), and S-100 (+) but not CD1a (-) (Table 2).
The patient diagnosed with isolated RDD in the left lobe of the liver was a 66-year-old woman. Results from the Ultrasound showed a hypoechoic mass in the left lobe of the liver with clear boundaries. Color Doppler flow imaging (CDFI) revealed a small amount of blood flow signal around the mass (Figure 1A), and CT results showed an exogenous nodule of approximately 3.0 cm x 2.9 cm in size in the left outer lobe of the liver, with smooth edges, clear boundaries, and uniform density. Furthermore, the enhanced scan showed mild enhancement in the arterial phase, sustained weak enhancement in the portal phase, and slightly reduced enhancement in the delayed phase. There was no sign of cystic necrosis or external invasion within the lesion, and the adjacent perihepatic space was clear (Figures 1B–E). Significant lymphoid tissue proliferation was observed under the microscope, along with the development of different-sized lymphoid follicles. In the interstitial region of the follicles, a large number of plasma cells and an abundance of rich, pale cytoplasm were visible, and huge, round-nucleated cells were clustered together. Figure 2 shows the presence of lymphatic cells in the cytosol. The affected cells expressed CD68 (+) and S-100 (+), but not CD1a (-) as illustrated by immunohistochemistry analysis.
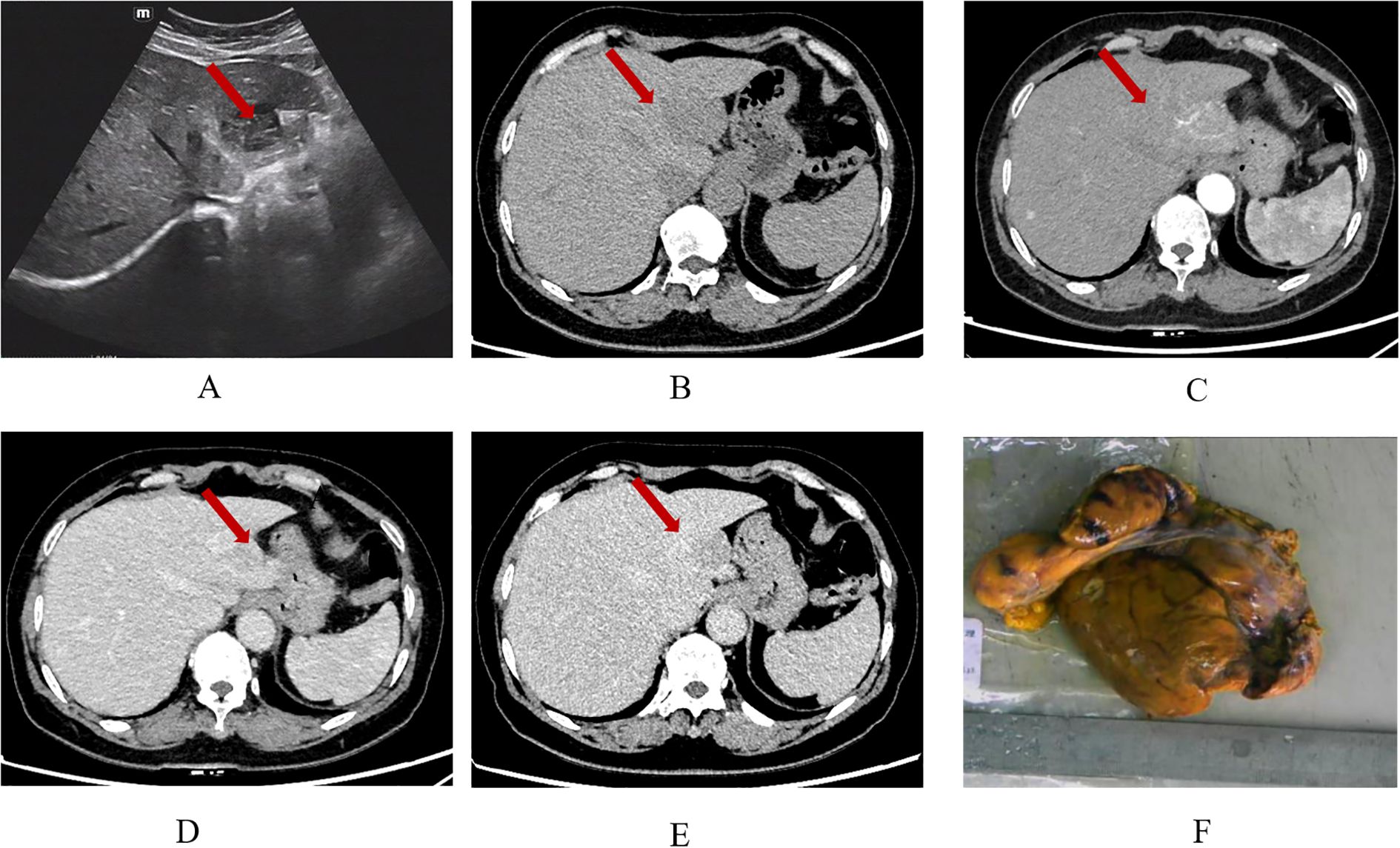
Figure 1. Ultrasound shows a hypoechoic nodule in the left outer lobe of the liver, with clear boundaries and uneven internal echoes (A); CT plain scan (B) shows a low-density shadow of about 3.0 x 2.9 cm in size in the left lobe of the liver, with smooth edges and clear boundaries. The plain scan density is uniform, with a CT value of about 49 HU. In the arterial phase (C) of the enhanced scan, the lesion shows moderate and uneven enhancement, with a CT value of about 77 HU. The accessory hepatic artery originating from the left gastric artery can be seen to participate in blood supply. In the portal phase (D), the lesion is further enhanced, with a CT value of about 87 HU. In the delayed phase (E), the enhancement slightly decreases, with a CT value of about 75 HU. Gross examination of the tumor specimen (F).
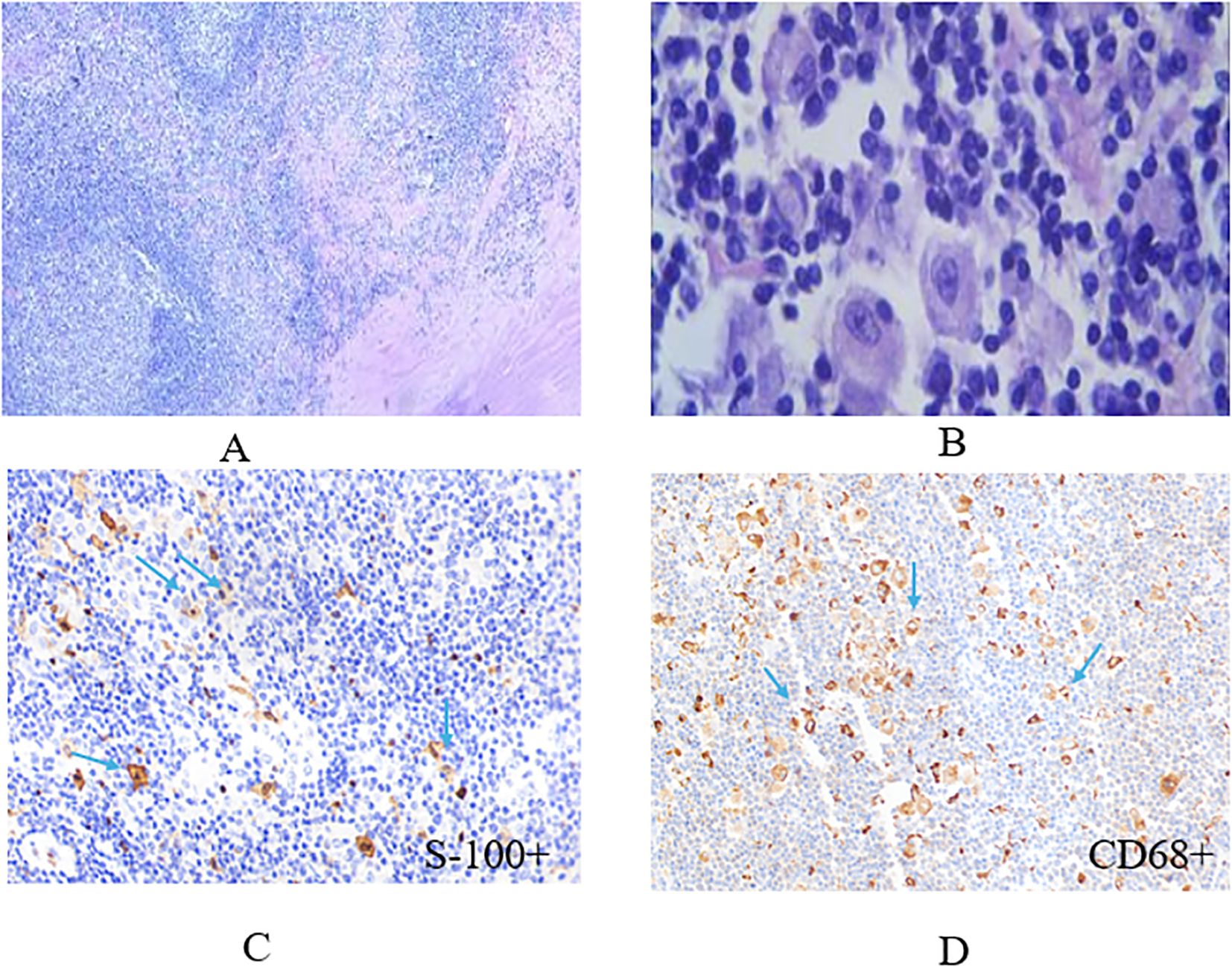
Figure 2. The representative image of histiocytes with eosinophilic nucleolus stained by hematoxylin and eosin (HE) method. Some dual or multinucleated occasionally represent a lymphophagocytosis (emperipolesis, left image: x10, right image: x40) (A, B), histiocytes stained positive for S-100 marker and CD68 with immunohistochemistry (IHC) technique (x40) (C, D).
Rosai Dorfman disease was defined as a lymphohematopoietic system malignancy by the WHO in 2006. In the 2016 updated classification of tissue cells and tumors, RDD is categorized as the R group, which includes familial, classical (lymph node type), extranodal, tumor-related, and autoimmune disease-related types (6–8). Numerous theories regarding the pathophysiology of RDD have been established, but its etiology remains unclear. Various etiologies have been speculated to cause RDD and these include immunodeficiency, infectious factors, autoimmune diseases, instability in the cellular microenvironment, and genetic mutations (9–11).
RDD can occur at any age but is more common in children and adolescents. This disease mainly affects African American males with a male-to-female ratio of 3:1 (12). From our findings, the 66-year-old woman presented with a classic type of RDD, belonging to the extranodal type. The lymph nodes were affected bilaterally, they were large painless cervical lymph node disease with or without intermittent fever. The patient experienced night sweats and weight loss. In some cases, mediastinal, axillary, and inguinal lymph nodes may also be affected, but retroperitoneal lymph nodes are rarely affected. Externally, the skin, soft tissue, upper respiratory tract, bones, orbit, nasopharynx, salivary glands, central nervous system, testes, and endocrine glands were the most severely affected areas (4, 13).
With non-specific imaging features, liver RDD is frequently misinterpreted as granulomatous lesions or as other tumor-related lesions. It may show up as isolated or many, single or multiple diffuse liver lesions. Six of the 17 cases in this group had masses or nodules in the liver that were slightly low-density; the isolated liver nodules were all located in the left lobe of the liver. Three of the cases had liver lesions that were isolated, two cases presented with multiple liver lesions (3 or more), and one case had diffuse liver lesions (reduced T2WI signal and narrowing of the internal bile duct). Among them, 4 cases underwent enhanced scanning, of which 2 cases showed low enhancement, and the other 2 cases showed no significant enhancement.
It is necessary to differentiate RDD from the following illnesses when it manifests as single nodules in the liver; (1) Mass or nodular lymphoma: As a common hypovascular tumor, liver lymphoma often exhibits gradually mild to moderate enhancement on dynamic contrast-enhanced scans, with a rather uniform enhancement. Since lymphoma develops in the liver interstitium, certain cancers may exhibit intrinsic liver blood vessels on contrast-enhanced imaging, which frequently results in the so-called “vascular floating syndrome (14, 15).” The surrounding blood vessels around the lesion are primarily characterized by narrowing, compression, and deformation with visible continuous vascular shadows (a classic manifestation of lymphoma. (2) Intrahepatic cholangiocarcinoma: This cancer manifests as low-density on plain scan. It causes adjacent liver capsule retraction, surrounding bile duct dilation, arterial phase peripheral ring enhancement, and gradual centripetal enhancement at the lesion center. The DWI shows target signs, indicating inconsistency between peripheral and internal information. Gadolinium acid cloud, with a high signal in the center and low signal in the periphery of liver and gallbladder lesions, represent a large number of live tumor cells in the surrounding area and varying degrees of fibrosis in the center (16). (3) Atypical hepatocellular carcinoma: When hepatocellular carcinoma does not have fast in and out typical enhancement features, it is difficult to diagnose. In China, liver cancer often occurs based on the development of hepatitis and cirrhosis. The combined effect of a medical history and AFP examination, can to successfully differentiate this disease (17).
To diagnose RDD, histopathological/immunohistochemical investigations are regarded as the gold standard. The findings from these analyses demonstrate that the afflicted liver’s overall mass is solid, with gray, white-gray cross-sections and a relatively hard texture. Certain areas of the liver may have a capsule. Low magnification reveals an uneven distribution of light and dark in the lesion area, while high magnification reveals tissue and cell proliferation in the bright area, with cells showing up as oval or polygonal in form, with plenty of cytoplasm, light staining, or eosinophilic red. A significant amount of atypia is present in the cells, and distinct binucleated cells and nucleoli are apparent. significant tissue cells also show different-sized vacuoles (18). In all 17 cases of this article, a significant amount of lymphoid tissue proliferation along with the development of lymphoid follicles was observed. Between the follicles, there were numerous plasma cells, a significant amount of cytoplasm, and large, oval-shaped cells with nuclei clustered together. Neutrophils, lymphocytes, and plasma cells with full phagocytic morphology were locally observed, and lymphocytes were discovered in the cytoplasm. For the diagnosis of RDD, immunohistochemical testing is crucial. While CD1a, CD207, and CD34 are not expressed by tissue cells, they frequently express S-100, CD68, and other proteins. S-100 protein (+) plays a significant role in the development of this disease (19). In 17 RDD tissues examined in this investigation, S-100, CD68, and CD163 were all positive whereas CD1a was negative.
In conclusion, extramedullary RDD of the liver is rare and only a few reports have been documented in the literature. Most of these reports have only provided a single aspect of either pathology or imaging. This study combines imaging and pathology to introduce the case and compares it with previous literature reports to comprehensively evaluate the disease and enhance our understanding of it.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
The studies involving humans followed the Declaration of Helsinki and it was approved by the Ethics Committee of the Baoji Central Hospital (ethical approval number: BZYL2022-14). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. The manuscript presents research on animals that do not require ethical approval for their study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
XZ: Data curation, Funding acquisition, Writing – original draft. HT: Formal Analysis, Investigation, Writing – review & editing. WL: Conceptualization, Data curation, Writing – review & editing. MY: Conceptualization, Writing – review & editing. CJ: Writing – review & editing. YP: Funding acquisition, Investigation, Writing – original draft.
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Baoji Health Committee Foundation of China (Grant Number 2024-035).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Rosai J, Dorfman RF. Sinus histiocytosis with massive lymphadenopathy. A newly recognized benign clinicopathological entity. Arch Pathol. (1969) 87:63–70.
2. Gazia C, Giordano L, Diodoro MG, Compalati I, Avantifiori R, Grazi GL. Differential diagnosis in Rosai-Dorfman disease: A rare case of isolated hepatic presentation mimicking a metastatic tumor with positive 18-FDG uptake. Intractable Rare Dis Res. (2022) 11:90–2. doi: 10.5582/irdr.2022.01037
3. Roosta Y, Esfahani A, Vahedi A, Tarvirizadeh K, Asoubar S, Boroofeh B, et al. Rosai-Dorfman disease presenting with solitary liver mass without lymphadenopathy: A case report. Clin Case Rep. (2021) 9:e04709. doi: 10.1002/ccr3.4709
4. Arabadzhieva E, Yonkov A, Bonev S, Bulanov D, Taneva I, Pirdopska T, et al. Rosai-Dorfman disease involving gallbladder and liver-Report of a case. Int J Surg Case Rep. (2015) 12:140–2. doi: 10.1016/j.ijscr.2015.06.001
5. Dong R, Gong L, Zhang W, et al. [Primary intraosseous Rosai-Dorfman disease: a clinicopathological analysis of fourteen cases].[J]. Zhonghua bing li xue za zhi = Chinese J Pathol. (2020) 49:904–9. doi: 10.3760/cma.j.cn112151-20191202-00772
6. Gwin K, Cipriani N, Zhang X, Schmidt R, Hyjek E. Bilateral breast involvement by disseminated extranodal Rosai-Dorfman disease. Breast J. (2011) 17:309–11. doi: 10.1111/j.1524-4741.2011.01072.x
7. Al-Daraji W, Anandan A, Klassen-Fischer M, Auerbach A, Marwaha JS, Fanburg-Smith JC. Soft tissue Rosai-Dorfman disease: 29 new lesions in 18 patients, with detection of polyomavirus antigen in 3 abdominal cases. Ann Diagn Pathol. (2010) 14:309–16. doi: 10.1016/j.anndiagpath.2010.05.006
8. Emile JF, Abla O, Fraitag S, Horne A, Haroche J, Donadieu J, et al. Revised classification of histiocytoses and neoplasms of the macrophage-dendritic cell lineages. Blood. (2016) 127:2672–81. doi: 10.1182/blood-2016-01-690636
9. Bruce-Brand C, Schneider JW, Schubert P. Rosai-Dorfman disease: an overview. J Clin Pathol. (2020) 73:697–705. doi: 10.1136/jclinpath-2020-206733
10. Vyas M, Deshmukh M, Shet T, Jambhekar N. Splenic angiomatoid nodular transformation in child with inflammatory pseudotumor-like areas. Indian J Pathol Microbiol. (2011) 54:829–31. doi: 10.4103/0377-4929.91543
11. di Dio F, Mariotti I, Coccolini E, Bruzzi P, Predieri B, Iughetti L. Unusual presentation of Rosai-Dorfman disease in a 14-month-old Italian child: a case report and review of the literature. BMC Pediatr. (2016) 16:62. doi: 10.1186/s12887-016-0595-9
12. Cai Y, Shi Z, Bai Y. Review of rosai-dorfman disease: new insights into the pathogenesis of this rare disorder. Acta Haematol. (2017) 138:14–23. doi: 10.1159/000475588
13. Alruwaii ZI, Zhang Y, Larman T, Miller JA, Montgomery EA. Rosai-dorfman disease of the digestive system-beware vasculopathy: A clinicopathologic analysis. Am J Surg Pathol. (2019) 43:1644–52. doi: 10.1097/PAS.0000000000001343
14. Betianu CI, Dima A, Pavaloiu G. Primary hepatic mucosa-associated lymphoid tissue lymphoma in a patient with no chronic liver disease: Case report. Radiol Case Rep. (2017) 12:715–9. doi: 10.1016/j.radcr.2017.08.004
15. Ippolito D, Porta M, Maino C, Pecorelli A, Ragusi M, Giandola T, et al. Diagnostic approach in hepatic lymphoma: radiological imaging findings and literature review. J Cancer Res Clin Oncol. (2020) 146:1545–58. doi: 10.1007/s00432-020-03205-x
16. Colagrande S, Calistri L, Grazzini G, Nardi C, Busoni S, Morana G, et al. MRI features of primary hepatic lymphoma. Abdom Radiol (NY). (2018) 43:2277–87. doi: 10.1007/s00261-018-1476-5
17. Liu Y, Guo D, He X, Liu X, Chen W, Chen L, et al. The MR imaging of primary intrahepatic lymphoepithelioma-like cholangiocarcinoma: A diagnostic challenge. Diagnostics (Basel). (2023) 13. doi: 10.3390/diagnostics13182998
18. Goyal G, Ravindran A, Young JR, Shah MV, Bennani NN, Patnaik MM, et al. Clinicopathological features, treatment approaches, and outcomes in Rosai-Dorfman disease. Haematologica. (2020) 105:348–57. doi: 10.3324/haematol.2019.219626
19. Tang M, Gu XZ, Wu PC, Yang XT. Clinicopathological and gene mutation analysis of 27 cases with extranodal rosai-dorfman disease. J Inflammation Res. (2022) 15:2775–87. doi: 10.2147/JIR.S365098
20. Liu C, Liu P. Extranodal Rosai Dorfman disease mimicking multiple liver metastases. Hepatobiliary Surg Nutr. (2021) 10:297–8. doi: 10.21037/hbsn-20-642
21. Yoshida M, Zoshima T, Hara S, Takahashi Y, Nishioka R, Ito K, et al. Case report: Rosai-Dorfman disease with rare extranodal lesions in the pelvis. heart liver skin Front Oncol. (2022) 12:1083500. doi: 10.3389/fonc.2022.1083500
22. Iwabuchi H, Kakihara T, Tanaka A, Uchiyama M, Shibuya H, Umezu H. Congenital Rosai-Dorfman disease without lymphadenopathy. Pediatr Pathol Mol Med. (2003) 22:399–403. doi: 10.1080/pdp.22.5.399.403
23. Shaikh F, Awan O, Mohiuddin S, Farooqui S, Khan SA, McCartney W. 18F-FDG PET/CT imaging of extranodal rosai-dorfman disease with hepatopancreatic involvement - A pictorial and literature review. Cureus. (2015) 7:e392. doi: 10.7759/cureus.392
24. Di Tommaso L, Rahal D, Bossi P, Roncalli M. Hepatic rosai-dorfman disease with coincidental lymphoma: report of a case. Int J Surg Pathol. (2010) 18:540–3. doi: 10.1177/1066896908329590
25. Chen Z, Xue Q, Yang Y, Shun H, Miao W. 68Ga-FAPI and 18F-FDG PET/CT images of a patient with rosai-dorfman disease with liver involvement. Clin Nucl Med. (2022) 47:1079–81. doi: 10.1097/RLU.0000000000004367
26. Maheshwari A, Seth A, Choudhury M, Aggarwal V, Patra B, Aggarwal S, et al. Rosai-Dorfman disease: a case with lymphadenopathy and liver involvement. J Pediatr Hematol Oncol. (2009) 31:200–2. doi: 10.1097/MPH.0b013e31818e5369
Keywords: Rosai Dorfman disease, liver, imaging manifestations, pathological correlation analysis, enhanced scanning
Citation: Zheng X, Tian H, Li W, Yang M, Jin C and Pang Y (2025) Case Report: Radiological characteristics and pathological correlation analysis of extranodal Rosai-Dorfman disease of liver. Front. Oncol. 15:1484820. doi: 10.3389/fonc.2025.1484820
Received: 08 November 2024; Accepted: 19 March 2025;
Published: 07 April 2025.
Edited by:
Cyrus Khandanpour, Universitätsklinikum Schleswig-Holstein, GermanyReviewed by:
Alessandro D’Amuri, Azienda Sanitaria Locale di Brindisi, ItalyCopyright © 2025 Zheng, Tian, Li, Yang, Jin and Pang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chenwang Jin, amluY2hlbndhbmcyMDI0MDZAMTYzLmNvbQ==; Yuhui Pang, cGFuZ3l1aHVpMTk5NEAxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.