
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 06 March 2025
Sec. Hematologic Malignancies
Volume 15 - 2025 | https://doi.org/10.3389/fonc.2025.1468373
 Sylwia Popek-Marciniec1*
Sylwia Popek-Marciniec1* Wojciech Styk2
Wojciech Styk2 Sylwia Chocholska3
Sylwia Chocholska3 Aneta Szudy-Szczyrek3
Aneta Szudy-Szczyrek3 Katarzyna Sidor2
Katarzyna Sidor2 Grazyna Swiderska-Kolacz4
Grazyna Swiderska-Kolacz4 Marek Hus3
Marek Hus3 Joanna Czerwik-Marcinkowska4
Joanna Czerwik-Marcinkowska4 Szymon Zmorzynski1
Szymon Zmorzynski1The growth of blood vessels from the existing vasculature has a significant impact on the course of multiple myeloma (MM). The ANGPT2 (angiopoietin-2) protein is encoded by the ANGPT2 gene and plays an important role in angiogenesis. The expression of proangiogenic proteins is influenced not only by microenvironmental factors but also by genetic changes. We analyzed two variants/polymorphisms of the ANGPT2 gene, rs1868554 (T>A) and rs7825407 (G>C). Both are located in the intron sequence and can affect the final mRNA sequence by modifying splicing.
Purpose: Therefore, we assessed the impact of selected variants on ANGPT2 gene expression at the mRNA and protein levels. Additionally, we evaluated the associations of the analyzed genetic changes with the clinical and laboratory parameters of the disease and the response to bortezomib/thalidomide-based therapies. We hypothesize that variants and expression of the ANGPT2 gene may be associated with a greater risk of MM development and may also affect the response to treatment in MM patients.
Patients and methods: Genomic DNA extracted from 103 newly diagnosed MM patients and 120 healthy blood donors was used to analyze ANGPT2 variants (via automated DNA sequencing). RNA was subjected to real-time PCR to determine ANGPT2 expression at the mRNA level. The concentration of angiopoietin-2 (in MM sera) was determined by ELISA.
Results: The results of our study showed that individuals with the AA genotype of rs1868554 and the CC genotype of rs7825407 had a greater risk of developing MM (OR=6.12, p=0.02 and OR=6.01, p=0.02, respectively). The ANGPT2 gene variants did not affect ANGPT2 expression at the mRNA level. However, ANGPT2 expression was positively correlated with CRP (Spearman’s rho 0.26, p<0.05) and negatively correlated with LDH (Spearman’s rho -0.25, p<0.05) in MM patients.
Conclusion: Our results showed that ANGPT2 expression at the mRNA level correlates with CRP, a negative prognostic factor in MM. The ANGPT2 protein is a proangiogenic factor, and its concentration is significantly greater in MM patients than in healthy individuals, which was also confirmed in our research. Therefore, this protein with VEGF and HB-EGF, should be considered in the future as a markers of angiogenesis in MM.
Angiogenesis is the most important condition for malignant cell development and proliferation. Tumors larger than 1-2 mm will not be able to continue growing if blood vessels do not supply them with nutrients (1, 2). This indicates that the process of tumor angiogenesis is essential for tumor growth, expansion and overall progression. The above changes are also specific to multiple myeloma (MM). The development of blood vessels from the existing vasculature also contributes to the progression of this disease (2). MM is a hematologic malignancy characterized by clonal expansion of plasma cells in the bone marrow (BM), osteolytic bone disease and monoclonal gammopathy (3). In the course of MM, angiogenesis can be assessed by measuring microvascular density (MVD). The mean number of immunohistochemically stained microvessels in paraffin-embedded BM sections was counted (4, 5). The bone marrow microenvironment plays an important role in angiogenesis, MM progression and response to therapeutic agents (6). One of the factors involved in the process of BM angiogenesis is angiopoietin-2 (ANGPT2, Ang-2) (7, 8). It is a protein that regulates vessel growth and maturation during angiogenesis. ANGPT2 binds to the TIE2 receptor and cooperates with the VEGF pathway to maintain physiological functions. Furthermore, in previous studies, a significant increase in circulating angiopoietin-2 in MM patients compared to healthy individuals was observed (4, 9). A hypoxic microenvironment is a common and important feature of most malignancies. Hypoxia affects the cell phenotype and mediates the response to the effects of chemotherapy, radiotherapy and immunotherapy on tumor cells (10). Under hypoxic conditions, HIF-1 induces increased expression of the ANGPT2 gene (8). In addition, to date, ANGPT2 expression has been demonstrated to be associated with disease severity (4, 9). ANGPT2 produced in the BM microenvironment may contribute to the development of MM angiogenesis, and this molecule may be further useful as both a biomarker of angiogenesis and a potential therapeutic target (11). The involvement of MM cells in ANGPT2 synthesis is still controversial. Hoffamn et al. showed that ANGPT2 has prognostic significance. Three angiogenesis markers, EGF, HGF and ANGPT2, are associated with progression from monoclonal gammopathy of undetermined significance (MGUS) to MM (12).
The ANGPT2 gene is located on chromosome 8 (locus 8p23.1) and is known to be mostly expressed by activated endothelial cells under physiological conditions (13). Lohr et al. observed homozygous deletions of the 8p23.1 locus in MM patients (14). Considering these findings, we analyzed the ANGPT2 gene located in this region, whose product may be related to angiogenesis. The ANGPT2 protein is a key mediator of angiogenesis. Studies on lung adenocarcinoma have shown that the ANGPT2 protein level is directly proportional to the size of the tumor and is correlated with the presence of metastases. More importantly, a higher concentration of angiopoietin-2 was negatively correlated with the overall survival (OS) of individuals with lung adenocarinoma (8). Increased ANGPT2 expression has also been described as a potential prognostic factor in colorectal carcinoma (13), gastric cancer (15), hepatocellular cancer (16, 17), lung cancer (18), and chronic lymphocytic leukemia (19). In MM, the serum ANGPT2 level is correlated with disease progression and response to therapy (9, 11).
Very little is known about the effects of single-nucleotide polymorphisms/variants (SNPs) in angiogenesis-related genes on treatment outcomes in MM patients. Genetic variants in the ANGPT2 gene may lead to its altered expression and can affect protein activity. For our research, we selected two variants, rs1868554 (T>A) and rs7825407 (G>C), which are located in the intron of the ANGPT2 gene. Variants that are present in introns do not necessarily directly affect the amount of transcript but can affect the final mRNA sequence by modifying splicing (20). The selected variants have not been studied in MM. Meyer and colleagues demonstrated that rs1868554 alters the ratio of ANGPT2 isoforms (20). Several studies have shown that ANGPT2 gene variants are associated with the course and effects of treatment for colorectal cancer (21), lung cancer (22), head and neck squamous cell carcinoma (23), hepatocellular carcinoma (24) and diseases other than cancer, such as rheumatoid arthritis (25) and systemic sclerosis (26).
Therefore, we wanted to determine whether the selected variants of the ANGPT2 gene have an impact on ANGPT2 expression. Moreover, we hypothesize that the rs1868554 and rs7825407 variants may be associated with a greater risk of developing MM and may also affect the response of MM patients to treatment. The aim of our research was also to determine whether ANGPT2 variants have a significant impact on the course of the disease, including clinical and laboratory MM parameters, as well as the response to bortezomib/thalidomide-based therapies.
The study enrolled 223 unrelated individuals, including 103 newly diagnosed MM patients and 120 healthy blood donors. The study participants were selected from the same ethnicity (Caucasian population). Between 2013 and 2020, material (bone marrow aspirates, peripheral blood and plasma) from MM patients was obtained from the Chair and Department of Hemato-Oncology and Bone Marrow Transplantation (Medical University of Lublin, Poland) by clinicians. The characteristics of the MM patients are presented in Table 1. MM bone marrow aspirates were used for cytogenetic analysis and ANGPT2 gene expression determination at the mRNA level; sera were used for ANGPT2 protein concentration analysis; and peripheral blood was used for ANGPT2 gene genotyping.
The control group (for genotyping analysis) consisted of healthy blood donors (60 men and 60 women, with a mean age of 37.9 years) from the Regional Blood Donation and Blood Treatment Center in Kielce, Poland.
Bone marrow aspirates obtained from 18 non-neoplastic patients with orthopedic injuries who were hospitalized at the Department of Orthopedic and Trauma Surgery of Jan Kochanowski University of Kielce were used for the expression study. Nucleated cells were isolated from bone marrow aspirates for gene expression analysis. In this regard, plasma cells were isolated from MM bone marrow aspirates via a magnetic method with CD138+ beads according to the manufacturer’s protocol (Miltenyi Biotec, Bergisch Gladbach, Germany). From bone marrow aspirates obtained from nonneoplastic patients with orthopedic injuries, mononuclear cells were isolated with Lymphoprep (Serumwerk, Bernburg, Germany) and used to determine ANGPT2 gene expression at the mRNA level. The experimental overflow is shown in Figure 1.
The study obtained positive opinions from the Bioethics Committee at the Medical University of Lublin (No. KE-0254/165/2013, No. KE-0254/337/2016) and the Bioethics Committee at Jan Kochanowski University of Kielce (No. KB-41/2016), according to the ethical standards established by the Helsinki Declaration. All methods were performed in accordance with the relevant guidelines and regulations. All study participants provided written informed consent. The inclusion and exclusion criteria for all individuals in the study are described in Table 2.
DNA was isolated from the peripheral blood of healthy blood donors and MM patients. For this purpose, a commercial kit (Qiagen, Velno, Netherlands) was used according to the manufacturer’s recommendations. The concentration and quality of the obtained nucleic acid were then checked using a NanoDrop One device (Thermo Fisher Scientific, Waltham, MA, USA).
DNA obtained from peripheral blood samples (from MM patients and healthy blood donors) was used to analyze the ANGPT2 rs1868554 and rs7825407 variants. ANGPT2 status was determined by automated DNA sequencing. A fragment of the intron (of the ANGPT2 gene) was amplified by PCR using a T100 Thermal Cycler™ (Bio-Rad, California, USA) with the following primers: forward, 5′-CAGTTAACTTGGGAGGCTTAGTG-3′; reverse, 5′-TGGCCTACGTCTTCTTGAGTC-3′. Each PCR mixture (10 µl) contained 100 ng of genomic DNA, RUN reaction buffer (A&A Biotechnology), a dNTP mixture (0.25 mM), RUN polymerase (0.25 U) (A&A Biotechnology) and primers (10 µM each). The mixture was heated at 95°C for 5 min, after which 35 amplification cycles were performed: denaturation at 95°C for 20 s, annealing at 60°C for 20 s, and elongation at 72°C for 30 s. The final elongation time was 5 min at 72°C. Sequencing PCR and analysis of the results were performed as previously described by using the BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems) (27). The results were analyzed with the use of Applied Biosystems software – Data Collection Software version 3.1 (Figures 2, 3).
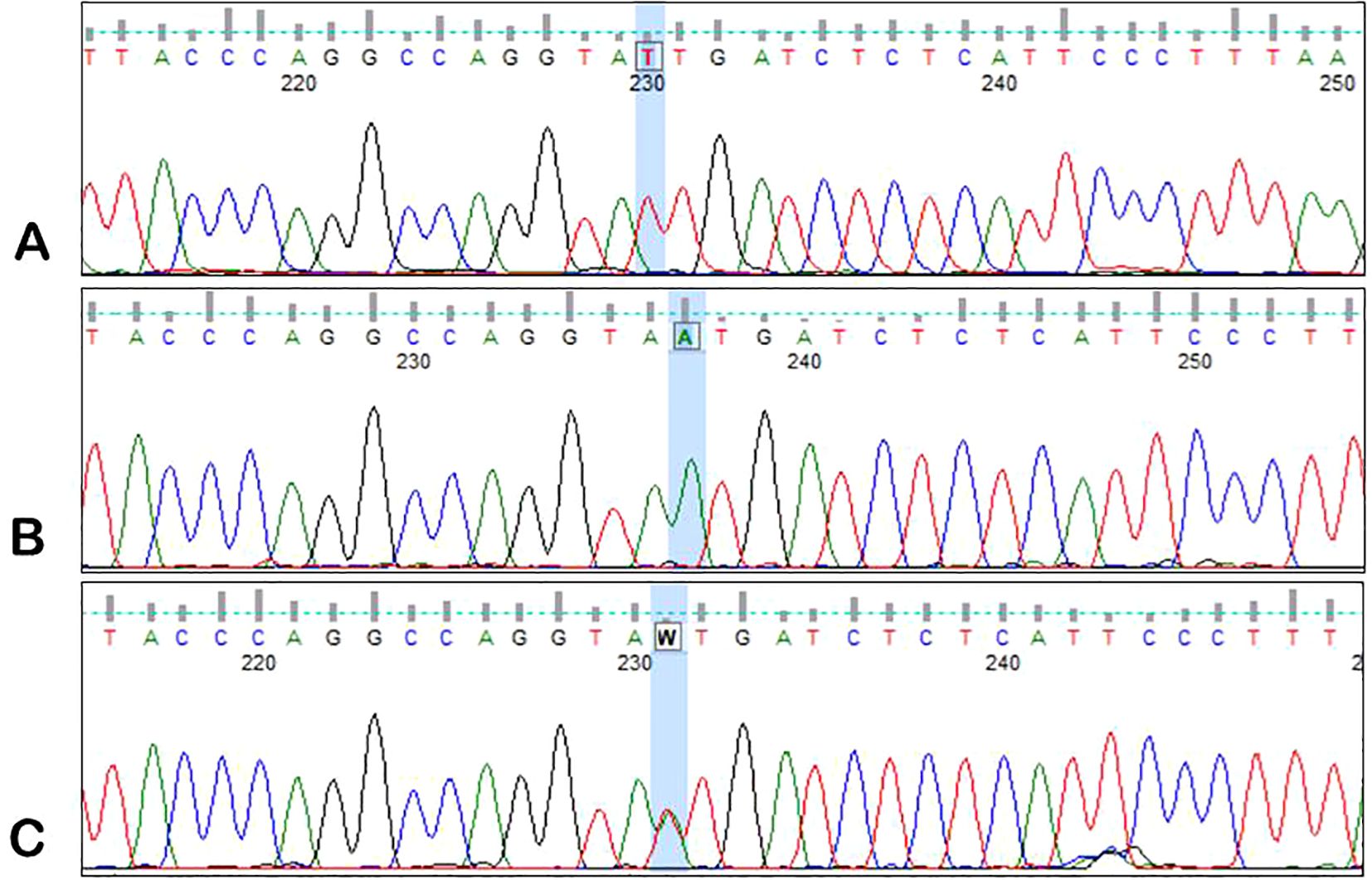
Figure 2. Sample electrophoregrams showing individual variants of the rs1868554 polymorphism of the AGPT2 gene. (A) – T/T variant, (B) – A/A variant, (C) – T/A variant.
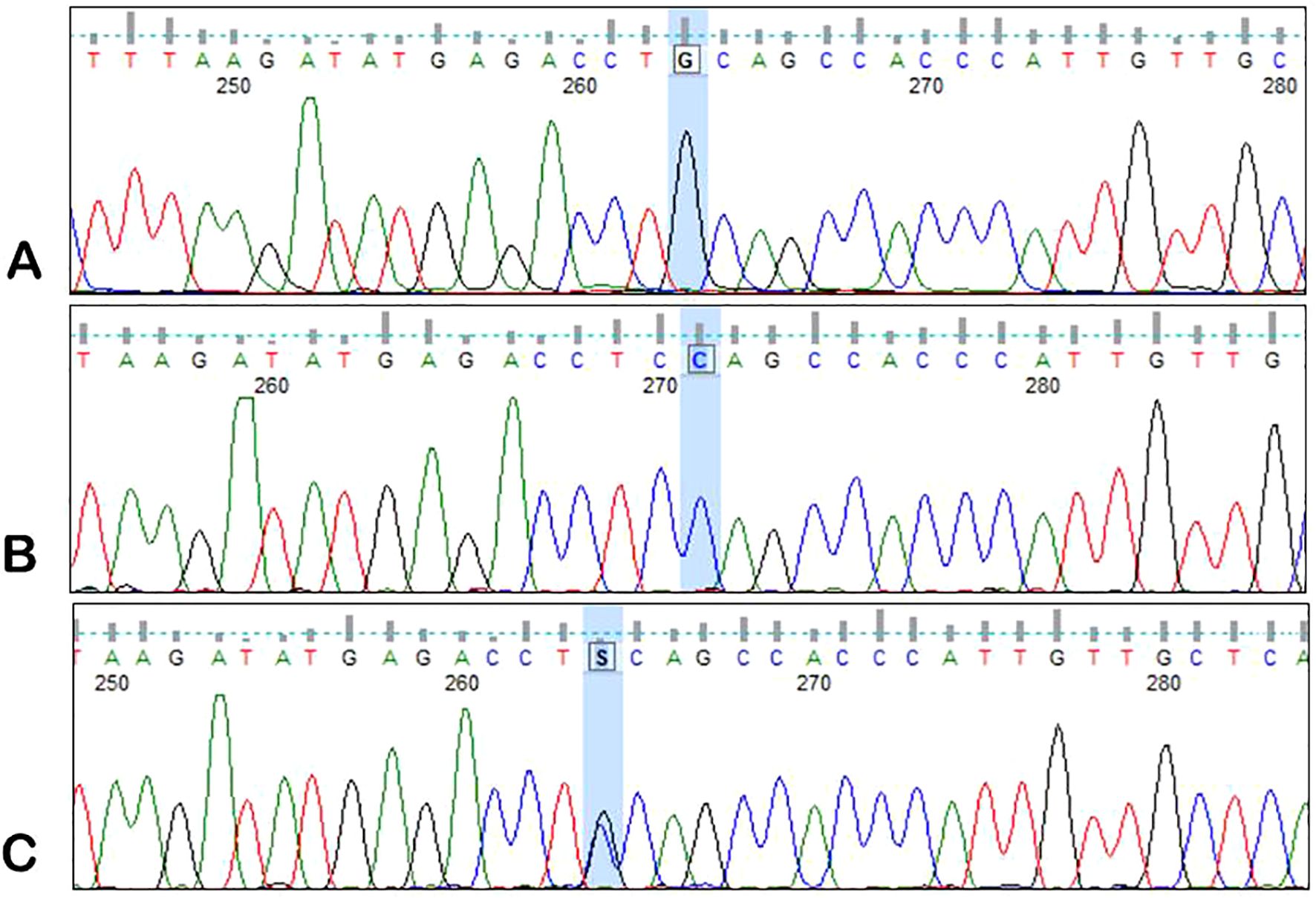
Figure 3. Sample electrophoregrams showing individual variants of the rs7825407 polymorphism of the AGPT2 gene. (A) – G/G variant, (B) – C/C variant, (C) – G/C variant.
Chromosomal aberrations with prognostic significance in MM were tested using cytoplasmic immunoglobulin and FISH (cIg-FISH) in accordance with the recommendations of Ross et al., 2012 (28). The culture and staining of malignant plasma cells were performed according to a previously described protocol with modifications (29, 30). The analysis of the results with the probes used was carried out as previously described (31).
RNA isolation from mononuclear bone marrow cells was carried out with a total RNA midi kit (Aabiot, Gdansk, Poland). The concentration of isolated RNA was checked spectrophotometrically using a NanoDrop One device (Thermo Scientific, Waltham, MA, USA). The quality of these nucleic acids was checked via electrophoresis on a 2% agarose gel. The RNA was stored at −80°C.
A reverse transcription reaction (RT−PCR) was performed after RNA isolation. RT−PCR was performed using a T100™ Thermal Cycler (Bio-Rad, California, USA). Real-time PCR was carried out on the cDNA (100 ng) template. Real-time PCR was performed using SYBR Green RT−PCR Mix (Aabiot, Poland), an annealing temperature of 60°C, and 100 μM primers (Genomed, Warszawa, Poland): forward, 5′-CAGTTAACTTGGGAGGCTTAGTG-3′; reverse, 5′-TGGCCTACGTCTTCTTGAGTC-3′. Twenty microliters of the reaction mixture was transferred to each well of the PCR plate. The plate was centrifuged and placed in a CFX Opus 96 Real-Time PCR System (Bio-Rad, California, USA). The qRT−PCR mixture was prepared according to the manufacturer’s protocol (A&A Biotechnology, Gdansk, Poland). Every sample was assayed in duplicate, and expression was calculated according to the 2−ΔΔCt method—normalized expression (32). The expression values are presented as the logarithm of R to base 2, where R was calculated as follows: R = 2−ΔΔCt, ΔΔCt = ΔCt of the control −ΔCt of the analyzed gene, and every ΔCt = Ct of the analyzed gene − Ct of the endogenous control. The expression of GAPDH served as a control. For this purpose, 100 μM primers (Genomed, Warszawa, Poland) were used with an annealing temperature of 60°C: forward, 5′-CAACGGATTTGGTCGTATTG-3′; reverse, 5′-GGATCTCGCTCCTGGAAG-3′.
A normalized expression (R) in the range of 0.8–1.2 indicated a normal level of gene expression, R < 0.8 indicated low expression, and R > 1.2 indicated high expression (33, 34).
A specific ELISA kit (Invitrogen, Waltham, MA, USA) was used (according to the manufacturer’s protocol) to determine the level of angiopoietin-2 in serum samples collected from 70 MM patients. A Multiskan FC plate reader (Thermo Scientific, Waltham, MA, USA) at a wavelength of 450 nm was used for the measurement of angiopoietin-2. The serum samples were diluted 20 times. The concentration read from the standard curve was multiplied by the dilution factor (2×).
The laboratory results of MM patients were compared with those of patients with the studied ANGTPT2 gene genotypes using t tests (for continuous variables) and chi-square tests (for categorical variables). The associations between ANGTPT2 genotypes and clinical data were assessed using the chi-square test or Fisher’s exact test (when one expected value was <5). Quantitative data are presented as frequencies or percentages. Hardy–Weinberg equilibrium (HWE) was assessed using the chi-square test with Yates correction for groups of fewer than five individuals. For the 95% confidence intervals (CIs), we assumed p = 0.05 and χ2 = 3.84; thus, if χ2 ≤ 3.84 and corresponding p ≥ 0.05, then the population was in HWE. The Cox proportional hazard model was used for univariate and multivariate analyses of OS and progression-free survival (PFS). The Kaplan–Meier method and log-rank test were used for survival analysis. Pearson correlation analysis was used to evaluate the associations between the ANGTPT2 expression level and laboratory/clinical data. We assumed a 5% error of inference and an associated significance level of p < 0.05, indicating the existence of statistically significant differences. Statistical analyses were performed using Statistica ver. 12.5 (StatSoft, Krakow, Poland).
The present study included 103 MM patients (52 males and 51 females). The rs1868554 and rs7825407 variants and the expression of the ANGPT2 gene at the mRNA and protein levels were analyzed.
Genotyping was successful for all the individuals investigated within the study. This was one of the inclusion criteria for MM patients and healthy blood donors. In MM patients, the ANGPT2 variants were not in the Hardy−Weinberg equilibrium (Table 3). The population of healthy blood donors was in HWE. In the dominant and recessive models, AA homozygotes of rs1868554 and CC homozygotes of rs7825407 were associated with a greater risk of MM development (Table 4). We did not observe statistically significant differences between allele frequencies among MM patients and healthy blood donors (Table 4).
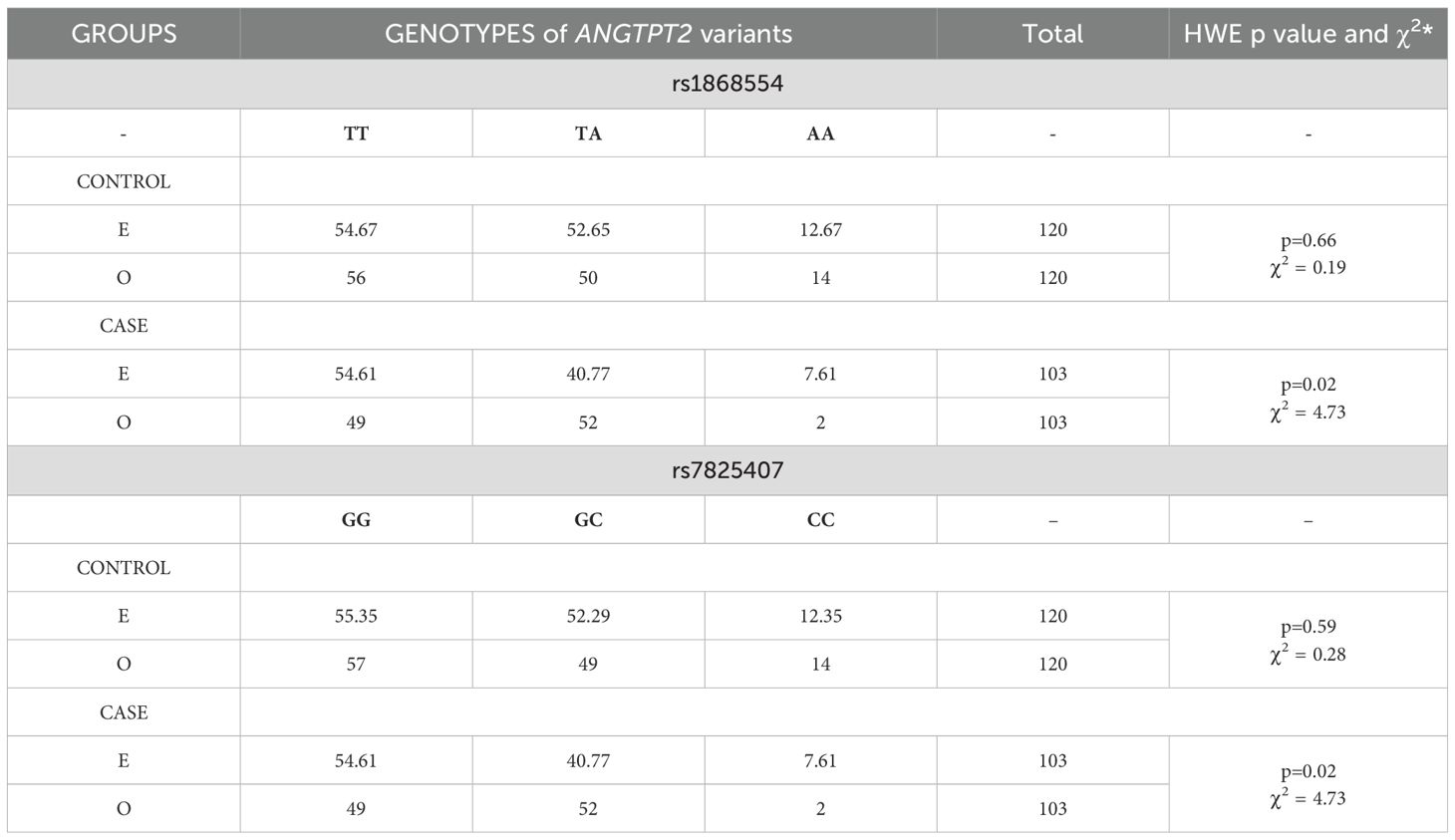
Table 3. Hardy−Weinberg equilibrium (HWE) for ANGTPT2 variants in the case and control groups according to expected (E) and observed (O) values.
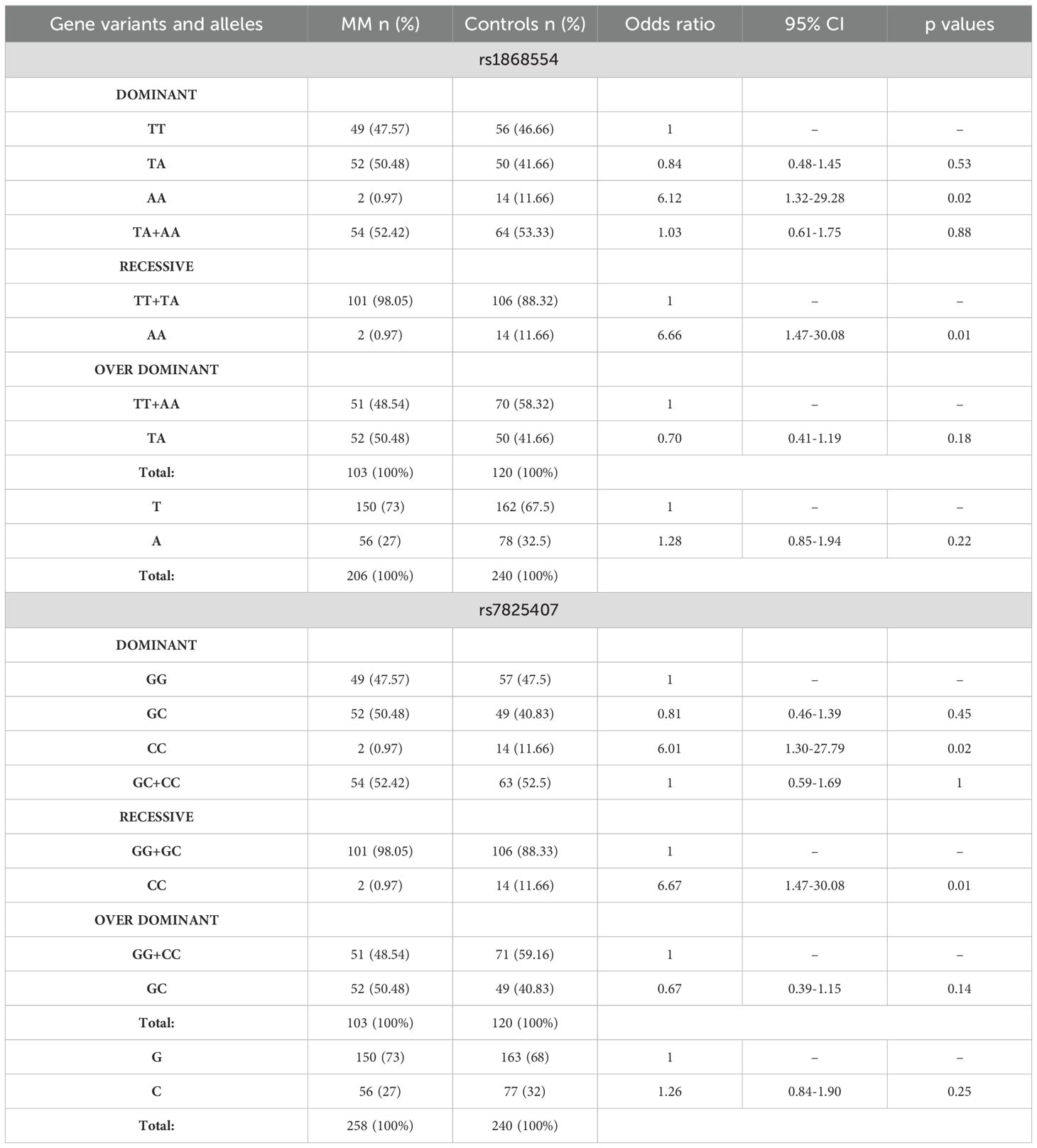
Table 4. Comparison of the allele frequency and distribution of ANGTPT2 variants among MM patients and controls.
The numbers of individuals with deletions of 17p13.1 and with chromosome 17 aneuploidies were low: n = 18 and n = 16, respectively. Deletion of 17p13.1 co-occurred with chromosome 17 aberrations. Among these changes, we observed chromosome 17 trisomy (n = 11), chromosome 17 tetrasomy (n = 1), chromosome 17 tetrasomy and deletion of two TP53 alleles (n = 2), chromosome 17 trisomy and deletion of one TP53 allele (n = 1), and chromosome 17 monosomy (n = 1). Due to the small number of particular chromosome 17 mutations, these changes were grouped and analyzed together. We did not observe an association between the studied variants and the presence of chromosome 17 aberrations—rs1868554, p=0.45 (OR=1.51, 95% CI 0.51-4.42); rs7825407, p=0.84 (OR=1.12, 95% CI 0.38-3.25). Due to the small number of individuals with structural cytogenetic changes, MM patients with del(17)(p13.1) containing the TP53 gene locus and 14q32.2 translocations (with the IgH gene locus) were grouped and analyzed together. We did not observe an association between cytogenetic structural aberrations and the studied ANGPT2 variants (p=0.33, OR=1.52, 95% CI 0.64-3.62).
Minor genotypes were analyzed together with heterozygotes due to their small sample size. Univariate Cox analysis revealed that patients with ISS stage III disease had a 2-fold (p = 0.001) increased risk of death (Table 5). In the case of MM patients who underwent autoHSCT, a lower risk of death was observed (HR = 0.238, p = 0.001). Similar findings were observed for disease relapse or progression in MM patients at stage III according to ISS (HR = 1.8, p = 0.001) and with autoHSCT (HR = 0.347, p = 0.001) (Table 5). Multivariate Cox regression analysis confirmed that patients who underwent autoHSCT had a decreased risk of death and disease relapse or progression (Table 6). In contrast, patients with stage III disease according to the ISS had an increased risk of death and disease relapse or progression. Univariate and multivariate Cox analyses did not reveal an impact of the studied genotypes on the risk of death, disease relapse or disease progression in MM patients.
We analyzed the potential relationships between clinical/laboratory results and selected genotypes of the ANGPT2 gene. The comparative analysis did not reveal any statistically significant differences in laboratory or clinical parameters between patients with particular variants of polymorphisms rs1868554 and rs7825407 (Table 7).
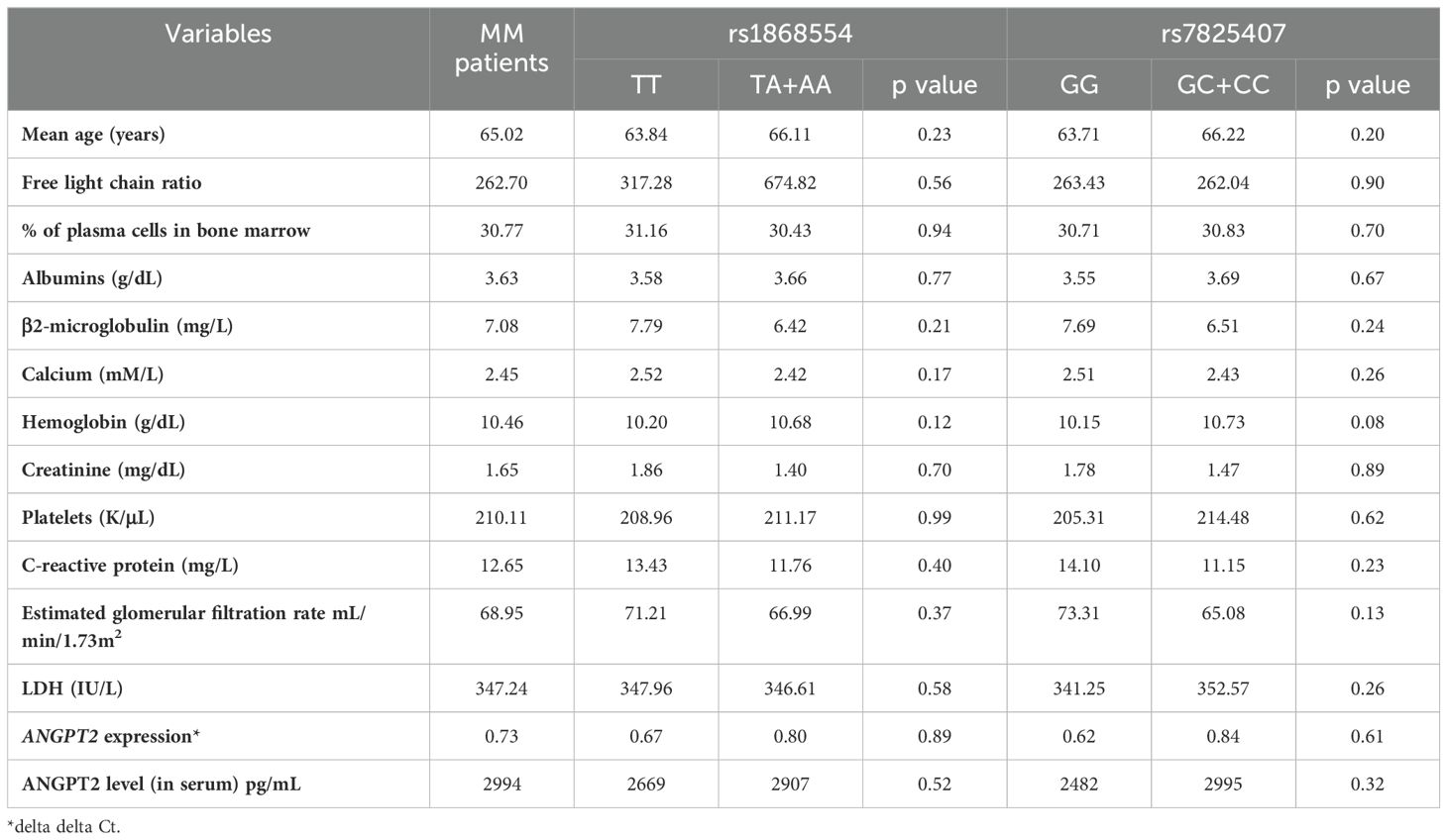
Table 7. The clinical value of MM patients (at diagnosis) included in the study took into account the studied variants.
The delta delta Ct method and log2-fold change methods were used to analyze ANGPT2 expression. We observed a positive correlation between ANGPT2 expression and CRP (C-reactive protein) levels (Spearman’s rho 0.26, p<0.05). A statistically significant negative correlation was found between ANGPT2 expression and LDH levels (Spearman’s rho -0.25, p<0.05). In the case of other clinical/laboratory values, we did not observe statistically significant differences.
The mean ANGPT2 concentration (pg/mL) in MM patients was greater than that in control individuals (2994 vs. 616, p < 0.001). Considering the analyzed variants, we did not observe an association between ANGPT2 concentration (pg/ml) and rs1868554 or rs7825407 (p = 0.52 or p = 0.32, respectively) (Table 7). Considering the rs1868554 and rs7825407 haplotypes, we observed a difference in ANGPT2 concentration at the level of tendency (p = 0.08) (Table 8). The analyzed haplotypes did not affect OS in MM patients (Table 9).
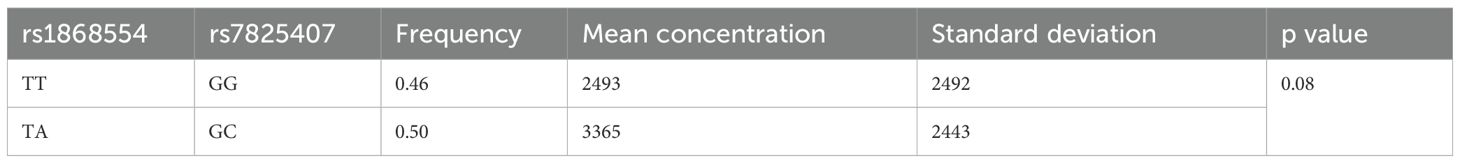
Table 8. Haplotypes comprising ANGPT2 gene variants and their protein concentrations in serum (pg/mL).
We analyzed the associations between the studied genotypes and the survival of MM patients (by log rank test). The rs1868554 and rs7825407 variants did not affect OS (log rank test p=0.82, p=0.79) or PFS (log rank test p=0.39 and p=0.36), respectively. Figure 4 shows an example of the OS analysis including the ANGPT2 variants (without considering the type of treatment).
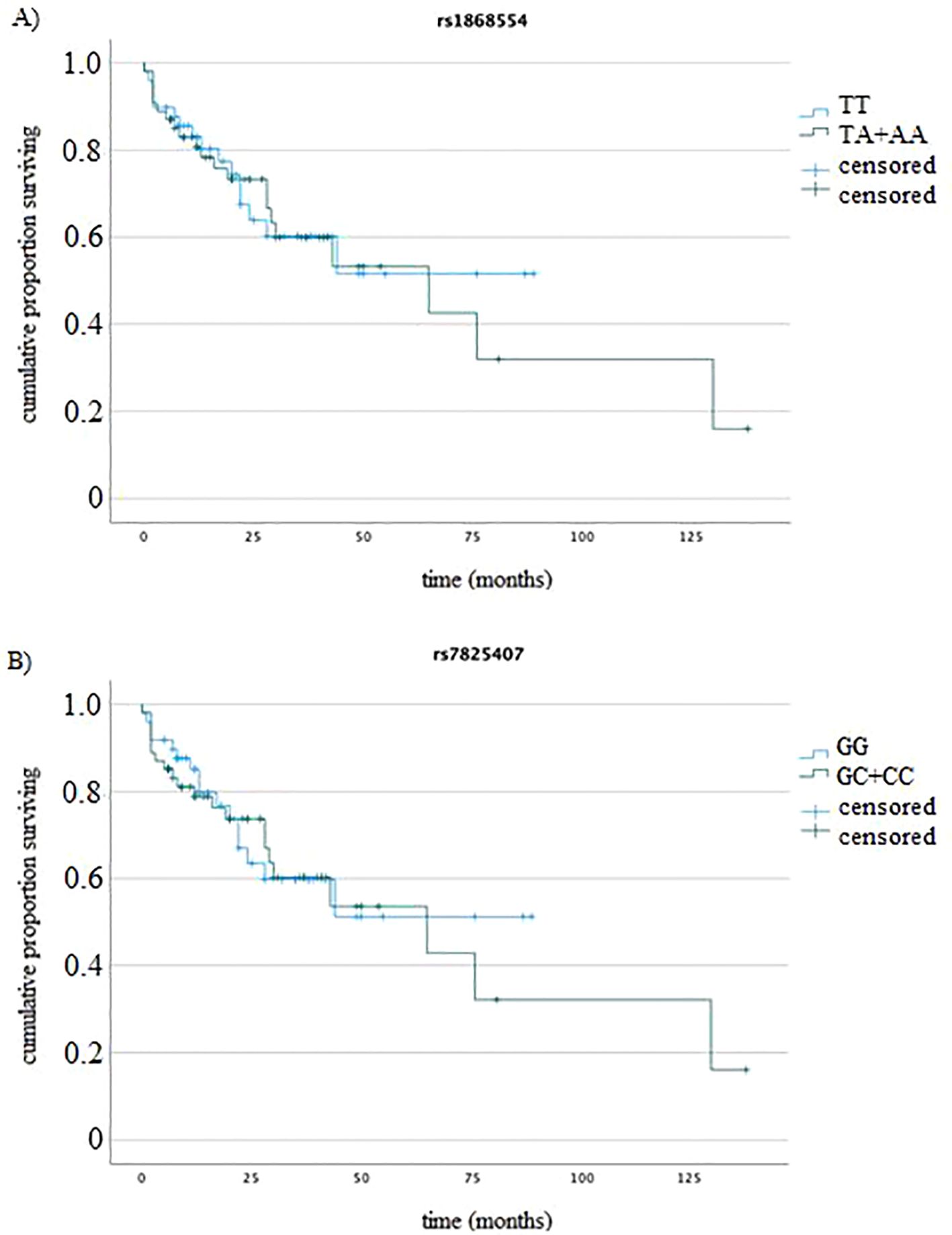
Figure 4. K−M analysis of OS (A) for rs1868554 (log-rank test p = 0.82) and (B) rs7825407 (log-rank test p = 0.79) according to genotype.
Angiogenesis is very important for the development and progression of malignant tumors. The formation of new blood vessels depends on the presence of proangiogenic factors. In MM, these compounds can be produced by both malignant cells and microenvironment bone marrow cells (35, 36).
In the course of multiple myeloma, the ANGPT2 factor plays an important role. Numerous studies indicate an increase in the concentration of ANGPT2 protein in the serum of MM patients compared to that in the serum of healthy donors (4, 9, 37). Our research aimed to first assess the serum ANGPT2 concentration in MM patients and healthy donors. Second, we investigated the effect of selected variants (rs1868554 and rs7825407) on ANGPT2 expression at mRNA/the protein levels. Then, we checked whether the analyzed variants, gene expression and protein levels were related to the course of MM and the response to treatment.
In our study, we showed a significantly greater concentration of ANGPT2 protein in the serum of MM patients than in that of healthy blood donors. To date, several studies have been published with similar results (4, 9). However, we did not observe a correlation between the analyzed variants and ANGPT2 gene expression at the mRNA or protein level. Considering the rs1868554 and rs7825407 haplotypes, we observed a difference in ANGPT2 concentration at the tendency level. The results of our study showed that individuals with the AA genotype of rs1868554 and the CC genotype of rs7825407 had a greater risk of developing MM. To date, no studies have been conducted to assess the contribution of these variants to MM progression. Two variants of the ANGPT2 gene, rs1868554 and rs2442598, were significantly associated with acute lung injury at the IInd clinical stage (20). Researchers are considering variants (other than those we analyzed) of the ANGTPT2 gene that may have clinical relevance. Hu and colleagues examined five ANGPT2 variants, rs2442598, rs734701, rs1823375, rs11137037, and rs12674822, and their impact on the development and course of lung cancer (38). They showed that carriers of the GT allele (rs12674822) had a greater risk of lung cancer than did carriers of the wild type, GG. The presence of the CC genotype in rs11137037 was associated with a greater degree of clinical disease advancement in comparison to carriers of the AA genotype (38). Hu and colleagues examined the effect of ANGPT2 variants on susceptibility to developing breast cancer in a Chinese Han population. Five ANGPT2 variants were selected: rs2442598, rs734701, rs1823375, rs11137037 and rs12674822. Based on the research results, the authors concluded that the T-T-C-A-T ANGPT2 haplotype significantly increased the risk of breast cancer development by almost 1.39 times (39). Our next study can be expanded to include ANGPT2 variants, which we have not yet analyzed. It is possible that they are related to elevated angiopoietin-2 levels in MM patients.
In the present study, genetic changes did not affect the expression of the ANGPT2 gene at the mRNA or protein level. However, ANGPT2 expression (mRNA level) was positively correlated with CRP and negatively correlated with LDH in our MM patients. Several acute phase proteins, e.g., CRP or IL-6, predict MM prognosis and may influence the survival of MM patients (40, 41). Additionally, it has been shown that a high CRP level at the time of MM diagnosis is a factor increasing the risk of cachexia during treatment (42).
According to the observations of Pappa et al., ANGPT2 seems to play a crucial role in the angiogenic process in MM patients, with a very important impact on their prognosis (4). They showed that the ANGPT2 concentration and BM MVD were greater in MM patients than in controls (4). In our study, we did not examine the MVD or ANGPT2 levels in the bone marrow plasma. MVD is not routinely performed in patients with MM, and it is currently impossible to perform this test because most of the examined patients died. We collected bone marrow aspirates and sera from the time of disease progression to determine changes in ANGPT2 expression (at the mRNA and protein levels). Saltarella et al. reported no significant differences between the blood and bone marrow plasma levels of proangiogenic cytokines, e.g., ANGPT2, HGF, VEGF or TNF-α, in MM patients (43). This is inconsistent with our results of ANGPT2 serum levels in MM patients. Joshi et al. reported significant increases in ANGPT2 and VEGF concentrations and their correlation with disease severity in patients with MM (9).
The protein ANGPT2 is a proangiogenic factor. This means that it supports the development of new blood vessels, or the process of neoangiogenesis. The formation of blood vessels in niches where MM cells are located is a necessary process for their proliferation and spread. Higher levels of ANGPT2 protein in people with multiple myeloma have a negative effect on the general condition of the patient because it promotes disease progression. Survival analysis of MM patients, conducted by Pappa et al. showed that patients with higher serum ANGPT2 levels before treatment were accompanied by shorter survival compared to those with lower values (62 vs. 36 months) (p < 0,04) (4).
One of the more important findings described in the literature is that ANGPT2 gene expression is largely dependent on the DNA methylation state ANGPT2 gene expression could be directly regulated by DNA methylation. The increase in the methytulation of the regulatory part of the gene can cause inhibition of its expression. Methylation levels of promoter region in ANGPT2 gene have been reported to be negatively correlated with its mRNA expression in CLL patients. This finding suggesting that ANGPT2 promoter methylation leads to gene silencing (44, 45). The group of epigenetic factors that affect gene expression also includes microRNA. miR-135b, miR-16 and miR-15a are involved in the regulation of the expression of genes involved in angiogenesis (46, 47). miRNA molecules attach to mRNA molecule and, as a consequence, completely or partially block protein translation. miR-16 and miR-15a are involved in the regulation of VEGF-A expression and miR-135b are involved in the regulation of the HIF-1 factor (46, 47). miRNA molecules that can regulate ANGPT2 gene expression include miR-542-3p (48). He and coworkers described, that miR-542-3p inhibit translation of Angpt2 mRNA by binding to its 3-UTR, and addition of miR-542-3p to in vitro cultured endothelial cells attenuate angiogenesis. Another miRNA molecule that can inhibit ANGPT2 gene expression is miR−135a−5p (49). Using a luciferase activity assay, Diao et coworkers identified that ANGPT2 is a potential target gene of miR−135a−5p in gallbladder cancer cells (49).
Furthermore, the expression of the ANGPT2 gene in MM may be affected by many factors: signals from plasma cells, bone marrow microenvironmental cells, ANGPT2 gene variants and epigenetic modifications (11, 20, 44). This system is very complex, and much research is still needed to elucidate the biology of MM cells.
The group of patients included in our study were diagnosed between 2013 and 2020, when standard treatment included the use of thalidomide and bortezomib. The antiangiogenic and immunomodulatory effects of thalidomide resulted in the inclusion of this drug in the therapy of MM patients several years ago. Thalidomide belongs to a group of immunomodulatory drugs (IMiDs). IMiDs play a crucial role in the treatment landscape across various stages of MM. The mechanism of action of IMiDs is still unclear. Thalidomide and its analogues affect angiogenesis indirectly. Ito and coworkers identified cereblon as a teratogenic target of thalidomide (50). Cereblon is the substrate receptor of the E3 ubiquitin ligase. Inhibition of cereblon expression in human multiple myeloma cell lines significantly reduces cell growth and viability. This data suggesting a key role of E3 ubiquitin ligase in mediating the IMiDs activity (50, 51). Research data on IMiDs and bone marrow angiogenesis in patients with MM are contradictory. Data from the publications Kumar et al., and Hatjiharissi et al., showed that MVD in bone marrow is significantly reduced in patients treated with thalidomide alone or in combination with dexamethasone, without a decrease serum levels of angiogenic cytokines e.g. VEGF, b-FGF, IL-6 (52, 53). Contrary results were presented in publication by Cury and colleagues. These scientists demonstrated the absence of any decrease in bone marrow angiogenesis in patients treated with thalidomide (54). We were unable to find a publication in which the direct effect of thalidomide on the expression of the ANGPT2 gene was described. This gives us the opportunity to focus our research for the future. Especially considering functional studies in vitro.
In an in vitro study, Saha and colleagues reported that thalidomide has antiangiogenic effects by targeting VEGFR-2 Tyr-1175, a key autophosphorylation site that regulates proangiogenic responses (55). However, Peach and colleagues previously showed that the molecular target of IMiDs (including thalidomide) is the cereblon protein (56). Wang and colleagues used a model based on human umbilical vein endothelial cells (HUVECs) in their angiogenesis study (57). Researchers have shown that thalidomide can inactivate the endothelium by downregulating angiogenic factors. Thalidomide significantly decreased ANGPT2 and VEGF protein expression and reduced ANGPT2 expression at the mRNA level. However, ANGPT1 concentrations did not significantly change (50). In our study, we did not observe an association between ANGPT2 variants and OS or PFS considering treatment. Moreover, ANGPT2 haplotypes were not related to the OS of MM patients.
In summary, angiogenesis is an essential process for the development of multiple myeloma. Proangiogenic factors may predict the course of the disease toward progression. Detailed studies of the factors involved in this process are necessary to expand our knowledge of the biology of MM.
Aberrant angiogenesis in bone marrow is a key hallmark of multiple myeloma progression and this process is very complex. Both MM and BMSCs cells play essential role to shape the BM angiogenic niche. This leading to the formation of new blood vessels by recruiting endothelial progenitor cells (EPCs) (58). During the activation of angiogenesis, high expression of oncogenes such as c-myc and Jun-B induces plasma cells to secrete high amounts of pro-angiogenic cytokines, including: VEGF, FGF-2, HGF, ANGPT2 and IGF-1 (59). In response to released factors, ECs increase the expression of receptors including: VEGFR, HGFR, and FGFR2 (58). The migration of other immune system cells also increases (58). Already in 2013, Bhaskar and colleagues, based on their research, suggested the important role of angiopoietin in the etiology and course of MM and suggested ANGPT2 as a potential target for antiangiogenic therapy in the treatment of MM (60). ANGPT2 is the vascular growth factor, mediated through VEGF-independent pathways (61). However, in subsequent studies, the researchers showed that VEGF and ANGPT2 play synergistically, and their expression levels are predictive of PFS in MM patients (60). Rao and colleagues described the involvement of the HB-EGF-EGFR pathway in MM-associated angiogenesis (62). They demonstrated that HB-EGF and EGFR are highly expressed in bone marrow endothelial cells of MM patients and are potent inducers of angiogenesis (62). In addition, they showed that blockade of the HB-EGF–EGFR pathway using EGFR inhibitors (such as erlotinib) reduced angiogenesis and tumor growth in in vitro and in vivo models of MM. These results suggest that targeting multiple angiogenic pathways, including ANGPT2, VEGF, and HB-EGF–EGFR signaling, may provide a more comprehensive therapeutic strategy for MM. Vanucizumab (RO5520985) is a novel bispecific humanized immunoglobulin that acts as a dual targeting inhibitor of VEGF-A and ANGPT2 (63, 64). Clinical studies have been conducted on the efficacy of vanucizumab in solid tumors (64, 65). There are no clinical studies with vanucizumab in hematological malignancies.
In the expression of proangiogenic factors are involved microenvironment cells, genetic variants and epigenetic factors such as miRNA molecules. This process is very complex and it is incredibly difficult to find one pathway that plays a major role in the pathogenesis of BM angiogenesis in multiple myeloma. This shows the need to construct complex therapies that will target different points of the disease, which are the proliferation of plasmocyte cells and angiogenesis of the microenvironment of bone marrow niches. Analysis of 3 markers of angiogenesis: ANGPT2, VEGF and HB-EGF can become a prognostic panel to assess the course of MM and predict the use of angiogenesis-oriented therapy.
The limitation of our study is the relatively small sample size, which is partly due to the low incidence of MM. Further analysis with a larger cohort may help to better understand the significance of ANGPT2 variants in MM pathology. Another limitation is the small number of ANGPT2 variants that we selected for this study. This choice was made taking into account their sequence location. Studies with selected variants have not been conducted in MM thus far. In the present study, we did not include analyses of MVD, VEGF variants or expression (at the mRNA and protein levels), or other ANGPT2 variants with confirmed significance in hematologic malignancies. Moreover, we did not perform an analysis of ANGPT2 expression during MM progression or after treatment. Such an analysis would allow us to obtain much more data and could show how ANGPT2 gene expression changes depending on the applied therapy. Our study did not include a group of patients with relapsed or refractory MM. Including this group, could provide insight into how ANGPT2 variants affect disease behavior and treatment response at different stages of the disease. In the future, we plan to expand the study group to at least 250 patients. A good direction is to cooperate with centers from another part of Europe to obtain samples. This will create a more diverse group. We plan to study other variants of the ANGPT2 gene, include variants and expression of the VEGF gene. In addition, we would like to complete the research with a functional study on cell lines, for example to study the effect of angiogenesis inhibitors on MM cell survival, including vanucizumab.
Taken together, the results of our study suggest that ANGPT2 variants may have negative prognostic implications for the risk of developing MM. Moreover, our results showed that ANGPT2 expression at the mRNA level correlates with CRP, a negative prognostic factor in MM. The ANGPT2 protein is a proangiogenic factor, and its concentration is significantly greater in MM patients than in healthy individuals, which was also confirmed in our research. Therefore, this protein with VEGF and HB-EGF, should be considered in the future as a markers of angiogenesis in MM.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by Bioethics Committee at the Medical University of Lublin and Bioethics Committee at Jan Kochanowski University of Kielce. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
SP-M: Data curation, Formal analysis, Investigation, Writing – original draft, Writing – review & editing. WS: Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. SC: Data curation, Investigation, Methodology, Writing – original draft, Writing – review & editing. AS-S: Data curation, Resources, Writing – review & editing. KS: Writing – review & editing. GS-K: Funding acquisition, Writing – review & editing. MH: Funding acquisition, Resources, Writing – review & editing. JC-M: Funding acquisition, Writing – review & editing. SZ: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This project was supported by Ministry of Higher Education and Science, Poland - Grant No. NdS-II/SP/0066/2024/01.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Naumov GN, Akslen LA, Folkman J. Role of angiogenesis in human tumor dormancy: animal models of the angiogenic switch. Cell Cycle. (2006) 5:1779–87. doi: 10.4161/cc.5.16.3018
2. Sun M, Qiu S, Xiao Q, Wang T, Tian X, Chen C, et al. Synergistic effects of multiple myeloma cells and tumor-associated macrophages on vascular endothelial cells in vitro. Med Oncol. (2020) 37:1–11. doi: 10.1007/s12032-020-01426-1
3. Cowan AJ, Green DJ, Kwok M, Lee S, Coffey DG, Holmberg LA, et al. Diagnosis and management of multiple myeloma. JAMA. (2022) 327:1–11. doi: 10.1001/jama.2022.0003
4. Pappa CA, Alexandrakis MG, Boula A, Thanasia A, Konsolas I, Alegakis A, et al. Prognostic impact of angiopoietin-2 in multiple myeloma. J Cancer Res Clin Oncol. (2014) 140:1801–5. doi: 10.1007/s00432-014-1731-2
5. Sezer O, Niemöller K, Eucker J, Jakob C, Kaufmann O, Zavrski I, et al. Bone marrow microvessel density is a prognostic factor for survival in patients with multiple myeloma. Ann Hematol. (2000) 79:574–7. doi: 10.1007/s002770000236
6. Maiso P, Mogollón P, Ocio EM, Garayoa M. Bone marrow mesenchymal stromal cells in multiple myeloma: their role as active contributors to myeloma progression. Cancers (Basel). (2021) 13:2542. doi: 10.3390/cancers13112542
7. Rao L, Giannico D, Leone P, Solimando AG, Maiorano E, Capporuso C, et al. HB-EGF–EGFR signaling in bone marrow endothelial cells mediates angiogenesis associated with multiple myeloma. Cancers (Basel). (2020) 12:173. doi: 10.3390/cancers12010173
8. Qin S, Yi M, Jiao D, Li A, Wu K. Distinct roles of VEGFA and ANGPT2 in lung adenocarcinoma and squamous cell carcinoma. J Cancer. (2020) 11:153–67. doi: 10.7150/jca.34693
9. Joshi S, Khan R, Sharma M, Kumar L, Sharma A. Angiopoietin-2: A potential novel diagnostic marker in multiple myeloma. Clin Biochem. (2011) 44:590–5. doi: 10.1016/j.clinbiochem.2011.01.010
10. Chen Z, Han F, Du Y, Shi H, Zhou W. Hypoxic microenvironment in cancer: molecular mechanisms and therapeutic interventions. Signal Transduct Target Ther. (2023) 8:70. doi: 10.1038/s41392-023-01332-8
11. Belloni D, Marcatti M, Ponzoni M, Ciceri F, Veschini L, Corti A, et al. Angiopoietin-2 in Bone Marrow milieu promotes Multiple Myeloma-associated angiogenesis. Exp Cell Res. (2015) 330:1–12. doi: 10.1016/j.yexcr.2014.10.017
12. Hofmann JN, Landgren O, Landy R, Kemp TJ, Santo L, McShane CM, et al. A prospective study of circulating chemokines and angiogenesis markers and risk of multiple myeloma and its precursor. JNCI Cancer Spectr. (2020) 4:1–9. doi: 10.1093/jncics/pkz104
13. Jary M, Hasanova R, Vienot A, Asgarov K, Loyon R, Tirole C, et al. Molecular description of ANGPT2 associated colorectal carcinoma. Int J Cancer. (2020) 147:2007–18. doi: 10.1002/ijc.32993
14. Lohr JG, Stojanov P, Carter SL, Cruz-Gordillo C, Lawrence MS, Auclair D, et al. Widespread genetic heterogeneity in multiple myeloma: implications for targeted therapy. Cancer Cell. (2014) 25:91–101. doi: 10.1016/j.ccr.2013.12.015
15. Yang S, Zou X, Li J, Yang H, Zhang A, Zhu Y, et al. Immunoregulation and clinical significance of neutrophils/NETs-ANGPT2 in tumor microenvironment of gastric cancer. Front Immunol. (2022) 13:1010434. doi: 10.3389/fimmu.2022.1010434
16. Zhu J, Wu Y, Yu Y, Li Y, Shen J, Zhang R. MYBL1 induces transcriptional activation of ANGPT2 to promote tumor angiogenesis and confer sorafenib resistance in human hepatocellular carcinoma. Cell Death Dis. (2022) 13:727. doi: 10.1038/s41419-022-05180-2
17. Llovet JM, Peña CEA, Lathia CD, Shan M, Meinhardt G, Bruix J. Plasma biomarkers as predictors of outcome in patients with advanced hepatocellular carcinoma. Clin Cancer Res. (2012) 18:2290–300. doi: 10.1158/1078-0432.CCR-11-2175
18. Lin C-Y, Cho C-F, Bai S-T, Liu J-P, Kuo T-T, Wang L-J, et al. ADAM9 promotes lung cancer progression through vascular remodeling by VEGFA, ANGPT2, and PLAT. Sci Rep. (2017) 7:15108. doi: 10.1038/s41598-017-15159-1
19. Martinelli S, Maffei R, Castelli I, Santachiara R, Zucchini P, Fontana M, et al. Increased expression of angiopoietin-2 characterizes early B-cell chronic lymphocytic leukemia with poor prognosis. Leuk Res. (2008) 32:593–7. doi: 10.1016/j.leukres.2007.09.002
20. Meyer NJ, Li M, Feng R, Bradfield J, Gallop R, Bellamy S, et al. ANGPT2 genetic variant is associated with trauma-associated acute lung injury and altered plasma angiopoietin-2 isoform ratio. Am J Respir Crit Care Med. (2011) 183:1344–53. doi: 10.1164/rccm.201005-0701°C
21. Stremitzer S, Zhang W, Yang D, Ning Y, Stintzing S, Sebio A, et al. Genetic variations in angiopoietin and pericyte pathways and clinical outcome in patients with resected colorectal liver metastases. Cancer. (2015) 121:1898–905. doi: 10.1002/cncr.29259
22. Yao S, Dong S-S, Ding J-M, Rong Y, Zhang Y-J, Chen H, et al. Sex-specific SNP-SNP interaction analyses within topologically associated domains reveals ANGPT1 as a novel tumor suppressor gene for lung cancer. Genes Chromosom Cancer. (2020) 59:13–22. doi: 10.1002/gcc.22793
23. Butkiewicz D, Gdowicz-Kłosok A, Krześniak M, Rutkowski T, Krzywon A, Cortez AJ, et al. Association of genetic variants in ANGPT/TEK and VEGF/VEGFR with progression and survival in head and neck squamous cell carcinoma treated with radiotherapy or radiochemotherapy. Cancers (Basel). (2020) 12:1506. doi: 10.3390/cancers12061506
24. Marisi G, Petracci E, Raimondi F, Faloppi L, Foschi FG, Lauletta G, et al. ANGPT2 and NOS3 polymorphisms and clinical outcome in advanced hepatocellular carcinoma patients receiving sorafenib. Cancers (Basel). (2019) 11:1023. doi: 10.3390/cancers11071023
25. Dai C, Kuo S-J, Zhao J, Jin L, Kang L, Wang L, et al. Correlation between genetic polymorphism of angiopoietin-2 gene and clinical aspects of rheumatoid arthritis. Int J Med Sci. (2019) 16:331–6. doi: 10.7150/ijms.30582
26. Michalska-Jakubus M, Rusek M, Kowal M, Krasowska D. ANGPT1 and ANGPT2 polymorphisms in systemic sclerosis: ANGPT2 rs2442598 and rs3739390 are associated with disease susceptibility and diffuse subtype. Polish Arch Intern Med. (2021). doi: 10.20452/pamw.16121
27. Zmorzyński S, Popek-Marciniec S, Styk W, Wojcierowska-Litwin M, Korszeń-Pilecka I, Szudy-Szczyrek A, et al. The impact of the NOD2/CARD15 variant (3020insC) and PSMA6 polymorphism (-8C<G) on the development and outcome of multiple myeloma. BioMed Res Int. (2020) 2020:1–15. doi: 10.1155/2020/7629456
28. Ross FM, Avet-Loiseau H, Ameye G, Gutiérrez NC, Liebisch P, O'Connor S, et al. Report from the European Myeloma Network on interphase FISH in multiple myeloma and related disorders. Hematologica. (2012) 97:1272–7. doi: 10.3324/hematol.2011.056176
29. Ahmann GJ, Jalal SM, Juneau AL, Christensen ER, Hanson CA, Dewald GW, et al. A novel three-color, clone-specific fluorescence in situ hybridization procedure for monoclonal gammopathies. Cancer Genet Cytogenet. (1998) 101:7–11. doi: 10.1016/S0165-4608(97)00058-7
30. Dmoszyńska A, Chocholska S. Molecular biology methods in the diagnosis of multiple myeloma. Molecular Aspects Hematologic Malignancies. (2012), 443–9. doi: 10.1007/978-3-642-29467-9_27
31. Zmorzyński Sz, Popek-Marciniec S, Szudy-Szczyrek A, Wojcierowska-Litwin M, Korszeń-Pilecka I, Chocholska S, et al. The association of GSTT1, GSTM1, and TNF-α Polymorphisms with the risk and outcome in multiple myeloma. Front Oncol. (2019) 9:1056. doi: 10.3389/fonc.2019.01056
32. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2–ΔΔCT method. Methods. (2001) 25:402–8. doi: 10.1006/meth.2001.1262
33. Lin B, Pang Z. Stability of methods for differential expression analysis of RNA-seq data. BMC Genomics. (2019) 20:35. doi: 10.1186/s12864-018-5390-6
34. Tarazona S, García-Alcalde F, Dopazo J, Ferrer A, Conesa A. Differential expression in RNA-seq: A matter of depth. Genome Res. (2011) 21:2213–23. doi: 10.1101/gr.124321.111
35. Ribatti D, Vacca A. New insights in anti-angiogenesis in multiple myeloma. Int J Mol Sci. (2018) 19:2031. doi: 10.3390/ijms19072031
36. Hou J, Wei R, Qian J, Wang R, Fan Z, Gu C, et al. The impact of the bone marrow microenvironment on multiple myeloma (Review). Oncol Rep. (2019). doi: 10.3892/or.2019.7261
37. Terpos E, Anargyrou K, Katodritou E, Kastritis E, Papatheodorou A, Christoulas D, et al. Circulating angiopoietin-1 to angiopoietin-2 ratio is an independent prognostic factor for survival in newly diagnosed patients with multiple myeloma who received therapy with novel antimyeloma agents. Int J Cancer. (2012) 130:735–42. doi: 10.1002/ijc.26062
38. Hu W, Tang C-H, Chen H-T, Zhao J, Jin L, Kang L, et al. Correlations between angiopoietin-2 gene polymorphisms and lung cancer progression in a Chinese Han population. J Cancer. (2019) 10:2935–41. doi: 10.7150/jca.31134
39. Hu G-N, Wang Y, Tang C-H, Jin L-L, Huang B-F, Wang Q, et al. The impact of Angiopoietin-2 genetic polymorphisms on susceptibility for Malignant breast neoplasms. Sci Rep. (2022) 12:14522. doi: 10.1038/s41598-022-18712-9
40. Alexandrakis MG, Passam FH, Ganotakis ES, Sfiridaki K, Xilouri I, Perisinakis K, et al. The clinical and prognostic significance of erythrocyte sedimentation rate (ESR), serum interleukin-6 (IL-6) and acute phase protein levels in multiple myeloma. Clin Lab Hematol. (2003) 25:41–6. doi: 10.1046/j.1365-2257.2003.00492.x
41. Lust JA, Lacy MQ, Zeldenrust SR, Witzig TE, Moon-Tasson LL, Dinarello CA, et al. Reduction in C-reactive protein indicates successful targeting of the IL-1/IL-6 axis resulting in improved survival in early stage multiple myeloma. Am J Hematol. (2016) 91:571–4. doi: 10.1002/ajh.24352
42. Mallard J, Gagez AL, Baudinet C, Herbinet A, Maury J, Bernard PL, et al. C-reactive protein level: A key predictive marker of cachexia in lymphoma and myeloma patients. J Hematol. (2019) 8:55–9. doi: 10.14740/jh536
43. Saltarella I, Morabito F, Giuliani N, Terragna C, Omedè P, Palumbo A, et al. Prognostic or predictive value of circulating cytokines and angiogenic factors for initial treatment of multiple myeloma in the GIMEMA MM0305 randomized controlled trial. J Hematol Oncol. (2019) 12:4. doi: 10.1186/s13045-018-0691-4
44. Martinelli S, Kanduri M, Maffei R, Fiorcari S, Bulgarelli J, Marasca R, et al. ANGPT2 promoter methylation is strongly associated with gene expression and prognosis in chronic lymphocytic leukemia. Epigenetics. (2013) 8:720–9. doi: 10.4161/epi.24947
45. Chatzidavid S, Kontandreopoulou CN, Giannakopoulou N, Diamantopoulos PT, Stafylidis C, Kyrtsonis MC, et al. The role of methylation in chronic lymphocytic leukemia and its prognostic and therapeutic impacts in the disease: A systematic review. Kumar M Ed Adv Hematol. (2024) 2024:1–38. doi: 10.1155/2024/1370364
46. Umezu T, Tadokoro H, Azuma K, Yoshizawa S, Ohyashiki K, Ohyashiki JH. Exosomal miR-135b shed from hypoxic multiple myeloma cells enhances angigenesis by targeting factor-inhibiting HIF-1. Blood. (2014) 124:3748–59. doi: 10.1182/blood-2014-05-576116
47. Sun C-Y, She X-M, Qin Y, Chu Z-B, Chen L, Ai L-S, et al. miR-15a and miR-16 affect the angigenesis of multiple myeloma by targeting VEGF. Carcinogenesis. (2012) 34:426–35. doi: 10.1093/carcin/bgs333
48. He T, Qi F, Jia L, Wang S, Song N, Guo L, et al. MicroRNA-542-3p inhibits tumor angiogenesis by targeting Angiopoetin-2. J Pathol. (2014) 232:499–508. doi: 10.1002/path.4324
49. Diao H, Xu X, Zhao B, Yang G. miR=135a-5p inhibits tumor invasion by targeting ANGPT2 in gallbladder cancer. Mol Med Rep. (2021) 24:528. doi: 10.3892/mmr.2021.12167
50. Ito T, Ando H, Suzuki T, Ogura T, Hotta K, Imamura Y, et al. Identification of primaryTarget of thalidomide teratogenicity. Science. (2010) 327:1345–50. doi: 10.1126/science.1177319
51. Sartarella I, Altamura C, Campanale C, Laghetti P, Vacca A, Frassanito MA, et al. Anti-angiogenic acitivity of drugs in multiple myeloma. Cancers. (2023) 15:1990. doi: 10.3390/cancers15071990
52. Kumar S, Witzig TE, Dispenzieri A, Lacy MQ, Wellik LE, Fonseca R, et al. Effect of thalidomide therapy on bone marrow angiogenesis in multiple myeloma. Leukemia. (2004) 18:624–7. doi: 10.1038/sj.leu.2403285
53. Hatjiharissi E, Terpos E, Papaioannou M, Hatjileontis C, Kaloutsi V, Galaktidou G, et al. The combination of intermediate doses of Thalidomide and Dexamethasone reduces bone marrow micro-vessel density but not serum levels of angiogenic cytokines in patients with refractory/relapsed multiple myeloam, Hematol. Oncol. (2004) 22:159–68. doi: 10.1002/hon.738
54. Cury de Camargo Cury P, Higashi F, Fernandes Silva Zacchi F, Bacic Palhares R, Alvares Quero A, Silva Dia ALM, et al. Effect of thalidomide on bone marrow angiogenesis in multiple myeloma patients. Hematol Transfus Cell Ther. (2020) 42:159–63. doi: 10.1016/j.htct.2019.04.006
55. Abhijit S, Sarker K, Ghosh A, Mishra S, Sen S. Analog based design, synthesis, biological evaluation, and molecular docking of some thalidomide metabolites as selective cytotoxic and antiangiogenic agents against multiple myeloma. Russ J Bioorganic Chem. (2022) 48:115–24. doi: 10.1134/S1068162022010022
56. Peach ML, Beedie SL, Chau CH, Collins MK, Markolovic S, Luo W, et al. Antiangiogenic activity and in silico cereblon binding analysis of novel thalidomide analogs. Molecules. (2020) 25:5683. doi: 10.3390/molecules25235683
57. Wang L, Wang S, Xue A, Shi J, Zheng C, Huang Y. Thalidomide inhibits angiogenesis via downregulation of VEGF and angiopoietin-2 in crohn’s disease. Inflammation. (2021) 44:795–807. doi: 10.1007/s10753-020-01378-8
58. Reale A, Melaccio A, Lamanuzzi A, Saltarella I, Dammacco F, Vacca A, et al. Functional and biological role of endothelial precursor cells in tumor progression: A new potential therapeutic target in haematological Malignancies. Stem Cells Int. (2016) 2016:7954580. doi: 10.1155/2016/7954580
59. Fan F, Malvestiti S, Vallet S, Lind J, Garcia-Manteiga JM, Morelli E, et al. JunB is a key regulator of multiple myeloma bone marrow angiogenesis. Leukemia. (2021) 35:3509–25. doi: 10.1038/s41375-021-01271-9
60. Bhaskar A, Gupta R, Vishnubhatla S, Kumar L, Sharma A, Sharma MC, et al. Angiopoietins as biomarker of disease activity and response to therapy in multiple myeloma. Leukemia&Lymphoma. (2012) 54:1473–8. doi: 10.3109/10428194.2012.745523
61. Oliner J, Min H, Leal J, Yu D, Rao S, You E, et al. Supresion of angiogenesis and tumor growth by selective inhibition of angiopoietin-2. Cancer Cell. (2004) 6:507–16. doi: 10.1016/j.ccr.2004.09.030
62. Rao L, Giannico D, Leone P, Giovanni Solimando A, Maiorano E, Caporusso C, et al. HB-EGF-EGFR signaling in bone marrow endothelial cells mediates angiogenesis associated with multiple myeloma. Cancers. (2020) 12:173. doi: 10.3390/cancers12010173
63. Kienast Y, Klein C, Scheuer W, Raemsch R, Lorenzon E, Bernicke D, et al. Ang-2-VEGF-A crossMab, a nowel bispecific human igG1 antibody blocking VEGF-A and ang-2 functions simultaneously, mediates potent antitumor, atiangiogenic, and antimetastatic efficacy. Clin Cancer Res. (2013) 19:6730–41. doi: 10.1158/1078-0432.CCR-13-0081
64. Heil F, Babitzki G, Julien-Laferriere A, Ooi C-H, Hidalgo M, Massard C, et al. Vanucizumab mode of action: serial biomarkers in plasma, tumor, and skin-wound-healing biopsies. Transl Oncol. (2021) 14:100984. doi: 10.1016/j.tranon.2020.100984
65. Hidalgo M, Martinez-Gracia M, Le Tourneau C, Massard C, Garralda E, Boni V, et al. First-in-human phase I study of single-agent vanucizumab, A first-in class bispecific anti-angiopoietin-2/anti-VEGF-A antibody, in adult patients with advanced solid tumors. Clin Canc Res. (2018) 24:1536–6. doi: 10.1158/1078-0432.CCR-17-1588
Keywords: hematology malignancies, plasma cell, angiogenesis, polymorphisms, thalidomide, bortezomib
Citation: Popek-Marciniec S, Styk W, Chocholska S, Szudy-Szczyrek A, Sidor K, Swiderska-Kolacz G, Hus M, Czerwik-Marcinkowska J and Zmorzynski S (2025) Associations of ANGPT2 expression and its variants (rs1868554 and rs7825407) with multiple myeloma risk and outcome. Front. Oncol. 15:1468373. doi: 10.3389/fonc.2025.1468373
Received: 21 July 2024; Accepted: 17 February 2025;
Published: 06 March 2025.
Edited by:
Cyrus Khandanpour, Klinik für Hämatologie und Onkologie, GermanyReviewed by:
Antonio Giovanni Solimando, University of Bari Aldo Moro, ItalyCopyright © 2025 Popek-Marciniec, Styk, Chocholska, Szudy-Szczyrek, Sidor, Swiderska-Kolacz, Hus, Czerwik-Marcinkowska and Zmorzynski. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sylwia Popek-Marciniec, c3lsd2lhLnBvcGVrLW1hcmNpbmllY0Bha2FkZW1pYXphbW9qc2thLmVkdS5wbA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.